磷酸二氢钾的性质及其对植物和土壤的影响Properties of potassium dihydrogen phosphate and its effects on plants and soil
方案详情

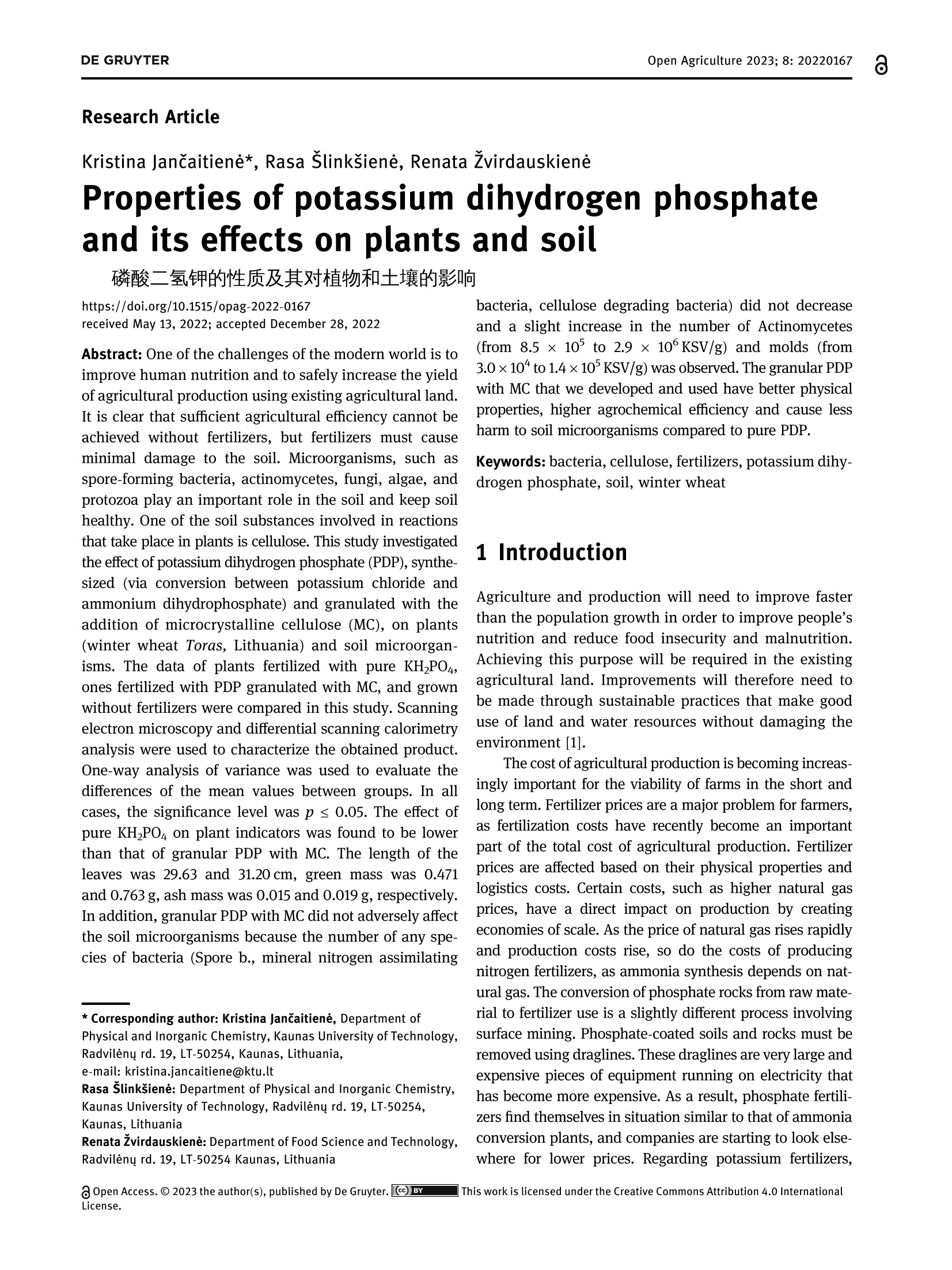
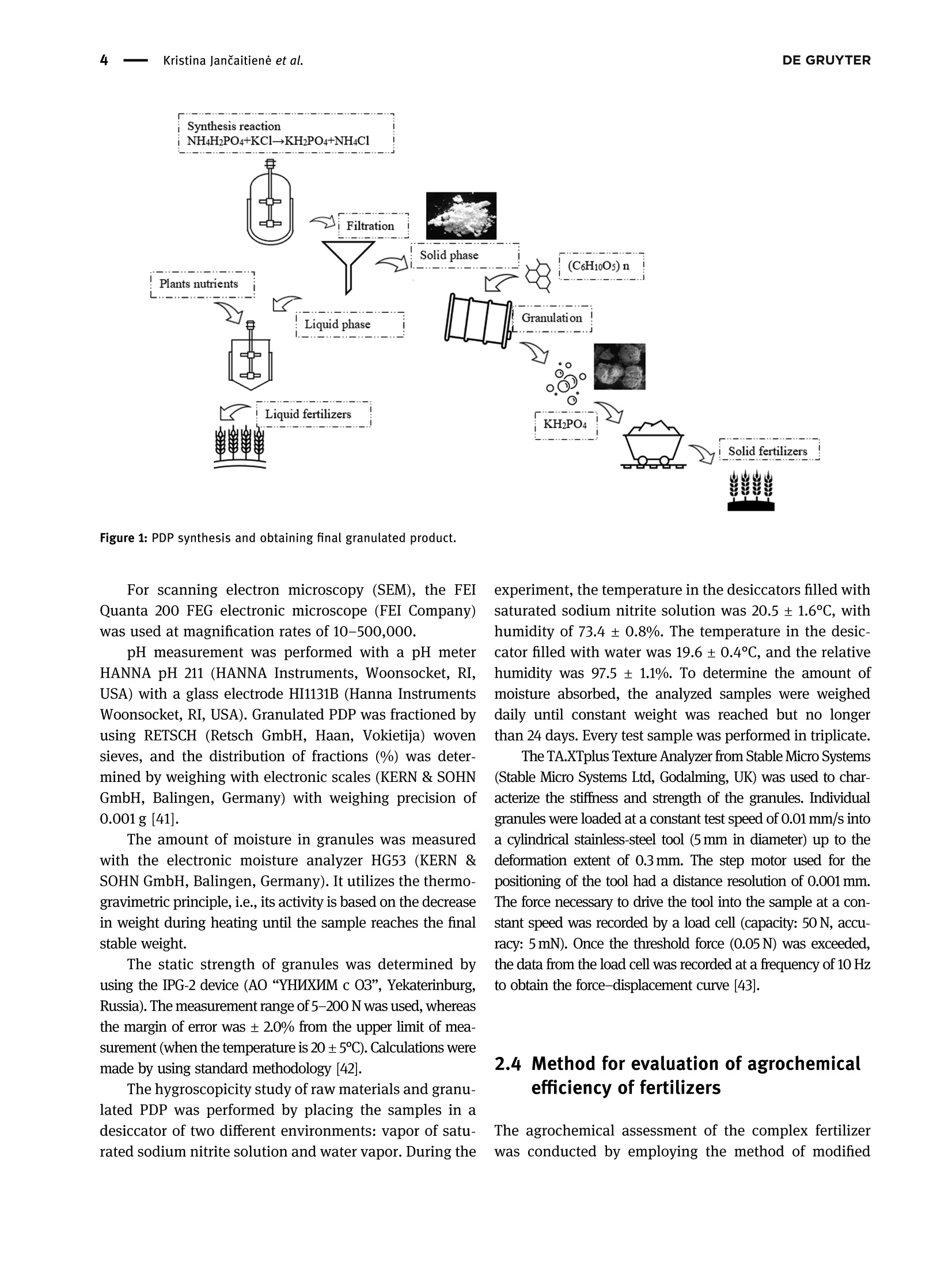
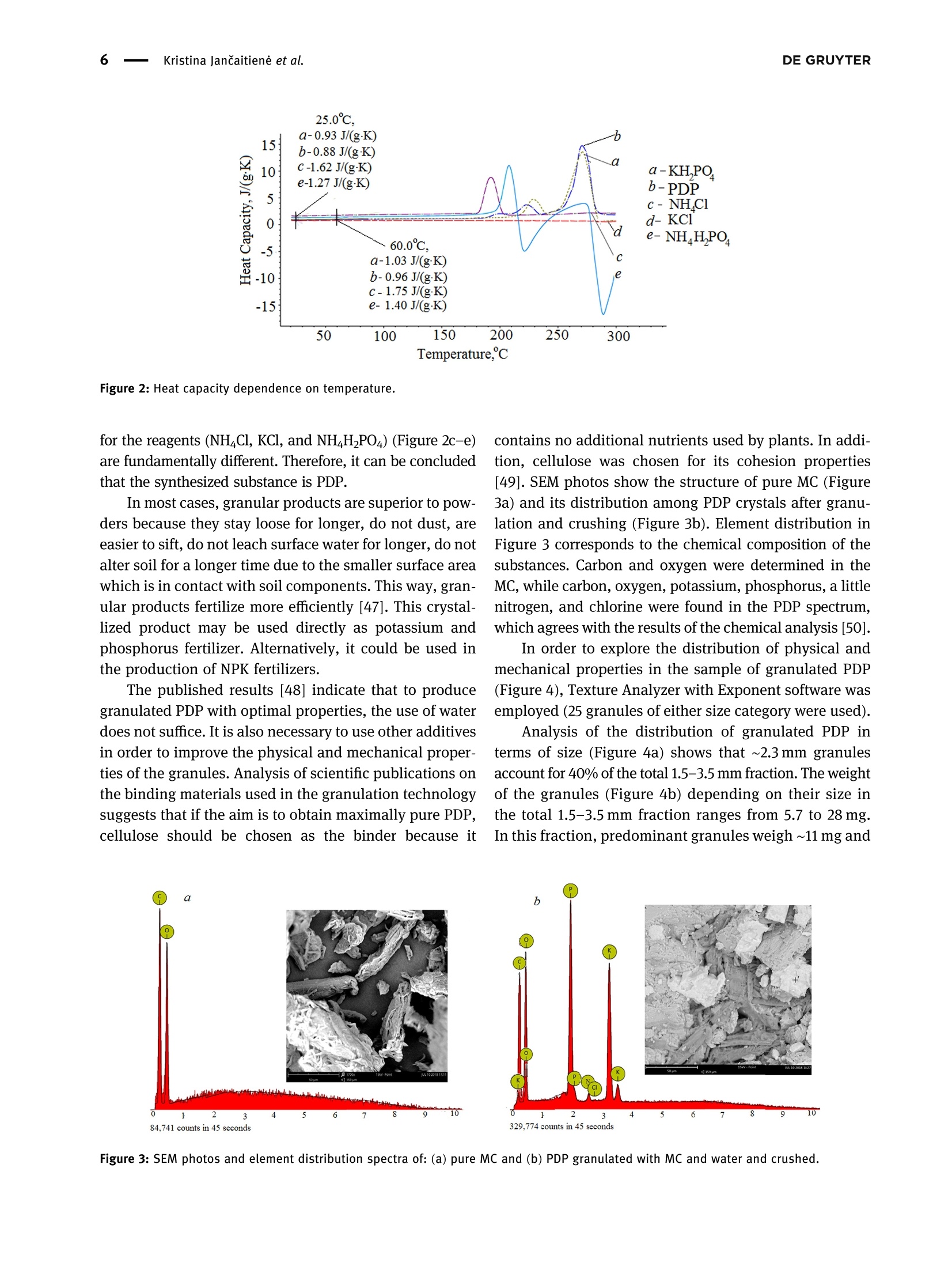
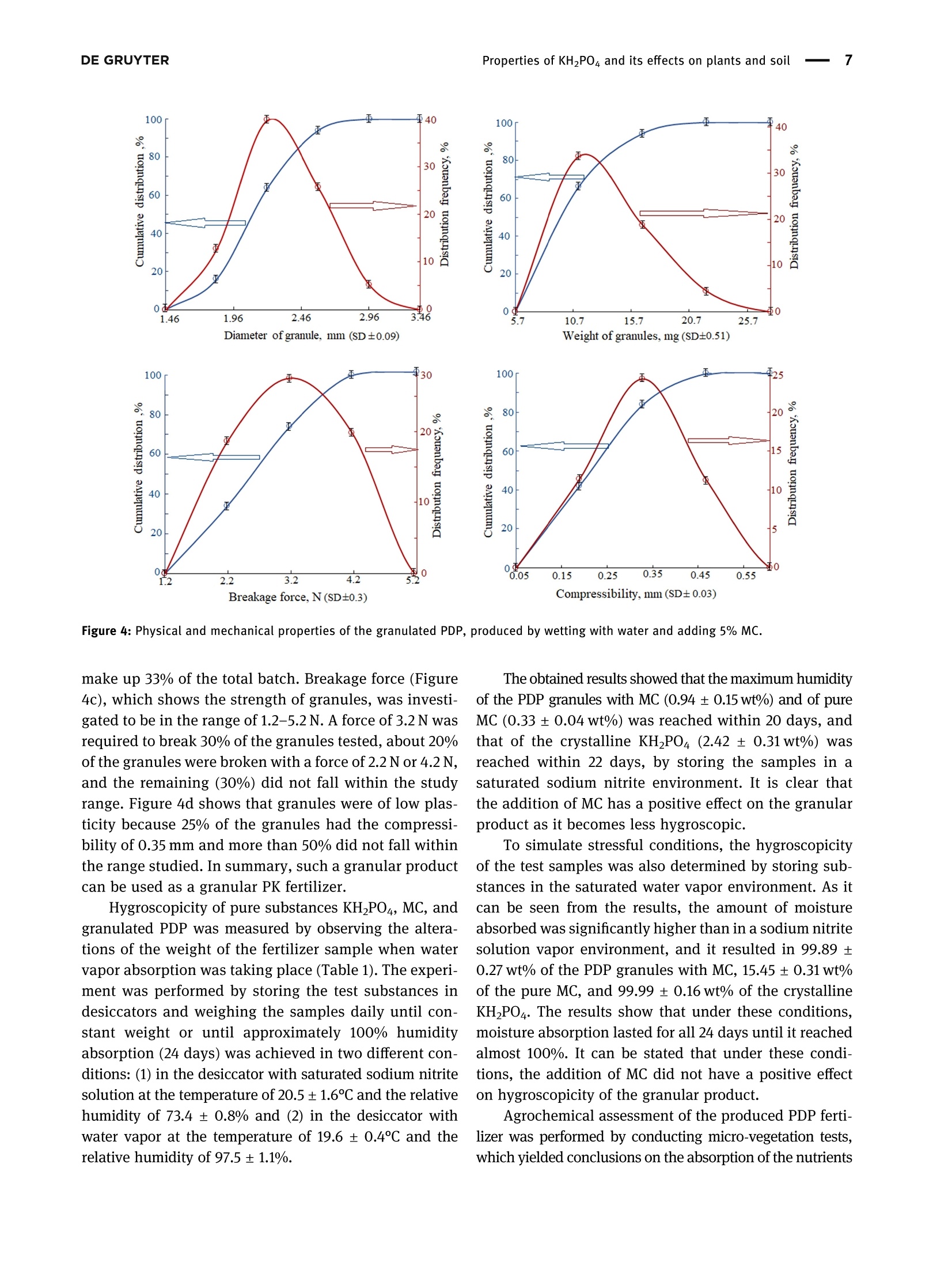
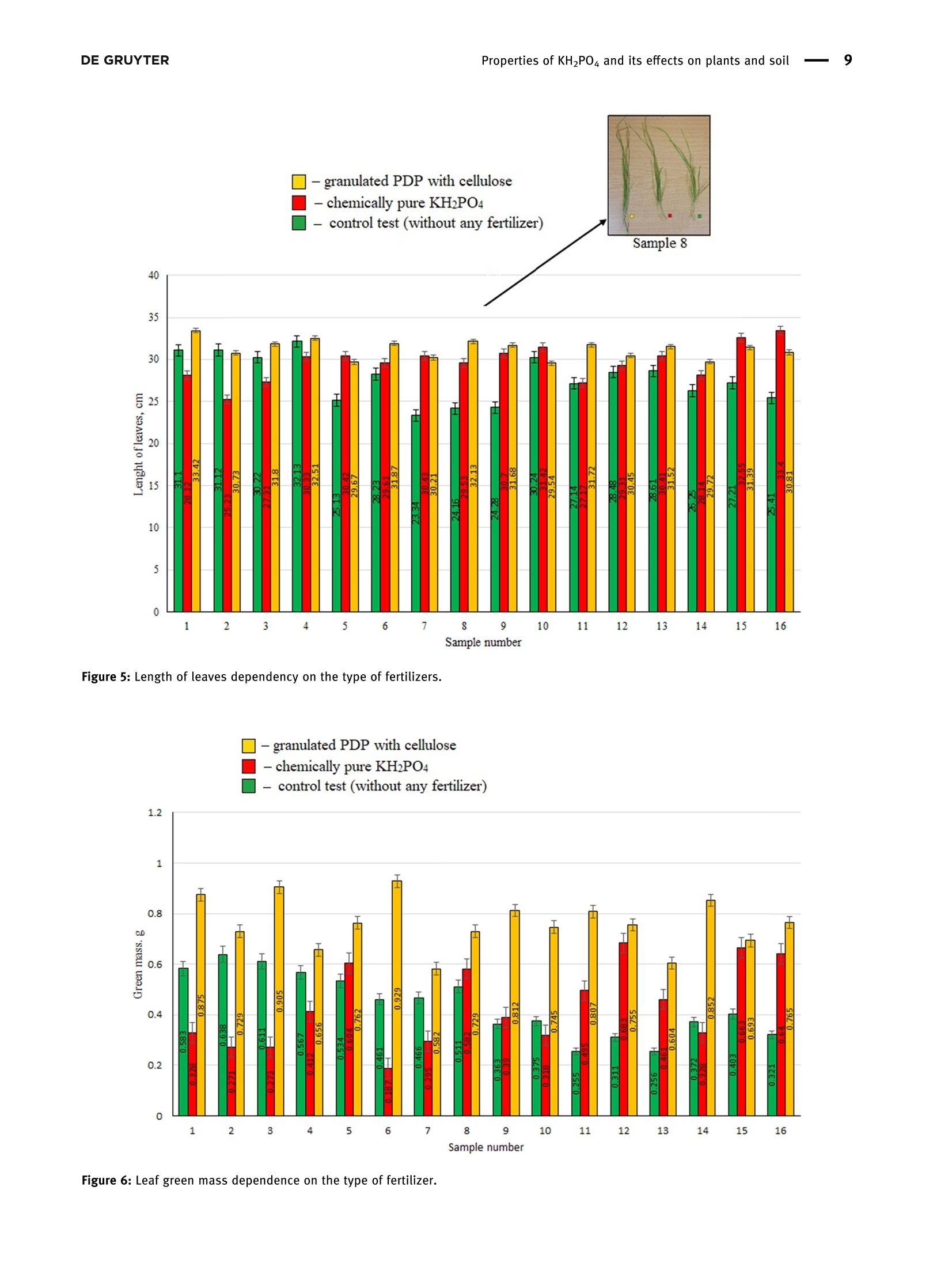
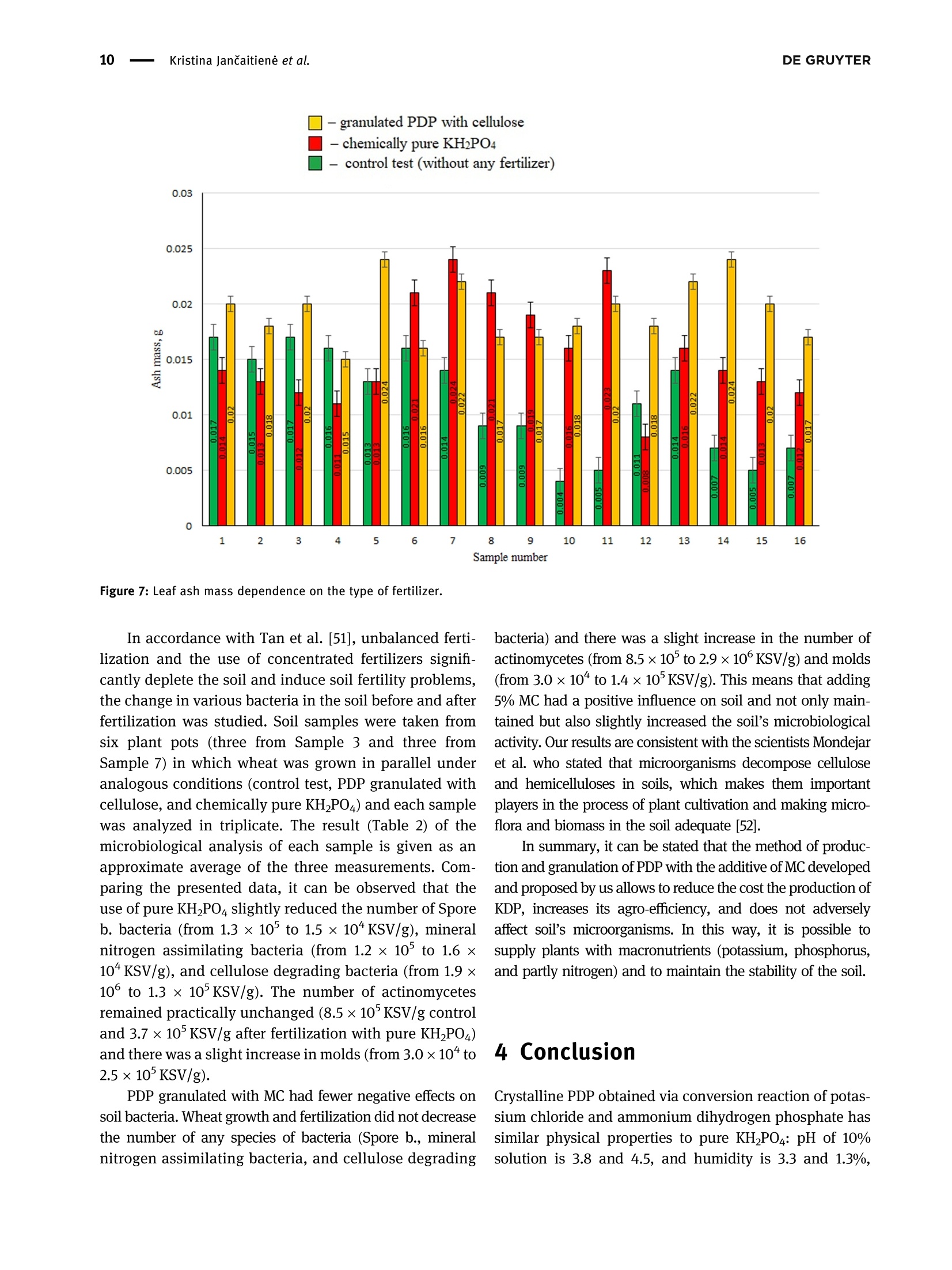
磷酸二氢钾的性质及其对植物和土壤的影响Properties of potassium dihydrogen phosphate and its effects on plants and soilDE GRUYTEROpen Agriculture 2023; 8: 20220167 DE GRUYTER2Kristina JanCaitiene et al. Research Article Kristina Jancaitiene*, Rasa Slinksiene, Renata Zvirdauskiene Properties of potassium dihydrogen phosphateand its effects on plants and soil 磷酸二氢钾的性质及其对植物和土壤的影响 https://doi.org/10.1515/opag-2022-0167 received May 13, 2022; accepted December 28,2022 Abstract: One of the challenges of the modern world is toimprove human nutrition and to safely increase the yieldof agricultural production using existing agricultural land.It is clear that sufficient agricultural efficiency cannot beachieved without fertilizers, but fertilizers must causeminimal damage to the soil. Microorganisms, such asspore-forming bacteria, actinomycetes, fungi, algae, andprotozoa play an important role in the soil and keep soilhealthy. One of the soil substances involved in reactionsthat take place in plants is cellulose. This study investigatedthe effect of potassium dihydrogen phosphate (PDP), synthe-sized (via conversion between potassium chloride andammonium dihydrophosphate) and granulated with theaddition of microcrystalline cellulose (MC), on plants(winter wheat Toras, Lithuania) and soil microorgan-isms. The data of plants fertilized with pure KH_PO4,ones fertilized with PDP granulated with MC, and grownwithout fertilizers were compared in this study. Scanningelectron microscopy and differential scanning calorimetryanalysis were used to characterize the obtained product.One-way analysis of variance was used to evaluate thedifferences of the mean values between groups. In allcases, the significance level was p < 0.05. The effect ofpure KH PO4 on plant indicators was found to be lowerthan that of granular PDP with MC. The length of theleaves was 29.63 and 31.20 cm, green mass was 0.471and 0.763 g, ash mass was 0.015 and 0.019 g, respectively.In addition, granular PDP with MC did not adversely affectthe soil microorganisms because the number of any spe-cies of bacteria (Spore b., mineral nitrogen assimilating * Corresponding author: Kristina Jancaitiene, Department of Physical and Inorganic Chemistry, Kaunas University of Technology,Radvileny rd. 19, LT-50254, Kaunas, Lithuania, e-mail: kristina.jancaitiene@ktu.It Rasa Slinksiene: Department of Physical and Inorganic Chemistry,Kaunas University of Technology, Radvileny rd. 19, LT-50254,Kaunas, Lithuania Renata Zvirdauskiene: Department of Food Science and Technology,Radvileny rd. 19, LT-50254 Kaunas, Lithuania bacteria, cellulose degrading bacteria) did not decreaseand a slight increase in the number of Actinomycetes(from 8.5 × 10 to 2.9×10°KSV/g) and molds (from3.0×10* to1.4×10KSV/g) was observed. The granular PDPwith MC that we developed and used have better physicalproperties, higher agrochemical efficiency and cause lessharm to soil microorganisms compared to pure PDP. Keywords: bacteria, cellulose, fertilizers, potassium dihy-drogen phosphate, soil, winter wheat 1 Introduction Agriculture and production will need to improve fasterthan the population growth in order to improve people'snutrition and reduce food insecurity and malnutrition.Achieving this purpose will be required in the existingagricultural land. Improvements will therefore need tobe made through sustainable practices that make gooduse of land and water resources without damaging theenvironment [1]. The cost of agricultural production is becoming increas-ingly important for the viability of farms in the short andlong term. Fertilizer prices are a major problem for farmers,as fertilization costs have recently become an importantpart of the total cost of agricultural production. Fertilizerprices are affected based on their physical properties andlogistics costs. Certain costs, such as higher natural gasprices, have a direct impact on production by creatingeconomies of scale. As the price of natural gas rises rapidlyand production costs rise, so do the costs of producingnitrogen fertilizers, as ammonia synthesis depends on nat-ural gas. The conversion of phosphate rocks from raw material to fertilizer use is a slightly different process involvingsurface mining. Phosphate-coated soils and rocks must beremoved using draglines. These draglines are very large andexpensive pieces of equipment running on electricity thathas become more expensive. As a result, phosphate fertili-zers find themselves in situation similar to that of ammoniaconversion plants, and companies are starting to look else-where for lower prices. Regarding potassium fertilizers, although potash is produced in potassium mines, it is alsoexposed to electricity. Everywhere, mines are up to a mileunderground. Roughly ten countries produce potassiumand even fewer countries export the product, making sup-plies even more limited [2,3]. One of the ways to increasethe efficiency and availability of fertilizers even inpoorer countries is the production of concentrated fer-tilizers using the cheapest available raw materials suchas potassium chloride [4] by the simplest method ofmanufacturing. Highly concentrated fertilizers frompotassium phosphates (potassium hydrogen phosphateand potassium dihydrogen phosphate (PDP)) are widelyknown and used. Unfortunately, they are usually expen-sive (KHPO4-968-1,162 E/t, KHPO4- 871-1,065 €/t) [5]due to excessive manufacturing costs or limited phosphaterock resources. As a result of high prices, PDP is usuallyused for highly chlorine-sensitive plants (e.g., tobacco,potato, a few fruits, berry, vegetables, some tree crops,and some soybean varieties) in greenhouses or hydropo-nics. Nevertheless, this sensitivity varies depending on thegrowing conditions [6]. Potassium is the major cation in the cytoplasm ofplants essential for the activity of various enzymes thatare involved in primary metabolism. Potassium is involvedin the regulation of turgor, protein synthesis, sugar trans-port, and photosynthesis [7-14]. Based on the above lit-erature, it can be stated that potassium has a significantinfluence on the uptake and use of other nutrients inplants. When a plant is deficient in potassium, it is likelythat it will activate a signaling mechanism that movesmotile potassium ions from old leaves to new ones to sup-port modulation of stomata diaphragm osmosis in thelatter [15,16]. Most of the potassium in the soil is insoluble.Therefore, potassium-dissolving microorganisms, a com-ponent of the soil microbial community, can colonize therhizosphere, promote crop yields, and enhance plantresponses to stress in adverse climates. Microorganismsplay an important role in providing plants with an avail-able form of potassium and ecologically improving soilfertility. Most scientists agree that it is important tostudy the effects of mineral potassium fertilizers on naturalsoil microorganisms, but this problem is still given littleattention, as most studies focus on nitrogen and phos-phorus fertilizers [17-20]. It should be noted that assimila-tion of mineral fertilizers depends on the activity of themicroorganisms living in the soil as well as their solubilityin the soil solution. Coatings of various films are used inproduction of fertilizers which plants can absorb over alonger period of time. Physical methods can be appliedfor coating fertilizers with a variety of coatings with addi-tives. Inorganic substances (phosphates, sulfur, oxides, clay, or gypsum), thermosetting or thermoplastic resins,synthetic organic polymers (alkyd), natural organic poly-mers (lignin and latex), mixed sulfur-polymer, and chit-osan are used as coatings. Thus, dissolution of suchfertilizers in the soil is prolonged, plants absorb them, andthereby, environmental pollution is reduced. Soluble nitrogenand phosphorus fertilizers are among the ones for which thesestatements can be applied [21,22]. Phosphorus performs various physiological and biochemical functions in plants. It is used for energy transfeiand storage through adenosine triphosphate in biologicalsystems and contributes to the synthesis and stability ofdeoxyribonucleic acid and ribonucleic acid. Phosphorusis required for the structural and functional integrity ofcell membranes and is a source of energy that promotesmany chemical reactions in the plant. In the presence ofphosphorus deficiency, structural disorders of cell mem-branes can be expected to adversely affect the transportof nutrients across root cell membranes. It enters theplant through root hair, root tips, and the outermostlayers of root cells. Phosphorus uptake is also facilitatedby mycorrhizal fungi, which grow along with the roots ofmany crops. Phosphorus is mainly absorbed as a primaryCorthophosphate ion (H POz), but some are also absorbedas a secondary orthophosphate (HPO), the latter form ofwhich increases with the increase in the soil ph. Once inthe root of the plant, phosphorus can be stored in the rootor transported to the upper parts of the plant. It is incorporated into organic compounds through various chemical reactions. These organic compounds are formedin the plant and, together with the inorganic phosphateions derived from the soil, transfer phosphorus throughoutthe plant where it is available for further reactions. Interms of plant energy reactions, phosphorus plays a vitalrole in almost all the plant processes where energy istransferred [23-25]. Cellulose microfibrils are a major component of thecell walls surrounding each cell. In the primary cell wallsof plants, cellulose accumulates in microfibrils having adiameter of few nanometers. The stiffness and orientatiorof these microfibrils control cell expansion; thereforecellulose synthesis is a major factor in plant growth andmorphogenesis. In order to expand, the primary cell wallsare nanocomposite materials in which long nanometersized cellulose microfibrils flow through a hydrated matrix ofxyloglucan, pectin, and other polymers [26-28]. Naturalcellulose microfibrils are partially crystalline, which are inso-luble cable-like structures typically consisting of approximately 36 hydrogen-bonded chains containing 500-14,000β-1,4-linked glucose molecules. About one-third of the massof most plants is cellulose [29-33]. The effectiveness of many of the substances neces-sary for the plant, which are present in the soil or addedwith fertilizers, depends on the number of microorgan-isms and their effects. The issue of microorganisms’dis-tribution in soil is not sufficiently analyzed and evaluated.Conventional microbiological studies of soil indicate thatthe composition of bacterial group in different soils is notthe same. Forms of bacteria that do not form spores pre-dominate in the soil. Spore-forming bacteria make upabout 10-20% of total bacterial count. Actinomycetes,fungi, algae, and protozoa also live in large quantities insoil. 1g of soil contains tens and hundreds of thousands,often millions, of fungi and actinomycetes. Ammonifyingbacteria, actinomycetes, microscopic fungi, and other micro-organisms cause mineralization of organic matter in the soiland the release of ammonium nitrogen from plants. Soil'smicroflora has varied composition and content because it isresponsible for conversion of mineral nitrogen to organicforms (immobilization process), to increase the uptake ofphosphorus, potassium, and other nutrients into plants.Formation processes of actinomycetes or radial fungi, whichare transient forms between bacteria and fungi, also play asignificant role in the soil. They are actively involved indecomposition of nitrogen and nitrogen-free organic matter,including the most persistent compounds that make up thesoil humus, or humus. Thus, the activity of soil’s microflorais crucial for soil's health and proper development ofplants [34-37]. From the review of the literature, it can be seen that itis difficult to maintain a healthy soil and not harm themicroorganisms in the soil when using concentrated fer-tilizers. Therefore, it is a challenge to select materials thatare friendly to the soil and the environment, are effectivefor plants, but do not reduce the effectiveness of fertilizers. The aim off this work is to evaluate the effect of PDP,synthesized in a simple way and then granulated withmicrocrystalline cellulose (MC), on plants and soil. 2 Material and methods 2.1 Materials Materials used in this work were chemically pure substancesof potassium chloride (KCl, 99-100.5% Sigma-Aldrich),ammonium dihydrogen phosphate (NH,HPO4, 99.0% FlukaAnalytical), pure PDP (KHPO4, 99-100.5% Sigma-Aldrich),ammonium chloride (NHCl, 99.0% Sigma-Aldrich), MC,manufactured by JRS Pharma GmbH & Co, Emcocel 90 M, and purified, partially depolymerized cellulose was used asa binder, which best suited for dry or wet granulation, with aparticle size of 90-60 um, density of 0.25-0.37 g/cm, andpH of 6.65 [38]. 2.2 Granulation methodology of PDP Fertilizers were granulated in the laboratory drum-typegranulator-dryer at a 5° tilt angle and constant (27 rpm)rotation speed (Figure 1) [39]. Raw materials were supplied to the granulator pre-heated up to 55-65℃, hot air for drying the granules wassupplied into the drum-type granulator by an air fan. Forirrigation, tap water was used, which was injected intothe raw material mixture in a separate mixture before themixture was placed into the drum-type granulator-dryer.By using the PDP (fraction <1mm), 21 samples weregranulated in the laboratory granulator repeating theprocedure two or three times. To aid granulation, MCwas used. The resultant granules were dried in an ovenfrom 8 to 16 h at 60°C, and then their physical and che-mical properties were assessed. 2.3 Analysis of the granular product The chemical composition of liquid and crystallized solidphases after synthesis between KCl and NH4H PO4 Wasanalyzed by employing methods of chemical analysis:concentration of ammonium nitrogen (NH4*) was estab-lished by the Kjeldahl method (Vapodest 45s, C. GerhardtGmbH & Co. KG, Konigswinter, Germany). The concentra-tion of phosphorus (P20s) was determined by using the photocolorimetric method (T70/T80 UV-VIS, PG InstrumentsLimited, Lutterworth, UK). The concentration of chlorine(Cl) was determined by employing the potentiometricmethod with the use of silver nitrate (TitroLine alphaplus, SI Analytics GmbH, Mainz, Germany). The concen-tration of potassium (K20) was discovered by employingthe marginal solutions method by flame photometric method(PFP-7, Cole-Parmer Ltd, Staffordshire, UK) [40]. Differential scanning calorimetry analysis was per-formed by using Netzsch DSC 214 Polyma thermal ana-lyzer (Mettler-Toledo, Greifensee, Schweiz) and settingthe following parameters: 10°C/min temperature-raisingspeed, 25-300°C temperature range, with weights ofstandard-blank Al crucible furnace atmosphere air sam-ples equaling 13 mg. Figure 1: PDP synthesis and obtaining final granulated product. experiment, the temperature in the desiccators filled withsaturated sodium nitrite solution was 20.5 ± 1.6°C, withhumidity of 73.4 ±0.8%. The temperature in the desiccator filled with water was 19.6±0.4C,and the relativehumidity was 97.5 ±1.1%. To determine the amount ofmoisture absorbed, the analyzed samples were weigheddaily until constant weight was reached but no longerthan 24 days. Every test sample was performed in triplicate. The TA.XTplus Texture Analyzer from Stable Micro Systems(Stable Micro Systems Ltd, Godalming, UK) was used to char-acterize the stiffness and strength of the granules. Individuagranules were loaded at a constant test speed of 0.01 mm/s intca cylindrical stainless-steel tool (5 mm in diameter) up to thedeformation extent of 0.3 mm. The step motor used for thepositioning of the tool had a distance resolution of 0.001 mmThe force necessary to drive the tool into the sample at a con-stant speed was recorded by a load cell (capacity: 50 N, accuracy: 5 mN). Once the threshold force (0.05N) was exceeded,the data from the load cell was recorded at a frequency of 10 Hzto obtain the force-displacement curve [43]. 2.4 Method for evaluation of agrochemicalefficiency of fertilizers The agrochemical assessment of the complex fertilizerwas conducted by employing the method of modified micro-vegetative experiments in vitro. The experimentwas conducted in plastic plant pots 4 cm × 4 cmx 4cmin size, filled with the same amount (32g) of mildly alka-line sandy loam. Four pre-sprouted seeds of winter wheat(Toras, Lithuania) were planted into each plant pot (16small pots, for each sample). Artificial 1,000 lx light ofdaylight lamps was used. Pure PDP and granulated PDPwere used for fertilization. The seedlings were fertilizedevery 4 days, six times, using 10 mL of 0.043 g/100 mL (K- 0.14%; P - 0.093%) concentration fertilizer solution foreach plant pot. Wheat was grown until the sprouts startedturning yellow because of almost exhaustive consump-tion of the nutrients (24 days). 2.5 Isolation and enumeration ofmicroorganisms To recover bacterial community, 10 g soil samples weresuspended in 90 mL of sterile water. The number of soilmicroorganisms of main groups were isolated and enum-erated for the serial dilution technique by applying 1 mLof soil suspension dilution on the surface of nutrientmedia for different microorganism groups with fourfoldrepetition. Meat-peptone agar (10.0g/L peptone, 2.0 g/LNa2HPO4, 0.1g/L KH PO4, 0.5g/L MgSO4,0.1g/L CaCO3,0.005 g/L FeClg, 10.0 g/L starch, and 15.0 g/L agar) wasused for ammonifying bacteria. Starch-ammonia agar(2.0g/L (NH4)2SO4, 1.0 g/L KHPO4, 1.0 g/L MgSO4, 1.0 g/LNaCl, 3.0 g/L CaCO3,10.0 g/L starch, and 15.0 g/L agar) wasused for bacteria assimilating mineral nitrogen. The modi-fied Han’s (MH) medium (1.88 g/L carboxymethyl cellulose,0.5 g/L sodium citrate, 2.0 g/L KHPO4, 7.0g/L KHPO4,0.1g/L MgSO47H20, 1.0g/L (NH4)2SO4, 10.0 g/L agar,and 0.2 g/L Congo red) was used for cellulose degradingbacteria. Potato-dextrose agar (Liofilhem, Italy) was usedfor molds [44]. The number of microorganisms in the soil sampleswas expressed as a number of colony-forming units (CFU)per gram of soil samples. 2.6 Statistical analysis The results were expressed as the arithmetic mean valueof no less than two measurements ± standard deviation(SD). The results were calculated with 95% probability. Inall cases, the significance level was pca R 吕 00 寸 N LZ0: 910: 品十++S7'S一TSZ0干690+8E0800o 6ON 8 LE1 O 07 寸 N 7 0 O + 0十 6 N 寸 的 一 970 T00 F E S E 9 N 尺 610+寸9 oo 寸6 O L 8 : 6 寸m 6 十 5 E EZ 06I : T 十6 N M + 十o MM 269 9T 一OToo*62 5 60¥0680 mO 寸M 0 十 +千寸 5 60 S T O F 7 6 0 2 2 0 .¥ 2 6 0 m L E O :寸 N N m N o N OM 20I 0 0 L刊¥ E98 O0三 o O小¥Z OL '0 00十+0 年 o 90 ¥06 80 8 十OO060¥899旦 寸210co:>cco0oo0I0o Ecoc旦Oa兰工兰 zoz contained in the fertilizer and their impact on the plants.Before conducting agrochemical tests, clean soil tests wereperformed and corresponding results were obtained: P-0.018% and K - 0.025%. In order to evaluate objectivelythe effect of the produced PDP with MC, pure KH PO4 Wasused for fertilization and no fertilizer was used for controfertilization. Different plant parameters (length of leavesgreen mass, ash mass) were investigated and the resultsare presented in Figures 5-7. As it can be seen from Figure 5, fertilization had someinfluence on the leaf length of the crop. In both cases.when pure KH PO4 and PDP were used, a similar averageof leaves length was determined: 29.63 and 31.20 cm,respectively. This leaf length average is higher comparedto control samples sprinkled with water only, which pro-duced an average of 27.69 cm. The results presented in Figure 6 show that the largestshare of the green mass of wheat leaves was obtainedwhen the PDP granulated with MC was used. In manycases, the lowest green mass of the wheat was obtainedwhen it was not fertilized at all (i.e., treated with wateronly). Fertilization with pure KH PO4 yielded almost thesame green mass (average value of 0.471g) as when treat-ment with water alone (average value of 0.441g), but lessthan fertilizing PDP with MC (average value of 0.763 g)This suggests that plants have better absorbed nutrientsfrom granular PDP. Analyzing the amount of ash formed by plants burningprocess at 900°℃ temperature (Figure 7), it can be noted thatin many cases, mass of the ash correlates with the greenmass. Ash mass, which corresponds to the mineral part ofthe plant, was larger when green mass was larger, andleaves were longer. The largest mass of ash (average valueof 0.019 g) was obtained when the PDP granulated with MCwas used. The least mass of the ash was obtained whenwheat was not fertilized at all. The pure KHPO4 did nothave any significant impact on the ash mass of the cropleaves, because the difference between the average valueof ash when pure KP was used and ash of untreated samples was only 0.003g. Summarizing the influence of pure KH PO4 and PDP,granulated with MC, on the growth of winter wheat seedlings, it can be stated that the effect of PDP is higherbecause the seedlings were longer, accumulated more greenmass and more mineral substances. It can be assumed thatthis was due to the chemical composition of the PDPobtained by conversion. In this case PDP contains notonly phosphorus and potassium but also a small amount(about 2%) of nitrogen, which is also a very importantmacronutrient. During the study period, 5% addition ofMC had a positive effect on the growth of winter wheat. -granulated PDP with cellulose Figure 5: Length of leaves dependency on the type of fertilizers. Figure 6: Leaf green mass dependence on the type of fertilizer. In accordance with Tan et al. [51], unbalanced ferti-lization and the use of concentrated fertilizers signifi-cantly deplete the soil and induce soil fertility problems,the change in various bacteria in the soil before and afterfertilization was studied. Soil samples were taken fromsix plant pots (three from Sample 3 and three fromSample 7) in which wheat was grown in parallel underanalogous conditions (control test, PDP granulated withcellulose, and chemically pure KH PO4) and each samplewas analyzed in triplicate. The result (Table 2) of themicrobiological analysis of each sample is given as anapproximate average of the three measurements. Com-paring the presented data, it can be observed that theuse of pure KHPO4 slightly reduced the number of Sporeb. bacteria (from 1.3 × 10 to 1.5×10*KSV/g), mineralnitrogen assimilating bacteria (from 1.2 × 10 to 1.6×10*KSV/g), and cellulose degrading bacteria (from 1.9×10° to 1.3×10KSV/g). The number of actinomycetesremained practically unchanged (8.5×10°KSV/g controland 3.7×10°KSV/g after fertilization with pure KHPO4)and there was a slight increase in molds (from 3.0×10“to2.5×10KSV/g). PDP granulated with MC had fewer negative effects onsoil bacteria. Wheat growth and fertilization did not decreasethe number of any species of bacteria (Spore b., mineralnitrogen assimilating bacteria, and cellulose degrading bacteria) and there was a slight increase in the number ofactinomycetes (from 8.5×10 to 2.9×10°KSV/g) and molds(from 3.0×10" to 1.4×10KSV/g). This means that adding5% MC had a positive influence on soil and not only maintained but also slightly increased the soil's microbiologicaactivity. Our results are consistent with the scientists Mondejaret al. who stated that microorganisms decompose celluloseand hemicelluloses in soils, which makes them importantplayers in the process of plant cultivation and making micro-flora and biomass in the soil adequate [52]. In summary, it can be stated that the method of production and granulation of PDP with the additive of MC developedand proposed by us allows to reduce the cost the production ofKDP, increases its agro-efficiency, and does not adverselyaffect soil's microorganisms. In this way, it is possible tosupply plants with macronutrients (potassium, phosphorus,and partly nitrogen) and to maintain the stability of the soil. 4 Conclusion Crystalline PDP obtained via conversion reaction of potas-sium chloride and ammonium dihydrogen phosphate hassimilar physical properties to pure KH PO4: pH of 10%solution is 3.8 and 4.5, and humidity is 3.3 and 1.3%, respectively. The results of this study show that the PDPproduced by the proposed method (which is enriched with5% MC additive) has a positive effect on the growth ofwinter wheat during the active period and improves soilhealth. The effect of granulated PDP with MC on winterwheat was higher than that of pure KH PO4 because wheatseedlings were longer (31.20 and 29.63 cm, respectively),leaf green mass was larger (0.763 and 0.471g, respec-tively), and ash mass was greater (0.015 and 0.019g,respectively). 5% MC additive had positive influence onsoil and not only maintained but also slightly increasedthe soil's microbiological activity, because applying PDPwith MC did not decrease the number of any species ofbacteria (spore b., mineral nitrogen assimilating bacteria,and cellulose degrading bacteria) and slightly increasedthe number of actinomycetes (from 8.5 × 10 to 2.9×10°KSV/g) and molds (from 3.0 × 10“ to 1.4×10KSV/g).Since the breakage force of the obtained granulated pro-duct is sufficient - 3.2 N, the obtained fertilizer could berecommended for use on winter wheat, especially grownin soils with low potassium and phosphorus, thus main-taining soil health [53]. For a more detailed study, nitrogencould be added to fertilizer and additional research couldbe carried out. Acknowledgments: The article was prepared based on thedata of my dissertation on the topic“Sustainable Technologyof PDP production and liquid waste recovery." This disserta-tion was defended in the field of technological sciences,che-mical engineering at Kaunas University of Technology.Funding information: The authors state no funding involved.Conflict of interest: The authors state no conflict ofinterest. Data availability statement: The datasets generated duringand/or analyzed during the current study are availablefrom the corresponding author on reasonable request. [1Bartley D, Batello C, Bernardi M, Biancalani R, Binswanger HP,Bonnal J, et al. The state of the world's land and waterresources for food and agriculture. New York, NY: Earthscan;2011. p. 10-20.[2]EU Agricultural Markets Briefs 2019. Fertilisers in the EU.Prices, trade and use; 2019 [cited 2022 March 4]. https://ec.europa.eu/info/sites/default/files/food-farming-fisheries/farming/documents/market-brief-fertilisers_june2019_en.pdf. OT> wxxxxr寸ooooxmXxx寸 OTXEE xxxxonowx十寸xxxxxxo 0一LXxxM6mOTo0oFxss,O0TOTI××xxm s0IXEZ oa wxxxxnooXs0T×xx0*8 。OT× 'Eo >s兰coEE aOO一m一aa工工oaaaaEcoa aEo aEo 70T×0E os0wTwxxxxXx寸LoZoo*wox0F,N0wxwIxxxxXO守*T >兰 0儿wxXxx8x寸rZ*o0Fs,00wTL×Xxx女0o7 s0T×Z't E o owcoc [3 Myers S, Nigh V. Too Many to Count: Factors Driving FertilizerPrices Higher and Higher; 2021 [cited 2022 February 22].https://www.fb.org/market-intel/too-many-to-count-factors-driving-fertilizer-prices-higher-and-higher. [4] Fotyma M. Fertilizer use by crop in Poland; 2003 [cited 2022January 14]. https://www.fao.org/3/y4620e/y4620e0a.htm. [5] ECHEMI 2022. Market Price & Insight; 2022 [cited 2022November 28]. https://www.echemi.com/productsInformation/temppid160705011376-potassium-dihydrogen-phosphate.html. [6 Eco City Hydroponics 2022. Potassium dihydrogen phosphate;2022 [cited 2022 November 18]. https://www.ecocityhydroponics.com/potassium-dihydrogen-phosphate.html. [7] Cotelle V, Leonhardt N. 14-3-3 proteins in guard cell signalling.Front Plant Sci. 2016;6:1-10. Blatt MR. Plant physiology: redefining the enigma of metabo-lism in stomatal movement. Curr Biol. 2016;26:107-24. [9] Takahashi K, Kinoshita T. The regulation of plant cell expan-sion: Auxin-induced turgor-driven cell elongation. In: Rose. Rj,editor. Molecular cell biology of the growth and differentiationof plant cells. Boca Raton, London, UK: CRC Press; 2016.p. 156-73. [10](Osakabe Y, Arinaga N, Umezawa T, Katsur S, Nagamachi K,Tanaka H, et al. Osmotic stress responses and plant growthcontrolled by potassium transporters in Arabidopsis. PlantCell. 2013;25:609-24. [11]Elumalai RP, Nagpal P, Reed jW. A mutation in the ArabidopsisKT2/KUP2 potassium transporter gene affects shoot cellexpansion. Plant Cell. 2002;14:119-31. [12][Liu Z, Persson S, Sanchez-Rodriguez C. At the border: Theplasma membrane-cell wall continuum. J Exp Bot.2015;66:1553-63. [13] Nieves-Cordones M, Andrianteranagna M, Cuellar T, CherelI,Gibrat R, Boeglin M, et al. Characterization of the grapevineShaker K* channel VvK3.1 supports its function in massivepotassium fluxes necessary for berry potassium loading andpulvinus-actuated leaf movements. N Phytol.2019;222:286-300. [14]\White PJ, Karley Aj. Potassium Cell Biology of Metals andNutrients. Berlin: Springer; 2010. p. 199-224. [15](Oosterhuis D, Loka D, Kawakami E, Pettigrew W. The physiology of potassium in crop production. Adv Agron.2014;126:203-234. [16] Hepler PK, Vidali L, Cheung AY. Polarized cell growth in higherplants. Annu Rev Cell Dev Biol. 2001;17:159-87. [17]FPettigrew WT. Potassium influences on yield and quality pro-duction for maize, wheat, soybean and cotton. Physiol Plant.2008;133:670-81. [18]Valentinuzzi F, Maver M, Fontanari S, Mott D, Savini G,Tiziani R, et al. Foliar application of potassium-based fertilizerimproves strawberry fruit quality. In: Mimmo T, Pii Y,Scandellari F, editors. VIII International Symposium on MineralNutrition of Fruit Crops. Vol. 1217. Book Series. Acta Hortic;2018. p. 379-84. [19] Holland JE, Hayes RC, Refshauge G, Poile GJ, Newell MT,Conyers MK. Biomass, feed quality, mineral concentration andgrain yield responses to potassium fertilizer of dual-purposecrops. N ZJ Agric Res. 2019;62:476-94. [20] Milford GFJ, Armstrong Mj, Jarvis Pj, Houghton Bj, Bellett-Travers DM, Jones j, et al. Effect of potassium fertilizer on the yield, quality and potassium offtake of sugar beet crops grownon soils of different potassium status. j Agric Sci.2000;135:1-10. [21] Jariwala H, Santos RM, Lauzon JD, Dutta A, Chiang YW.Controlled release fertilizers (CRFs) for climate-smart agricul-ture practices: A comprehensive review on releasemechanism, materials, methods of preparation, and effecton environmental parameters. Env Sci Pollut Res Int.2022;29:53967-95. [22] Lawrencia D, Wong SK, Low DYS, Goh BH, Goh JK,Ruktanonchai UR, et al. Controlled release fertilizers: A reviewon coating materials and mechanism of release. Plants.2021;10:1-25. [23] Hawkesford M, Horst W, Kichey T, Lambers H, Schjoerringl,Molle Sl, et al. Functions of macronutrients. Marschner'sMineral Nutrition of Higher Plants; 2012. p. 158-65. [24] Umar S, Bansal SK, Imas P, Magen H. Effect of foliar fertiliza-tion of potassium on yield, quality, and nutrient uptake ofgroundnut. J Plant Nutr. 2008;22:1785-95. [25] Kovarj, Cantarella H. Measuring Crop-Available. Better CropPlant Food. 2019;103:13-7. [26] Thomas LH, Forsyth VT, Sturcova A, Kennedy CJj, May RP,Altaner CM, et al. Structure of cellulose microfibrils in primarycell walls from collenchyma. Plant Physiol. 2013;161:465-76. [27] Szymanski DB, Cosgrove DJ. Dynamic coordination of cytos-keletal and cell wall systems during plant cell morphogenesis.Curr Biol. 2009;19:800-11. [28] Knox JP. Revealing the structural and functional diversity ofplant cell walls. Curr Opin Plant Biol. 2008;11:308-13. [29] Mohnen D. Pectin structure and biosynthesis. Curr Opin PlantBiol. 2008;11:266-77. [30] Scheller HV, Ulvskov P. Hemicelluloses. Annu Rev Plant Biol.2010;61:263-89. [31]NNishiyama Y. Structure and properties of the cellulose micro-fibril. J Wood Sci. 2009;255:241-9. [32] Fernandes AN, Thomas LH, Altaner CM, Callow P, Forsyth VT,Apperle DC, et al. Nanostructure of cellulose microfibrilsin spruce wood. Proc Natl Acad Sci U S A.2011;108:1195-203. [33] Cosgrove DJ. Growth of the plant cell wall. Nat Rev Mol CellBiol. 2005;6:850-61. [34] Baskin TI. Anisotropic expansion of the plant cell wall. AnnuRev Plant Biol. 2005;21:203-22. [35] Jacoby R, Peukert M, Succurro A, Koprivova A, Kopriva S. Therole of soil microorganisms in plant mineral nutrition - currentknowledge and future directions. Front Plant Sci. 2017;8:1-19. [36] Pasmionka IB, Bulski K, Boligtowa E. The participation ofmicrobiota in the transformation of nitrogen compounds in thesoil - A review. Agron (Basel). 2021;977:1-26. [37]BBhatti AA, Haq S, Bhat RA. Actinomycetes benefaction role insoil and plant health. Microb Pathog. 2017;111:458-67. [38] Pharmatrans SanaqAG MCC; 2018 [cited 2022 January 10].http://www.pharmatrans-sanaq.com/products/multifunctional-excipients/mcc-sanaq-burst/. [39] Paleckiene R, Sviklas AM, Slinksiene R, Streimikis V.Processing of rape straw ash into compound fertilizers usingsugar factory waste. Pol J Env Stud. 2012;21:993-9. [40] Gupta AP, Neue HU, Singh VP. Phosphorus determination inrice plants containing variable manganese content by thephospho-molybdo-vanadate (yellow) and phosphomolybdate (blue) colorimetric methods. Commun Soil Sci Plant Anal.1993;24:1309-18. [41]ISO 10390:2005. Soil Quality - Determination of pH; c2015[cited 2021 November 4]. https://www.iso.org/standard/40879.html. [42] LST CR 1233:2006. Fertilizers-Crushing StrengthDetermination on Fertilizer Grains. Vilnius, Lithuania:Lithuanian Standards Board; 2006. [43] Fries L, Antonyuk S, Heinrich S, Niederreitera G, Palzera S.Product design based on discrete particle modeling of a flui-dized bed granulator. Particoulogy. 2014;11:13-34. [44] Kasana RC, Salwan R, Dhar H, Dutt S, Gulati A. A rapid and easymethod for the detection of microbial cellulases on agar platesusing gram’s iodine. Curr Microbiol. 2008;5:503-7. [45] Statista 2022. Price for potassium chloride from 2015 to 2020with a forecast for 2021 to 2035; 2015 [cited 2021 November15]. https://www.statista.com/statistics/469705/potassium-chloride-price-forecast/. [46] Jancaitiene K, Slinksiene R. KHzPO4 crystallisation frompotassium chloride and ammonium dihydrogen phosphate.Pol J Chem Technol. 2016;18:1-8. [47] Flisyuk OM, Martsulevich NA, Shininov TN. Granulation ofpowdered materials in a high-speed granulator. Russ j ApplChem. 2016;89:603-10. [48] Jancaitiene K, Slinksiene R. Influence of cellulose additive onthe granulation process of potassium dihydrogen phosphate.Chem Ind Chem Eng Q. 2020;26:359-67. [49] Pato U, Ayu DF, Riftyan E, Restuhadi F, Pawenang WT,Firdaus R, et al. Cellulose microfiber encapsulated probiotic:Viability, acid and bile tolerance during storage at differenttemperature. Emerg Sci J. 2022;6:106-17. [50] Jancaitiene K, Slinksiene R. Solid-liquid equilibrium in liquidcompound fertilizers. Chem Ind Chem Eng Q. 2018;24:59-68. [51]11Tan ZX, Lal R, Wiebe KD. Global soil nutrient depletion andyield reduction. j Sustain Agric. 2008;26:123-46. [52] Mondejar RL, Zuhlke D, Becher D, Riedel K, Baldrian P.Cellulose and hemicellulose decomposition by forest soilbacteria proceeds by the action of structurally variable enzy-matic systems. Sci Rep. 2016;6:1-12. [53] Jabal ZK, Khayyun TS, Alwan IA. Impact of climate change oncrops productivity using MODIS-NDVI time series. Civ Eng J.2022;8:1136-56.
确定







还剩11页未读,是否继续阅读?
中国格哈特为您提供《氯化钾和磷酸一铵混合肥料中铵NH4+含量的检测》,该方案主要用于复合肥料中含量分析检测,参考标准--,《氯化钾和磷酸一铵混合肥料中铵NH4+含量的检测》用到的仪器有格哈特全自动凯氏定氮仪VAPODEST 450、凯氏消化管
推荐专场
















