土壤悬浮液使用格哈特Laboshake振荡器振荡30分钟Soil suspensions were gently shaken for 30 min at a Laboshake (Gerhardt GmbH &Co. KG, Ko¨nigswinter, Germany), and one microliter of the mixed soil
suspension was pipetted into each well of 96-well plates (Costar,Corning, New York, USA) containing Escherichia coli OP50 (E.coli) as food source.
方案详情

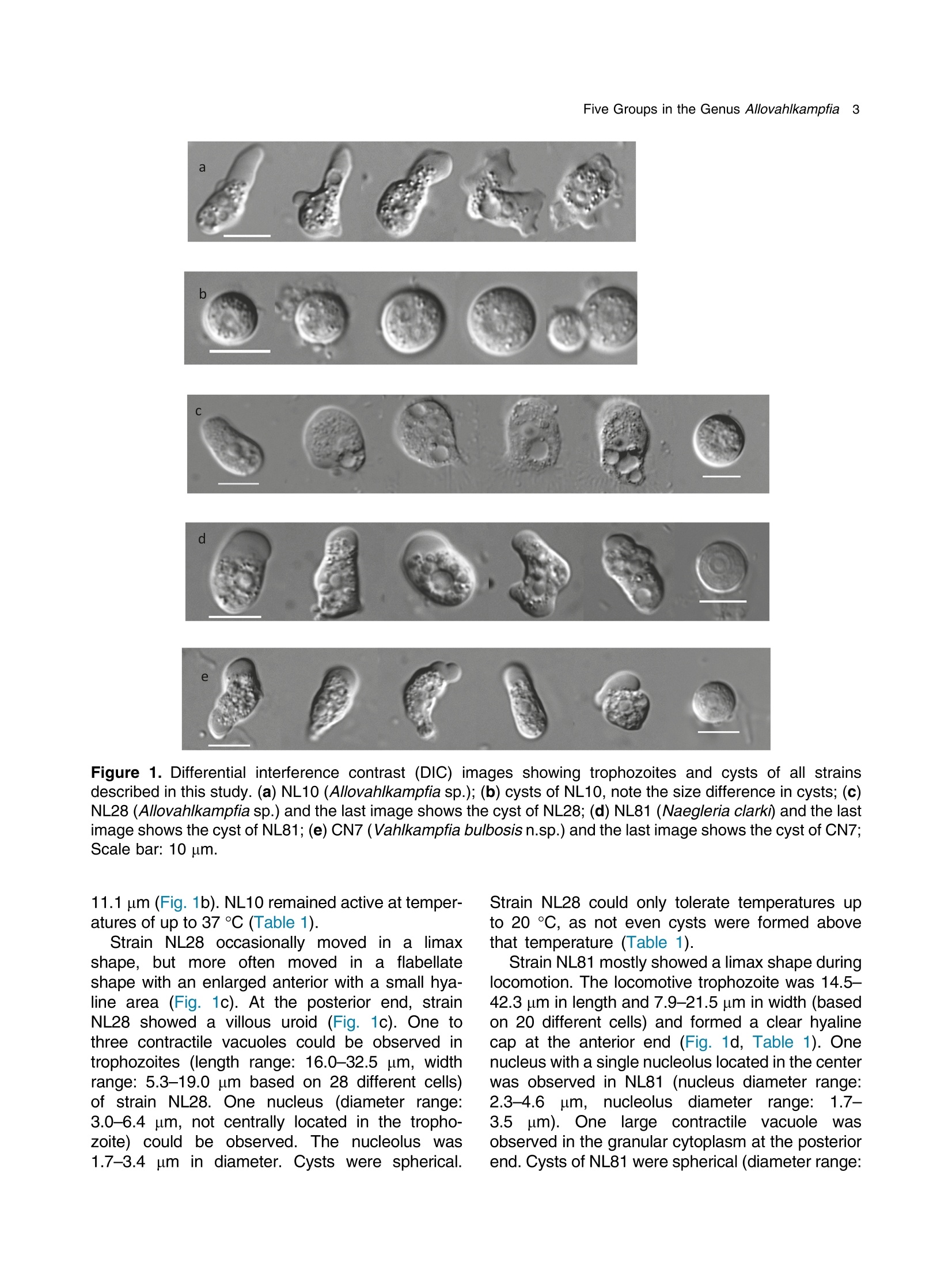
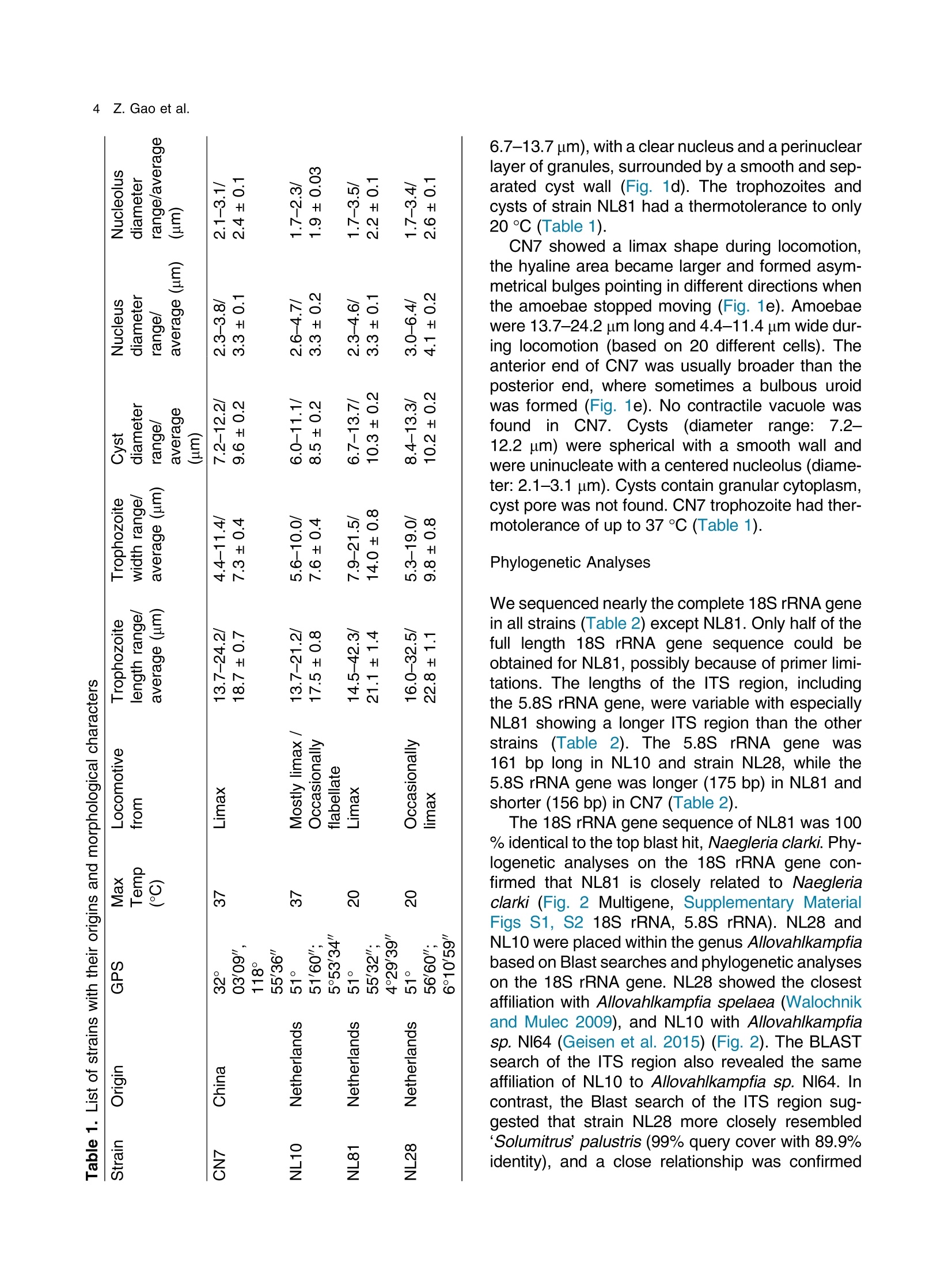
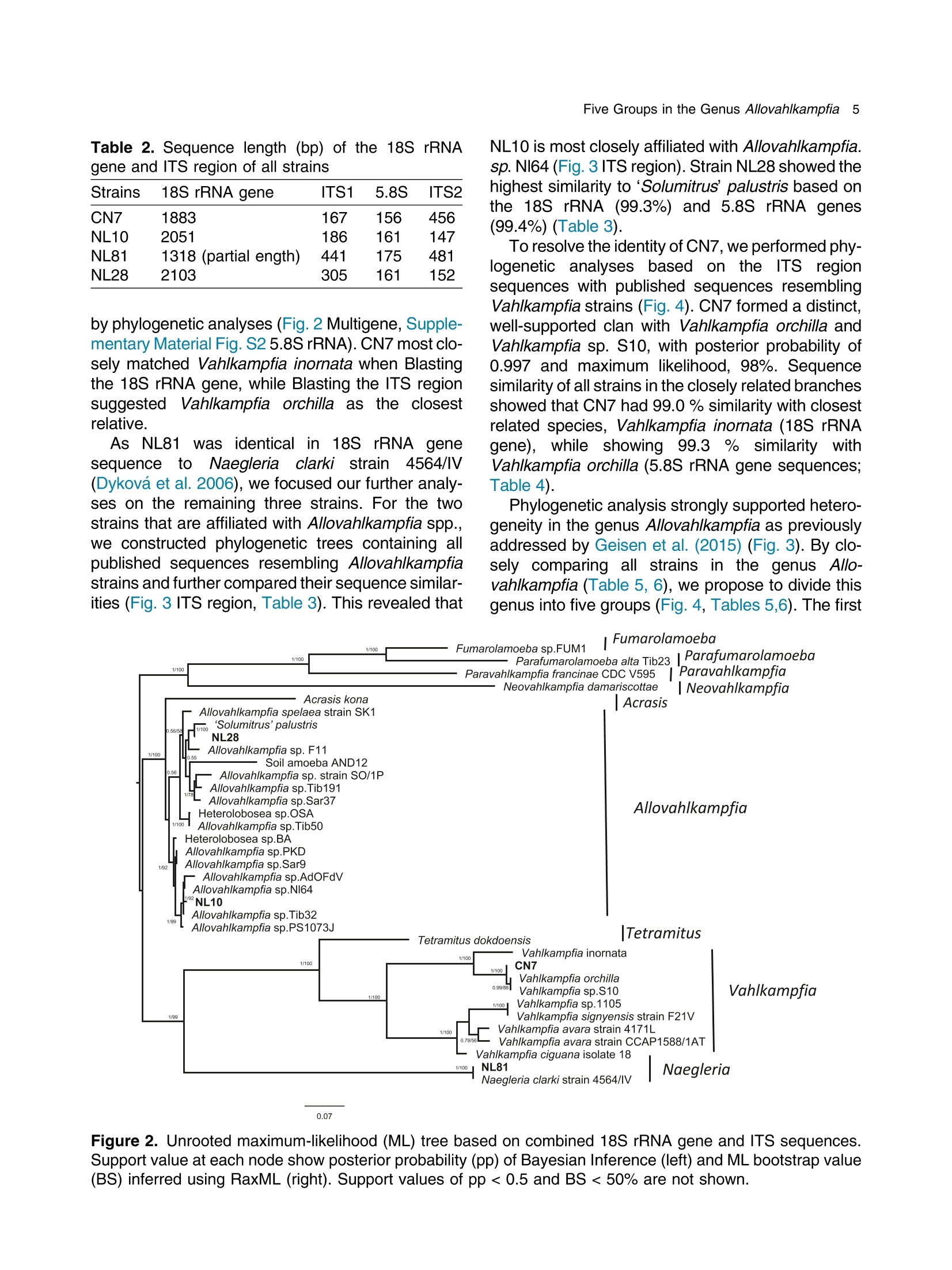
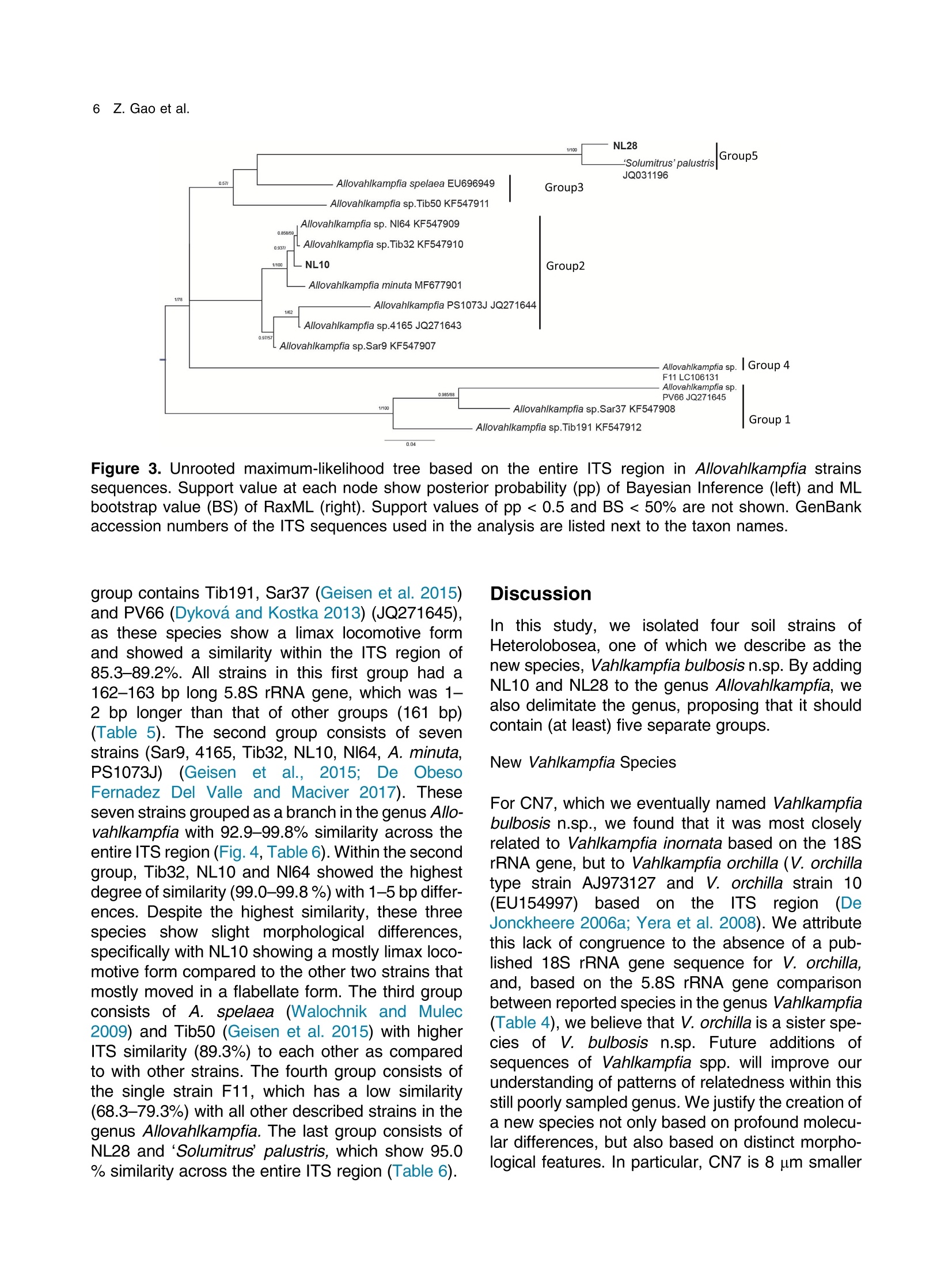
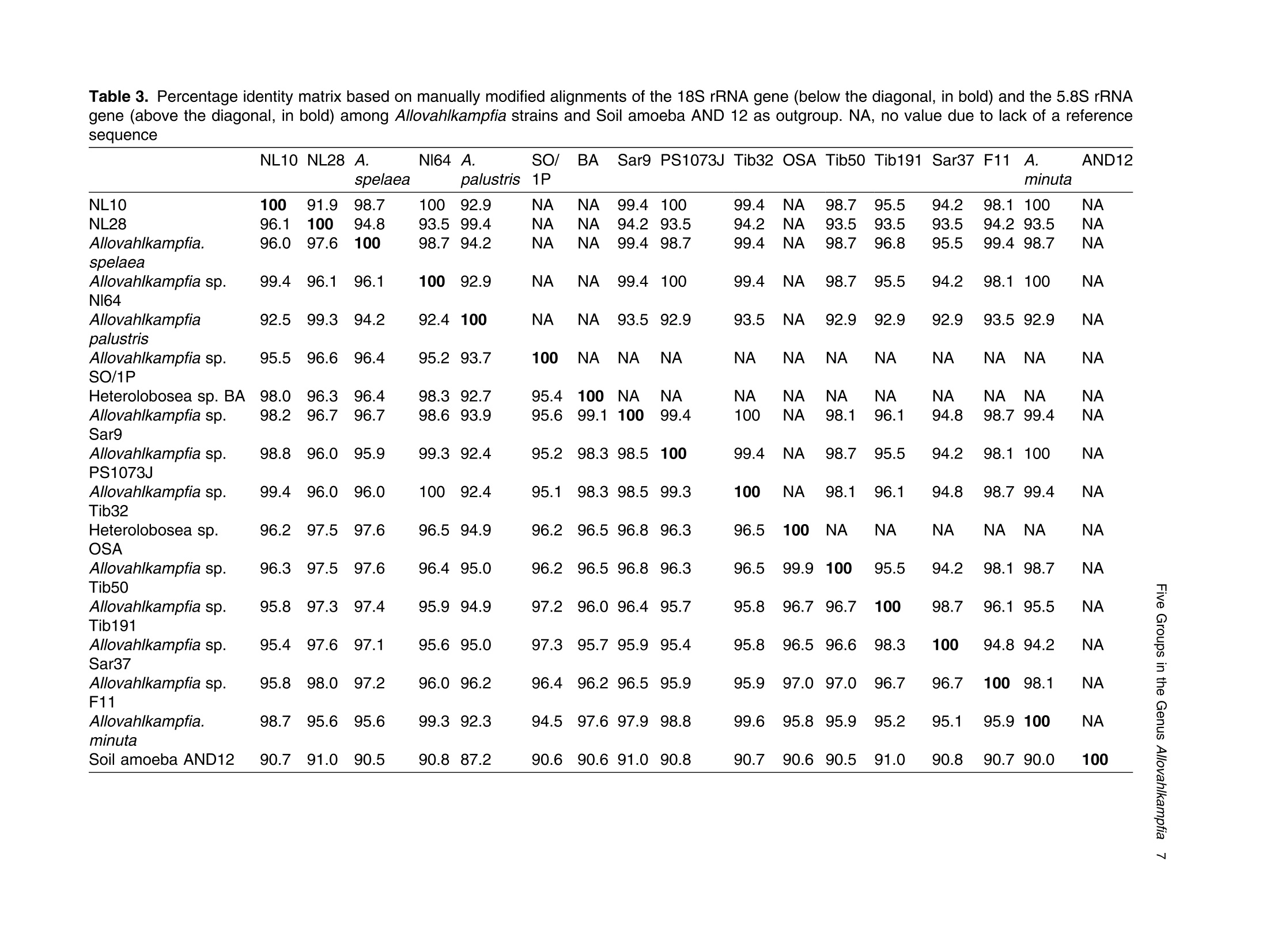
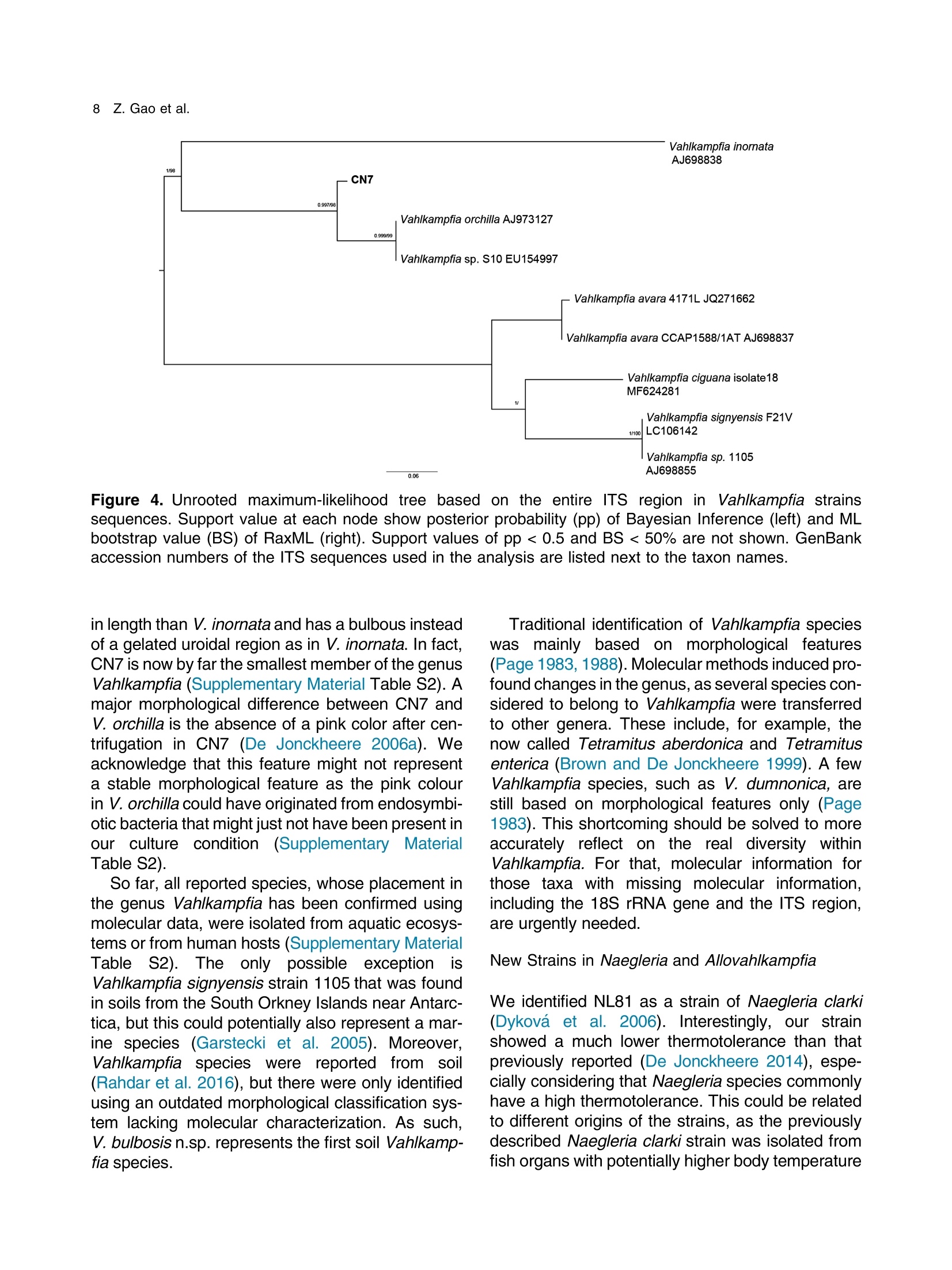
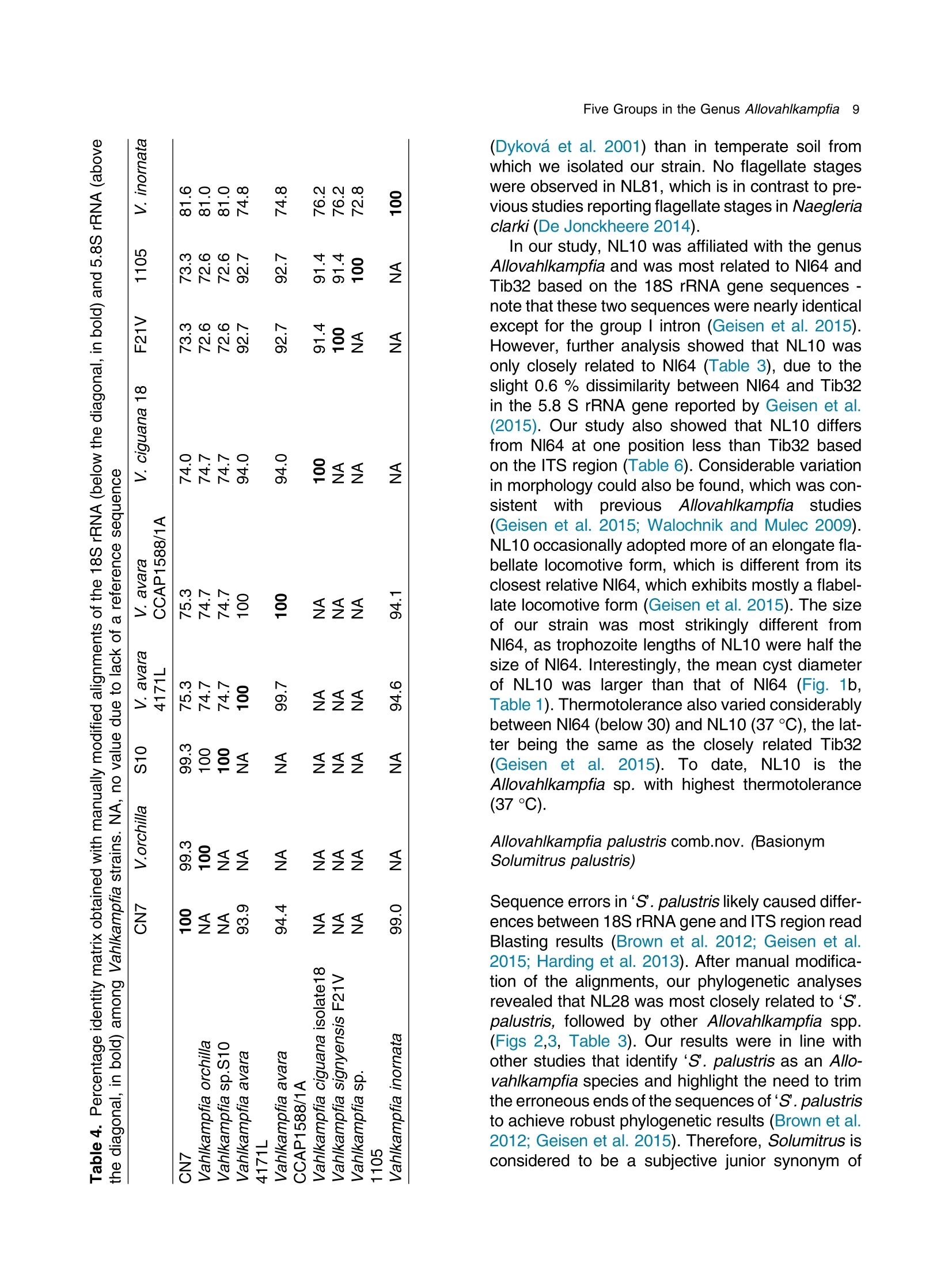
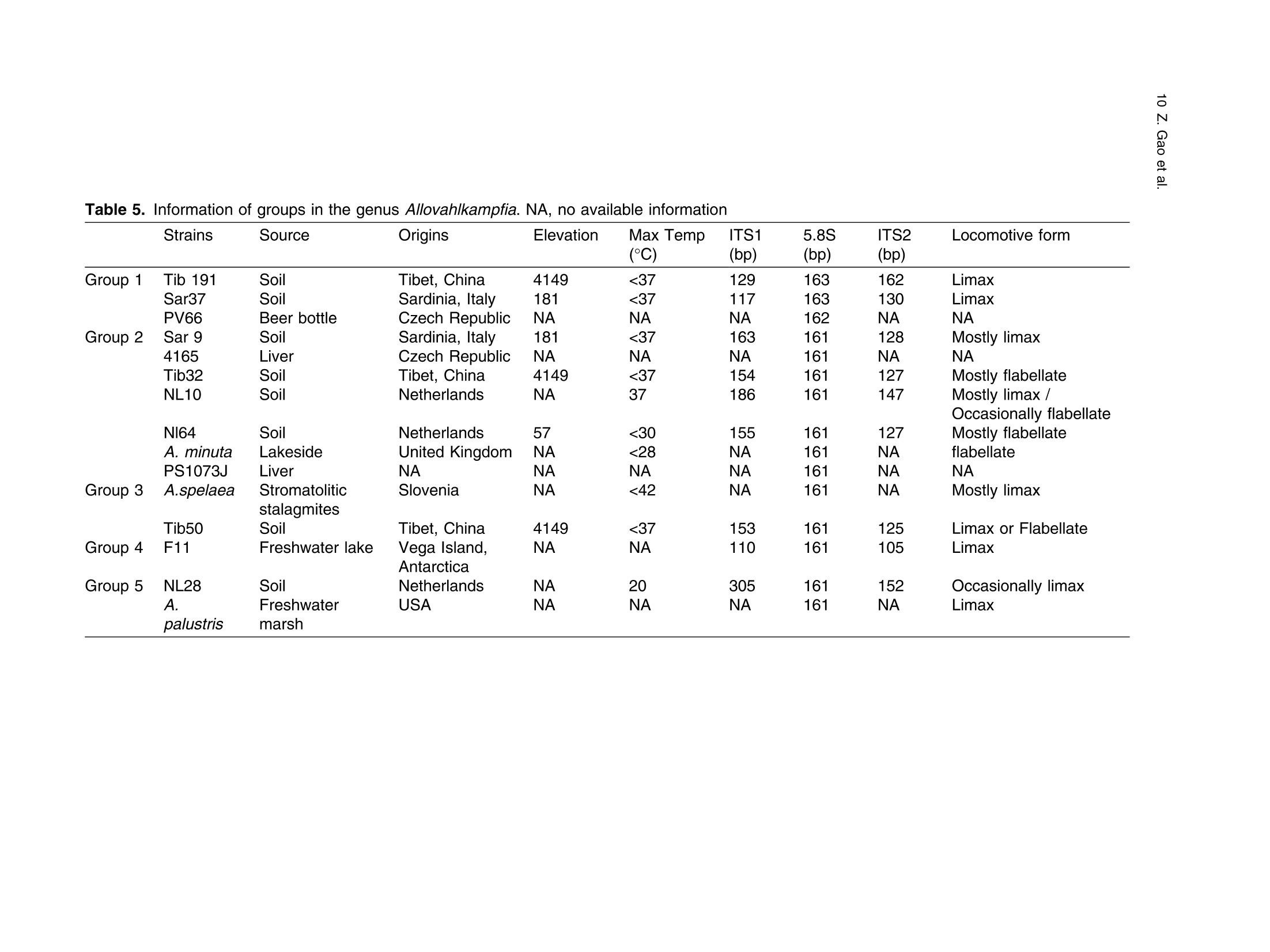
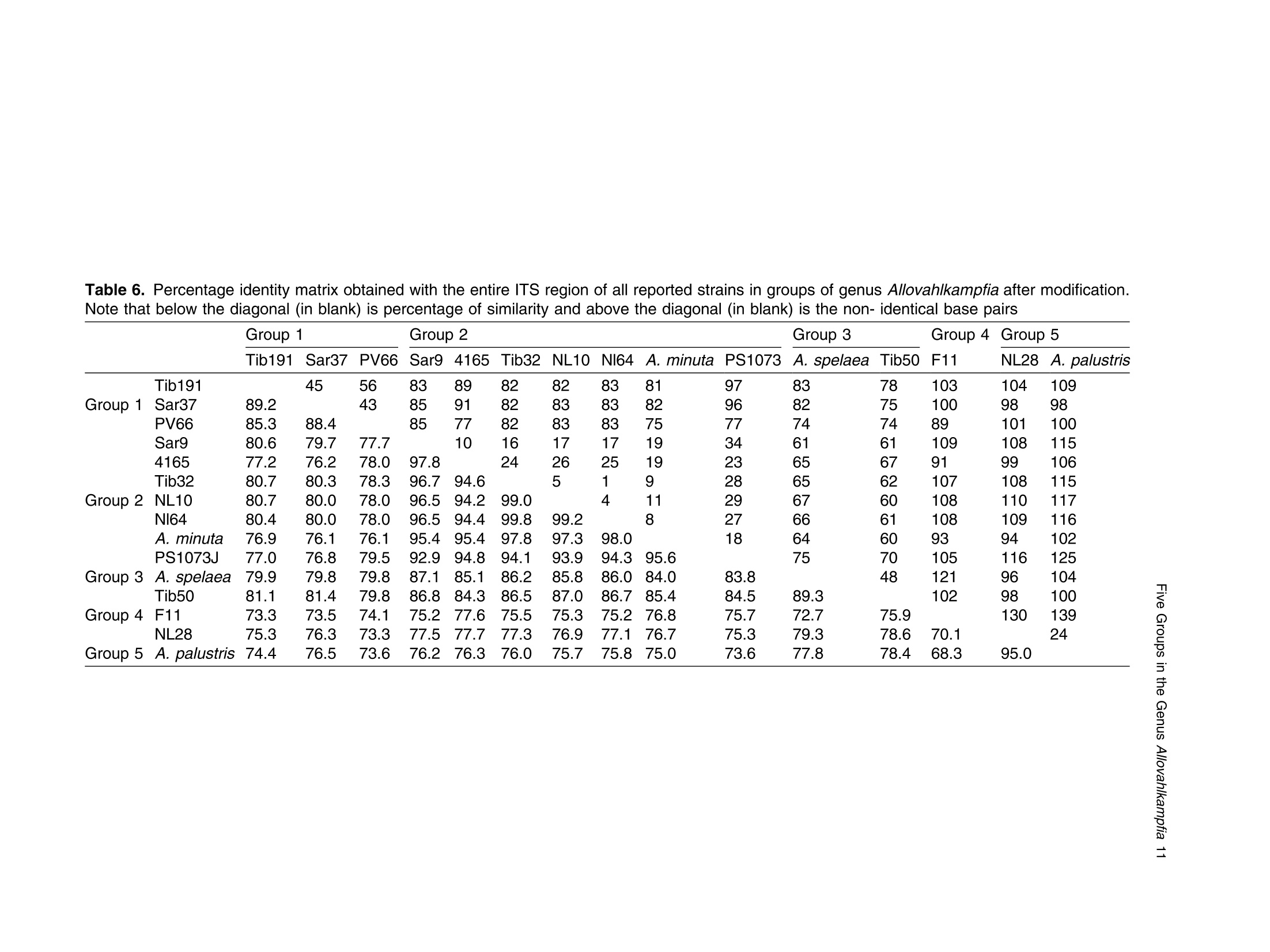
土壤悬浮液使用格哈特Laboshake振荡器振荡30分钟Soil suspensions were gently shaken for 30 min at a Laboshake (Gerhardt GmbH &Co. KG, Ko¨nigswinter, Germany), and one microliter of the mixed soilsuspension was pipetted into each well of 96-well plates (Costar,Corning, New York, USA) containing Escherichia coli OP50 (E.coli) as food source.Protist 173 (2022) 125870http://www.elsevier.de/protis Published online date 23 March 2022Protist 2 Z. Gao et al. ORIGINALPAPER 异球镰孢属五个类群及新种球镰孢的记述 Five Groups in the Genus Allovahlkampfia and the Description of the NewSpecies Vahlkampfia bulbosis n.sp. Zhilei Gao a ,1, Alexandre Jousset a , George A. Kowalchuk a , and Stefan Geisen b 乌得勒支大学环境生物学、生态和生物多样性研究所 aInstitue of Environmental Biology, Ecology&Biodiversity, Utrecht University, Padualaan 8, 3584 CHUtrecht, the Netherlands bDepartment ofPlantScience, Laboratory ofNematology,Wageningen University, 6700 ESWageningen, the Netherlands Submitted December 11, 2020; Accepted March 18, 2022 Monitoring Editor: Alastair Simpson 荷兰瓦赫宁根大学线虫病实验室植物科学系 Heterolobosea is one of themajor protist groups in soils.While an increasing number of soil heterolo-bosean species has been described, we have likely only scratched the surface of heterolobosean diversity in soils.Here,we expand this knowledge bymorphologically andmolecularly classifying four novel strains.Onewas identified as Naegleria clarki ,while the remaining three strains had no identical Blast hit againstGenBank and could only be reliably identified to the genus level: two strains as Allo-vahlkampfia spp. and one strain as Vahlkampfia sp.One Allovahlkampfia strainwasmost closely affil-iated with Allovahlkampfia sp. Nl64 and the other strain was affiliated with ‘Solumitrus ’ palustris , whichisnow named Allovahlkampfiapalustris comb.nov. As thereareonlytwo validspecies described within Allovahlkampfia , we combined all published sequences related to Allovahlkampfia and propose five new groups within this genus. The last strain was most closely related, but clearly distinct from,Vahlkampfia orchilla, based on DNA barcoding. As such, we propose this amoeba as a new species named Vahlkampfia bulbosis n.sp. Together, our study extends the described diversity of soil heteroloboseans through the description of a new Vahlkampfia species and by revising the morphologically and phylogenetically diverse genus Allovahlkampfia.◎ 2022 The Author(s). Published by Elsevier GmbH. This is an open access article under the CCBY license (http://creativecommons.org/licenses/by/4.0/). Key words: Allovahlkampfia ;Vahlkampfia bulbosis n.sp.; Heterolobosea; ITS region; 18S rRNA gene. Introduction Protistswithin theHeterolobosea have a global dis-tribution ranging from tropical to polar regions, 1434-4610/@ 2022 The Author(s). Published by Elsevier GmbH. This is an open access article under theCCBYlicense (http://creativecommons.org/licenses/by/4.0/). flagellates,amoeboflagellatesandacrasid“slime molds” (Brown et al. 2012; Brugerolle and Simpson 2004; Harding et al. 2013; Pa´nek et al.2017; Yubuki and Leander 2008). During locomo-tion, most heterolobosean amoebae adopt a cylin-drical limax shape with eruptive bulges (Page and Blanton 1985). Some heterolobosean species, including several Naegleria species (the mostwell-studied heterolobosean genus due to the particular attention paid to the human pathogenic Naegleria fowleri ), can shift between an amoeba and a flagel-late stage (De Jonckheere 2002, 2011, 2012; Visvesvara et al. 2007). Classical identification of heterolobosean species has relied on morphological characters (Page 1967).However,molecular tools have revealed that morphological characters can be ambiguous, with limited ability to distinguish between species (De Jonckheere and Brown 2005). Molecular sequenc-ing using barcoding regions such as the 18S rRNA gene and the ITS (internal transcribed spacer) region has substantially increased the taxonomic resolution within Heterolobosea and corrected the often erroneous taxon relatedness previously pro-posed based on morphological characters (Brown and De Jonckheere 1999; De Jonckheere and Brown 2005). For example, the genus Vahlkampfia has been shown to be genuinely polyphyletic, lead-ing to its subsequent division into several new gen-era including Neovahlkampfia , Paravahlkampfia, and reassignment of other species to Tetramitus (Brown andDe Jonckheere 1999). To date, approximately 150 heterolobosean spe-cies, including ca. 26 soil species, have been described (Anderson et al. 2011; Brown and De Jonckheere 2004; De Jonckheere 2002; De Jonckheere et al. 2011; De Obeso Fernadez Del Valle and Maciver 2017; Geisen et al. 2015; Murase et al. 2010; Sandon 1927). Soil isolates are underrepresented in the current phylogenies, with soil species described for seven out of the 35known heterolobosean genera: Naegleria , Allo-vahlkampfia, Fumarolamoeba, Parafumarolam-oeba, Paravahlkampfia, Tetramitus and Vrihiamoeba (Pa´nek et al. 2017). As explained above, due to its potential pathogenicity,Naegleria spp. have received a dis-proportionate amount of attention in research focused on (soil) Heterolobosea, which has led to a thorough molecular species definition within the genus Naegleria (De Jonckheere 2004). However, much less is known about other heterolobosean genera. Among Allovahlkampfia species, only two, A.spelaea (Walochnik andMulec 2009) and A.min-uta (De Obeso Fernadez Del Valle and Maciver 2017) have been formally described. A third spe-cies,whichwas originally described as ‘Solumitrus ’palustris (Anderson et al. 2011), has also repeatedly been shown to be placed within Allovahlkampfia (Brown et al. 2012;Geisen et al. 2015). The aim of the current studywas to increase the existing knowledgeconcerning the diversity and phylogeny of soil heterolobosean amoebae. To this end,we isolated four distinct heterolobosean amoe-bae from soils of different origins, examined each strain’smorphology and tested their ranges of ther-motolerance. We also sequenced the 18S rRNA gene and the ITS region, including the 5.8S rRNA gene, to allow for robust phylogenetic analysis of each strain. We could show that one strain was identical to Naegleria clarki,while two strains were affiliated to new species/strains of Allovahlkampfia and one strain represented a new species within Vahlkampfia. Results Cultivation and Morphological Identification Seven wells containingpotential heterolobosean amoebaewereobserved in 96-well plates.After puri-fying each strain, we isolated four heterolobosean strains.All strains exhibited a typical heterolobosean eruptive locomotion and showed an irregular shape with eruptive pseudopods projecting in various direc-tions during the non-locomotive stage (Fig. 1). All strains formed cysts (Fig. 1). No specific floating forms, flagellate stages, or formation of fruiting bodies could be observed across a range of test conditions for any of the strains examined. Strain NL10 mostly adopted an elongated limax locomotive form, but occasionally showed an elon-gated flabellate shape (Fig. 1a). A clear and large hyaline part was observed in the locomotive form. Trophozoites (length range: 13.7–21.2 m m, width range: 5.6–10.0 m m based on 11 different cells) of NL10were uninucleatewith one contractile vacuole being observed. One nucleus was observed that was not centrally located in the trophozoites (nu-cleus diameter range: 2.6–4.7 m m). The nucleolus was centrally located in the nucleus (diameter range: 1.7–2.3 m m). Cysts were spherical but pro-foundly differed in size, ranging from 6.0 to Figure1. Differentialinterferencecontrast(DIC) imagesshowingtrophozoitesandcystsofallstrains described in this study. (a )NL10 (Allovahlkampfia sp.); (b ) cysts ofNL10, note the size difference in cysts; (c ) NL28 (Allovahlkampfia sp.) and the last image shows the cyst ofNL28; (d )NL81 (Naegleria clarki ) and the last image shows the cyst ofNL81; (e )CN7 (Vahlkampfia bulbosis n.sp.) and the last image shows the cyst ofCN7; Scale bar: 10 m m. 11.1 m m(Fig. 1b).NL10 remained active at temper-atures of up to 37 ℃℃C (Table 1). Strain NL28 occasionally moved in a limax shape, but more often moved in a flabellate shape with an enlarged anterior with a small hya-line area(Fig. 1c). At the posterior end, strain NL28 showed a villous uroid (Fig. 1c). One to threecontractile vacuoles couldbeobservedin trophozoites (lengthrange:16.0–32.5 m m, width range: 5.3–19.0 m m based on 28 different cells) of strain NL28. One nucleus (diameter range:3.0–6.4 m m, not centrallylocatedinthetropho-zoite) could be observed. The nucleolus was 1.7–3.4m m indiameter. Cysts were spherical. Strain NL28 could only tolerate temperatures up to 20 °°C, as not even cysts were formed above that temperature (Table 1). StrainNL81mostly showed a limax shape during locomotion. The locomotive trophozoite was 14.5–42.3 m min length and 7.9–21.5 m minwidth (based on 20 different cells) and formed a clear hyaline cap at the anterior end (Fig. 1d, Table 1). One nucleuswith a single nucleolus located in the center was observed in NL81 (nucleus diameter range:2.3–4.6 m m, nucleolus diameter range: 1.7–3.5 m m). One large contractile vacuole was observed in the granular cytoplasm at the posterior end.Cysts ofNL81were spherical (diameter range: Z 80 寸 c ooao 6N6寸 a c c艹 coo CO o x一 coo c /11-09 o 寸NI IN oocao cc NIO c 一E> 寸 E三 >c 一Z I a CN7 showed a limax shape during locomotion, the hyaline area became larger and formed asym-metrical bulges pointing in different directionswhen the amoebae stopped moving (Fig. 1e). Amoebae were 13.7–24.2 m mlong and 4.4–11.4 m mwide dur-ing locomotion (based on 20 different cells). The anterior end of CN7 was usually broader than the posterior end, where sometimes a bulbous uroid was formed (Fig. 1e). No contractile vacuole was found in CN7. Cysts (diameter range: 7.2–12.2 m m) were spherical with a smooth wall and were uninucleatewith a centered nucleolus (diame-ter: 2.1–3.1 m m).Cysts contain granular cytoplasm, cyst porewas not found.CN7 trophozoite had ther-motolerance of up to 37 ℃℃C(Table 1). Phylogenetic Analyses Wesequenced nearly the complete 18SrRNAgene in all strains (Table 2) exceptNL81.Only half of the full length 18S rRNA gene sequence could be obtained for NL81, possibly because of primer limi-tations. The lengths of the ITS region, including the 5.8S rRNA gene, were variable with especially NL81 showing a longer ITS region than the other strains (Table 2). The 5.8S rRNA gene was 161 bp long in NL10 and strain NL28, while the 5.8S rRNA gene was longer (175 bp) in NL81 and shorter (156 bp) inCN7 (Table 2). The 18S rRNAgene sequence ofNL81was 100% identical to the top blast hit,Naegleria clarki .Phy-logeneticanalyses on the18S rRNA gene con-firmed that NL81 iscloselyrelatedto Naegleria clarki (Fig.2 Multigene, Supplementary Material Figs S1, S2 18S rRNA, 5.8S rRNA). NL28 and NL10were placedwithin the genus Allovahlkampfia based onBlast searches and phylogenetic analyses on the 18S rRNA gene. NL28 showed the closest affiliation with Allovahlkampfia spelaea (Walochnik and Mulec 2009), and NL10 with Allovahlkampfia sp.Nl64 (Geisen et al. 2015) (Fig. 2). The BLAST search of the ITS region also revealed the same affiliation of NL10 to Allovahlkampfia sp. Nl64. In contrast, the Blast search of the ITS region sug-gested that strain NL28 more closely resembled ‘Solumitrus ’ palustris (99% query cover with 89.9%identity), and a close relationship was confirmed Table2. Sequencelength(bp)ofthe18S rRNA gene and ITS region of all strains Strains 18S rRNA gene ITS1 5.8S ITS2 CN7 1883 167 156 456 NL10 2051 186 161 147 NL81 1318 (partial ength) 441 175 481 NL28 2103 305 161 152 by phylogenetic analyses (Fig. 2Multigene,Supple-mentaryMaterial Fig.S2 5.8SrRNA).CN7most clo-sely matched Vahlkampfia inornata when Blasting the 18S rRNA gene, while Blasting the ITS region suggested Vahlkampfia orchilla as the closest relative. As NL81 was identical in 18S rRNA gene sequence to Naegleria clarki strain 4564/IV (Dykova´ et al. 2006),we focused our further analy-ses on the remaining three strains. For the two strains that are affiliated with Allovahlkampfia spp., we constructedphylogenetictreescontainingall publishedsequencesresembling Allovahlkampfia strains and further compared their sequence similar-ities (Fig. 3 ITS region, Table 3). This revealed that NL10 ismost closely affiliatedwith Allovahlkampfia. sp .Nl64 (Fig. 3 ITSregion).StrainNL28 showed the highest similarity to ‘Solumitrus ’ palustris based on the 18S rRNA (99.3%) and 5.8S rRNA genes (99.4%) (Table 3). To resolve the identity ofCN7,weperformed phy-logenetic analyses based on the ITS region sequences with published sequences resembling Vahlkampfia strains (Fig. 4).CN7 formed a distinct, well-supported clan with Vahlkampfia orchilla and Vahlkampfia sp. S10, with posterior probability of 0.997 and maximum likelihood,98%. Sequence similarity of all strains in the closely related branches showed thatCN7 had 99.0% similaritywith closest related species,Vahlkampfia inornata (18S rRNA gene), while showing 99.3 % similarity with Vahlkampfia orchilla (5.8S rRNA gene sequences; Table 4). Phylogenetic analysis strongly supported hetero-geneity in the genus Allovahlkampfia as previously addressed by Geisen et al. (2015) (Fig. 3). By clo-sely comparing all strains in the genus Allo-vahlkampfia (Table 5, 6), we propose to divide this genus into five groups (Fig. 4, Tables 5,6). The first 0.07 Figure 2. Unrooted maximum-likelihood (ML) tree based on combined 18S rRNA gene and ITS sequences. Support value at each node show posterior probability (pp) ofBayesian Inference (left) andML bootstrap value (BS) inferred using RaxML (right). Support values of pp < 0.5 and BS < 50% are not shown. Figure3. Unrooted maximum-likelihoodtreebasedontheentireITS regionin Allovahlkampfia strains sequences. Support value at each node show posterior probability (pp) of Bayesian Inference (left) and ML bootstrap value (BS) of RaxML (right). Support values of pp < 0.5 and BS < 50% are not shown. GenBank accession numbers of the ITS sequences used in the analysis are listed next to the taxon names. group contains Tib191, Sar37 (Geisen et al. 2015) and PV66 (Dykov´a and Kostka 2013) (JQ271645), as these species show a limax locomotive form and showed a similarity within the ITS region of 85.3–89.2%. All strains in this first group had a 162–163 bp long 5.8S rRNA gene, which was 1–2bplongerthanthatofothergroups(161bp)(Table5).Thesecond groupconsistsofseven strains (Sar9, 4165, Tib32, NL10, Nl64,A.minuta , PS1073J) (Geisen et al., 2015; De Obeso Fernadez Del Valle and Maciver 2017). These seven strains grouped as a branch in the genus Allo-vahlkampfia with 92.9–99.8% similarity across the entire ITSregion (Fig. 4, Table 6).Within the second group, Tib32, NL10 and Nl64 showed the highest degree of similarity (99.0–99.8%)with 1–5 bp differ-ences. Despite the highest similarity, these three species show slight morphological differences, specificallywithNL10 showing amostly limax loco-motive form compared to the other two strains that mostly moved in a flabellate form. The third group consists of A. spelaea (Walochnik and Mulec 2009) and Tib50 (Geisen et al. 2015) with higher ITS similarity (89.3%) to each other as compared to with other strains. The fourth group consists of the single strain F11, which has a low similarity (68.3–79.3%)with all other described strains in the genus Allovahlkampfia. The last group consists of NL28 and ‘Solumitrus ’ palustris,which show 95.0% similarity across the entire ITS region (Table 6). Discussion In this study, we isolated four soil strains of Heterolobosea, one of which we describe as the new species,Vahlkampfia bulbosis n.sp.By adding NL10 and NL28 to the genus Allovahlkampfia , we also delimitate the genus, proposing that it should contain (at least) five separate groups. New Vahlkampfia Species For CN7,which we eventually named Vahlkampfia bulbosis n.sp., we found that it was most closely related to Vahlkampfia inornata based on the 18S rRNA gene, but to Vahlkampfia orchilla (V. orchilla type strain AJ973127 and V. orchilla strain 10(EU154997) based on the ITS region (De Jonckheere 2006a; Yera et al. 2008). We attribute this lack of congruence to the absence of a pub-lished 18S rRNA gene sequence for V. orchilla, and, based on the 5.8S rRNA gene comparison between reported species in the genus Vahlkampfia (Table 4),we believe that V. orchilla is a sister spe-cies of V. bulbosis n.sp. Future additions of sequences of Vahlkampfia spp. will improve our understanding of patterns of relatednesswithin this still poorly sampled genus .Wejustify the creation of a new species not only based on profoundmolecu-lar differences, but also based on distinct morpho-logical features. In particular, CN7 is 8 m m smaller Table 3. Percentage identity matrix based on manually modified alignments of the 18S rRNA gene (below the diagonal, in bold) and the 5.8S rRNA gene (above the diagonal, in bold) among Allovahlkampfia strains and Soil amoeba AND 12 as outgroup. NA, no value due to lack of a reference sequence NL10 NL28 A. Nl64 A. SO/ BA Sar9 PS1073J Tib32 OSA Tib50 Tib191 Sar37 F11 A. AND12 spelaea palustris 1P minuta NL10 100 91.9 98.7 100 92.9 NA NA 99.4 100 99.4 NA 98.7 95.5 94.2 98.1 100 NA NL28 96.1 100 94.8 93.5 99.4 NA NA 94.2 93.5 94.2 NA 93.5 93.5 93.5 94.2 93.5 NA Allovahlkampfia. 96.0 97.6 100 98.7 94.2 NA NA 99.4 98.7 99.4 NA 98.7 96.8 95.5 99.4 98.7 NA spelaea Allovahlkampfia sp. 99.4 96.1 96.1 100 92.9 NA NA 99.4 100 99.4 NA 98.7 95.5 94.2 98.1 100 NA Nl64 Allovahlkampfia 92.5 99.3 94.2 92.4 100 NA NA 93.5 92.9 93.5 NA 92.9 92.9 92.9 93.5 92.9 NA palustris Allovahlkampfia sp. 95.5 96.6 96.4 95.2 93.7 100 NA NA NA NA NA NA NA NA NA NA NA SO/1P Heterolobosea sp. BA 98.0 96.3 96.4 98.3 92.7 95.4 100 NA NA NA NA NA NA NA NA NA NA Allovahlkampfia sp. 98.2 96.7 96.7 98.6 93.9 95.6 99.1 100 99.4 100 NA 98.1 96.1 94.8 98.7 99.4 NA Sar9 Allovahlkampfia sp. 98.8 96.0 95.9 99.3 92.4 95.2 98.3 98.5 100 99.4 NA 98.7 95.5 94.2 98.1 100 NA PS1073J Allovahlkampfia sp. 99.4 96.0 96.0 100 92.4 95.1 98.3 98.5 99.3 100 NA 98.1 96.1 94.8 98.7 99.4 NA Tib32 Heterolobosea sp. 96.2 97.5 97.6 96.5 94.9 96.2 96.5 96.8 96.3 96.5 100 NA NA NA NA NA NA OSA Allovahlkampfia sp. 96.3 97.5 97.6 96.4 95.0 96.2 96.5 96.8 96.3 96.5 99.9 100 95.5 94.2 98.1 98.7 NA Tib50 Allovahlkampfia sp. 95.8 97.3 97.4 95.9 94.9 97.2 96.0 96.4 95.7 95.8 96.7 96.7 100 98.7 96.1 95.5 NA Tib191 Allovahlkampfia sp. 95.4 97.6 97.1 95.6 95.0 97.3 95.7 95.9 95.4 95.8 96.5 96.6 98.3 100 94.8 94.2 NA Sar37 Allovahlkampfia sp. 95.8 98.0 97.2 96.0 96.2 96.4 96.2 96.5 95.9 95.9 97.0 97.0 96.7 96.7 100 98.1 NA F11 Allovahlkampfia. 98.7 95.6 95.6 99.3 92.3 94.5 97.6 97.9 98.8 99.6 95.8 95.9 95.2 95.1 95.9 100 NA minuta Soil amoeba AND12 90.7 91.0 90.5 90.8 87.2 90.6 90.6 91.0 90.8 90.7 90.6 90.5 91.0 90.8 90.7 90.0 100 Figure 4. Unrooted maximum-likelihood tree based on the entire ITS region in Vahlkampfia strains sequences. Support value at each node show posterior probability (pp) of Bayesian Inference (left) and ML bootstrap value (BS) of RaxML (right). Support values of pp < 0.5 and BS < 50% are not shown. GenBank accession numbers of the ITS sequences used in the analysis are listed next to the taxon names. in length than V. inornata and has a bulbous instead of a gelated uroidal region as in V. inornata . In fact, CN7 is nowby far the smallestmember of the genus Vahlkampfia (SupplementaryMaterial TableS2).A major morphological difference between CN7 and V. orchilla is the absence of a pink color after cen-trifugation in CN7 (De Jonckheere 2006a ). We acknowledge that this feature might not represent a stable morphological feature as the pink colour in V. orchilla could have originated fromendosymbi-otic bacteria thatmight just not have been present in our culture condition (Supplementary Material TableS2). So far, all reported species, whose placement in the genus Vahlkampfia has been confirmed using molecular data,were isolated from aquatic ecosys-tems or from human hosts (SupplementaryMaterial Table S2). The only possible exception is Vahlkampfia signyensis strain 1105 that was found in soils from theSouthOrkney Islands nearAntarc-tica, but this could potentially also represent amar-ine species (Garstecki et al. 2005). Moreover, Vahlkampfia species were reported from soil (Rahdar et al. 2016), but there were only identified using an outdated morphological classification sys-tem lacking molecular characterization. As such, V. bulbosis n.sp. represents the first soil Vahlkamp-fia species. Traditional identification of Vahlkampfia species was mainly based on morphological features (Page 1983, 1988).Molecularmethods induced pro-found changes in the genus, as several species con-sidered to belong to Vahlkampfia were transferred to other genera. These include, for example, the now called Tetramitus aberdonica and Tetramitus enterica (Brown and De Jonckheere 1999). A few Vahlkampfia species, such as V. dumnonica, are still based on morphological features only (Page 1983). This shortcoming should be solved to more accurately reflect on the real diversity within Vahlkampfia.For that, molecular information for those taxa with missing molecular information, including the 18S rRNA gene and the ITS region, are urgently needed. NewStrains in Naegleria and Allovahlkampfia We identified NL81 as a strain of Naegleria clarki (Dykova´ et al. 2006). Interestingly, our strain showed a much lower thermotolerance than that previously reported (De Jonckheere 2014), espe-cially considering that Naegleria species commonly have a high thermotolerance. This could be related to different origins of the strains, as the previously described Naegleria clarki strain was isolated from fish organswith potentially higher body temperature Z c 令 c o Z c o c c o o 寸 寸 ¥66 O ZO o woawcO寸O ava wc> v 0t6 ZO 6 寸寸6 O ->N (Dykova´et al. 2001) than in temperate soil from which we isolated our strain. No flagellate stages were observed inNL81,which is in contrast to pre-vious studies reporting flagellate stages in Naegleria clarki (De Jonckheere 2014). In our study, NL10 was affiliated with the genus Allovahlkampfia and was most related to Nl64 and Tib32 based on the 18S rRNA gene sequences -note that these two sequenceswere nearly identical except for the group I intron (Geisen et al. 2015). However, further analysis showed that NL10 was only closely related to Nl64 (Table 3), due to the slight 0.6 % dissimilarity between Nl64 and Tib32in the 5.8 S rRNA gene reported by Geisen et al.(2015). Our study also showed that NL10 differs from Nl64 at one position less than Tib32 based on the ITS region (Table 6).Considerable variation inmorphology could also be found,whichwas con-sistent with previous Allovahlkampfia studies (Geisen et al. 2015; Walochnik and Mulec 2009). NL10 occasionally adoptedmore of an elongate fla-bellate locomotive form, which is different from its closest relativeNl64,which exhibitsmostly a flabel-late locomotive form (Geisen et al. 2015). The size of our strain was most strikingly different from Nl64, as trophozoite lengths of NL10 were half the size of Nl64. Interestingly, the mean cyst diameter of NL10 was largerthan thatof Nl64 (Fig.1b, Table 1). Thermotolerance also varied considerably betweenNl64 (below 30) andNL10 (37 ℃℃C), the lat-ter being the same as the closely related Tib32(Geisen et al. 2015). To date, NL10 is the Allovahlkampfia sp . with highest thermotolerance (37 ℃℃C). Allovahlkampfia palustris comb.nov. (Basionym Solumitrus palustris) Sequence errors in ‘S ’. palustris likely caused differ-ences between 18SrRNAgene and ITSregion read Blasting results (Brown et al. 2012; Geisen et al.2015; Harding et al. 2013). After manual modifica-tion of the alignments, our phylogenetic analyses revealed thatNL28wasmost closely related to ‘S ’. palustris,followed by other Allovahlkampfia spp.(Figs 2,3, Table 3). Our results were in line with other studies that identify ‘S ’. palustris as an Allo-vahlkampfia species and highlight the need to trim the erroneous ends of the sequences of ‘S ’. palustris to achieve robust phylogenetic results (Brown et al.2012; Geisen et al. 2015). Therefore,Solumitrus is considered to be a subjective junior synonym of Table 5. Information of groups in the genus Allovahlkampfia . NA, no available information Strains Source Origins Elevation Max Temp ITS1 5.8S ITS2 Locomotive form (C) (bp) (bp) (bp) Group 1 Tib 191 Soil Tibet, China 4149 <37 129 163 162 Limax Sar37 Soil Sardinia, Italy 181 <37 117 163 130 Limax Group 2 PV66 Beer bottle Czech Republic NA NA NA 162 NA NA Sar 9 Soil Sardinia, Italy 181 <37 163 161 128 Mostly limax 4165 Liver Czech Republic NA NA NA 161 NA NA Tib32 Soil Tibet, China 4149 <37 154 161 127 Mostly flabellate NL10 Soil Netherlands NA 37 186 161 147 Mostly limax / Occasionally flabellate Nl64 Soil Netherlands 57 <30 155 161 127 Mostly flabellate A.minuta Lakeside United Kingdom NA <28 NA 161 NA flabellate PS1073J Liver NA NA NA NA 161 NA NA Group 3 A.spelaea Stromatolitic Slovenia NA <42 NA 161 NA Mostly limax stalagmites Tib50 Soil Tibet, China 4149 <37 153 161 125 Limax or Flabellate Group 4 F11 Freshwater lake Vega Island, NA NA 110 161 105 Limax Antarctica Group 5 NL28 Soil Netherlands NA 20 305 161 152 Occasionally limax A. Freshwater USA NA NA NA 161 NA Limax palustris marsh Table 6. Percentage identity matrix obtained with the entire ITS region of all reported strains in groups of genus Allovahlkampfia after modification. Note that below the diagonal (in blank) is percentage of similarity and above the diagonal (in blank) is the non- identical base pairs Group 1 Group 2 Group 3 Group 4 Group 5 Tib191 Sar37 PV66 Sar9 4165 Tib32 NL10 Nl64 A.minuta PS1073 A. spelaea Tib50 F11 NL28 A. palustris Tib191 45 56 83 89 82 82 83 81 97 83 78 103 104 109 Group 1 Sar37 89.2 43 85 91 82 83 83 82 96 82 75 100 98 98 PV66 85.3 88.4 85 77 82 83 83 75 77 74 74 89 101 100 Sar9 80.6 79.7 77.7 10 16 17 17 19 34 61 61 109 108 115 4165 77.2 76.2 78.0 97.8 24 26 25 19 23 65 67 91 99 106 Tib32 80.7 80.3 78.3 96.7 94.6 5 1 9 28 65 62 107 108 115 Group 2 NL10 80.7 80.0 78.0 96.5 94.2 99.0 4 11 29 67 60 108 110 117 Nl64 80.4 80.0 78.0 96.5 94.4 99.8 99.2 8 27 66 61 108 109 116 A.minuta 76.9 76.1 76.1 95.4 95.4 97.8 97.3 98.0 18 64 60 93 94 102 PS1073J 77.0 76.8 79.5 92.9 94.8 94.1 93.9 94.3 95.6 75 70 105 116 125 Group 3 A. spelaea 79.9 79.8 79.8 87.1 85.1 86.2 85.8 86.0 84.0 83.8 48 121 96 104 Tib50 81.1 81.4 79.8 86.8 84.3 86.5 87.0 86.7 85.4 84.5 89.3 102 98 100 Group 4 F11 73.3 73.5 74.1 75.2 77.6 75.5 75.3 75.2 76.8 75.7 72.7 75.9 130 139 NL28 75.3 76.3 73.3 77.5 77.7 77.3 76.9 77.1 76.7 75.3 79.3 78.6 70.1 24 Group 5 A. palustris 74.4 76.5 73.6 76.2 76.3 76.0 75.7 75.8 75.0 73.6 77.8 78.4 68.3 95.0 Allovahlkampfia , and Solumitrus palustris is trans-ferred as Allovahlkampfia palustris comb.nov. ASubdivision of the Genus Allovahlkampfia Based upon ITSsequence analyses that include two new Allovahlkampfia strains,wepropose five groups of described Allovahlkampfia strains (Fig. 3). This proposal reflects previous observations of hetero-geneity within the genus Allovahlkampfia (Geisen et al. 2015).Strains from the first group always har-bored 5.8S rRNAgenes that are 1–2 bp longer than those observed for the other groups (Table 6), and both of the two described strains (Tib191 and Sar37) were morphologically similar and displayed the limax locomotive form (Geisen et al. 2015). The secondgroupincludedsevenstrains, in which Nl64, NL10 and Tib32 showed only 1–5 bp length differences in the 5.8SrRNAgene.However, these three strains displayed slight differences in their locomotive form; Tib32 and Nl64 showed mostly a flabellate shape, while NL10 showed mostly a limax shape and occasionally flabellate and Sar 9 moved entirely in a limax shape (Fig. 1a, Table 5).NL10might represent an interme-diatemorphotype between limax and flabellate. The fourth group was composed of the single Antarctic strain, F11, that potentially is unique to extreme conditionsand mightrepresentapolar group such as found for the Naegleria polar cluster (De Jonckheere 2006b; Tyml et al. 2016).However additional polar Allovahlkampfia strains would be required before such a polar Allovahlkampfia group might be identified. Allovahlkampfia palustris comb.nov. and NL28arestrainsinthefifthgroupofthegenus Allo-vahlkampfia , as these two strains showed the high-est similarity with each other (Tables 5,6). We suggested the five groups mainly based on ITS sequences, however, species that lack reference ITS sequences were not included in this group. For example, our phylogenetic analysis also con-firmed that Heterolobosea OSA had 94.9–97.6%similaritywith other Allovahlkampfia species. Future studies utilizing new molecular data for identifying the groups within the genus Allovahlkampfia are needed to confirm the group definition. Conclusions We report strainCN7 as a new species,Vahlkamp-fia bulbosis n.sp. Furthermore, we identified two novel strains that allowed us to subdivide the genus Allovahlkampfia into 5 groups based onmorpholog-ical and (mainly) molecular information. Together, our study extends the knowledge on soil Heterolo-bosea, but also suggests that we are still far from having captured a complete inventory of this group of soil protists. Future studies should prioritize the discovery of the unknown diversity amongHeterolo-bosea,which seemespecially abundant and diverse in soils. These should include the taxa described in pre-molecular times to infermore accurate phyloge-nies, while additionally describing novel taxa that seemto bewidespread.Additional gene information to the ITS and 18S rRNA genewill also be needed (such as with single-cell transcriptomics (Kang et al. 2017; Tice et al. 2016)) tomore deeply under-stand the evolutionary origin of this group and the relationships between members within Heterolo-bosea (Burki et al. 2020;Simpson et al. 2006). Taxonomic Summary Phylum:Heterolobosea. Clade: Tetramitia. Clade:Eutetramitia. Family:Vahlkampfiidae. Genus:Vahlkampfia. Species:Vahlkampfia bulbosis n.sp. Diagnosis: Trophozoites 13.7–24.2 m m (average 18.7m m) inlength and 4.4–11.4m m (average 7.3 m m) inwidth; limax locomotionwith highly erup-tive pseudopodia; broader anterior than posterior end,where sometimes a bulbous uroidwas formed; no contractile vacuole visible.Spherical cystswith a smoothwall and uninucleatewith a centered nucle-olus (nucleus diameter: 3.3 ± 0.1 m m, nucleolus diameter: 2.4 ± 0.1 m m); Thermotolerance of up to 37 °°C. Etymology: bulbosis referring to the bulbous uroid formed byCN7. Type locality: Qilin, Jiangsu, China (320°0300900,118°°5503600). Typematerial:A permanentmicroscopic slide of strainCN7 fixed byBouin-Hollande’s fluid following themethod of Pa´nek et al. (2014), including tropho-zoites and cysts, has been deposited at Utrecht University,Department ofBiology,Group ofEcology and Biodiversity (Accession number EB-P-001). This slide represents the name-bearing type of the species (an hapantotype). A culture of the strain CN7 has been deposited at the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures GmbH under the accession number DSM 113307. Phylum:Heterolobosea. Clade:Tetramitia. Clade:Eutetramitia. Order:Acrasida. Family:Acrasidae. Genus: Allovahlkampfia Walochnikand Mulec 2009. Species: Allovahlkampfia palustris (Anderson et al. 2011) comb.nov. (basionym Solumitrus palustris ). Remarks: Solumitrus was suggested to sub-sumed to Allovahlkampfia in Brown et al. 2012 and Geisen et al. 2015 and it is nested in Allovahlkamp-fia in phylogenetic trees (Fig. 2). Therefore,Solu-mitrus is considered to be a subjective junior synonymof Allovahlkampfia.Species nowassigned to Allovahlkampfia are: Allovahlkampfia spelaea (Walochnik and Mulec 2009); Allovahlkampfia palustris (Andersonetal.2011;basionym Solu-mitrus palustris); Allovahlkampfia minuta (De Obeso FernadezDelValle andMaciver 2017); Strains included: Group1: Tib 191, Sar 37(Geisen et al. 2015), PV66 (Dykova´and Kostka 2013) (JQ271645); Group2: Sar9, Tib32, Nl64(Geisen et al. 2015), NL10, 4165 (JQ271643), PS1073J (JQ271644); Group3: Tib50 (Geisen et al. 2015); Group4: F11 (Tyml et al. 2016); Group5:NL28. Methods Isolation and cultivation: Four strains were isolated from three different soils;NL81was isolated from a green house inRotterdam, the Netherlands (51 w 5503200; 4d 2903900); CN7 was isolated from the rhizosphere of tomato in Qilin town, Jiangsu province, China (32°°0300900, 118of 5503600);NL28was isolated from a grassland soil in the province Friesland, theNetherlands (51d 5606000; 6ss 1005900);NL10was isolated from rhizosphere soil of Centaurea stoebe in the Nether-lands (51la 5106000; 5rh 5303400). Isolations used one gramof soil sample suspended in 20mLPAS(Page’sAmoebaSaline).Soil suspensions were gently shaken for 30 min at a Laboshake (Gerhardt GmbH&Co.KG,Ko¨nigswinter,Germany), and onemicroliter of themixed soil suspension was pipetted into each well of 96-well plates (Costar, Corning, NewYork, USA) containing Escherichia coli OP50 (E.coli ) as foodsource. After severaldaysofincubationat15 5 C, we screened each well to select protists under an inverted microscope Nikon Eclipse TS100-F (NIKON, Tokyo, Japan). Wells containing potentially pure protist strains were further diluted several times in order to purify a single protist strain.Note that this isolationmethod is dependent on many factors, for instance, dilution, medium and site characteristics (such as fungal load). All protists strains were main-tained at 15 °℃Cand regularly transferred to newmediumwith E.coli . In order to examine thermotolerance, we grew each protist spe-cieswith E. coli in 96-well plates and incubated them at 15, 20, 25,28, 32, 37 ℃℃C.Each temperature assaywas replicated five-fold.We then trackedthenumber of activeprotistscellseachdayuntil encystment or extinction. This took 7–10 days, depending on the species.We defined thermotolerance as themaximum temperature atwhich presence of protistswas observed. Morphological characterization:All four strainswere identified under the locomotion, stationary and cyst stages. Tenmicroliters of each active protist or protist cysts were deposited on a glass slide, immediately covered by the glass slip, and the edges were sealed using nail polish. The images of each protist trophozoite and cyst wereacquiredusinga Nikon EclipseTi-E inverted microscope (NIKON, Tokyo, Japan) with Differential interference contrast (DIC) using thePlan Fluor 40x 1.30N.A. oil objective (Nikon) and aCool-SNAPHQ2 camera (Photometrics). The 16-bit images were pro-jected onto the CCD chip at a magnification of 107.5 nm/pixel with intermediatemagnification 1.5X (Nikon). The imageswere captured with 50 ms exposure time using MicroManager (v.1.4.22. ImageJ 1.48v). Time-lapse movies were generated with a time interval 1second to determine the locomotive form.Photos of each strainwere also taken under inverted microscope Nikon Eclipse TS100-F (NIKON, Tokyo,Japan).Lengthand width were measuredfor trophozoites or cysts per strain in ImageJ (1.48v). NL10 measure-mentwas based on 11 different cells,NL28measurementwas based on 28 different cells,NL81measurementwas based on 20 different cells,CN7measurementwas based on 20 different cells. In short, the scalewas set by the image scale (1 pixel = 107.5 nm), andwe further measured the length orwidth by drawing a line fromthe top to bottom of the protist trophozoite. The presence of floating and flagellate forms of each protistwere investigated. The ability to formflagellates was examined by adding strains to distilledwater for ca. 90mins. The formation of fruiting bodieswas investigated by following themethod in (Brown et al. 2012), in short, autoclave-sterilized Quercus alba bark togetherwith sterileDIH 2Oslurry of Rhodotorulamucilaginosa were added to the agar dishes containing actively growing strains.A permanentmicroscopic slide of strainCN7was prepared by fixation using Bouin-Hollande’s fluid, following the method of Pa´nek et al.(2014). DNA extraction and amplification:Protist DNAwas extracted from 100 m L of protist culture using the E.Z.N.A Bacterial DNA extraction Kit (Omega, Bio-Tek Inc., Georgia, USA) and DNeasy Blood & Tissue Kit (QIAGENN.V., Maryland, USA) following the manufacturer’s instructions, with an additional 2 min bead-beating step at maximum speed to improve the DNA yield. The extracted DNAswere stored at -20℃℃Cbefore use in polymerase chain reaction (PCR). The ITS region, including the 5.8S rRNA gene, was amplified using the primers JITS-F and JITS-R (De Jonckheere and Brown,2005),PCRamplificationswere run in aVeriti 96-well thermal cycler (Applied Biosystems, California, USA) with the following program:95 ℃℃C for 5 min, followed by 30 cycles with 95 ℃℃C 30 s, 50 ℃℃C for 60 s, and endingwith 72 °℃Cfor 120 s. PCR products (25 m L) were subjected to gel electrophoresis in 1%agarose dissolved in Tris-borate buffer (2.5mMdisodiumEDTA,89mM Tris base, and 8.9 mM boric acid). The band containing the PCR product of interest was then excised from the gel and purified using the QIAquick Gel Extraction Kit (QIAGEN N.V., Maryland, USA). The cleanedPCRproductswere then sequenced (BaseClear B.V., Leiden, theNetherlands) using appropriate primers. Phylogenetic analysis: The taxonomic affiliation of each strain was first determined by comparison against theNCBIdatabase using a BLAST search. To obtain resolved phylogenetic affiliations of our strains, the sequences of closely affiliated species were also down-loaded for further analysis. In total, we generated the following five datasets, one including 30 18SrRNAgene sequences; one including the ITS regions for the same set of 30 species; onemultigenematrix including both 18SrRNAgene and ITSregions that assembled using SequenceMatrix-Window-1.7.8 (Vaidya et al. 2011), one including the ITS region for 18 species in the genus Allovahlkampfia ; and one including the same region for 9 species in the genus Vahlkamp-fia . All datasets were aligned within MAFFT (version 7) using the FFT-NS-2method (Katoh et al. 2017) for the ITSandG-INS-imethod for the 18S, and furthermanually adjusted inSEAVIEW(version 4.7) to remove any poorly aligned regions base pairs. In order to assess the stability of the clades, phylogenetic anal-ysis of all datasetswas performed based onBayesian analysis using MrBayes 3.2 (Huelsenbeck andRonquist 2001) andmaximumlikeli-hood using RAxML (v0.9.0) (Kozlov et al. 2019). Bayesian analysis was conducted under 6General TimeReversible (GTR) substitution types with assumptions of rate variations across sites according to gamma + invariable distribution. Markov chain MonteCarlo simula-tions were performed for 100,000 generations, sampled every 100generations. The first 100 sampleswere discarded as burnin.Maxi-mum likelihood analysis was also based on GTR + GAMMA + I model for all datasets with rapid bootstraps. All phylogenetic trees were illustrated in FigTree (V1.4.4). We also compared similarities of published species sequences with the genera Allovahlkampfia and Vahlkampfia based on the manually modified alignments of the 18S rRNA and the 5.8S rRNA gene sequences.All sequences described in this study are available in GenBank, NL81 as MT739326, NL10 as MT739327, NL28 as MT739328, CN7as MT739329 for 18S rRNA gene; NL81 as MW031117, NL10 as MW031118, NL28 as MW031119, CN7 as MW031120 for the ITSregion including the 5.8SrDNA.All strains are deposited at the Leibniz Institute DSMZ-German Collection of Microorganisms andCellCulturesGmbH under accession numbers DSM 113306 (NL81), DSM 113307 (CN7), DSM 113308 (NL28), DSM113309 (NL10). CRediT authorship contribution statement ZhileiGao:Methodology,Validation, Investigation, Visualization,Formal analysis, Writing – original draft, Writing – review& editing.Alexandre Jous-set:Writing – review&editing,Supervision.George A. Kowalchuk: Supervision, Writing–review &editing.StefanGeisen:Methodology, Formal anal-ysis,Writing – original draft,Writing – review&edit-ing,Supervision. Acknowledgements Z.G.was supported by Chinese Scholarship Council (CSC),S.G. by a NWO-VENI grant from the Nether-lands Organization for Scientific Research (016. Veni.181.078). Moreover, we thank Xueyang Sun forthehelpofextracting DNA of protistsstrains and prof. ZhongWei for providing the soil fromChina. We appreciate Dr.Xuankun Li (Gainesville) for com-ments and suggestions on the paper. We appreciate Dr. Ilya Grigoriev and Biology Imaging Center (Utrecht University, the Netherlands) for the help of taking protist images and videos. Appendix A. Supplementary Data Supplementary data to this article can be found online at https://doi.org/10.1016/j.protis.2022.125870. References Anderson OR, Wand W, Faucher SP, Bi K, Shuman HA (2011)A new heterolobosean amoeba Solumitrus palustris n. g., n. sp. isolated from freshwater marsh soil. J Eukaryot Microbiol 58:60–67 Brown S, De Jonckheere JF (1999) A reevaluation of the amoeba genus Vahlkampfia based onSSUrDNAsequences. Europ J Protistol 35:49–54 Brown S, De Jonckheere JF (2004)Isolation of a new vahlkampfiid amoeba from soil:Paravahlkampfia lenta n. sp. Europ J Protistol 40:289–294 Brown MW, Silberman JD, Spiegel FW (2012) A contemporary evaluation of the acrasids (Acrasidae, Heterolobosea, Excavata). Europ J Protistol 48:103–123 BrugerolleG,SimpsonAGB (2004) The flagellar apparatus of Heteroloboseans. J Eukaryot Microbiol 51:96–107 Burki F, Roger AJ,BrownMW,SimpsonAGB (2020) The new tree of eukaryotes. Trends Ecol Evol 35:43–55 Dykova´ I, Kostka M (2013)Illustrated Guideto Culture Collection of Free-livingAmoebae.Academia;Prague, 363 p Dykova´I, Peckov´a H, Fiala I, Dvoˇr´akov´a H (2006) Fish-isolated Naegleria strains and their phylogeny inferred from ITS and SSU rDNA sequences. Folia Parasitol 53:172–180 Dykova´ I, Kyselov´a I, Peckov´a H, Obornı´k M, Lukeˇs J (2001) Identity of Naegleria strains isolated from organs of freshwater fishes. Dis Aquat Org 46:115–121 Garstecki T, Brown S, De Jonckheere JF (2005) Description of Vahlkampfia signyensis n. sp.(Heterolobosea), based on morphological, ultrastructural and molecular characteristics. Europ J Protistol 41:119–127 Geisen S, Bonkowski M, ZhangJ, De JonckheereJF (2015) Heterogeneity in the genus Allovahlkampfia and the description of the new genus Parafumarolamoeba (Vahlkampfiidae; Heterolobosea). Europ J Protistol 51:335–349 Harding T, Brown MW, Plotnikov A, Selivanova E, Park JS,Gunderson JH,BaumgartnerM,Silberman JD,Roger AJ, Simpson AGB (2013) Amoeba stages in the deepest branching heteroloboseans, including Pharyngomonas :Evolutionary and systematic implications. Protist 164:272–286 Huelsenbeck JP, Ronquist F (2001) MRBAYES:Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755 Larsen J, Patterson DJ (1990) Some flagellates (Protista) from tropical marine sediments. J Nat Hist 24:801–937 De JonckheereJF (2002) A centuryofresearchonthe amoeboflagellate genus Naegleria . Acta Protozool 41:309–342 De Jonckheere JF (2004) Molecular definition and the ubiquity of species in the genus Naegleria .Protist 155:89–103 De Jonckheere JF (2006a) Isolation and molecular identification of vahlkampfiid amoebae from an Island (Tenerife, Spain). Acta Protozool 45:91–96 De Jonckheere JF (2006b) Isolation and molecular identification of free-living amoebae of the genus Naegleria from Arctic and sub-Antarctic regions. Europ J Protistol 42:115–123 De Jonckheere JF (2011) Origin and evolution of the worldwide distributed pathogenic amoeboflagellate Naegleria fowleri . Infect Genet Evol 11:1520–1528 De Jonckheere JF (2012) The impact of man on the occurrence ofthe pathogenic free-living amoeboflagellate Naegleria fowleri . Future Microbiol 7:5–7 De Jonckheere JF (2014) What do we know by now about the genus Naegleria ? Exp Parasitol 145:S2–S9 De Jonckheere JF, Brown S (2005) The identification of vahlkampfiid amoebae by ITS sequencing.Protist 156:89–96 De Jonckheere JF,Murase J,Opperdoes FR (2011)A new thermophilic heterolobosean amoeba, Fumarolamoeba ceborucoi, gen. nov., sp. nov., isolated near a fumarole at a Volcano in Mexico. Acta Protozool 50:41–48 Kang S, Tice AK, Spiegel FW, Silberman JD, Pa´nek T, ˇC epiˇcka I, Kostka M, Kosakyan A, Alcˆantara DM, Roger AJ, Shadwick LL, Smirnov A, Kudryavtsev A, Lahr DJG, BrownMW (2017) Between a pod and a hard test: the deep evolution of amoebae. Mol Biol Evol 34:2258–2270 Katoh K, Rozewicki J, Yamada KD (2017) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20:1160–1166 Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A (2019) RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihoodphylogenetic inference. Bioinformatics 35:4453–4455 Murase J,KawasakiM,De Jonckheere JF (2010) Isolation of a new heterolobosean amoeba from a rice field soil:Vrihiamoeba italica gen. nov., sp. nov. Europ J Protistol 46:164–170 De Obeso Fernadez Del Valle A, Maciver SK (2017) Allovahlkampfia minuta nov. sp., (Acrasidae, Heterolobosea, Excavata) a new soil amoeba at the boundary of the acrasid cellular slime moulds. Acta Protozool 56:181–189 Page FC, Blanton RL (1985) The Heterolobosea (Sarcodina: Rhizopoda), a new class uniting the Schizopyrenida and the Acrasidae (Acrasida). Protistologica 21:121–132 Page FC (1967) Taxonomic criteria for limax amoebae, with descriptions of 3new species of Hartmannella and 3 of Vahlkampfia . J Protozool 14:499–521 Page FC (1983) Marine Gymnamoebae. Institute of Terrestrial Ecology, Natural Environment Research Council. LevenhamPress; Lavenham, Sulffolk, UK, 56 p Page FC (1988) New Key to Freshwater and Soil Gymnamoebae. Freshwater Biological Association; Ambleside, UK, 122 p P´anek T, Simpson AGB, Brown MW, Dyer BD (2017) Heterolobosea. In Slamovits C,Archibald JM,Simpson AGB (eds) Handbook of the Protists. Springer International Publishing; Cham, pp 1005–1046 P´anekT, Simpson AGB, Hampl V, ˇCepiˇckaI (2014) Creneis carolina gen. et sp. nov. (Heterolobosea), a novel marine anaerobic protist with strikingly derived morphology and life cycle. Protist 165:542–567 Park JS, Simpson AGB (2011) Characterization of Pharyngomonas kirbyi (= ‘Macropharyngomonas halophila’nomen nudum), a very deep-branching, obligately halophilic heterolobosean flagellate. Protist 162:691–709 Rahdar M, Tavalla M, Ebadi S, Salehi M (2016) The identification of free-living amoebae in water and soil from in and around Ahvaz, Southwest Iran. Biochem Cell Arch 16:379–382 Reeder WHH, Sanck J, Hirst M, Dawson SC, Wolfe GV (2015) The food web of Boiling Springs Lake appears dominated by the heterolobosean Tetramitus thermacidophilus strain BSL. J Eukaryot Microbiol 62:374–390 Sandon H (1927) The Composition and Distribution of the Protozoan Fauna of the Soil. In Crew FAE, Cutler DW, editors. Biological Monographs andManuals Vol. 7. Oliver &Boyd; Edinburgh, 239 p SimpsonAGB, InagakiY,RogerAJ (2006)Comprehensive multigene phylogenies of excavate protists reveal the evolutionary positions of “primitive” eukaryotes. Mol Biol Evol 23:615–625 Tice AK,Shadwick LL, Fiore-Donno AM,GeisenS,Kang S,SchulerGA,Spiegel FW,WilkinsonKA,BonkowskiM, DumackK, LahrDJ,VoelckerE,ClaußS, Zhang J,Brown MW (2016) Expansion of the molecular and morphological diversity of Acanthamoebidae (Centramoebida, Amoebozoa) and identification of a novel life cycle type within the group. Biol Direct 11:1–21 Tyml T, Skulinov´a K, Kavan J, Ditrich O, Kostka M, Dykova´ I (2016) Heterolobosean amoebae from Arctic and Antarctic extremes: 18 novel strains of Allovahlkampfia , Vahlkampfia and Naegleria . Europ J Protistol 56:119–133 Vaidya G, Lohman DJ, Meiern R (2011) SequenceMatrix:concatenation software for the fast assembly of multi-gene datasetswith character set and codon information.Cladistics 27:171–180 Visvesvara GS, Moura H, Schuster FL (2007) Pathogenic and opportunistic free-living amoebae:Acanthamoeba spp., Balamuthia mandrillaris , Naegleria fowleri , and Sappinia diploidea . FEMS Immunol Med Microbiol 50:1–26 Walochnik J, Mulec J (2009) Free-living amoebae in carbonate precipitating microhabitats of karst caves and a new vahlkampfiid amoeba, Allovahlkampfia spelaea gen. nov., sp. nov. Acta Protozool 48:25–33 Yera H, Zamfir O, BourcierT, Viscogliosi E, No¨el C, Dupouy-Camet J, Chaumeil C (2008) The genotypic characterisation of Acanthamoeba isolates from human ocular samples. Brit J Ophthalmol 92:1139–1141 Yubuki N, Leander BS (2008) Ultrastructure and molecular phylogeny of Stephanopogon minuta : An enigmatic microeukaryote frommarine interstitial environments. Europ J Protistol 44:241–253 Available online at:www.sciencedirect.com ScienceDirect
确定







还剩14页未读,是否继续阅读?
中国格哈特为您提供《土壤悬浮液的振荡》,该方案主要用于其他中土壤悬浮液的振荡检测,参考标准--,《土壤悬浮液的振荡》用到的仪器有格哈特强力高重现振荡器LS500/RO500、格哈特快速干燥仪STL56、德国移液器MM
推荐专场
快速干燥仪
更多
相关方案
更多
该厂商其他方案
更多


















