混合Na-A沸石氧切薄膜复合纳滤膜去除Cr(III)三价铬Hybrid Na-A Zeolite Oxycut Residue Thin Film Composite Nanofiltration Membrane for Cr (Iii) Removal
使用格哈特振荡器100rpm振荡24小时
方案详情

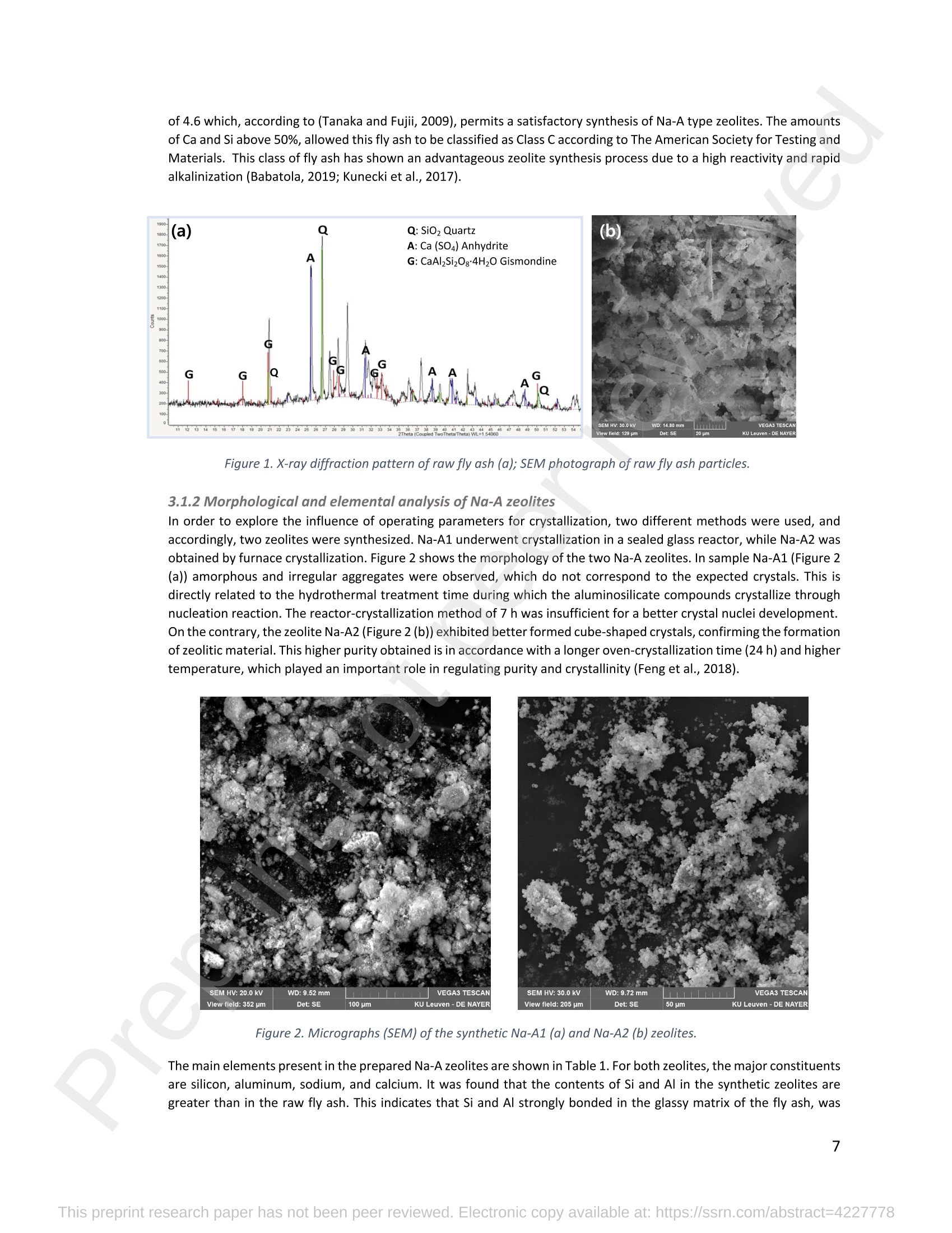
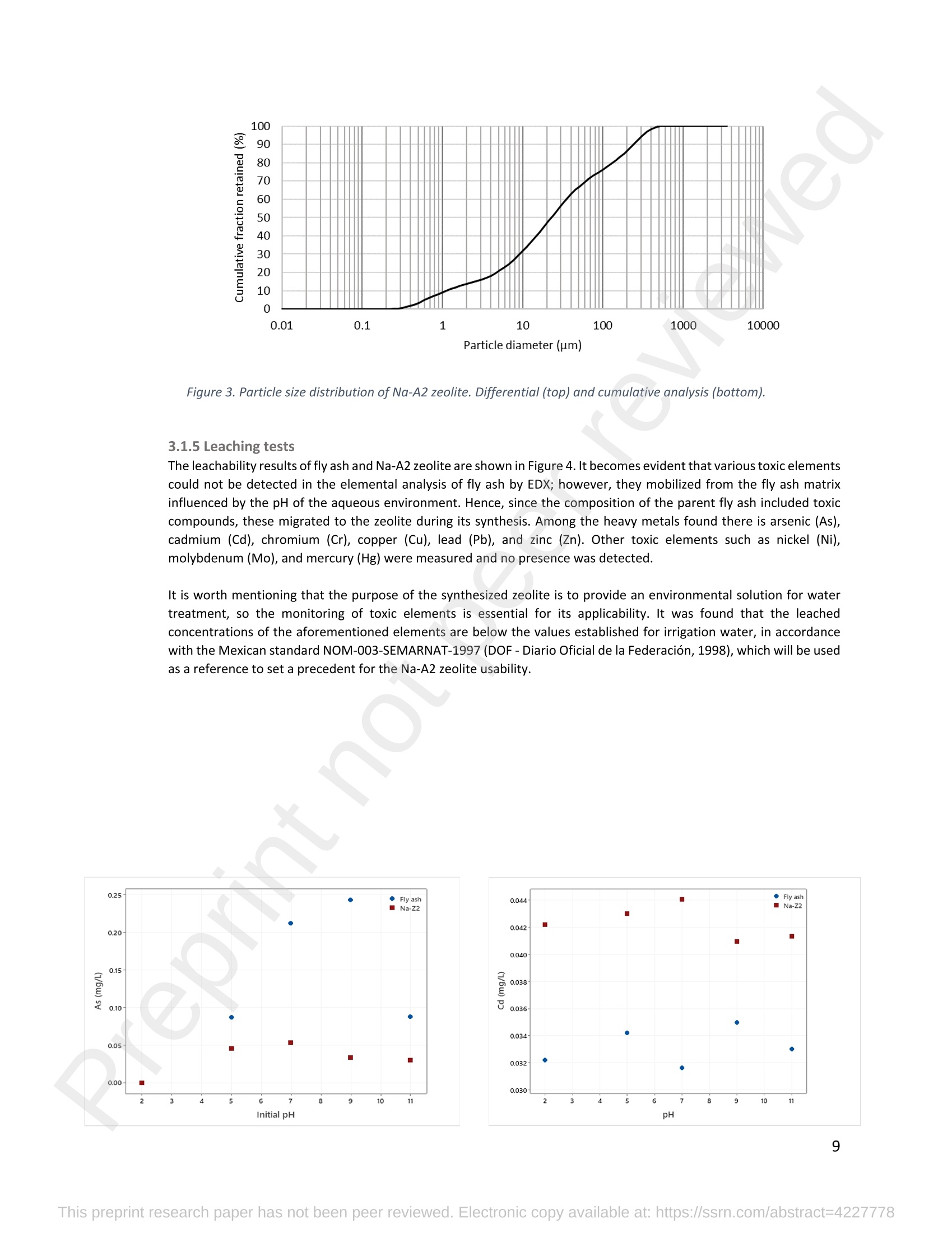
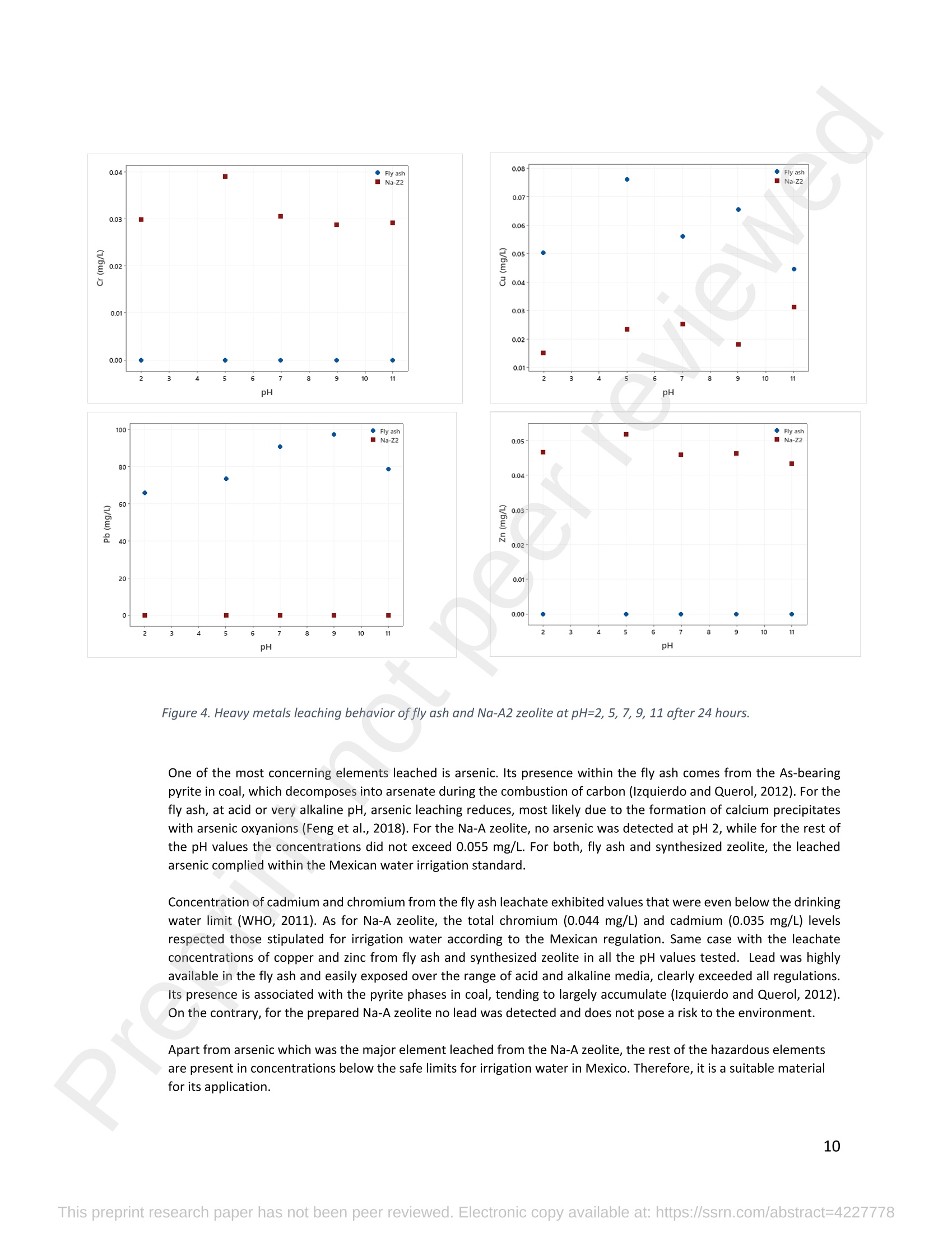
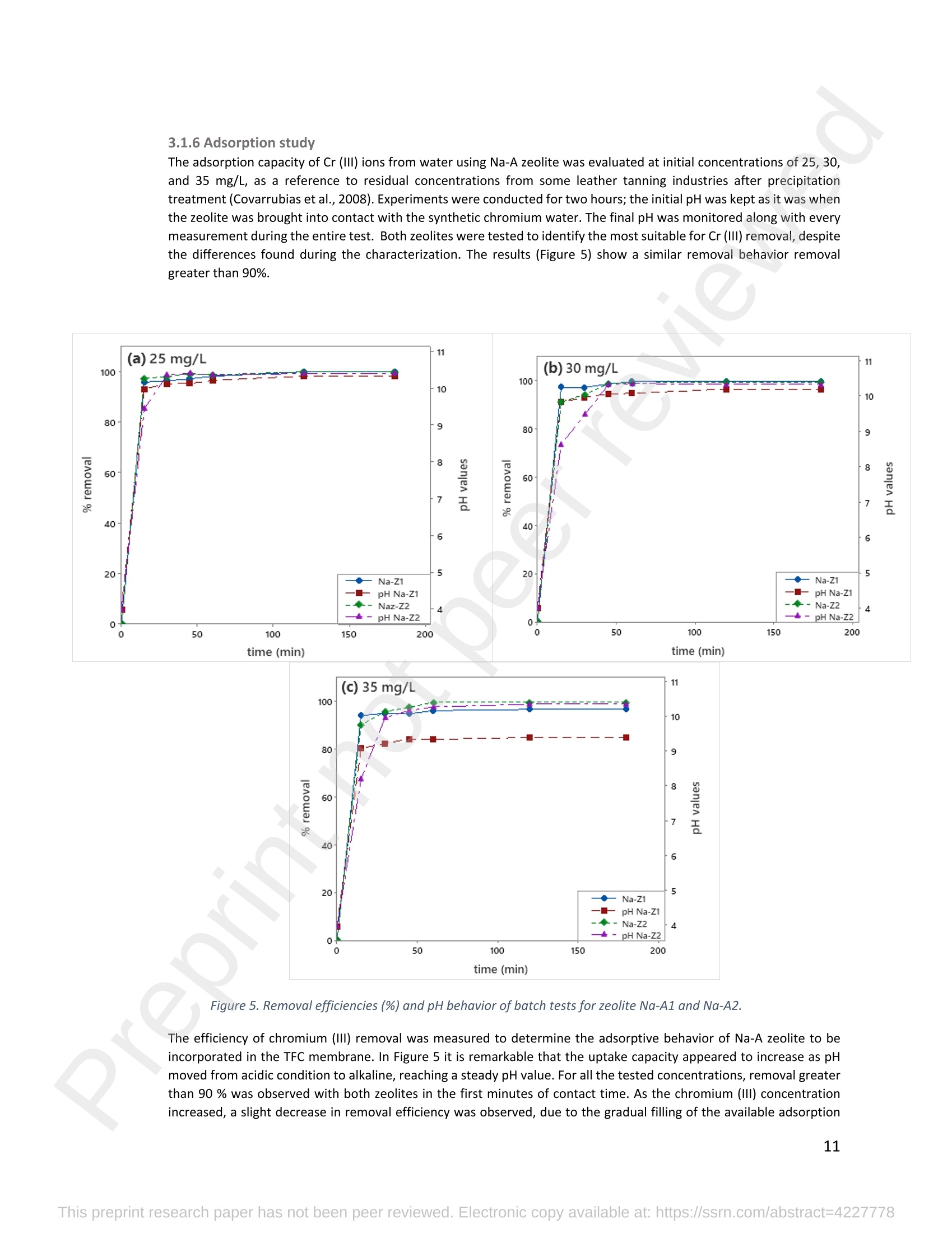
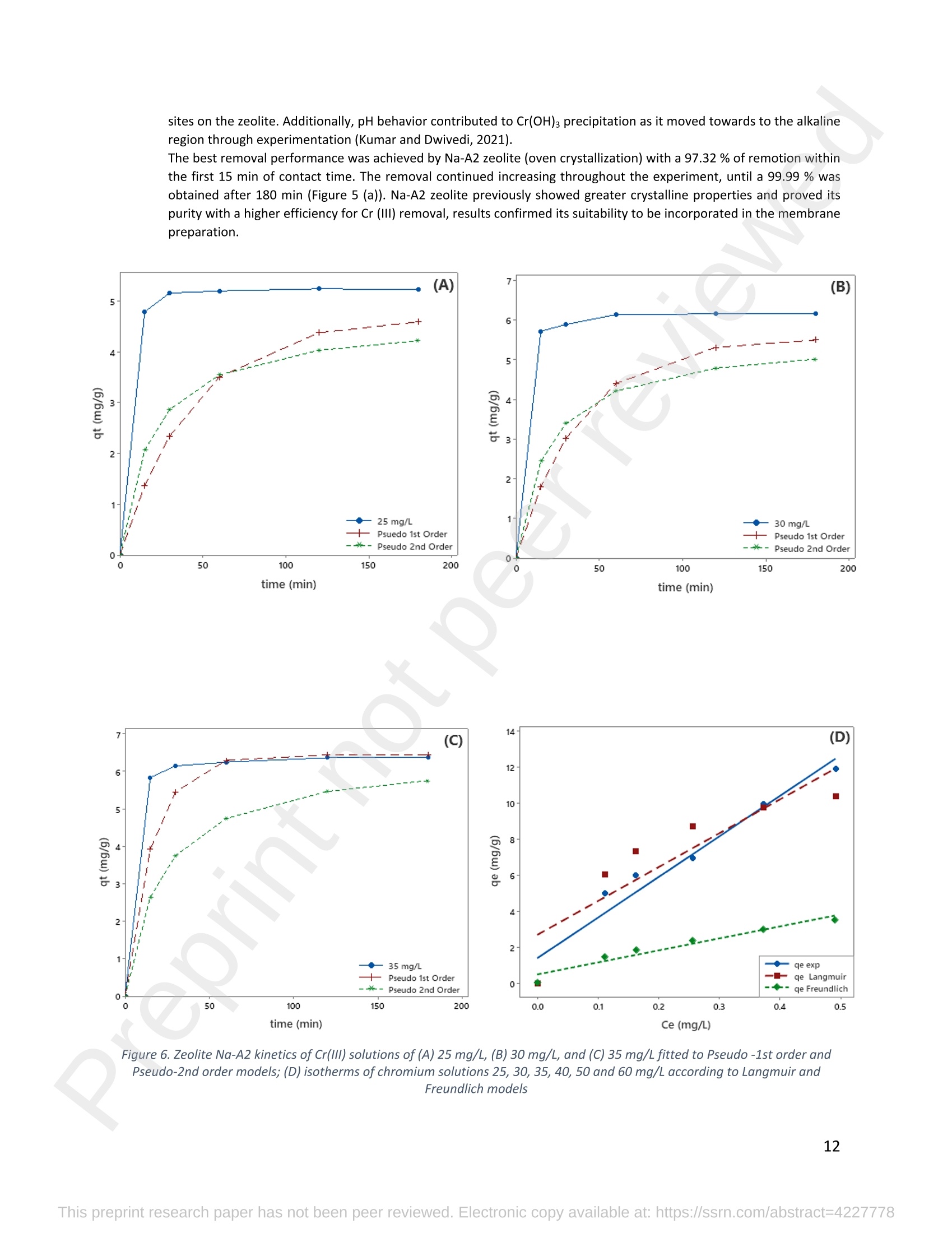
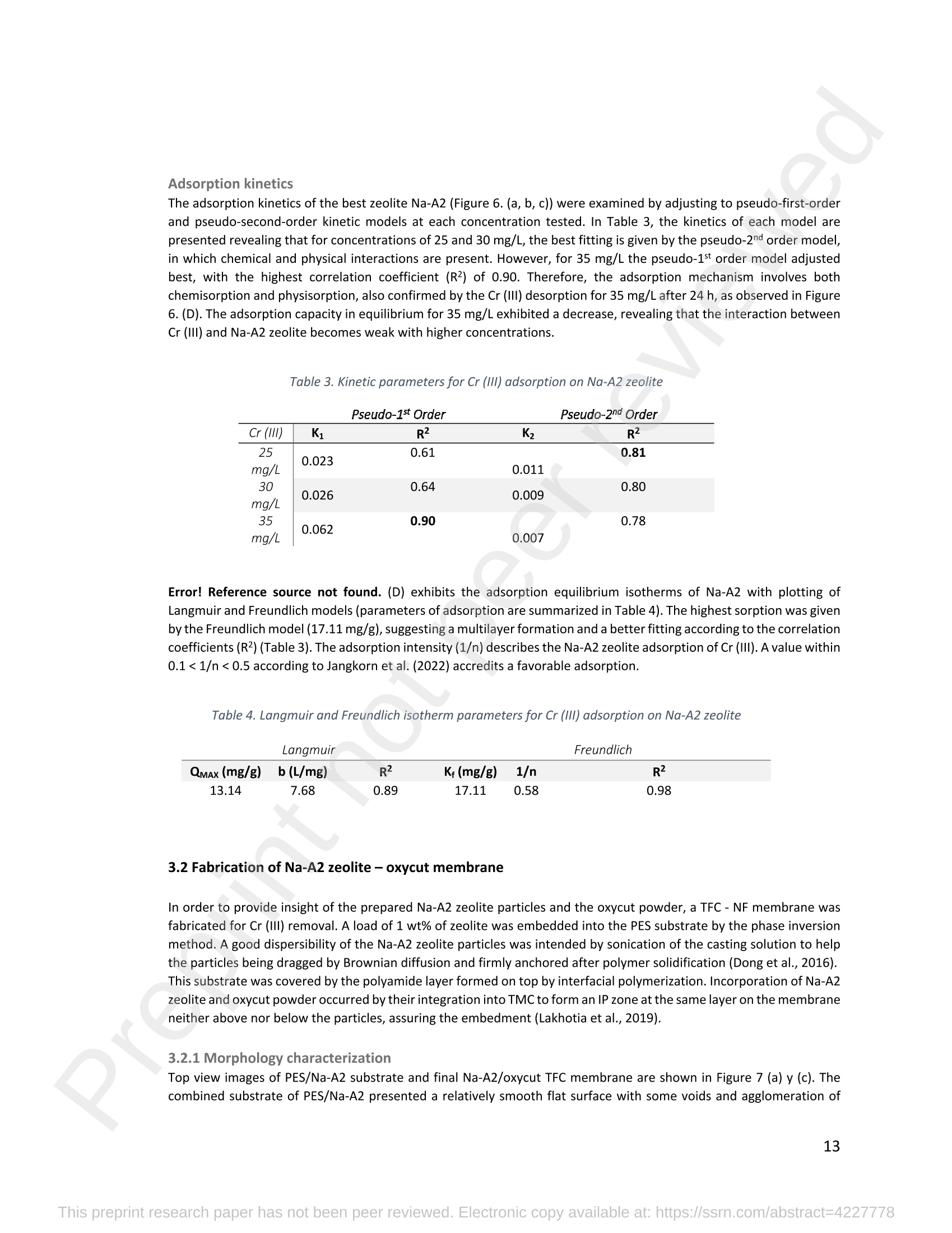
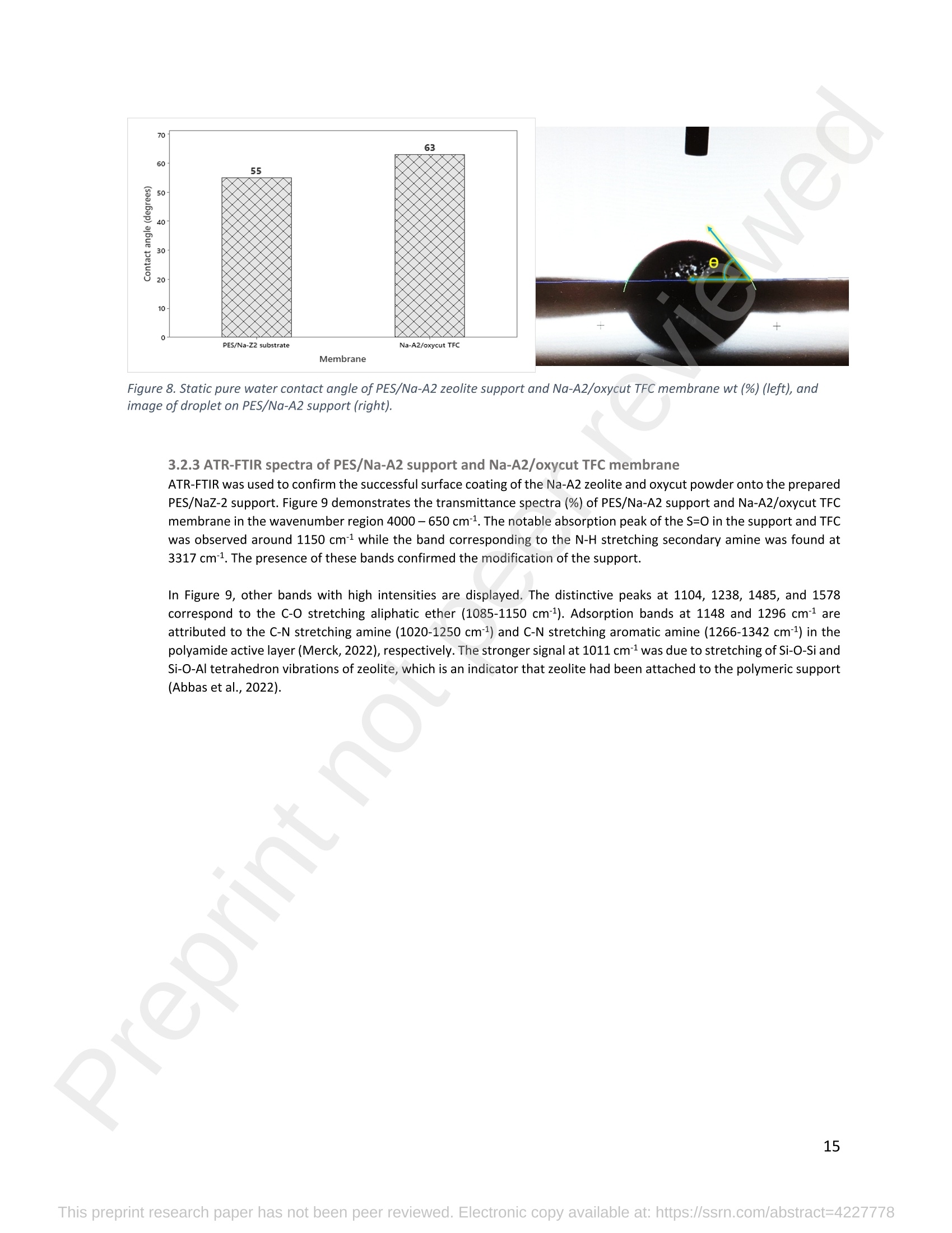
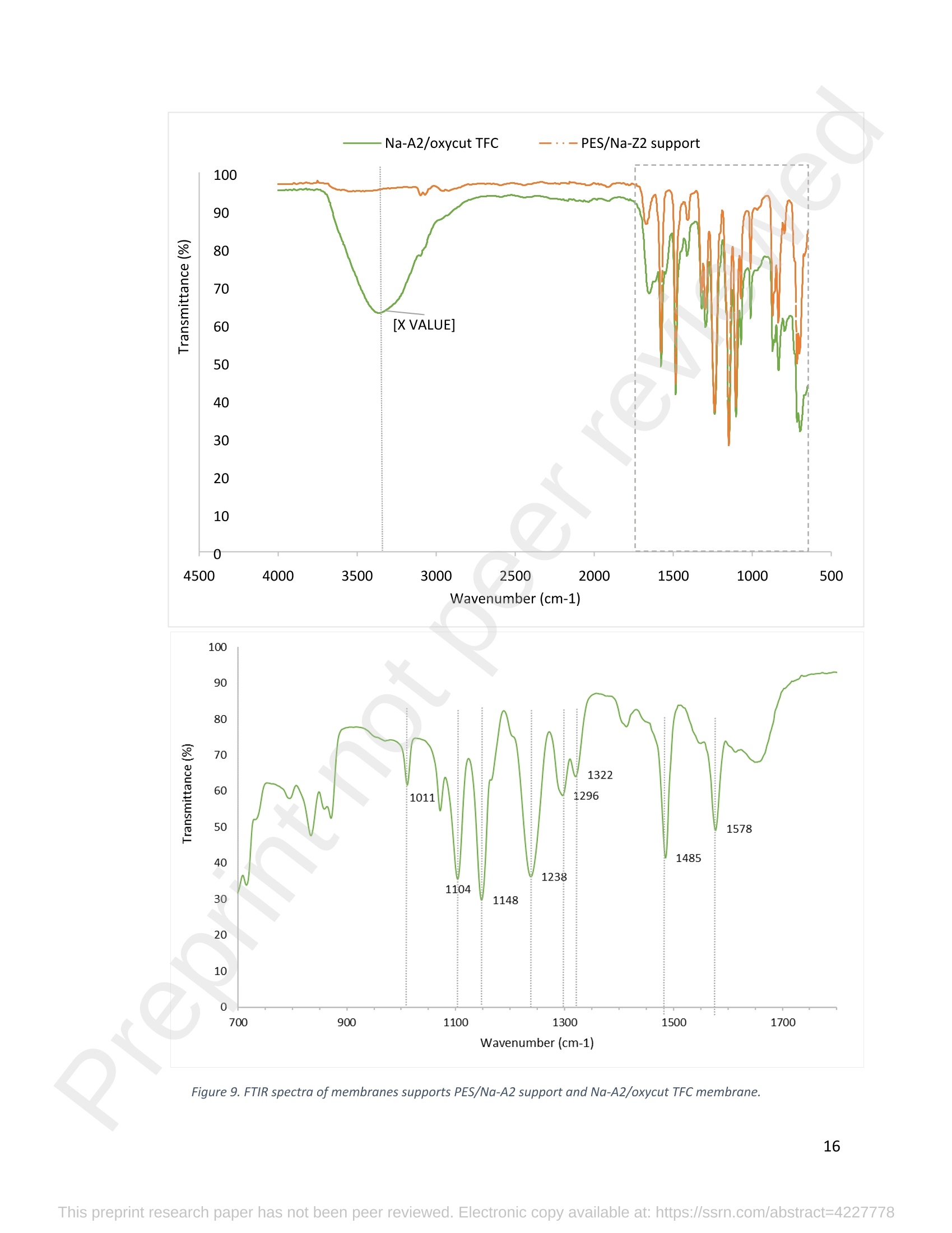
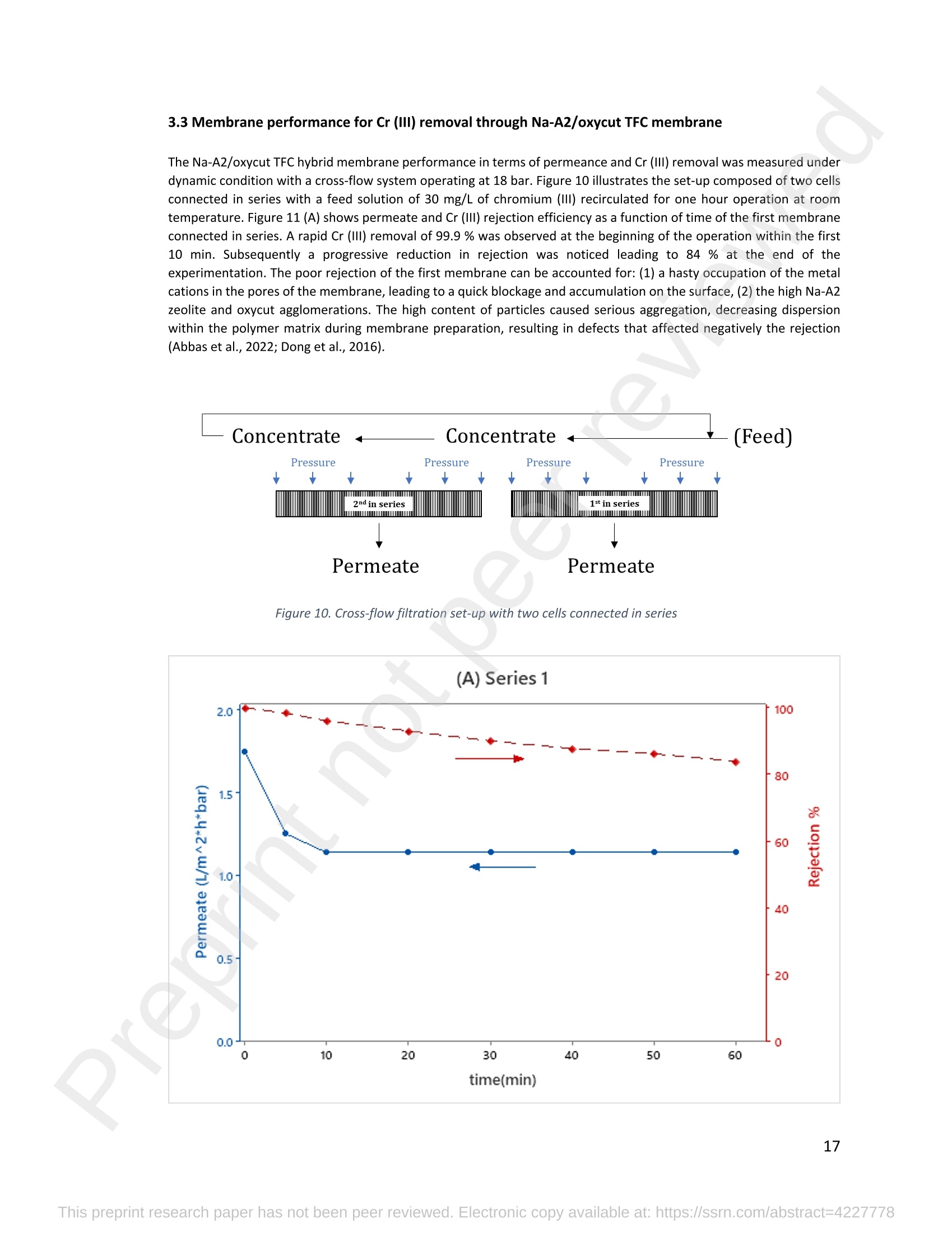
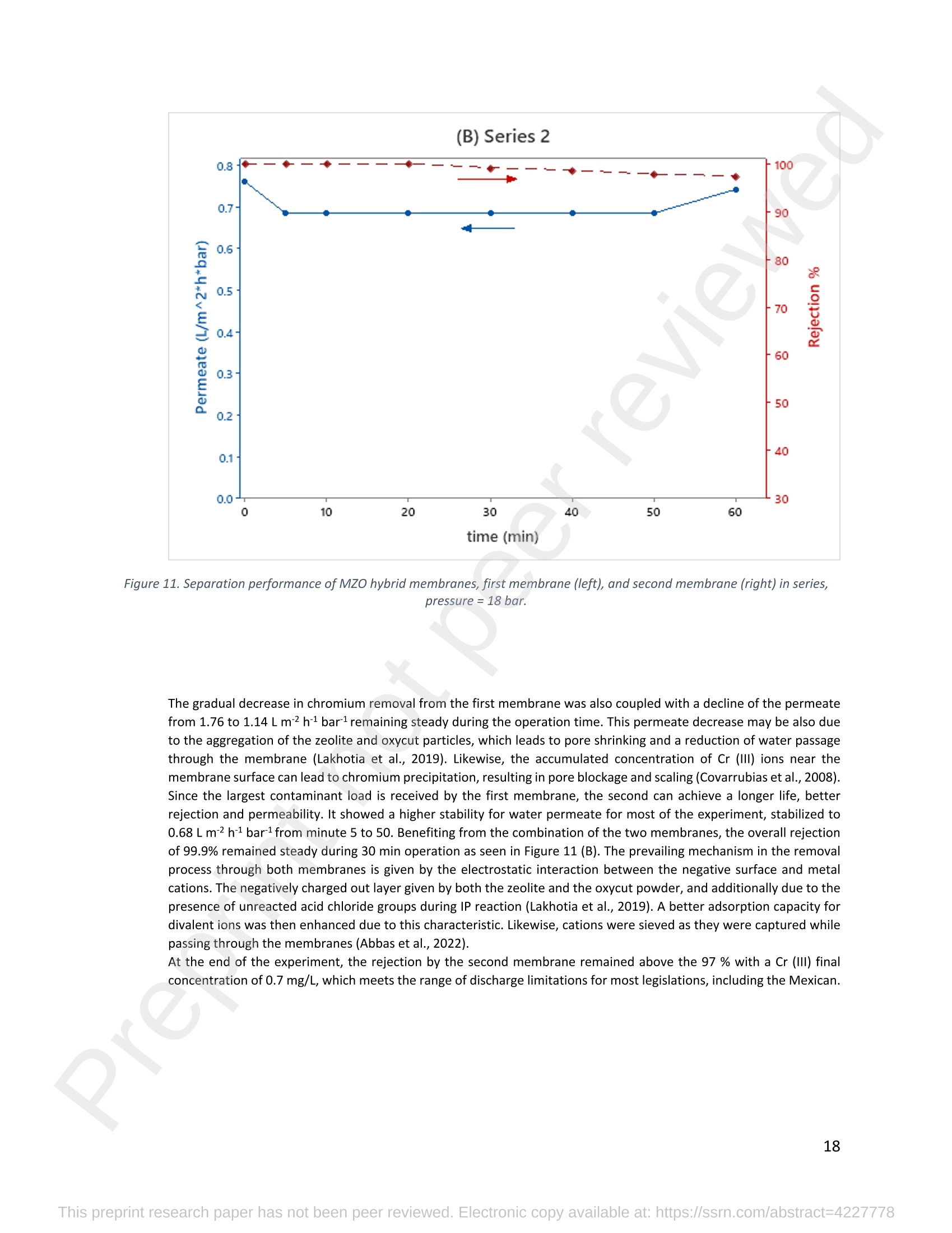
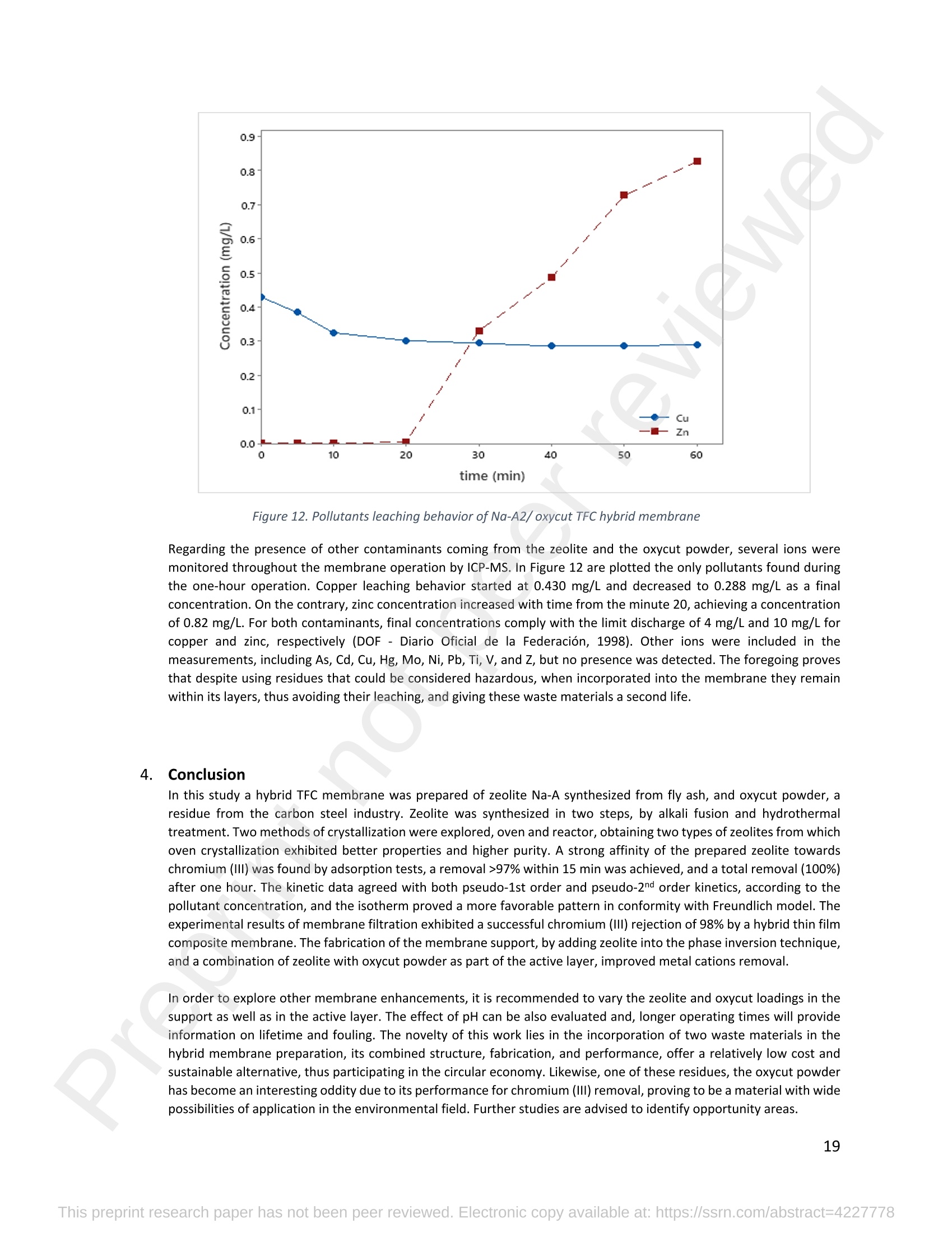
混合Na-A沸石氧切薄膜复合纳滤膜去除Cr(III)三价铬Hybrid Na-A Zeolite Oxycut Residue Thin Film Composite Nanofiltration Membrane for Cr (Iii) Removal2.3.2 Leaching tests A heavy metal leaching test from fly ash and Na-A zeolite was investigated in aqueous solutions in both acid and alkaline media, adjusted by hydrochloric acid and sodium hydroxide solutions. Suspensions containing 0.05 g of zeolite and fly ash, in 10 ml of solution at pH values of 2, 5, 7, 9, and 11, were agitated for 24 h at 100 rpm (Gerhardt Laboshake). The pH of the leachate was measured using a pH meter (Mettler-Toledo), and samples were filtered and analyzed by ICP-MS (Perkin Elmer ICP-MS, Optima 8300). 使用格哈特振荡器100rpm振荡24小时2.3.3 Adsorption studies of Cr (III) Batch tests were conducted to determine the metal adsorption by shaking at 100 rpm (Gerhardt Laboshake), 0.05 g of zeolite, and 10 ml of Cr3+ solutions with different concentrations. The experiments were performed in closed flasks at room temperature (25 +- 2⁰ C) in duplicate, pH values (Mettler Toledo, pH-meter) were monitored at each sample withdrawal. Isotherm data were obtained after reaching an equilibrium time of 24 h. All samples were filtered using a membrane of 0.45 m pore size prior to measurements of Cr3+ concentrations by ICP-MS (Perkin Elmer ICP-MS, Optima 8300). The adsorption capacity (qe) of zeolite was calculated by Eq. (1) where C0 and Cf are the initial and final metal ion concentrations, respectively, Va is the aqueous phase volume and M is the weight of zeolite used. 混合Na-A沸石/氧切薄膜复合纳滤膜去除Cr(III)三价铬 Hybrid Na-A zeolite/oxycut residue thin film composite nanofiltration membrane for Cr (III) removal Julieta García-Chirinoa*, Alicia Dáder Jiméneza, Bart Van der Bruggena a Department of Chemical Engineering, KU Leuven, Celestijnenlaan 200F, B-3001 Leuven, Belgium 比利时鲁汶大学化学工程系 *Corresponding author Tel.: +32 474080252 E-mail: julieta.garciachirino@kuleuven.be Hybrid Na-A zeolite/oxycut residue thin film composite nanofiltration membrane for Cr (III) removal Abstract: An alternative approach to fabricating a hybrid thin-film composite (TFC) nanofiltration membrane involving industrial residues is reported in this study. Na-A zeolite was synthesized from coal fly ash by alkali fusion and hydrothermal treatment., to be embedded into a polyethersulfone (PES) support by phase inversion. Oxycut powder, an unexplored carbon steel residue, and prepared zeolite were incorporated in the polyamide layer by interfacial polymerization. Zeolite was characterized via energy-dispersive X-ray spectroscopy (EDX), scanning electron microscopy (SEM), and leaching tests. Zeolite adsorption capacity achieved a total removal (100%) of chromium (III), experimental data were reliably fitted by Freundlich adsorption isotherms. The theoretical maximum adsorption capacity was 17.11 mg/g, confirming zeolite suitability as membrane filler. Zeolite was then impregnatedwithinthePESmembranematrix,followedbyamixtureofoxycut-zeoliteincorporatedintheinterfacial polymerization to form the selective layer. The effect of the modified membrane and the particles addition was evaluated through scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy (FT-IR) and water contact angle measurements. The hybrid membrane performance for chromium (III) removal was tested in a cross-flow setup with two membranes in series. Results disclosed a chromium (III) rejection of 98%, higher than the obtained by previous related studies, which neither incorporated zeolite in both support and active layers nor contained oxycut powders. Considering the nature of the wastes, the leaching of other contaminants was constantly monitored; the results exhibited no presence of other pollutants in the permeate, proving the success in the applicability of these residues. Keywords: waste valorization, thin film composite membrane, zeolite synthesis, chromium (III) removal, oxycut powder. 1. Introduction Chromium-containing wastewater is one of the major concerns of the last decade, its discharge at high concentrations into the environment poses life-threatening consequences (Ali et al., 2019). Significant environmental and health problems are directly related to chromium compounds widely used in industrial and manufacturing processes related to the production of chemicals, electroplating, textile dyeing, leather tanning, wood preservation, metallurgy, and minerals, among others (Kaźmierczak et al., 2021; Prasad et al., 2021). Chromium occurs in different oxidation states ranging from Cr (II) to Cr (VI), the most stable and common forms being Cr(III) and Cr(VI) (Sharma et al., 2021). Hexavalent chromium is well-known as a top priority pollutant due to its high toxicity, solubility, and mobility. It is classified as a human carcinogen according to the IARC (International Agency for Research on Cancer) (Siciliano et al.,2021; Qiu et al., 2020). On the contrary, Cr (III) is 500 times less harmful than Cr (VI) and plays an essential role as a micronutrient for the human body in metabolic activities and biochemical processes (Ali et al., 2019; Rojas-Romero et al.,2015).Nevertheless,atelevatedconcentrationsandhighexposureconditions,ithasgenotoxiceffects, mutagenicity, and causes DNA cell damage. Furthermore, inorganic and organic Cr (III) complexes can be oxidized to virulent Cr (VI) (Eastmond et al., 2008; Song et al., 2022). In the understanding that it is a less toxic compound, Cr (III) utilization in the industry has considerably increased. The release of wastewaters with concentrations between 3,000and 10,000 mg/L has been reported (Bonola et al., 2022). Hence, the accumulation of large amounts in soils, food crops, and irrigation water has been exacerbated and rapidly reached populations (Chow et al., 2018; Ramalingam et al.,2021). International standards suggest a maximum permissible concentration for total chromium of 0.5 – 1.5 ppm (Tumolo et al., 2020). In order to pursue these values, many treatment methods are available. Among them, nanofiltration (NF) membrane technologies have attracted wide attention due to their variety of applications, high removal efficiencies, cost-effectiveness, and environmental friendship approach (Li et al., 2021; Yang et al., 2017; Xu et al., 2022). Many NF membranes are fabricated as thin-film composites (TFC), formed of a film composed of two or more layered materials: a polyester non-woven fabric that acts as the structural support, a porous interlayer, and an ultrathin polyamide layer (Alsayed and Ashraf, 2021; Nambi Krishnan et al., 2022). There are many ways to prepare a TFC membrane such as interfacial polymerization (IP), solution mixing, coating technology, and post-modification treatment (Liu et al., 2019). Recently, interfacial polymerization has become the most competitive method due to its stability, industrialization expectations, and various applications (Li et al., 2022). Ounifi et al. (2022) successfully removed malachite green and Congo red by preparing a NF-TFC of synthetic cellulose acetate as support, and IP with methylamine and trimesoyl chloride (TMC). Rejection reached 89% for malachite green and 85% for Congo red dye (Ounifi et al., 2022) . Rahimi et al. (2022) reported a 94% removal of copper with a TFC fabricated with cellulose/copper oxide nanoparticles. Several studies have investigated the addition of these kinds of nanomaterials for the creation of thin-film (TFN) membranes. This method disperses the nanoparticles into an aqueous or organic phase, to embed them into the polyamide layer during the IP process to improve the separation performance (Yang et al., 2017; Wu et al., 2021). Graphene oxide nanoparticles were added to the preparation of a TFN membrane, achieving a 97% cobalt removal (Lari et al., 2022). Wu et al. (2021) incorporated zeolite imidazolate nanoparticles, which yielded an increased negative charge, higher water permeability, and enhanced rejection of Na2SO4. The incorporation of expensive commercial nanoparticles (NPs) such as TiO2, SiO2, AgNPs, (Zhao et al., 2021) can be changed by preparation of these materials by other sources such as residues. Spherical silica NPs were prepared to be incorporated into the active layer, increasing the water permeability and rejection of salts (Yin et al., 2012). Abbas et al. (2022) synthesized NaY zeolite by a hydrothermal method, to impregnate it within the polyethersulfone matrix membrane. This preparation method yielded a removal rate of 90.2% of isotope Cesium-137 from aqueous radioactive waste. The encouraging performance attained by some materials like zeolites makes their preparation from residues an interesting asset in terms of a circular economy, in which the value of products and materials is kept inside the economy as much as possible, and waste generation is minimized (Macedonio and Drioli, 2022). During the last decades, a number of alternative materials for water treatment have been studied. Minerals, agricultural waste, industrial byproducts, organic or biological compounds (Mercado-Borrayo et al., 2018) have attracted the attention due to their local availability, low cost and applicability. Waste management has been a crucial challenge, mostly in low-income countries, due to the associated treatment costs and lack of regulations (Kwikima et al., 2021). Thus, valorization of waste materials needs further exploration in order to encourage a wider use. 2. Experimental: materials and methods 2.1 Materials Non-woven polypropylene/polyethylene fabric (Novatex-2471) with a thickness of 0.18 mm (Freudenberg Group, Germany) was used as membrane support. Polyethersulfone (PES, BASF Co. Ltd., Germany). N, N-Dimethylacetamide (DMAC, 99.5%, Fischer Scientific), and polyvinylpyrrolidone (PVP, Merck), were used for the preparation of the phase inversion substrate. M -phenylenediamine (MPD, 99%, Merck), trimesoyl chloride (TMC, 98%, Sigma-Aldrich), n-hexane (99%, Chem-Lab Analytical) were used to create the polyamide layer by interfacial polymerization. Chromium (III) oxide (98%, Merck) was used for preparing solutions to test the Na-A zeolite and membrane performance. Deionized water (DI) was used in all experiments. A sample of fly ashes from a cogeneration plant was retrieved from a waste recovery company located in the south of Belgium. Hydrochloric acid (HCl, 37%, Chem-Lab Analytical), sodium hydroxide pellets (NaOH, 98%, Thermo Fischer), and sodium aluminate (NaAlO2, Thermo Fischer) were used for zeolite synthesis. Oxycut powder is a waste from the carbon steel industry, generated during the scrap cutting by an oxygen lancet. The resulting powders are either reincorporated into the scrap melting process or discarded, depending on each country legislation. Since this material has not yet been studied, this work seeks to investigate its possible applications. This sample was produced in Monterrey, Mexico. Table 1 shows the bulk chemical composition according to XRF, in which a high amount of iron oxides is noticeable. Therefore, it is expected to have a behavior similar to that of commercial iron oxides in the removal of contaminants. Its incorporation into the membrane aims to prove its usefulness and provide a waste valorization. The presence of other contaminants was monitored from the effluent produced by the membrane. Table 1. XRF chemical composition of Oxycut powder (wt%) Chemical Fe2O3 ZnO MnO SO3 NiO CaO P2O5 Cr2O3 CuO MoO3 71.9 26.2 0.28 0.23 0.23 0.21 0.12 0.1 0.1 0.1 composition (wt. %) 2.2 Na- A zeolite synthesis from fly ashes Prior to zeolite synthesis, a pretreatment was performed, consisting of calcination of the fly ash at 800⁰ C for one hour and an alkaline fusion. Calcinated fly ash was washed with a solution of HCl 10% v/v under a ratio of 15 ml of acid/g of fly ash. The mixture was stirred at 300 rpm at 80⁰ C for 2 hours (Fisherbrand Isotemp Hot Plate Stirrer). Once stirred, the mixture was left to sediment during a certain time and decanted; this step was repeated until the decanted water lost coloration. The obtained solid was filtered and repeatedly washed with distilled water until the filtrated solution reached neutral pH. The neutral solid sample was dried overnight at 90⁰ C (Memmert UF160), then mixed with NaOH pellets in a weight ratio of 1 NaOH: 1.2 fly ash. The ground mixture was placed in a nickel crucible for fusion at 550⁰ C for one hour (Navertherm Muffle Furnace). Hydrothermal synthesis was conducted once the mixture was cooled down. Sodium aluminate (NaAlO2) with a molar ratio SiO2/Al2O3 of 0.5, was added to the product and mixed with water in a ratio of 10 g per 100 ml. This mixture was stirred (Fisherbrand Isotemp Hot Plate Stirrer) at room temperature for 20 h to obtain an aluminosilicate gel. The slurry was crystallized by two methods. The first one consisted of placing the slurry in a sealed glass reactor at 90⁰ C for 7 h, while the second took place in the oven for 24 h at 100⁰ C, resulting in zeolites Na-A1 and Na-A2, respectively. For both crystallizations, after the slurry cooled down at room temperature, the suspensions were filtered and washed with 1 L of DI water to achieve a pH solution of 10. The wet solid was dried overnight at 100⁰ C (Memmert UF160). Subsequently, the synthesized zeolite was ball milled (Pulverisette 7, Fritsch) at 500 rpm for 5 minutes. The method for Na-A zeolite synthesis was inspired by the literature (Pérez et al., 2016), although this work utilized a sedimentation/decantation step before the washing during the alkaline fusion. Besides, a glass reactor was used instead of an autoclave for zeolite crystallization. 2.3 Na-A zeolite characterization and performance 2.3.1 Elemental composition and morphology of fly ash and Na-A zeolite Raw fly ash and synthesized zeolite were characterized by scanning electron microscopy SEM (Tescan Vega 3), elements were recognized by energy-dispersive X-ray spectroscopy (EDX, Bruker EDX analyzer), and particle size was determined with a Malvern Mastersizer 3000, using a Hydro EV dispersing unit with a stirrer. 2.3.2 Leaching tests A heavy metal leaching test from fly ash and Na-A zeolite was investigated in aqueous solutions in both acid and alkaline media, adjusted by hydrochloric acid and sodium hydroxide solutions. Suspensions containing 0.05 g of zeolite and fly ash, in 10 ml of solution at pH values of 2, 5, 7, 9, and 11, were agitated for 24 h at 100 rpm (Gerhardt Laboshake). The pH of the leachate was measured using a pH meter (Mettler-Toledo), and samples were filtered and analyzed by ICP-MS (Perkin Elmer ICP-MS, Optima 8300). 2.3.3 Adsorption studies of Cr (III) Batch tests were conducted to determine the metal adsorption by shaking at 100 rpm (Gerhardt Laboshake), 0.05 g of zeolite, and 10 ml of Cr3+ solutions with different concentrations. The experiments were performed in closed flasks at room temperature (25 +- 2⁰ C) in duplicate, pH values (Mettler Toledo, pH-meter) were monitored at each sample withdrawal. Isotherm data were obtained after reaching an equilibrium time of 24 h. All samples were filtered using a membrane of 0.45 m pore size prior to measurements of Cr3+ concentrations by ICP-MS (Perkin Elmer ICP-MS, Optima 8300). The adsorption capacity (qe) of zeolite was calculated by Eq. (1) where C0 and Cf are the initial and final metal ion concentrations, respectively, Va is the aqueous phase volume and M is the weight of zeolite used. Pseudo-first-order and pseudo-second-order kinetic models were used to analyze the experimental data. The Lagergren equation was used for accurate pseudo-first-order modeling as described by Eq. (2): where q e is the equilibrium adsorption capacity (mg/g), q t is the adsorption capacity (mg/g) at different times t (min), and k1 velocity constant (min-1). The pseudo-second-order was used to analyze the chemisorption kinetic from the liquid solution, and it can be expressed by the following equation Eq. (3): where k2 is the pseudo-second-order rate constant (g / (mg min)). 2.4 Preparation of membrane substrate The substrate was prepared via the phase inversion method. The casting solution was prepared by dispersing Na-A zeolite (1 wt%) in DMAc (80 wt%). The casting solution was sonicated (VWR Ultrasonic Cleaner) for 30 min, then PES (18 wt%) and PVP (1 wt%) were incorporated and continuously stirred at 500 rpm for 24 h. The casting solution was then cast onto a nonwoven fabric using a doctor blade with a gap thickness of 100 m. The membranes were immersed in a water bath for 2 h, then air-dried for 2 h, and dried overnight at 60⁰ C in the oven to finally be stored in deionized water for further modifications. 2.5 Thin-film composite nanofiltration fabrication The thin-film composite polyamide layer was fabricated using interfacial polymerization reaction by MPD and TMC on the zeolite substrates. Prior to performing interfacial polymerization, 0.25% (w/v) of Na-A zeolite and oxycut powder were incorporated into an organic phase solution of TMC (0.15% w/v), this solution was later mixed with hexane. The substrates were kept in a glass holder and first exposed to MPD (3% w/v) for 6 minutes. The solution was poured out and subsequently, the substrate was impregnated with the organic phase solution. After 1 minute, the excess solution was poured off, and membranes were dried at 60⁰ C for 7 minutes, then placed in DI water for storage. 2.6 Membrane characterization Prepared membranes were dried in a vacuum oven at 40⁰ C overnight. The chemical composition of the membranes was measured by ATR-FTIR (PerkinElmer Spectrum 100, Germany). Surface and morphology micrographs of membranes were obtained with a scanning electron microscope (Tescan Vega 3 with Bruker EDX analyzer). The contact angle of all membrane samples was measured with a Krüss (DSA 10-Mk2) Drop Shape Analysis System. 2.7 Membrane performance Theperformanceofthemembranewasevaluatedusingacross-flowsystemapparatus(EvonikStainless-steel, membrane module, UK) with an effective area of 14.6 cm2 and a trans-membrane pressure of 2 bar. A 30 min pre-pressurization of membranes was performed between 18 and 20 bar with DI water to attain a steady water flux. The pressure was subsequently set at 18 bar and the pure water flux was calculated by the following Eq. (4): where V represents the volume of permeate liquid (L) collected over a period of sampling time Δt (h), A is the effective membrane area (m2), Δp is the trans-membrane pressure (bar). After pre-pressurization, the rejection of Cr (III) was determined by the Eq. (5), during a one-hour operation. where Cp (mg/L) and Cf (mg/L) are the chromium concentration in the permeate and in the feed solutions, respectively. The set-up consisted of a stainless-steel feed tank (800 ml), a high-pressure pump (Lafert, micropump), and two filtration cells connected in series, with a constant volume fed and recirculated, during one-hour operation. Samples were withdrawn at the second membrane cell at different times and were analyzed. 3. Results and discussion 3.1 Na-A zeolite synthesis and characterization 3.1.1 Fly ash characterization XRD and SEM-EDX measurements were carried out to characterize the raw fly ash material for zeolite synthesis. Composition and morphology were monitored to determine the quality of zeolite to be obtained since the Si/Al molar ratio and alkalinity influence its formation. The X-ray diffractogram of raw fly ash is shown in Figure 1 (a). The presence ofsiliconandaluminumwasobserved,intheformsofquartz(Si02),anhydrite(Ca(SO)4)andgismondine (CaAl2Si2O8∙4H2O), as identified by the sharp peaks. The existence of these crystalline phases indicates that this fly ash is suitable for zeolite formation (Inada et al., 2005). The morphology of the fly ash is shown in Figure 1 (b), where blunt and irregular shapes predominate. These angular particles correspond to the ones produced by the fluidized bed combustion (FBC) where low combustion temperatures (700-900 ⁰C) are not sufficient to melt ash particles into the typical spherical shapes produced by PCC (pulverized coal combustion) (Ohenoja et al., 2020). The elemental composition of the raw fly ash (Table 2) indicates a Si/Al molar ratio of 4.6 which, according to (Tanaka and Fujii, 2009), permits a satisfactory synthesis of Na-A type zeolites. The amounts of Ca and Si above 50%, allowed this fly ash to be classified as Class C according to The American Society for Testing and Materials. This class of fly ash has shown an advantageous zeolite synthesis process due to a high reactivity and rapid alkalinization (Babatola, 2019; Kunecki et al., 2017). Figure 1. X-ray diffraction pattern of raw fly ash (a); SEM photograph of raw fly ash particles. 3.1.2 Morphological and elemental analysis of Na-A zeolites In order to explore the influence of operating parameters for crystallization, two different methods were used, and accordingly, two zeolites were synthesized. Na-A1 underwent crystallization in a sealed glass reactor, while Na-A2 was obtained by furnace crystallization. Figure 2 shows the morphology of the two Na-A zeolites. In sample Na-A1 (Figure 2(a)) amorphous and irregular aggregates were observed, which do not correspond to the expected crystals. This is directly related to the hydrothermal treatment time during which the aluminosilicate compounds crystallize through nucleation reaction. The reactor-crystallization method of 7 h was insufficient for a better crystal nuclei development. On the contrary, the zeolite Na-A2 (Figure 2 (b)) exhibited better formed cube-shaped crystals, confirming the formation of zeolitic material. This higher purity obtained is in accordance with a longer oven-crystallization time (24 h) and higher temperature, which played an important role in regulating purity and crystallinity (Feng et al., 2018). Figure 2. Micrographs (SEM) of the synthetic Na-A1 (a) and Na-A2 (b) zeolites. dissolved into NaOH in the alkaline fusion (Inada et al., 2005). During hydrothermal process the elements recombined to form aluminosilicate gel, precursor of zeolitization, generating an increase in Si and Al (Lin and Chen, 2021). No presence of heavy metals was observed by the raw fly ash (Table 2). However, for the prepared Na-A zeolites trace amounts of various toxic elements are present. The migration of some heavy metals was due to the high temperature fusion and the strong alkaline media, making available the trapped elements of the fly ash. The presence of arsenic can be associated to the dissolution of arsenate complexes embedded in the fly ash, which were released under alkaline condition. Pb, Cu and Cr, are amphoteric elements able to react in the alkali media to form soluble anionic hydroxo-complexes (Feng et al., 2018). The concentrations of the contaminants were monitored in the leaching and adsorption tests. Table 2. Elemental analysis of fly ash and synthetized Na-A zeolites in atomic % by EDX FA Na-A1 Na-A2 FA Na-A1 Na-A2 Si 12.46 33.79 33.91 Ti - 11.58 7.06 Al 2.7 36.41 31.71 V - - - Ca 50.96 5.89 5.16 Cr - 0.02 0.15 Fe 5.59 1.76 1.47 Mn 1.5 0.31 0.22 K 19.06 - 0.32 As - 0.41 - Na 3.22 9.18 18.36 Ni - - 0.55 Mg 4.52 0.57 0.07 Cu - - 0.2 Zn - - 0.81 Pb - 0.09 - 3.1.3 Particle size distribution of synthesized zeolite In Figure 3, differential and cumulative curves for the particle size distribution of the most crystalline sample (Na-A2) are displayed. After milling for 5 min at 500 rpm, the proportion of material obtained was mainly distributed around one peak value, corresponding to 1 m. The percentile obtained D10=1.26 µm, corresponded to a 10% of the sample being smaller than 1.2 µm. The percentile D50=25.8 1 µm indicated a 50% of the distribution smaller than 25.8 µm, and D90=274.1 µm meant that 90% of the sample is smaller than 274.1 µm. Although it appears that the sample is mostly larger than 200 microns, this may be due to the particle’s agglomerations as observed in the SEM micrograph Figure 2(b). This can be explained by the water molecules surrounding the large particles and penetrating into them, enduring adsorption on the particle surface. This phenomenon created and enlargement effect of the particles, hindering the exact measurement which is presumed to be 1 m (Wang et al., 2016). Figure 3. Particle size distribution of Na-A2 zeolite. Differential (top) and cumulative analysis (bottom). 3.1.5 Leaching tests The leachability results of fly ash and Na-A2 zeolite are shown in Figure 4. It becomes evident that various toxic elements could not be detected in the elemental analysis of fly ash by EDX; however, they mobilized from the fly ash matrix influenced by the pH of the aqueous environment. Hence, since the composition of the parent fly ash included toxic compounds, these migrated to the zeolite during its synthesis. Among the heavy metals found there is arsenic (As), c c a a d d m m i i u u m m ((C C d d )),, c c h h r r o o m m i i u u m m ((C C r r )),, c c o o p p p p e e r r ((C C u u )),, l l e e a a d d ((P P b b )),, a a n n d d z z i i n n c c ((Z Z n n )).. O O t t h h e e r r t t o o x x i i c c e e l l e e m m e e n n t t s s s s u u c c h h a a s s n n i i c c k k e e l l ((N N i i )),, molybdenum (Mo), and mercury (Hg) were measured and no presence was detected. It is worth mentioning that the purpose of the synthesized zeolite is to provide an environmental solution for water treatment,sothemonitoringoftoxicelementsisessentialforitsapplicability.Itwasfoundthattheleached concentrations of the aforementioned elements are below the values established for irrigation water, in accordance with the Mexican standard NOM-003-SEMARNAT-1997 (DOF - Diario Oficial de la Federación, 1998), which will be used as a reference to set a precedent for the Na-A2 zeolite usability. Figure 4. Heavy metals leaching behavior of fly ash and Na-A2 zeolite at pH=2, 5, 7, 9, 11 after 24 hours. One of the most concerning elements leached is arsenic. Its presence within the fly ash comes from the As-bearing pyrite in coal, which decomposes into arsenate during the combustion of carbon (Izquierdo and Querol, 2012). For the fly ash, at acid or very alkaline pH, arsenic leaching reduces, most likely due to the formation of calcium precipitates with arsenic oxyanions (Feng et al., 2018). For the Na-A zeolite, no arsenic was detected at pH 2, while for the rest of the pH values the concentrations did not exceed 0.055 mg/L. For both, fly ash and synthesized zeolite, the leached arsenic complied within the Mexican water irrigation standard. Concentration of cadmium and chromium from the fly ash leachate exhibited values that were even below the drinking water limit (WHO, 2011). As for Na-A zeolite, the total chromium (0.044 mg/L) and cadmium (0.035 mg/L) levels respected those stipulated for irrigation water according to the Mexican regulation. Same case with the leachate concentrations of copper and zinc from fly ash and synthesized zeolite in all the pH values tested. Lead was highly available in the fly ash and easily exposed over the range of acid and alkaline media, clearly exceeded all regulations. Its presence is associated with the pyrite phases in coal, tending to largely accumulate (Izquierdo and Querol, 2012). On the contrary, for the prepared Na-A zeolite no lead was detected and does not pose a risk to the environment. Apart from arsenic which was the major element leached from the Na-A zeolite, the rest of the hazardous elements are present in concentrations below the safe limits for irrigation water in Mexico. Therefore, it is a suitable material for its application. 3.1.6 Adsorption study The adsorption capacity of Cr (III) ions from water using Na-A zeolite was evaluated at initial concentrations of 25, 30, and 35 mg/L, as a reference to residual concentrations from some leather tanning industries after precipitation treatment (Covarrubias et al., 2008). Experiments were conducted for two hours; the initial pH was kept as it was when the zeolite was brought into contact with the synthetic chromium water. The final pH was monitored along with every measurement during the entire test. Both zeolites were tested to identify the most suitable for Cr (III) removal, despite the differences found during the characterization. The results (Figure 5) show a similar removal behavior removal greater than 90%. (a) 25 mg/L 11 100 10 80 9 60 7 40 6 20 5 Na-Z 1 pH Na-Z 1 卜Naz -Z2 pH Na-Z 2 0 50 100 150 200 time (m i n ) (C) 35 mg/L 1 100 一 . 10 80 9 8 60 40 6 20 5 Na-Z 1 pH Na-Z 1 Na-Z2 4 -pH Na-Z2 0 50 100 150 200 time (min) Figure 5. Removal efficiencies (%) and pH behavior of batch tests for zeolite Na-A1 and Na-A2. The efficiency of chromium (III) removal was measured to determine the adsorptive behavior of Na-A zeolite to be incorporated in the TFC membrane. In Figure 5 it is remarkable that the uptake capacity appeared to increase as pH moved from acidic condition to alkaline, reaching a steady pH value. For all the tested concentrations, removal greater than 90 % was observed with both zeolites in the first minutes of contact time. As the chromium (III) concentration increased, a slight decrease in removal efficiency was observed, due to the gradual filling of the available adsorption sites on the zeolite. Additionally, pH behavior contributed to Cr(OH)3 precipitation as it moved towards to the alkaline region through experimentation (Kumar and Dwivedi, 2021). The best removal performance was achieved by Na-A2 zeolite (oven crystallization) with a 97.32 % of remotion within the first 15 min of contact time. The removal continued increasing throughout the experiment, until a 99.99 % was obtained after 180 min (Figure 5 (a)). Na-A2 zeolite previously showed greater crystalline properties and proved its purity with a higher efficiency for Cr (III) removal, results confirmed its suitability to be incorporated in the membrane preparation. Ce (mg/L) Figure 6. Zeolite Na-A2 kinetics of Cr(III) solutions of (A) 25 mg/L, (B) 30 mg/L, and (C) 35 mg/L fitted to Pseudo -1st order and Pseudo-2nd order models; (D) isotherms of chromium solutions 25, 30, 35, 40, 50 and 60 mg/L according to Langmuir and Freundlich models Adsorption kinetics The adsorption kinetics of the best zeolite Na-A2 (Figure 6. (a, b, c)) were examined by adjusting to pseudo-first-order and pseudo-second-order kinetic models at each concentration tested. In Table 3, the kinetics of each model are presented revealing that for concentrations of 25 and 30 mg/L, the best fitting is given by the pseudo-2nd order model, in which chemical and physical interactions are present. However, for 35 mg/L the pseudo-1st order model adjusted best,withthehighestcorrelationcoefficient(R2)of0.90.Therefore,theadsorptionmechanisminvolvesboth chemisorption and physisorption, also confirmed by the Cr (III) desorption for 35 mg/L after 24 h, as observed in Figure 6. (D). The adsorption capacity in equilibrium for 35 mg/L exhibited a decrease, revealing that the interaction between Cr (III) and Na-A2 zeolite becomes weak with higher concentrations. Table 3. Kinetic parameters for Cr (III) adsorption on Na-A2 zeolite Cr (III) K1 R2 K2 R2 25 0.023 0.61 0.81 mg/L 0.011 30 mg/L 0.026 0.64 0.009 0.80 35 0.90 0.78 mg/L 0.062 0.007 Error! Reference source not found. (D) exhibits the adsorption equilibrium isotherms of Na-A2 with plotting of Langmuir and Freundlich models (parameters of adsorption are summarized in Table 4). The highest sorption was given by the Freundlich model (17.11 mg/g), suggesting a multilayer formation and a better fitting according to the correlation coefficients (R2) (Table 3). The adsorption intensity (1/n) describes the Na-A2 zeolite adsorption of Cr (III). A value within 0.1 < 1/n < 0.5 according to Jangkorn et al. (2022) accredits a favorable adsorption. Table 4. Langmuir and Freundlich isotherm parameters for Cr (III) adsorption on Na-A2 zeolite QMAX (mg/g) b (L/mg) R2 Kf (mg/g) 1/n R2 13.14 7.68 0.89 17.11 0.58 0.98 3.2 Fabrication of Na-A2 zeolite – oxycut membrane In order to provide insight of the prepared Na-A2 zeolite particles and the oxycut powder, a TFC - NF membrane was fabricated for Cr (III) removal. A load of 1 wt% of zeolite was embedded into the PES substrate by the phase inversion method. A good dispersibility of the Na-A2 zeolite particles was intended by sonication of the casting solution to help the particles being dragged by Brownian diffusion and firmly anchored after polymer solidification (Dong et al., 2016). This substrate was covered by the polyamide layer formed on top by interfacial polymerization. Incorporation of Na-A2zeolite and oxycut powder occurred by their integration into TMC to form an IP zone at the same layer on the membrane neither above nor below the particles, assuring the embedment (Lakhotia et al., 2019). 3.2.1 Morphology characterization Top view images of PES/Na-A2 substrate and final Na-A2/oxycut TFC membrane are shown in Figure 7 (a) y (c). The combined substrate of PES/Na-A2 presented a relatively smooth flat surface with some voids and agglomeration of zeolite. This can be attributed to the size of the particles and also the delayed exchange of the solvent and non-solvent when phase inversion process occurred, due to the effect of an increase of solid material on the solution’s viscosity (Abbas et al., 2022). The TFC layer was synthesized on top of the PES/Na-A2 substrate via interfacial polymerization with a loading of Na-A2 (0.25 wt%) and oxycut powder (0.25 wt%). The latter was added in a particle size range of 1 –63 m as seen in the Figure 7 (b) after being manually sieved and not pre-treated in any way. This decision was made arbitrarily since the sample received was only 5 g. Figure 7 (c) depicts the PES/Na-A2 support with the Na-A2/oxycut powder embedded in the polyamide layer formed. Particles were not uniformly distributed on the surface, several agglomerations occurred mainly due to the oxycut size. It was also possible that few particles either oxycut or zeolite could stick out of the selective layer. Figure 7. SEM surface images for (a) PES membrane support with 0.1% Na-A2 zeolite, (b) oxycut powder, (c) PES – Na-A2 support with Na-A2 and oxycut power in the polyamide layer. 3.2.2 Hydrophilicity analysis of PES/Na-A2 substrate and Na-A2/oxycut TFC membrane The hydrophilic character of the membrane support and final membrane was evaluated by measuring the contact angle as shown in Figure 8 (A). The hydrophilicity of the substrate is critical for polyamide formation and water transport, which have an impact on membranes performance since rejection is enhanced when substrates are highly hydrophilic (Peng et al., 2022). The PES/Na-A2 substrate exhibited a contact angle of 55⁰ that can be credited by the natural hydrophilicity of the Na-A zeolite particles which absorb water through their pores (Abbas et al., 2022). The hybrid prepared Na-A2/oxycut TFC membrane indicated a lower hydrophilicity than the support with a value of 63⁰. This could be a consequence of the formation of additional polyamide chains inside the hydrophilic substrate which iinnccrreeaasseess tthhee hhyyddrraauulliicc rreessiissttaannccee ((PPeenngg eett aall..,, 22002222)).. HHoowweevveerr,, iitt eexxhhiibbiittss aa ggoooodd wweettttaabbiilliittyy aaccccoorrddiinngg ttoo tthhee hydrophilicity classification in which values between 10⁰<<90⁰ correspond to hydrophilic membranes. Figure 8. Static pure water contact angle of PES/Na-A2 zeolite support and Na-A2/oxycut TFC membrane wt (%) (left), and image of droplet on PES/Na-A2 support (right). 3.2.3 ATR-FTIR spectra of PES/Na-A2 support and Na-A2/oxycut TFC membrane ATR-FTIR was used to confirm the successful surface coating of the Na-A2 zeolite and oxycut powder onto the prepared PES/NaZ-2 support. Figure 9 demonstrates the transmittance spectra (%) of PES/Na-A2 support and Na-A2/oxycut TFC membrane in the wavenumber region 4000 – 650 cm-1. The notable absorption peak of the S=O in the support and TFC was observed around 1150 cm-1 while the band corresponding to the N-H stretching secondary amine was found at 3317 cm-1. The presence of these bands confirmed the modification of the support. In Figure 9, other bands with high intensities are displayed. The distinctive peaks at 1104, 1238, 1485, and 1578correspond to the C-O stretching aliphatic ether (1085-1150 cm-1). Adsorption bands at 1148 and 1296 cm-1 are attributed to the C-N stretching amine (1020-1250 cm-1) and C-N stretching aromatic amine (1266-1342 cm-1) in the polyamide active layer (Merck, 2022), respectively. The stronger signal at 1011 cm-1 was due to stretching of Si-O-Si and Si-O-Al tetrahedron vibrations of zeolite, which is an indicator that zeolite had been attached to the polymeric support (Abbas et al., 2022). 100 Figure 9. FTIR spectra of membranes supports PES/Na-A2 support and Na-A2/oxycut TFC membrane. 3.3 Membrane performance for Cr (III) removal through Na-A2/oxycut TFC membrane The Na-A2/oxycut TFC hybrid membrane performance in terms of permeance and Cr (III) removal was measured under dynamic condition with a cross-flow system operating at 18 bar. Figure 10 illustrates the set-up composed of two cells connected in series with a feed solution of 30 mg/L of chromium (III) recirculated for one hour operation at room temperature. Figure 11 (A) shows permeate and Cr (III) rejection efficiency as a function of time of the first membrane connected in series. A rapid Cr (III) removal of 99.9 % was observed at the beginning of the operation within the first 10min.Subsequentlyaprogressivereductioninrejectionwasnoticedleadingto84%attheendofthe experimentation. The poor rejection of the first membrane can be accounted for: (1) a hasty occupation of the metal cations in the pores of the membrane, leading to a quick blockage and accumulation on the surface, (2) the high Na-A2zeolite and oxycut agglomerations. The high content of particles caused serious aggregation, decreasing dispersion within the polymer matrix during membrane preparation, resulting in defects that affected negatively the rejection (Abbas et al., 2022; Dong et al., 2016). (Feed) Figure 10. Cross-flow filtration set-up with two cells connected in series Figure 11. Separation performance of MZO hybrid membranes, first membrane (left), and second membrane (right) in series, pressure = 18 bar. The gradual decrease in chromium removal from the first membrane was also coupled with a decline of the permeate from 1.76 to 1.14 L m-2 h-1 bar-1 remaining steady during the operation time. This permeate decrease may be also due to the aggregation of the zeolite and oxycut particles, which leads to pore shrinking and a reduction of water passage through the membrane (Lakhotia et al., 2019). Likewise, the accumulated concentration of Cr (III) ions near the membrane surface can lead to chromium precipitation, resulting in pore blockage and scaling (Covarrubias et al., 2008). Since the largest contaminant load is received by the first membrane, the second can achieve a longer life, better rejection and permeability. It showed a higher stability for water permeate for most of the experiment, stabilized to 0.68 L m-2 h-1 bar-1 from minute 5 to 50. Benefiting from the combination of the two membranes, the overall rejection of 99.9% remained steady during 30 min operation as seen in Figure 11 (B). The prevailing mechanism in the removal process through both membranes is given by the electrostatic interaction between the negative surface and metal cations. The negatively charged out layer given by both the zeolite and the oxycut powder, and additionally due to the presence of unreacted acid chloride groups during IP reaction (Lakhotia et al., 2019). A better adsorption capacity for divalent ions was then enhanced due to this characteristic. Likewise, cations were sieved as they were captured while passing through the membranes (Abbas et al., 2022). At the end of the experiment, the rejection by the second membrane remained above the 97 % with a Cr (III) final concentration of 0.7 mg/L, which meets the range of discharge limitations for most legislations, including the Mexican. Figure 12. Pollutants leaching behavior of Na-A2/ oxycut TFC hybrid membrane Regarding the presence of other contaminants coming from the zeolite and the oxycut powder, several ions were monitored throughout the membrane operation by ICP-MS. In Figure 12 are plotted the only pollutants found during the one-hour operation. Copper leaching behavior started at 0.430 mg/L and decreased to 0.288 mg/L as a final concentration. On the contrary, zinc concentration increased with time from the minute 20, achieving a concentration of 0.82 mg/L. For both contaminants, final concentrations comply with the limit discharge of 4 mg/L and 10 mg/L for copperandzinc,respectively(DOF-DiarioOficialdelaFederación,1998).Otherionswereincludedinthe measurements, including As, Cd, Cu, Hg, Mo, Ni, Pb, Ti, V, and Z, but no presence was detected. The foregoing proves that despite using residues that could be considered hazardous, when incorporated into the membrane they remain within its layers, thus avoiding their leaching, and giving these waste materials a second life. 4. Conclusion In this study a hybrid TFC membrane was prepared of zeolite Na-A synthesized from fly ash, and oxycut powder, a residue from the carbon steel industry. Zeolite was synthesized in two steps, by alkali fusion and hydrothermal treatment. Two methods of crystallization were explored, oven and reactor, obtaining two types of zeolites from which oven crystallization exhibited better properties and higher purity. A strong affinity of the prepared zeolite towards chromium (III) was found by adsorption tests, a removal >97% within 15 min was achieved, and a total removal (100%) after one hour. The kinetic data agreed with both pseudo-1st order and pseudo-2nd order kinetics, according to the pollutant concentration, and the isotherm proved a more favorable pattern in conformity with Freundlich model. The experimental results of membrane filtration exhibited a successful chromium (III) rejection of 98% by a hybrid thin film composite membrane. The fabrication of the membrane support, by adding zeolite into the phase inversion technique, and a combination of zeolite with oxycut powder as part of the active layer, improved metal cations removal. In order to explore other membrane enhancements, it is recommended to vary the zeolite and oxycut loadings in the support as well as in the active layer. The effect of pH can be also evaluated and, longer operating times will provide information on lifetime and fouling. The novelty of this work lies in the incorporation of two waste materials in the hybrid membrane preparation, its combined structure, fabrication, and performance, offer a relatively low cost and sustainable alternative, thus participating in the circular economy. Likewise, one of these residues, the oxycut powder has become an interesting oddity due to its performance for chromium (III) removal, proving to be a material with wide possibilities of application in the environmental field. Further studies are advised to identify opportunity areas. Declaration of Competing Interest The authors report no declaration of interest. Credit authorship contribution statement J. García-Chirino: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – Original draft and Editing. A. Dáder Jiménez : Methodology, Formal analysis, Investigation, Data curation, Writing – Review and Editing. B. Van der Bruggen : Resources, Supervision, Writing – Review and Editing. Acknowledgements J.García-ChirinogratefullyacknowledgesthefinancialsupportthroughthePhDscholarshipfromCONACyT“Estudiosde Doctorado en el Extranjero”. We thank Christine Wouters for her technical assistance performing ICP-MS measurements. References Abbas, T. K., Rashid, K. T. and Alsalhy, Q. F.: NaY zeolite-polyethersulfone-modified membranes for the removal of cesium-137 from liquid radioactive waste, Chem. Eng. Res. Des., 179, 535–548, doi:10.1016/j.cherd.2022.02.001,2022. Ali, J., Tuzen, M., Citak, D., Uluozlu, O. D., Mendil, D., Kazi, T. G. and Afridi, H. I.: Separation and preconcentration of trivalent chromium in environmental waters by using deep eutectic solvent with ultrasound-assisted based dispersive liquid-liquid microextraction method, J. Mol. Liq., 291, 111299, doi:10.1016/j.molliq.2019.111299, 2019. Alsayed, A. F. M. and Ashraf, M. A.: Modified nanofiltration membrane treatment of saline water, INC., 2021. Babatola: Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International, West Conshohocken, PA., ASTM Stand., , C618-19, 2019. Bonola, B., Sosa-Rodríguez, F., Lara, R. H., García-Solares, S. M., Mena-Cervantes, V. Y., Lartundo-Rojas, L., Vazquez-Arenas, J. and Hernández-Altamirano, R.: Sustainable and fast elimination of high Cr(III) concentrations from real tannery wastewater using an electrochemical-chemical process forming Cr2FeO4, Sep. Purif. Technol.,294(May), 121211, doi:10.1016/j.seppur.2022.121211, 2022. Chow, Y. N., Lee, L. K., Zakaria, N. A. and Foo, K. Y.: Phytotoxic effects of trivalent chromium-enriched water irrigation in Vigna unguiculata seedling, J. Clean. Prod., 202, 101–108, doi:10.1016/j.jclepro.2018.07.144, 2018. Covarrubias, C., García, R., Arriagada, R., Yánez, J., Ramanan, H., Lai, Z. and Tsapatsis, M.: Removal of trivalent chromium contaminant from aqueous media using FAU-type zeolite membranes, J. Memb. Sci., 312(1–2), 163–173, doi:10.1016/j.memsci.2007.12.052, 2008. DOF - Diario Oficial de la Federación: NORMA Oficial Mexicana NOM-003-ECOL-1997, Secr. Medio Ambient. Recur. Nat. y Pesca, 1998. Dong, L., Huang, X., Wang, Z., Yang, Z., Wang, X. and Tang, C. Y.: A thin-film nanocomposite nanofiltration membrane prepared on a support with in situ embedded zeolite nanoparticles, Sep. Purif. Technol., 166, 230–239, doi:10.1016/j.seppur.2016.04.043, 2016. Eastmond, D. A., MacGregor, J. T. and Slesinski, R. S.: Trivalent chromium: Assessing the genotoxic risk of an essential trace element and widely used human and animal nutritional supplement, Crit. Rev. Toxicol., 38(3), 173–190, doi:10.1080/10408440701845401, 2008. Feng, W., Wan, Z., Daniels, J., Li, Z., Xiao, G., Yu, J., Xu, D., Guo, H., Zhang, D., May, E. F. and Li, G. (Kevin): Synthesis of high quality zeolites from coal fly ash: Mobility of hazardous elements and environmental applications, J. Clean. Prod., 202, 390–400, doi:10.1016/j.jclepro.2018.08.140, 2018. Inada, M., Eguchi, Y., Enomoto, N. and Hojo, J.: Synthesis of zeolite from coal fly ashes with different silica-alumina composition, Fuel, 84(2–3), 299–304, doi:10.1016/j.fuel.2004.08.012, 2005. Izquierdo, M. and Querol, X.: Leaching behaviour of elements from coal combustion fly ash: An overview, Int. J. Coal Geol., 94, 54–66, doi:10.1016/j.coal.2011.10.006, 2012. Jangkorn, S., Youngme, S. and Praipipat, P.: Comparative lead adsorptions in synthetic wastewater by synthesized zeolite A of recycled industrial wastes from sugar factory and power plant, Heliyon, 8(4), e09323, doi:10.1016/j.heliyon.2022.e09323, 2022. Kaźmierczak, B., Molenda, J. and Swat, M.: The adsorption of chromium (III) ions from water solutions on biocarbons obtained from plant waste, Environ. Technol. Innov., 23, 101737, doi:10.1016/j.eti.2021.101737, 2021. Kumar, V. and Dwivedi, S. K.: A review on accessible techniques for removal of hexavalent Chromium and divalent Nickel from industrial wastewater: Recent research and future outlook, J. Clean. Prod., 295, doi:10.1016/j.jclepro.2021.126229, 2021. Kunecki, P., Panek, R., Wdowin, M. and Franus, W.: Synthesis of faujasite (FAU) and tschernichite (LTA) type zeolites as a potential direction of the development of lime Class C fly ash, Int. J. Miner. Process., 166, 69–78, doi:10.1016/j.minpro.2017.07.007, 2017. Kwikima, M. M., Mateso, S. and Chebude, Y.: Potentials of agricultural wastes as the ultimate alternative adsorbent for cadmium removal from wastewater. A review, Sci. African, 13, e00934, doi:10.1016/j.sciaf.2021.e00934, 2021. Lakhotia, S. R., Mukhopadhyay, M. and Kumari, P.: Iron oxide (FeO) nanoparticles embedded thin-film nanocomposite nanofiltration (NF) membrane for water treatment, Sep. Purif. Technol., 211(March 2018), 98–107, doi:10.1016/j.seppur.2018.09.034, 2019. Lari, S., Parsa, S. A. M., Akbari, S., Emadzadeh, D. and Lau, W. J.: Fabrication and evaluation of nanofiltration membrane coated with amino-functionalized graphene oxide for highly efficient heavy metal removal, Int. J. Environ. Sci. Technol., 19(6), 4615–4626, doi:10.1007/s13762-021-03464-2, 2022. Li, P., Lan, H., Chen, K., Ma, X., Wei, B., Wang, M., Li, P., Hou, Y. and Jason Niu, Q.: Novel high-flux positively charged aliphatic polyamide nanofiltration membrane for selective removal of heavy metals, Sep. Purif. Technol.,280(July 2021), 119949, doi:10.1016/j.seppur.2021.119949, 2022. Li, T., Yang, T., Yu, Z., Xu, G., Han, Q., Luo, G., Du, J., Guan, Y. and Guo, C.: Trivalent chromium removal from tannery wastewater with low cost bare magnetic Fe3O4 nanoparticles, Chem. Eng. Process. - Process Intensif.,169(30), 108611, doi:10.1016/j.cep.2021.108611, 2021. Lin, Y. J. and Chen, J. C.: Resourcization and valorization of waste incineration fly ash for the synthesis of zeolite and applications, J. Environ. Chem. Eng., 9(6), 106549, doi:10.1016/j.jece.2021.106549, 2021. Liu, F., Wang, L., Li, D., Liu, Q. and Deng, B.: A review: The effect of the microporous support during interfacial polymerization on the morphology and performances of a thin film composite membrane for liquid purification, RSC Adv., 9(61), 35417–35428, doi:10.1039/c9ra07114h, 2019. Macedonio, F. and Drioli, E.: Circular economy in selected wastewater treatment techniques, INC., 2022. Mercado-Borrayo, B. M., González-Chávez, J. L., Ramírez-Zamora, R. M. and Schouwenaars, R.: Valorization of Metallurgical Slag for the Treatment of Water Pollution: An Emerging Technology for Resource Conservation and Re-utilization, J. Sustain. Metall., 4(1), 50–67, doi:10.1007/s40831-018-0158-4, 2018. Merck: IR Spectrum Table & Chart, Sigma-Aldrich, n.d. Nambi Krishnan, J., Venkatachalam, K. R., Ghosh, O., Jhaveri, K., Palakodeti, A. and Nair, N.: Review of Thin Film Nanocomposite Membranes and Their Applications in Desalination, Front. Chem., 10(January), 1–22, doi:10.3389/fchem.2022.781372, 2022. Ohenoja, K., Pesonen, J., Yliniemi, J. and Illikainen, M.: Utilization of fly ashes from fluidized bed combustion: A review, Sustain., 12(7), 1–26, doi:10.3390/su12072988, 2020. Ounifi, I., Guesmi, Y., Ursino, C., Castro-Muñoz, R., Agougui, H., Jabli, M., Hafiane, A., Figoli, A. and Ferjani, E.:Synthesis and Characterization of a Thin-Film Composite Nanofiltration Membrane Based on Polyamide-Cellulose Acetate: Application for Water Purification, J. Polym. Environ., 30(2), 707–718, doi:10.1007/s10924-021-02233-z,2022. Peng, L. E., Yang, Z., Long, L., Zhou, S., Guo, H. and Tang, C. Y.: A critical review on porous substrates of TFC polyamide membranes: Mechanisms, membrane performances, and future perspectives, J. Memb. Sci.,641(September 2021), 119871, doi:10.1016/j.memsci.2021.119871, 2022. Pérez, N. W. F., Lázaro, W. R., Camacho, J. D., Bobadilla, M., Infantes, B. C. and Yaipén, C. A.: Influencia de la proporción de hidróxido de sodio a cenizas volantes en la obtención de zeolitas para purificar efluentes acuosos contaminados con metales pesados, Rev. Cienc. Y Tecnol., 11, 127–140, doi:10.1120/3093-1-PB, 2016. Prasad, S., Yadav, K. K., Kumar, S., Gupta, N., Cabral-Pinto, M. M. S., Rezania, S., Radwan, N. and Alam, J.:Chromium contamination and effect on environmental health and its remediation: A sustainable approaches, J. Environ. Manage., 285(August 2020), 112174, doi:10.1016/j.jenvman.2021.112174, 2021. Qiu, Y., Zhang, Q., Gao, B., Li, M., Fan, Z., Sang, W., Hao, H. and Wei, X.: Removal mechanisms of Cr(VI) and Cr(III) by biochar supported nanosized zero-valent iron: Synergy of adsorption, reduction and transformation, Environ. Pollut., 265, 115018, doi:10.1016/j.envpol.2020.115018, 2020. Rahimi, M., Tabrizi, S. A. H. and Aminsharei, F.: Fabrication and antibacterial properties of TFC membrane modified with cellulose/copper oxide nanoparticles for removal of cadmium from water, Sep. Sci. Technol., 57(11), doi:10.1080/01496395.2021.2002893, 2022. Ramalingam, B., Venkatachalam, S. S., Kiran, M. S. and Das, S. K.: Rationally designed Shewanella oneidensis Biofilm Toilored Graphene-Magnetite Hybrid Nanobiocomposite as Reusable Living Functional Nanomaterial for Effective Removal of Trivalent Chromium, Environ. Pollut., 278, 116847, doi:10.1016/j.envpol.2021.116847, 2021. Rojas-Romero, J. E., Rincón-Ramírez, J. E., Marín-Leal, J. C., Buonocore.Tovar, R., Colina, M. and Brinolfo-Montilla, J.: Toxicidad y bioacumulación de Cromo (Cr6+) en la almeja Polymesoda solida del sistema estuarino Lago de Maracaibo, Boletín del Cent. Investig. Biológicas, 49(1), 5–25, 2015. Sharma, N., Sodhi, K. K., Kumar, M. and Singh, D. K.: Heavy metal pollution: Insights into chromium eco-toxicity and recent advancement in its remediation, Environ. Nanotechnology, Monit. Manag., 15(July 2020), 100388, doi:10.1016/j.enmm.2020.100388, 2021. Siciliano, A., Curcio, G. M. and Limonti, C.: Hexavalent chromium reduction by zero-valent magnesium particles in column systems, J. Environ. Manage., 293(April), 112905, doi:10.1016/j.jenvman.2021.112905, 2021. Song, L., Jing, S., Qiu, Y., Liu, F. and Li, A.: Efficient removal of Cr(III)-carboxyl complex from neutral and high-salinity wastewater by nitrogen doped biomass-based composites, Chinese Chem. Lett., (Iii), doi:10.1016/j.cclet.2022.01.073, 2022. Tanaka, H. and Fujii, A.: Effect of stirring on the dissolution of coal fly ash and synthesis of pure-form Na-A and -X zeolites by two-step process, Adv. Powder Technol., 20(5), 473–479, doi:10.1016/j.apt.2009.05.004, 2009. Tumolo, M., Ancona, V., De Paola, D., Losacco, D., Campanale, C., Massarelli, C. and Uricchio, V. F.: Chromium pollution in European water, sources, health risk, and remediation strategies: An overview, Int. J. Environ. Res. Public Health, 17(15), 1–25, doi:10.3390/ijerph17155438, 2020. Wang, X., Wang, K., Plackowski, C. A. and Nguyen, A. V.: Sulfuric acid dissolution of 4A and Na-Y synthetic zeolites and effects on Na-Y surface and particle properties, Appl. Surf. Sci., 367, 281–290, doi:10.1016/j.apsusc.2016.01.103, 2016. WHO: Water quality for drinking: WHO guidelines, Encycl. Earth Sci. Ser., 876–883, doi:10.1007/978-1-4020-4410-6_184, 2011. Wu, X., Yang, L., Meng, F., Shao, W., Liu, X. and Li, M.: ZIF-8-incorporated thin-film nanocomposite (TFN) nanofiltration membranes: Importance of particle deposition methods on structure and performance, J. Memb. Sci., 632(April), 119356, doi:10.1016/j.memsci.2021.119356, 2021. Xu, D., Luo, X., Jin, P., Zhu, J., Zhang, X., Zheng, J., Yang, L., Zhu, X., Liang, H. and Van der Bruggen, B.: A novel ceramic-based thin-film composite nanofiltration membrane with enhanced performance and regeneration potential, Water Res., 215(November 2021), 118264, doi:10.1016/j.watres.2022.118264, 2022. Yang, L., Wang, Z. and Zhang, J.: Zeolite imidazolate framework hybrid nanofiltration (NF) membranes with enhanced permselectivity for dye removal, J. Memb. Sci., 532(December 2016), 76–86, doi:10.1016/j.memsci.2017.03.014, 2017. Yin, J., Kim, E. S., Yang, J. and Deng, B.: Fabrication of a novel thin-film nanocomposite (TFN) membrane containing MCM-41 silica nanoparticles (NPs) for water purification, J. Memb. Sci., 423–424, 238–246, doi:10.1016/j.memsci.2012.08.020, 2012. Zhao, B., Long, X., Wang, H., Wang, L., Qian, Y., Zhang, H., Yang, C., Zhang, Z., Li, J., Ma, C. and Shi, Y.: Polyamide thin film nanocomposite membrane containing polydopamine modified ZIF-8 for nanofiltration, Colloids Surfaces A Physicochem. Eng. Asp., 612(November 2020), 125971, doi:10.1016/j.colsurfa.2020.125971, 2021.
确定













还剩22页未读,是否继续阅读?
中国格哈特为您提供《酸性和碱性介质水溶液中粉煤灰和钠沸石的重金属浸出试验中样品的24小时振荡》,该方案主要用于薄膜材料中酸性和碱性介质水溶液中粉煤灰和钠沸石的重金属浸出试验检测,参考标准《HJ 557-2010 固体废物 浸出毒性浸出方法 水平振荡法》,《酸性和碱性介质水溶液中粉煤灰和钠沸石的重金属浸出试验中样品的24小时振荡》用到的仪器有格哈特强力高重现振荡器LS500/RO500、格哈特快速干燥仪STL56、德国移液器MM
推荐专场
快速干燥仪
更多
相关方案
更多
该厂商其他方案
更多

















