方案详情
文
比较有机海藻生产的营养来源对糖藻孵化阶段发育的影响Comparing effects of nutrient sources approved for organic seaweed production on hatchery stage development of sugar kelp, Saccharina latissima 使用格哈特Laboshake振荡器振荡海藻孢子
方案详情

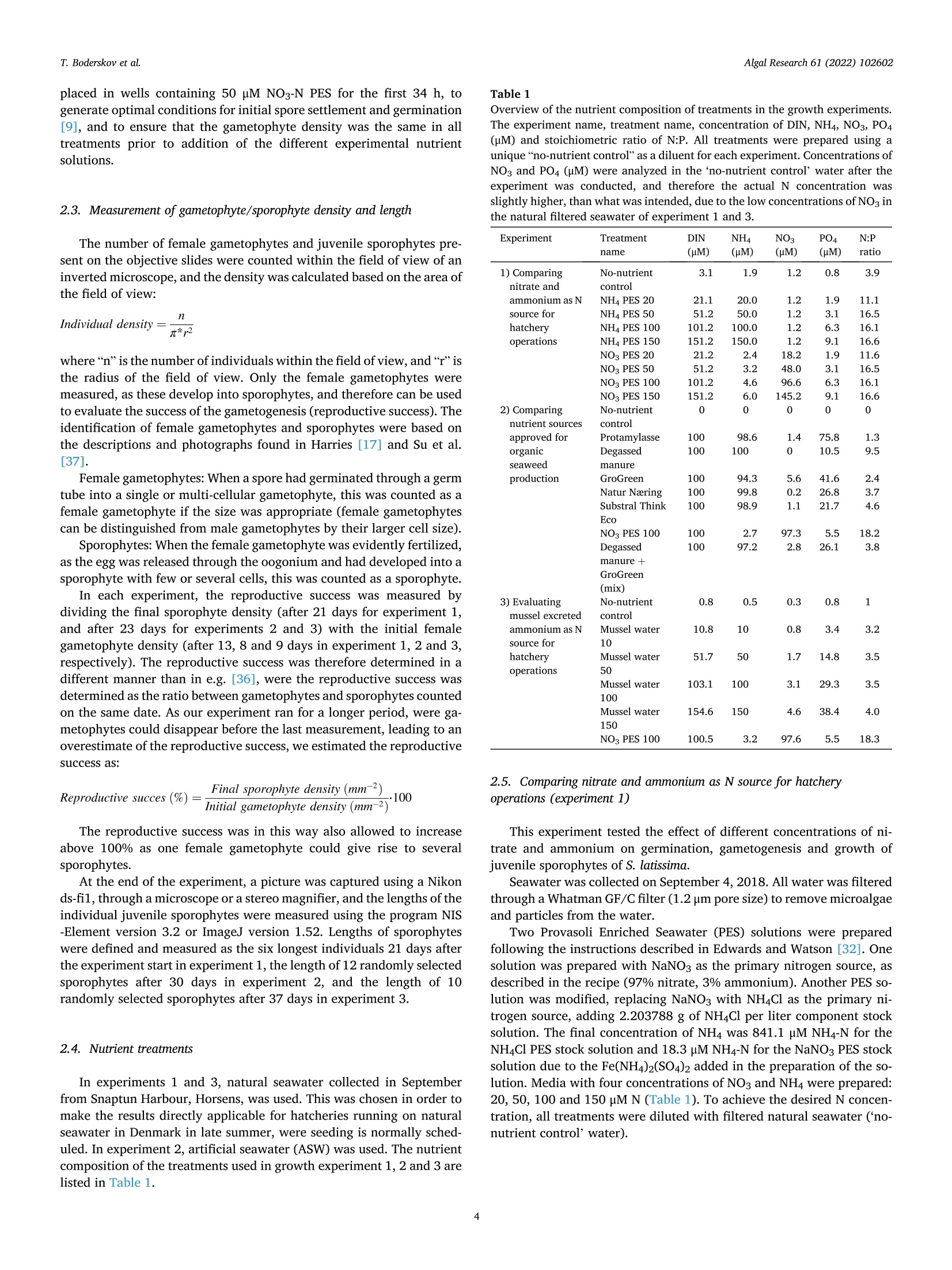
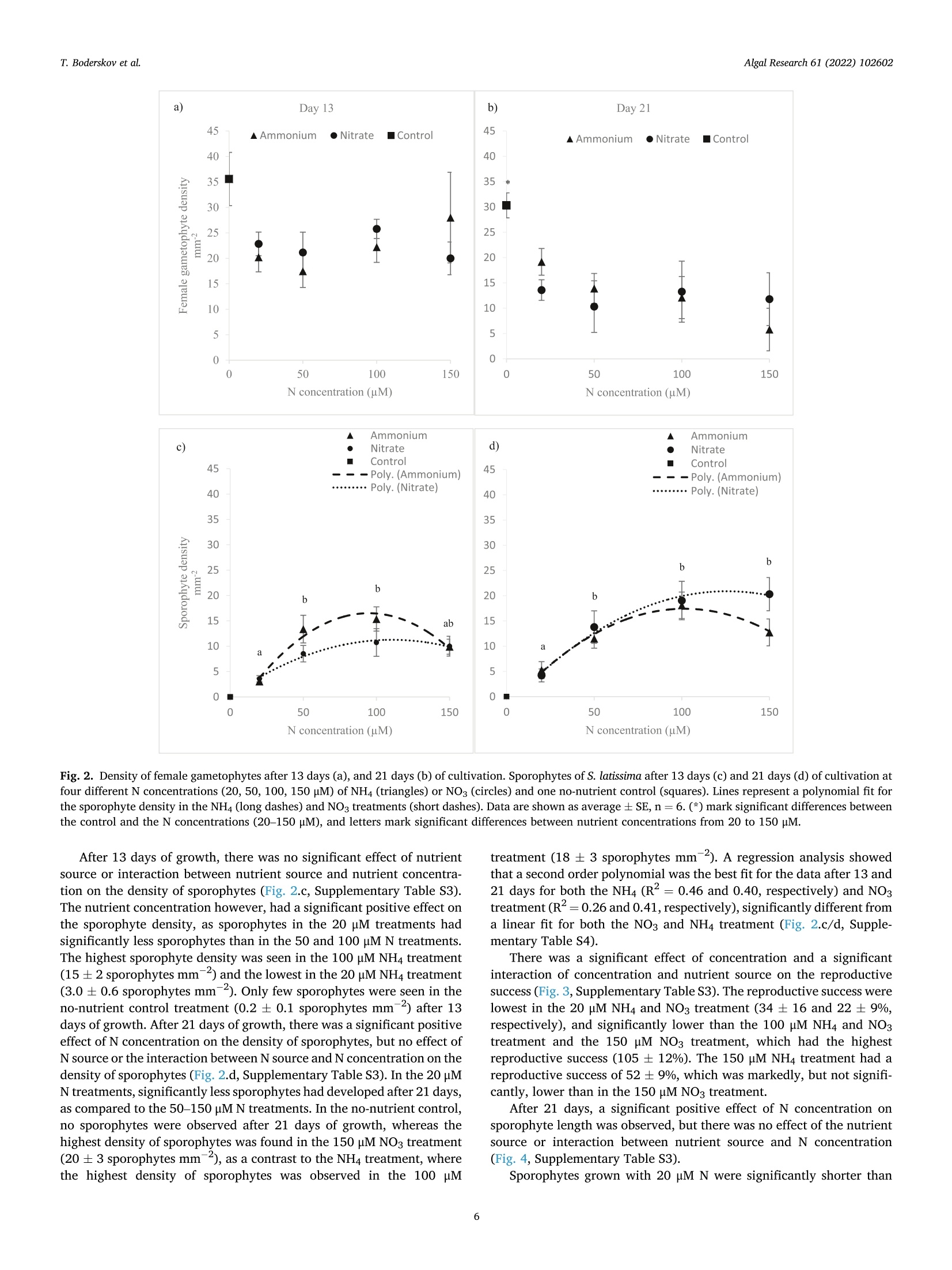
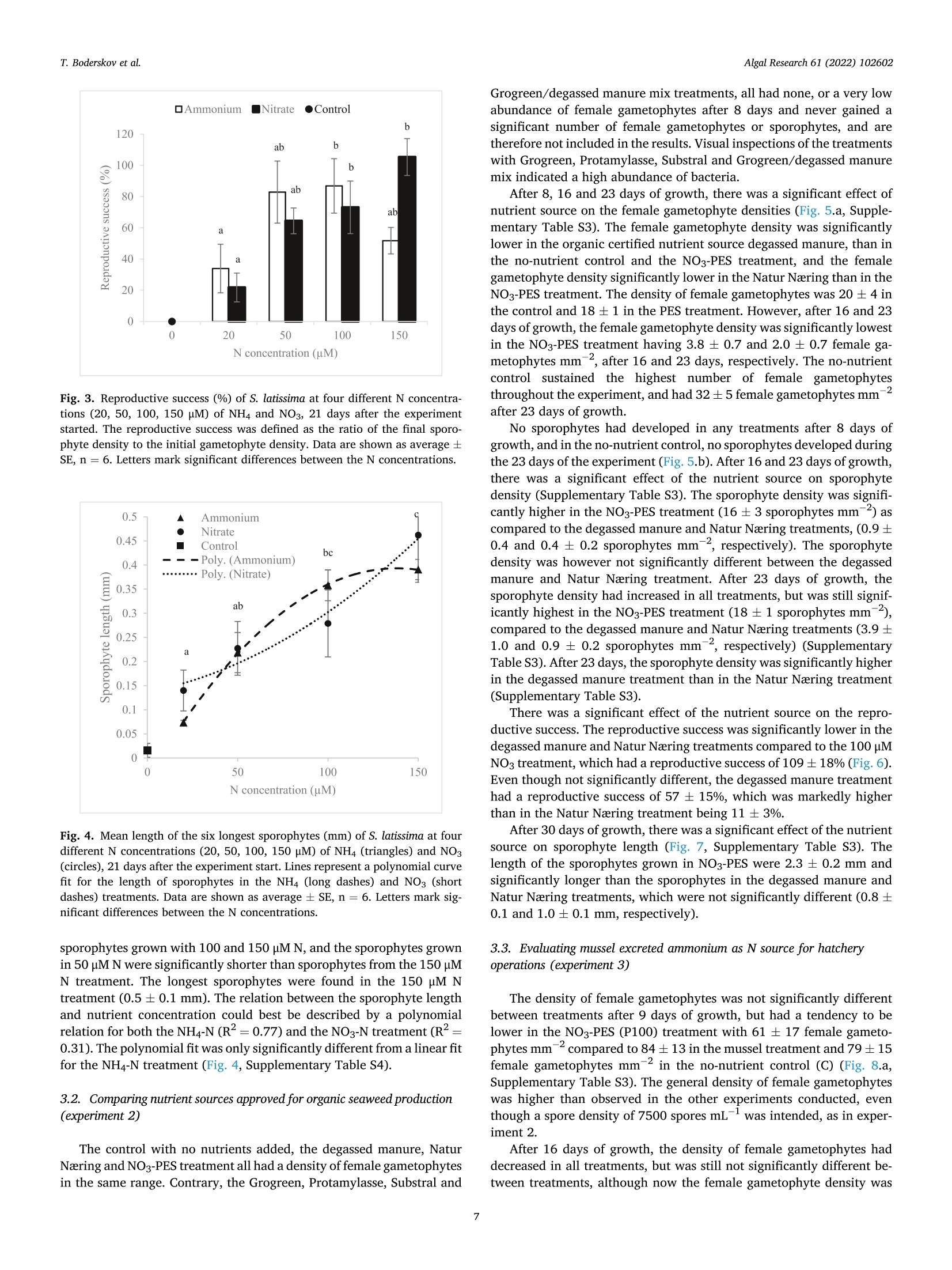
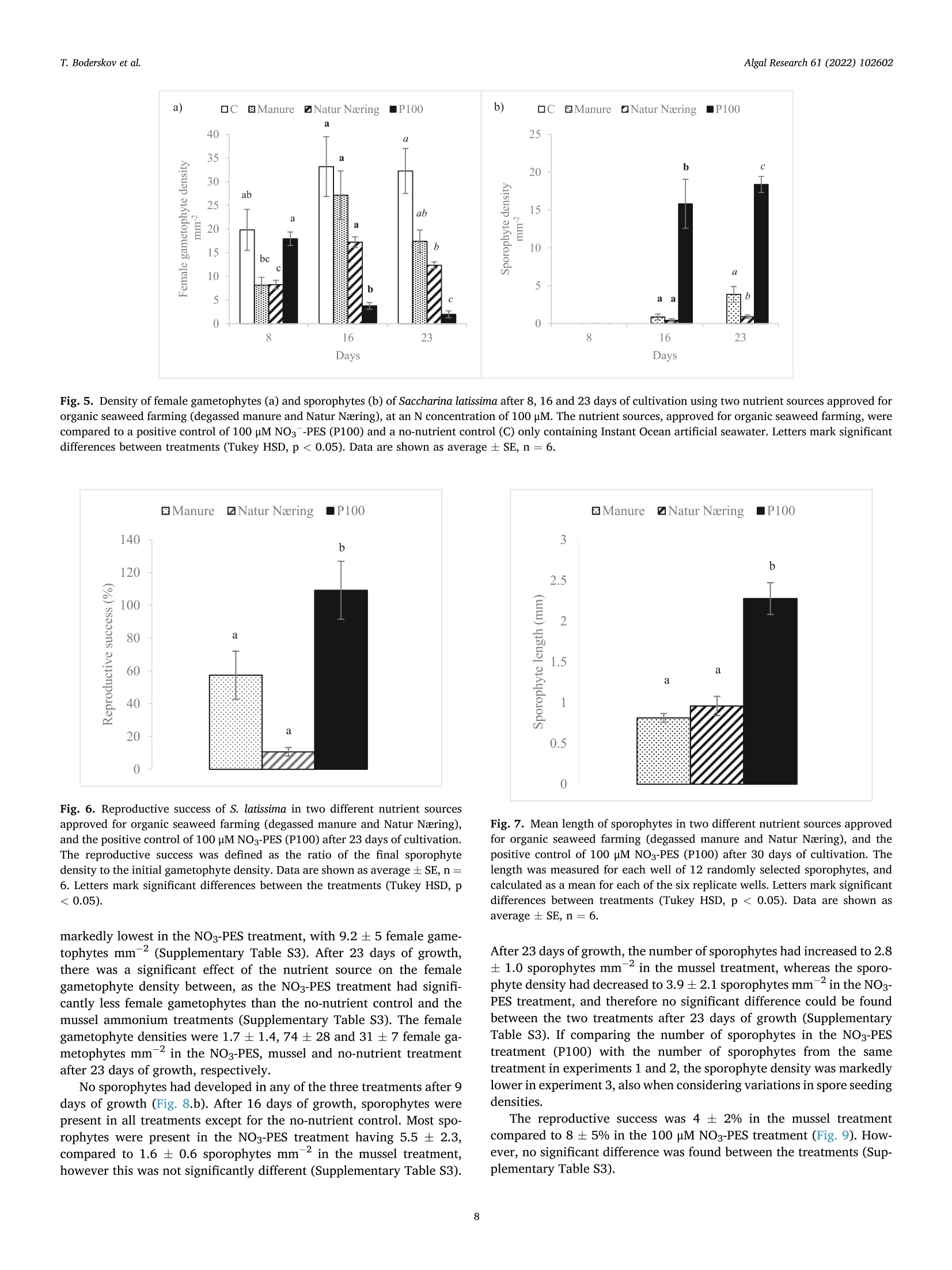
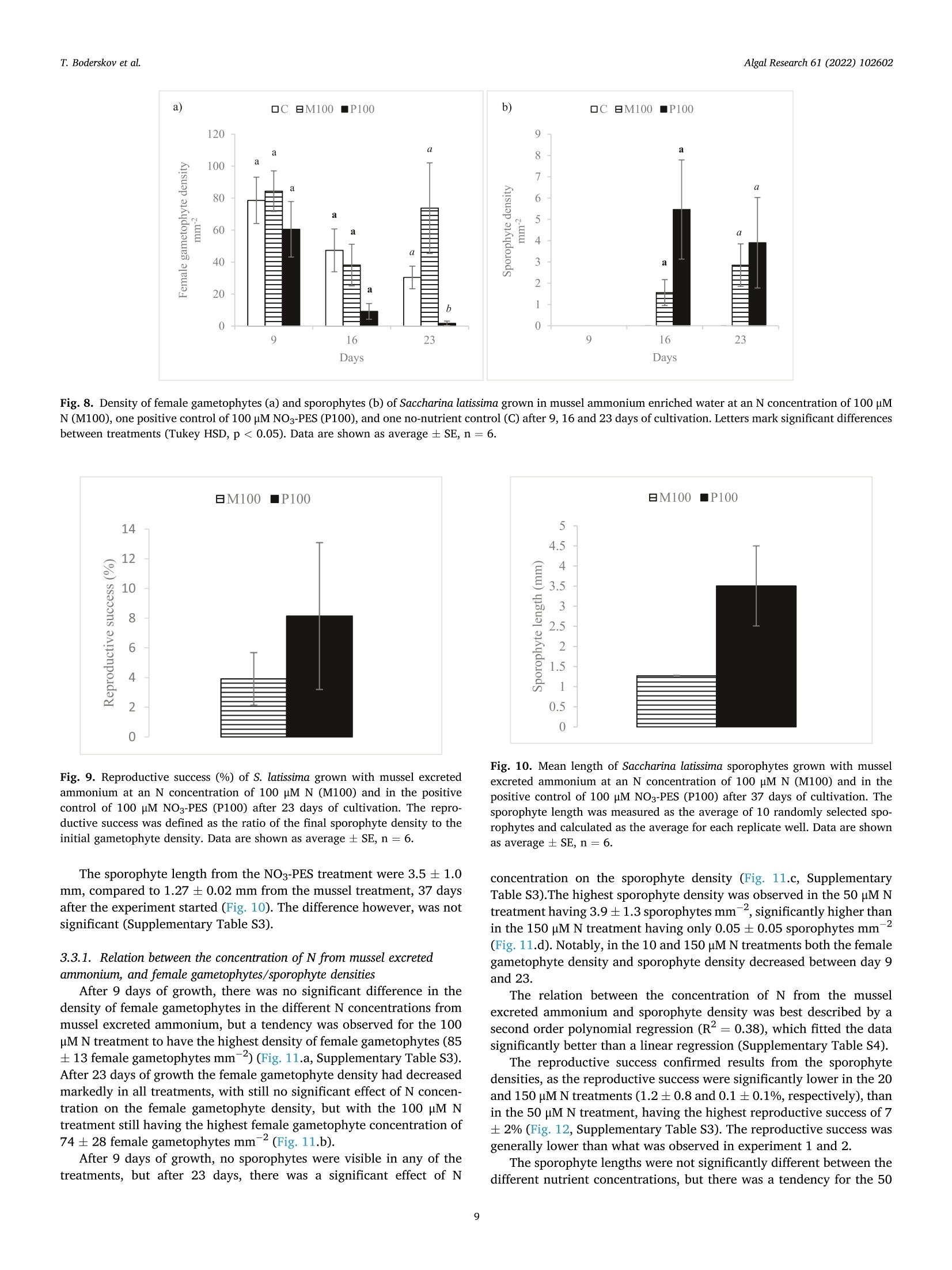
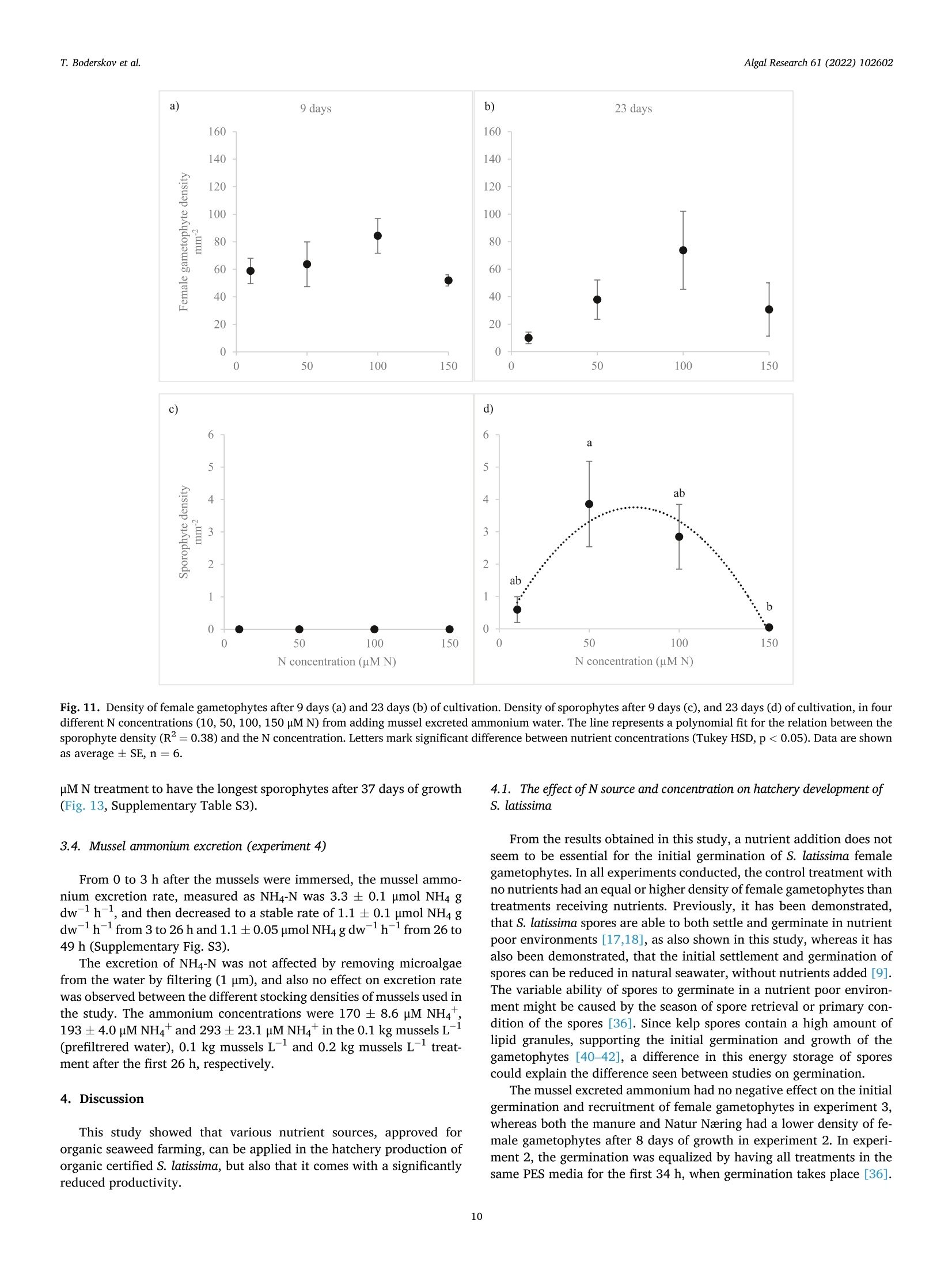
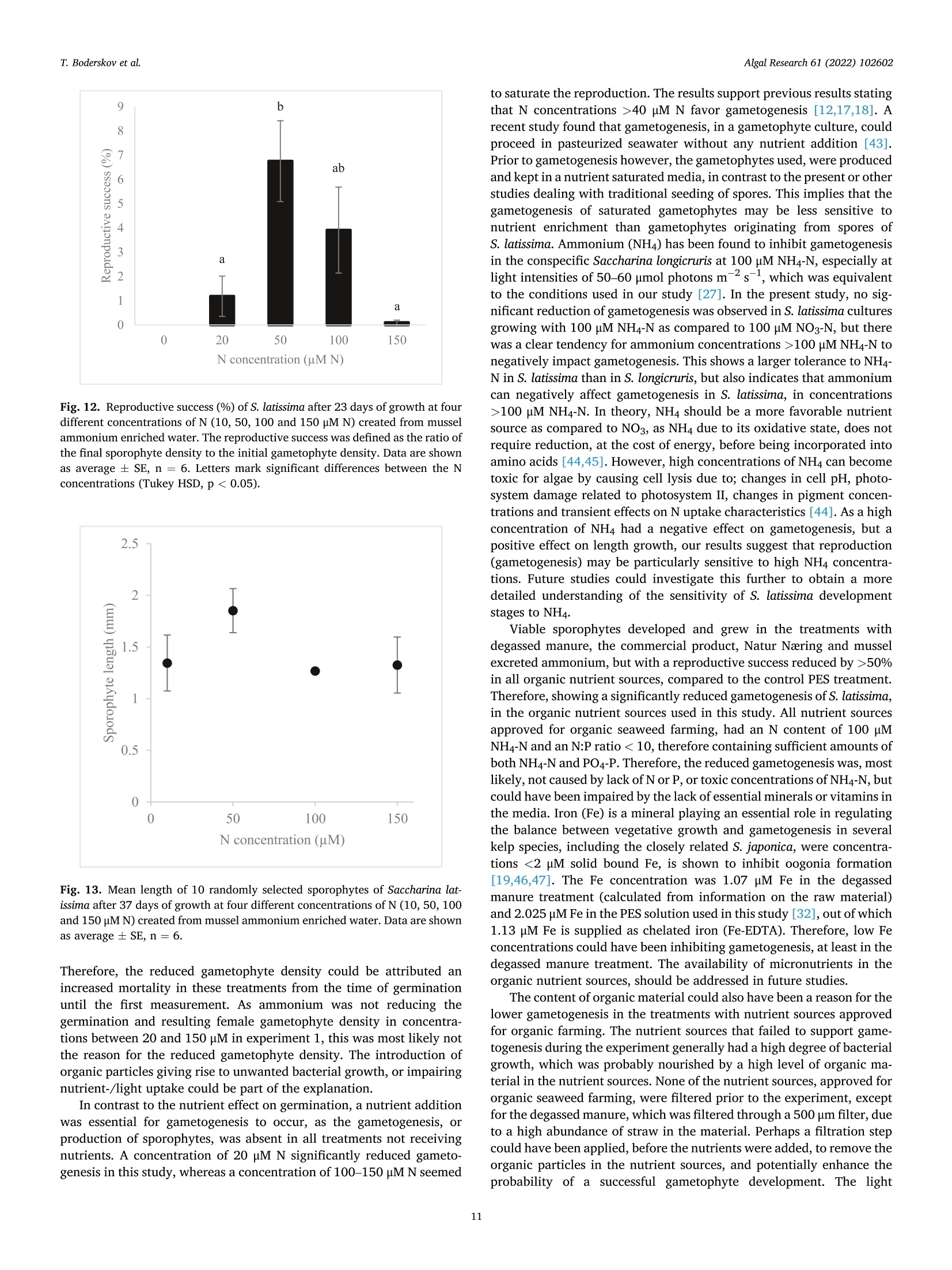
比较有机海藻生产的营养来源对糖藻孵化阶段发育的影响Comparing effects of nutrient sources approved for organic seaweed production on hatchery stage development of sugar kelp, Saccharina latissima使用格哈特Laboshake振荡器振荡海藻孢子Algal Research 61 (2022) 102602Contents lists available at ScienceDirectAlgal Research Algal Research 61 (2022) 102602T. Boderskov et al. journal homepage: www.elsevier.com/locate/algal 比较有机海藻生产的营养来源对糖藻孵化阶段发育的影响 Comparing effects of nutrient sources approved for organic seaweed production on hatchery stage development of sugar kelp, Saccharina latissima Teis Boderskov a ,b ,c ,*, Michael Bo Rasmussen a ,b , Christian Hein Cassard a , Julie Svensgaard d , Laurids N ø rskov Enevoldsen a , Annette Bruhn a ,b a Aarhus University, Department of Ecoscience, Vejls ø vej 25, Silkeborg, Denmark b Centre for Circular Bioeconomy, Aarhus University (CBIO), Denmark c Hjarn ø Havbrug A/S, Snaptunvej 57B, 7130 Juelsminde, Denmark d Copenhagen University, Ole Maal ø es Vej 5, 2200 Copenhagen, Denmark 丹麦奥尔胡斯大学生态科学系丹麦奥尔胡斯大学循环生物经济中心 丹麦哥本哈根大学 Keywords:Degassed manure Mussel ammonia excretion Ammonium Gametogenesis Sporophyte development Organic fertilizers A B S T R A C T The demand for organic certifeid seaweed is growing in European countries, but only few studies have inves-tigated how nutrient sources approved for organic seaweed farming affect the growth and development of macroalgae. Through a series of four experiments, this study documented the effects of nitrogen (N) source and concentration on the key hatchery processes of meiospore germination, gametogenesis and initial sporophyte growth of Saccharina latissima . The nutrient sources were Provasoli Enriched Seawater (PES) with NO3-N or NH4-N and six potential organic certifeid growth media with N predominantly as NH4-N, all with N concentrations up to 150 u M N. The potential organic certifeid N sources were: Degassed manure, protamylasse, three commercial liquid fertilizers and mussel excreted ammonium. In contrast to gametogenesis and initial sporophyte growth, the germination of S. latissima meiospores was generally not affected by neither N source nor concentration. Gametogenesis was the process being most sensitive to N source and concentration: In PES media, the N con-centration had an overall positive effect on gametogenesis, albeit at concentrations of 150 u M NH4, gameto-genesis was impaired. The degassed manure, one liquid fertilizer and mussel excreted ammonium supported successful gametogenesis and sporophyte growth, but resulted in a 4.7, 20 and 1.3 fold lower sporophyte density after 23 days of growth, respectively, as compared to cultures growing with NO3-PES. Optimum N concentrations were 50–100 u M N. We conclude that degassed manure and mussel excreted ammonium in concentrations <100u M NH4-N, are useful, but suboptimal N sources, for an organic hatchery production of S. latissima . 1. Introduction Seaweed aquaculture has expanded globally from a production of 10.6 million tonnes fresh weight (FW) in year 2000 to a production of 32.4 million tonnes FW in year 2018, with 99% of the production in Asia, compared to an estimated <600 t FW production in Europe in 2020[1–3]. Seaweeds are a well-established part of the human diet in Asia, and worldwide 77.6% of the produced seaweed was used for human consumption in 2018 [2]. In Europe, the species most produced is Sac-charina latissima (Linnaeus) C.E.Lane, C.Mayes, Druehl & G.W.Saunders [1]. Although the main market for seaweed remains in Asia, there is a growing interest for seaweed products in Europe. Parallel to the increase in retail sales of organic food products in Europe from a turnover of 7billion Euros in 2000 to 40.7 billion Euros in 2018 [4], the demand for organic certifeid seaweed products is increasing in Europe [5]. When producing or selling organic seaweed in Europe, the producer needs to comply with the EU regulation 2018/848 [6] and 889/2008[7], in which two of the most important demands for the production are, that an organic seaweed producer cannot: 1) use conventional fertilizers in the hatchery, and 2) discharge nutrient enriched water from the hatchery. When producing seaweed in hatcheries, only limited amounts of nutrients are needed, but when producing organic certifeid seaweed in Europe, the producer is limited to using specifci nutrients listed in annex 1 of the EU regulation 889/2008, or alternatively excluding the * Corresponding author at: Aarhus University, Department of Ecoscience, Vejls ø vej 25, Silkeborg, Denmark. E-mail address: tebo@ecos.au.dk (T. Boderskov). 2211-9264/© 2021 The Authors. Published by Elsevier B.V. This is an open access article under the CCBY license (http://creativecommons.org/licenses/by/4.0/). use of external nutrients in the hatchery and relying on the nutrients present in the ambient seawater. Addition of fertilizers is needed for a fast and effciient production of seeding materials in a hatchery producing S. latissima [8,9]. The pres-ence of dissolved inorganic N (DIN) can be limited in natural seawater, with concentrations <1 u M N, depending on location and time of year [10,11], and therefore the growth and reproduction of S. latissima can be restricted in a hatchery based on using only natural seawater with no nutrient addition [9,12–14]. Brinkhuis et al. [56] and R o o ß ner et al. [14] found a reduced, but successful, reproduction of S. latissima in ambient seawater, whereas for instance in Norway, the use of external nutrients can be omitted, where nutrient rich bottom water can be pumped into the hatchery from depths below the pycnocline [16]. Sites that do not have access to nutrient rich water during the hatchery period, would depend on external nutrient sources to complete a successful hatchery operation. While S. latissima meiospores (spores) readily germinate in an environment without nitrate (NO3) and phosphate (PO4), the develop-ment of gametophytes and gametogenesis, as well as the morphology of gametophytes, depend on the presence and concentration of these two essential macronutrients [17,18]. Without NO3 and PO4, the female gametophytes only develop few cells and are unable to produce oogonia, whereas the male gametophytes, to a limited extend, are capable of producing antheridia, when just one of the two macronutrients is pre-sent in the right quantity [18]. Harries [17] documented, that especially nitrogen (N) is a limiting factor for growth and gametogenesis of S. latissima gametophytes. Hsiao [18] found that the development of oogonia and antheridia was positively related to the concentration of NO3 and PO4 up to a concentration of 42 u M NO3 and 0.4 p M PO4, after which increasing concentrations had a negative effect on the develop-ment. However, later studies have shown that PO4 concentrations up to 23 u M PO4 can be benefciial for the growth of S. latissima gametophytes [12]. Another consideration when operating an organic seaweed hatchery is, that the seawater discharged from the hatchery, cannot contain more nutrients (regulated as DIN in DK) than the intake water. In a conven-tional hatchery operation, the nutrient concentrations are kept at a level saturating growth and development (typically >400 u M N) regardless of maintaining gametophyte cultures or operating a traditional sporophyte seedling production from spores [8,32]. Therefore, excess nutrients will be present in the cultivation water, and will need to be removed before discharging the water from an organic certifeid hatchery. The process of nutrient removal would require e.g. an additional land-based production of macroalgae to take up the excess nutrients or a sewage treatment system, which in either case would demand for additional space and operational costs. As an alternative to the addition and subsequent removal of external nutrients, fliter-feeding organisms such as bivalves could be co-cultivated in the hatchery. Filter-feeders, such as mussels, are known to positively affect the growth of macroalgae beds [55] partly by their ability to transform dissolved organic N (DON) and P (DOP) to DIN and DIP. Mussels excrete nitrogenous waste products mainly as un-ionized soluble ammonia (NH3) and ammonium ions (NH43) [54], which dissociate in the surrounding water to achieve an equilibrium, determined by environmental factors such as temperature, salinity and pH [33]. In marine waters with a typical pH of 8.1, 97.7% of the total ammonia (TA) will be in the form of ammonium, NH4m , which can be assimilated by S. latissima , and only 2.3% of the TA will be present as ammonia, NH3 [34]. Hence, in the following, ammonia-N (NH3/NH4N) will be addressed as NH4-N. Mussel excretion rates of TA varies from 0.1–1 u mol TA g dry weight (dw)- 1 h - 1 depending on temperature, but can increase up to 2.5 u mol TA g dw - 1 h - 1 in a post-harvest situation where the mussels have been stored without water [35,52]. The co-cultivation of mussels and seaweed has been tested before in a single study by R o o ß ner et al. [14] demonstrating that co-cultivation of mussels with S. latissima seeded lines advanced the abundancy of sporophytes produced as compared to a no-nutrient control [14]. This study by R o o ß ner et al. [14] is presently the only study investigating the effect of nutrient sources, approved for organic seaweed farming, on the hatchery production of S. latissima , and to our knowledge also other macroalgae species. When considering the large increase in demand for organic certifeid seaweed, there is a need to optimize and develop the organic hatchery production of kelp. The aims of this study were to compare the effect on three different life stages, spore germination, gametogenesis and early sporophyte growth of S. latissima , of 1) the two inorganic N sources, NO3 and NH4, and 2) N sources, approved for organic seaweed production, and 3) mussel excreted ammonium. We hypothesized that gametogene is of S. latissima sporophytes would be inhibited by ammonium concentrations higher than 100 u M, in accordance with what Yarish et al. [27] found for gametophytes of Saccharina longicruris , and that the nutrient sources approved for organic seaweed farming, and mussel excreted ammonium, would result in an impaired gametogenesis and a slower growth of S. latissima as compared to growth with growth media specifcially developed for optimal growth of algae, such as Provasolis Enriched Solution (PES). 2. Materials and methods This study is composed of three experiments with the purpose of testing the effect of organic certifeid sources of nutrients on three different early developmental stages of S. latissima ; spore germination, gametogenesis, and early sporophyte growth. We also tested the use of mussel excreted NH4 as a nutrient source, for which an additional experiment was included in order to evaluate and control the rate of mussel ammonium excretion. All experiments were inspired by the setup described by Lee and Brinkhuis [36], but conducted with modifciations as described in detail in the following. Key parameters regarding seeding and measurements differed between the three growth experiments, as described in the following and summarized in Table S1. Due to the Covid-19 pandemic, local restrictions interfered with the planned measurement program of experiment 1, and therefore the densities of female gametophytes and sporophytes were not measured after 8 or 9 days, but only after 13 and 21 days in this experiment. 2.1. Sporophyte collection and spore release Adult and intact sporophytes of S. latissima were collected in Mid-delfart, Denmark (55°30'33.7""N, 9°43'12.4E) at 1–3 m depth on March 9 2018, February 19 2019 and March 5 2020 (Fig. 1). Following collection, the fertile blades were transported to the lab, in a dark plastic bag without water, and kept in seawater for up to 17 days at 10 °C and 100 u mol m - 2 s - 1 using a 8:16 light:dark cycle before the meiospores (spores) were released. Sori from more than three different fertile sporophytes were excised, cleaned thoroughly with artifciial seawater and dried with paper towel to remove epiphytes as described in Edwards and Watson [32]. The clean and dry sori were stored at 10 °C between layers of paper towel in trays covered with a layer of plastic to prevent desiccation. The following day, the sori were rehydrated in 10 °C seawater to induce the release of spores (in ambient light). The solution of spores was passed through a 41 u m fliter to remove larger tissue fragments/other larger organisms in order to minimize contamination at later stages. The density of spores was determined by counting spores in a Thoma haemocytometer (0.1 mm depth, 0.0025 mm2 Hawksley, UK). 2.2. General experimental setup The extracted spores were added to six well plates, achieving the specifci concentration of spores required in each experiment (Supple-mentary Table S1) to reach a fnial water volume of 10 mL per well. The six-well plates were placed in a randomized design (randomized at the beginning of each experiment), to account for minor light and temper-ature differences, on a shaker table (Gerhardt, Laboshake RO 300/08) at 100–150 rpm (Supplementary Fig. S1). Within each well, a 22 22 mm square glass coverslip was placed for adhesion of spores. The well plates were provided light with an average irradiance of 50–60 u mol photons m - 2 s - 1 in a 16:8 light:dark photoperiod, and at a constant temperature of 10 °C. In experiment 1, all treatments were exposed to light straight after seeding, whereas in experiment 2 and 3, the coverslips were placed in darkness for the frist 34 h before being placed in the respective treat-ments receiving light. Further, in experiment 2, the coverslips were Fig. 1. Intact and adult sporophytes for all experiments were collected in Little Belt at Middelfart Old Harbour, Denmark. The site is indicated with a black circle. placed in wells containing 50 u M NO3-N PES for the frist 34 h, to generate optimal conditions for initial spore settlement and germination [9], and to ensure that the gametophyte density was the same in all treatments prior to addition of the different experimental nutrient solutions. 2.3. Measurement of gametophyte/sporophyte density and length The number of female gametophytes and juvenile sporophytes pre-sent on the objective slides were counted within the feild of view of an inverted microscope, and the density was calculated based on the area of the feild of view: n Individual density =元 *r 2 Female gametophytes: When a spore had germinated through a germ tube into a single or multi-cellular gametophyte, this was counted as a female gametophyte if the size was appropriate (female gametophytes can be distinguished from male gametophytes by their larger cell size). Sporophytes: When the female gametophyte was evidently fertilized, as the egg was released through the oogonium and had developed into a sporophyte with few or several cells, this was counted as a sporophyte. In each experiment, the reproductive success was measured by dividing the fnial sporophyte density (after 21 days for experiment 1, and after 23 days for experiments 2 and 3) with the initial female gametophyte density (after 13, 8 and 9 days in experiment 1, 2 and 3, respectively). The reproductive success was therefore determined in a different manner than in e.g. [36], were the reproductive success was determined as the ratio between gametophytes and sporophytes counted on the same date. As our experiment ran for a longer period, were ga-metophytes could disappear before the last measurement, leading to an overestimate of the reproductive success, we estimated the reproductive success as: The reproductive success was in this way also allowed to increase above 100% as one female gametophyte could give rise to several sporophytes. At the end of the experiment, a picture was captured using a Nikon ds-f1i, through a microscope or a stereo magnifeir, and the lengths of the individual juvenile sporophytes were measured using the program NIS -Element version 3.2 or ImageJ version 1.52. Lengths of sporophytes were defnied and measured as the six longest individuals 21 days after the experiment start in experiment 1, the length of 12 randomly selected sporophytes after 30 days in experiment 2, and the length of 10randomly selected sporophytes after 37 days in experiment 3. 2.4. Nutrient treatments In experiments 1 and 3, natural seawater collected in September from Snaptun Harbour, Horsens, was used. This was chosen in order to make the results directly applicable for hatcheries running on natural seawater in Denmark in late summer, were seeding is normally sched-uled. In experiment 2, artifciial seawater (ASW) was used. The nutrient composition of the treatments used in growth experiment 1, 2 and 3 are listed in Table 1. Table 1 Overview of the nutrient composition of treatments in the growth experiments. The experiment name, treatment name, concentration of DIN, NH4, NO3, PO4(u M) and stoichiometric ratio of N:P. All treatments were prepared using a unique “no-nutrient control ” as a diluent for each experiment. Concentrations of NO3 and PO4 (u M) were analyzed in the ‘no-nutrient control ’ water after the experiment was conducted, and therefore the actual N concentration was slightly higher, than what was intended, due to the low concentrations of NO3 in the natural flitered seawater of experiment 1 and 3. Experiment Treatment DIN NH4 NO3 PO4 N:P name (DM) (HM) (NM) (PM) ratio 1) Comparing No-nutrient 3.1 1.9 1.2 0.8 3.9 nitrate and control ammonium as N NH4 PES 20 21.1 20.0 1.2 1.9 11.1 source for NH4 PES 50 51.2 50.0 1.2 3.1 16.5 hatchery NH4 PES 100 101.2 100.0 1.2 6.3 16.1 operations NH4 PES 150 151.2 150.0 1.2 9.1 16.6 NO3 PES 20 21.2 2.4 18.2 1.9 11.6 NO3 PES 50 51.2 3.2 48.0 3.1 16.5 NO3 PES 100 101.2 4.6 96.6 6.3 16.1 NO3 PES 150 151.2 6.0 145.2 9.1 16.6 2) Comparing No-nutrient 0 0 0 0 0 nutrient sources control approved for Protamylasse 100 98.6 1.4 75.8 1.3 organic Degassed 100 100 0 10.5 9.5 seaweed manure production GroGreen 100 94.3 5.6 41.6 2.4 Natur Næring 100 99.8 0.2 26.8 3.7 Substral Think 100 98.9 1.1 21.7 4.6 Eco NO3 PES 100 100 2.7 97.3 5.5 18.2 Degassed 100 97.2 2.8 26.1 3.8 manure 十 GroGreen (mix) 3) Evaluating No-nutrient 0.8 0.5 0.3 0.8 1 mussel excreted control ammonium as N Mussel water 10.8 10 0.8 3.4 3.2 source for 10 hatchery Mussel water 51.7 50 1.7 14.8 3.5 operations 50 Mussel water 103.1 100 3.1 29.3 3.5 100 Mussel water 154.6 150 4.6 38.4 4.0 150 NO3 PES 100 100.5 3.2 97.6 5.5 18.3 2.5. Comparing nitrate and ammonium as N source for hatchery operations (experiment 1) This experiment tested the effect of different concentrations of ni-trate and ammonium on germination, gametogenesis and growth of juvenile sporophytes of S. latissima . Seawater was collected on September 4, 2018. All water was flitered through a Whatman GF/C fliter (1.2 u m pore size) to remove microalgae and particles from the water. Two Provasoli Enriched Seawater (PES) solutions were prepared following the instructions described in Edwards and Watson [32]. One solution was prepared with NaNO3 as the primary nitrogen source, as described in the recipe (97% nitrate, 3% ammonium). Another PES so-lution was modifeid, replacing NaNO3 with NH4Cl as the primary ni-trogen source, adding 2.203788 g of NH4Cl per liter component stock solution. The fnial concentration of NH4 was 841.1 u M NH4-N for the NH4Cl PES stock solution and 18.3 p M NH4-N for the NaNO3 PES stock solution due to the Fe(NH4)2(SO4)2 added in the preparation of the so-lution. Media with four concentrations of NO3 and NH4 were prepared:20, 50, 100 and 150 u M N (Table 1). To achieve the desired N concen-tration, all treatments were diluted with flitered natural seawater (‘no-nutrient control ’ water). 2.6. Comparing nutrient sources approved for organic seaweed production (experiment 2) This experiment tested the effect of fvie nutrient sources, approved for organic seaweed farming, on gametogenesis and growth of juvenile sporophytes of S. latissima . The germination phase was equal between treatments in this experiment, as all experiments had the same nutrient source during the germination phase, as previously stated. All experimental treatments used in experiment 2, were based on artifciial seawater (ASW), and hence the water was not flitered for this experiment. The concentration of NH4, NO3 and PO4of all nutrient sources were analyzed prior to the experiment (Supplementary Table S2). The growth media had different colors, as the nutrient sources con-tained varying contents of organic material (Supplementary Fig. S2). The darkest appearing growth media were the ones containing Gro-Green. The light extinction was not measured for the different nutrient treatments. Additional information on the degassed manure was provided by the company, Kroghsminde biogas, from an analysis made on Feb 52,018. From this analysis, the concentrations of Iron (Fe), Molybdenum (Mo), Cobalt (Co) and Sulphur (S) were determined in the raw material to be 106 mg Fe L - 1, 0.32 mg Mo L - 1, 0.068 mg Co L - 1 and 40 g S L - 1 giving concentrations of 1.08 u M Fe, 0.0019 u M Mo, 0.00065 u M Co and 708u M S in the fnial experimental treatment. 2.7. Evaluating mussel excreted ammonium as N source for hatchery operations (experiment 3) This experiment compared gametogenesis and growth of S. latissima sporophytes in a nutrient range made from mussel excreted ammonium. Four concentrations of ammonium were prepared based on water, from the mussel excretion experiment (experiment 4), following a gradient with increasing N concentration: 10, 50, 100 and 150 u M N (Table 1). To achieve the right N concentration, all treatments were diluted with natural flitered seawater from the no-nutrient control in the mussel excretion experiment. 2.8. Mussel ammonium excretion (experiment 4) To evaluate the potential of using the blue mussel (Mytilus edulis ) as a relevant provider of NH4 enriched water for a hatchery operation of S. latissima , an additional experiment was made evaluating the NH4excretion of mussels in different stocking densities. To test whether removing microalgae from the water was affecting the ammonium excretion, one of the treatments was made also in pre-flitered water. Mussels were collected September 24, 2018 at an organic certifeid production site in Horsens fjord owned by Hjarn ø Havbrug A/S, and kept for 22 h at 15 °C without water to promote the following excretion of NH4 [35]. After 22 h, the mussels were placed in 5 L buckets containing flitered (0.5 u m and UV-treated) or unflitered seawater at a stocking density of 0.2 or 0.1 kg mussels L - 1. The mussels had an average length of 5.4 0.13 cm and an average dry meat weight of 33.4 ±1.6 g (5.7%dry meat per live fresh weight). After 3 h, the water was renewed to evaluate the initial ammonium excretion rate, and again, after 26 h, the water was changed until the experiment was terminated after 49 h. Water samples were taken from each replicate bucket upon each water change. The NH4 concentrations were determined in all water samples. Concentrations of PO4 were only determined on the water used in experiment 3. The water used in growth experiment 3, was derived from treatment 1 (0.1 kg L - 1 and flitered seawater) after the initial 26 h of the experiment. 2.9. Water analysis The raw nutrient sources, used in growth experiment 2, was analyzed for NH4-N and PO4, according to Danish Standard number 224 and 291, respectively, using a Shimadzu 1800UV Spectrophotometer. NO3-N were analyzed according to the FOSS FIASTAR 5000 protocol using a FOSS FIASTAR 5000 and FOSS auto sampler. The experimental seawater concentrations of NH4-N, was analyzed by standard spectrometry methods [38], according to the salicylate-hypochlorite method for determining ammonia in seawater [39]. The experimental seawater concentrations of NO3-N were analyzed using a NO –NO2–NOx analyzer (Thermo Environmental Instruments Inc. 42C), and concentrations of PO4-P were measured by standard spectrometry methods [38]. 2.10. Statistics All data analyses were performed in R. All data were tested for normality by Shapiro Wilks test, and for homoscedasticity by Levene's test. If assumption were met, data were analyzed using either a two way or one-way ANOVA followed by Tukey's HSD post hoc test. If the data did not meet the assumption of normality or homoscedasticity, a log transformation was applied before analysis. If the assumptions were not met by log-transformation, a Kruskall Wallis (KW) test was used fol-lowed by a Wilcoxon (W) post hoc test to test for signifciant differences b b e e t t w w e e e e n n g g r r o o u u p p s s u u s s i i n n g g t t h h e e B B e e n n j j a a m m i i n n i i H H o o c c h h b b e e r r g g ((B B H H )) a a d d j j u u s s t t m m e e n n t t method. When producing different regressions, a linear model (lm) was fti to the data in R, and compared using a one-way ANOVA. A signifi cance level of 0.05 was used as the level of signifciance in all experi-ments and data are presented as average ,standard error (AV ± SE), unless specifcially mentioned otherwise. 3. Results 3.1. Comparing nitrate and ammonium as N source for hatchery operations (experiment 1) After 13 days of growth, the number of female gametophytes was not signifciantly different between the nutrient treatments or N concentra-tions (Fig. 2.a, Supplementary Table S3). The densities of female ga-metophytes in the NO3 or NH4 treatments was also not signifciantly different from the no-nutrient control, even though the highest density of female gametophytes were observed in the no-nutrient control treatment, averaging 36 ±13 female gametophytes mm - 2 after 13 days. After 21 days of growth, there was no signifciant effect of nutrient source or concentration on the gametophyte density (Fig. 2.b, Supplementary Table S3). The gametophyte density in the no-nutrient control however, was signifciantly higher than the gametophyte densities in all of the nutrient concentrations tested, as the number of female gametophytes remained high in this treatment (30 ± 3 female gametophytes mm = 2), whereas it was lower in all other treatments. Fig. 2. Density of female gametophytes after 13 days (a), and 21 days (b) of cultivation. Sporophytes of S. latissima after 13 days (c) and 21 days (d) of cultivation at four different N concentrations (20, 50, 100, 150 p M) of NH4 (triangles) or NO3 (circles) and one no-nutrient control (squares). Lines represent a polynomial fti for the sporophyte density in the NH4 (long dashes) and NO3 treatments (short dashes). Data are shown as average SE, n =6. (*) mark signifciant differences between the control and the N concentrations (20–150 u M), and letters mark signifciant differences between nutrient concentrations from 20 to 150 p M. After 13 days of growth, there was no signifciant effect of nutrient source or interaction between nutrient source and nutrient concentra-tion on the density of sporophytes (Fig. 2.c, Supplementary Table S3). The nutrient concentration however, had a signifciant positive effect on the sporophyte density, as sporophytes in the 20 p M treatments had signifciantly less sporophytes than in the 50 and 100 p M N treatments. The highest sporophyte density was seen in the 100 u M NH4 treatment (15 ±2 sporophytes mm 2) and the lowest in the 20 u M NH4 treatment (3.0 ± 0.6 sporophytes mm - 2). Only few sporophytes were seen in the no-nutrient control treatment (0.2 ± 0.1 sporophytes mm - 2) after 13days of growth. After 21 days of growth, there was a signifciant positive effect of N concentration on the density of sporophytes, but no effect of N source or the interaction between N source and N concentration on the density of sporophytes (Fig. 2.d, Supplementary Table S3). In the 20 u M N treatments, signifciantly less sporophytes had developed after 21 days, as compared to the 50–150 u M N treatments. In the no-nutrient control, no sporophytes were observed after 21 days of growth, whereas the highest density of sporophytes was found in the 150 u M NO3 treatment (20 ± 3 sporophytes mm - 2), as a contrast to the NH4 treatment, where the highest density of sporophytes was observed in the 100 treatment (18 ± 3 sporophytes mm - 2). A regression analysis showed that a second order polynomial was the best fti for the data after 13 and 21 days for both the NH4 (R2 = 0.46 and 0.40, respectively) and NO3treatment (R2 =0.26 and 0.41, respectively), signifciantly different from a linear fti for both the NO3 and NH4 treatment (Fig. 2.c/d, Supple-mentary Table S4). There was a signifciant effect of concentration and a signifciant interaction of concentration and nutrient source on the reproductive success (Fig. 3, Supplementary Table S3). The reproductive success were lowest in the 20 u M NH4 and NO3 treatment (34 ± 16 and 22 ± 9%, respectively), and signifciantly lower than the 100 p M NH4 and NO3treatment and the 150 p M NO3treatment, which had the highest reproductive success (105 ± 12%). The 150 u M NH4 treatment had a reproductive success of 52 ± 9%, which was markedly, but not signif-i cantly, lower than in the 150 u M NO3 treatment. After 21 days, a signifciant positive effect of N concentration on sporophyte length was observed, but there was no effect of the nutrient source or interaction between nutrient source and N concentration (Fig. 4, Supplementary Table S3). Sporophytes grown with 20 p M N were signifciantly shorter than Fig. 3. Reproductive success (%) of S. latissima at four different N concentra-tions (20, 50, 100, 150 p M) of NH4 and NO3, 21 days after the experiment started. The reproductive success was defined as the ratio of the final sporo-phyte density to the initial gametophyte density. Data are shown as average ±SE, n = 6. Letters mark signifciant differences between the N concentrations. Fig. 4. Mean length of the six longest sporophytes (mm) of S. latissima at four different N concentrations (20, 50, 100, 150 u M) of NH4 (triangles) and NO3(circles), 21 days after the experiment start. Lines represent a polynomial curve fti for the length of sporophytes in the NH4 (long dashes) and NO3 (short dashes) treatments. Data are shown as average ± SE, n = 6. Letters mark sig-nifciant differences between the N concentrations. sporophytes grown with 100 and 150 u M N, and the sporophytes grown in 50 u M N were signifciantly shorter than sporophytes from the 150 p M N treatment. The longest sporophytes were found in the 150 u M N treatment (0.5 ± 0.1 mm). The relation between the sporophyte length and nutrient concentration could best be described by a polynomial relation for both the NH4-N (R2 =0.77) and the NO3-N treatment (R2 =0.31). The polynomial fti was only signifciantly different from a linear fi for the NH4-N treatment (Fig. 4, Supplementary Table S4). 3.2. Comparing nutrient sources approved for organic seaweed production (experiment 2) The control with no nutrients added, the degassed manure, Natur Næring and NO3-PES treatment all had a density of female gametophytes in the same range. Contrary, the Grogreen, Protamylasse, Substral and Grogreen/degassed manure mix treatments, all had none, or a very low abundance of female gametophytes after 8 days and never gained a signifciant number of female gametophytes or sporophytes, and are therefore not included in the results. Visual inspections of the treatments with Grogreen, Protamylasse, Substral and Grogreen/degassed manure mix indicated a high abundance of bacteria. After 8, 16 and 23 days of growth, there was a signifciant effect of nutrient source on the female gametophyte densities (Fig. 5.a, Supple-mentary Table S3). The female gametophyte density was signifciantly lower in the organic certifeid nutrient source degassed manure, than in the no-nutrient control and the NO3-PES treatment, and the female gametophyte density signifciantly lower in the Natur Næring than in the NO3-PES treatment. The density of female gametophytes was 20 ± 4 in the control and 18 ± 1 in the PES treatment. However, after 16 and 23days of growth, the female gametophyte density was signifciantly lowest in the NO3-PES treatment having 3.8 ± 0.7 and 2.0 ± 0.7 female ga-metophytes mm - 2, after 16 and 23 days, respectively. The no-nutrient control sustained the highest number of female gametophytes throughout the experiment, and had 32 ±5 female gametophytes mm 2after 23 days of growth. No sporophytes had developed in any treatments after 8 days of growth, and in the no-nutrient control, no sporophytes developed during the 23 days of the experiment (Fig. 5.b). After 16 and 23 days of growth, there was a signifciant effect of the nutrient source on sporophyte density (Supplementary Table S3). The sporophyte density was signif-i cantly higher in the NO3-PES treatment (16 ± 3 sporophytes mm - 2) as compared to the degassed manure and Natur Næring treatments, (0.9 ±0.4 and 0.4 ± 0.2 sporophytes mm - 2, respectively). The sporophyte density was however not signifciantly different between the degassed manure and Natur Næring treatment. After 23 days of growth, the sporophyte density had increased in all treatments, but was still signif-icantly highest in the NO3-PES treatment (18 ± 1 sporophytes mm 2), compared to the degassed manure and Natur Næring treatments (3.9 ±1.0 and 0.9 ± 0.2 sporophytes mm - 2, respectively) (Supplementary Table S3). After 23 days, the sporophyte density was signifciantly higher in the degassed manure treatment than in the Natur Næring treatment (Supplementary Table S3). There was a signifciant effect of the nutrient source on the repro-ductive success. The reproductive success was signifciantly lower in the degassed manure and Natur Næring treatments compared to the 100 p M NO3 treatment, which had a reproductive success of 109 ±18% (Fig. 6). Even though not signifciantly different, the degassed manure treatment had a reproductive success of 57 ± 15%, which was markedly higher than in the Natur Næring treatment being 11 ± 3%. After 30 days of growth, there was a signifciant effect of the nutrient source on sporophyte length (Fig. 7, Supplementary Table S3). The length of the sporophytes grown in NO3-PES were 2.3 ± 0.2 mm and signifciantly longer than the sporophytes in the degassed manure and Natur Næring treatments, which were not signifciantly different (0.8 ±0.1 and 1.0 ± 0.1 mm, respectively). 3.3. Evaluating mussel excreted ammonium as N source for hatchery operations (experiment 3) The density of female gametophytes was not signifciantly different between treatments after 9 days of growth, but had a tendency to be lower in the NO3-PES (P100) treatment with 61 ± 17 female gameto-phytes mm - 2 compared to 84 ±13 in the mussel treatment and 79 ±15female gametophytes mm - 2in the no-nutrient control (C) (Fig. 8.a, Supplementary Table S3). The general density of female gametophytes was higher than observed in the other experiments conducted, even though a spore density of 7500 spores mL - 1 was intended, as in exper-iment 2. After 16 days of growth, the density of female gametophytes had decreased in all treatments, but was still not signifciantly different be-tween treatments, although now the female gametophyte density was Fig. 5. Density of female gametophytes (a) and sporophytes (b) of Saccharina latissima after 8, 16 and 23 days of cultivation using two nutrient sources approved for organic seaweed farming (degassed manure and Natur Næring), at an N concentration of 100 p M. The nutrient sources, approved for organic seaweed farming, were compared to a positive control of 100 p M NO3-PES (P100) and a no-nutrient control (C) only containing Instant Ocean artifciial seawater. Letters mark signifciant differences between treatments (Tukey HSD, p < 0.05). Data are shown as average ± SE, n = 6. Fig. 6. Reproductive success of S. latissima in two different nutrient sources approved for organic seaweed farming (degassed manure and Natur Næring), and the positive control of 100 p M NO3-PES (P100) after 23 days of cultivation. The reproductive success was defined as the ratio of the final sporophyte density to the initial gametophyte density. Data are shown as average SE, n =6. Letters mark signifciant differences between the treatments (Tukey HSD, p < 0.05). markedly lowest in the NO3-PES treatment, with 9.2 ± 5 female game-tophytes mm y 2(Supplementary Table S3). After 23 days of growth, there was a signifciant effect of the nutrient source on the female gametophyte density between, as the NO3-PES treatment had signifi cantly less female gametophytes than the no-nutrient control and the mussel ammonium treatments (Supplementary Table S3). The female gametophyte densities were 1.7 ± 1.4, 74 ± 28 and 31 ± 7 female ga-metophytes mm - 2 in the NO3-PES, mussel and no-nutrient treatment after 23 days of growth, respectively. No sporophytes had developed in any of the three treatments after 9days of growth (Fig. 8.b). After 16 days of growth, sporophytes were present in all treatments except for the no-nutrient control. Most spo-rophytes were present in the NO3-PES treatment having 5.5 ± 2.3, compared to 1.6 ± 0.6 sporophytes mm 2 2in the mussel treatment, however this was not signifciantly different (Supplementary Table S3). Fig. 7. Mean length of sporophytes in two different nutrient sources approved for organic seaweed farming (degassed manure and Natur Næring), and the positive control of 100 p M NO3-PES (P100) after 30 days of cultivation. The length was measured for each well of 12 randomly selected sporophytes, and calculated as a mean for each of the six replicate wells. Letters mark signifciant differences between treatments (Tukey HSD, p < 0.05). Data are shown as average ± SE, n = 6. After 23 days of growth, the number of sporophytes had increased to 2.8± 1.0 sporophytes mm " 2 in the mussel treatment, whereas the sporo-phyte density had decreased to 3.9 ±2.1 sporophytes mm 2 in the NO3-PES treatment, and therefore no signifciant difference could be found between the two treatments after 23 days of growth (Supplementary Table S3). If comparing the number of sporophytes in the NO3-PES treatment (P100) with the number of sporophytes from the same treatment in experiments 1 and 2, the sporophyte density was markedly lower in experiment 3, also when considering variations in spore seeding densities. The reproductive success was 4 ± 2% in the mussel treatment compared to 8 ± 5% in the 100 p M NO3-PES treatment (Fig. 9). How-ever, no signifciant difference was found between the treatments (Sup-plementary Table S3). Fig. 8. Density of female gametophytes (a) and sporophytes (b) of Saccharina latissima grown in mussel ammonium enriched water at an N concentration of 100 p M N (M100), one positive control of 100 p M NO3-PES (P100), and one no-nutrient control (C) after 9, 16 and 23 days of cultivation. Letters mark signifciant differences between treatments (Tukey HSD, p < 0.05). Data are shown as average ± SE, n = 6. Fig. 9. Reproductive success (%) of S. latissima grown with mussel excreted ammonium at an N concentration of 100 p M N (M100) and in the positive control of 100 p M NO3-PES (P100) after 23 days of cultivation. The repro-ductive success was defined as the ratio of the final sporophyte density to the initial gametophyte density. Data are shown as average ± SE, n = 6. The sporophyte length from the NO3-PES treatment were 3.5 ± 1.0mm, compared to 1.27 ± 0.02 mm from the mussel treatment, 37 days after the experiment started (Fig. 10). The difference however, was not signifciant (Supplementary Table S3). 3.3.1. Relation between the concentration of N from mussel excreted ammonium, and female gametophytes/sporophyte densities After 9 days of growth, there was no signifciant difference in the density of female gametophytes in the different N concentrations from mussel excreted ammonium, but a tendency was observed for the 100u M N treatment to have the highest density of female gametophytes (85±13 female gametophytes mm - 2) (Fig. 11.a, Supplementary Table S3). After 23 days of growth the female gametophyte density had decreased markedly in all treatments, with still no signifciant effect of N concen-tration on the female gametophyte density, but with the 100 p M N treatment still having the highest female gametophyte concentration of 74 ± 28 female gametophytes mm - 2 (Fig. 11.b). After 9 days of growth, no sporophytes were visible in any of the treatments, but after 23 days, there was a signifciant effect of N Fig. 10. Mean length of Saccharina latissima sporophytes grown with mussel excreted ammonium at an N concentration of 100 u M N (M100) and in the positive control of 100 u M NO3-PES (P100) after 37 days of cultivation. The sporophyte length was measured as the average of 10 randomly selected spo-rophytes and calculated as the average for each replicate well. Data are shown as average ± SE, n = 6. concentration on the sporophyte density (Fig. 11.c, Supplementary Table S3).The highest sporophyte density was observed in the 50 p M N treatment having 3.9 ±1.3 sporophytes mm - 2, signifciantly higher than in the 150 u M N treatment having only 0.05 0.05 sporophytes mm 2(Fig. 11.d). Notably, in the 10 and 150 u M N treatments both the female gametophyte density and sporophyte density decreased between day 9and 23. The relation between the concentration of N from the mussel excreted ammonium and sporophyte density was best described by a second order polynomial regression (R2 ² 0.38), which ftited the data signifciantly better than a linear regression (Supplementary Table S4). The reproductive success confrimed results from the sporophyte densities, as the reproductive success were signifciantly lower in the 20and 150 u M N treatments (1.2 ±0.8 and 0.1 ±0.1%, respectively), than in the 50 p M N treatment, having the highest reproductive success of 7±2% (Fig. 12, Supplementary Table S3). The reproductive success was generally lower than what was observed in experiment 1 and 2. The sporophyte lengths were not signifciantly different between the different nutrient concentrations, but there was a tendency for the 50 Fig. 11. Density of female gametophytes after 9 days (a) and 23 days (b) of cultivation. Density of sporophytes after 9 days (c), and 23 days (d) of cultivation, in four different N concentrations (10, 50, 100, 150 p M N) from adding mussel excreted ammonium water. The line represents a polynomial fti for the relation between the sporophyte density (R2 =0.38) and the N concentration. Letters mark signifciant difference between nutrient concentrations (Tukey HSD, p <0.05). Data are shown as average ± SE, n = 6. u M N treatment to have the longest sporophytes after 37 days of growth (Fig. 13, Supplementary Table S3). 3.4. Mussel ammonium excretion (experiment 4) From 0 to 3 h after the mussels were immersed, the mussel ammo-nium excretion rate, measured as NH4-N was 3.3 ± 0.1 u mol NH4 g dw - 1 h - 1, and then decreased to a stable rate of 1.1 ± 0.1 u mol NH4 g dw - 1 h - 1 from 3 to 26 h and 1.1 ±0.05 u mol NH4 g dw - 1 h - 1 from 26 to 49 h (Supplementary Fig. S3). The excretion of NH4-N was not affected by removing microalgae from the water by flitering (1 u m), and also no effect on excretion rate was observed between the different stocking densities of mussels used in the study. The ammonium concentrations were 170 ± 8.6 p M NH4193 H 4.0 u M NH44and 293 ±23.1 u M NH40 in the 0.1 kg mussels L - 1(preflitrered water), 0.1 kg mussels L - 1 and 0.2 kg mussels L - 1 treat-ment after the frist 26 h, respectively. 4. Discussion T T h h i i s s s s t t u u d d y y s s h h o o w w e e d d t t h h a a t t v v a a r r i i o o u u s s n n u u t t r r i i e e n n t t s s o o u u r r c c e e s s ,, a a p p p p r r o o v v e e d d f f o o r r organic seaweed farming, can be applied in the hatchery production of organic certifeid S. latissima , but also that it comes with a signifciantly reduced productivity. 4.1. The effect of N source and concentration on hatchery development of S. latissima From the results obtained in this study, a nutrient addition does not seem to be essential for the initial germination of S. latissima female gametophytes. In all experiments conducted, the control treatment with no nutrients had an equal or higher density of female gametophytes than treatments receiving nutrients. Previously, it has been demonstrated, that S. latissima spores are able to both settle and germinate in nutrient poor environments [17,18], as also shown in this study, whereas it has also been demonstrated, that the initial settlement and germination of spores can be reduced in natural seawater, without nutrients added [9]. The variable ability of spores to germinate in a nutrient poor environ-ment might be caused by the season of spore retrieval or primary con-dition of the spores [36]. Since kelp spores contain a high amount of lipid granules, supporting the initial germination and growth of the gametophytes [40–42], a difference in this energy storage of spores could explain the difference seen between studies on germination. The mussel excreted ammonium had no negative effect on the initial germination and recruitment of female gametophytes in experiment 3, whereas both the manure and Natur Næring had a lower density of fe-male gametophytes after 8 days of growth in experiment 2. In experi-ment 2, the germination was equalized by having all treatments in the same PES media for the frist 34 h, when germination takes place [36]. Fig. 12. Reproductive success (%) of S. latissima after 23 days of growth at four different concentrations of N (10, 50, 100 and 150 u M N) created from mussel ammonium enriched water. The reproductive success was defined as the ratio of the final sporophyte density to the initial gametophyte density. Data are shown as average ± SE, n = 6. Letters mark signifciant differences between the N concentrations (Tukey HSD, p < 0.05). Fig. 13. Mean length of 10 randomly selected sporophytes of Saccharina lat-issima after 37 days of growth at four different concentrations of N (10, 50, 100and 150 u M N) created from mussel ammonium enriched water. Data are shown as average ± SE, n = 6. Therefore, the reduced gametophyte density could be attributed an increased mortality in these treatments from the time of germination until the frist measurement. As ammonium was not reducing the germination and resulting female gametophyte density in concentra-tions between 20 and 150 u M in experiment 1, this was most likely not the reason for the reduced gametophyte density. The introduction of organic particles giving rise to unwanted bacterial growth, or impairing nutrient-/light uptake could be part of the explanation. In contrast to the nutrient effect on germination, a nutrient addition was essential for gametogenesis to occur, as the gametogenesis, or production of sporophytes, was absent in all treatments not receiving nutrients. A concentration of 20 p M N signifciantly reduced gameto-genesis in this study, whereas a concentration of 100–150 u M N seemed to saturate the reproduction. The results support previous results stating that N concentrations >40 u M N favor gametogenesis [12,17,18]. A recent study found that gametogenesis, in a gametophyte culture, could proceed in pasteurized seawater without any nutrient addition [43]. Prior to gametogenesis however, the gametophytes used, were produced and kept in a nutrient saturated media, in contrast to the present or other studies dealing with traditional seeding of spores. This implies that the gametogenesis of saturated gametophytes may be less sensitive to nutrient enrichment than gametophytes originating from spores of S. latissima . Ammonium (NH4) has been found to inhibit gametogenesis in the conspecifci Saccharina longicruris at 100 p M NH4-N, especially at light intensities of 50–60 u mol photons m - 2 s - 1, which was equivalent to the conditions used in our study [27]. In the present study, no sig-nifciant reduction of gametogenesis was observed in S. latissima cultures growing with 100 p M NH4-N as compared to 100 p M NO3-N, but there was a clear tendency for ammonium concentrations >100 u M NH4-N to negatively impact gametogenesis. This shows a larger tolerance to NH4-N in S. latissima than in S. longicruris , but also indicates that ammonium can negatively affect gametogenesis in S. latissima , in concentrations >100 u M NH4-N. In theory, NH4 should be a more favorable nutrient source as compared to NO3, as NH4 due to its oxidative state, does not require reduction, at the cost of energy, before being incorporated into amino acids [44,45]. However, high concentrations of NH4 can become toxic for algae by causing cell lysis due to; changes in cell pH, photo-system damage related to photosystem II, changes in pigment concen-trations and transient effects on N uptake characteristics [44]. As a high concentration of NH4 had a negative effect on gametogenesis, but a positive effect on length growth, our results suggest that reproduction (gametogenesis) may be particularly sensitive to high NH4 concentra-tions. Future studies could investigate this further to obtain a more detailed understanding of the sensitivity of S. latissima development stages to NH4. Viable sporophytes developed and grew in the treatments with degassed manure, the commercial product, Natur Næring and mussel excreted ammonium, but with a reproductive success reduced by >50%in all organic nutrient sources, compared to the control PES treatment. Therefore, showing a signifciantly reduced gametogenesis of S. latissima , in the organic nutrient sources used in this study. All nutrient sources approved for organic seaweed farming, had an N content of 100 p M NH4-N and an N:P ratio <10, therefore containing suffciient amounts of both NH4-N and PO4-P. Therefore, the reduced gametogenesis was, most likely, not caused by lack of N or P, or toxic concentrations of NH4-N, but could have been impaired by the lack of essential minerals or vitamins in the media. Iron (Fe) is a mineral playing an essential role in regulating the balance between vegetative growth and gametogenesis in several kelp species, including the closely related S. japonica , were concentra-tions <2 p M solid bound Fe, is shown to inhibit oogonia formation [19,46,47]. The Fe concentration was 1.07 u M Fe in the degassed manure treatment (calculated from information on the raw material) and 2.025 u M Fe in the PES solution used in this study [32], out of which 1.13 u M Fe is supplied as chelated iron (Fe-EDTA). Therefore, low Fe concentrations could have been inhibiting gametogenesis, at least in the degassed manure treatment. The availability of micronutrients in the organic nutrient sources, should be addressed in future studies. The content of organic material could also have been a reason for the lower gametogenesis in the treatments with nutrient sources approved for organic farming. The nutrient sources that failed to support game-togenesis during the experiment generally had a high degree of bacterial growth, which was probably nourished by a high level of organic ma-terial in the nutrient sources. None of the nutrient sources, approved for organic seaweed farming, were flitered prior to the experiment, except for the degassed manure, which was flitered through a 500 u m fliter, due to a high abundance of straw in the material. Perhaps a flitration step could have been applied, before the nutrients were added, to remove the organic particles in the nutrient sources, and potentially enhance the probability of a successful gametophyte development. The light conditions were poorer in the nutrient sources approved for organic seaweed farming, as the nutrient sources colored the water. The water volume and hence light extinction, however, was low in this study, but in hatchery tanks with a larger water depth, this could probably further reduce the effciiency of the nutrient sources. In the mussel excretion experiment, this was not a problem, as the mussels in contrast cleared the water before use. In all treatments receiving organic nutrient sources, the sporophytes were >50% shorter than in the positive control treatment containing PES. This could have been caused by a slower gametogenesis or/and a lower capacity for growth. As seen in experiment 1, NH4 was not reducing the sporophytes capacity to grow, so the NH4 concentration was not the reason for the impaired growth. In the degassed manure and Natur Næring treatments, the reduced growth could have been caused by organic particles lowering the light availability, which is important to apply in right quantities, to obtain optimal initial growth of the sporo-phyte [48]. However, also a lower availability of micronutrient and vitamins could have caused the reduced capacity to grow in these treatments. 4.2. Application of the results in commercial seaweed farming of S. latissima The setup of the hatchery could potentially affect the concentration optimal for gametophyte development. It is important to consider, that the concentrations found to be optimal in this study, were determined in a small-scale batch setup, were some compounds, which are not measured, might be interacting with the nutrient concentrations tested. Other factors important for nutrient uptake and growth such as folw conditions and concentration of micronutrients such as Fe can be different in a large-scale hatchery situation [49,50]. In a large-scale folw-through hatchery in China, producing S. japonica , an N concen-tration < 11.8 p M NO3-N is strived for during the initial development phase when gametogenesis occurs [37], which underlines that optimal nutrient concentrations can be signifciantly different in a large scale folw-through setup. The general recommendation of nutrient addition in western based manuals, is to use half or full strength PES giving an N concentration of >400 or >800 p M N (primarily NO3-N), respectively [8,32]. These high concentrations are probably neither necessary to achieve an optimal production of sporophytes from the hatchery, nor optimal from an environmentally or economically point of view. A more nuanced approach could be considered, were the nutrients are added in increasing amounts to counteract initial fouling of the growth lines, and support later gametogenesis and sporophyte growth, as also applied in China [37]. The growth response on natural seawater, seen in this study, could be different between hatcheries, as both regional and seasonal differences in the nutrient content in the ambient seawater could change the response. Hatcheries situated in e.g. deep fjord areas, were nutrient rich waters are present year-round, could be alleviated from the demand of nutrient addition, because of the natural elevated concentration of nu-trients in the water [16]. F F r r o o m m t t h h e e r r e e s s u u l l t t s s o o b b t t a a i i n n e e d d i i n n t t h h i i s s s s t t u u d d y y ,, a a h h a a t t c c h h e e r r y y p p r r o o d d u u c c i i n n g g S. latissima should preferentially use NO3 over NH4 as N-source, and the hatchery effciiency will benefti from using a NO3 concentration of 150u M NO3, whereas a hatchery using NH4 as N-source should not use an NH4 concentration higher than 100 u M-N. Degassed manure was the best performing organic nutrient source used in this study, as a concentration of 100 u M-N could support gametogenesis and thereby a production of sporophytes from an organic hatchery, but with a signifciantly reduced effectivity. As degassed manure contain organic particles, the use of this nutrient source will require flitering, prior to being applied in the hatchery. The degassed manure can also vary in composition depending on farm-origin and season, and therefore, the use of this nutrient source would demand for insight into the composition, before being applied. Further it is impor-tant to notice, that even if using this organic nutrient source, it will be necessary according to organic regulations, to subsequently treat the hatchery water to remove remaining nutrients, such as NH4, before disposing the water [6]. As mussels excrete both N and P in the form of primarily inorganic NH4-N and PO4-P, the coupling of the nutrient excretion of mussel species such as M. edulis and kelp in an organic certifeid seaweed hatchery, is evident, and has been proposed before [14]. As no nutrients are being added, when using mussel excreted water, there will be no need for subsequent treatment of the water, if using this nutrient source. Using mussels together with seaweed in an organic hatchery will further enable the capture of nutrients from the sea, already at the hatchery stage production of S. latissima , and add on to the positive case of using the production of seaweed as an instrument for circular nutrient man-agement [51]. In 2014, Ro o ß ner et al. found, that mussels via ammonium excretions, could increase the gametogenesis and growth of S. latissima both in the hatchery and in a grow-out facility at sea compared to a control in ambient seawater, but also that the sporophytes developed slower than in a conventional hatchery. The mussels in the study by R B o ß ner et al. [14] were kept in the same container as the seedling lines, which could impose a contamination risk in a productive hatchery, and nutrient levels are hard to control. The present study seeked to increase the control of the process, and t t h h e e r r e e f f o o r r e e t t h h e e m m u u s s s s e e l l c c u u l l t t i i v v a a t t i i o o n n w w a a s s d d e e c c o o u u p p l l e e d d f f r r o o m m t t h h e e s s e e a a w w e e e e d d hatchery. This ensured the potential to increase both the N and P con-centrations to levels >200 and >50 times, respectively, the ambient concentrations in 23 h, and the opportunity to adjust the nutrient con-centration by dilution in the hatchery, and fliter the water before entering the hatchery, which is vulnerable to even minute introductions of microalgae. The excretion rates of the mussels used in this study were 1.1 ±0.1 u mol NH4 g dw - 1 h - 1 similar to what is found in other studies [35,52], and was not affected by mussel stocking densities. Therefore, a reliable calculation can be applied to an upscaling scenario, were 38.8kg (wet weight) of mussels would be needed to increase the NH4-N concentration to 100 u M NH4-N in 1000 L of seawater during 23 h. From the growth trials of this study, an NH4 concentration of 50 u M NH4 was optimal to obtain both the highest reproduction and subsequent growth of the sporophytes in an organic hatchery using mussel excreted water. 4.3. Considerations for the organic hatchery production of seaweed in EU Using nutrient sources approved for organic seaweed farming, will from the results obtained in this study, inevitably reduce the effective-ness of the hatchery and introduce an extra workload to the hatchery operation. Inarguably, nutrient waste from seaweed hatchery processes should be minimized from environmentally as well as economical points of view. Considering however, that only minute absolute amounts of nutrients are used at the hatchery stage of S. latissima , the actions needed in the hatchery, to live up to an organic certifciation are drastic considering the doubtful beneftis obtained at this stage of the produc-tion. If conventional seedstock could be approved for organic seaweed production, this could ease the transition/incorporation of the organic seaweed cultivation in EU. In the European commission regulation no.889/2008, L250 (29) it is stated that “currently for many species there is still not enough organic seed and vegetative propagating material available and, in those cases, the use of non-organic seed and vegetative propagating material should be allowed ”. Considering that, to our knowledge, only the present study has used and tested organic nutrient sources approved for organic hatchery production of S. latissima , organic seeds of S. latissima would only be available from areas where ambient nutrient levels are suffciient to support the development of S. latissima gametophytes to sporophytes. Therefore, non-organic seeding material, could be allowed, and therefore the use of minute nutrient amounts in hatcheries could be evaluated, to sustain and develop the production of o o r r g g a a n n i i c c c c e e r r t t i i f f ie ei d d S S .. l l a a t t i i s s s s i i m m a a ,, a a s s t t h h e e a a v v a a i i l l a a b b i i l l i i t t y y o o f f o o r r g g a a n n i i c c s s e e e e d d // vegetative propagating material are currently limited. Future studies could further investigate the use of gametophytes as seed stock for the production of organic certifeid seaweed and/or additionally investigate the possibilities of tailoring nutrient sources suitable for organic certi-feid seaweed production. 5. Conclusion The present study confrimed the hypothesis, that the gametogenesis of S. latissima would be negatively affected at NH4 concentrations >100u M, although not to the same extend as previously described for the closely related S. longicruris . The study also confrimed the hypothesis that hatchery operations based on organic nutrient sources resulted in both an impaired gametogenesis and slower growth of S. latissima ju-veniles. However, two of the organic nutrient sources, degassed manure and mussel excreted ammonium, demonstrated a potential as nutrient sources for the production of organic certifeid S. latissima . Still, further development is needed before the organic nutrient sources investigated can substitute conventional fertilizers in an effciient commercial hatchery. CRediT authorship contribution statement Teis Boderskov: Conceptualization, Methodology, Investigation, Data Curation, Writing - Original Draft, Writing - Review & Editing. Michael Bo Rasmussen: Conceptualization, Methodology, Writing -Review & Editing. Christian Cassard: Conceptualization, Methodology, Investigation, Data Curation, Writing - Review & Editing. Julie Svensgaard: Conceptualization, Methodology, Investigation, Data Curation, Writing - Review & Editing. Laurids N ø rskov Enevoldsen: Conceptualization, Methodology, Investigation, Data Curation, Writing - Review & Editing. Annette Bruhn: Conceptualization, Methodology, Data Curation, Writing - Review & Editing. Declaration of competing interest The authors declare that they have no known competing fniancial interests or personal relationships that could have appeared to infulence the work reported in this paper. Acknowledgements The work was supported by the Innovation Fund Denmark, and Hjarn ø Havbrug A/S through the commercial PhD project “Ø kologisk Sukkertang – industriel produktion af en ny dansk bioressource ”, grant no 7038-00133B. Statement of informed consent and human/animal rights No confilcts, informed consent, or human or animal rights are applicable to this study. Appendix A. Supplementary data Supplementary data to this article can be found online at https://doi. org/10.1016/j.algal.2021.102602. References [1] R. Araújo, F.V. Caldero n n, J.S. Lo ó pez, I. Azevedo, A. Bruhn, S. Fluch, M. García-Tasende, T. Ilmja, F. Ghaderiardakani, M. Laurans, M. MacMonagail, S. Mangini, C. Peteiro, C. Rebours, T. Stef a ansson, J. Ullmann, Current status of the algae production industry in Europe: an emerging sector of the blue bioeconomy, Front. Mar. Sci. 7 (2021), 626389, https://doi.org/10.3389/fmars.2020.626389. [2] T. Chopin, A.G.J. Tacon, Importance of seaweeds and extractive species in global aquaculture production, Rev. Fish Sci. Aquac (2020), https://doi.org/10.1080/23308249.2020.1810626. [3] FAO. The State of World Fisheries and Aquaculture 2018 - Meeting the Sustainable Development Goals, FAO, Rome, 2018, pp. 1–227. https://www.fao.org/doc uments/card/en/c/I9540EN/. [4] H. Willer, B. Schlatter, J. Tr a avní i cek, L. Kemper, J. Lernoud. The World of Organic Agriculture 2020, FiBL, IFOAM, 2020, pp. 1–333. https://www.fbil.org/en/sh op-en/5011-organic-world-2020. [5] S.F. Pedersen, M. Meland, C. Rebours, Macroalgae for an increasing organic market, Bioforsk Focus 8 (2013) 337–338, https://doi.org/10.1007/s10811-008-9326-4.Mattilsynet . [6] European Commission, Regulation (EU) 2018/848 of the European parliament and of the council of 30 May 2018 on organic production and labelling of organic products and repealing council regulation (EC) No 834/2007, Off. J. Eur. Union.(2018) 1–92. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri =CELEX%3A32018R0848. [7] European Commission, Commission regulation (EC) no 889/2008, Off. J. Eur. Union 1–132 (2017), https://doi.org/10.1037/0022-3514.51.6.1173. [8] K. Flavin, N. Flavin, B. Flahive, Kelp farming manual, Ocean Approved 130 (2013). https://maineaqua.org/wp-content/uploads/2020/06/OceanApproved_Kelp ManualLowRez.pdf . [9] P. Kerrison, M.S. Stanley, M. Kelly, A. Macleod, K.D. Black, A.D. Hughes, Optimising the settlement and hatchery culture of Saccharina latissima (Phaeophyta) by manipulation of growth medium and substrate surface condition, J. Appl. Phycol. 28 (2) (2015) 1181–1191, https://doi.org/10.1007/s10811-015- 0621-6. [10] J.W. Hansen, S. H ø gslund, Marine områder 2018, NOVANA. Aarhus University, DCE – National center for environment and Energy, Denmark, 2019, 156 p. –Scientifci report from DCE nr. 355. [11] C. Peteiro, N. S a anchez, B. Martínez, Mariculture of the asian kelp Undaria pinnatifdia and the native kelp Saccharina latissima along the Atlantic coast of southern Europe: an overview, Algal Res. 15 (2016) 9–23, https://doi.org/10.1016/j.algal.2016.01.012. [12] M.M. Nielsen, J.P. Kumar, A. Soler-Vila, M.P. Johnson, A. Bruhn, Early stage growth responses of Saccharina latissima spores and gametophytes. Part 1: inclusion of different phosphorus regimes, J. Appl. Phycol. 28 (2016) 387–393, https://doi. org/10.1007/s10811-015-0547-z . [13] J.J. Ratcliff, A. Soler-Vila, D. Hanniffy, M.P. Johnson, M.D. Edwards, Optimisation of kelp (Laminaria digitata ) gametophyte growth and gametogenesis: effects of photoperiod and culture media, J. Appl. Phycol. 29 (2017) 1957–1966, https://doi.org/10.1007/s10811-017-1070-1. [14] Y. R d o ß ner, P. Krost, C. Schulz, Increasing seaweed crop yields through organic fertilisation at the nursery stage, J. Appl. Phycol. 26 (2014) 753–762, https://doi. org/10.1007/s10811-014-0269-7. [16] K. Lüning, L. Mortensen, European aquaculture of sugar kelp (Saccharina latissima ) for food industries: iodine content and epiphytic animals as major problems, Bot. Mar. 58 (2015) 449–455, https://doi.org/10.1515/bot-2015-0036. [17] R. Harries, An investigation by cultural methods of some of the factors infulencing the development of the gametophytes and the early stages of the sporophytes of Laminaria digitata , L. saccharina , and L. Cloustoni , Ann. Bot. 46 (184) (1932)893–928. Published by: Oxford University Press . [18] S.I. Hsiao, Nutritional requirements for gametogenesis in Laminaria saccharina (L.), Lamouroux (1968) 1–189. [19] C.L. Hurd, C.S. Lobban, K. Bischof, P.J. Harrison, Nutrients, in: C.L. Hurd, C. S. Lobban, K. Bischof, P.J. Harrison (Eds.), Seaweed Ecology and Physiology, 2nd edn., Cambridge University Press, Cambridge, 2014, pp. 238–293. [20] M.Y. Roleda, C.L. Hurd, Seaweed nutrient physiology: application of concepts to aquaculture and bioremediation, Phycologia 58 (2019) 552–562, https://doi.org/10.1080/00318884.2019.1622920. [21] M.F. Pedersen, J. Borum, F.L. Fotel, Phosphorus dynamics and limitation of fast-and slow-growing temperate seaweeds in oslofjord, Norway, Mar. Ecol. Prog. Ser.399 (2010) 103–115, https://doi.org/10.3354/meps08350. [22] O. Ahn, R.J. Petrell, P.J. Harrison, Ammonium and nitrate uptake by Laminaria saccharina and Nereocystis luetkeana originating from a salmon sea cage farm, J. Appl. Phycol. 10 (1998) 333–340, https://doi.org/10.1023/A:1008092521651. [23] A. Subandar, R.J. Petrell, P.J. Harrison, Laminaria culture for reduction of dissolved inorganic nitrogen in salmon farm effulent, J. Appl. Phycol. 5 (1993) 455–463, https://doi.org/10.1007/BF02182738. [24] M.T. Ale, J.D. Mikkelsen, A.S. Meyer, Differential growth response of Ulva lactuca to ammonium and nitrate assimilation, J. Appl. Phycol. 23 (2011) 345–351, https://doi.org/10.1007/s10811-010-9546-2. [25] M.M. Nielsen, A. Bruhn, M.B. Rasmussen, B. Olesen, M.M. Larsen, H.B. M ø ller, Cultivation of Ulva lactuca with manure for simultaneous bioremediation and biomass production, J. Appl. Phycol. 24 (2012) 449–458, https://doi.org/10.1007/s10811-011-9767-z . [26] E.B. Young, J.A. Berges, M.J. Dring, Physiological responses of intertidal marine brown algae to nitrogen deprivation and resupply of nitrate and ammonium, Physiol. Plant. 135 (2009) 400–411, https://doi.org/10.1111/j.1399- 3054.2008.01199.x . [27] C. Yarish, C.A. Penniman, B. Egan, Growth and reproductive responses of Laminaria longicruris (Laminariales, Phaeophyta) to nutrient enrichment, Hydrobiologia 204–205 (1990) 505–511, https://doi.org/10.1007/BF00040278. [28] L.A. Green, C.D. Neefus, Effects of temperature, light level, photoperiod, and ammonium concentration on Pyropia leucosticta (Bangiales, Rhodophyta) from the Northwest Atlantic, J. Appl. Phycol. 27 (2015) 1253–1261, https://doi.org/10.1007/s10811-014-0421-4. [29] H. Mizuta, S. Ogawa, H. Yasui, Phosphorus requirement of the sporophyte of Laminaria japonica (Phaeophyceae), Aquat. Bot. 76 (2003) 117–126, https://doi. org/10.1016/S0304-3770(03)00034-2. [30] J.C. Philips, C.L. Hurd, Kinetics of nitrate, ammonium, and urea uptake by four intertidal seaweeds from New Zealand, J. Phycol. 40 (2004) 534–545, https://doi. org/10.1111/j.1529-8817.2004.03157.x . [31] J.M. Smith, M.A. Brzezinski, J.M. Melack, R.J. Miller, D.C. Reed, Urea as a source of nitrogen to giant kelp (Macrocystis pyrifera ), Limnol. Oceanogr. Lett. 3 (2018)365–373, https://doi.org/10.1002/lol2.10088. [32] M. Edwards, L. Watson, Aquaculture explained no. 26 cultivating Laminaria digitata , Aquaculture 26 (2011) 1–71. [33] T.G. Bell, M.T. Johnson, T.G. Jickells, P.S. Liss, Ammonia/ammonium dissociation coeffciient in seawater: a signifciant numerical correction, Environ. Chem. 4(2007) 183–186, https://doi.org/10.1071/EN07032. [34] ANZECC, ARMCANZ, in: Australian and New Zealand Guidelines for Fresh and Marine Water Quality 2, Australian Water Association, 2000, p. 161. Table 8.3.7. [35] S. Barrento, I. Lupatsch, A. Keay, G. Christophersen, Metabolic rate of blue mussels (Mytilus edulis ) under varying post-harvest holding conditions, Aquat. Living Resour. 26 (2013) 241–247, https://doi.org/10.1051/alr/2013050. [36] J.-A. Lee, B.H. Brinkhuis, Seasonal light and temperature interaction effects on development of Laminaria saccharina (Phaeophyta) gametophytes and juvenile sporophytes, J. Phycol. 24 (1988) 181–191, https://doi.org/10.1111/j.1529-8817.1988.tb04232.x . [37] L. Su, S.J. Pang, T.F. Shan, X. Li, Large-scale hatchery of the kelp Saccharina japonica : a case study experience at Lvshun in northern China, J. Appl. Phycol. 29(2017) 3003–3013, https://doi.org/10.1007/s10811-017-1154-y . [38] K. Grasshoff, M. Erhardt, K. Kremling, Methods of Seawater Analysis, Verlag Chemie, Weinheim, 1983. [39] C.E. Bower, T.H. Hansen, A salicylate-hypochlorite method for determining ammonia in seawater, Can. J. Fish. Aquat. Sci. 37 (1980) 794–798, https://doi. org/10.1139/f80-106. [40] M.A. Brzezinski, D.C. Reed, C.D. Amsler, Neutral lipids as major storage products in zoospores of the giant kelp Macrocystis pyrifera (Phaeophyceae), J. Phycol. 29(1993) 16–23, https://doi.org/10.1111/j.1529-8817.1993.tb00275.x . [41] T. Motomura, Ultrastructural and immunofulorescence studies of zoosporogenesis in Laminaria angustata , Hokkaido Univ. Collect Sch. Acad. Pap. 3 (1993) 1–32. [42] F.S. Steinhoff, M. Graeve, C. Wiencke, A. Wulff, K. Bischof, Lipid content and fatty acid consumption in zoospores/developing gametophytes of Saccharina latissima (Laminariales, Phaeophyceae) as potential precursors for secondary metabolites as phlorotannins, Polar Biol. 34 (2011) 1011–1018, https://doi.org/10.1007/s00300-011-0960-y . [43] A. Ebbing, R. Pierik, T. Bouma, J.C. Kromkamp, K. Timmermans, How light and biomass density infulence the reproduction of delayed Saccharina latissima gametophytes (Phaeophyceae), J. Phycol. 56 (2020) 709–718, https://doi.org/ 10.1111/jpy.12976. [44] Y. Collos, P.J. Harrison, Acclimation and toxicity of high ammonium concentrations to unicellular algae, Mar. Pollut. Bull. 80 (2014) 8–23, https://doi. org/10.1016/j.marpolbul.2014.01.006. [45] C.S. Lobban, P.J. Harrison, Seaweed Ecology and Physiology, in: Cambridge University Press, Cambridge, London, 1994, pp. 163–209. [46] R.J. Lewis, M.K. Green, M.E. Afzal, Effects of chelated iron on oogenesis and vegetative growth of kelp gametophytes (Phaeophyceae), Phycol. Res. 61 (2013)46–51, https://doi.org/10.1111/j.1440-1835.2012.00667.x . [47] Y. Suzuki, K. Kuma, K. Matsunaga, Effect of iron on oogonium formation, growth rate and pigment synthesis of Laminaria japonica (Phaeophyta), Fish. Sci. 60 (1994)373–378, https://doi.org/10.2331/fsihsci.60.373. [48] S. Augyte, C. Yarish, C.D. Neefus, Thermal and light impacts on the early growth stages of the kelp Saccharina angustissima (Laminariales, Phaeophyceae), Algae 34(2019) 153–162, https://doi.org/10.4490/algae.2019.34.5.12. [49] J.A. Berges, D.J. Franklin, P.J. Harrison, Evolution of an artifciial seawater medium: improvements in enriched seawater, artifciial water over the last two decades, J. Phycol. 37 (2001) 1138–1145, https://doi.org/10.1046/j.1529-8817.2001.01052.x . [50] C.L. Hurd, Water motion, marine macroalgal physiology, and production, J. Phycol. 36 (2000) 453–472, https://doi.org/10.1046/j.1529-8817.2000.99139. x . [51] M. Seghetta, D. T ø rring, A. Bruhn, M. Thomsen, Bioextraction potential of seaweed in Denmark – an instrument for circular nutrient management, Sci. Total Environ.563 (564) (2016) 513–529, https://doi.org/10.1016/j.scitotenv.2016.04.010. [52] H.F. Vinther, M. Holmer, Experimental test of biodeposition and ammonium excretion from blue mussels (Mytilus edulis ) on eelgrass (Zostera marina ) performance, J. Exp. Mar. Biol. Ecol. 364 (2008) 72–79, https://doi.org/10.1016/j.jembe.2008.07.003. [54] J. Thomsen, N. Himmerkus, N. Holland, F.J. Sartoris, M. Bleich, M. Tresguerres, Ammonia excretion in mytilid mussels is facilitated by ciliary beating, J. Exp. Biol.219 (15) (2016) 2300–2310, https://doi.org/10.1242/jeb.139550. [55] B. Santelices, E. Martínez, Effects of fliter-feeders and grazers on algal settlement and growth in mussel beds, J. Exp. Mar. Biol. Eco. 118 (3) (1988) 281–306, https://doi.org/10.1016/0022-0981(88)90079-2. [56] B.H. Brinkhuis, E.C. Mariana, V.A. Breda, M.M. Brady-Campbell, Cultivation of Laminaria saccharina in the New York Marine Biomass Program, Hydrobiologica 116/117 (1984) 266–271, https://doi.org/10.1007/BF00027682.
确定






还剩12页未读,是否继续阅读?
中国格哈特为您提供《海藻孢子的振荡》,该方案主要用于生物农业中海藻孢子的振荡检测,参考标准--,《海藻孢子的振荡》用到的仪器有格哈特强力高重现振荡器LS500/RO500、格哈特快速干燥仪STL56、德国移液器MM
推荐专场
快速干燥仪
更多
相关方案
更多
该厂商其他方案
更多


















