
沉积物掺入的光度测量:来自卡纳维拉尔港(美国)试验地点的天然沙沉积物被高压灭菌并悬浮在无菌过滤海水(佛罗里达州,浓度为37.5g·L−1)中。聚合物涂层的载玻片水平放置在一个线性摇床上(Laboshake,格哈特公司)。在60 rpm下搅拌2小时后,将载玻片在无沉淀物的盐水中垂直摇晃两次2 min,以去除不粘附的颗粒。在每个样品表面测量440 nm处的光度吸收,并与原始载玻片的吸光度进行比较。
方案详情

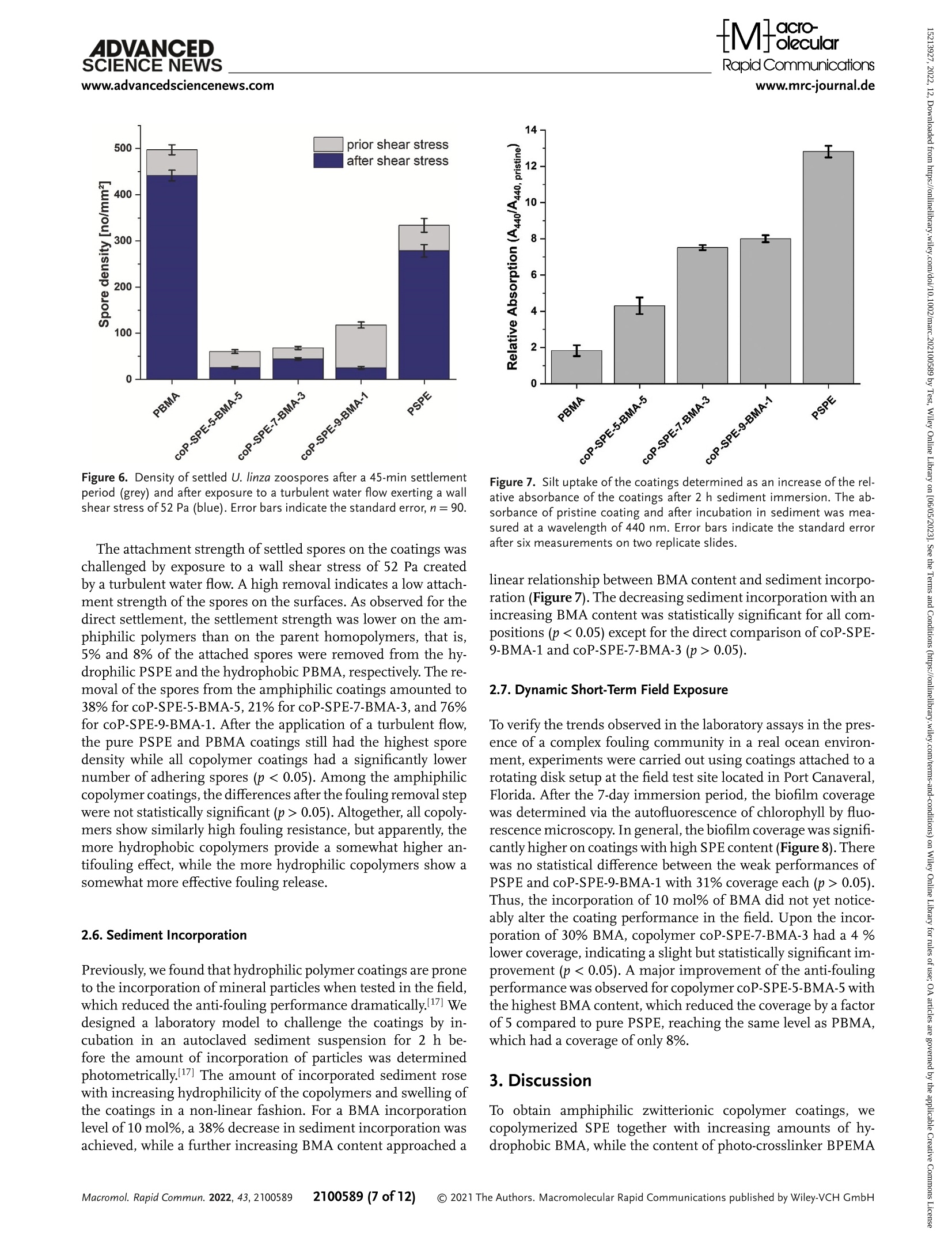
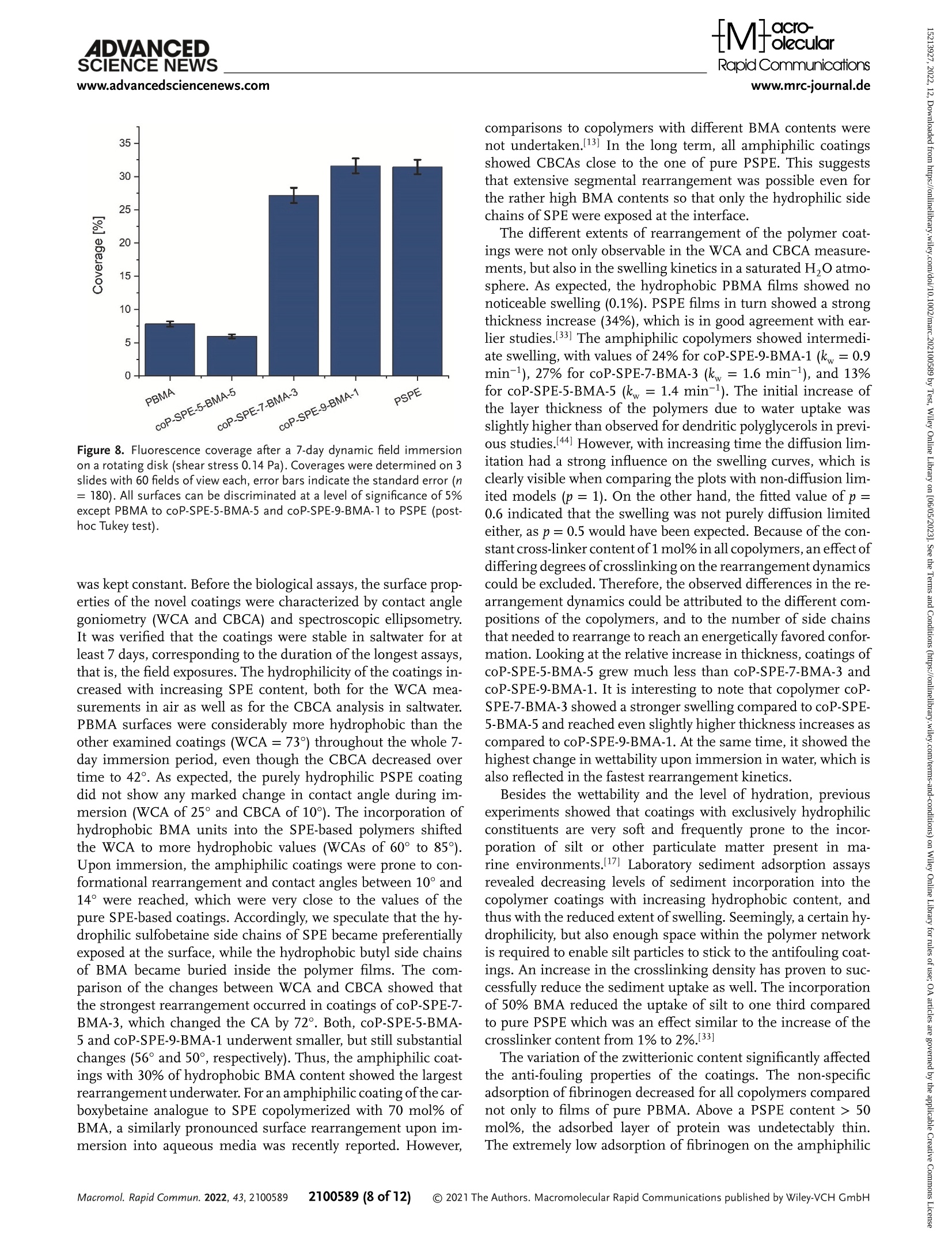
沉积物掺入的光度测量:来自卡纳维拉尔港(美国)试验地点的天然沙沉积物被高压灭菌并悬浮在无菌过滤海水(佛罗里达州,浓度为37.5g·L−1)中。聚合物涂层的载玻片水平放置在一个线性摇床上(Laboshake,格哈德公司)。在60 rpm下搅拌2小时后,将载玻片在无沉淀物的盐水中垂直摇晃两次2 min,以去除不粘附的颗粒。在每个样品表面测量440 nm处的光度吸收,并与原始载玻片的吸光度进行比较。Check for updatesRESEARCH ARTICLERapid Communicationswww.mrc-journal.de ADVANCED SCIENCE NEWSRapid Communicationswww.advancedsciencenews.comwww.mrc-journal.de Low Fouling Polysulfobetaines with Variable Hydrophobic Content Lisa Schardt, AlejandroMartínez Guajardo, Julian Koc, Jessica L. Clarke, John A. Finlay, Anthony S. Clare, Harrison Gardner, GeofreyW. Swain, Kelli Hunsucker, André Laschewsky,* and Axel Rosenhahn* Amphiphilic polymer coatings combining hydrophilic elements, in particular zwitterionic groups, and hydrophobic elements comprise a promising strategy to decrease biofouling. However, the influence of the content of the hydrophobic component in zwitterionic coatings on the interfacialmolecular reorganization dynamics and the anti-fouling performance is not well understood. Therefore, coatings of amphiphilic copolymers of sulfobetaine methacrylate 3-[N -2’-(methacryloyloxy)ethyl-N,N -dimethyl]-ammonio propane-1-sulfonate (SPE) are prepared which contain increasing amounts of hydrophobic n -butylmethacrylate (BMA). Their fouling resistance is compared to that of their homopolymers PSPE and PBMA. The photo-crosslinked coatings formhydrogel films with a hydrophilic surface. Fouling by the proteins fibrinogen and lysozyme as well as by the diatom Navicula perminuta and the green algae Ulva linza is assessed in laboratory assays.While biofouling is strongly reduced by all zwitterionic coatings, the best fouling resistance is obtained for the amphiphilic copolymers. Also in preliminary field tests, the anti-fouling performance of the amphiphilic copolymer films is superior to that of both homopolymers.When the coatings are exposed to amarine environment, the reduced susceptibility to silt incorporation, in particular compared to themost hydrophilic polyzwitterion PSPE, likely contributes to the improved fouling resistance. animals, on man-made surfaces that are exposed to aqueous media. The accumu-lation of these organisms impacts most severely the surface and interface proper-ties of objects exposed to the sea, which thus lose their original function and per-formance. For instance, ships, marine en-ergyturbines,harborinstallations,and oceanographic sensors are prone to ma-rine biofouling leading to high mainte-nance costs.[n] Ships, for example, experi-ence higher shear forces due to rougher surfaces resulting frombiofouling, increas-ing fuel consumption, and imposing ex-tra costs for hull cleaning.[d] The current strategy to control biofouling is the use of coatings releasing biocides such as copper compounds,[e] which are increasingly re-stricted, thus creating the demand for non-toxic alternatives.[r,a] An ideal coating com-bines an inhibiting e]ect that reduces ini-tial settlement of foulers, and/or facilitates aneasyreleaseofattachedorganismsunder low shear stress (fouling-release).[e] Many of the hydrophilic coatingmateri-als explored for antifouling use have been 1. Introduction The termbiofouling denotes the unwanted accumulation of bio-logicalmatter, including proteins, (micro)organisms, plants, and L. Schardt, J. Koc, A. Rosenhahn Analytical Chemistry – Biointerfaces Ruhr University Bochum 44801 Bochum, Germany E-mail: axel.rosenhahn@rub.de 波鸿鲁尔大学 生物表面分析化学 The ORCID identification number(s) for the author(s) of this article can be found under https://doi.org/10.1002/marc.202100589 ©2021 The Authors.Macromolecular Rapid Communications published byWiley-VCH GmbH. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in anymedium, provided the original work is properly cited, the use is non-commercial and nomodifications or adaptations aremade. DOl: 10.1002/marc.202100589 chosen due to their performance in the biomedical field, where they were known to improve hemocom-patibility, and/or to reduce toxicity, inflammatory, or immune responses. These investigations have mostly focused on non-ionic hydrophilic polymers such as polyethylenglycol (PEG), polysarcosine, or more recently poly(2-hydroxypropylmethacrylamide) in order to minimize, or even suppress biofouling.[1,.] Alternatively, hydrophilic zwitterionic surfaces have also demonstrated high resistance to biofouling and high biocompatibility for both technical and biomedical uses.[9–13] Zwitterions are charge-neutralmolecules that contain pairs of positive and negative charges, which allow electrostatic interactions with surrounding watermolecules.[14] As a result, a stable hydration shell is formed, which is assumed to counteract the adsorption and binding of proteins and the settlement of micro- and macrofoulers,[9,10,14,15] because the bond formation between the organisms’adhesives and the surface is inhibited.[16] However, the performance of such polymeric coatings in the field is often limited due to a lack of resilience.[9,17,18]While the chemical stability seems to be the lesser problem when appropriate polymers are employed, for example, such having a polymethacrylate backbone,[18,19] the mechanical strength is generally low due to their hydrogel character. Based on many laboratory model studies, very hydrophilic and highly swollen zwitterionic coatings confer high fouling resistance, but the extensive swelling tends to impair themechanical properties. Besides hydrophilic polymers, amphiphilic polymers contain-ingadjacenthydrophilicandhydrophobicmoietieshaveemerged as promising candidates formarine antifouling coatings.[4,20–23] Despite the low surface energy, hydrophobic polymers, in gen-eral, show little resistance against non-specific adhesion,[24] but their combination with hydrophilic compounds can lead to in-ert surfaces with superior fouling-release properties.[25] For am-phiphilic systems, not only the averaged content of hydrophilic and hydrophobic moieties is relevant, but also the distance between the two diferent groups which can organize into nanodomains.[26] Coatings with a compositional control over hy-drophilic and hydrophobic componentswere shown to exhibit re-sistanceagainstabroaderrangeofspecies,whichwasassignedto theformationofmicrodomainsofdiferentpropertiesonthesur-face of the polymer films.[21,22,27] Such amphiphilic systems can be conveniently prepared by copolymerization of hydrophilic and hydrophobicmonomers. Seemingly, compositional andmorpho-logical heterogeneity at the nanoscale is crucial for a successful amphiphilic low-fouling coating, whilemicrophase segregation should be avoided.[28] Possibly beneficial properties of zwitterionic amphiphilic coat-ings for fighting marine biofouling have occasionally been explored. For instance, the incorporation of 1–20 mol% of thesulfobetaine3-[N -2’-(methacryloyloxy)ethyl-N,N -dimethyl]-ammonio propane-1-sulfonate (SPE) into a hydrophobic lauryl methacrylate-based coating increased the anti-fouling resistance slightly.[29] The increase in surface roughness was, however, too great to form efective coatings,most likely due to phase sepa-ration during the preparation of the films. Also, low adhesion of proteins was reported for mixed amphiphilic surfaces con-taining carboxybetaine methacrylates and hydrophobic n -butyl methacrylate (BMA).[30] The incorporation of 5–15 wt% of car-boxybetainemethacrylate into a hydrophobic ethylene glycol di-cyclopentenyl ether acrylatematrix improved the anti-fouling ef-ficiency significantly.[23] The addition of zwitterionic additives including carboxybetainemethacrylate to hydrophobic ethylene glycoldicyclopentenylethermethacrylatesandtheanalogueacry-lates showed that the choice of the hydrophobic component is important for anti-fouling behavior. The added carboxybetaine methacrylate outperformed its acrylic analogue in the field.[31] Recently, copolymers of BMA with various ionic comonomers were prepared and characterized, among them poly(BMA-co-SPE) at a molar ratio of 9:1.[32] Relatively high amounts of non-freezable water strongly bound to the polymer were found for these zwitterionic amphiphilic copolymers. Such coatings showed significantly lower barnacle settlement and a high re-lease of fouling organisms at relatively low shear forces com-pared to pure BMA. The authors speculated that the swelling of the polymers in seawatermight contribute to the high fouling-release eicacy. Note in this context, that the fouling resistance of SPE-based coatings was also improved with increasing amounts of the hydrophobic photo-crosslinker 2-(4’-benzoylphenoxy)ethyl methacrylate (BPEMA).[33] It is not clear yet, to what extent the improvement derives from the higher crosslink density, which seems highly efective for, for example, reducing the protein fouling of nano carriers,[8] or to the increasingly amphiphilic character of these polymers. Also, statistical copolymers of BMA containing up to 0 mol% of SPE on poly(methylmethacrylate)(PMMA) were reported to reduce the adhesion ofmacrophages and fibroblast cells as well as somepathogenic bacteria compared to uncoated PMMA, the efects increasing with the sulfobetaine content.[34] Analogously in the biomedical context, zwitterionic copolymers of BMA with a phosphobetaine monomer, namely phosphatidylcholine methacrylate (MPC), have been used suc-cessfully formore than two decades for coatingmedical devices and implants, excelling in their high hemocompatibility and re-sistance against the adhesion of proteins, bacteria, and cells.[35] Although the success of the latter copolymers is often credited to the unique structure of the building blockMPCand its particular interaction with watermolecules,[36] the amphiphilic character of these copolymersmay also play an important role in their per-formance. Note that also copolymers of BMA with sulfobetaine comonomers (instead ofMPC) seem to exhibit comparably ad-vantageous polymer–water interactions.[12] In contrast, a recent study on coatings of copolymers of BMA with the analogous car-boxybetaine comonomerreportedlowadhesion of fibroblastcells but strong fouling by proteins such as fibrinogen, which was at-tributed to an overall positive ζ -potential.[13] Such studies demonstrated improved fouling resistance of hydrophobic polymer coatings when rendering them partially hydrophilic. Still, in reverse, for strongly hydrophilic polymers and hydrogels, the general perception is that the fouling resis-tance ismore or less compromised when introducing additional hydrophobic building blocks, not only in the biomedical field.[37] Nevertheless, considering these observations, amphiphilic copolymers of SPE with BMA seem to be attractive candidates formarine fouling-resistant coatings.While the phosphobetaine moiety inMPC-based polymers is rather sensitive to hydrolytic degradation inmarine environments, sulfobetaines (as in SPE) aremuchmore resilient.[38] Still, up to now, a systematic study of the anti-fouling potential of copolymers of SPE and BMA, in particular with high zwitterion contents, has been missing. In this study, we prepared amphiphilic coatings based on the sulfobetainemonomer SPE, which contain 10, 30, and 0 mol%of hydrophobic BMA (see Figure 1). All copolymers also contain 1 mol% of the photo cross-linker BPEMA, which assured the stability of the coatings in aqueous media after a photo-curing www.mrc-journal.de n Figure 1. Chemical structure and abbreviations of the monomers used (left) and the copolymers produced (right). For the photo-reactive pseudo-homopolymers PSPE (x = r.ct, y = n) and PBMA (x = o, y = o.er) as well as the terpolymers coP-SPE-d-BMA-h (x = r.th, y = o.o-), coP-SPE-s-BMA-3(x = 0.69, y = 0.30), and coP-SPE-5-BMA-5 (x = 0.495, y = 0.495), the content of BPEMA is z = 0.01. Table 1. Polymer composition,molarmasses, and sample codes. Sample BMA content [mol%] SPE content [mol%] BPEMA content [mol%] Yield [%] M w app [kgmol−1]a) Ðb) PBMAc) 99 (99)° – 1±0.3 77 140 2.8 coP-SPE-5-BMA-5 46”(49.5)° 53(49.5) 1±0.3 84 240 3.1 coP-SPE-7-BMA-3 27((30)e) 72(69) 1±0.3 73 320 3.3 coP-SPE-9-BMA-1 11”(10)° 88°(89)° 1±0.3 63 420 4.7 PSPEc) – 99(99) 1±0.3 72 490 5.4 a)Apparent weight averagemolarmass by SEC, eluent 0 mM CFCOONa in HFIP, calibration by PMMA standards;b)Apparent dispersity (M w app/M n app) by SEC, eluent COONa in HFIP, calibration by PMMA standards;c)Data from ref. [9]; d)Values are precise within ± 10 rel% (for details see Supporting Information);e)Values put in parentheses indicate the comonomer content in the original reaction feed (see Supporting Information). step.[9,18]Identically photo-cured coatings of the underlying photo-reactive “homopolymers” PSPE and PBMA served as references. The fouling resistance was evaluated in laboratory assays using proteins and themodel fouling organisms Navicula perminuta (N. perminuta ) and Ulva linza (U. linza ). The swelling of the coatings and the surface rearrangement of the hydrophilic and hydrophobicmolecular fragments weremonitored with cap-tive bubble contact angle (CBCA)measurements. To assess a po-tential use in themarine environment, the inertness against sed-iment was tested additionally by a short-termfield experiment. 2. Results 2.1. Design and Synthesis of the Amphiphilic Copolymers The amphiphilic SPE/BMA copolymers were obtained by free radical copolymerization of the zwitterionic methacrylate monomer SPE in trifluoroethanol (TFE) with varying amounts of hydrophobic butylmethacrylate BMA and a fixed amount of the benzophenone-bearing methacrylate BPEMA (Figure 1) as photo cross-linker. Detailed procedures for the synthesis and an-alytical data are provided in the Supporting Information. The content of the photo cross-linker BPEMA was kept constant at mol%in the reactionmixture, so that the crosslinking density in all photo-cured polymer coatings should be similar. The con-tent of the hydrophobic comonomer BMA in the reactionmix-ture was increased from 10 to 30, and eventually to 0 mol% in the copolymer series. The compositional analysis of the ternary copolymers, which are all hygroscopic, was not trivial. The com-bined data fromelemental analysis and the integration of consti-tutional repeat unit (CRU)-specific signals in the HNMRspec-tra revealed that the contents of BMAand BPEMAinthe zwitteri-onic copolymers corresponded to the composition of the copoly-merizationmixtures within the precision of the data, as summa-rized together with other keymolecular parameters in Table 1. As the monomer conversions were high, the analytical data cannotrigorouslyprove,thatallcopolymerizationreactivityratios are virtually at unity, that is, that the systemundergoes truly ideal azeotropic copolymerization. Only in this case, the copolymers formed at diferent reaction times have a uniform composition, andthedistributionoftheCRUswithinagivenchai followsran-domstatistics. To achieve this, comonomers were selected which bear the identical polymerizable moiety, namely methacrylate, and the copolymerization was conducted under homogeneous conditions in a good solvent for all components, namely TFE, as all thesemeasures a priori favor ideal azeotropic copolymeriza-tion. Nevertheless, the large diferences inmonomer polarities must be kept inmind. Studies of copolymerization of SPE and BMAinwell-compatible solvents including ionic liquids revealed deviations fromideal behavior when kept at low conversion rates, indicating a tendency for micro blockiness in the sequence of zwitterionic and hydrophobic monomers within the polymer chains.[39]Highmolar mass copolymers were synthesized (Ta-ble 1), with broadmolarmass distributions as is typical for free radical polymerizations carried to high conversions. Accordingly, 2100589 (3 of 12) Table 2.Wettability of the coatings by water. Static water contact angles (WCA) were determined on the dry polymers in air, the captive bubble wa-ter contact angles (CBCA) were determined after immersion in salt wa-ter. The contact angle decrease (|m CA|) is the dimerence between WCA and CBCA. Reported errors are the standard errors after 0 measurements(WCA, dry) and measurements after 7 days immersion time (CBCA, un-derwater). The APTMS-pretreated support is included as reference sub-strate prior to polymer coating. WCA [°] CBCA [°] |ACA|[°] PBMA 73±2 42±2 31 coP-SPE-5-BMA-5 70±1 14±2 56 coP-SPE-7-BMA-3 85±2 13±1 72 coP-SPE-9-BMA-1 60±1 10±1 50 PSPE 25±3 10±1 15 APTMS 56±4 – – most copolymer chains contain several photo-crosslinker groups as is needed to prepare stable films with a low leachable content after curing, despite the average content of only about mol%of crosslinker. Thermogravimetric analysis of the copolymers showed the onset of degradation at about 240 °C, as well as 5–10% of weight loss around 100 °C indicative of 1-– molecules of water tightly bound to the sulfobetaine moiety. Diferential scanning calorimetry run up to 220 °C did not indicate any glass transition. Upon increasing incorporation of the hydrophobic BMA units into the copolymers, the solubility of the copolymers was somewhat altered compared to the solubility of the ho-mopolymer PSPE.While all copolymers were still soluble in TFE andHFIP, they did not dissolve anymore in hot water, but only in brine, and this only if the BMA content did not exceed 0 mol%. Simple alcohols such as methanol or ethanol, chloroform, mixtures of the latter, and all aprotic solvents tested (including DMF, NMP, DMSO, acetonitrile, acetone, ethyl acetate, THF, CH,Cl2, pyridine, or benzene) remain non-solvents, as noted before for other hydrophobically modified polysulfobetaines with a substantial content of betaine groups.[40] 2.2. Preparation of Thin FilmPolymer Coatings and Surface Characterization The polymers were applied as thin coatings to surfaces by spin coating their solutions in TFE following previously published protocols.[9,18] Thin, homogeneous, and smooth coatings with reproducible properties were obtained. The coatings were stabi-lized by photo-curing, exploiting the photo-reactivity of the ben-zophenonemoiety in the comonomer BPEMA. This enabled si-multaneous cross-linking of the polymer chains within the coat-ings as well as their covalent anchoring to surfaces containing aliphatic CHmoieties via the so-called C,H-insertion crosslink-ingmechanism.[9,41] The wettability of the coatings was charac-terized by water contact angle (WCA) goniometry (Table 2). The coatingofthepurepolyzwitterionPSPEwasthemosthydrophilic one, exhibiting a dry contact angle of 25 ± 3°. For the copoly-mercoatings,thewettabilitywaslowerresultingincontactangles of 60 ± 1° for coP-SPE-9-BMA-1, 85 ± 2° for coP-SPE-7-BMA-3, and 70 ± 1° for coP-SPE-5-BMA-5. PBMA produced hydropho-bic coatings with aWCA of 73 ± 2°. Interestingly, a content of Figure 2.Water contact angle of the coatings. Captive bubble contact an-gle (CBCA) goniometry of air bubbles on the polymercoatings in saltwater over 7 days of immersion. Error bars indicate the standard error obtained from 5 air bubbles on the same slide. For comparison, squares indicate the static water contact angles in air with the standard error after 0 mea-surements on diferent slides. Full and dashed lines aremeant as guide to the eye. only 0 mol% of hydrophobic BMA in the copolymers was suf-ficient to raise the WCA values substantially compared to the purePSPEcoatings.Wenotedalsothataccordingtoexpectations, WCA values increased with increasing content of hydrophobic comonomer BMA. To our surprise, the somewhat higherWCAs found for coP-SPE-7-BMA-3 compared to pure PBMA and coP-SPE-5-BMA-5 deviated fromthis relation. In order to understand the extent of polymer surfaces rear-rangement in aqueous environments, CBCA weremeasured un-der water. PBMA had a contact angle of 70° immediately af-ter the immersion in saltwater, which decreased slowly stabi-lizing at around 42° (Figure 2). This evolution of CBCA was most likely caused by a slow rearrangement of the polymer to ex-pose preferentially themore hydrophilic carboxyl groups toward the polymer–artificial seawater (ASW) interface, thereby bury-ing the hydrophobic butyl side chains inside the polymer film. In contrast, themost hydrophilic polyzwitterions, namely PSPE and coP-SPE-9-BMA-1, exposed hydrophilic surfaces immedi-ately after immersion with a CBCA of around 10° through the whole immersion period. The copolymers coP-SPE-BMA with higher BMA contents showed intermediate behavior. Sample coP-SPE-7-BMA-3 exhibited an initial CBCA of 22°, which de-creased within 5 min to 13°. Sample coP-SPE-5-BMA-5 with a further increased BMAcontent hadaWCAof70° andrearranged within the firstminute of immersion in ASWtoformarather hy-drophilic surface with a CBCA of 14°. The analysis of the contact angle changes (Table 2) showed that theWCAs strongly decreased upon immersion in saltwater (CBCA).While for samples with lower SPE content, the change in contact angle increased with an increasing amount of incor-porated SPE, the change decreased again after reaching itsmaxi-mumof72° for coP-SPE-7-BMA-3. PBMAwithout SPE units can 2100589 (4 of 12) only rearrange bymoving themore polar ester group close to the backbonetotheoutside.Thisconformationalrearrangementhad only a limited efect, resulting in a decrease of the contact angle by 31°. Sample coP-SPE-5-BMA-5 showed a more pronounced decrease of the contact angle by 56° upon immersion in saltwa-ter due to the presence of the SPE side chains. These allowed for rearrangements that led to an exposure of the hydrophilic SPE groups toward the aqueous phase, resulting inmuch lower final contact angles (14° compared to 40° for PBMA). This trend con-tinuedwithcoP-SPE-7-BMA- featuringa A CAof72°.Byincreas-ing the SPE content further in coP-SPE-9-BMA-1, the change in contact angle was less prominent. The polymer films showed a muchmore hydrophilic surface in their dry state, indicating that manySPE groups were already exposed at the interface. This lim-ited the possibilities to further decrease the contact angle by seg-mental rearrangements when immersed in water. Nevertheless, even for the polyzwitterion PSPE that lacked hydrophobic side chains, amoderate, but notable reduction in contact angle by 15°was observed when exposed to saltwater. To ensure that the coatings were stable during the biological measurements, all samples were immersed in saltwater, which contained all essential salts but no organic components.[42] There was no substantial decrease in the thickness of the dry coating during 7 days of immersion of the copolymers. These findings obtained by spectroscopic ellipsometry were supported by the CBCAmeasurements as there was no significant change in the contact angle after the initial rearrangement discussed above. As all copolymer coatings retained their thickness and contact an-gle over time, they were suited for biological testing without any risk of delamination or disintegration during the duration of the tests. The water uptake of the polymer coatings in an atmosphere saturatedwithwatervaporwasstudiedbyspectroscopicellipsom-etry. Such ameasurement characterizes the water ainity of the coatings.[43,44] PBMA did not show any significant swelling due to its hydrophobic nature, whichmade water uptake unfavorable (Figure 3). Therefore, it was not possible to determine the rate constant of water uptake. PSPE films increased their thickness to 134%within the first 0 min, without reaching full saturation. Allmixed amphiphilic coatings swelled less than PSPE, reaching relativethicknessesof127%forcoP-SPE-7-BMA-3,124%forcoP-SPE-9-BMA-1,and113%forcoP-SPE-5-BMA-5.Theswellingwas close to itsmaximumafter the first 0 min of exposure to water-saturated air. The fitted kinetic constant k w was between 0.92 for coP-SPE-9-BMA-1 and 1.63 for coP-SPE-7-BMA-3 (Table 3) with-out a clear trend regarding the composition of the polymers. The exponent p was fitted globally due to the assumption that the de-gree of difusion limitation[45] was equal for all polymers, giving p=0.60±0.01. 2.3. Protein Adsorption The resistance of amphiphilic copolymers coP-SPE-BMAagainst non-specific adsorption of proteins was evaluated by surface plas-mon resonance spectroscopy (SPR, Figure 4). The shift in plas-mon resonance before and after the injection of fibrinogen and lysozyme provides insight into the amount of irreversibly bound Figure 3. Exemplary time-dependent relative swelling curves of the poly-mer coatings in a saturated water atmospheremeasured by spectroscopic ellipsometry (symbols). The lines represent the fit of the swelling using Equation (1). Table 3. Uptake of water froma water-saturated atmospheremeasured by spectroscopic ellipsometry.Kinetic constantswere derived assumingadif-fusion limited pseudo first-order kinetics. The reported relative increases are the average of threemeasurements on three replicate slides. p was fit-ted globally for all curves resulting in p = e.re ± c.te. PBMA did not show enough swelling to fit a rate constant. Polymer %increase of the layer thickness kw[min−-] t R2 PBMA 0.1±0,5 – – – coP-SPE-5-BMA-5 13±1 1.353±0.004 0.039±0.002 0.996 coP-SPE-7-BMA-3 27±6 1.637±0.009 -0.410±0.008 0.926 coP-SPE-9-BMA-1 24±5 0.922±0.002 0.007±0.002 0.999 PSPE 34±6 1.290±0.004 -0.041±0.003 0.998 protein. The two model proteins used difer in size and net-charge: fibrinogen has a slightly negative charge at pH7. (pI 5.5, molarmass40 kDa),[46] whereas lysozymehasadistinct positive charge (pI 10.9,molarmass 14 kDa).[47] For fibrinogen, the ad-sorption was highest on the hydrophobic reference PBMA, while coP-SPE-5-BMA-5 exhibited amore than 200-fold lower protein adsorption. The protein adlayers on the polymers PSPE, coP-SPE-7-BMA-3,andcoP-SPE-9-BMA- werenegligibleandtoolow to allow discrimination between these surfaces at a level of sig-nificance of 5%. Compared to fibrinogen, the adsorption of lysozyme on the surfaces was slightly more diferentiated. While PBMA was again contaminated to the largest extent, PSPE exhibited a sig-nificantly higher protein resistance (Figure 4, p < 0.05, post-hoc Tukey test). The amphiphilic copolymers showed the highest re-sistance against lysozyme. The coating of coP-SPE-7-BMA-3 had no detectable protein adsorption layer, while the adlayers on coP-SPE-5-BMA-5 and coP-SPE-9-BMA-1 were already very small. Remarkably, the transient adsorption during the injection phase oftheproteinswasnotparticularlylow,butthesubsequentbufer injection at a flow speed of ly l L ·min −t removed the lysozyme nearly completely from the coated surfaces (Figure S1, Support-ingInformation).Thus,theamountofirreversiblyboundprotein wasmuch lower than for pure PBMA and even pure PSPE coat-ings. This finding compares well with a recent report onmicellar nanoparticles of copolymers of BMA and SPE, for which the sta-tistical copolymers bound proteins and hydrophobic compounds much weaker than both the PBMA core and the PSPE shell of micellar nanoparticles of the analogous block copolymers.[36] The extent of protein adsorption was not statistically signifi-cantly diferent for the various copolymers coP-SPE-5-BMA-5, coP-SPE-7-BMA-3, and coP-SPE-9-BMA-1 (p > 0.05). However, the diferences between the copolymers and the pure PSPE and PBMA polymers were statistically significant (p < 0.05). Figure 5. Dynamicmicrofluidic diatomattachment assay on the polymer coatings, normalized to the attachment on PBMA, at a wall shear stress of0.16 Pa for 0 min. Error bars indicate the standard error for measure-ments on 3 replicates with 30 fields of view each. 2.4.Marine DiatomAccumulation The marine diatom N. perminuta is a frequently occurring fouling organismin themarine environment and an established model organism for biofouling studies.[48,49] The diatom is non-motile and when exposed to surfaces under dynamic conditions, the relative diatom densities provide an estimate of the fouling release properties of the surface.[48] Figure 5 summarizes the relative attachment of N. perminuta after a 90-min dynamic microfluidic accumulation assay at a wall shear stress of 0.16 Pa. All data were normalized to PBMA, which had the highest density of attached diatoms. The increasing incorporation of zwitterionic SPE units into the coatings reduced the accumu-lation of the diatoms, with PSPE showing the lowest number of attached cells (Figure 5). The mixed copolymers showed a significant (p < 0.05) decrease of the relative diatomdensity from coP-SPE-5-BMA-5 to coP-SPE-7-BMA-3 and coP-SPE-9-BMA-1, but the small diference between the latter two copolymers was not statistically significant (p > 0.05). 2.5. U. linza Zoospore Settlement and Removal In contrast tomarine diatoms, zoospores of the green algae U. linza actively explore surfaces to settle on a favorable spot.[50,51] Figure 6 summarizes the results of settlement and removal ex-periments with U. linza on the series of amphiphilic copolymers. SettlementwashighestonthehydrophobicPBMAbutwassignif-icantly reduced on the hydrophilic PSPE. Allmixed amphiphilic polymers had a significantly lower settlement compared to both homopolymers(p <0.05,post-hocTukeytest).Amongthecopoly-mer coatings, the settlement was highest on coP-SPE-9-BMA-1, while settlement was lowest and not significantly diferent (p >0.05) on copolymers coP-SPE-7-BMA-3 and coP-SPE-5-BMA-5. www.mrc-journal.de Figure 6. Density of settled U. linza zoospores after a 45-min settlement period (grey) and after exposure to a turbulent water flow exerting a wall shear stress of nd Pa (blue). Error bars indicate the standard error, n = al. Figure 7. Silt uptake of the coatings determined as an increase of the rel-ative absorbance of the coatings after 2 h sediment immersion. The ab-sorbance of pristine coating and after incubation in sediment wasmea-sured at a wavelength of 440 nm. Error bars indicate the standard error after sixmeasurements on two replicate slides. The attachment strength of settled spores on the coatings was challenged by exposure to a wall shear stress of 52 Pa created by a turbulent water flow. A high removal indicates a low attach-ment strength of the spores on the surfaces. As observed for the direct settlement, the settlement strength was lower on the am-phiphilic polymers than on the parent homopolymers, that is,5% and 8% of the attached spores were removed from the hy-drophilic PSPE and the hydrophobic PBMA, respectively. The re-moval of the spores fromthe amphiphilic coatings amounted to 38%for coP-SPE-5-BMA-5, 1%%for coP-SPE-7-BMA-3, and 76%for coP-SPE-9-BMA-1. After the application of a turbulent flow, the pure PSPE and PBMA coatings still had the highest spore density while all copolymer coatings had a significantly lower number of adhering spores (p < 0.05). Among the amphiphilic copolymercoatings,thediferencesafterthefoulingremovalstep were not statistically significant (p > 0.05). Altogether, all copoly-mers show similarly high fouling resistance, but apparently, the more hydrophobic copolymers provide a somewhat higher an-tifouling efect, while themore hydrophilic copolymers show a somewhatmore efective fouling release. linear relationship between BMAcontent and sediment incorpo-ration (Figure 7). The decreasing sediment incorporation with an increasing BMA content was statistically significant for all com-positions (p < 0.05) except for the direct comparison of coP-SPE-9-BMA-1 and coP-SPE-7-BMA-3 (p > 0.05). 2.7. Dynamic Short-TermField Exposure 2.6. Sediment Incorporation To verify the trends observed in the laboratory assays in the pres-ence of a complex fouling community in a real ocean environ-ment, experiments were carried out using coatings attached to a rotating disk setup at the field test site located in Port Canaveral, Florida. After the 7-day immersion period, the biofilm coverage was determined via the autofluorescence of chlorophyll by fluo-rescencemicroscopy.Ingeneral,thebiofilmcoveragewassignifi-cantlyhigheroncoatingswithhighSPEcontent(Figure8).There was no statistical diference between the weak performances of PSPE and coP-SPE-9-BMA-1 with 31%coverage each (p > 0.05). Thus, the incorporation of 0 mol% of BMA did not yet notice-ably alter the coating performance in the field. Upon the incor-poration of 30% BMA, copolymer coP-SPE-7-BMA-3 had a 4%lower coverage, indicating a slight but statistically significant im-provement (p < 0.05). Amajor improvement of the anti-fouling performancewas observed for copolymercoP-SPE-5-BMA- with the highest BMAcontent, which reduced the coverage by a factor of 5 compared to pure PSPE, reaching the same level as PBMA, which had a coverage of only 8%. Previously, we found that hydrophilic polymercoatings are prone to the incorporation ofmineral particles when tested in the field, which reduced the anti-fouling performance dramatically.[17]We designed a laboratory model to challenge the coatings by in-cubation in an autoclaved sediment suspension for 2 h be-fore the amount of incorporation of particles was determined photometrically.[17] The amount of incorporated sediment rose with increasing hydrophilicity of the copolymers and swelling of the coatings in a non-linear fashion. For a BMA incorporation level of 0 mol%, a 38%decrease in sediment incorporation was achieved, while a further increasing BMA content approached a 3. Discussion Toobtainamphiphiliczwitterioniccopolymer coatings,we copolymerized SPE together with increasing amounts of hy-drophobic BMA, while the content of photo-crosslinker BPEMA 2100589 (7 of 12) Figure 8. Fluorescence coverage after a 7-day dynamic field immersion on a rotating disk (shear stress 0.14 Pa). Coverages were determined on 3slides with 60 fields of view each, error bars indicate the standard error (n = 180). All surfaces can be discriminated at a level of significance of 5%except PBMA to coP-SPE-5-BMA-5 and coP-SPE-9-BMA-1 to PSPE (post-hoc Tukey test). was kept constant. Before the biological assays, the surface prop-erties of the novel coatings were characterized by contact angle goniometry (WCA and CBCA) and spectroscopic ellipsometry. It was verified that the coatings were stable in saltwater for at least 7 days, corresponding to the duration of the longest assays, that is, the field exposures. The hydrophilicity of the coatings in-creased with increasing SPE content, both for the WCA mea-surements in air as well as for the CBCA analysis in saltwater. PBMA surfaces were considerably more hydrophobic than the other examined coatings (WCA = 73°) throughout the whole 7-day immersion period, even though the CBCA decreased over time to 42°. As expected, the purely hydrophilic PSPE coating did not show any marked change in contact angle during im-mersion (WCA of 25° and CBCA of 10°). The incorporation of hydrophobic BMA units into the SPE-based polymers shifted the WCA to more hydrophobic values (WCAs of 60° to 85°). Upon immersion, the amphiphilic coatings were prone to con-formational rearrangement and contact angles between 10° and 14° were reached, which were very close to the values of the pure SPE-based coatings. Accordingly, we speculate that the hy-drophilic sulfobetaine side chains of SPE became preferentially exposed at the surface, while the hydrophobic butyl side chains of BMA became buried inside the polymer films. The com-parison of the changes between WCA and CBCA showed that the strongest rearrangement occurred in coatings of coP-SPE-7-BMA-3, which changed the CA by 72°. Both, coP-SPE-5-BMA-5 and coP-SPE-9-BMA-1 underwent smaller, but still substantial changes (56° and 50°, respectively). Thus, the amphiphilic coat-ings with 30% of hydrophobic BMA content showed the largest rearrangementunderwater.Foranamphiphiliccoatingofthecar-boxybetaine analogue to SPE copolymerized with 70 mol% of BMA, a similarly pronounced surface rearrangement upon im-mersion into aqueous media was recently reported. However, comparisons to copolymers with diferent BMA contents were not undertaken.[13] In the long term, all amphiphilic coatings showed CBCAs close to the one of pure PSPE. This suggests that extensive segmental rearrangement was possible even for the rather high BMA contents so that only the hydrophilic side chains of SPE were exposed at the interface. The diferent extents of rearrangement of the polymer coat-ings were not only observable in theWCA and CBCAmeasure-ments, but also in the swelling kinetics in a saturatedH,O atmo-sphere. As expected, the hydrophobic PBMA films showed no noticeable swelling (0.1%). PSPE films in turn showed a strong thickness increase (34%), which is in good agreement with ear-lier studies.[33] The amphiphilic copolymers showed intermedi-ate swelling, with values of 24% for coP-SPE-9-BMA-1 (k w = 0.9min −l), 27% for coP-SPE-7-BMA-3 (k w = 1.6 min −l), and 13%for coP-SPE-5-BMA-5 (k w = 1.4 min −1). The initial increase of the layer thickness of the polymers due to water uptake was slightly higher than observed for dendritic polyglycerols in previ-ous studies.[44]However, with increasing time the difusion lim-itation had a strong influence on the swelling curves, which is clearly visible when comparing the plots with non-difusion lim-itedmodels (p = m). On the other hand, the fitted value of p =0.6 indicated that the swelling was not purely difusion limited either, as p = 0.5 would have been expected. Because of the con-stantcross-linkercontentof mol%inallcopolymers,anefectof diferingdegreesofcrosslinkingontherearrangementdynamics could be excluded. Therefore, the observed diferences in the re-arrangement dynamics could be attributed to the diferent com-positions of the copolymers, and to the number of side chains that needed to rearrange to reach an energetically favored confor-mation. Looking at the relative increase in thickness, coatings of coP-SPE-5-BMA-5 grew much less than coP-SPE-7-BMA-3 and coP-SPE-9-BMA-1. It is interesting to note that copolymer coP-SPE-7-BMA-3 showed a stronger swelling compared to coP-SPE-5-BMA-5 and reached even slightly higher thickness increases as compared to coP-SPE-9-BMA-1. At the same time, it showed the highest change in wettability upon immersion in water, which is also reflected in the fastest rearrangement kinetics. Besides the wettability and the level of hydration, previous experiments showed that coatings with exclusively hydrophilic constituents are very soft and frequently prone to the incor-poration of silt or other particulate matter present in ma-rine environments.[ 1] Laboratory sediment adsorption assays revealed decreasing levels of sediment incorporation into the copolymer coatings with increasing hydrophobic content, and thus with the reduced extent of swelling. Seemingly, a certain hy-drophilicity, but also enough space within the polymer network is required to enable silt particles to stick to the antifouling coat-ings. An increase in the crosslinking density has proven to suc-cessfully reduce the sediment uptake as well. The incorporation of 50% BMA reduced the uptake of silt to one third compared to pure PSPE which was an efect similar to the increase of the crosslinker content from1%to 2%.[33] The variation of the zwitterionic content significantly afected the anti-fouling properties of the coatings. The non-specific adsorption of fibrinogen decreased for all copolymers compared not only to films of pure PBMA. Above a PSPE content > 50mol%, the adsorbed layer of protein was undetectably thin. The extremely low adsorption of fibrinogen on the amphiphilic polymers is in line with previous reports.[32,52] Also, the ad-hesion of lysozyme was significantly lower on all amphiphilic copolymers than on the pure PBMA and PSPE coatings. The settlement of themarine diatom N. perminuta decreased with increasing hydrophilicity of the coatings. Pure PSPE films had the lowest settlement density. Yet, in experiments with zoospores of the green algae U. linza , the amphiphilic coatings outperformed the ones of pure PSPE and PBMA polymer coat-ings. Similar observations weremade previously for amphiphilic PEG block copolymers.[20,32,52] The good performance of am-phiphilic zwitterionic polymers is in line with many results in literature showing high resistance against cell adhesion,[30] at-tachment of N. incertia ,[29] and a good anti-fouling performance against zoospores of U. linza .[53] In recent studies, the formation of domains within am-phiphilic coatings was described and it was pointed out that it could be energetically favorable if hydrophilic and hydrophobic islandsareformed.[21] Inourstudy,noindicationswerefoundfor such phase segregation, but future work to precisely understand the interplay of chemical composition and domain size would be very interesting. In preliminary field tests, the highest fouling was accumulated on coatings with high SPE content (cf. Figure 8). This trend co-incides well with the tendency of the coatings to accumulate silt (cf. Figure 7). Accumulation of biomass in the field exposures de-creased with increasing hydrophobic content. Sample coP-SPE-5-BMA-5 performed similar to the hydrophobic control PBMA, and it is not clear where would be the optimumBMA content. It may be speculated whether coP-SPE-BMAcopolymers with even lower levels of SPE incorporation (i.e., copolymers with x < y in Figure 1)might further improve the anti-fouling efect in the field. The comonomer limited swelling due to the reduced hy-drophilicity of such zwitterionic coatings, for instance, might enhance their underwater mechanical strength. Previous stud-ies have shown that very soft and highly swollen hydrogels are prone to the accumulation of silt on which biomass can settle.[17] Also, low amounts of carboxybetaines in a DCPEAmatrix were suicient to strongly improve themarine antifouling properties of the purely hydrophobic parent coatings.[23] Similar observa-tions weremade for diferent hydrophilic functionalities, includ-ing zwitterionic ones, in a BMA-dominated polymermatrix.[32] 4. Conclusion A series of photo-crosslinked amphiphilic coatings fromcopoly-mers of the zwitterionic sulfobetainemethacrylate SPE with con-tents of hydrophobic butyl methacrylate BMA up to 50 mol%were prepared and characterized in detail. In comparison to not only the hydrophobic coating of homopolymer PBMA, which is known to show substantial fouling, but also to the hydrophilic zwitterionic coating of the homopolymerPSPE, which is thought to produce low-fouling surfaces, the incorporation of the hy-drophobic comonomer enhanced the fouling resistance notably. This contrasts with the widespread perception that the incorpo-ration of additional hydrophobic groups in strongly hydrophilic polymers and hydrogels is a priori detrimental to their high foul-ing resistance. The amphiphilic copolymers were equally efec-tive against both proteins and fouling organisms in laboratory assays, as well as in short-term dynamic field tests in the ocean. Upon exposure to a water saturated atmosphere, all polyzwitte-rion filmsswelledmarkedly, and the surfaces rearranged to form hydrogel coatings with amostly hydrophilic surface, in contrast to the reference of pure PBMA. The extent of rearrangement and its kinetics changed slightly for diferent SPE:BMA ratios. The presence of hydrophobic BMA units in the coatings significantly improved the anti-fouling properties, resulting in lower protein adsorption, and lower settlement of U. linza zoospores than on coatings from both parent polymers. Further, the attachment of diatom N. perminuta was decreasedmore efectively. The incor-poration of increasing amounts of hydrophobic BMA within the series also decreased the sediment uptake, which hypothetically is a consequence of the reduced swelling of such coatings, thus increasing their hardness in aqueous environments.When the amphiphilic coatings were challenged by a complex fouling com-munity in the field, the amphiphilic copolymersshowed substan-tially reduced biofouling compared to the zwitterionic homopoly-merPSPE. Presumably, the lower sensitivity of the copolymersto silt uptake contributes crucially to the improved antifouling per-formance in the field. Notwithstanding this important efect, the incorporationofacertainamountofmoderatelyhydrophobicele-ments into polyzwitterion coatings seems in general to boost the fouling resistance. 5. Experimental Section PolymerSynthesis : Copolymersof3-[N-2’-(methacryloyloxy)ethyl-N,N-dimethyl]-ammonio propane-1-sulfonate (SPE), n -butyl methacrylate (BMA), and 2-(4’-benzoylphenoxy)ethylmethacrylate (BPEMA) were syn-thesized as described previously.[9,38,54] A standard procedure[38] of free radical copolymerization in solution was used employing the comonomer ratios specified in Table 1. Details are provided in the Supporting Informa-tion, including the 1H NMR spectra (Figures Sn–Sh, Supporting Informa-tion). The polymerswere purified by dialysis (membrane’s nominalmolec-ular weight cut-olMWCO = ne's gmol −e). Chemicals and Sample Preparation : Glass substrates were modified with amonolayer of 3-aminopropyltrimethoxysilane (APTMS, 97%,Sigma Aldrich) to promote the adhesion of the polymer coatings on the sur-face following a previously described procedure.[9,55] The substrates were treated in oxygen plasma for min (0. mbar, 0 W, 22 kHz,miniFlecto-PC-MFC, Gala Instruments GmbH) and immersed into a 5% APTMS solution (v/v) under inert atmosphere (nitrogen, 99%, Air Liquide). Af-terward, the substrates were immersed successively for 20 s into ethyl acetate (>99.8%, Fisher Scientific), ethanol (p.a.,>99.8%, Fisher Scien-tific), andMilli-Q (MerckMillipore, Darmstadt, Germany) and ultrason-icated. Gold-coated glass substrates with a thin interlayer of titanium to promote adhesion were functionalized by a self-assembled mono-layer of 11-amino-1-undecanthiol (ProChimia) following published proto-cols including a 24 h immersion and subsequent cleaning steps to re-move non-covalently bound molecules.[9] The polymers were dissolved in TFE (Roth) and spin-coated on themodified substrates (WS-650MZ-23NPP/Lite, Laurell Technologies Corporation) by following previously es-tablished protocols.[9] The concentration of the polymers in the solutions was generally 1 wt% (0.5 wt% for SPR) to achieve thicknesses of the fi-nal coatings of 150 ± 8 nm. For SPR studies, polymer concentrations of the coating solutions were F.r wt%, yielding thicknesses of ra ± n nm. Thepolymercoatings were covalently crosslinked by curing under UV-light (Uvacube 100, Dr. Hönle AG, 00 Wiron-dopedmercury vapor lamp, H1Filter >00, nm, intensity at sample memWcm − ) for lamin. Static Water Contact Angle (WCA) Goniometry : Static WCAs of the polymer coatings were determined using a custom-built goniometer equipped with a CCD camera. A droplet of triply distilled water was ap-plied on the coating and its shape was fitted using Young’s equation. Captive Bubble Contact Angle (CBCA) Goniometry : Under water con-tact anglemeasurements were performed using a custom-build setup[5y] with a CCD camera and a glass cell filled with saltwater as described by Kester.[42] A slide with the coated side facing down was placed in the cell, and a 20 u L air bubble was deposited using a U-shaped syringe from un-derneath the coated surface at ambient temperature. The water/solid con-tact angle at the substrate–air–water interface was fittedmanually.[56] The reported values were averages of fivemeasurements. Tomonitor the rear-rangement of the polymer surfaces, contact angles weremeasured after 1,5, 10, 15, 30, 45, 60, and 20 minandafter 1, 2, 4, and 7 daysof immersion. Spectroscopic Ellipsometry : The thickness of dry polymer films was measured by spectroscopic ellipsometry (M-2000,Woollam,USA with the CompleteEase software package) at incidence angles of 65°, 70°, and 75°. A single layer of polymerwasmodeled using an absorbing filmmodel. The water uptake from a saturated vapor atmosphere in a humidity chamber was also followed by spectroscopic ellipsometry. As HzO was present with a large excess compared to the volume of the coating, the water concen-tration was assumed to be constant to apply pseudo first-order kinetics given by Equation (1), where k w represents the rate of water uptake, t o the inset time of the swelling, d(t) the timedependent thickness at time t and de the final thick-ness in equilibrium with water. Beyond a normal Langmuir kinetics, the parameter p was used to account for a difusion limited water uptake of the part of the polymer filmcloser to the substrate.[45]While for very thin films p should converge to 1 (Langmuir case), thicker films face an ad-ditional time delay due to the difusion of water leading to values <1. To account for this difusion, a formalismrecently developed was applied to describe adsorption processes fromvery diluted solutions using a fixed ex-ponent of 0.5.[45] To account for intermediate behavior, the exponent was fitted globally for themeasured samples between the extremes of 0.5 and 1 Stability Test : The stability of the polymer coatings in saltwater was monitored by the dry filmthickness after l and 2 h and 1, 2, 3, and 7 days of immersion in freshly prepared seawater.[42,57] Before themeasurement the coatings were rinsed withMilli-Q water to remove the remaining salt fromthe surface. Surface Plasmon Resonance (SPR) Spectroscopy : The protein adsorp-tion on the diferent coatings was analyzed by SPR (SRC7000DC, pump SR7500, autosampler SR7100, Reichert). After the injection of PBS bufer with a flow rate of SR7 0L ·min −c until a stable baseline was obtained, the flow rate was reduced to 00 u L ·min −i. Protein solutions (omg ·mL −d, in PBS) were injected for pLmin at a flow rate of ti n L ·min −g.Washing with PBS for another 0 min at 1a p L ·min −w removed reversibly attached pro-teins. The diference in the shift of the SPR signal before and after protein adsorption correlated with the amount of protein irreversibly attached to the surface. DynamicAttachment Assays of Navicula perminuta : Dynamicmicroflu-idic measurements of the accumulation of diatoms followed previously published protocols.[48,58] A suspension with a concentration of 3.0 ×bli cells ·mL −c in filtered seawater (FSW, CaribSea, USA, pH 3.0) was prepared from a continuous N. perminuta culture. IBIDI sticky slides 0.1(IBIDI) were placed onto the substrates coated with the polymers to form a microfluidic channel (150 u m height). The suspension was pumped through the channel for 0 min exerting a constant wall shear stress of0.16 Pa followed by a 0 min rinsing step with FSWto remove non-settled diatoms. For quantification, 30 fields of view from the central part of the channel were acquired by a phase-contrast microscope (Nikon Eclipse TE2000-U, 10× phase contrast objective Nikon CFI Plan Fluor DLL NA 0.3). Automatic diatom counting was conducted using the NIS Elements software (Nikon). Statistical analysis was performed by ANOVA with post-hoc Tukey test at a significance level of α = 0.05. Settlement and Removal of Zoospores of Ulva linza : Zoospores were collected frommature plants of U. linza from Craster, U.K. Following the method of Callow et al.,[II] a zoospore suspension (0 mL; 1× 101 spores mL −1) was added to the compartments of quadriPERM dishes (Sarstedt Ltd.) containing 6 replicates of each sample. After 5 min in the dark at20 °C, the slides were passed backward and forward 10 times through a beaker of 0.22 u mfiltered ASW(TropicMarin salts) to wash of unsettled (i.e., swimming) spores. Slides were fixed with ASWcontaining 2.5%glu-taraldehyde (Thermo Fisher Scientific, USA). The density of zoospores on the surface per slide was counted using an imageanalysissystemattached to a fluorescencemicroscope (Zeiss Axioscope 2 plus). Spores were visu-alized and counted using the autofluorescence of chlorophyll (excitation and emission: 546 and 590 nm) on 30 fields of view of 0.5 mm² each per slide. Zoospore removal was determined using another three slides with zoospores settled for 5 min by themethod above. Slides were ex-posedtoashearstressof2 Painaspeciallydesignedturbulentflowwater channel.[59] The slides were then fixed and the number of spores remain-ing attached was counted. The counts were averaged over all replicates and statistical analysis was performed by ANOVA with post-hoc Tukey test at a significance level of α = 0.05. Photometric Measurement of Sediment Incorporation : Natural sand sediment from the test site at Port Canaveral (USA) was autoclaved and suspendedinsterilefilteredseawater(CaribSeaPure, FortPierce, FL,USA) at a concentration of lt.r g ·L −a. The polymer-coated slides were placed horizontally on a linear shaker (Laboshake Heavy Load, Gerhardt Analyt-ical Systems). After 2 h at 60 rpm, the slides were shaken twice vertically for min in sediment-free saltwater to remove non-sticking particles. On each sample surface the photometric absorption at 440 nmwasmeasured and compared to the absorbance of the pristine slide. Dynamic Short-Term Field Exposure on a Rotating Disk : Field test-ing was executed on a custom-built rotating disk at Port Canaveral, Florida,USA(28°24’29.4’’N80°37’37,7’’W)followingpreviouslypublished protocols.[60] The calculated shear force at the center of the samples was 0.14 Pa. After a 7-days immersion, the absorbedmaterial was treated with 2.5 wt%glutaraldehyde (Fisher Scientific, Pittsburg PA, USA) for fixation. The absorbed material was quantified using the fluorescence coverage of 60 fields of view (light source: ExFO XCite-120, Excelitas Technologies Corp., filter: BV-2A, excitation: 400–440 nm, dichroicmirror: 455, barrier filter: 470, Nikon Instruments). Supporting Information Supporting Information is available fromtheWiley Online Library or from the author. Acknowledgements The authors gratefully acknowledge funding by the Deutsche Forschungs-gemeinscha f t (DFG) GRK2376/331085229, LA 611/14, and RO 2524/4. Funding was additionally provided by the Oice of Naval Research (ONR) N0014-20-12244 (A.R.), N00014-16-1-2988 (A.S.C.), N00014-16-1-3125(A.S.C. and J.A.F.), and N0014-20-12243 (K.H. and G.W.S.). Open access funding enabled and organized by Projekt DEAL. Conflict of Interest The authors declare no conflict of interest. Data Availability Statement Research data are not shared. Keywords amphiphilic, field tests, fouling release,marine biofouling, Navicula per-minuta , polyzwitterion, Ulva linza Published online: November 20, 2021 [1] L. D. Chambers, K. R. Stokes, F. C.Walsh, R. J. K.Wood, Surf. Coat. Technol. 2006, 201,3642. [2] a) Swain, J. Ship Prod. 2007, 23, 164; b)M. P. Schultz, J. A. Bendick, E. R. Holm,W.M. Hertel, Biofouling 2011, 27, 87; [3] I. K. Konstantinou, Antifouling Paint Biocides , Springer-Verlag GmbH, Berlin 2006. [4] A. K. Leonardi, C. K. Ober, Annu. Rev. Chem. Biomol. Eng. 2019, 10,241. [] L. Tian, Y. Yin,W. Bing, E. Jin, J. Bionic Eng. 2021, 18, 239. [] a)M. Lejars, A.Margaillan, C. Bressy, Chem. Rev. 2012, 112, 4347; b) D.M. Yebra, S. Kiil, K. Dam-Johansen, Prog. Org. Coat. 2004, 50, 75; [7] a) E. Ostuni, R. G. Chapman, R. E. Holmlin, S. Takayama, G. M. Whitesides, Langmuir 2001, 17, 5605; b)M. Barz, R. Luxenhofer, R. Zentel,M. J. Vicent, Polym. Chem. 2011, 2, 1900; c) E. Roeven, A. R. Kuzmyn, L. Scheres, J. Baggerman,M.M. J. Smulders, H. Zuilhof, Langmuir 2020, 36,10187; [8] I. Alberg, S. Kramer, M. Schinnerer, Q. Hu, C. Seidl, C. Leps, N. Drude, D. Möckel, C. Rijcken, T. Lammers, M. Diken, M.Maskos, S.Morsbach, K. Landfester, S. Tenzer,M.Barz, R. Zentel, Small 2020,16,1907574. [9] a) J. Koc, E. Schönemann, A. Amuthalingam, J. Clarke, J. A. Finlay, A. S. Clare, A. Laschewsky, A. Rosenhahn, Langmuir. 2019, 35, 1552; b) E. Schönemann, J. Koc, N. Aldred, A. S. Clare, A. Laschewsky, A. Rosenhahn, E.Wischerhof,Macromol. Rapid Commun. 2020, 41,1900447. [10] S. Jiang, Z. Cao, Adv.Mater. 2010, 22, 920. [11] a) A. Laschewsky, A. Rosenhahn, Langmuir 2019, 35, 1056; b) J. B. Schlenoff, Langmuir 2014, 30, 9625; [12] H. Kitano, T.Mori,Y. Takeuchi, S. Tada,M.Gemmei-Ide, Y. Yokoyama, M. Tanaka,Macromol. Biosci. 2005, 5, 314. [13] J. Lee, S. Yi, K. D. Hong, J.-H. Seo, J. Ind. Eng. Chem. 2021, 96, 284.[14] S. Chen, S. Jiang, Adv.Mater. 2008, 20, 335. [15] J. Baggerman, M. M. J. Smulders, H. Zuilhof, Langmuir 2019, 35,1072. [16] a) C. Leng, H.-C. Hung, S. Sun, D.Wang, Y. Li, S. Jiang, Z. Chen, ACS Appl.Mater. Interfaces 2015, 7, 16881; b) A. Rosenhahn, S. Schilp, H. J. Kreuzer,M. Grunze, Phys. Chem. Chem. Phys. 2010, 12, 4275; [17] J. Koc, T. Simovich, E. Schönemann, A. Chilkoti, H. Gardner, G.W. Swain, K. Hunsucker, A. Laschewsky, A. Rosenhahn, Biofouling 2019,35,454. [18] E.Schönemann,A.Laschewsky,E.Wischerhof,J.Koc,A.Rosenhahn, Polymers 2019,11,1014. [19] C.-C. Yang, C.-T. Lo, Y.-L. Luo, A. Venault, Y. Chang, ACS Biomater. Sci. Eng. 2021, 7, 1031. [20] S.Krishnan,N.Wang,C.K.Ober,J.A.Finlay,M.E.Callow,J.A.Callow, A. Hexemer,K. E. Sohn, E. J. Kramer,D. A. Fischer, Biomacromolecules 2006,7,1449. [21] G. Galli, E.Martinelli,Macromol. Rapid Commun. 2017, 38, 1600704. [22] C. S. Gudipati, C.M. Greenlief, J. A. Johnson, P. Prayongpan, K. L. Wooley, J. Polym. Sci., Part A: Polym. Chem. 2004, 42, 6193. [23] F. Koschitzki, R. Wanka, L. Sobota, J. Koc, H. Gardner, K. Z. Hun-sucker, G.W. Swain, A. Rosenhahn, ACS Appl.Mater. Interfaces 2020,12,34148. [24] S. Herrwerth, W. Eck, S. Reinhardt, M. Grunze, J. Am. Chem. Soc.2003,125,9359. [25] a) S. Colak, G. N. Tew, Biomacromolecules 2012, 13, 1233; b) S. Co-lak, G. N. Tew, Langmuir 2012, 28, 666; c) V. Jakobi, J. Schwarze, J. A. Finlay, K. A. Nolte, S. Spöllmann, H.-W. Becker, A. S. Clare, A. Rosen-hahn, Biomacromolecules 2018, 19, 402; [26] J. A. Finlay, S. Krishnan,M.E. Callow, J. A. Callow, R. Dong, N. Asgill, K.Wong, E. J. Kramer, C. K. Ober, Langmuir 2008, 24, 503. [27] Z. Zhang, J. A. Finlay, L.Wang, Y. Gao, J. A. Callow,M. E. Callow, S. Jiang, Langmuir 2009, 25,13516. [28] a) J.W. Bartels, C. Cheng, K. T. Powell, J. Xu, K. L.Wooley,Macromol. Chem. Phys. 2007, 208, 1676; b) D. Gan, A.Mueller, K. L.Wooley, J. Polym. Sci., Part A: Polym. Chem. 2003, 41, 3531; [29] C. Ventura, A. J. Guerin, O. El-Zubir, A. J. Ruiz-Sanchez, L. I. Dixon, K. J. Reynolds,M.L. Dale, J. Ferguson, A. Houlton, B. R. Horrocks, A. S. Clare, D. A. Fulton, Biofouling 2017, 33, 892. [30] X. Lin, P. Jain, K.Wu, D. Hong, H.-C. Hung,M. B. O’kelly, B. Li, P. Zhang, Z. Yuan, S. Jiang, Langmuir 2019, 35, 1544. [31] F. Koschitzki, R.Wanka, L. Sobota, H. Gardner, K. Z. Hunsucker, G. W. Swain, A. Rosenhahn, Langmuir 2021, 37, 5591. [32] J. H. Kardela, I. S. Millichamp, J. Ferguson, A. L. Parry, K. J. Reynolds, N. Aldred, A. S. Clare, ACS Appl.Mater. Interfaces 2019, 11,29477. [33] J. Koc, E. Schönemann, R.Wanka, N. Aldred, A. S. Clare, H. Gardner, G.W. Swain, K. Hunsucker, A. Laschewsky, A. Rosenhahn, Biofouling 2020,36,646. [34] A. B. Lowe,M. Vamvakaki,M. A.Wassall, L.Wong, N. C. Billingham, S. P. Armes, A.W. Lloyd, J. Biomed.Mater. Res. 2000, 52, 88. [] K.Ishihara,H. Oshida, Y. Endo, A. Watanabe, T.Ueda, N. Nakabayashi, J. Biomed.Mater. Res. 1993, 27, 1309. [36] M.Mu, T. Konno, Y. Inoue, K. Ishihara, Colloids Surf., B 2017, 158,249. [37] a) X. Li, C. Tang, D. Liu, Z. Yuan, H.-C. Hung, S. Luozhong,W. Gu, K. Wu, S. Jiang, Adv.Mater. 2021, 33, 2102479; b) C. Li,M. Li,W. Qi, R. Su, J. Yu, Langmuir. 2021, 37, 8455; c) D.Mitra, E. N.-T. Kang, K. G. Neoh, ACS Appl. Polym.Mater. 2021, 3, 2233; [38] E. Schönemann, A. Laschewsky, A. Rosenhahn, Polymers 2018, 10,639. [39] a) V. Strehmel, A. Laschewsky, H.Wetzel, e-Polym. 2006, 6, 11; b) V. Strehmel, H.Wetzel, A. Laschewsky, E.Moldenhauer, T. Klein, Polym. Adv. Technol. 2008,19,1383; [40] a) P. Köberle, A. Laschewsky, D. van den Boogaard, Polymer 1992,33, 4029; b) P. Anton, A. Laschewsky,Makromol. Chem. 1993, 194,601: [41] O. Prucker, T. Brandstetter, J. Rühe, Biointerphases 2017, 13, 010801. [42] D. R. Kester, I.W. Duedall, D. N. Connors, R.M. Pytkowicz, Limnol. Oceanogr. 1967, 12,176. [43] B. T. Koziara, N. Akkilic, K. Nijmeijer, N. E. Benes, J.Mater. Sci. 2016,51,1074. [44] R.Wanka, N. Aldred, J. A. Finlay, A. Amuthalingam, J. L. Clarke, A. S. Clare, A. Rosenhahn, Langmuir 2019, 35, 16568. [45] O. Dannenberger,M. Buck,M. Grunze, J. Phys. Chem. B 1999, 103,2202. [46] J. Kim, G. A. Somorjai, J. Am. Chem. Soc. 2003, 125, 3150. [47] I. J. Colton, J. R. Anderson, J. Gao, R. G. Chapman, L. Isaacs, G.M. Whitesides, J. Am. Chem. Soc. 1997, 119, 12701. [48] K. A. Nolte, J. Schwarze, A. Rosenhahn, Biofouling 2017, 33, 531. [49] A. Rosenhahn, T. Ederth,M. E. Pettitt, Biointerphases 2008, 3, IR1. [50] M.E. Callow, J. A. Callow, J. D. Pickett-Heaps, R.Wetherbee, J. Phycol.1997,33,938. [51] a) L. K. Ista,M. E. Callow, J. A. Finlay, S. E. Coleman, A. C. Nolasco, R. H. Simons, J. A. Callow, G. P. Lopez, Appl. Environ.Microbiol. 2004,70, 4151; b)M.Heydt,M.E. Pettitt, X. Cao,M.E. Callow, J. A. Callow, M. Grunze, A. Rosenhahn, Biointerphases 2012, 7, 33; [52] S.Krishnan,C.J.Weinman,C.K.Ober,J.Mater.Chem.20 2,08,318,. [53] C. S. Gudipati, J. A. Finlay, J. A. Callow,M. E. Callow, K. L.Wooley, Langmuir 2005, 21,3044. [54] J. Buller, A. Laschewsky, E.Wischerhof, SoftMatter 2013, 9, 929. [55] a) X. Cao,M.E.Pettit, S. L. Conlan,W.Wagner, A. D. Ho, A. S. Clare, J. A.Callow,M.E.Callow,M.Grunze,A.Rosenhahn,Biomacromolecules 2009, 10, 907; b) S. Bauer,M.Alles,M.P. Arpa-Sancet, E. Ralston, G. W.Swain, N. Aldred, A. S. Clare, J. A. Finlay,M.E. Callow, J. A. Callow, A. Rosenhahn, Biomacromolecules 2016, 17, 897; [56] R.Wanka, F. Koschitzki, V. Puzovic, T. Pahl, E.Manderfeld, K. Z. Hun-sucker, G.W. Swain, A. Rosenhahn, ACS Appl.Mater. Interfaces 2021,13,6659. [57] I.Thome,M.E.Pettitt,M.E.Callow,J.A.Callow,M.Grunze,A.Rosen-hahn, Biofouling 2012, 28, 501. [58] K. A. Nolte, J. Schwarze, C. D. Beyer, O. Özcan, A. Rosenhahn, Bioin-terphases 2018, 13,041007. [59] M. P. Schultz, J. A. Finlay,M. E. Callow, J. A. Callow, Biofouling 2000,15,243. [60] K. A. Nolte, J. Koc, J.M. Barros, K. Hunsucker,M. P. Schultz, G.W. Swain, A. Rosenhahn, Biofouling 2018, 34, 398.
确定






还剩10页未读,是否继续阅读?
中国格哈特为您提供《天然砂质沉积物渗入涂层的振荡》,该方案主要用于涂料中沉积物渗入的光度测量检测,参考标准--,《天然砂质沉积物渗入涂层的振荡》用到的仪器有格哈特强力高重现振荡器LS500/RO500、格哈特快速干燥仪STL56、德国移液器MM
推荐专场
快速干燥仪
更多
相关方案
更多

















