黄粉虫蛋白的热诱导凝胶作用:pH和锌浓度的影响Heat-induced gelation of protein from mealworm (Tenebrio molitor) influence of pH and zinc concentration
方案详情

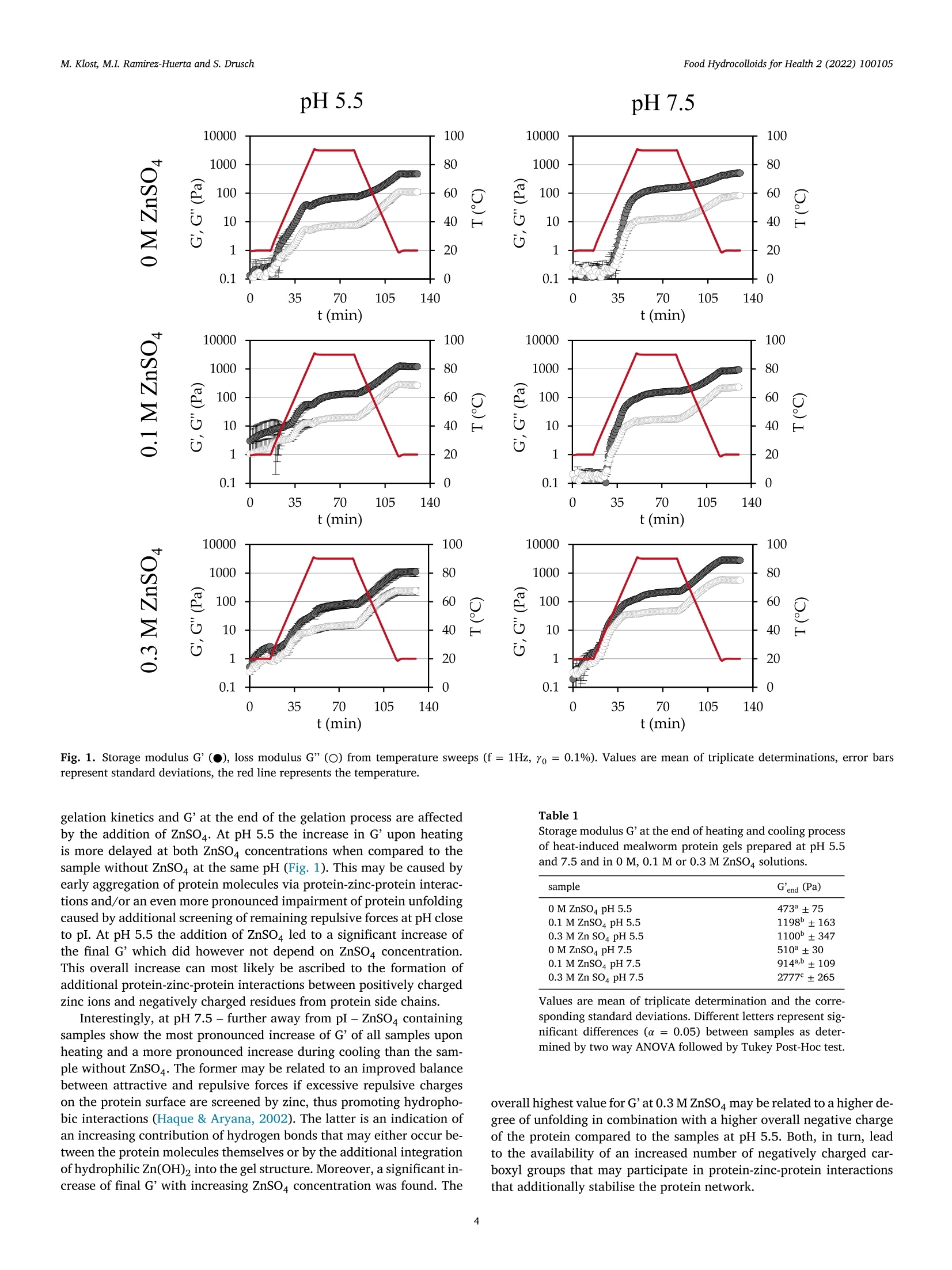
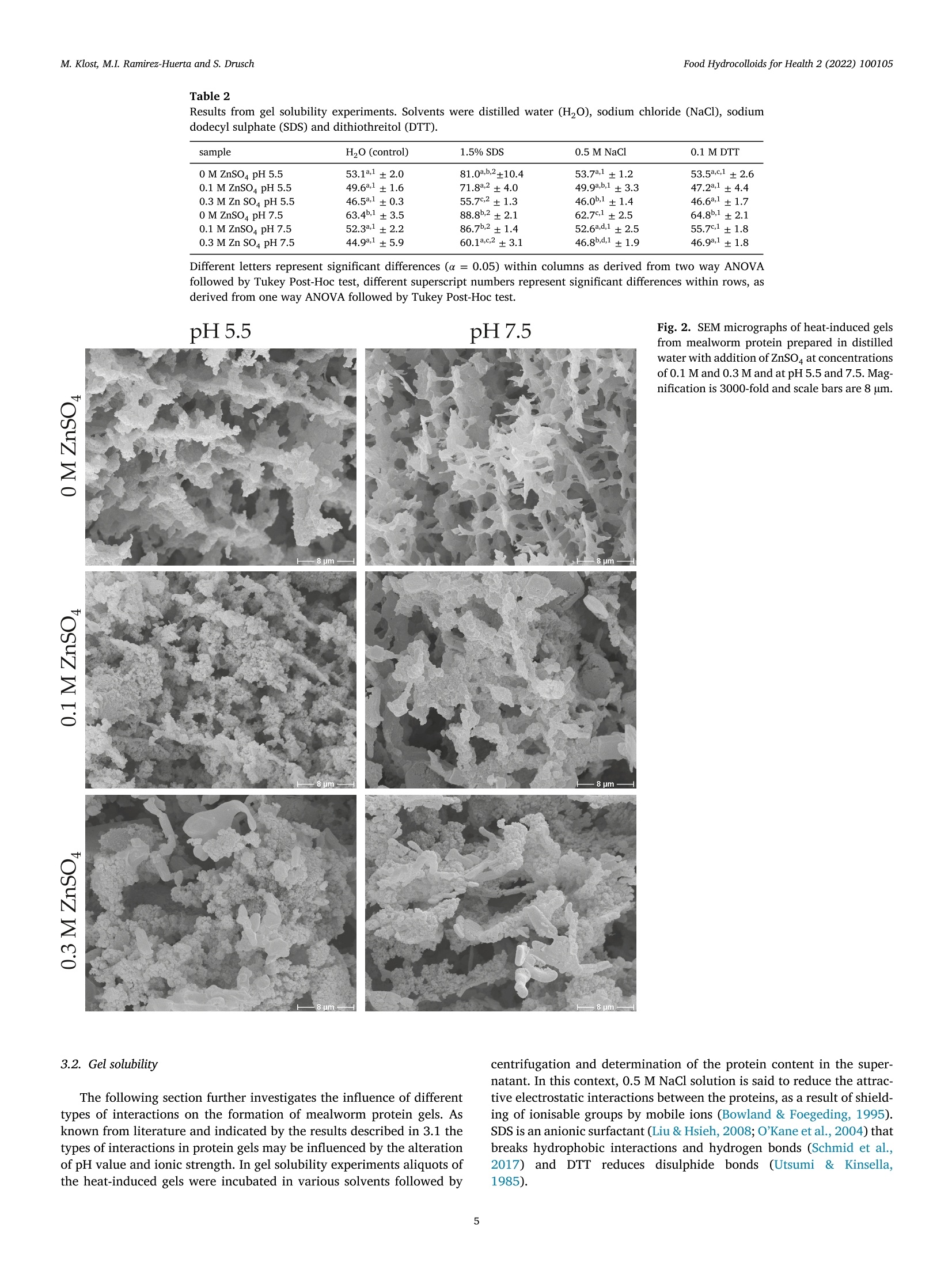
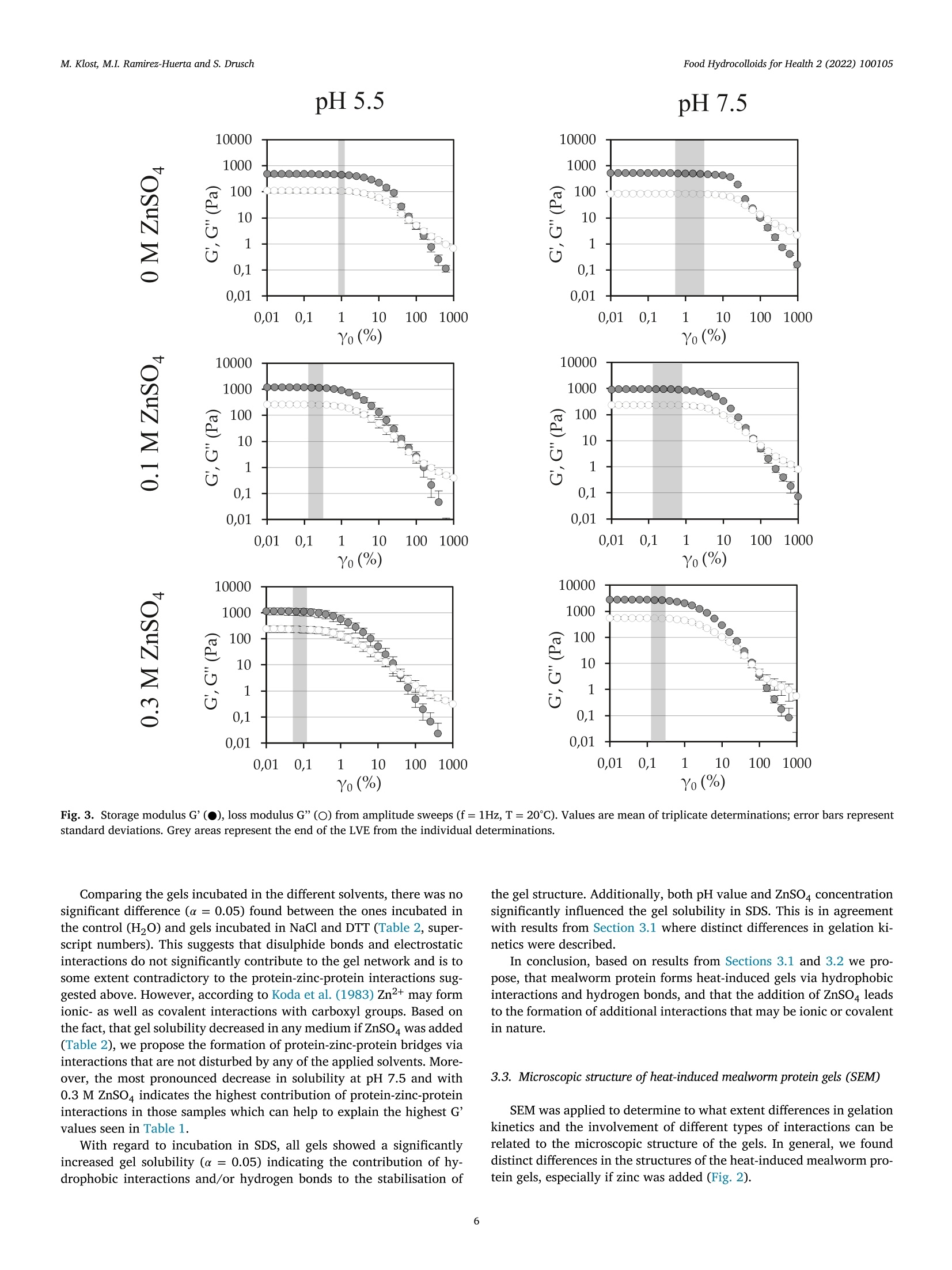
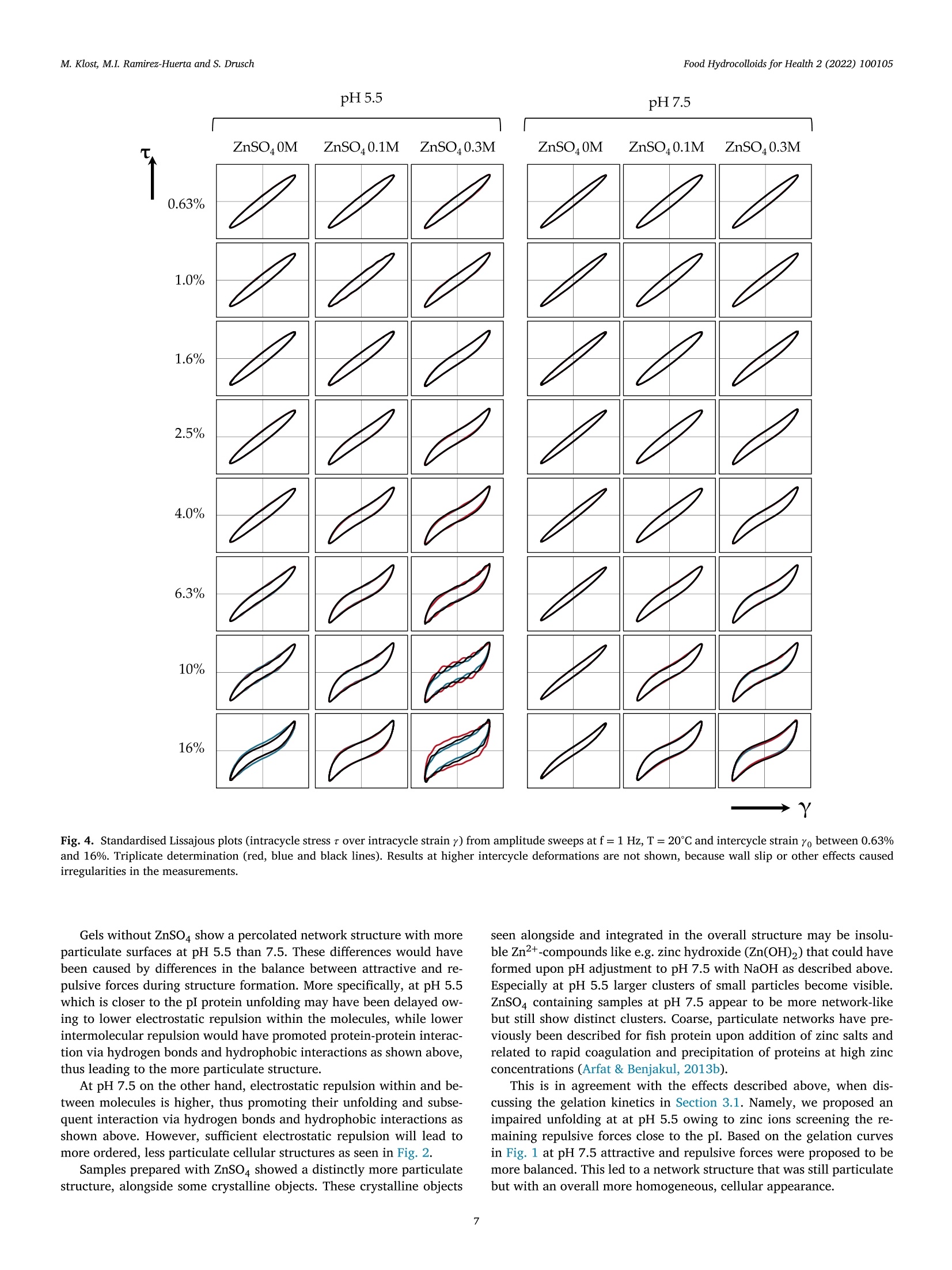
黄粉虫蛋白的热诱导凝胶作用:pH和锌浓度的影响Heat-induced gelation of protein from mealworm (Tenebrio molitor) influence of pH and zinc concentrationFood Hydrocolloids for Health 2 (2022) 100105 Food Hydrocolloids for Health 2 (2022) 100105M. Klost, M.I. Ramirez-Huerta and S. Drusch Contents lists available at ScienceDirect Food Hydrocolloids for Health ELSEVIER journal homepage: www.elsevier.com /locate/fhfh 黄粉虫蛋白的热诱导凝胶作用: pH和锌浓度的影响 Heat-induced gelation of protein from mealworm (Tenebrio molitor):influence of pH and zinc concentration Martina Klost*, Maria Isabel Ramirez-Huerta, Stephan Drusch Technische Universitat Berlin, Faculty IlI Process Sciences, Institute for Food Technology and Food Chemistry, Department of Food Technology and Food MaterialScience, Strae des 17. Juni 135, Berlin 10623, Germany ARTICLE INFO ABSTRACT Keywords: Mealworm protein has the general potential to substitute environmentally less favourable, animal derived raw-Rheology materials as a gelling biomaterial for zinc enriched wound healing ointments. However, various factors affectingGel solubility heat-induced gelation of mealworm protein have not yet been studied in detail. Therefore, we investigated theWound healing influence of pH (pH 5.5 and 7.5) and zinc concentration (0 M, 0.1 M and 0.3 M) on rheological properties and gelZincstructure of heat-induced mealworm protein gels. Additionally, we used gel solubility experiments and rheologicalDivalent cationsiInsectsnvestigations to study the gels with regard to the involved types of interactions. Generally, all examined samplesThermal gelation were able to form heat-induced gels. From gel solubility experiments and temperature sweeps, hydrophobicinteractions and hydrogen bonds were found to be dominant in all samples. From higher storage moduli we derivethe contribution of additional electrostatic and/or covalent interactions in samples with ZnSO4, while shorterlinear viscoelastic regimes and more pronounced intracycle strain stiffening were related to less homogenousand more particulate gel structures. From our results strategies for the customisation of gel properties and gelcomposition may be deduced, that may advance the potential for future utilisation of mealworm protein in wound dressings. 1. Introduction Protein gelation can be influenced by various extrinsic factors suchas ionic strength, type of ions and pH value. The effect of ionic strengthand pH on gel structure has been described by Foegeding et al. (1995):Close to the isoelectric point (pI) higher salt concentrations decrease the protein’s solubility by increasing intermolecular interactions, therebydetermining the dispersed/aggregated state of the proteins in solution.The amount and extent of aggregated proteins determines the type ofgel microstructure. Gel microstructure can either be a fine-stranded ma-trix caused by low ionic strength of monovalent cations, or a mix be-tween a fine-stranded and a particulate matrix under conditions withhigher ionic strength. In the same manner matrix formation can also beachieved as a cause of low concentrations of divalent salt in protein sus-pensions. Analogously to the effect of ionic strength, the gel microstruc-ture becomes more particulate when the pH approaches the pI. Conse-quently, the gel matrix can be manipulated by altering pH and ionicstrength, however, the manipulations required to produce one specificmicrostructure depend on the hydrophobicity and charged groups of theprotein (Foegeding et al., 1995). In biomedical applications, proteins can be used as biocompatible,bioresorbable and biodegradable raw materials that can aid the recon-struction tissue without the chronic inflammation that is often related tosynthetic polymers (Mano et al., 2007). Currently, collagen from cattleand pigs is one of the often-used biopolymers in biomedical applications(Irastorza et al., 2021). However, even though collagen is obtained asa side-stream from animal farming, the fact remains, that the increas-ing demand for livestock (mainly for the food industry) contributes to https://doi.org/10.1016/j.fhfh.2022.100105 Received 30 September 2022;Received in revised form 9 November 2022; Accepted 14 November 2022 2667-0259/O 2022 The Author(s). Published by Elsevier B.V. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/) the constant increase of global environmental pressure (Evans, 1998;FAO, 2015) and results in a loss of biodiversity, waste of energy and in-crease of climate issues (Aiking, 2011). Therefore, it is desirable to lookinto alternative, more sustainable raw materials in all areas of daily live.Besides plant derived proteins, insects are gaining relevance as a sustain-able protein source. Mealworms for example need little water and canbe reared on organic side streams (Ramos-Elorduy et al., 2002) whichhas the potential to actively contribute to low waste concepts and in-creased sustainability. The protein content of mealworm varies depend-ing on feed and rearing conditions (Bordiean et al., 2020). It can reachbetween 51% and 63% of dry matter in commercial dried mealworms(Kulma et al., 2016; Marono et al., 2015). However, it should be noted,that protein contents may be overestimated depending on the protein tonitrogen conversion factor applied (Janssen et al., 2017). So far, research on mealworm protein mainly focuses on the char-acterization of the protein itself and the optimization of the proteinextraction process (Azagoh et al., 2016; L. Yi et al., 2017; Yi et al.,2013). About 35% of the corresponding publications origin in the areaof Food Science and Technology (as determined from web of sciencein September 2022). Research on gelation properties of mealworm pro-tein is scarce (Gkinali et al., 2022). The few existing publications onheat-induced gelation of mealworm protein show an influence of tem-perature, sample concentration, addition of salt and enzyme and pHalterations on rheological properties (Kim et al., 2020; Lee et al., 2019;Yi et al., 2013; Zhao et al.,2016). However, with regard to perspectivebiomedical applications like ointments or wound dressings, specific pHvalues like the pH of skin (around pH 5.5) and wound secret (pH 7.5),as well as the incorporation of supplements that are potentially beneficial to wound healing need to be looked into. One type of ions thathas been described repeatedly to be beneficial to wound healing is Zn2+(Lansdown et al.,2007). However, to date there is no literature, thatdescribes the impact of zinc on mealworm protein gelation - informa-tion clearly needed to assess the suitability of mealworm protein forcorresponding applications. With our study we aim to generate a general understanding on theheat-induced gelation behaviour of mealworm protein with and with-out the addition of ZnSO4 at pH 5.5 and pH 7.5. We describe the gela-tion kinetics and involved types of interactions of these gels as well astheir microstructures and link the latter to the rheological behaviourof the different gels. As derived from literature, mealworm protein isexpected to form heat-induced gels. The addition of ZnSO may leadto more pronounced aggregation as charges on the protein surface arescreened. However, it may enhance crosslinking via the formation ofprotein-zinc-protein bridges, which in turn may influence both, rheo-logical behaviour and gel structure. 2. Materials and methods 2.1. Materials Freeze dried mealworms were provided by the Institute of FoodTechnology and Bioprocess Engineering at the University of Applied Sciences Bremerhaven where they were produced according toKroncke et al. (2019).ZnSO4, SDS, NaCl, NaOH, HCl and dithiothreitolwere purchased from Carl Roth (Karlsruhe, Germany) or VWR (Darm-stadt, Germany) and were of analytical grade. 2.2. Mealworm protein extraction process Mealworms were ground for 1 min at 10000 rpm with a food proces-sor (Grindomix GM 200, Retch GmbH, Germany). Ground mealwormswere defatted by mixing them with hexane, at a sample to solvent ratioof 1:5 (w/v) in Schott glass bottles. Samples were stirred for 1 h witha magnetic stirrer under a fume hood. After 1 h rest without stirring,hexane excess was decanted, and sample residue was thinly spread on apetri dish and left to dry overnight under the fume hood. Defatted worm meal was mixed with distilled water at a ratio of 1:10 (w/v). The sus-pension was adjusted to a pH of 8 by addition of 1 M sodium hydroxide(NaOH) and stirred for 4 h with a magnetic stirrer at room tempera-ture. pH was controlled regularly for the first hour with a pH-meter(Lab865, SI Analytics, Germany) and readjusted if necessary. An AvantiJ-E with JA-10 fixed angle rotor (Beckman Coulter Brea CA, USA) wasused to centrifuge the sample at 1850 g for 20 min at 20℃. The super-natant was then collected and distributed in round-bottomed flasks andfrozen in an ethanol bath at a temperature of -39℃ to -41℃ (K40/FOC-1, Christ Gefriertrocknungsanlagen, Osterode am Harz Deutschland).Lastly the samples were freeze-dried for approximately 48 h (Beta 1-8LSCplus Christ Gefriertrocknungsanlagen, Osterode am Harz, Deutsch-land). The extracted mealworm protein powder was stored at 4℃. Theprotein content of the extracted mealworm protein was 58.4% as deter-mined by the Dumas method (DUMATHERM DT & software DumathermV4.17, C. Gerhardt, Konigswinter, Germany) with protein factor 5.60(Janssen et al., 2017). According to Janssen et al. the general composi-tion of mealworm involves fat, ash, carbohydrates and protein. There-fore, we assume the non-protein dry matter of our extracted mealwormprotein to be constituted from ash, residual moisture and soluble con-stituents like carbohydrates and possibly a remainder of fat. The pres-ence of chitin is unlikely, since chitin is insoluble in water and mildbasic solutions (Roy et al., 2017) and should have been retained in thepellet after centrifugation. 2.3. Preparation of mealworm protein suspensions To examine the influence of salt addition on the gelation, solutionswith 9% protein concentration were prepared by dispersing mealwormprotein in distilled water, 0.1 M ZnSOa solution or 0.3 M ZnSOa solution.All solutions were prepared at two different pH values (5.5 and 7.5) toexamine the influence of pH on the gels. pH values were chosen to reflectthe pH of skin (pH 5.5) and wound secret (pH 7.5) respectively and pHwas adjusted with NaOH / HCl (concentrations of 0.1 M, 0.5 M and1M). Samples were stirred at room temperature for 30 min with a mag-netic stirrer. Part of the sample was used for gel solubility experiments.The rest was stored at 4℃ overnight and subsequently used for the rhe-ological characterisation. 2.4. S-Potential of protein suspensions All samples were analyzed with a particle electrophoresis instrument(Nano ZS and software Zetasizer, Malvern Pananalytical,Malvern, UK).The samples were diluted to a concentration of 0.3% with the respectivesolvent (Section 2.3). -potential was measured at 25℃ and was deter-mined in triplicate. Due to a lack of solubility and therefore a remainderof particles in the dispersions, samples containing ZnSO4 (pH 5.5 and7.5) were inadequate for the analysis. 2.5. Investigation of gel formation and gelation kinetics 2.6. Gel solubility of mealworm protein gels Gelation was carried out in a water bath at 90℃ with a holding timeof 30 min. Subsequently the gels were stored at 4℃ overnight. Afterstorage, 0.3 g of the gels were incubated with 1.5 mL of various sol-vents in 2 mL Eppendorf caps. The following solutions were used assolvents to identify different types of interactions responsible for thegel formation: distilled water as a control, 0.5 M NaCl to decrease thenumber of electrostatic interactions, 1.5 % SDS to reduce hydrophobicinteractions and hydrogen bonds, 0.1 M dithiothreitol (DTT) to disruptdisulphide bonds. All solutions were previously adjusted to pH 5.5 andpH 7.5 with 0.1 M HCl/ NaOH. Gel solubility experiments were doneas previously adapted from O'Kane et al. (2004) and Utsumi & Kin-sella (1985) by Klost et al. (2020). In brief, the samples were left inan agitator (Celloshaker Variospeed, MALTA Chermetron, Italy) withgentle agitation for 6 h at room temperature. All suspensions and gelswere then centrifuged at 10,000 g for 15 min at room temperature (MiniSpin Eppendorf SE, Hamburg, Germany). The protein content of the su-pernatant was determined with the Dumas method and calculated aspercent of the protein content of the undissolved gel. 2.7. Scanning electron microscopy Protein suspensions were prepared as described in 2.3 and gels (10g)were formed in 20 mL plastic containers as described in Section 2.6.Subsequently, approx. 2 to 3 g of each sample were filled into fresh20 mL plastic containers, frozen in liquid nitrogen, stored at -20℃ andlyophilized. The lyophilized samples were then broken into pieces andthe breakage site was sputtered with gold (sputter coater SCD 030,Balz-ers, Wiesbaden-Nordenstadt, Germany). Sputtering and SEM analysiswas carried out by the Center for Electron Microscopy (ZELMI), Tech-nische Universitat Berlin, Berlin, Germany with a S-2700 scanning electron microscope (Hitachi, Tokyo, Japan) at magnifications of 1000x and3000x. 2.8. Rheological characterisation of mealworm protein gels For characterisation of the viscoelastic behaviour of the gels, sub-sequent to the time sweeps amplitude sweeps were performed at 20℃,f=1 Hz and yo=0.01%-1000%. To determine the limit of the linearviscoelastic range (LVE), a tolerated deviation of 5% was defined. Forfurther analysis, results were plotted in Lissajous plots (shear stress rover intracycle strain y). 2.9. Statistical evaluation Experiments and measurements were carried out in triplicate fromthe same solutions, except for SEM for which only a single determi-nation was performed. The statistical significance of the results fromgel solubility experiments, storage moduli and linear viscoelastic rangeswere analysed by two-way ANOVA (p <0.05) with Turkey Post-Hoc test(α=0.05). Additionally, a one-way ANOVA (p<0.05) with Turkey Post-Hoc test (α=0.05) was carried out to test for significant differences be-tween gel solubility of each sample in the different solvents (OriginPro2020b software (OriginLab Corp., Northampton, USA)). 3. Results and discussion The first part of results and discussion section covers the gelationkinetics of the mealworm protein in dependence of pH and ZnSO4 con-centration. Subsequently, we will relate the gelation kinetics to the typesof protein-protein interactions involved. To further characterise the gel,we then additionally look into the microstructure and further rheologi-cal properties of the gels. To investigate and describe the gelation behaviour, all samples wereheated and subsequently cooled in a rheometer. Results are presentedas storage modulus G'and loss modulus G” (Fig. 1). The investigation of gelation kinetics, and more precisely the in-crease in G'during heating and cooling can be used as an indicator forthe type of involved protein-protein interactions. Generally, an increasein G'during heating is associated with hydrophobic interactions and /orthe formation of disulphide bonds that form when hydrophobic patchesbecome exposed and intramolecular disulphide bonds get disrupted dur-ing protein unfolding (Kim et al., 2016). During cooling of the gels, shortranged interactions such as hydrogen bonds (Chronakis, 2001) and vander Waals forces begin to additionally contribute to the stabilisation ofgels, thus causing the second increase in Fig. 1. Consequently, the in-crease in G’during both, heating and cooling indicates the contributionof both, hydrogen bonds, hydrophobic interactions and possibly disul-phide bonds to the stabilisation of the gel structure in all samples. However, there are distinct differences between the gelation curvesof the samples (Fig. 1) as well as between the G'values at the end of gela-tion (Table 1). Gels without ZnSO4 at pH 5.5 showed a lower increaseof G'and G” during heating and a steeper increase during the coolingphase, compared to the corresponding gels prepared at pH 7.5. Thisindicates a shift in the ratio between hydrogen bonds and hydropho-bic interactions and/or disulphide bonds towards a lower contributionof hydrophobic interactions and/or disulphide bonds at pH 5.5, whichmay be caused by a delay in protein-unfolding owing to a more stableconformation under conditions of lower electrostatic repulsion betweenand within molecules (i.e. at pH closer to the isoelectric point (pI) of themealworm protein at pH 4-5, (Borremans et al., 2020; Lee et al., 2019;L. Yi et al., 2017)). Delayed or even impaired unfolding will in turn re-duce the number of newly exposed hydrophobic patches and/or brokendisulphide bonds and consequently the formation of hydrophobic in-teractions and new intermolecular disulphide bonds. However, despitethese differences in gelation kinetics, there was no significant influenceon G. In sum, the addition of ZnSO4 can be expected to change the electro-static properties of the system. Consequently, it is not surprising, that gelation kinetics and G'at the end of the gelation process are affectedby the addition of ZnSO4. At pH 5.5 the increase in G'upon heatingis more delayed at both ZnSO4 concentrations when compared to thesample without ZnSO4 at the same pH (Fig. 1). This may be caused byearly aggregation of protein molecules via protein-zinc-protein interac-tions and/or an even more pronounced impairment of protein unfoldingcaused by additional screening of remaining repulsive forces at pH closeto pI. At pH 5.5 the addition of ZnSO led to a significant increase ofthe final G' which did however not depend on ZnSO4 concentration.This overall increase can most likely be ascribed to the formation ofadditional protein-zinc-protein interactions between positively chargedzinc ions and negatively charged residues from protein side chains. Interestingly, at pH 7.5 - further away from pI -ZnSO4 containingsamples show the most pronounced increase of G’ of all samples uponheating and a more pronounced increase during cooling than the sample without ZnSO4. The former may be related to an improved balancebetween attractive and repulsive forces if excessive repulsive chargeson the protein surface are screened by zinc, thus promoting hydropho-bic interactions (Haque & Aryana, 2002). The latter is an indication ofan increasing contribution of hydrogen bonds that may either occur be-tween the protein molecules themselves or by the additional integrationof hydrophilic Zn(OH), into the gel structure. Moreover, a significant in-crease of final G' with increasing ZnSO4 concentration was found. The Table 1 Storage modulus G'at the end of heating and cooling processof heat-induced mealworm protein gels prepared at pH 5.5and 7.5 and in 0 M, 0.1 M or 0.3 M ZnSOa solutions. sample G'end (Pa) 0 M ZnSO4 pH 5.5 473±75 0.1 M ZnSO4 pH 5.5 1198±163 0.3 M Zn SO4 pH 5.5 1100±347 0 M ZnSO4 pH 7.5 510°±30 0.1 M ZnSO4 pH 7.5 914a,b±109 0.3 M Zn SO4 pH 7.5 2777°±265 Values are mean of triplicate determination and the corre-sponding standard deviations. Different letters represent sig-nificant differences (α = 0.05) between samples as deter-mined by two way ANOVA followed by Tukey Post-Hoc test. overall highest value for G’at 0.3 M ZnSO may be related to a higher de-gree of unfolding in combination with a higher overall negative chargeof the protein compared to the samples at pH 5.5. Both, in turn, leadto the availability of an increased number of negatively charged car-boxyl groups that may participate in protein-zinc-protein interactionsthat additionally stabilise the protein network. Results from gel solubility experiments. Solvents were distilled water (HO), sodium chloride (NaCl), sodiumdodecyl sulphate (SDS) and dithiothreitol (DTT). sample H,O (control) 1.5% SDS 0.5 M NaCl 0.1 M DTT 0 M ZnSO4 pH 5.5 53.1a.1±2.0 81.0a,b,2±10.4 53.7a.1±1.2 53.5a.c,1±2.6 0.1 M ZnSO4 pH 5.5 49.6a.1±1.6 71.88.2±4.0 49.9a,b,1±3.3 47.2a.1±4.4 0.3 M Zn SO4 pH 5.5 46.5a.1±0.3 55.7c.2±1.3 46.0b.1±1.4 46.6a.1±1.7 0 M ZnSO4 pH 7.5 63.4b,1±3.5 88.86.2±2.1 62.7c1±2.5 64.8b.1±2.1 0.1 M ZnSO4 pH 7.5 52.3a.1±2.2 86.76.2±1.4 52.6a.d,1±2.5 55.7c1±1.8 0.3 M Zn SO4 pH7.5 44.9a.±5.9 60.1a,c2±3.1 46.8b,d,1±1.9 46.9a.±1.8 Different letters represent significant differences (a=0.05) within columns as derived from two way ANOVAfollowed by Tukey Post-Hoc test, different superscript numbers represent significant differences within rows, asderived from one way ANOVA followed by Tukey Post-Hoc test. Fig. 2. SEM micrographs of heat-induced gelsfrom mealworm protein prepared in distilledwater with addition of ZnSO at concentrationsof 0.1 M and 0.3 M and at pH 5.5 and 7.5. Mag-nification is 3000-fold and scale bars are 8 um. -8 3.2. Gel solubility The following section further investigates the influence of differenttypes of interactions on the formation of mealworm protein gels. Asknown from literature and indicated by the results described in 3.1 thetypes of interactions in protein gels may be influenced by the alterationof pH value and ionic strength. In gel solubility experiments aliquots ofthe heat-induced gels were incubated in various solvents followed by centrifugation and determination of the protein content in the super-natant. In this context, 0.5 M NaCl solution is said to reduce the attrac-tive electrostatic interactions between the proteins, as a result of shield-ing of ionisable groups by mobile ions (Bowland & Foegeding, 1995).SDS is an anionic surfactant (Liu & Hsieh, 2008;O'Kane et al., 2004) thatbreaks hydrophobic interactions and hydrogen bonds (Schmid et al.,2017))andDTT reduces disulphide bonds(Utsumi&Kinsella.1985). Comparing the gels incubated in the different solvents, there was nosignificant difference (α=0.05) found between the ones incubated inthe control (H20) and gels incubated in NaCl and DTT (Table 2, super-script numbers). This suggests that disulphide bonds and electrostaticinteractions do not significantly contribute to the gel network and is tosome extent contradictory to the protein-zinc-protein interactions sug-gested above. However, according to Koda et al. (1983) Zn2+ may formionic- as well as covalent interactions with carboxyl groups. Based onthe fact, that gel solubility decreased in any medium if ZnSO was added(Table 2), we propose the formation of protein-zinc-protein bridges viainteractions that are not disturbed by any of the applied solvents. More-over, the most pronounced decrease in solubility at pH 7.5 and with0.3 M ZnSO4 indicates the highest contribution of protein-zinc-proteininteractions in those samples which can help to explain the highest G'values seen in Table 1. With regard to incubation in SDS, all gels showed a significantlyincreased gel solubility (a=0.05) indicating the contribution of hydrophobic interactions and/or hydrogen bonds to the stabilisation of the gel structure. Additionally, both pH value and ZnSO4 concentrationsignificantly influenced the gel solubility in SDS. This is in agreementwith results from Section 3.1 where distinct differences in gelation ki-netics were described. In conclusion, based on results from Sections 3.1 and 3.2 we pro-pose, that mealworm protein forms heat-induced gels via hydrophobicinteractions and hydrogen bonds, and that the addition of ZnSO leadsto the formation of additional interactions that may be ionic or covalentin nature. 3.3. Microscopic structure of heat-induced mealworm protein gels (SEM) SEM was applied to determine to what extent differences in gelationkinetics and the involvement of different types of interactions can berelated to the microscopic structure of the gels. In general, we founddistinct differences in the structures of the heat-induced mealworm pro-tein gels, especially if zinc was added (Fig. 2). pH5.5 pH7.5 飞 →Y Fig. 4. Standardised Lissajous plots (intracycle stress t over intracycle strain y) from amplitude sweeps at f=1 Hz, T=20℃ and intercycle strain yo between 0.63%and 16%. Triplicate determination (red, blue and black lines). Results at higher intercycle deformations are not shown, because wall slip or other effects causedirregularities in the measurements. Gels without ZnSO show a percolated network structure with moreparticulate surfaces at pH 5.5 than 7.5. These differences would havebeen caused by differences in the balance between attractive and re-pulsive forces during structure formation. More specifically, at pH 5.5which is closer to the pI protein unfolding may have been delayed ow-ing to lower electrostatic repulsion within the molecules, while lowerintermolecular repulsion would have promoted protein-protein interac-tion via hydrogen bonds and hydrophobic interactions as shown abovethus leading to the more particulate structure. At pH 7.5 on the other hand, electrostatic repulsion within and between molecules is higher, thus promoting their unfolding and subsequent interaction via hydrogen bonds and hydrophobic interactions asshown above. However, sufficient electrostatic repulsion will lead tomore ordered, less particulate cellular structures as seen in Fig. 2. Samples prepared with ZnSO4 showed a distinctly more particulatestructure, alongside some crystalline objects. These crystalline objects seen alongside and integrated in the overall structure may be insolu-ble Zn2+-compounds like e.g. zinc hydroxide (Zn(OH),) that could haveformed upon pH adjustment to pH 7.5 with NaOH as described above.Especially at pH 5.5 larger clusters of small particles become visible.ZnSO4 containing samples at pH 7.5 appear to be more network-likebut still show distinct clusters. Coarse, particulate networks have pre-viously been described for fish protein upon addition of zinc salts andrelated to rapid coagulation and precipitation of proteins at high zincconcentrations (Arfat & Benjakul,2013b). This is in agreement with the effects described above, when dis-cussing the gelation kinetics in Section 3.1. Namely, we proposed animpaired unfolding at at pH 5.5 owing to zinc ions screening the re-maining repulsive forces close to the pI. Based on the gelation curvesin Fig. 1 at pH 7.5 attractive and repulsive forces were proposed to bemore balanced. This led to a network structure that was still particulatebut with an overall more homogeneous, cellular appearance. While the microscopic structure mirrors and supports the proposedgelation mechanism, only limited information can be drawn from it withregard to rheological properties, because these do not only depend onthe structure (e.g. its homogeneity) but also on the strength and numberof the involved bonds (Nicolai & Chassenieux, 2019). 3.4. Rheological characterisation of mealworm protein gels To further characterise the rheological behaviour of the gels - es-pecially outside the linear viscoelastic regime (LVE) - we performedamplitude sweeps subsequent to the temperature sweeps. In amplitudesweeps samples showed intercycle strain softening behaviour as indi-cated by a decrease in both, G'and G" at the end of the LVE (Hyun et al.,2011; Mezger, 2020). The intercycle strain at end of the LVE (Yo.end LVE)- derived from a 5% deviation of G’- was significantly lower with theaddition of ZnSO4 and decreased at lower pH (Fig. 3). It ranged fromYo= 0.09%± 0.02 in samples prepared in 0.3 M ZnSO solution atpH 5.5 to 1.57%± 0.94 in samples prepared without ZnSO4 at pH 7.5.Overall, all gels showed a fairly low stability against shearing. Van Kleef (1986) described heat-induced ovalbumin protein gels atpH 5 (close to the pI=4.5) to have a protein distribution that is lessuniform which leads to regions with higher and lower protein concen-tration. The regions with lower protein concentration act as weak pointsand result in low breaking-stress. Considering the clustery, particulateand non-uniform structure (Fig. 2) of our gels with added ZnSO4, thedecrease in yo,end Lve may very well be caused by similar effects. Onthe same note, findings by Arfat & Benjakul (2013a) support the resultsfrom our study by showing that the addition of higher concentrationsof ZnSO4 in stripe trevally protein gels, led to coagulation of proteinsresulting in a particulate gel with decreasing breaking point and defor-mation. Outside the LVE G'and G"are no longer sufficient for the descriptionof the rheological behaviour of the gels owing to the increasing influenceof higher harmonics in the stress response (Hyun et al., 2011). There-fore, Lissajous plots (Fig.4) of intracycle stress t over intracycle strain yare used to describe rheological properties outside the LVE. Within theLVE the elastic Lissajous plots exhibit ellipsoid shapes, which are char-acteristic for predominantly elastic properties (Ewoldt et al., 2010). InFig.4 at yo =0.63% Lissajous plots of all samples are ellipsoid or nearlyellipsoid with a slightly narrower shape of the Lissajous plots at pH 7.5and without the addition of ZnSO4. At higher Yo the Lissajous Plots begin to deviate from their ellipsoidshapes (Fig.4). This becomes visible sooner and is more pronounced ingels prepared with ZnSO4, more so at pH 5.5 than pH 7.5 and reflectsthe extent of the LVE regimes as shown above (Fig. 3). The deviationtowards an inversed sigmoidal shape indicates a transition from intra-cycle linear viscoelastic behaviour towards intracycle strain stiffeningfor all samples. Generally, this type of intracycle behaviour is causedby the beginning disruption of rigid clusters which leads to a reduc-tion in size and volume fraction thus causing a decrease in the slopeat low intracycle strain y. The stretching of the remaining clusters inturn leads to an increase in intracycle stress r at high intracycle strain y(Park et al., 2015). With regard to our samples, the more pronounced in-tracycle strain stiffening behaviour in ZnSO4-containing samples can berelated to the less homogenous, more particulate gel structure (Fig. 2).This less homogenous structure leads to an earlier rupture in areas withlower concentration (Van Kleef, 1986) which is reflected in decreasedslopes of intracycle stress t over intracycle strain y at low y, while retain-ing fairly stable clusters that lead to the pronounced increase in r at highy. Especially samples prepared at pH 7.5 without the addition of ZnSO4showed distinctly less pronounced intracycle strain stiffening behavioureven at yo =16%. In agreement with the proposed enhancement of in-tracycle strain stiffening behaviour with increasing inhomogeneity, thiscan be related to the more homogenous cellular structure of these sam-ples. More specifically, more homogenous (and less compact) structuresare usually more flexible owing to fewer overall protein-protein interac- tions (as seen in results from gel solubility experiments (Table 2)) andtherefore lack the “weak points”described to be responsible for earlyrupture of the gel network. 4. Conclusions With the utilisation of mealworm protein for wound healing oint-ments in mind, the aim of our study was to investigate the influence ofpH (pH 5.5 and 7.5) and zinc concentration (0 M, 0.1 M and 0.3 M) onrheological properties and gel structure of heat-induced mealworm pro-tein gels. Under pH conditions relevant to biomedical applications (i.e.pH 5.5 (pH value of human skin) and pH 7.5 (pH value of wound secret))mealworm protein was able to form heat-induced gels with and withoutthe addition of ZnSO4. We showed, that the gels were mainly stabilisedvia non-covalent interactions such as hydrogen bonds and hydrophobicinteractions. The addition of ZnSO, led to a significant increase in stor-age modulus which can be related to the formation of additional interac-tions, which are most likely of ionic or covalent nature and may involveprotein-zinc-protein interactions. This effect was most pronounced at0.3 M ZnSO4 and pH 7.5 and was reflected in a more particulate gelstructure and lower gel solubility with addition of zinc. These findings are a first step toward gaining a deeper understand-ing of the gel properties and underlying mechanisms in the gelation ofmealworm protein especially with regard to the addition of ZnSO4.Fromour results, some parameters (like the influence of divalent cations) forthe customisation of mealworm protein gels towards their utilisationin wound dressings may be deduced. However, additional research isneeded, e.g., with regard to improving the purity of the raw material, toelucidating the influence of any other substances e.g. from the ash pro-portion and to understanding and counteracting the underlying mech-anisms for the gels'low stability against shearing. Therefore, from amechanistic point of view, experiments with different protein concen-trations should be done to elucidate on the contribution of intramolec-ular interactions vs. intermolecular interactions. This will help to gaina deeper understanding on the intracycle behaviour outside the LVEand therefore on the breaking or yielding behaviour of the gels. From abiomedical point of view, future research should focus on interactionswith wound secrete, the release of zinc from the gels and the applicationof the gels to skin cells. Funding This research was funded by Technische Universitat Berlin, TU-internal research funding: Strategic Call“Pro Nachhaltigkeit”. Ethical statement The authors declare, that the presented research did not involve hu-mans or living animals. Declaration of Competing Interest The authors declare no conflict of interest. CRediT authorship contribution statement Martina Klost: Conceptualization, Investigation, Writing - origi-nal draft, Visualization, Supervision, Funding acquisition. Maria IsabelRamirez-Huerta: Conceptualization, Formal analysis, Investigation,Writing-original draft. Stephan Drusch: Resources, Writing-review& editing, Funding acquisition. Data availability Data will be made available on request. Acknowledgments We thank out project partner Claudia Keil from the Department ofFood Chemistry and Toxicology, Technische Universitat Berlin for fruit-ful discussions and funding acquisition. We further thank Silvia Heimand Elena Koster from the Department of Food Technology and FoodMaterial Science, Technische Universitat Berlin, for their skillful lab-work and Jorg Nissen from the Center for Electron Microscopy (ZELMI),Technische Universitat Berlin for carrying out the SEM investigations. References Aiking, H. (2011). Future protein supply. Trends in Food Science and Technology, 22(2-3),112-120.10.1016/j.tifs.2010.04.005. Arfat, Y. A., & Benjakul, S. (2013a). Effect of zinc sulphate on gelling properties of phos-phorylated protein isolate from yellow stripe trevally. Food Chemistry, 141(3),2848-2857.10.1016/j.foodchem.2013.05.112. Arfat, Y. A., & Benjakul, S. (2013b). Gel strengthening effect of zinc salts in surimi fromyellow stripe trevally. Food Bioscience, 3, 1-9. 10.1016/j.fbio.2013.04.009.y·Arntfield, S. D., Murray, E. D., & Ismond, M. A. H. (1990). Influence of salts on themicrostructural and rheological properties of heat-induced protein networks fromovalbumin and vicilin. Journal of Agricultural and Food Chemistry, 38(6), 1335-1343.10.1021/jf00096a008. HIPe Azagoh, C., Ducept, F., Garcia, R., Rakotozafy, L., Cuvelier, M. E., Keller, S.,Lewandowski, R., & Mezdour, S. (2016). Extraction and physicochemical char-acterization of Tenebrio molitor proteins. Food Research International, 88, 24-31.10.1016/j.foodres.2016.06.010. Bordiean, A., Krzyzaniak, M., Stolarski, M. J., Czachorowski, S., & Peni, D. (2020). Willyellow mealworm become a source of safe proteins for Europe? Agriculture (Switzer-land), 10(6),1-30. 10.3390/agriculture10060233. Borremans, A., BuBler, S., Sagu, T. S., Rawel, H. M., Schliiter, O., & Campenhout, L. V.(2020). Functional properties of powders produced from either or not fermented meal-worm (Tenebrio molitor) paste. 10.1101/2020.04.17.042556. Bowland, E. L., & Foegeding, E. A. (1995). Effects of anions on thermally in-duced whey protein isolate gels. Topics in Catalysis, 9(1), 47-56. 10.1016/S0268-005X(09)80193-8. Chronakis, I. S. (2001). Gelation of edible blue-green algae protein isolate (Spirulinaplatensis strain Pacifica): Thermal transitions, rheological properties, and molec-ular forces involved. Journal of Agricultural and Food Chemistry, 49(2), 888-898.10.1021/jf0005059. Clark, A. H. (1991). Structural and mechanical properties of Biopolymer gels. In Struc-tural and mechanical properties of biopolymer gels (pp. 322-338). Woodhead PublishingLimited. 10.1533/9781845698331.322. Evans, L. T.(1998). Feeding the ten billion: Plants and population growth. Cambridge Uni-versity Press. Ewoldt, R. H., Winter, P., Maxey,J., & McKinley,G. H. (2010). Large amplitude oscillatoryshear of pseudoplastic and elastoviscoplastic materials. Rheologica Acta, 49(2),191-212. 10.1007/s00397-009-0403-7. FAO. (2015). Climate change and food security: Risks and responses. FAO http://www.fao.org/3/a-i5188e.pdf. Foegeding, E. A., Bowland, E. L., & Hardin, C. C. (1995). Factors that determine the frac-ture properties and microstructure of globular protein gels. Topics in Catalysis, 9(4),237-249.10.1016/S0268-005X(09)80254-3. Gkinali, A. A., Matsakidou, A., Vasileiou, E., & Paraskevopoulou, A. (2022). Potentialityof Tenebrio molitor larva-based ingredients for the food industry: A review. Trends inFood Science and Technology, 119, 495-507.10.1016/j.tifs.2021.11.024. Haque, Z. Z., & Aryana, K. J. (2002). Effect of copper, iron, zinc and magnesium ionson bovine serum albumin gelation. Food Science and Technology Research, 8(1),1-3.10.3136/fstr.8.1. Hyun, K., Wilhelm, M., Klein, C., Cho, K., Nam, J., Ahn, K., Lee, S., Ewoldt, R., & McKin-ley, G. (2011). A review of nonlinear oscillatory shear tests: Analysis and applicationoorVf large amplitude oscillatory shear (LAOS). Progress in Polymer Science, 36(12),1697-1753 (Oxford). 10.1016/j.progpolymsci.2011.02.002. Irastorza, A., Zarandona, I., Andonegi, M., Guerrero, P., & de la Caba, K. (2021). Theversatility of collagen and chitosan: From food to biomedical applications. Food Hy-drocolloids, 116.10.1016/j.foodhyd.2021.106633. Janssen, R. H., Vincken, J. P., Van Den Broek, L. A. M., Fogliano, V., & Lakemond,C. M.M(2017).Nitrogen-to-protein conversion factors for three edible insects: Tenebrio moli-tor, alphitobius diaperinus, and hermetia illucens. Journal of Agricultural and FoodChemistry, 65(11), 2275-2278.10.1021/acs.jafc.7b00471. Jurkiewicz, K. (1990). The removal of zinc from solutions by foam separation, II. Precip-itate flotation of zinc hydroxide. International Journal of Mineral Processing, 29(1-2),1-15.10.1016/0301-7516(90)90002-G. Kim, J. H., Varankovich, N. V., Stone, A. K., & Nickerson, M.T. (2016). Nature of protein-protein interactions during the gelation of canola protein isolate networks. Food Re-search International, 89,408-414.10.1016/j.foodres.2016.08.018. Kim, T. K., Lee, M. H., Yu, M. H., Yong, H. I., Jang, H. W., Jung, S., & Choi, Y. S. (2020).Thermal stability and rheological properties of heat-induced gels prepared us-ing edible insect proteins in a model system. LWT, 134(August), Article 110270.10.1016/j.lwt.2020.110270. Klost, M., Gimenez-Ribes, G., & Drusch, S. (2020). Enzymatic hydrolysis of pea pro-tein: Interactions and protein fractions involved in fermentation induced gels andtheir influence on rheological properties. Food Hydrocolloids, 105, Article 105793.10.1016/j.foodhyd.2020.105793. Koda, S., Nomura, H., & Nagasawa,M. (1983). Raman spectroscopic studies on the inter-action between divalent counterion and polyion. Biophysical Chemistry, 18,361-367. Kroncke, N., Grebenteuch, S., Keil, C., Demtroder, S., Kroh, L., Thiinemann, A. F., Ben-ning, R., & Haase, H. (2019). Effect of different drying methods on nutrient qual-ity of the yellow mealworm (Tenebrio molitor L.). Insects, 10(4), 1-13.10.3390/in-sects10040084. Kulma, M., Plachy, V., Koufimska, L., Vrabec, V., Bubová, T., Adámkova, A., &Hucko,B. (2016). Nutritional value of three Blattodea species used as feed for animals.Journal of Animal and Feed Sciences, 25(4), 354-360.10.22358/jafs/67916/2016. Lakshmi, R. V., & Basu, B. J. (2009). Fabrication of superhydrophobic sol-gel composite films using hydrophobically modified colloidal zinc hydroxide. Journal of Colloid andInterface Science, 339(2), 454-460. 10.1016/j.jcis.2009.07.064. Lansdown, A. B. G., Mirastschijski, U., Stubbs, N., Scanlon, E., & Agren, M. S. (2007). Zincin wound healing: Theoretical, experimental, and clinical aspects. Wound Repair andRegeneration, 15(1), 2-16.10.1111/j.1524-475X.2006.00179.x. Lee, H. J., Kim, J. H., Ji, D. S., & Lee, C. H. (2019). Effects of heating time and temperatureon functional properties of proteins of yellow mealworm larvae (Tenebrio molitor L.).Food Science of Animal Resources, 39(2), 296-308. 10.5851/kosfa.2019.e24. Liu, K. S., & Hsieh, F. H. (2008). Protein-protein interactions during high-moisture extru-sion for fibrous meat analogues and comparison of protein solubility methods usingdifferent solvent systems. Journal of Agricultural and Food Chemistry, 56(8), 2681-2687.10.1021/jf073343q. Mano, J. F., Silva, G. A., Azevedo, H. S., Malafaya, P. B., Sousa, R. A., Silva, S. S.,Boesel, L. F., Oliveira, J. M., Santos, T. C., Marques, A. P., Neves, N. M., &Reis, R. L. (2007). Natural origin biodegradable systems in tissue engineering andregenerative medicine: Present status and some moving trends. Journal of the RoyalSociety Interface, 4(17), 999-1030. 10.1098/rsif.2007.0220. Marono, S., Piccolo, G., Loponte, R., Meo, C. Di, Attia, Y. A., Nizza, A., & Bovera, F (2015).In vitro crude protein digestibility of tenebrio molitor and hermetia illucens insectmeals and its correlation with chemical composition traits. Italian Journal of AnimalScience, 14(3),338-343.10.4081/ijas.2015.3889. Mezger, T. (2020). The rheology handbook (5th ed.). Vincentz Network GmbH & Co. KG.Nakai, S. (1983). Structure-function relationships of food proteins with an emphasis onthe importance of protein hydrophobicity. Journal of Agricultural and Food Chemistry,31(4), 676-683.10.1021/jf00118a001. Nicolai, T., & Chassenieux, C. (2019). Heat-induced gelation of plant globulins. CurrentOpinion in Food Science, 27, 18-22.10.1016/j.cofs.2019.04.005. O'Kane, F., Happe, R., Vereijken, J., Gruppen, H., & Van Boekel, M. (2004). Heat-inducedgelation of pea legumin: Comparison with soybean glycinin. Journal of Agriculturaland Food Chemistry, 52(16), 5071-5078.10.1021/jf035215h. Park, J. D., Ahn, K. H., &Lee, S. J.(2015). Structural change and dynamics of colloidal gelsunder oscillatory shear flow. Soft Matter, 11(48),9262-9272. 10.1039/c5sm01651g. Ramos-Elorduy, J., Gonzalez, E. A., Hernandez, A. R., & Pino, J. M. (2002). Useof Tenebrio molitor (Coleoptera: Tenebrionidae) to recycle organic wastes andas feed for broiler chickens. Journal of Economic Entomology, 95(1), 214-220.10.1603/0022-0493-95.1.214. Roy, J. C., Salaiin, F., Giraud, S., Ferri, A., Chen, G., & Guan, J.(2017). Solubility of Chitin:Solvents, solution behaviors and their related mechanisms. Solubility of polysaccharides(Issue November). INTECH. 10.5772/intechopen.71385. Schmid, M., Prinz, T. K., Stabler, A., & Sangerlaub, S. (2017). Effect of sodium sul-fite, sodium dodecyl sulfate, and urea on the molecular interactions and prop-erties of whey protein isolate-based films. Frontiers in Chemistry, 5(JAN), 1-15.10.3389/fchem.2016.00049. Scholliers, J., Steen, L., Glorieux, S., Van de Walle, D., Dewettinck, K., & Fraeye,I. (2019).The effect of temperature on structure formation in three insect batters. Food ResearchInternational, 122(February), 411-418.10.1016/j.foodres.2019.04.033. Totosaus, A., Montejano, J. G., Salazar, J. A., & Guerrero, I. (2002). A review of physicaland chemical protein-gel induction. International Journal of Food Science and Technol-ogy, 37(6), 589-601.10.1046/j.1365-2621.2002.00623.x. Utsumi, S., & Kinsella, J. E. (1985). Forces involved in soy protein gelation: Effectsof various reagents on the formation, hardness and solubility of heat-induced gelsmade from 7S, 11S, and soy isolate. Journal of Food Science, 50(5), 1278-1282.10.1111/j.1365-2621.1985.tb10461.x. Van Kleef, F. S. M. (1986). Thermally induced protein gelation: Gelation and rheologicalcharacterization of highly concentrated ovalbumin and soybean protein gels. Biopoly-mers, 25(1), 31-59.10.1002/bip.360250105. Yi, L., Van Boekel, M. A. J. S., & Lakemond, C. M. M (2017). Extracting tenebrio molitorprotein while preventing browning: Effect of pH and NaCL on protein yield. Journalof Insects as Food and Feed, 3(1), 21-31.10.3920/JIFF2016.0015. Yi, Liya, Lakemond, C. M. M., Sagis, L. M. C., Eisner-Schadler, V., Huis, A. Van,& Boekel, M. A. J. S. VV ((22001133)).. EExxttrraaccttiioonn and characterisation of pro-tein fractions from five insect species. Food Chemistry, 141(4), 3341-3348.10.1016/j.foodchem.2013.05.115. 7Zhao, X., Vazquez-Gutierrez, J. L., Johansson, D. P., Landberg, R., & Langton, M. (2016).Yellow mealworm protein for food purposes - extraction and functional properties.PLoS ONE, 11(2),1-17.10.1371/journal.pone.0147791. Ziegler, G. R., & Foegeding, E. A. (1990). The gelation of proteins. Advances in Food andNutrition Research, 34(C), 203-298.10.1016/S1043-4526(08)60008-X.
确定





还剩7页未读,是否继续阅读?
中国格哈特为您提供《伤口愈合膏胶凝胶生物材料-黄粉虫蛋白粉蛋白质含量的检测》,该方案主要用于生物医用材料中蛋白质检测,参考标准--,《伤口愈合膏胶凝胶生物材料-黄粉虫蛋白粉蛋白质含量的检测》用到的仪器有格哈特杜马斯定氮仪DT N Pro
推荐专场
相关方案
更多
该厂商其他方案
更多
















