次生有机质对暴露在气-液界面的重组人支气管上皮中烟灰颗粒毒性的作用Role of Secondary Organic Matter on Soot Particle Toxicity in Reconstituted Human Bronchial Epithelia Exposed at the Air–Liquid Interface
使用格哈特公司全自动快速索氏萃取系统 索克森Soxtherm Sox 416 Macro以二氯甲烷作为溶剂从滤器样品中萃取多环芳烃PAHs,使用安捷伦公司的气质联用仪6890N and 5973检测。
Polycyclic aromatic hydrocarbons (PAH) were extracted from the filter samples using an automated Soxhlet extraction (Soxtherm Sox 416 Macro, C. Gerhardt GmbH &Co. KG, Germany) with dichloromethane and analyzed using a gas chromatograph−mass spectrometer (GC-MS, Agilent
6890N and 5973).
方案详情

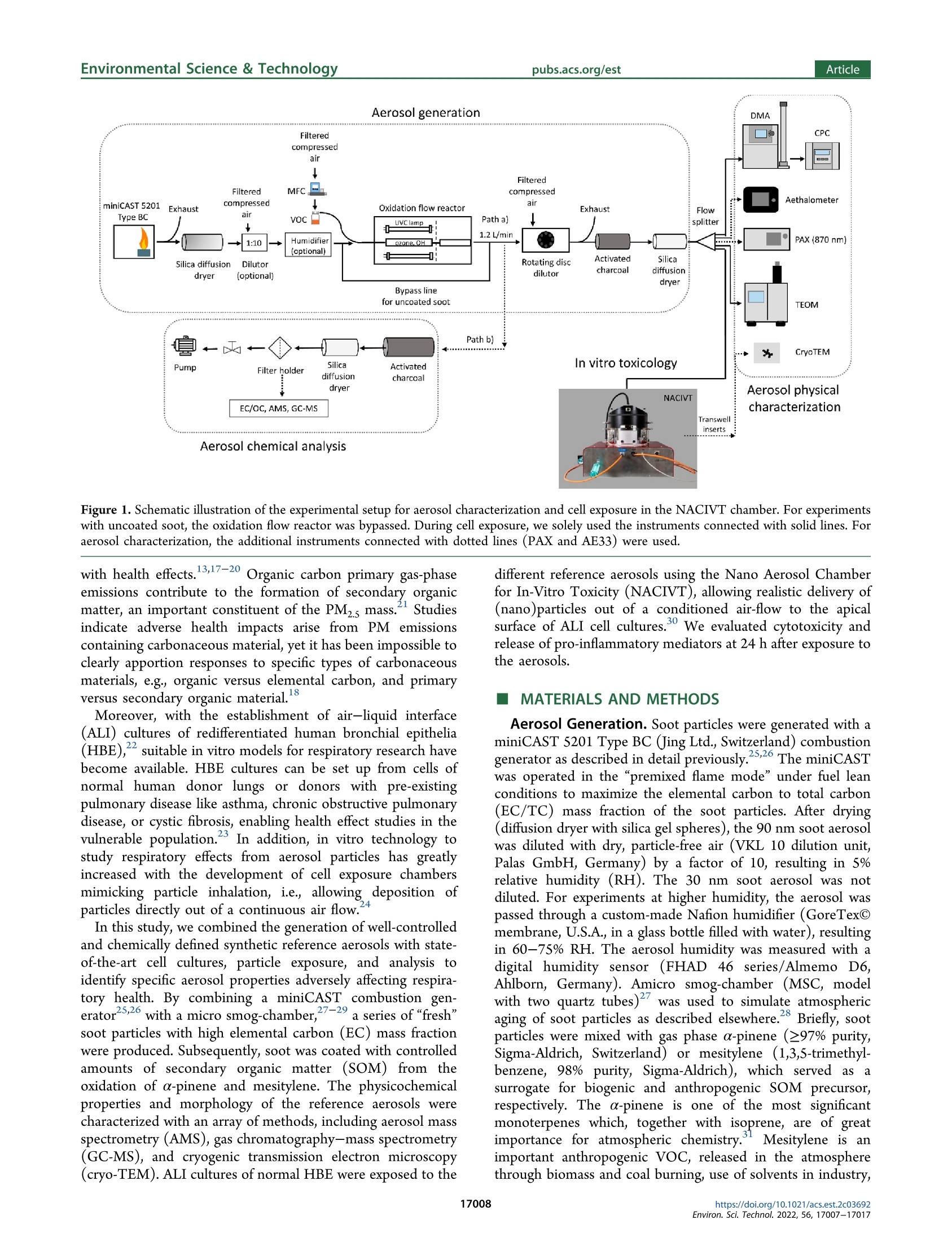
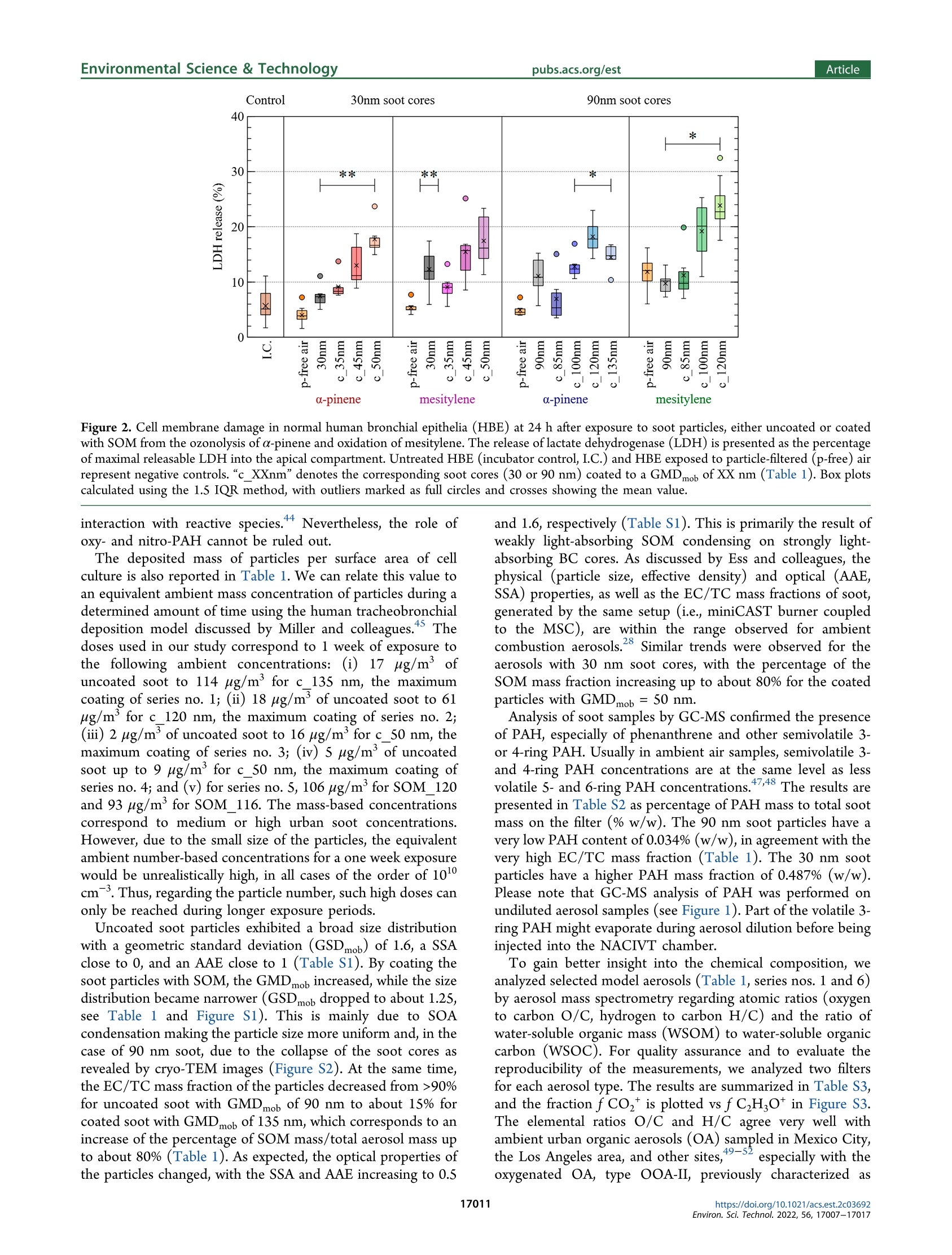
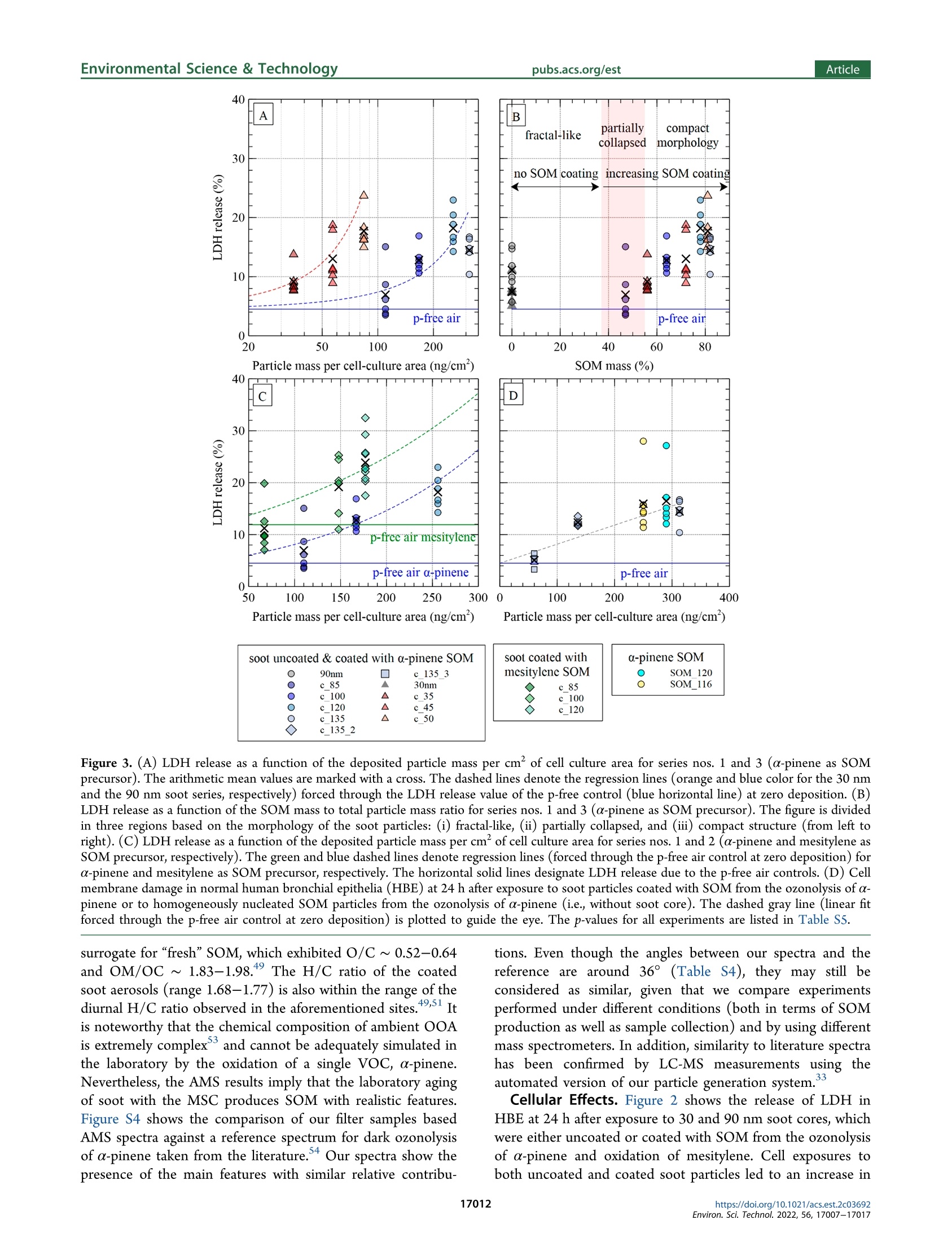
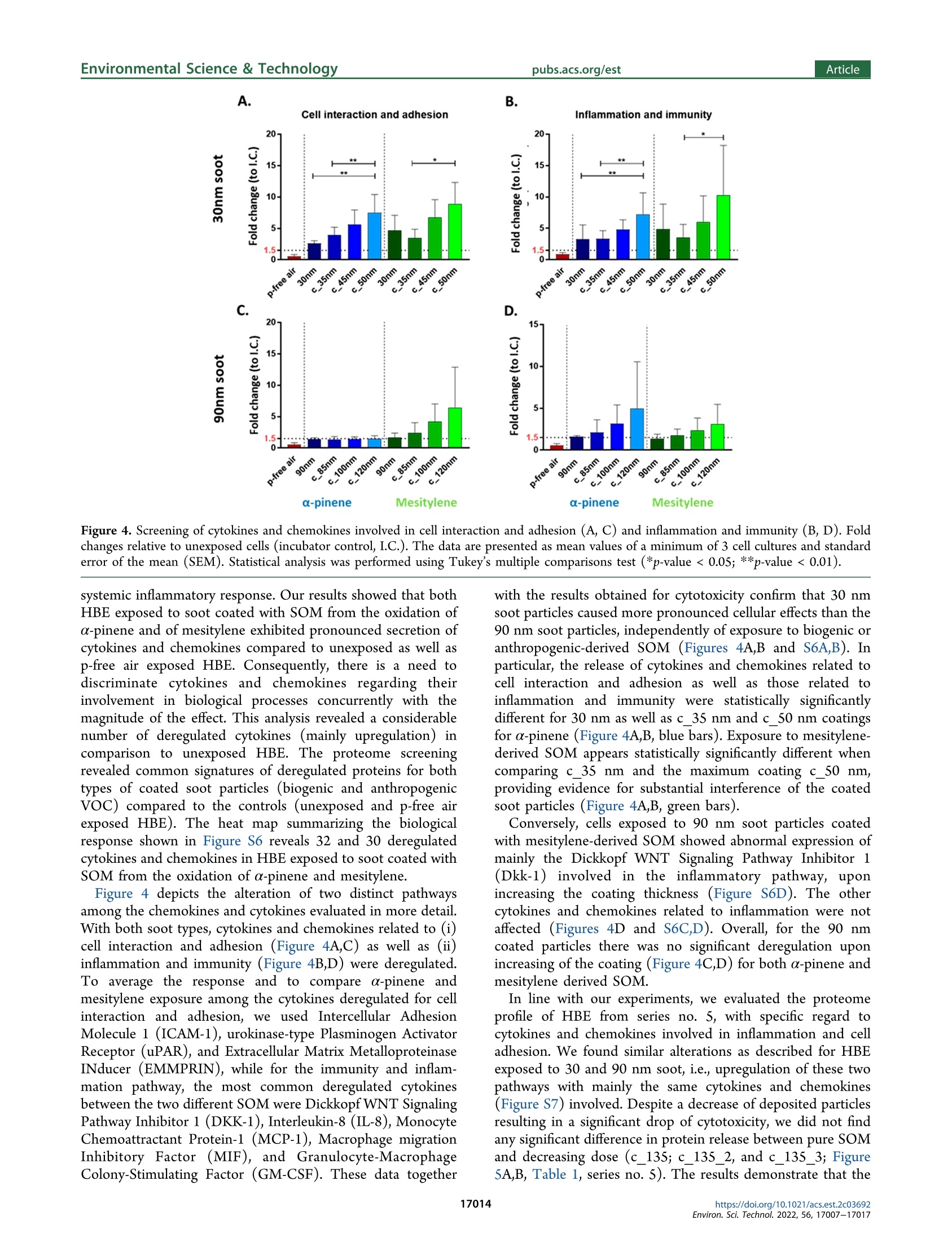
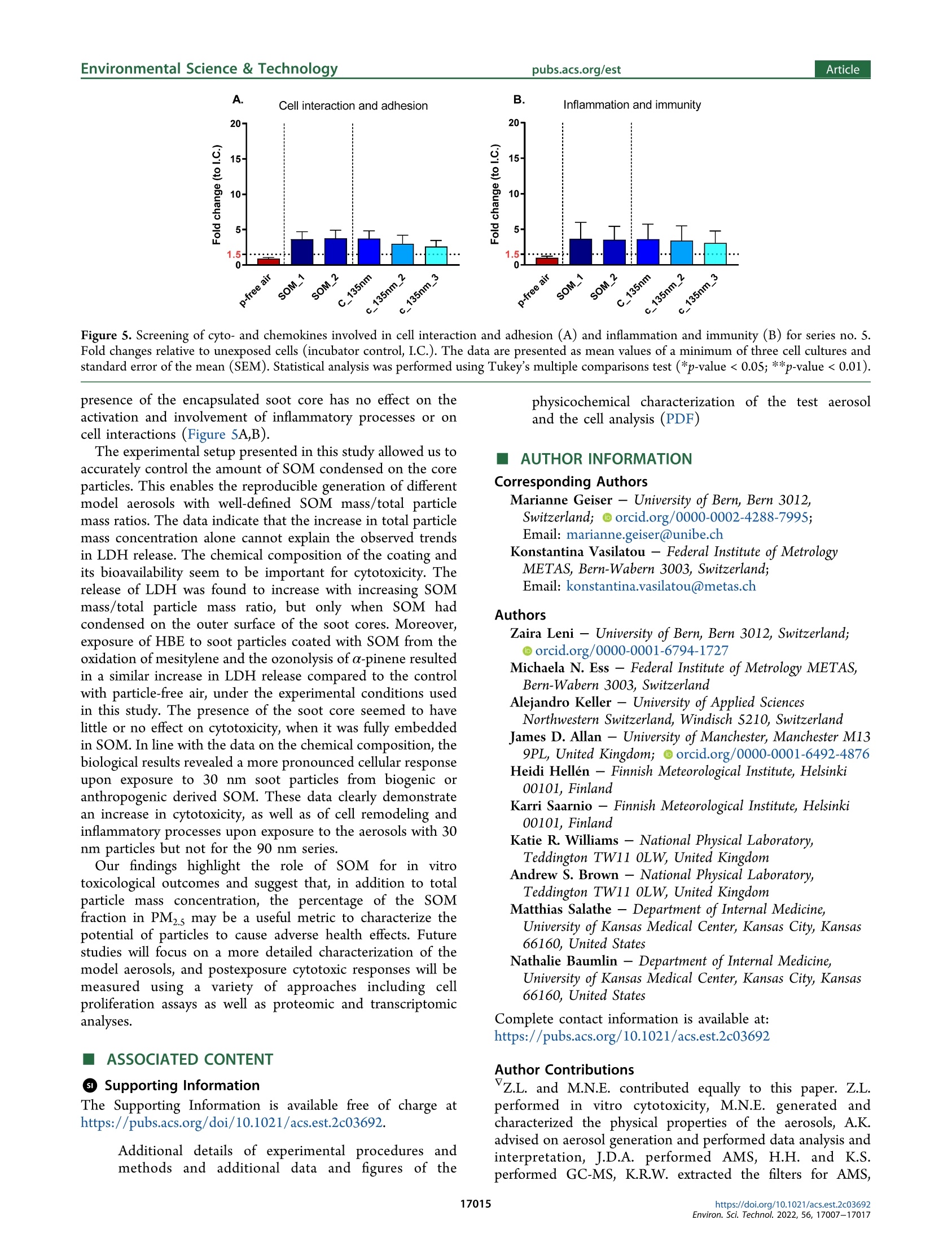
次生有机质对暴露在气-液界面的重组人支气管上皮中烟灰颗粒毒性的作用Role of Secondary Organic Matter on Soot Particle Toxicity in Reconstituted Human Bronchial Epithelia Exposed at the Air–Liquid Interface使用格哈特公司全自动快速索氏萃取系统 索克森Soxtherm Sox 416 Macro以二氯甲烷作为溶剂从滤器样品中萃取多环芳烃PAHs,使用安捷伦公司的气质联用仪6890N and 5973检测。Polycyclic aromatic hydrocarbons (PAH) were extracted from the filter samples using an automated Soxhlet extraction (Soxtherm Sox 416 Macro, C. Gerhardt GmbH &Co. KG, Germany) with dichloromethane and analyzed using a gas chromatograph−mass spectrometer (GC-MS, Agilent6890N and 5973).FOVIROILMENTALScience &lechnoloouArticle ArticleEnvironmental Science & Technologypubs.acs.org/est pubs.acs.org/est 次生有机质对暴露在气-液界面的重组人支气管上皮中烟灰颗粒毒性的作用 Role of Secondary Organic Matter on Soot Particle Toxicity inReconstituted Human Bronchial Epithelia Exposed at the Air-LiquidInterface Zaira Leni, Michaela N. Ess, Alejandro Keller, James D. Allan, Heidi Hellen, Karri Saarnio,Katie R. Williams, Andrew S. Brown, Matthias Salathe, Nathalie Baumlin, Konstantina Vasilatou,*and Marianne Geiser* Cite This: Environ. Sci. Technol. 2022,56,17007-17017 Read Online ACCESS| 二 INTRODUCTION Atmospheric particulate pollution has been linked to a broadspectrum of adverse health effects, such as respiratory andcardiovascular diseases, lung cancer, and dementia.1一-8Atmospheric aerosols vary significantly in their compositionand size distribution. Due to this complexity and theircontinuous temporal and spatial variations, it has beenimpossible to clearly identify which metric(s) dominate theetiology and progression of detrimental health effects.Atmospheric aerosols have been regulated for human healthpurposes by the mass concentration of the size fractions, forexample, PMio (particulate matter with aerodynamic diameterbelow 10 pm) and PMs (particulate matter with aerodynamicdiameter below 2.5 um, generally described as fine particles,European Parliament Directive 2008/50/EC). This metric,which is used in most epidemiologic studies, is suitable tocapture the effects of large particles with considerable mass butcannot account for the effects of ultrafine particles (UFP,particulate matter with aerodynamic diameter ≤100 nm),which only negligibly contribute to the total aerosol mass concentration of ambient air. Therefore, it has been suggestedthat PM mass concentration, while useful, is not the mostinformative metric to characterize the potential of particles tocause the detrimental health effects reported. Additional air-quality metrics, such as particle numberconcentration, oxidative potential, and chemical composition,have been proposed to disentangle the health effects ofcombustion particles,,9,12-144tthe mass of which is too small tobe efficiently regulated by mass-based metrics. Several studiesshow that fresh and mature diesel exhaust soot and carbonblack soot model particles strongly produce reactive oxygenspecies and are genotoxic.15-17'There is also growinginformation on the association of organic carbon fractions Received:May 23, 2022 Revised: November 13, 2022 Accepted::November 14,2022 Published: November 23, 2022 Figure 1. Schematic illustration of the experimental setup for aerosol characterization and cell exposure in the NACIVT chamber. For experimentswith uncoated soot, the oxidation flow reactor was bypassed. During cell exposure, we solely used the instruments connected with solid lines. Foraerosol characterization, the additional instruments connected with dotted lines (PAX and AE33) were used. with health effects. 3,17-20 Organic carbon primary ga aseemissions contribute to the formation of secondary organicmatter, an important constituent of the PMs mass.Studiesindicate adverse health impacts arise from PM emissionscontaining carbonaceous material, yet it has been impossible toclearly apportion responses to specific types of carbonaceousS1Smaterials, e.g., organic versus elemental carbon, and primaryversus secondary organic material. Moreover, with the establishment of air-liquid interface(ALI) cultures of redifferentiated human bronchial epithelia(HBE)," suitable in vitro models for respiratory research havebecome available. HBE cultures can be set up from cells ofnormal human donor lungs or donors with pre-existingpulmonary disease like asthma, chronic obstructive pulmonarydisease, or cystic fibrosis, enabling health effect studies in thevulnerable population.3 In addition, in vitro technology tostudy respiratory effects from aerosol particles has greatlyincreased with the development of cell exposure chambersmimicking particle inhalation, i.e., allowing deposition ofparticles directly out of a continuous air flow.24 In this study, we combined the generation of well-controlledand chemically defined synthetic reference aerosols with state-of-the-art cell cultures, particle exposure, and analysis toidentify specific aerosol properties adversely affecting respira-tory health. By combining a miniCAST combustion gen-erator25.26 with a micro smog-chamber,?727-29 a series of "freshsoot particles with high elemental carbon (EC) mass fractionwere produced. Subsequently, soot was coated with controlledamounts of secondary organic matter (SOM) from theoxidation of a-pinene and mesitylene. The physicochemicalproperties and morphology of the refereOrpnce aerosols werecharacterized with an array of methods, including aerosol massspectrometry (AMS), gas chromatography-mass spectrometry(GC-MS), and cryogenic transmission electron microscopy(cryo-TEM). ALI cultures of normal HBE were exposed to the different reference aerosols using the Nano Aerosol Chamberfor In-Vitro Toxicity (NACIVT), allowing realistic delivery of(nano)particles out of a conditioned air-flow to the apicalsurface of ALI cell cultures. We evaluated cytotoxicity andrelease of pro-inflammatory mediators at 24 h after exposure tothe aerosols. MATERIALS AND METHODS Aerosol Generation. Soot particles were generated with aminiCAST 5201 Type BC (Jing Ltd., Switzerland) combustiongenerator as described in detail previously.25,26 The miniCASTwas operated in the “premixed flame mode” under fuel leanconditions to maximize the elemental carbon to total carbon(EC/TC) mass fraction of the soot particles. After drying(diffusion dryer with silica gel spheres), the 90 nm soot aerosolwas diluted with dry, particle-free air (VKL 10 dilution unit,Palas GmbH, Germany) by a factor of 10, resulting in 5%relative humidity (RH). The 30 nm soot aerosol was notdiluted. For experiments at higher humidity, the aerosol waspassed through a custom-made Nafion humidifier (GoreTexOmembrane, U.S.A., in a glass bottle filled with water), resultingin 60-75% RH. The aerosol humidity was measured with adigital humidity sensor(FHAD 46 series/Almemo D6,Ahlborn, Germany). Amicro smog-chamber (MSC, modelwith two quartz tubes)27 was used to simulate atmosphericaging of soot particles as described elsewhere.28 Briefly, sootparticles were mixed with gas phase a-pinene (297% purity,(Sigma-Aldrich, 1.3.5-trirSwitzerland) or mesitylene (1,3,S-trimethyl-benzene,998% purity, Sigma-Aldrich), which served asSasurrogate for biogenic and anthropogenic SOM precursor,respectively. The a-pinene is one of the most significantmonoterpenes which, together with isoprene, are of greatimportance for atmospheric chemistry. Mesitylene is animportant anthropogenic VOC, released in the atmospherethrough biomass and coal burning, use of solvents in industry, and diesel and (to a lesser extent) gasoline exhaust. TheVOC concentration was controlled by adjusting the flow ofzero-air through the VOC container (cylindrical gas bubbler)using a mass flow controller (V-red-y Compact with handvalve, 1-100 mL/min, Vogtlin, Switzerland). In the MSC, thea-pinene or mesitylene vapors were oxidized by OH and/or Osradicals, yielding SOM, part of which condensed on the sootparticles as coating.2 More specifically, ozonolysis of a-pinenewas performed under dry conditions (5% relative humidity),while oxidation of mesitylene by O and OH radicals wasperformed under humid conditions (60-75% RH). In thisstudy, only four out of five UVC lamps (4 W UVC with 254and 185 nm emission lines) were used, leading to a maximumO3 concentration of 120 mg/m. The UVA lamp located abovethe second quartz chamber in the MSC was switched off. Thesetup for coating a particle with SOM has been automated andminiaturized after this study through a novel system called anorganic coating unit (OCU). quartz filters for reliable assessment of EC/TC ratios. Opticalproperties, such as the single scattering albedo (SSA) andAngstrom absorption exponent (AAE) wweerree mmoonnitored forquality assurance as explained in the Supporting Information(Text S1). Polycyclic aromatic hydrocarbons (PAH) wereextracted from the filter samples using an automated Soxhletextraction (Soxtherm Sox 416 Macro, C. Gerhardt GmbH &Co. KG, Germany) with dichloromethane and analyzed using agas chromatograph-mass sspectrometer (GC-MS, Agilent6890N and 5973). Aerosol mass spectrometry (AMS) wasperformed offline using a high-resolution AMs34 operatingunder default configuration with the vaporizer at 600°℃ andusing 70 eV electron ionization. Each sample was measured forat least 300 s, with data recorded every 30 s. A dwell time of7.5 s was used. More information on filter materials, extraction,measurement procedure, and data analysis for EC/OC, GC-MS, and AMS can be found in the Supporting Information(Text S2). Cell Cultures, Aerosol Exposure, and Cell AnalysisNormal human bronchial epithelial cells were isolated fromlungs deemed unsuitable for transplant. Lungs were donatedfor research purposes and recovered by the Life Alliance OrganRecovery Agency (LAORA) at the University of Miami. TheInstitutional Review Board (IRB) declared that consent fororgan donation for research covers the use of these cells and noother approval was needed for the here carried out experiments. Cells were collected from the proximal conductingairways, and ALI cultures of redifferentiated HBE weregenerated as previously described.23,35-37 After approximately28days, the cells revealedaPpseudostratified ciliatedepithelium. Cell morphology, mucus secretion, and ciliarybeating were evaluated visually and by phase-contrast lightmicroscopy. Apical cell surfaces were washed with Dulbecco’sphosphate-buffered saline (DPBS, with Cat and Mg,Invitrogen, Lucerne, Switzerland) 2 h before exposure. Fullydifferentiated HBEwere exposed to the aerosols in theNACIVT chamber23,24,30), allowing efficient deposition ofparticles directly out of a conditioned air flow (RH 85-95%,5% CO,) under physiological conditions (37℃) onto 24 ALIcell cultures simultaneously. Cell cultures were exposed to theparticles for 60 min at comparable aerosol particle numberconcentrations within one series of aerosols. Apical cellsurfaces were washed with DPBS 4 h after aerosol exposureto remove unbound particles. Cell analyses were performed at24 h after exposure. We conducted at least three independentexperiments with each aerosol, with a minimum of triplicateHBE cultures in each experiment. Cytotoxicity was assessed bymeasuring lactate dehydrogenase (LDH) in apical washes, aspreviously described.3 The LDH values obtained from cellsexposed to either aerosol or particle-filtered (p-free) air werethen compared to those of untreated cells (negative control).The p-free air control was carried out by inserting a HEPAfilter upstream of the NACIVT chamber; i.e., the cells wereexposed to the gas phase of the synthetic aerosols. Accordingto gas phase analysis with the Model 49C Ozone Calibrator(Thermo Scientific, U.S.A.), the ozone amount fractions werelower than 100 ppb throughout the experiment. The NOxamount fraction was measured directly at the miniCASTexhaust with a portable emission analyzer (Model 350, Testo,Germany) and found to be about 2 ppm. The NOx amountfraction in the model aerosols delivered to NACIVT wastherefore <70 ppb depending on the aerosol dilution ratio. Theinflammatory response was evaluated by measuring the release Table 1. GMDmob, GSDmob (Geometric Standard Deviation), EC/TC Mass Fraction, SOM Mass/Total Particle Mass, ParticleNumber Concentration C, and Total Particle Mass Concentration Cm" Series Label VOC GMDmob (nm) GSDmob (-) EC/TC (%) C(cm-) C (ug/m) SOM mass/ total mass (%) Estimated deposition (Naep/cm²) Estimated deposition (ng/cm) 1 90 nm N/A 91.5±0.3 1.61 99±10 (1.3±0.1)×10° 62±1 0 1.5×10° 53 1 c_85 nm a-pinene 88.4±0.6 1.47 54±5 (1.3±0.1)×10° 116±1 47 1.5×10° 110 1 c 100 nm 103.3±1.5 1.34 31±3 (1.3±0.1)×10° 173±1 64 1.4×10° 167 1 c_120 nm 121.8±2.1 1.28 26±2 (1.3±0.1)×10° 279±2 78 1.3×10% 256 2 90 nm N/A 94.3±0.2 1.61 92±10 (1.4±0.1)×10° 64±1 0 1.5×10° 54 2 c 85 nm mesitylene 84.4±0.6 1.55 74±6 (1.3±0.1)×10 73±3 19 1.5×10° 67 2 c_100 nm 102.4±1.7 1.37 51±4 (1.3±0.1)×10% 154±2 61 1.4×10 148 2 c_120 nm 119.0±0.4 1.33 ≈12° (1.3±0.1)×105 192±1 69 1.3×10° 177 3 30 nm N/A 28.7±0.3 1.59 ≈76° (3.6±0.2)×10° 12±3 5.9×10° 16 3 c_35 nm a-pinene 37.5±0.4 1.42 (3.4±0.2)×10° 26±1 56 5.2×10° 35 3 c 45 nm 45.4±0.3 1.37 (3.7±0.1)×10° 44±1 72 5.4×10° 57 3 c 50 nm 51.7±0.6 1.33 一 (3.7±0.1)×10° 66±1 81 5.2×10° 84 4 30 nm N/A 28.6±0.5 1.69 心76° (2.7±0.2)×10° 9±2 4.5×10% 12 4 c 35 nm mesitylene 36.7±0.5 1.45 一 (2.5±0.2)×10° 17±1 51 3.9×10° 23 4 c_45 nm 44.6±0.6 1.41 (2.5±0.1)×10% 25±1 67 3.8×10° 32 4 c 50 nm 50.5±0.3 1.41 14±3 (2.9±0.1)×105 38±1 74 4.1×10° 47 5,1 c_135 nm a-pinene 134.5±2.0 1.25 17±1 (1.3±0.1)×105 351±2 82 1.3×10 313 c_135 nm_2 135.6±1.6 1.25 (6.5±0.1)×104 153±1 ~82 6.3×10' 136 5 c_135 nm_3 135.0±0.9 1.25 - (3.2±0.1)×10+ 77±4 ~82 3.1×10' 69 6 SOM_120 120.5±2.7 1.56 ≈0.3 (1.3±0.1)×10° 338±1 100 1.3×10% 290 6 SOM_116 116.4±5.9 1.58 (1.0±0.1)×10° 287±2 100 1.0×10° 250 “The deposited particle number per cm"of cell culture area (Ndep/cm) and deposited particle mass per cm" of cell culture area (ng/cm²) have alsobeen evaluated. Measurement uncertainties correspond to one standard deviation. Uncoated soot particles are labeled by their nominal mobilitydiameter, e.g.,“90 nm" stands for uncoated soot particles with a nominal GMDmob of 90 mm. Coated soot particles are distinguished by the prefix“c"; e.g., “c_120 nm"refers to coated soot particles with nominal GMDmob of 120 mm. Particles formed by homogeneous nucleation of SOM arelabeled as SOM_120 and SOM _116, with the index indicating measured GMDmob. Indicative value. Because of the low mass of material on thefilter, the measurement is subject to high uncertainty. “Soot particles with GMDmob of 30 nm contain primary organic carbon (POC) fromincomplete combustion. POC was not volatile and could not be removed with thermal treatment of the soot aerosol at 300 °℃.25,46 eCalculated as100·(Cm,coated - Cm,uncoated)/Cm,coated based on the mass concentrations measured by the TEOM (listed in the eighth column of the table).Whenever the particle number concentrations C of the uncoated and coated particles were not identical (see 7th column of the table), Cm,uncoatedwas scaled accordingly. The method to evaluate the number of particles deposited per cm of cell culture area is described in the SupportingInformation (Text S3). of 102 cytokines and chemokines from cells by high-throughput screening using the Proteome profiler HumanXL Cytokine Array (ARY022, R&D Systems, Minneapolis,MN, U.S.A.). Data Analysis. The results are presented as mean values ±standard deviation (SD) or standard error of the mean (SEM).Statistical analyses were performed using GraphPad Prism 7.04(GraphPad Software Inc., San Diego, U.S.A.). Mean valueswere compared using Multiple comparison one-way ANOVA,Bonferroni's multiple comparison test with **p<0.00S, ***p<0.0005, and ****p<0.0001. The data passed the Brown-Forsythe normality test (alpha =0.05). ■RESULTS AND DISCUSSION count median diameter CMD < 60 nm. Furthermore, petrol,ethanol, and compress natural gas engines have been shown toproduce soot particles with modal sizes around 20 to 30 nm fora variety of engine conditions and different fuel injectiontechnologies.,41,42In series no. S, soot particles coated withSOM from the ozonolysis of a-pinene were generated at threedifferent concentrations to evaluate any dose-responserelationship. Finally, in series no. 6, particles consisting ofpure SOM (i.e., particles formed by homogeneous nucleation)were generated aiming at “decoupling" the toxic effects ofSOM from those of the soot core. Note that the 30 nm sootparticles were generated at higher number concentrations thantheir 90 nm counterparts, enabling mass concentrationmeasurements by TEOM within a reasonable time frame. ItWe generated six series of model aerosols (Table 1). In seriesis reasonable to assume that changes in toxic effects duringnos. 1-4, soot particles with geometric mean mobilitycoating experiments will mainly come from SOM rather thandiameter (GMDmob) of 30 or 90 nm were coated withfrom oxidation of the core due to several reasons. Fdifferent amounts of SOM first, gasrom the photo-oxidation of α-phase reactions arpinene or mesitylene. Particles with GMe much faster thaDmob = 90 nm aren heterogeneousrepresentative of the main particle size mode (i.e., accumu-reactions.43This favors the formation of coating prior to38,39lation mode) of soot emitted by diesel engines.The interaction with surface-bound species like PAH, preventingsmaller size mode of GMDmob=30 nm covers emissions from formation of toxic materials like oxy- or nitro-PAH.other engine operation points, injection technologies, and/or Furthermore, SOM coating has been shown to protect thefuels. A review by Giechaskiel and colleaguesshows that both particle-bound PAH, reducing their interaction with gas-phasediesel and petrol engines can emit nonvolatile particles with a reactive species and lowering the overallFparticle surface Figure 2. Cell membrane damage in normal human bronchial epithelia (HBE) at 24 h after exposure to soot particles, either uncoated or coatedwith SOM from the ozonolysis of a-pinene and oxidation of mesitylene. The release of lactate dehydrogenase (LDH) is presented as the percentageof maximal releasable LDH into the apical compartment. Untreated HBE (incubator control, I.C.) and HBE exposed to particle-filtered (p-free) airrepresent negative controls. “c_XXnm" denotes the corresponding soot cores (30 or 90 nm) coated to a GMD mob of XX nm (Table 1). Box plotscalculated using the 1.5 IQR method, with outliers marked as full circles and crosses showing the mean value. interaction with reactive species.Nevertheless, the role ofoxy- and nitro-PAH cannot be ruled out. The deposited mass of particles per surface area of cellculture is also reported in Table 1. We can relate this value toan equivalent ambient mass concentration of particles during adetermined amount of time using the human tracheobronchialdeposition model discussed by Miller and colleagues. Thedoses used in our study correspond to 1 week of exposure tothe following ambient concentrations: (i) 17 ug/m’ ofuncoated soot to 114 ug/m’ for c_135 nm, the maximumcoating of series no. 1; (ii) 18 ug/m’ of uncoated soot to 61ug/m’ for c_120 nm, the maximum coating of series no. 2;(iii) 2 ug/m’of uncoated soot to 16 ug/m’ for c _50 nm, themaximum coating of series no. 3; (iv) 5 ug/m’ of uncoatedsoot up to 9 ug/m’ for c_50 nm, the maximum coating ofseries no. 4; and (v) for series no. 5, 106 ug/m' for SOM_120and 93 ug/m’for SOM_116. The mass-based concentrationscorrespond to medium or high urban soot concentrations.However, due to the small size of the particles, the equivalentambient number-based concentrations for a one week exposurewould be unrealistically high, in all cases of the order of 10cm. Thus, regarding the particle number, such high doses canonly be reached during longer exposure periods. Uncoated soot particles exhibited a broad size distributionwith a geometric standard deviation (GSDmob) of 1.6, a SSAclose to 0, and an AAE close to 1 (Table S1). By coating thesoot particles with SOM, the GMDmob increased, while the sizedistribution became narrower (GSDmob dropped to about 1.25,see Table 1 and Figure S1). This is mainly due to SOAcondensation making the particle size more uniform and, in thecase of 90 nm soot, due to the collapse of the soot cores asrevealed by cryo-TEM images (Figure S2). At the same time,the EC/TC mass fraction of the particles decreased from >90%for uncoated soot with GMDmob of 90 nm to about 15% forcoated soot with GMDmob of 135 nm, which corresponds to anincrease of the percentage of SOM mass/total aerosol mass upto about 80% (Table 1). As expected, the optical properties ofthe particles changed, with the SSA and AAE increasing to 0.5 and 1.6, respectively (Table S1). This is primarily the result ofweakly light-absorbing SOM condensing on strongly light-absorbing BC cores. As discussed by Ess and colleagues, thephysical (particle size, effective density) and optical (AAE,SSA) properties, as well as the EC/TC mass fractions of soot,generated by the same setup (i.e., miniCAST burner coupledto the MSC), are within the range observed for ambientcombustion aerosols. Similar trends were observed for theaerosols with 30 nm soot cores, with the percentage of theSOM mass fraction increasing up to about 80% for the coatedparticles with GMDmob =50 nm. Analysis of soot samples by GC-MS confirmed the presenceof PAH, especially of phenanthrene and other semivolatile 3-or 4-ring PAH. Usually in ambient air samples, semivolatile 3-and 4-ring PAH concentrations are at the same level as lessvolatile S- and 6-ring PAH concentrations. The results arepresented in Table S2 as percentage of PAH mass to total sootmass on the filter (% w/w). The 90 nm soot particles have avery low PAH content of 0.034% (w/w), in agreement with thevery high EC/TC mass fraction (Table 1). The 30 nm sootparticles have a higher PAH mass fraction of 0.487%(w/w).Please note that GC-MS analysis of PAH was performed onundiluted aerosol samples (see Figure 1). Part of the volatile 3-ring PAH might evaporate during aerosol dilution before beinginjected into the NACIVT chamber. c 85 30nm c 100 c 35 c 120 c 45 c 135 c 50 c_135 2 Figure 3. (A) LDH release as a function of the deposited particle mass per cm² of cell culture area for series nos. 1 and 3 (a-pinene as SOMprecursor). The arithmetic mean values are marked with a cross. The dashed lines denote the regression lines (orange and blue color for the 30 nmand the 90 nm soot series, respectively) forced through the LDH release value of the p-free control (blue horizontal line) at zero deposition. (B)LDH release as a function of the SOM mass to total particle mass ratio for series nos. I and 3 (a-pinene as SOM precursor). The figure is dividedin three regions based on the morphology of the soot particles: (i) fractal-like, (ii) partially collapsed, and (iii) compact structure (from left toright).(C) LDH release as a function of the deposited particle mass per cm’ of cell culture area for series nos. 1 and 2 (a-pinene and mesitylene asIture:SOM precursor, respectively). The green and blue dashed lines denote regression lines (forced through the p-free air control at zero deposition) fora-pinene and mesitylene as SOM precursor, respectively. The horizontal solid lines designate LDH release due to the p-free air controls. (D) Cellmembrane damage in normal human bronchial epithelia (HBE) at 24 h after exposure to soot particles coated with SOM from the ozonolysis of a-pinene or to homogeneously nucleated SOM particles from the ozonolysis of a-pinene (i.e., without soot core). The dashed gray line (linear fitforced through the p-free air control at zero deposition) is plotted to guide the eye. The p-values for all experiments are listed in Table SS. surrogate for “fresh"SOM, which exhibited O/C~0.52-0.64and OM/OC ~ 1.83-1.98.4 The H/C ratio of the coatedsoot aerosols (range 1.68-1.77) is also within the range of thediurnal H/C ratio observed in the aforementioned sites.49,51 Itis noteworthy that the chemical composition of ambient OOAis extremely complexand cannot be adequately simulated inthe laboratory by the oxidation of a single VOC, a-pinene.Nevertheless, the AMS results imply that the laboratory agingof soot with the MSC produces SOM with realistic features.Figure S4 shows the comparison of our filter samples basedAMS spectra against a reference spectrum for dark ozonolysisof a-pinene taken from the literature.+ Our spectra show thepresence of the main features with similar relative contribu- tions. Even though the angles between our spectra and thereference are around 36°(Table S4), they may still beconsidered as similar, given that we compare experimentsperformed under different conditions (both in terms of SOMproduction as well as sample collection) and by using differentmass spectrometers. In addition, similarity to literature spectrahas been confirmed by LC-MS measurements using theautomated version of our particle generation system. Cellular Effects. Figure 2 shows the release of LDH inHBE at 24 h after exposure to 30 and 90 nm soot cores, whichwere either uncoated or coated with SOM from the ozonolysisof a-pinene and oxidation of mesitylene. Cell exposures toboth uncoated and coated soot particles led to an increase in LDH release in comparison to the incubator control (I.C.). Inmost experiments, there was hardly any increase in LDHrelease in HBE exposed to particle-free air compared to theincubator control, indicating negligible effects by the gas phasecomponents of the aerosols. The only exception was series no.2, where 90 nm soot cores were coated with SOM from theoxidation of mesitylene. In this case, the particle-free aircontrol resulted in higher LDH release compared to theincubator control, whereby effects by gas phase componentsmay be ruled out. The variability in the release of LDH fromHBE cells in response to aerosol exposure may be allocated tocell culture heterogeneity. There was no statistical significancebetween I.C. and p-free air. The cytotoxicity of uncoated soot particles with GMDmob of30 nm was higher for series no. 4 (mesitylene as VOC) thanfor no. 3 (a-pinene as VOC), despite the lower particlenumber concentration (Table 1). This is probably due toinstabilities in soot generation at this small mobility diameter,which is at the lowest limit of the miniCAST 5201 BC burner.Even though GMDmobb wWas set to 30 nm during theseexperiments, instabilities in the flame might have influencedthe chemical composition (e.g., PAH amount) of the sootsurface, resulting in the differences in LDH release for 30 nmsoot in these two experimental series. Figure 2 shows a general increase in LDH release, when sootparticles are coated with either type of SOM. This is inagreement with previous studies, where SOA compoundsinduced higher toxicity than uncoated soot particles, when cellcultures were exposed at ALI.2 More specifically, in Figure 3A,LDH release has been plotted as a function of the depositedparticle mass per cm’ of cell culture area, for series nos. l and 3(a-pinene as SOM precursor). The data indicate that increasein total mass concentration alone cannot explain the observedtrends in LDH release. For instance, soot coated with SOMfrom the ozonolysis of a-pinene with a GMDmob of 50 nm(c _50 nm, light orange triangles) led to higher LDH releasethan the coated 85 nm soot particles (dark blue dots)corresponding to a higher mass loading. Similar results wereobtained with mesitylene as SOM-precursor (series nos. 2 and4) as shown in Figure S5. In Figure 3B, LDH release is plotted as a function of theSOM mass to total particle mass ratio. Three different regionsare shown: (i) uncoated particles (90 nm soot, light gray dots;30 nm soot, dark gray triangles) have a fractal-like morphology,and (ii) soot coated with a small amount of SOM (c_85 nm,dark blue dots) have a more compact structure due to a partialcollapse of the soot core, while (iii) all the other coated sootparticles have a compact morphology (see TEM images inFigure S2). According to previous studies, upon condensationof SOM, the major morphological transformation occursstepwise, by filling of void space and subsequent growth ofdiameter. This might explain why the soot particles withpartially collapsed soot structure (c_85 nm) result in similarLDH release as the uncoated soot particles (90 and 30 nm)despite a SOM/total mass ratio of about 47%. Part of the SOMhas filled the open voids of the soot with minimum change inparticle size. At higher SOM mass/total mass ratios (>55%),the particles have already become compact and SOMcondenses increasingly on the outer surface of the sootparticles, thus being bioavailable to interact with the epithelialcells. Indeed, in this case, LDH release increases as a functionof the SOM mass/total mass ratio. Another explanation forthese findings may be a threshold in dose-response. Further studies are necessary to clarify the observed trend in LDHrelease presented in Figure 3B. Note that the coated particles labeled “c _120 nm”and“c_50 nm", which both have about 80% SOM mass/total mass,induce a very similar LDH release despite the fact that particleswith a GMDmob of 120 nm have an about 6 times larger surfacearea (assuming spherical particle morphology) than those witha GMDmob of 50 nm. This is largely compensated by the factthat the estimated number of deposited particles per cm² ofcell culture area in the case of the “c 50 nm"aerosol is about 4times higher than that of the “c_120 nm"ones (Table 1). As aresult, the total surface area of the “c_120 nm"particles as anensemble is only 1.5 times higher than that of the “c_50 nm”aerosol. In future experiments, it would be interesting to repeata similar series of experiments by adjusting the particle numberconcentration of the “c_50 nm"aerosol in such a way that thetotal aerosol surface area matches that of the “c _120 nm”aerosol. This would allow investigating whether LDH releasedepends on particle size, when the SOM mass and total aerosolsurface area remain the same. A recent study has reported more pronounced adverseeffects for anthropogenic SOM (naphthalene as precursor)compared to biogenic SOM (B-pinene as precursor). In ourstudy, to compare the cytotoxicity of SOM from the oxidationof a-pinene (biogenic VOC) and mesitylene (anthropogenicVOC), LDH release was plotted as a function of the depositedparticle mass per cm’of cell culture area for series nos. l and 2(Figure 3C). The particle number concentration in series no. 1(a-pinene as SOM precursor) and no. 2 (mesitylene as SOMprecursor) was kept constant at (1.3±0.1)×10% cm-. Inaddition, the GMDmob values of the uncoated soot cores werein both cases very similar (91.5 and 94.3 nm, respectively). Asa result, the deposited mass per cm’ of cell culture area of theuncoated 90 nm soot particles (Table 1) was almost identical(~62 ng/cm²) in both experiments. This implies that theincrease in deposited mass was solely due to the increasingamount of SOM condensing on the soot particle surface. Atfirst sight, it might look as if LDH release is higher for seriesno. 2 (dashed green line, mesitylene as SOM precursor)compared to series no. 1 (dashed blue line, a-pinene as SOMprecursor). However, the p-free air control led to aconsiderably higher LDH release (11.9%, solid green line) inthe first case than in the latter (4.2%, solid blue line).Considering the control tests, there is no significant evidencethat SOM from the oxidation of mesitylene causes higher levelsof cell membrane damage than that from the ozonolysis of apinene under the experimental conditions used in our studyFinally, we evaluated in series no. 6 the dose effect on HBEregarding particle number concentration and SOM particlemass concentration of one type of coated particles, thuseliminating any particle size effect (Figure 3D). SOM_120 hadalmost identical deposited particle number and mass per cmof cell culture as c 135. The LDH release remained constant,showing that the presence/absence of a fully embedded sootcore has little to no effect on cytotoxicity. Moreover, thestepwise aerosol dilution of c_135 and therefore the decreaseof the deposited particle mass from 313 ng/cm’ to 69 ng/cm²(series no. 5, Table 1) resulted in a significant drop in LDHrelease, nearly to the level of the p-free air control. In addition, we assessed the inflammatory response of HBEby semiquantitatively measuring the release of 102 cytokinesand chemokines into the basal media at 24 h post-exposure.The activation of pro-inflammatory mediators may lead to a Figure 4. Screening of cytokines and chemokines involved in cell interaction and adhesion (A, C) and inflammation and immunity (B, D). Foldchanges relative to unexposed cells (incubator control, I.C.). The data are presented as mean values of a minimum of 3 cell cultures and standarderror of the mean (SEM). Statistical analysis was performed using Tukey's multiple comparisons test (*p-value <0.05; **p-value <0.01). systemic inflammatory response. Our results showed that bothHBE exposed to soot coated with SOM from the oxidation ofa-pinene and of mesitylene exhibited pronounced secretion ofcytokines and chemokines compared to unexposed as well asp-free air exposed HBE. Consequently, there is a need todiscriminate cytokines and chemokines regarding theirinvolvement in biological processes concurrently with themagnitude of the effect. This analysis revealed a considerablenumber of deregulated cytokines (mainly upregulation) incomparison to unexposed HBE. The proteome screeningrevealed common signatures of deregulated proteins for bothtypes of coated soot particles (biogenic and anthropogenicVOC) compared to the controls (unexposed and p-free airexposed HBE). The heat map summarizing the biologicalresponse shown in Figure S6 reveals 32 and 30 deregulatedcytokines and chemokines in HBE exposed to soot coated withSOM from the oxidation of a-pinene and mesitylene. Figure 4 depicts the alteration of two distinct pathwaysamong the chemokines and cytokines evaluated in more detail.With both soot types, cytokines and chemokines related to (i)cell interaction and adhesion (Figure 4A,C) as well as (ii)inflammation and immunity (Figure 4B,D) were deregulated.To average the response and to compare a-pinene andmesitylene exposure among the cytokines deregulated for cellinteraction and adhesion, we used Intercellular AdhesionMolecule 1 (ICAM-1), urokinase-type Plasminogen ActivatorReceptor (uPAR), and Extracellular Matrix MetalloproteinaseINducer (EMMPRIN), while for the immunity and inflam-mation pathway, the most common deregulated cytokinesbetween the two different SOM were Dickkopf WNT SignalingPathway Inhibitor 1 (DKK-1), Interleukin-8 (IL-8), MonocyteChemoattractant Protein-1 (MCP-1), Macrophage migrationInhibitory Factor (MIF), and Granulocyte-MacrophageColony-Stimulating Factor (GM-CSF). These data together with the results obtained for cytotoxicity confirm that 30 nmsoot particles caused more pronounced cellular effects than the90 nm soot particles, independently of exposure to biogenic oranthropogenic-derived SOM (Figures 4A,B and S6A,B). Inparticular, the release of cytokines and chemokines related tccell interaction and adhesion as well as those related toinflammation and immunity were statistically significantlydifferent for 30 nm as well as c _35 nm and c_50 nm coatingsfor a-pinene (Figure 4A,B, blue bars). Exposure to mesitylene-derived SOM appears statistically significantly different whencomparing c_35 nm and the maximum coating c _5o nm,providing evidence for substantial interference of the coatedsoot particles (Figure 4A,B, green bars). Conversely, cells exposed to 90 nm soot particles coatedwith mesitylene-derived SOM showed abnormal expression ofmainly the Dickkopf WNT Signaling Pathway Inhibitor 1(Dkk-1) involved in the inflammatory pathway, uponincreasing the coating thickness (Figure S6D). The othercytokines and chemokines related to inflammation were notaffected (Figures 4D and S6C,D). Overall, for the 90 nmcoated particles there was no significant deregulation uponincreasing of the coating (Figure 4C,D) for both a-pinene andmesitylene derived SOM. In line with our experiments, we evaluated the proteomeprofile of HBE from series no. 5, with specific regard tocytokines and chemokines involved in inflammation and celladhesion. We found similar alterations as described for HBEexposed to 30 and 90 nm soot, i.e., upregulation of these twopathways with mainly the same cytokines and chemokines(Figure S7) involved. Despite a decrease of deposited particlesresulting in a significant drop of cytotoxicity, we did not findany significant difference in protein release between pure SOMand decreasing dose (c_135; c_135 _2, and c_135_3; Figure5A,B, Table 1, series no. 5). The results demonstrate that the Figure 5. Screening of cyto- and chemokines involved in cell interaction and adhesion (A) and inflammation and immunity (B) for series no. 5.Fold changes relative to unexposed cells (incubator control, I.C.). The data are presented as mean values of a minimum of three cell cultures andstandard error of the mean (SEM). Statistical analysis was performed using Tukey’s multiple comparisons test (*p-value <0.05; **p-value <0.01). presence of the encapsulated soot core has no effect on theactivation and involvement of inflammatory processes or oncell interactions (Figure SA,B). The experimental setup presented in this study allowed us toaccurately control the amount of SOM condensed on the coreparticles. This enables the reproducible generation of differentmodel aerosols with well-defined SOM mass/total particlemass ratios. The data indicate that the increase in total particlemass concentration alone cannot explain the observed trendsin LDH release. The chemical composition of the coating andits bioavailability seem to be important for cytotoxicity. Therelease of LDH was found to increase with increasing SOMmass/total particle mass ratio, but only when SOM hadcondensed on the outer surface of the soot cores. Moreover,exposure of HBE to soot particles coated with SOM from theoxidation of mesitylene and the ozonolysis of a-pinene resultedin a similar increase in LDH release compared to the controlwith particle-free air, under the experimental conditions usedin this study. The presence of the soot core seemed to havelittle or no effect on cytotoxicity, when it was fully embeddedin SOM. In line with the data on the chemical composition, thebiological results revealed a more pronounced cellular responseupon exposure to 30 nm soot particles from biogenic oranthropogenic derived SOM. These data clearly demonstratean increase in cytotoxicity, as well as of cell remodeling andinflammatory processes upon exposure to the aerosols with 30nm particles but not for the 90 nm series. Our findings highlight the role of SOM for in vitrotoxicological outcomes and suggest that, in addition to totalparticle mass concentration, the percentage of the SOMfraction in PM.s may be a useful metric to characterize thepotential of particles to cause adverse health effects. Futurestudies will focus on a more detailed characterization of themodel aerosols, and postexposure cytotoxic responses will bemeasured using a variety of approaches including cellproliferation assays as well as proteomic and transcriptomicanalyses. ASSOCIATED CONTENT ⑤ Supporting Information The Supporting Information is available free of charge athttps://pubs.acs.org/doi/10.1021/acs.est.2c03692. Additional details of experimental procedures andmethodIsSandadditional data and figures of the physicochemical characterization of the test aerosoland the cell analysis (PDF) AUTHOR INFORMATION Corresponding Authors Marianne Geiser - University of Bern, Bern 3012,Switzerland;@ orcid.org/0000-0002-4288-799S;Email: marianne.geiser@unibe.chKonstantina Vasilatou - Federal Institute of MetrologyMETAS, Bern-Wabern 3003, Switzerland;Email: konstantina.vasilatou@metas.ch Authors Zaira Leni - University of Bern, Bern 3012, Switzerland;D orcid.org/0000-0001-6794-1727 Michaela N. Ess - Federal Institute of Metrology METAS,Bern-Wabern 3003, Switzerland Alejandro Keller - University of Applied SciencesNorthwestern Switzerland, Windisch 5210, Switzerland James D. Allan - University of Manchester, Manchester M139PL, United Kingdom;D orcid.org/0000-0001-6492-4876 Heidi Hellen - Finnish Meteorological Institute, Helsinki00101, Finland Karri Saarnio - Finnish Meteorological Institute, Helsinki00101, Finland Katie R. Williams - National Physical Laboratory,Teddington TW11 0LW, United Kingdom Andrew S. Brown - National Physical Laboratory,Teddington TW11 0LW, United Kingdom Matthias Salathe - Department of Internal Medicine,University of Kansas Medical Center, Kansas City, Kansas66160, United States Nathalie Baumlin - Department of Internal Medicine,University of Kansas Medical Center, Kansas City, Kansas66160, United States Complete contact information is available at:https://pubs.acs.org/10.1021/acs.est.2c03692 Author Contributions Z.L. and M.N.E. contributed equally to this paper. Z.L.performed in vitro cytotoxicity, M.N.E. generated andcharacterized the physical properties of the aerosols, A.Kadvised on aerosol generation and performed data analysis andinterpretation, J.D.A. performed AMS, H.H. and K.S.performed GC-MS, K.R.W. extracted the filters for AMS, A.S.B. performed AMS data interpretation, M.S. and N.B.provided the HBE cells, and K.V. and M.G. designed,coordinated, and supervised the study and performed dataanalysis and interpretation. All authors contributed to thewriting of the manuscript and approved the final version. Notes The authors declare no competing financial interest. FACKNOWLEDGMENTS This study was supported by the Swiss Federal Office for theEnvironment (FOEN), Project No. 17.0094.PJ (fundinggranted to M.G., K.V., and A.K.). This work has also receivedfunding from the 18HLT02 AeroTox project of the EuropeanUnion through the European Metrology Programme forInnovation and Research (EMPIR). EMPIR is jointly fundedby the EMPIR participating countries within EURAMET andthe European Union. Further, M.S. and N.B. acknowledgefunding by the National Institute of Health, NIH (R01HL133240 and R01 HL157942). We thank BarbaraKupferschmid for help with the cell cultures and MarekKaminek (Institute of Anatomy, University of Bern) for cryo-TEM preparation and imaging of the particles. REFERENCES (1) Chen, H.; Kwong, J. C.; Copes, R.; Hystad, P.; van Donkelaar,A.; Tu, K.; Brook, J. R.; Goldberg, M. S.; Martin, R. V.; Murray, B. J.;et al. Exposure to Ambient Air Pollution and the Incidence ofDementia : A Population-Based Cohort Study. Environ. Int. 2017, 108,271-277. (2) Kim, K.-H.; Kabir, E.; Kabir, S. A Review on the Human HealthImpact of Airborne Particulate Matter. Environ. Int. 2015,74,136一143. (3) Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; Ghissassi, F. El;Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.;Straif, K. The Carcinogenicity of Outdoor Air Pollution. Lancet Oncol.2013,14(13),1262-1263. (4) Peters, R.; Ee, N.; Peters, J.; Booth, A.; Mudway, I.; Anstey, K. J.Air Pollution and Dementia: A Systematic Review. J. Alzheimer’s Dis2019, 70, S145-S163. (5) WHO. Preventing disease through healthy environments: a globalassessment of the burden of disease from environmental risks. https://www.who.int/publications/i/item/9789241565196 (accessed Oct. 7,2022). (6) Pope, C. A.; Coleman, N.; Pond, Z. A.; Burnett, R. T. FineParticulate Air Pollution and Human Mortality: 25+ Years of CohortStudies. Environ. Res. 2020, 183, 108924. (7) Cohen, A. J.; Brauer, M.; Burnett, R.; Anderson, H. R.; Frostad,J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona,R.; et al. Estimates and 25-Year Trends of the Global Burden ofDisease Attributable to Ambient Air Pollution: An Analysis of Datafrom the Global Burden of Diseases Study 2015. Lancet 2017, 389(10082),1907-1918. (8) EEA. Europe's air quality status 2022. https://www.eea.europa.eu//publications/status-of-air-quality-in-Europe-2022 (accessed Oct.7,2022). (9) Cassee, F. R.; Heroux, M.-E.; Gerlofs-Nijland, M. E.; Kelly, F. J.Particulate Matter beyond Mass: Recent Health Evidence on the Roleof Fractions, Chemical Constituents and Sources of Emission. Inhal.Toxicol. 2013, 25(14), 802-812. (10) Park, M.; Joo, H. S.; Lee, K.; Jang, M.; Kim, S. D.; Kim, I.;Borlaza, L. J. S.; Lim, H.; Shin, H.; Chung, K. H.; et al. DifferentialToxicities of Fine Particulate Matters from Various Sources. Sci. Rep.2018,8(1),17007. (11) Wyzga, R. E.; Rohr, A. C. Long-Term Particulate MatterExposure: Attributing Health Effects to Individual PM Components.J. Air Waste Manag. Assoc. 2015, 65 (5), 523-543. (12) Daellenbach, K. R.; Uzu, G.; Jiang, J.; Cassagnes, L.-E.; Leni, Z.;Vlachou, A.; Stefenelli, G.; Canonaco, F.; Weber, S.; Segers, A.; et al.Sources of Particulate-Matter Air Pollution and Its Oxidative Potentialin Europe. Nature 2020, S87, 414-419. (13) Delfino, R. J.; Staimer, N.; Tjoa, T.; Arhami, M.; Polidori, A.;Gillen, D. L.; George, S. C.; Shafer, M. M.; Schauer, J. J.; Sioutas, C.Associations of Primary and Secondary Organic Aerosols With Airwayand Systemic Inflammation in an Elderly Panel Cohort. Epidemiology2010,21(6),892-902. (14) Trojanowski, R.; Fthenakis, V. Nanoparticle Emissions fromResidental Wood Combustion:A Critical Literature Review,Characterization and Recommendations. Renew. Sustain. Energy Rev.2019,103,515-528. (15) Gren, L.; Malmborg, V. B.; Jacobsen, N. R.; Shukla, P. C.;Bendtsen, K. M.; Eriksson, A. C.; Essig, Y.J.; Krais, A. M.; Loeschner,K.; Shamun, S. Effect of Renewable Fuels and Intake O2Concentration on Diesel Engine Emission Characteristics andReactive Oxygen Species (ROS) Formation. Atmosphere (Basel)2020,11(6),641. (16) Modrzynska, J.; Berthing, T.; Ravn-Haren, G.; Jacobsen, N. R.;Weydahl, I. K.; Loeschner, K.; Mortensen, A.; Saber, A. T.; Vogel, U.Primary Genotoxicity in the Liver Following Pulmonary Exposure toCarbon Black Nanoparticles in Mice. Part. Fibre Toxicol.2018,15(1),2. (17) Hakkarainen, H.; Salo, L.; Mikkonen, S.; Saarikoski, S.; Aurela,M.; Teinila, K.; Ihalainen, M.; Martikainen, S.; Marjanen, P.; Lepisto,T.; et al. Black Carbon Toxicity Dependence on Particle Coating:Measurements with a Novel Cell Exposure Method. Sci. Total Environ.2022,838,156543. (18) Rohr, A.; McDonald, J. Health Effects of Carbon-ContainingParticulate Matter: Focus on Sources and Recent Research ProgramResults. Crit. Rev. Toxicol. 2016, 46(2),97. (19) Almeida, A. S.; Ferreira, R. M. P.; Silva, A. M. S.; Duarte, A. C.;Neves, B. M.; Duarte, R. M. B. O. Structural Features and Pro-Inflammatory Effects of Water-Soluble Organic Matter in InhalableFine Urban Air Particles. Environ. Sci. Technol. 2020, S4 (2),1082-1091. (20) Offer, S.; Hartner, E.; Di Bucchianico, S.; Bisig, C.; Bauer, S.;Pantzke, J.; Zimmermann, E. J.; Cao, X.; Binder, S.; Kuhn, E.; et al.Effect of Atmospheric Aging on Soot Particle Toxicity in Lung CellModels at the Air-Liquid Interface: Differential Toxicological Impactsof Biogenic and Anthropogenic Secondary Organic Aerosols (SOAs).Environ. Health Perspect. 2022, 130(2),027003. (21) Docherty, K. S.; Stone, E. A.; Ulbrich, I. M.; DeCarlo, P. F.;Snyder, D. C.; Schauer, J. J.; Peltier, R. E.; Weber, R. J.; Murphy, S.M.; Seinfeld, J. H.; et al. Apportionment of Primary and SecondaryOrganic Aerosols in Southern California during the 2005 Study ofOrganic Aerosols in Riverside (SOAR-1). Environ. Sci. Technol. 2008,42(20),7655-7662. (22) Fulcher, M. L.; Gabriel, S.; Burns, K. A.; Yankaskas, J. R.;Randell, S. H. Well-Differentiated Human Airway Epithelial CellCultures. In Methods in Molecular Medicine; Picot, J., Ed.; HumanaPress Inc.: Totowa, NJ, 2005; Vol. 107, pp 183-206. (23) Kunzi, L.; Krapf, M.; Daher, N.; Dommen, J.; Jeannet, N.;Schneider, S.; Platt, S.; Slowik, J. G.; Baumlin, N.; Salathe, M.; et al.Toxicity of Aged Gasoline Exhaust Particles to Normal and DiseasedAirway Epithelia. Sci. Rep. 2015, S, 11801. (24) Geiser, M.; Jeannet, N.; Fierz, M.; Burtscher, H. EvaluatingAdverse Effects of Inhaled Nanoparticles by Realistic in VitroTechnology. Nanomaterials 2017, 7 (2), 49. (25) Ess, M. N.; Vasilatou, K. Characterization of a New MiniCASTwith Diffusion Flame and Premixed Flame Options: Generation ofParticles with High EC Content in the Size Range 30 Nm to 200 Nm.Aerosol Sci. Technol. 2019, 53 (1), 29-44. (26) Ess, M. N.; Berto, M.; Irwin, M.; Modini, R. L.; Gysel-Beer, M.;Vasilatou, K. Optical and Morphological Properties of Soot ParticlesGenerated by the MiniCAST 5201 BC Generator. Aerosol Sci. Technol.2021, 55(7),828-847. (27) Keller, A.; Burtscher, H. A Continuous Photo-Oxidation FlowReactor for a Defined Measurement of the SOA Formation Potentialof Wood Burning Emissions. J. Aerosol Sci. 2012, 49 (12),9-20. (28) Ess, M. N.; Berto, M.; Keller, A.; Gysel-Beer, M.; Vasilatou, K.Coated Soot Particles with Tunable, Well-Controlled PropertiesGenerated in the Laboratory with a MiniCAST BC and a Micro SmogChamber. J. Aerosol Sci. 2021, 157,105820. (29) Kalbermatter, D. M.; Mocnik, G.; Drinovec, L.; Visser, B.;Rohrbein, J.; Oscity, M.; Weingartner, E.; Hyvarinen, A.-P.; Vasilatou,K. Comparing Black-Carbon- and Aerosol-Absorption-MeasuringInstruments - a New System Using Lab-Generated Soot Coatedwith Controlled Amounts of Secondary Organic Matter. Atmos. Meas.Technol. 2022, 15 (2),561-572. (30) Jeannet, N.; Fierz, M.; Kalberer, M.; Burtscher, H.; Geiser, M.Nano Aerosol Chamber for In-Vitro Toxicity (NACIVT) Studies.Nanotoxicology 2015,9(1),34-42. (31) Kesselmeier, J.; Staudt, M. An Overview on Emission,Physiology and Ecology.J. Atmos. Chem. 1999, 33, 23-88. (32) Zeng, P.; Guo, H.; Cheng, H.; Wang, Z.; Zeng, L.; Lyu, X.;Zhan, L.; Yang, Z. Aromatic Hydrocarbons in Urban and SuburbanAtmospheres in Central China: Spatiotemporal Patterns, SourceImplications, and Health Risk Assessment. Atmosphere (Basel) 2019,10(10), S65. (33) Keller, A.; Kalbermatter, D. M.; Specht, P.; Steigmeier, P.;Resch, J; Kalberer, M.; Hammer, T.; Vasilatou, K.; Wolfer, K. TheOrganic Coating Unit, an All-in-One System for ReproducibleGeneration of Secondary Organic Matter Aerosol. Aerosol Sci. Technol.2022,56(10), 947-958. (34) DeCarlo, P. F.; Kimmel, J. R.; Trimborn, A.; Northway, M. J.;Jayne, J. T.; Aiken, A. C.; Gonin, M.; Fuhrer, K.; Horvath, T.;Docherty, K. S.; et al. Field-Deployable, High-Resolution, Time-of-Flight Aerosol Mass Spectrometer. Anal. Chem. 2006, 78 (24),8281-8289. (35) Schmid, A.; Sutto, Z.; Schmid, N.; Novak, L.; Ivonnet, P.;Horvath, G.; Conner, G.; Fregien, N.; Salathe, M. Decreased SolubleAdenylyl Cyclase Activity in Cystic Fibrosis Is Related to DefectiveApical Bicarbonate Exchange and Affects Ciliary Beat FrequencyRegulation. J. Biol. Chem. 2010, 285 (39),29998-30007. (36) Kuinzi, L.; Mertes, P.; Schneider, S.; Jeannet, N.; Menzi, C.;Dommen, J.; Baltensperger, U.; Prevot, A. S. H.; Salathe, M.;Kalberer, M.; et al. Responses of Lung Cells to Realistic Exposure ofPrimary and Aged Carbonaceous Aerosols. Atmos. Environ. 2013, 68,143-150. (37) Delaval, M.; Egli, D.; Schiipfer, P.; Benarafa, C.; Geiser, M.;Burtscher, H. Novel Instrument to Generate Representative E-Cigarette Vapors for Physicochemical Particle Characterization andin-Vitro Toxicity. J. Aerosol Sci. 2019, 129, 40-52. (38) Burtscher, H. Physical Characterization of ParticulateEmissions from Diesel Engines: A Review. J. Aerosol Sci. 2005, 36(7),896-932. (39) Rana, S.; Saxena, M. R.; Maurya, R. K. A Review onMorphology, Nanostructure, Chemical Composition, and NumberConcentration of Diesel Particulate Emissions. Environmental Scienceand Pollution Research. 2022, 29,15432-15489. (40) Giechaskiel, B.; Mamakos, A.; Andersson, J.; Dilara, P.; Martini,G.; Schindler, W.; Bergmann, A. Measurement of AutomotiveNonvolatile Particle Number Emissions within the EuropeanLegislative Framework: A Review. Aerosol Sci. Technol. 2012,46(7),719-749. (41) Samaras, Z.; Rieker, M.; Papaioannou, E.; van Dorp, W. F.;Kousoulidou, M.; Ntziachristos, L.; Andersson, J.; Bergmann, A.;Hausberger, S.; Keskinen, J.; et al. Perspectives for Regulating 10 NmParticle Number Emissions Based on Novel Measurement Method-ologies. J. Aerosol Sci. 2022, 162,105957. (42) Di Iorio, S.; Catapano, F.; Magno, A.; Sementa, P.; Vaglieco, B.M. Investigation on Sub-23 nm Particles and Their Volatile OrganicFraction (VOF) in PFI/DI Spark Ignition Engine Fueled withGasoline, Ethanol and a 30% v/v Ethanol Blend. J. Aerosol Sci. 2021,153,105723. (43) Donahue, N. M.; Chuang, W.; Epstein, S. A.; Kroll, J. H.;Worsnop, D. R.; Robinson, A. L.; Adams, P. J; Pandis, S. N. Why DoOrganic Aerosols Exist? Understanding Aerosol Lifetimes Using theTwo-Dimensional Volatility Basis Set. Environ. Chem. 2013, 10 (3),151-157. (44) Zhou, S.; Shiraiwa, M.; McWhinney, R. D.; Poschl, U.; Abbatt,J. P. D. Kinetic Limitations in Gas-Particle Reactions Arising fromSlow Diffusion in Secondary Organic Aerosol. Faraday Discuss. 2013,165,391-406. (45) Miller, F. J.; Asgharian, B.; Schroeter, J. D.; Price, 0.Improvements and Additions to the Multiple Path Particle DosimetryModel. J. Aerosol Sci.2016, 99, 14-26. (46) Maricq, M. M. Examining the Relationship between BlackCarbon and Soot in Flames and Engine Exhaust. Aerosol Sci. Technol.2014,48(6),620-629. (47) Dat, N.-D.; Chang, M. B. Science of the Total EnvironmentReview on Characteristics of PAHs in Atmosphere, AnthropogenicSources and Control Technologies. Sci. Total Environ. 2017, 609,682-693. (48) Vestenius, M.; Leppanen, S.; Anttila, P.; Kyllonen, K.; Hatakka,J; Hellen, H.; Hyvarinen, A.-P.; Hakola, H. Background Concen-trations and Source Apportionment of Polycyclic Aromatic Hydro-carbons in South-Eastern Finland. Atmos. Environ. 2011, 45(20),3391-3399. (49) Aiken, A. C.; DeCarlo, P. F.; Kroll, J. H.; Worsnop, D. R.;Huffman, J. A.; Docherty, K. S.; Ulbrich, I. M.; Mohr, C.; Kimmel, J.R.; Sueper, D.; et al. O/C and OM/OC Ratios of Primary, Secondary,and Ambient Organic Aerosols with High-Resolution Time-of-FlightAerosol Mass Spectrometry. Environ. Sci. Technol. 2008, 42(12),4478-4485. (50) Chen, Q.; Heald, C. L.; Jimenez, J. L.; Canagaratna, M. R.;Zhang Q.; He, L.; Huang, X.; Campuzano-Jost, P; Palm, B. B.;Poulain, L.; et al. Elemental Composition of Organic Aerosol: TheGap between Ambient and Laboratory Measurements. Geophys. Res.Lett. 2015, 42, 4182-4189. (51) Ortega, A. M.; Hayes, P. L.; Peng, Z.; Palm, B. B.; Hu, W.; Day,D. A.; Li, R.; Cubison, M. J.; Brune, W. H.; Graus, M.; et al. Real-Time Measurements of Secondary Organic Aerosol Formation andAging from Ambient Air in an Oxidation Flow Reactor in the LosAngeles Area. Atmos. Chem. Phys. 2016,16(11),7411-7433. (52) Shah, R. U.; Robinson, E. S.; Gu, P.; Robinson, A. L.; Apte,J.S.; Presto, A. A. High-Spatial-Resolution Mapping and SourceApportionment of Aerosol Composition in Oakland, California,Using Mobile Aerosol Mass Spectrometry. Atmos. Chem. Phys. 2018,18(22), 16325-16344. (S3) Hallquist, M.; Wenger, J. C.; Baltensperger, U.; Rudich, Y.;Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N. M.; George, C.;Goldstein, A. H.; et al. The Formation, Properties and Impact ofSecondary Organic Aerosol: Current and Emerging Issues. Atmos.Chem. Phys. 2009,9,5155-5236. (54) Bahreini, R.; Keywood, M. D.; Ng, N. L.; Varutbangkul, V.Gao, S.; Flagan, R. C.; Seinfeld, J. H.; Worsnop, D. R.; Jimenez, J. L.Measurements of Secondary Organic Aerosol from Oxidation ofCycloalkenes, Terpenes, and m-Xylene Using an Aerodyne AerosolMass Spectrometer. Environ. Sci. Technol. 2005, 39 (15), 5674-5688. (55) Pei, X.; Hallquist, M.; Eriksson, A. C.; Pagels, J.; Donahue, N.M.; Mentel, T.; Svenningsson, B.; Brune, W.; Pathak, R. K.Morphological Transformation of Soot: Investigation of Micro-physical Processes during the Condensation of Sulfuric Acid andLimonene Ozonolysis Product Vapors. Atmos. Chem. Phys. 2018,18,9845-9860.
确定






还剩9页未读,是否继续阅读?
产品配置单
中国格哈特为您提供《气溶胶颗粒中多环芳烃PAHs的测定》,该方案主要用于废气中有机污染物检测,参考标准--,《气溶胶颗粒中多环芳烃PAHs的测定》用到的仪器有格哈特全自动快速溶剂萃取仪Sox416、格哈特快速干燥仪STL56、格哈特强力高重现振荡器LS500/RO500、德国加液器MM、玻璃滤筒、棕色避光防紫外线萃取杯、滤纸筒
相关方案
更多
该厂商其他方案
更多