一种基于聚乙烯胺的植物酸基叠层棉花阻燃涂料A Flame-Retardant Phytic-Acid-Based LbL-Coating for Cotton Using Polyvinylamine
方案详情

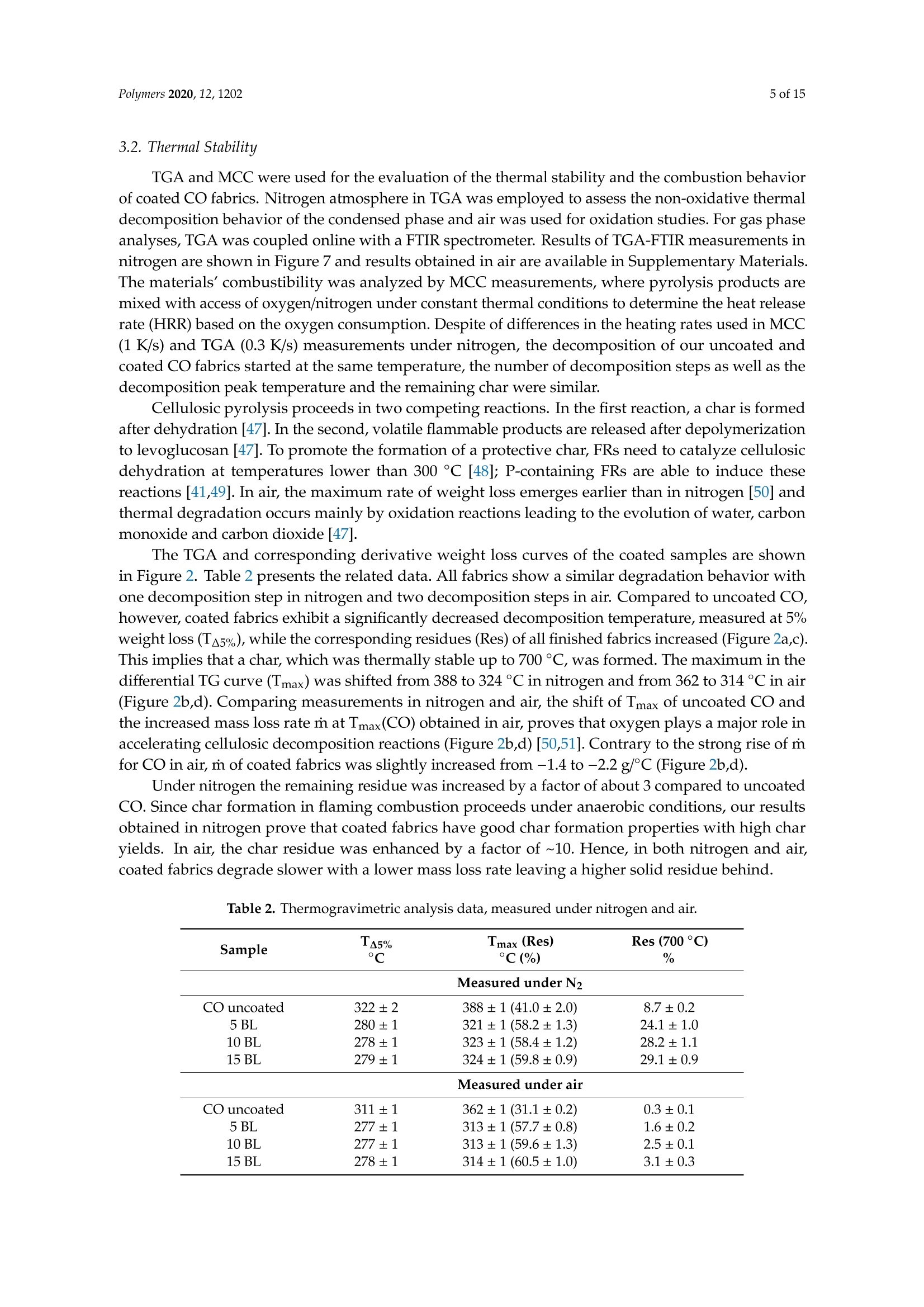
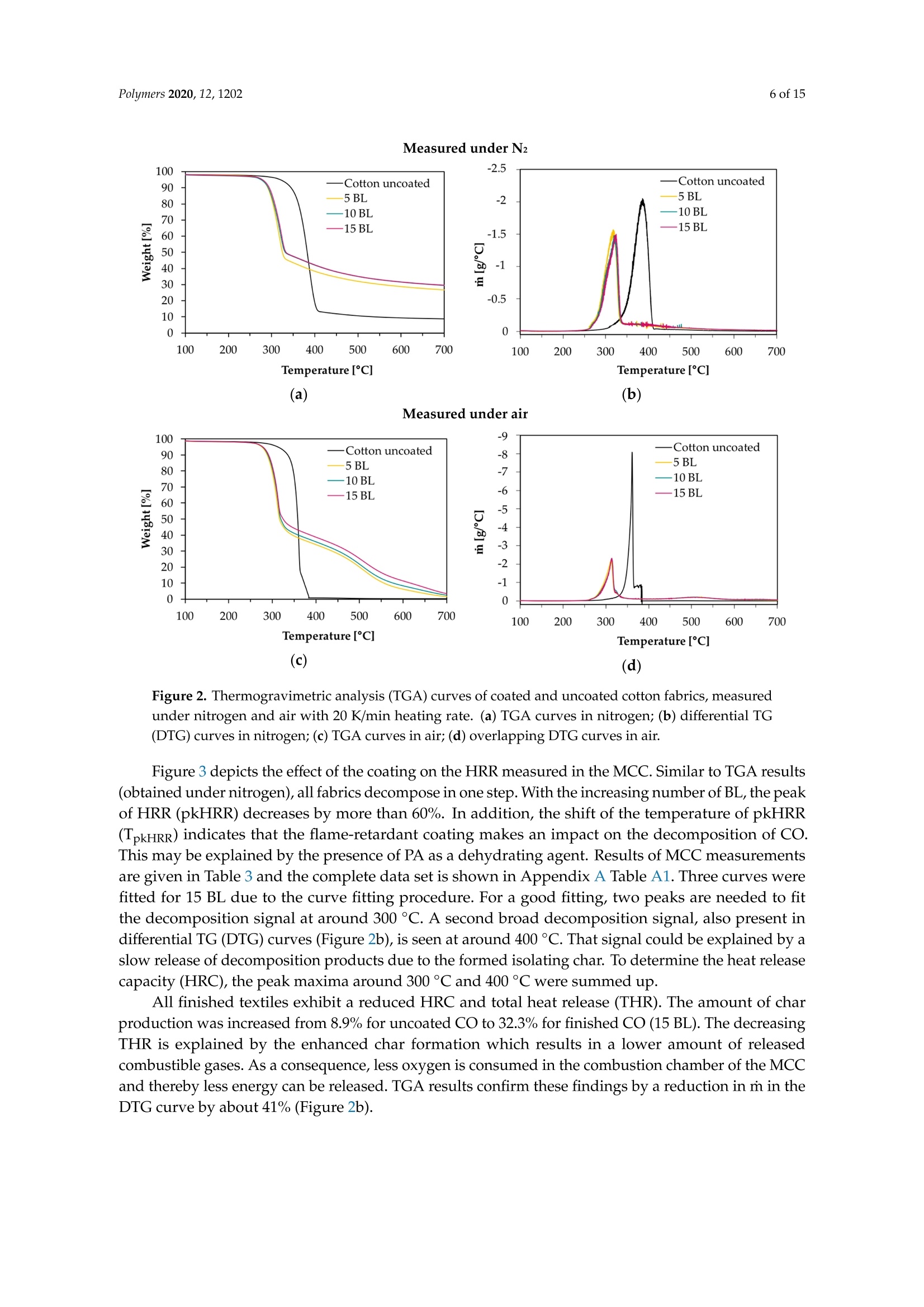
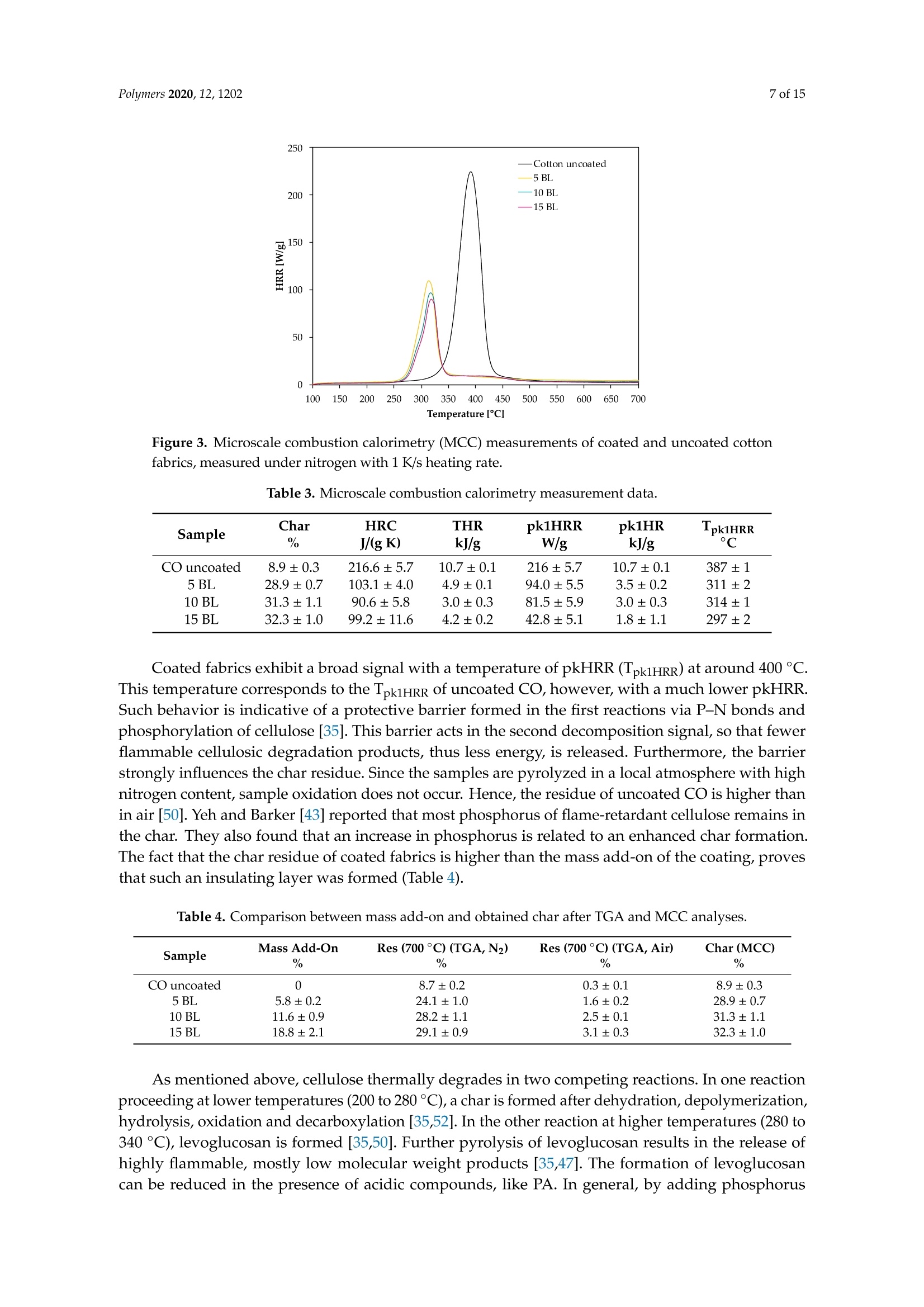
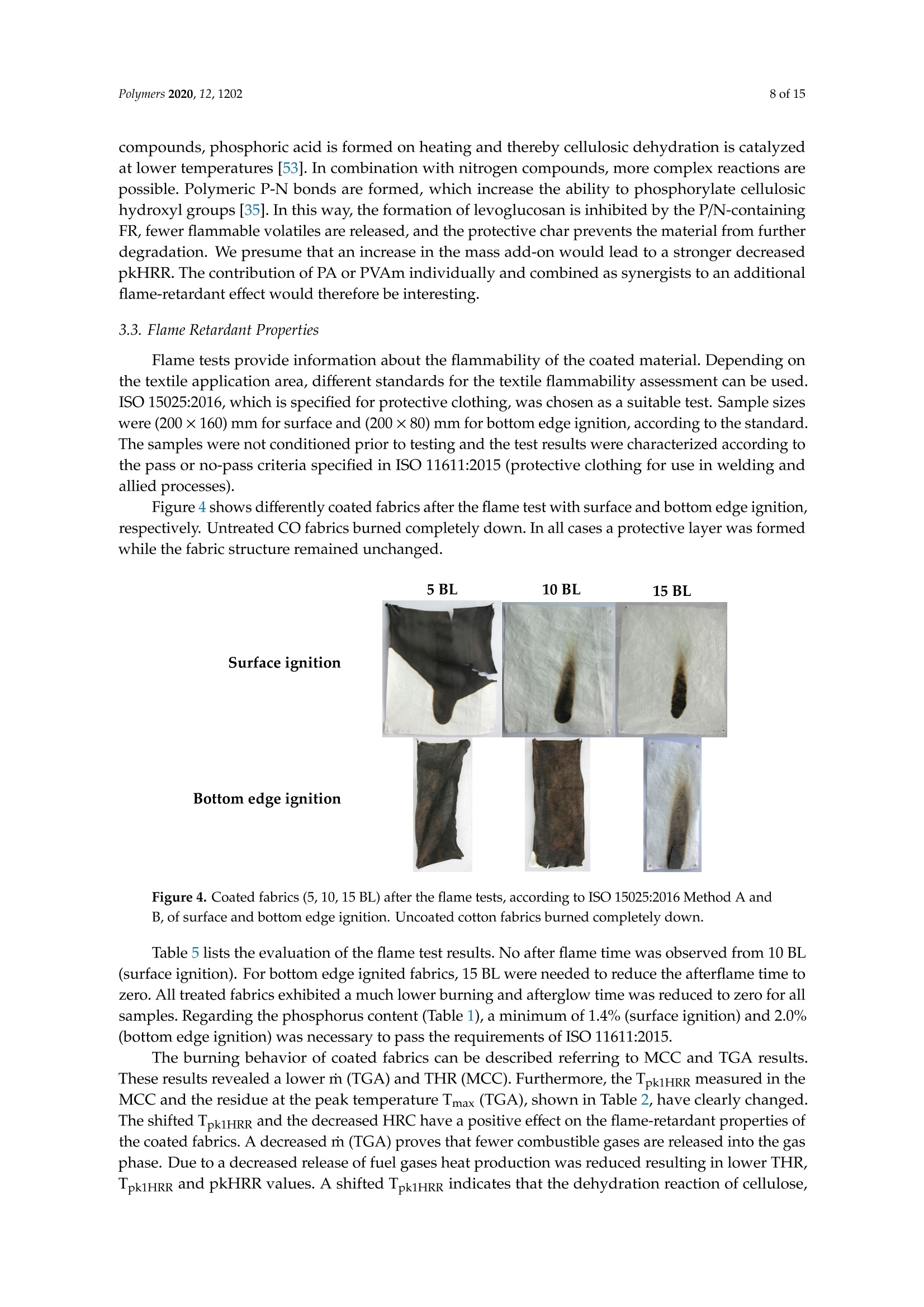
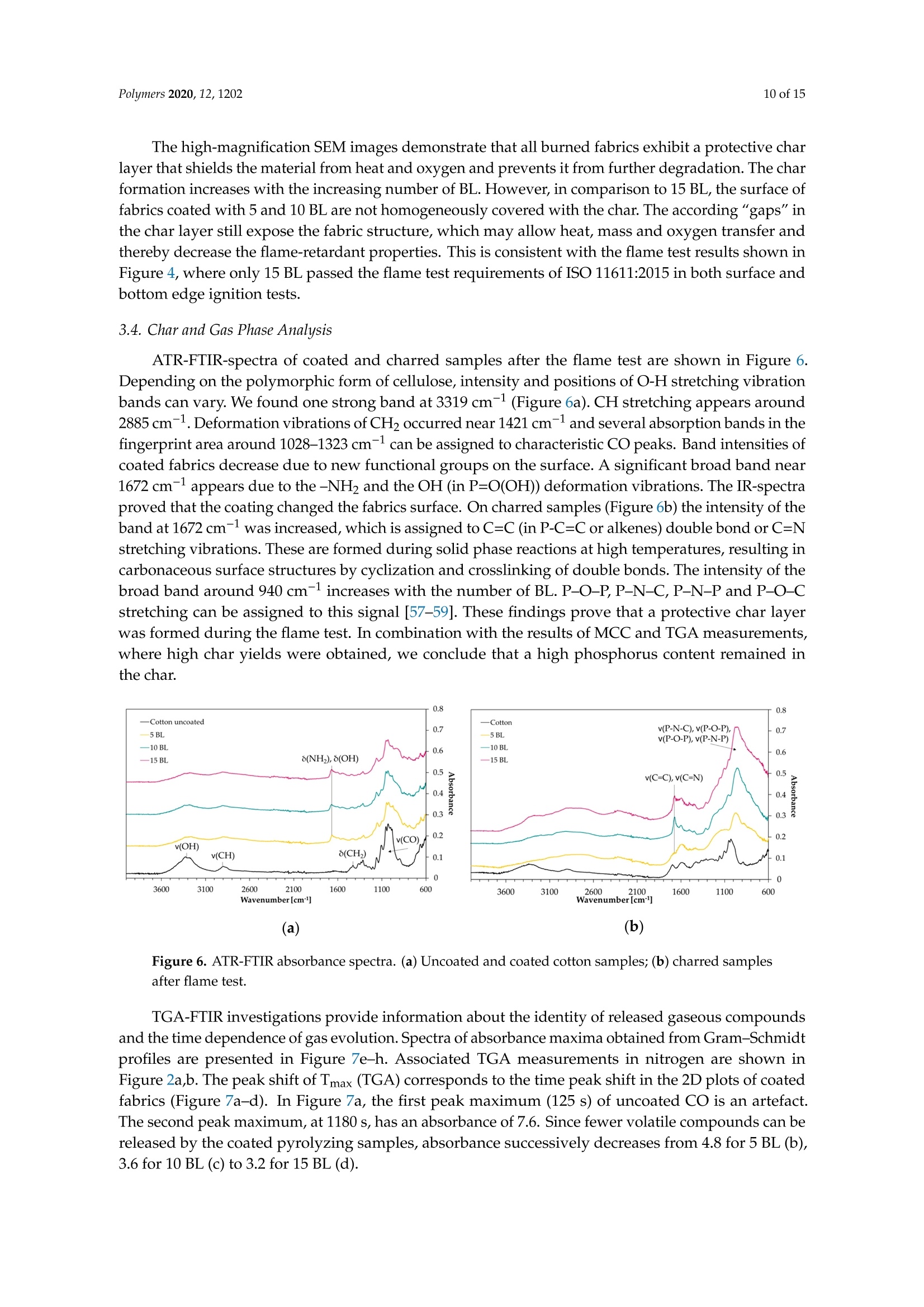
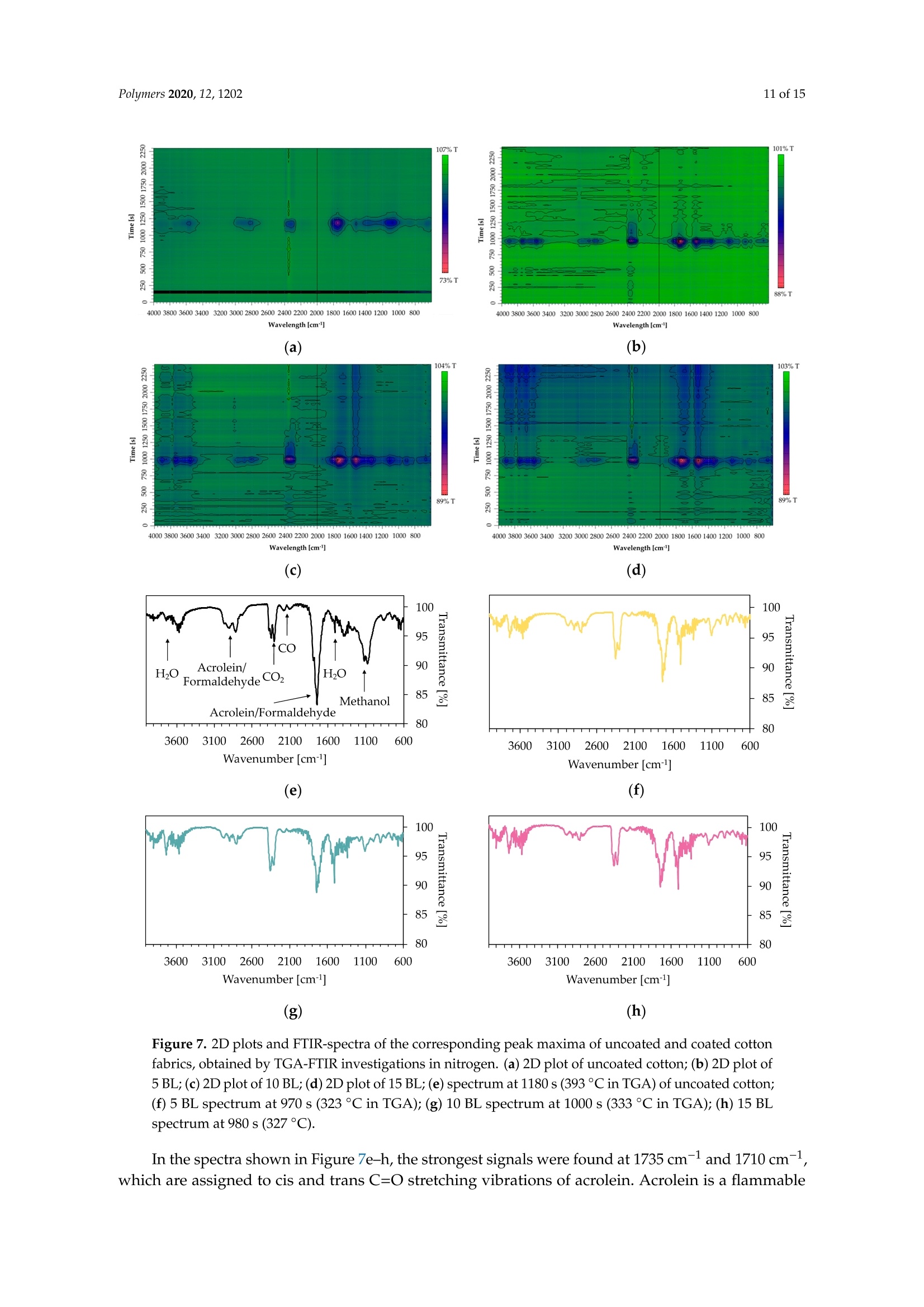
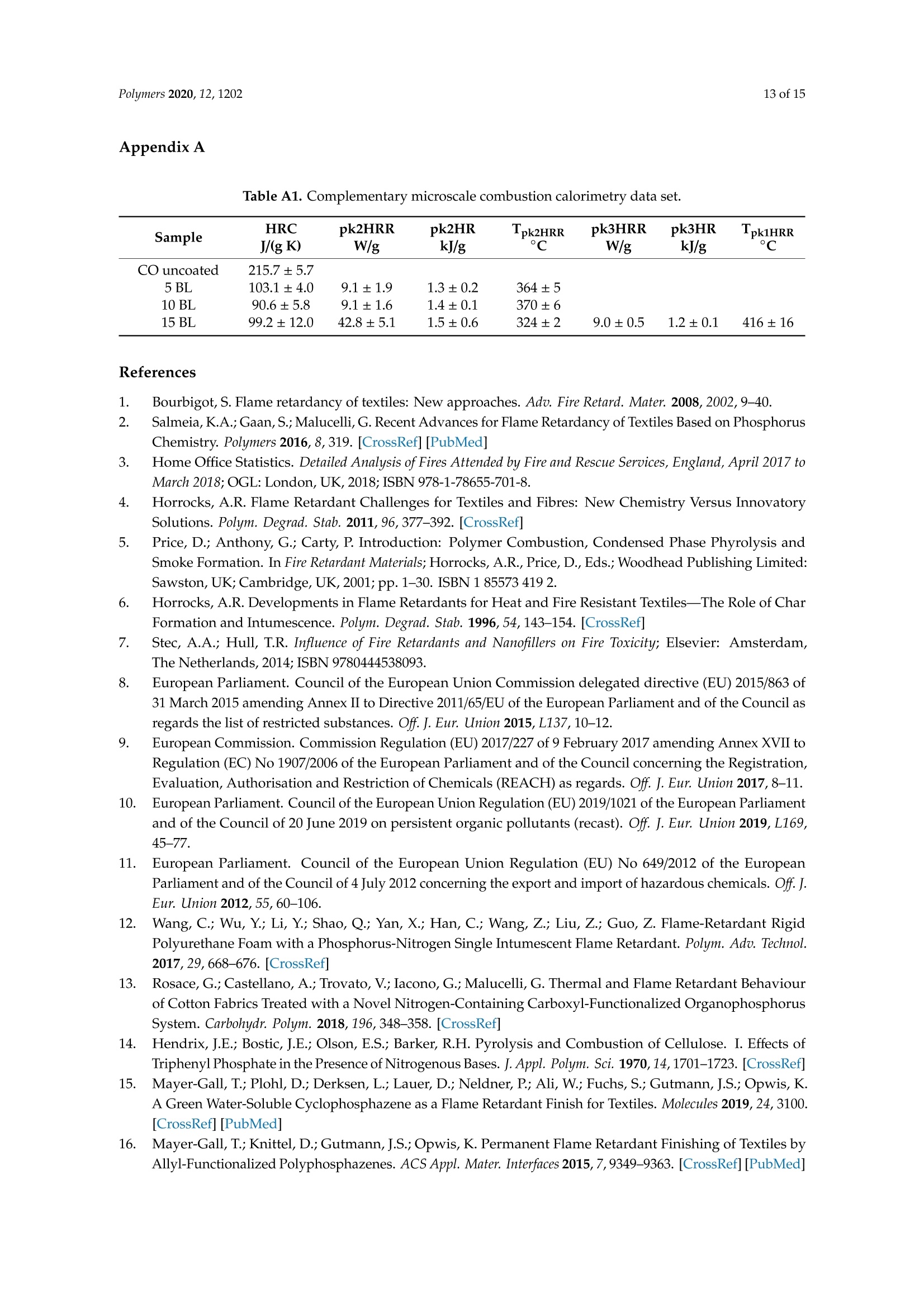
一种基于聚乙烯胺的植物酸基叠层棉花阻燃涂料A Flame-Retardant Phytic-Acid-Based LbL-Coating for Cotton Using Polyvinylamine使用格哈特 特伯森+维普得 凯氏定氮仪测定带叠层阻燃涂料棉花的氮含量。MDPIpolymers 2 of 15Polymers 2020,12,1202 一种基于聚乙烯胺的植物酸基叠层棉花阻燃涂料 Article A Flame-Retardant Phytic-Acid-Based LbL-Coatingfor Cotton Using Polyvinylamine Olga Zilke 1*, Dennis Plohl 1, Klaus Opwis 1, Thomas Mayer-Gall 1.2iDand Iochen Stefan Gutmann 1,2,*iD Deutsches Textilforschungszentrum Nord-West gGmbH, Adlerstrasse 1, D-47798 Krefeld, Germany; dennis.plohl@dtnw.de (D.P.); opwis@dtnw.de (K.O.); mayer-gall@dtnw.de (T.M.-G.) 2 Physical Chemistry & CENIDE, University Duisburg-Essen, Universitatsstrasse 5, D-45117 Essen, Germany Correspondence: zilke@dtnw.de (O.Z.); jochen.gutmann@uni-due.de (J.S.G.) Received: 5 April 2020; Accepted: 20 May 2020; Published: 25 May 2020 check forupdates Abstract: Phytic acid (PA), as a natural source of phosphorus, was immobilized on cotton (CO) in alayer-by-layer (LbL) approach with polyvinylamine (PVAm) as the oppositely charged electrolyteto create a partly bio-based flame-retardant finish. PVAm was employed as a synthetic nitrogensource with the highest density of amine groups of all polymers. Vertical flame tests revealed aflame-retardant behavior with no afterflame and afterglow time for a coating of 15 bilayers (BL)containing 2% phosphorus and 1.4% nitrogen. The coating achieved a molar P:N ratio of 3:5. Microscalecombustion calorimetry (MCC) analyses affirmed the flame test findings by a decrease in peak heatrelease rate (pkHRR) by more than 60% relative to unfinished CO. Thermogravimetric analyses (TGA)and MCC measurements exhibited a shifted CO peak to lower temperatures indicating proceedingreactions to form an isolating char on the surface. Fourier transform infrared spectroscopy (FTIR)coupled online with a TGA system, allowed the identification of a decreased amount of acrolein,methanol, carbon monoxide and formaldehyde during sample pyrolysis and a higher amount ofreleased water. Thereby the toxicity of released volatiles was reduced. Our results prove that PAenables a different reaction by catalyzing cellulosic dehydration, which results in the formation of aprotective char on the surface of the burned fabric. Keywords: textiles; cotton; phytic acid; polyvinylamine; flame retardant finishing; layer-by-layer 1. Introduction Textiles are used in a wide variety of applications, including carpets, clothing, upholstery orfurnishing fabrics. They are made of synthetic and natural polymers, have a large surface area and anorganic origin [1]. Therefore most of them are flammable and thereby potentially hazardous unlesssuitable protective coatings are applied [2]. In 2017/18 about 25% of household fires were caused bytextiles, upholstery and furnishings, while 46% of all fire-related fatalities were associated with thesefires [3]. Phytic acid (PA) is a low molecular weight antinutrient [20] and has a strong chelating abilityto form cation-phytate complexes [21]. In plant seeds and grains, phytate is present as the mainphosphorus storage form [22]. O'Dell et al.[23] found the major proportion of all analyzed elementsin the outer layers of wheat and rice kernels whereas phytates were mainly found in the germ.Since these parts are removed by milling during cereal grain processing [23], phytate can be producedfrom these food industry waste products [24]. Several studies have indicated that PA can reducetextile flammability [25-28]. This is due to the ability of acidic phosphorus-based FRs to enhancethe cellulosic carbonization by dehydration and to decrease the release of flammable gases [29-31].In combination with nitrogen-compounds, phosphorus-based FRs can improve their efficiency by aP-N-synergism on cellulose [32-34]. Initial findings, summarized by Horrocks [35], suggested thatnitrogen forms polymeric compounds containing P-N bonds by nucleophilic attack on the phosphategroup. In the study of Cheng et al.[25] PA was used for the synthesis of a phosphorus-based FR whichwas covalently linked to wool. They investigated a self-extinguishing effect in the vertical flame testafter 20 washing cycles. As an alternative to covalent linkage, layer-by-layer (LbL) assembly is anotherstrategy of textile surface modification by PA due to its highly charged phosphate groups [36,37].LbL assembly involves alternate deposition of positively and negatively charged electrolytes to buildmultilayered films on the substrate (Figure 1) [38-40]. Laufer et al. [26] immobilized PA and chitosan asan environmental-friendly flame-retardant finishing for cotton (CO) by LbL technique. The compositionof the coating was influenced by varying the pH of the deposition solutions. Fabrics coated with30 bilayers (BL) exhibited a self-extinguishing effect in a vertical flame test. For LbL coatings water can be used as solvent, no additional crosslinking agent is required andthe deposition solution can be recycled [41]. These characteristics make the process sustainable. 1 cycle =1 bilayer (BL) Figure 1. Schematic of LbL assembly using phytic acid and polyvinylamine. The cycles are repeateduntil the desired number of bilayers is obtained. Given this background, our work focuses on the immobilization of phosphorus-containing PA onCO using an LbL approach with polyvinylamine (PVAm) to create a partially bio-based flame-retardantfinish. Since phosphorus- and nitrogen-containing FRs often act as flame-retardant synergists, a highnitrogen content potentially leads to a favorable flame-retardant performance. PVAm contains thehighest amount of primary amine groups of all polymers with a nitrogen content of 32.5%, whereaschitosan as a biopolymer alternative has 8.7% nitrogen content. Hence, the commercially availablePVAm acts as a cationic polyelectrolyte and synthetic nitrogen-rich source in our coating. Stable ion pairs were generated by LbL assembly and varying number of BL. Flame-retardantproperties were investigated according to ISO 15025:2016. In addition, the thermal stability wasexamined by means of thermogravimetric analysis (TGA) and microscale combustion calorimetry(MCC). The surface topography was studied by use of scanning electron microscopy (SEM).Fourier transform infrared spectroscopy (FTIR) was employed for chemical analysis of fabrics beforeand after flame test. TGA coupled with a FTIR spectrometer was used for gas phase investigations. 2. Materials and Methods 2.1. Materials Woven CO fabric (plain weave, 170 g/m², white) was purchased from wfk Testgewebe GmbH,Briggen, Germany. PA (50% in water) was obtained from TCI Deutschland GmbH, Eschborn,Germany. Technical PVAm, sourced from Lupamin@ 9095 (L9095, >90% degree of hydrolysis,solid content 20-22%), and branched polyethylenimine (BPEI), sourced from Lupasol@ WF (99%BPEI),were purchased from BASF. Nitric acid (69%, supra-quality ROTIPURAN@, Carl Roth GmbH+ Co. KG, Karlsruhe, Germany)was used for microwave-assisted digestion of textile samples for phosphorus determination byinductively coupled plasma optical emission spectroscopy (ICP-OES). For the potentiometric determination of Kjeldahl nitrogen H2SO4 (ROTIPURAN@ 98%),NaOH (50%, extra pure) and HCl (0.05 mol/L, volumetric solution) were purchased from CarlRoth GmbH+ Co. KG, Karlsruhe, Germany, HzBO3 (≥99.5%, Ph.Eur., USP, BP) from Bernd KraftGmbH, Duisburg, Germany and Kjeltabs Auto, used as a digestion catalyst, from Thompson andCapper Ltd, Runcorn, UK. 2.2. Instrumentation 2.3. LbL Assembly All reagents were used as received. Demineralized water (DW,1.8MQ,pH~6.8) was used toprepare deposition solutions. A 5% PA solution (pH ~ 0.7) and 5% L9095 solution (pH~8.7) wereused for the coating of the textiles. The deposition procedure is shown in Figure 1. First, the textilesurface was activated using a 1% BPEI solution (pH~11.7), according to Laufer et al. [26]. Then thesubstrates were alternately dipped into the PA and L9095 solution. The first immersion steps lasted for5 min each, subsequent steps 1 min. Each immersion step was followed by rinsing with DW (2 min) toremove unbonded residues. Subsequently, textiles were wrung out. After the desired number of BLwas obtained, the samples were dried at 80 ℃. 2.4. Characterization of Immobilized Compounds The phosphorus content of ffinished textiles was quantitatively determined using ICP-OES of200 mg samples digested with 8 mL HNO3 in a microwave. The samples were then diluted withultrapure water to a volume of 25 mL, filtered and analyzed. The mass add-on A (%) was calculated using where mo is the mass before treatment (g) and mj is the mass after treatment (g). Nitrogen content was determined by potentiometric Kjeldahl titration. For that reason, the samples(200 mg) were digested in 20 mL sulfuric acid. Then, during the steam distillation NaOH was added tothe digested sample. The formed ammonia was distilled off and transferred into the receiver vesselwith aqueous H3BO3 solution as absorbing solution. The captured ammonium ions were determinedby potentiometric titration with HCl. 3. Results and Discussion 3.1. Coating Properties Many phosphorus-and nitrogen-containing FRs in cellulose are able to promote char formation [42].That was revealed by detailed studies of formed char, which showed that most phosphorus-containingFRs were not volatilized, but remained in the char instead [43]. An increase in the flame-retardantcontent, enhances the char formation and thus the flame-retardant characteristics of the coating.However, the effectiveness of phosphorus compounds differs from each other, depending on theirchemical structure. Hendrix and coworkers [14] suggested that a phosphorus content of at least 1.5%(w/w) is required for the finishing to be efficient. Later studies by Tesoro [44] found a minimum of 2-4%(w/w) phosphorus content, whereas some nitrogen compounds were able to decrease the minimumphosphorus amount required. For that reason, nitrogen-rich polymers such as PVAm can be used toachieve better flame-retardant properties. The synergism of phosphorus- and nitrogen-containing FRsin cellulose can be explained by an enhancement of phosphorylation of cellulose and an increasedcharring and gas phase mode of action [35,45]. PA has a phosphorus content of about 28% (w/w),therefore add-ons of 7%-14%(w/w) are needed to achieve phosphorus levels of 2%-4%(w/w). Results of determined phosphorus and nitrogen mass fractions x, the molar P:N ratio as well asthe mass add-on are summarized in Table 1. As expected, add-on increases linearly with increasingnumber of BL. After deposition of 15 BL the phosphorus content was up to 2% and the nitrogen content1.4%. Considering the synergistic effect of phosphorus and nitrogen in flame-retardant finishings and arequired phosphorus content of at least 1.5%, samples with 10 and 15 BL are expected to have goodflame-retardant properties. Furthermore, several studies, reviewed by Heywood et al.[46], have shownthat the P:N molar ratio influences the flame-retardant performance of the coating. Our samples coatedwith 15 BL had a P:N molar ratio of ~3:5. Table 1. Summary of determined mass add-on, phosphorus- and nitrogen-mass fractions x and molaiP/N ratio of coated cotton fabrics. Sample Mass Add-On xp Measured xN Measured P/N Molar Ratio % % % mol/mol 5 BL 5.8±0.2 0.8±0.04 0.7±0.01 1:2 10 BL 11.6±0.9 1.4±0.08 1.0±0.03 3:5 15 BL 18.8±2.1 2.0±0.08 1.4±0.13 3:5 1 Measured quantitatively by ICP-OES. 2 Measured quantitatively by Kjeldahl nitrogen determination. 3.2. Thermal Stabilitu TGA and MCC were used for the evaluation of the thermal stability and the combustion behaviorof coated CO fabrics. Nitrogen atmosphere in TGA was employed to assess the non-oxidative thermaldecomposition behavior of the condensed phase and air was used for oxidation studies. For gas phaseanalyses, TGA was coupled online with a FTIR spectrometer. Results of TGA-FTIR measurements innitrogen are shown in Figure 7 and results obtained in air are available in Supplementary Materials.The materials' combustibility was analyzed by MCC measurements, where pyrolysis products aremixed with access of oxygen/nitrogen under constant thermal conditions to determine the heat releaserate (HRR) based on the oxygen consumption. Despite of differences in the heating rates used in MCC(1 K/s) and TGA (0.3 K/s) measurements under nitrogen, the decomposition of our uncoated andcoated CO fabrics started at the same temperature, the number of decomposition steps as well as thedecomposition peak temperature and the remaining char were similar. The TGA and corresponding derivative weight loss curves of the coated samples are shownin Figure 2. Table 2 presents the related data. All fabrics show a similar degradation behavior withone decomposition step in nitrogen and two decomposition steps in air. Compared to uncoated CO,however, coated fabrics exhibit a significantly decreased decomposition temperature, measured at 5%weight loss (TA5%), while the corresponding residues (Res) of all finished fabrics increased (Figure 2a,c).This implies that a char, which was thermally stable up to 700 °℃, was formed. The maximum in thedifferential TG curve (Tmax) was shifted from 388 to 324 °℃ in nitrogen and from 362 to 314°C in air(Figure 2b,d). Comparing measurements in nitrogen and air, the shift of Tmax of uncoated CO andthe increased mass loss rate m at Tmax(CO) obtained in air, proves that oxygen plays a major role inaccelerating cellulosic decomposition reactions (Figure 2b,d) [50,51]. Contrary to the strong rise of mfor CO in air, m of coated fabrics was slightly increased from -1.4 to -2.2 g/C (Figure 2b,d). Under nitrogen the remaining residue was increased by a factor of about 3 compared to uncoatedCO. Since char formation in flaming combustion proceeds under anaerobic conditions, our resultsobtained in nitrogen prove that coated fabrics have good char formation properties with high charyields. In air, the char residue was enhanced by a factor of ~10. Hence, in both nitrogen and air,coated fabrics degrade slower with a lower mass loss rate leaving a higher solid residue behind. Table 2. Thermogravimetric analysis data, measured under nitrogen and air. Sample TA5% Tmax (Res) Res (700°C) Measured under N2 CO uncoated 322±2 388±1(41.0±2.0) 8.7±0.2 5 BL 280±1 321±1(58.2±1.3) 24.1±1.0 10 BL 278±1 323±1(58.4±1.2) 28.2±1.1 15 BL 279±1 324±1(59.8±0.9) 29.1±0.9 Measured under air CO uncoated 311±1 362±1(31.1±0.2) 0.3±0.1 5 BL 277±1 313±1(57.7±0.8) 1.6±0.2 10 BL 277±1 313±1(59.6±1.3) 2.5±0.1 15 BL 278±1 314±1(60.5±1.0) 3.1±0.3 Measured under N2 Figure 2. Thermogravimetric analysis (TGA) curves of coated and uncoated cotton fabrics, measuredunder nitrogen and air with 20 K/min heating rate. (a) TGA curves in nitrogen; (b) differential TG(DTG) curves in nitrogen; (c) TGA curves in air; (d) overlapping DTG curves in air. Figure 3 depicts the effect of the coating on the HRR measured in the MCC. Similar to TGA results(obtained under nitrogen), all fabrics decompose in one step. With the increasing number of BL, the peakof HRR (pkHRR) decreases by more than 60%. In addition, the shift of the temperature of pkHRR(TpkHRR) indicates that the flame-retardant coating makes an impact on the decomposition of CO.This may be explained by the presence of PA as a dehydrating agent. Results of MCC measurementsare given in Table 3 and the complete data set is shown in Appendix A Table A1. Three curves werefitted for 15 BL due to the curve fitting procedure. For a good fitting, two peaks are needed to fitthe decomposition signal at around 300 °C. A second broad decomposition signal, also present indifferential TG (DTG) curves (Figure 2b), is seen at around 400 C. That signal could be explained by aslow release of decomposition products due to the formed isolating char. To determine the heat releasecapacity (HRC), the peak maxima around 300°C and 400 ℃ were summed up. All finished textiles exhibit a reduced HRC and total heat release (THR). The amount of charproduction was increased from 8.9% for uncoated CO to 32.3% for finished CO (15 BL). The decreasingTHR is explained by the enhanced char formation which results in a lower amount of releasedcombustible gases. As a consequence, less oxygen is consumed in the combustion chamber of the MCCand thereby less energy can be released. TGA results confirm these findings by a reduction in m in theDTG curve by about 41% (Figure 2b). Figure 3. Microscale combustion calorimetry (MCC) measurements of coated and uncoated cottonfabrics, measured under nitrogen with 1 K/s heating rate. Table 3. Microscale combustion calorimetry measurement data. Sample Char HRC THR pk1HRR pk1HR Lpk1HRR CO uncoated 8.9±0.3 216.6±5.7 10.7±0.1 216±5.7 10.7±0.1 387±1 5 BL 28.9±0.7 103.1±4.0 4.9±0.1 94.0±5.5 3.5±0.2 311±2 10 BL 31.3±1.1 90.6±5.8 3.0±0.3 81.5±5.9 3.0±0.3 314±1 15 BL 32.3±1.0 99.2±11.6 4.2±0.2 42.8±5.1 1.8±1.1 297±2 Coated fabrics exhibit a broad signal with a temperature of pkHRR (Tpk1HRR) at around 400°℃.This temperature corresponds to the Tpk1HRR of uncoated CO, however, with a much lower pkHRR.Such behavior is indicative of a protective barrier formed in the first reactions via P-N bonds andphosphorylation of cellulose [35]. This barrier acts in the second decomposition signal, so that fewerflammable cellulosic degradation products, thus less energy, is released. Furthermore, the barrierstrongly influences the char residue. Since the samples are pyrolyzed in a local atmosphere with highnitrogen content, sample oxidation does not occur. Hence, the residue of uncoated CO is higher thanin air [50]. Yeh and Barker [43] reported that most phosphorus of flame-retardant cellulose remains inthe char. They also found that an increase in phosphorus is related to an enhanced char formation.The fact that the char residue of coated fabrics is higher than the mass add-on of the coating, provesthat such an insulating layer was formed (Table 4). Table 4. Comparison between mass add-on and obtained char after TGA and MCC analyses. Sample Mass Add-On Res (700°C) (TGA,N2) Res (700 °C) (TGA, Air) Char (MCC) CO uncoated 0 8.7±0.2 0.3±0.1 8.9±0.3 5 BL 5.8±0.2 24.1±1.0 1.6±0.2 28.9±0.7 10 BL 11.6±0.9 28.2±1.1 2.5±0.1 31.3±1.1 15 BL 18.8±2.1 29.1±0.9 3.1±0.3 32.3±1.0 As mentioned above, cellulose thermally degrades in two competing reactions. In one reactionproceeding at lower temperatures (200 to 280 °C), a char is formed after dehydration, depolymerization,hydrolysis, oxidation and decarboxylation [35,52]. In the other reaction at higher temperatures (280 to340°C), levoglucosan is formed [35,50]. Further pyrolysis of levoglucosan results in the release ofhighly flammable, mostly low molecular weight products [35,47]. The formation of levoglucosancan be reduced in the presence of acidic compounds, like PA. In general, by adding phosphorus compounds, phosphoric acid is formed on heating and thereby cellulosic dehydration is catalyzedat lower temperatures [53]. In combination with nitrogen compounds, more complex reactions arepossible. Polymeric P-N bonds are formed, which increase the ability to phosphorylate cellulosichydroxyl groups [35]. In this way, the formation of levoglucosan is inhibited by the P/N-containingFR, fewer flammable volatiles are released, and the protective char prevents the material from furtherdegradation. We presume that an increase in the mass add-on would lead to a stronger decreasedpkHRR. The contribution of PA or PVAm individually and combined as synergists to an additionalflame-retardant effect would therefore be interesting. 3.3. Flame Retardant Properties Flame tests provide information about the flammability of the coated material. Depending onthe textile application area, different standards for the textile flammability assessment can be used.ISO 15025:2016, which is specified for protective clothing, was chosen as a suitable test. Sample sizeswere (200×160) mm for surface and (200×80) mm for bottom edge ignition, according to the standard.The samples were not conditioned prior to testing and the test results were characterized according tothe pass or no-pass criteria specified in ISO 11611:2015 (protective clothing for use in welding andallied processes). Figure 4 shows differently coated fabrics after the flame test with surface and bottom edge ignition,respectively. Untreated CO fabrics burned completely down. In all cases a protective layer was formedwhile the fabric structure remained unchanged. Figure 4. Coated fabrics (5, 10, 15 BL) after the flame tests, according to ISO 15025:2016 Method A andB, of surface and bottom edge ignition. Uncoated cotton fabrics burned completely down. Table 5 lists the evaluation of the flame test results. No after flame time was observed from 10 BL(surface ignition). For bottom edge ignited fabrics, 15 BL were needed to reduce the afterflame time tozero. All treated fabrics exhibited a much lower burning and afterglow time was reduced to zero for allsamples. Regarding the phosphorus content (Table 1), a minimum of 1.4% (surface ignition) and 2.0%(bottom edge ignition) was necessary to pass the requirements of ISO 11611:2015. The burning behavior of coated fabrics can be described referring to MCC and TGA results.These results revealed a lower m (TGA) and THR (MCC). Furthermore, the Tpk1HRR measured in theMCC and the residue at the peak temperature Tmax (TGA), shown in Table 2, have clearly changed.The shifted Tpk1HRR and the decreased HRC have a positive effect on the flame-retardant properties ofthe coated fabrics. A decreased m (TGA) proves that fewer combustible gases are released into the gasphase. Due to a decreased release of fuel gases heat production was reduced resulting in lower THR,Tpk1HRR and pkHRR values. A shifted Tpk1HRR indicates that the dehydration reaction of cellulose, caused by phosphorus catalysis, occurs at lower temperatures. The carbonized layer is formed andthus shields the combustible material below. In this way the fire spread can be inhibited or delayed.These changes were confirmed in the flame tests (Figure 4) and the SEM study (Figure 5), as in all casesthe textile structure remained unchanged. HRC values can also be correlated to a UL 94 flame test,as suggested by Lyon et al. [54]. With a HRC lower than 100 J/(g K), our samples coated with 10 and15 BL could achieve a UL 94 V0 rating or better (no ignition, no afterflame). Table 5. Flame test results of coated cotton samples according to ISO 15025:2016. The stated rating-pass,no pass-is based on two criteria specified in ISO 11611:2015 Sample Surface Ignition Bottom Edge Ignition Afterflame Flame Reaches Upper or Test Passed Afterflame Flame Reaches Upper or Test Passed Burns Burns CO uncoated completely yes completely yes no down within no down within 5 BL 64 s 17s 10 BL 57 s yes no 18 s yes no 0s no yes 14s yes no 15 BL 0s no yes 0s no yes The surface morphologies of untreated, coated and burned fabrics were investigated by SEM(Figure 5). Compared to uncoated CO, the surface of coated fabrics was changed by dispersion of theflame-retardant coating on and around the fiber, without affecting the textile structure. Figure 5. Scanning electron microscopy top view images of untreated, treated and burned cottonfabrics. Top row: fabrics before flame test; middle and bottom row: charred samples after flame test indifferent magnifications. Contrary to 10 and 15 BL, samples finished with 5 BL formed blisters on the surface after burning.For 10 BL blisters were found on fibers exposed by gaps in the char layer (see high magnificationimages in Figure 5, 10 BL). Phosphorus and nitrogen compounds are able to catalyze the dehydrationof CO [41,49] and can act in both, gas and condensed phase [55,56]. It may be assumed that the blistersform as a result of gases formed during the thermal decomposition of PVAm and PA on CO. The high-magnification SEM images demonstrate that all burned fabrics exhibit a protective charlayer that shields the material from heat and oxygen and prevents it from further degradation. The charformation increases with the increasing number of BL. However, in comparison to 15 BL, the surface offabrics coated with 5 and 10 BL are not homogeneously covered with the char. The according “gaps"inthe char layer still expose the fabric structure, which may allow heat, mass and oxygen transfer andthereby decrease the flame-retardant properties. This is consistent with the flame test results shown inFigure 4, where only 15 BL passed the flame test requirements of ISO 11611:2015 in both surface andbottom edge ignition tests. 3.4. Char and Gas Phase Analysis ATR-FTIR-spectra of coated and charred samples after the flame test are shown in Figure 6.Depending on the polymorphic form of cellulose, intensity and positions of O-H stretching vibrationbands can vary. We found one strong band at 3319 cm-(Figure 6a). CH stretching appears around2885 cm-1. Deformation vibrations of CH2 occurred near 1421 cm-l and several absorption bands in thefingerprint area around 1028-1323 cm-1 can be assigned to characteristic CO peaks. Band intensities ofcoated fabrics decrease due to new functional groups on the surface. A significant broad band near1672 cm- appears due to the -NH2 and the OH (in P=O(OH)) deformation vibrations. The IR-spectraproved that the coating changed the fabrics surface. On charred samples (Figure 6b) the intensity of theband at 1672 cm-1 was increased, which is assigned to C=C (in P-C=C or alkenes) double bond or C=Nstretching vibrations. These are formed during solid phase reactions at high temperatures, resulting incarbonaceous surface structures by cyclization and crosslinking of double bonds. The intensity of thebroad band around 940 cm-1 increases with the number of BL. P-O-P, P-N-C, P-N-P and P-O-Cstretching can be assigned to this signal [57-59]. These findings prove that a protective char layerwas formed during the flame test. In combination with the results of MCC and TGA measurements,where high char yields were obtained, we conclude that a high phosphorus content remained inthe char. Figure 6. ATR-FTIR absorbance spectra. (a) Uncoated and coated cotton samples; (b) charred samplesafter flame test. TGA-FTIR investigations provide information about the identity of released gaseous compoundsand the time dependence of gas evolution. Spectra of absorbance maxima obtained from Gram-Schmidtprofiles are presented in Figure 7e-h. Associated TGA measurements in nitrogen are shown inFigure 2a,b. The peak shift of Tmax (TGA) corresponds to the time peak shift in the 2D plots of coatedfabrics (Figure 7a-d). In Figure 7a, the first peak maximum (125 s) of uncoated CO is an artefact.The second peak maximum, at 1180 s, has an absorbance of 7.6. Since fewer volatile compounds can bereleased by the coated pyrolyzing samples, absorbance successively decreases from 4.8 for 5 BL (b),3.6 for 10 BL (c) to 3.2 for 15 BL (d). 107%1 101%T (g) (h) Figure 7. 2D plots and FTIR-spectra of the corresponding peak maxima of uncoated and coated cottonfabrics, obtained by TGA-FTIR investigations in nitrogen. (a)2D plot of uncoated cotton;(b)2D plot of5 BL; (c) 2D plot of 10 BL; (d) 2D plot of 15 BL; (e) spectrum at 1180 s (393°Cin TGA) of uncoated cotton;(f) 5 BL spectrum at 970 s (323°C in TGA); (g) 10 BL spectrum at 1000 s (333℃ in TGA); (h) 15 BLspectrum at 980 s (327°C). In the spectra shown in Figure 7e-h, the strongest signals were found at 1735 cm-= and 1710 cm-1,which are assigned to cis and trans C=O stretching vibrations of acrolein. Acrolein is a flammable and toxic compound by all exposure routes. Furthermore, the band at 1735 cm-appears due tothe C=O stretching of formaldehyde. CH2 stretching vibrations of formaldehyde at 2800 cm- werefound, its intensity decreased with the flame-retardant coating. The release of CO2 (2349 cm-)andwater (~1500 cm-1) of the coated fabrics increase in comparison with uncoated fabrics, while theevolution of carbon monoxide (stretching vibrations at 2171 cm-l) decreases. Transmittance of C-Ostretching vibrations around 1070 cm- was enhanced with growing number of BL. This band reflectsmethanol evolution. On basis of FTIR char analyses and TGA-FTIR results we conclude that organic components werebound in the char as a result of a PA-induced dehydration reaction leading to a reduced release of fuelgases (volatile compounds). The reduction in the released flammable and toxic pyrolysis productsproves the effectiveness of the flame-retardant coating. 4. Summary Phytic acid and polyvinylamine bilayers were deposited on cotton fabrics using layer-by-layerassembly. Increasing the number of bilayers resulted in an increasing phosphorus and nitrogencontent. The finishing led to a flame-retardant behavior (no afterflame and afterglow time, no ignition).Cotton finished with 15 bilayers—with a mass add-on of around 19%, a phosphorus content of 2%anda nitrogen content of 1.4%-passed the bottom edge ignition flame test according to ISO 15025:2016.Microscale combustion calorimetry and thermogravimetric analysis (TGA) measurements showed ashift of the decomposition temperatures in combination with high char yields. From Fourier transforminfrared spectroscopy (FTIR) and scanning electron microscopy analyses, cellulosic dehydration byphytic acid as an acid catalyst and the formation of a protective char layer, as a physical barrier onthe surface of the fabric, were proven. More water was released during pyrolysis in the TGA-FTIRinvestigations confirming the assumption of acid catalyzed dehydration. The lower carbon monoxide,formaldehyde, methanol and acrolein release, shown by TGA-FTIR, proves that the flame-retardantcoatings also reduce the toxicity of the pyrolysis gases. In conclusion, we achieved excellent flame-retardant properties of cotton fabrics with a coating of15 bilayers containing polyvinylamine, which is produced on industrial scale, and phytic acid as agrain processing by-product. With our partially bio-based coating fewer procedure steps are neededto accomplish the same effect as a chitosan/phytic acid coating, requiring 30 bilayers [26]. However,flame-retardant textile finishes usually have to fulfill challenging parameters, e.g., durability andapplicability [60]. These requirements can limit the industrial usage as layer-by-layer coatings are mostlynon-durable and time- and labor-intensive [60,61]. Hence, Chang et al. [61] developed a continuouslayer-by-layer deposition process proving the layer-by-layer scale-up viability. For industrial andtechnical applications future studies will be necessary to investigate textile properties such as thewashing stability of the coating, the tensile strength of the fabric and the scale-up applicability. Supplementary Materials: The following are available online at http://www.mdpi.com/2073-4360/12/5/1202/s1,Figure S1: 2D plots and FTIR-spectra of the corresponding first peak maxima of uncoated and coated cottonfabrics, obtained by TGA-FTIR investigations in air. (a) 2D plot of uncoated cotton;(b) 2D plot of 5 BL; (c) 2D plotof 10 BL;(d) 2D plot of 15 BL;(e) spectrum at 1070 s (357℃ in TGA) of uncoated cotton; (f) 5 BL spectrum at 950 s(317°C in TGA); (g) 10 BL spectrum at 970 s (323°℃ in TGA); (h) 15 BL spectrum at 950 s (317°C). Author Contributions: Conceptualization, K.O.; methodology, O.Z., K.O. and T.M.-G.; investigation, O.Z., D.P;writing-original draft preparation, O.Z.; writing—review and editing, K.O.,T.M.-G. and J.S.G.; supervision,J.S.G.;funding acquisition, K.O. and J.S.G. All authors have read and agreed to the published version of the manuscript. Funding: The R&D project"NPBioPhos" (grant 031B0668B) is funded by the German Federal Ministry ofEducation and Research (BMBF). Acknowledgments: We want to thank DTNW Offentliche Prufstelle GmbH for the ICP-OES measurements.Support by the Open Access Publication Fund of the University Duisburg-Essen is gratefully acknowledged. Conflicts of Interest: The funders had no role in the design of the study; in the collection, analyses, or interpretationof data; in the writing of the manuscript, or in the decision to publish the results. Appendix A Table A1. Complementary microscale combustion calorimetry data set. Sample HRC pk2HRR pk2HR ·pk2HRR pk3HRR pk3HR Lpk1HRR CO uncoated 215.7±5.7 5 BL 103.1±4.0 9.1±1.9 1.3±0.2 364±5 10 BL 90.6±5.8 9.1±1.6 1.4±0.1 370±6 15 BL 99.2±12.0 42.8±5.1 1.5±0.6 324±2 9.0±0.5 1.2±0.1 416±16 References 1. Bourbigot, S. Flame retardancy of textiles: New approaches. Adv. Fire Retard. Mater. 2008,2002,9-40. 2. Salmeia, K.A.; Gaan,S.;Malucelli, G. Recent Advances for Flame Retardancy of Textiles Based on PhosphorusChemistry. Polymers 2016, 8,319. [CrossRef] [PubMed] 3. Home Office Statistics. Detailed Analysis of Fires Attended by Fire and Rescue Services, England, April 2017 toMarch 2018;OGL: London, UK, 2018; ISBN 978-1-78655-701-8. 4. Horrocks, A.R. Flame Retardant Challenges for Textiles and Fibres: New Chemistry Versus InnovatorySolutions. Polym. Degrad. Stab. 2011, 96,377-392. [CrossRef] 5. Price, D.; Anthony, G.; Carty, P. Introduction: Polymer Combustion, Condensed Phase Phyrolysis andSmoke Formation. In Fire Retardant Materials; Horrocks, A.R., Price, D., Eds.; Woodhead Publishing Limited:Sawston, UK; Cambridge, UK, 2001; pp. 1-30. ISBN 1 85573 419 2. 6. Horrocks,A.R. Developments in Flame Retardants for Heat and Fire Resistant Textiles-The Role of CharFormation and Intumescence. Polym. Degrad. Stab. 1996,54,143-154. [CrossRef] 7. Stec, A.A.; Hull, T.R. Influence of Fire Retardants and Nanofillers on Fire Toxicity; Elsevier: Amsterdam,The Netherlands, 2014; ISBN 9780444538093. 8. European Parliament. Council of the European Union Commission delegated directive (EU) 2015/863 of31 March 2015 amending Annex II to Directive 2011/65/EU of the European Parliament and of the Council asregards the list of restricted substances. Off J. Eur. Union 2015,L137,10-12. 9. European Commission. Commission Regulation (EU) 2017/227 of 9 February 2017 amending Annex XVII toRegulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration,Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards. Of. J. Eur. Union 2017, 8-11. 10. European Parliament. Council of the European Union Regulation (EU) 2019/1021 of the European Parliamentand of the Council of 20 June 2019 on persistent organic pollutants (recast). Off. J. Eur. Union 2019, L169,45-77. 11. European Parliament. Council of the European Union Regulation (EU) No 649/2012 of the EuropeanParliament and of the Council of 4 July 2012 concerning the export and import of hazardous chemicals. OffJEur. Union 2012,55,60-106. 12. Wang, C.; Wu, Y.; Li, Y.; Shao, Q.; Yan, X.; Han, C.; Wang, Z.; Liu, Z.; Guo, Z. Flame-Retardant RigidPolyurethane Foam with a Phosphorus-Nitrogen Single Intumescent Flame Retardant. Polym. Adv. Technol.2017,29,668-676. [CrossRef 13. Rosace, G.; Castellano, A.; Trovato, V.;Iacono, G.; Malucelli, G. Thermal and Flame Retardant Behaviourof Cotton Fabrics Treated with a Novel Nitrogen-Containing Carboxyl-Functionalized OrganophosphorusSystem. Carbohydr. Polym. 2018,196,348-358. [CrossRef] 14 Hendrix, J.E.; Bostic, J.E.; Olson, E.S.; Barker, R.H. Pyrolysis and Combustion of Cellulose. I. Effects ofTriphenyl Phosphate in the Presence of Nitrogenous Bases. J. Appl. Polym. Sci. 1970,14,1701-1723.[CrossRef] 17. Cheng, X.W.; Guan, J.P.; Yang, X.H.; Tang, R.C.; Yao, F. A Bio-Resourced Phytic Acid/Chitosan PolyelectrolyteComplex for the Flame Retardant Treatment of Wool Fabric. J. Clean. Prod. 2019, 223, 342-349. [CrossRef] 18. Thota, S.; Somisetti, V.; Kulkarni, S.; Kumar, J.; Nagarajan, R.; Mosurkal, R. Covalent Functionalization ofCellulose in Cotton and a Nylon-Cotton Blend with Phytic Acid for Flame Retardant Properties. Cellulose2019,27,11-24. [CrossRef] 19. Sonnier,R.; Taguet, A.; Ferry, L.;Lopez-Cuesta,J.-M. Towards Bio-based Flame Retardant Polymers;Navard,P.,Ed.;Springer International Publishing: Cham, Switzerland, 2017; ISBN 9783319670829. 20. Hobbs, C.E. Recent Advances in Bio-Based Flame Retardant Additives for Synthetic Polymeric Materials.Polymers 2019,11,224. [CrossRef| 21. Vohra, P.; Gray, G.A.; Kratzer, F.H. Phytic Acid-Metal Complexes. Exp. Biol. Med. 1965,120,447-449.[CrossRef 222..Reddy, N.R.; Sathe, S.K.; Salunkhe, D.K. Phytates in Legumes and Cereals. In Advances in Food Research;Chichester, C.O., Ed.; Academic Press: New York,NY, USA, 1982; ISBN 0120164280. 23. O'Dell, B.L.; De Boland, A.R.; Koirtyohann,S.R. Distribution of Phytate and Nutritionally Important Elementsamong the Morphological Components of Cereal Grains. J. Agric. Food Chem. 1972,20,718-721. [CrossRef] Graf, E. Applications of phytic acid. J. Am. Oil Chem25. Soc. 1983,60,1861-1867. [CrossRef] Cheng, X.W.; Guan, J.P.; Kiekens, P.; Yang, X.H.; Tang, R.C. Preparation and evaluation of an eco-friendly,reactive, and phytic acid-based flame retardant for wool. React. Funct. Polym. 2019,134,58-66. [CrossRef 26. Laufer, G.; Kirkland, C.; Morgan, A.B.; Grunlan, J.C. Intumescent Multilayer Nanocoating, Made withRenewable Polyelectrolytes, for Flame-Retardant Cotton. Biomacromolecules 2012, 13, 2843-2848. [CrossRef][PubMedl 277..Zhang, X.; Zhou, X.Y.; Cheng, X.W.; Tang, R.C. Phytic Acid as an Eco-Friendly Flame Retardant for Silk/WoolBlend: A Comparative Study with Fluorotitanate and Fluorozirconate. J. Clean. Prod. 2018,198,1044-1052[CrossRef| 28. Cheng, X.W.; Guan, J.P.; Chen, G.; Yang, X.H.; Tang, R.C. Adsorption and Flame Retardant Properties ofBio-Based Phytic Acid on Wool Fabric. Polymers 2016,8,122. [CrossRef] [PubMed] 29. Gaan, S.; Sun, G. Effect of Phosphorus and Nitrogen on Flame Retardant Cellulose: A Study of PhosphorusCompounds. J. Anal. Appl. Pyrolysis 2007, 78, 371-377. [CrossRef] 30. Gaan,S.; Sun, G.; Hutches, K.; Engelhard, M.H. Effect of Nitrogen Additives on Flame Retardant Action ofTributyl Phosphate: Phosphorus-Nitrogen Synergism. Polym. Degrad. Stab. 2008, 93,99-108. [CrossRef] 31. Gaan, S.;Rupper, P.; Salimova, V.; Heuberger, M.; Rabe, S.; Vogel, F. Thermal Decomposition and BurningBehavior of Cellulose Treated with Ethyl Ester Phosphoramidates: Effect of Alkyl Substituent on NitrogenAtom. Polym. Degrad. Stab. 2009, 94,1125-1134. [CrossRef] 32. Sharma, J.K.; Lal, K.; Bhatnagar, H.L. THPC/Thiourea Flame-Retardant Finish for Cotton Textiles. Indian J.Text. Res. 1977,2,116-118. 33. Feng, D.; Zhou, Z.; Bo, M. An Investigation of the Thermal Degradation of Melamine Phosphonite by XPSand Thermal Analysis Techniques. Polym. Degrad. Stab. 1995,50,65-70. [CrossRef] 34. Deh, S.;Gahr, F; Buchmeiser, M.R. Synergistic Effects in the Pyrolysis of Phosphorus-Based Flame-Retardants:The Role of Si- and N-Based Compounds. Polym. Degrad. Stab. 2016,130,155-164. [CrossRef] 35. Horrocks, A.R. An Introduction to the Burning Behaviour of Cellulosic Fibres. J. Soc. Dye. Colour. 1983,99,191-197. [CrossRef] 36. Laufer, G.; Kirkland, C.; Cain, A.A.; Grunlan, J.C. Clay-Chitosan Nanobrick Walls: Completely RenewableGas Barrier and Flame-Retardant Nanocoatings. ACS Appl. Mater. Interfaces 2012, 4, 1643-1649. [CrossRef] 37. Carosio, F; Fontaine, G.; Alongi, J.; Bourbigot, S. Starch-Based Layer by Layer Assembly: Efficient andSustainable Approach to Cotton Fire Protection. ACS Appl. Mater. Interfaces 2015, 7, 12158-12167. [CrossRef] 39. Carosio, F.;Di Blasio, A.; Cuttica,F; Alongi,J.;Frache, A.; Malucelli, G. Flame Retardancy of Polyester FabricsTreated by Spray-Assisted Layer-by-Layer Silica Architectures. Ind. Eng. Chem. Res. 2013, 52, 9544-9550.[CrossRef| 40. Carosio, F; Laufer, G.; Alongi, J.; Camino, G.; Grunlan, J.C. Layer-by-Layer Assembly of Silica-Based FlameRetardant Thin Film on PET Fabric. Polym. Degrad. Stab. 2011, 96,745-750. [CrossRef] 41. Alongi, J.; Horrocks, A.R.; Carosio, F; Malucelli, G. Update on Flame Retardant Textiles: State of theArt, Environmental Issues and Innovative Solutions; Smithers Rapra Technology: Shawbury, UK, 2013;ISBN 9781909030176. 42. Horrocks, A.R. Flame-Retardant Finishes and Finishing. In Textile Finishing; Heywood, D., Ed.; Society ofDyers and Colourists: Bradford, UK, 2003; pp.214-250. ISBN 0901956813. 43. Barker, R.H.; Yeh, K.-N. Pyrolysis and Combustion of Cellulose; Part IV: Thermochemistry of Cotton CelluloseTreated with Selected Phosphorus-Containing Flame Retardants. Text. Res. J. 1970, 41,932-938. 44. Tesoro, G. Current Research on Chemical Modification of Cellulose. Pure Appl. Chem. 1976, 46,239-245.[CrossRef 45. Lewin, M. Synergism and catalysis in flame retardancy of polymers. Polym. Adv. Technol. 2001, 12,215-222.|CrossRef 46. Heywood, D. Society of Dyers and Colourists. In Textile Finishing; Heywood, D., Ed.; Society of Dyers andColourists: Bradford, UK, 2003; ISBN 0901956813. Shafizadeh, F. Pyrolysis and Combustion of Cellulosic Materials. Adv. Carbohydr. Chem. 1968,23, 419-474. Aenishanslin, R.; Guth, C.; Hofmann,P.; Maeder, A.; Nachbur, H. A New Chemical Approach to DurableFlame-Retardant Cotton Fabrics. Text. Res. J. 1969,39,375-381. [CrossRef] 49. Schindler, W.D.; Hauser, P.J. Chemical Finishing of Textiles; Woodhead Publishing: Cambridge, UK; CRC Press:Boca Raton, FL, USA, 2004; ISBN 084932825X. 50. Shafizadeh,F; Bradbury, A.G.W. Thermal Degradation of Cellulose in Air and Nitrogen at Low Temperatures.J. Appl. Polym. Sci. 1979,23,1431-1442. [CrossRef] 511..Alongi, J.; Malucelli, G. Thermal Degradation of Cellulose and Cellulosic Substrates. React. Mech. Therm.Anal. Ado. Mater. 2015,301-332. 52. Kandola, B.K.; Horrocks, A.R.; Price, D.; Coleman, G.V. Flame-Retardant Treatments of Cellulose and TheirInfluence on the Mechanism of Cellulose Pyrolysis. J. Macromol. Sci.-Rev. Macromol. Chem. Phys. 1996,36,721-794. [CrossRef] 53. Price, D.; Horrocks, A.R.; Akalin, M. Use of Dta with Infrared Analysis of Evolved Gas to Investigate theEffect of Flame Retardants on Gas Evolution from Pyrolysed Cellulose (Cotton). Br. Polym. J. 1988,20,61-67.|CrossRef 54. Lyon, R.E.; Walters, R.N.; Stoliarov, S.I. Thermal Analysis of Flammability. J. Therm. Anal. Calorim. 2007,89,441-448. [CrossRef] Granzow, A. Flame Retardation by Phosphorus Compounds. Acc. Chem. Res. 1978, 11, 177-183. [CrossRef]55. Klatt, M. Nitrogen-based Flame Retardants. In Non-Halogenated Flame Retardant Handbook; Morgan, A.B.,Wilkie, C.A., Eds.; Scrivener Publishing: Salem, MA, USA, 2014; pp. 143-168. ISBN 9781118686249. 57. Shi, Y.; Xing, W.; Wang, B.; Hong, N.; Zhu, Y.; Wang, C.; Gui, Z.; Yuen, R.K.K.; Hu, Y. Synergistic Effectof Graphitic Carbon Nitride and Ammonium Polyphosphate for Enhanced Thermal and Flame RetardantProperties of Polystyrene. Mater. Chem. Phys. 2016,177,283-292. [CrossRef] 59. Zhang, P.; Song, L.; Lu, H.; Hu, Y.; Xing, W.;Ni, J; Wang, J. Synergistic Effect of Nanoflaky ManganesePhosphate on Thermal Degradation and Flame Retardant Properties of Intumescent Flame RetardantPolypropylene System. Polym. Degrad. Stab. 2009, 94,201-207. [CrossRef] 60. Malucelli, G. Flame Retarded Cotton Fabrics: Current Achievements, Open Challenges, and FuturePerspectives. In Advanced Functional Textiles and Polymers; Ul Islam, S., Butola, B.S., Eds.; Wiley & Sons:Hoboken, NJ, USA, 2019;pp. 1-32. ISBN 9783540773405. 61. Chang, S.; Slopek, R.P.; Condon, B.; Grunlan, J.C. Surface Coating for Flame-Retardant Behavior of CottonFabric Using a Continuous Layer-by-Layer Process. Ind. Eng. Chem. Res. 2014,53,3805-3812.[CrossRef @ 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open accessCC article distributed under the terms and conditions of the Creative Commons Attribution(CC BY) license (http://creativecommons.org/licenses/by/4.0/).
确定







还剩13页未读,是否继续阅读?
中国格哈特为您提供《带叠层阻燃涂料棉花的氮含量检测》,该方案主要用于涂料中含量分析检测,参考标准--,《带叠层阻燃涂料棉花的氮含量检测》用到的仪器有格哈特带自动进样器凯氏定氮仪VAP500C、格哈特红外加热消解快速消化系统TTs125、格哈特维克松废气实验室废物处理系统涤气VS、德国加液器MM、凯氏定氮催化片
推荐专场
定氮仪、凯氏定氮仪、Dumas定氮仪
更多
相关方案
更多