方案详情
文
从巴拉圭剩余农业工业生物质中提取的纳米纤维素:提取过程、理化和形态学表征Nanocellulose Extracted from Paraguayan Residual Agro-Industrial Biomass Extraction Process, Physicochemical and Morphological Characterization
方案详情

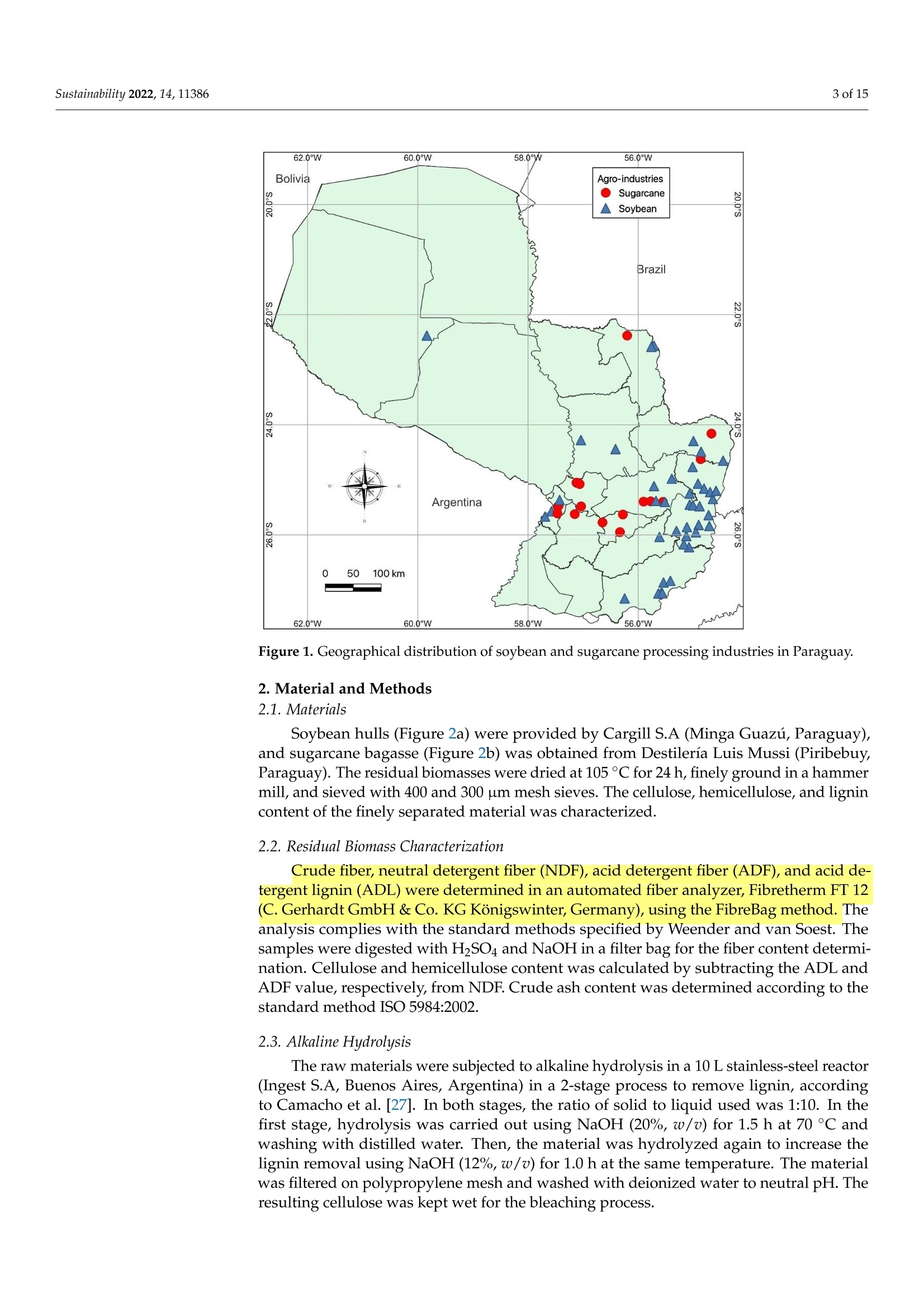
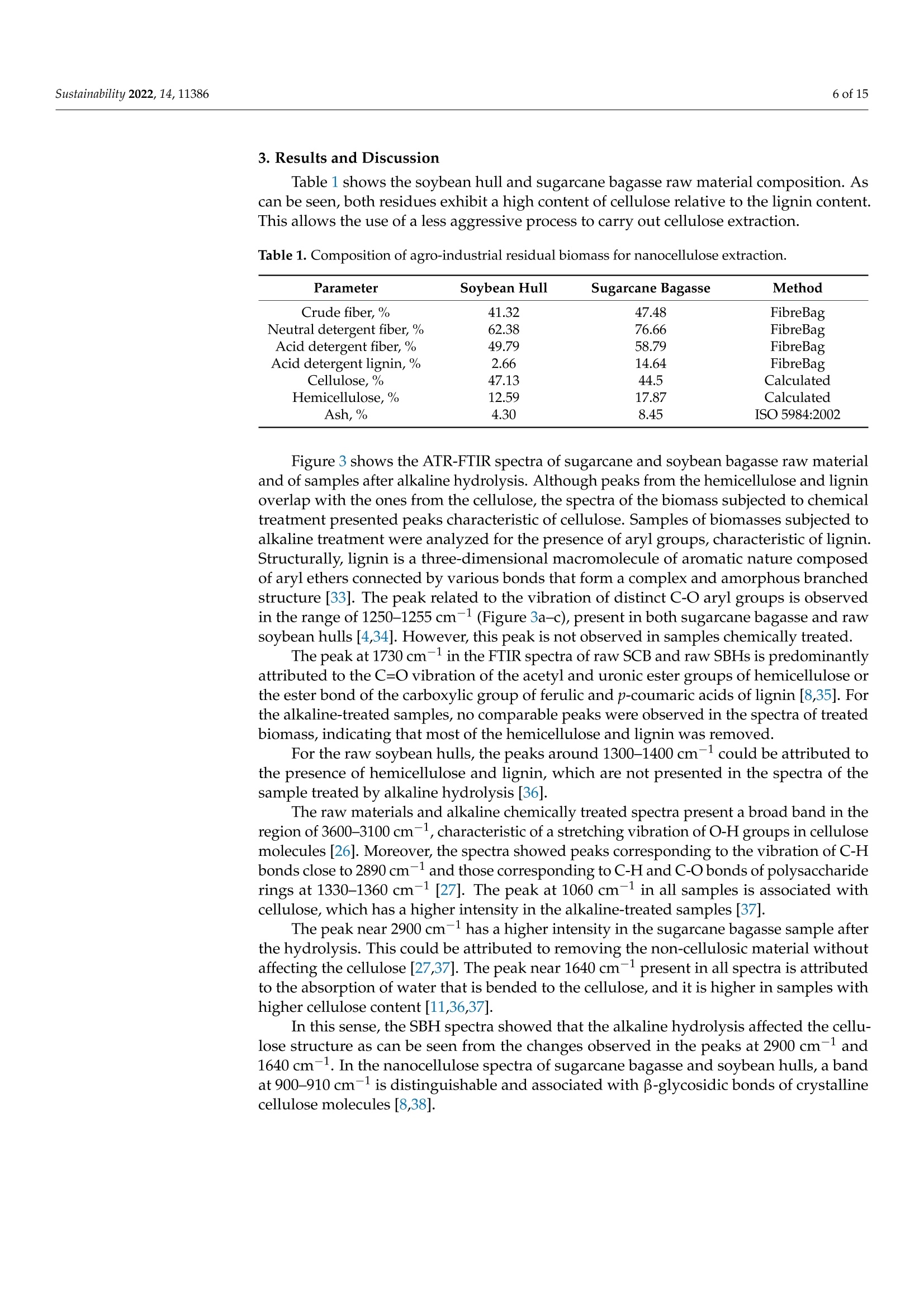
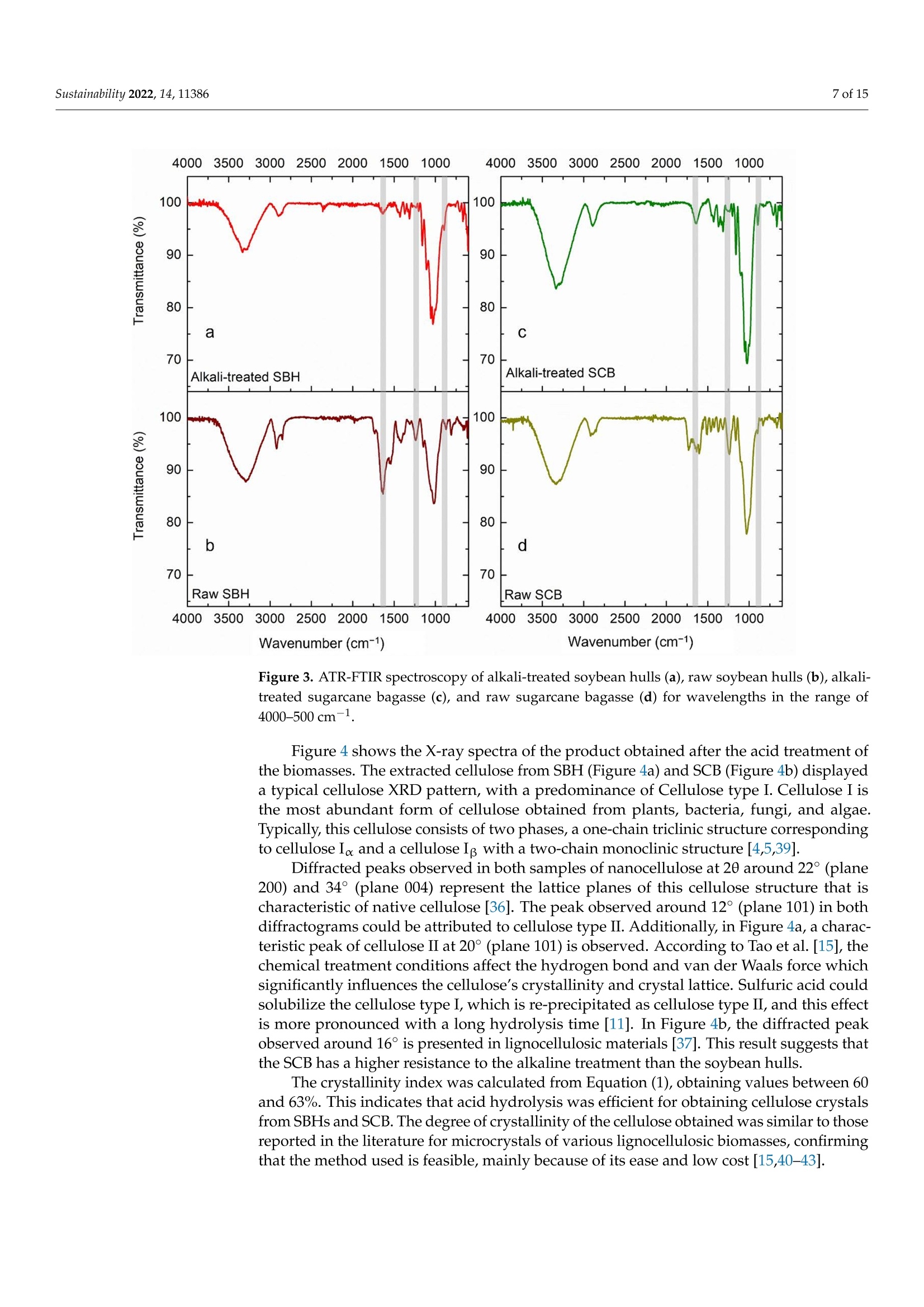
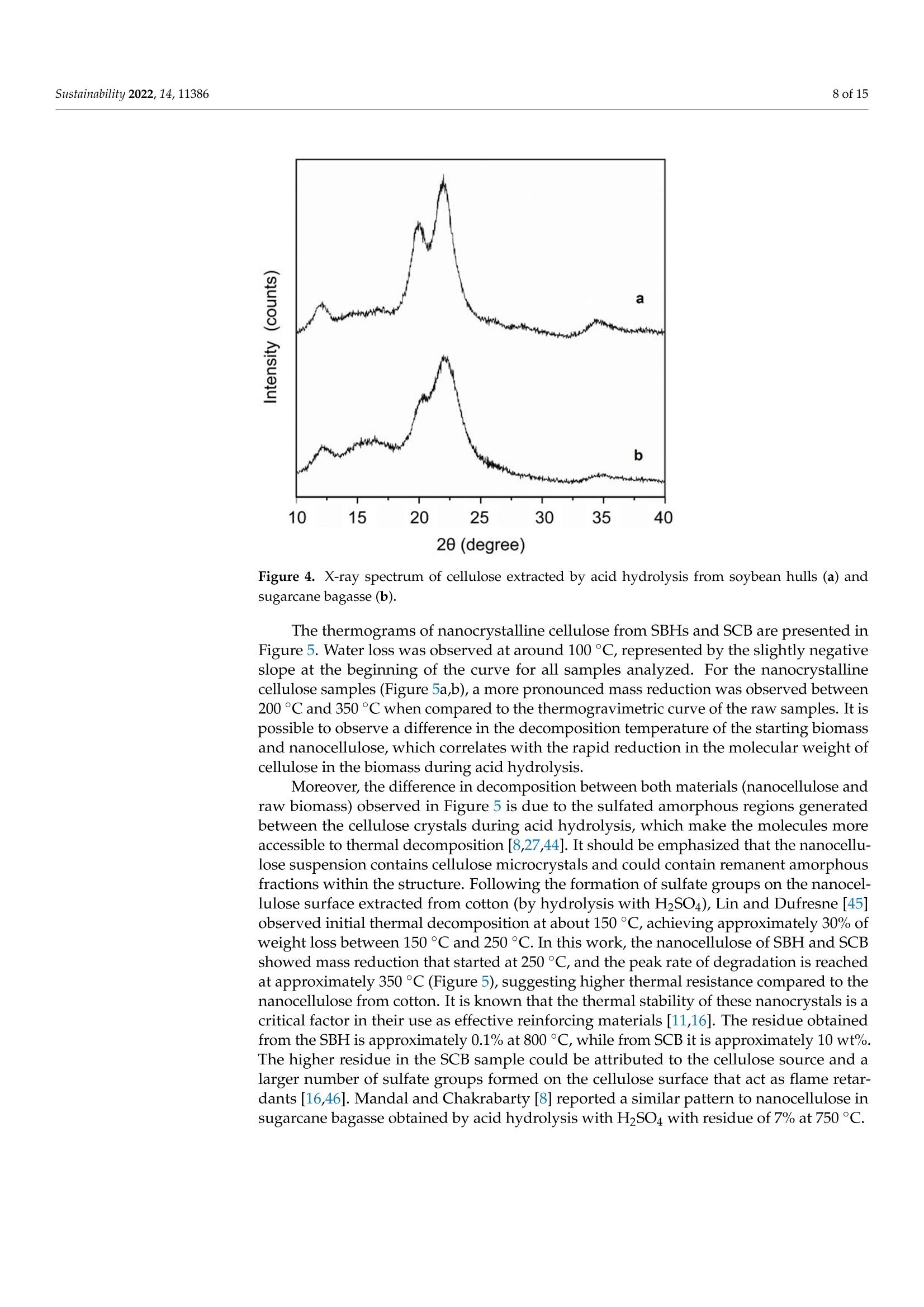
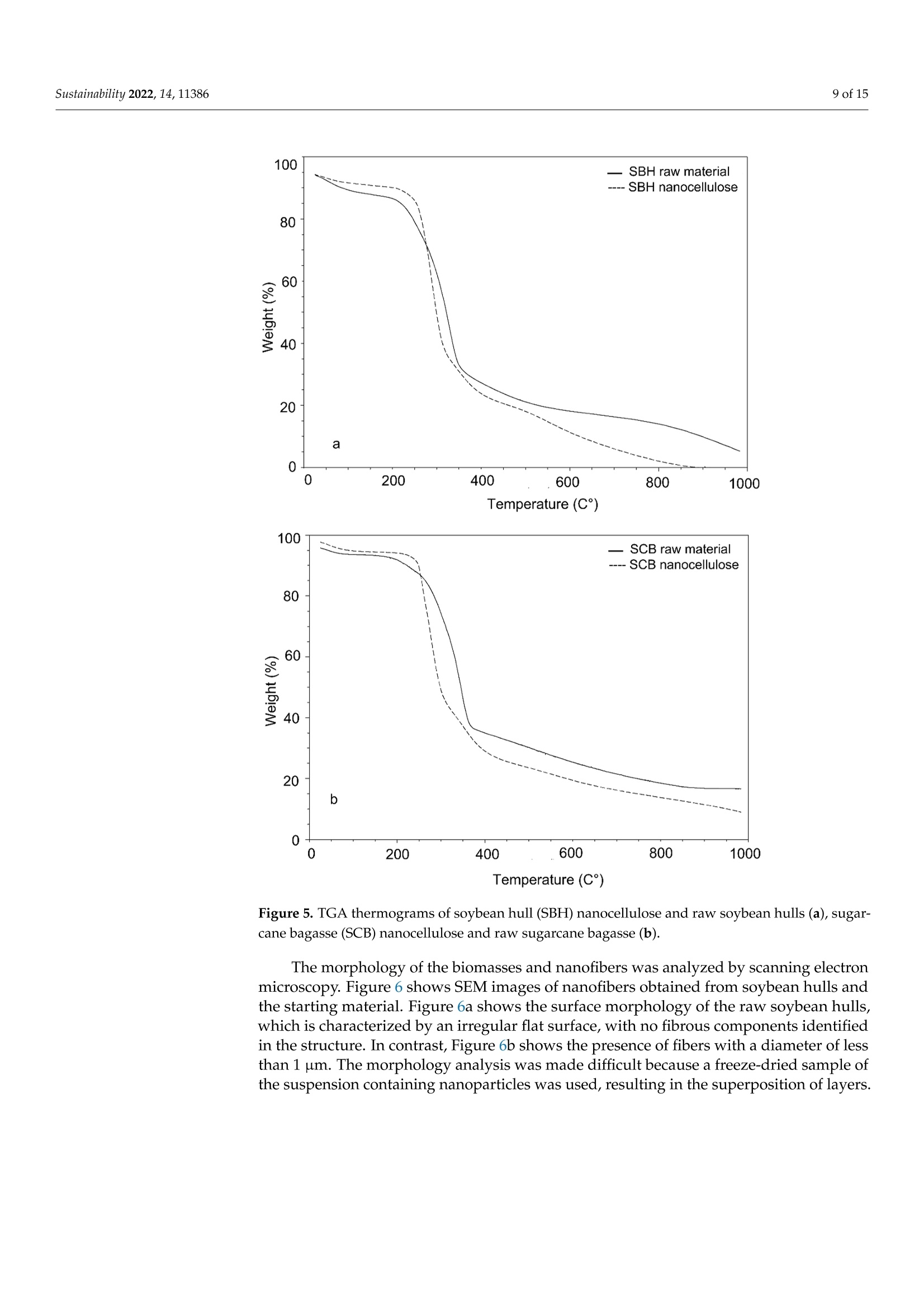
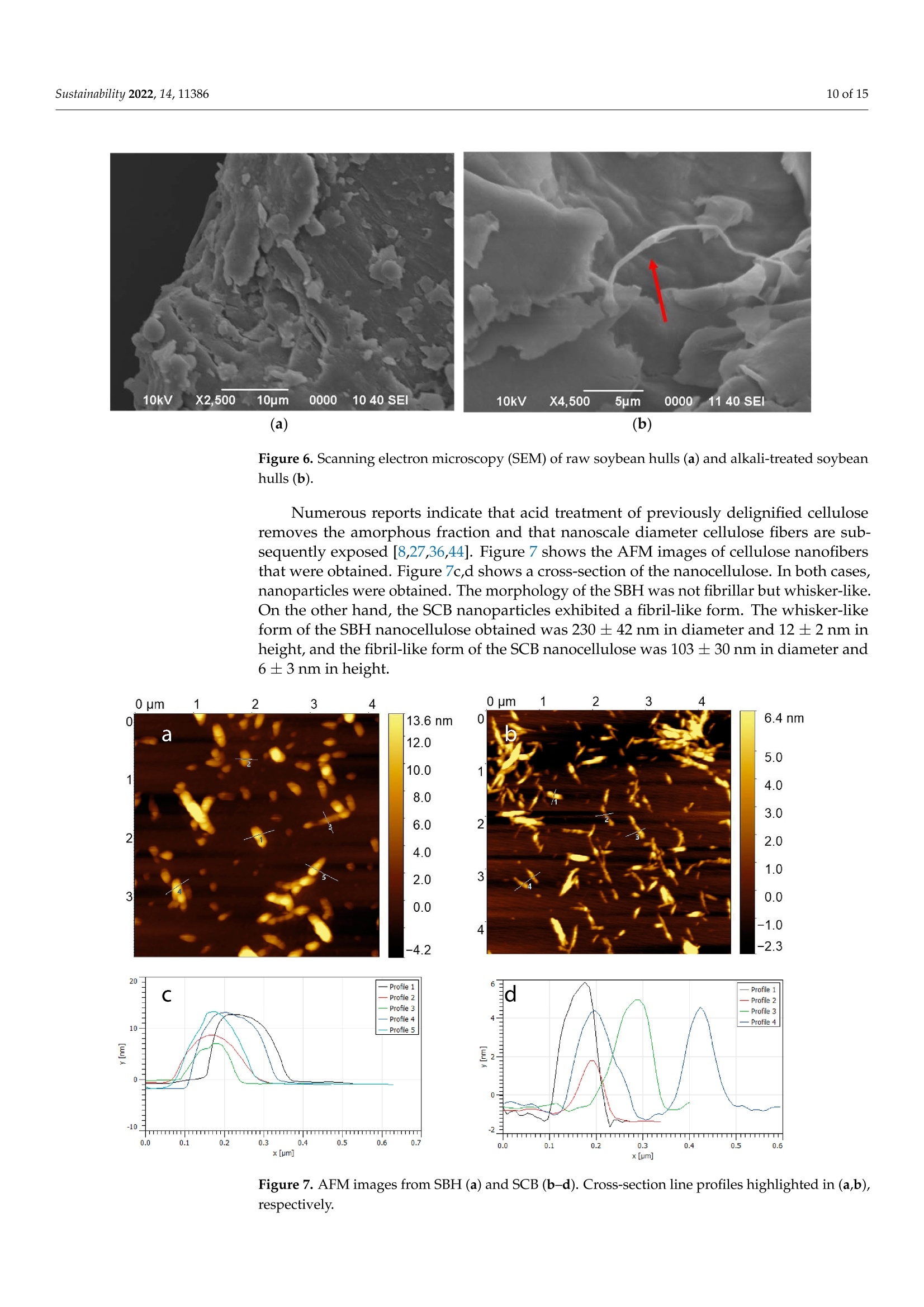
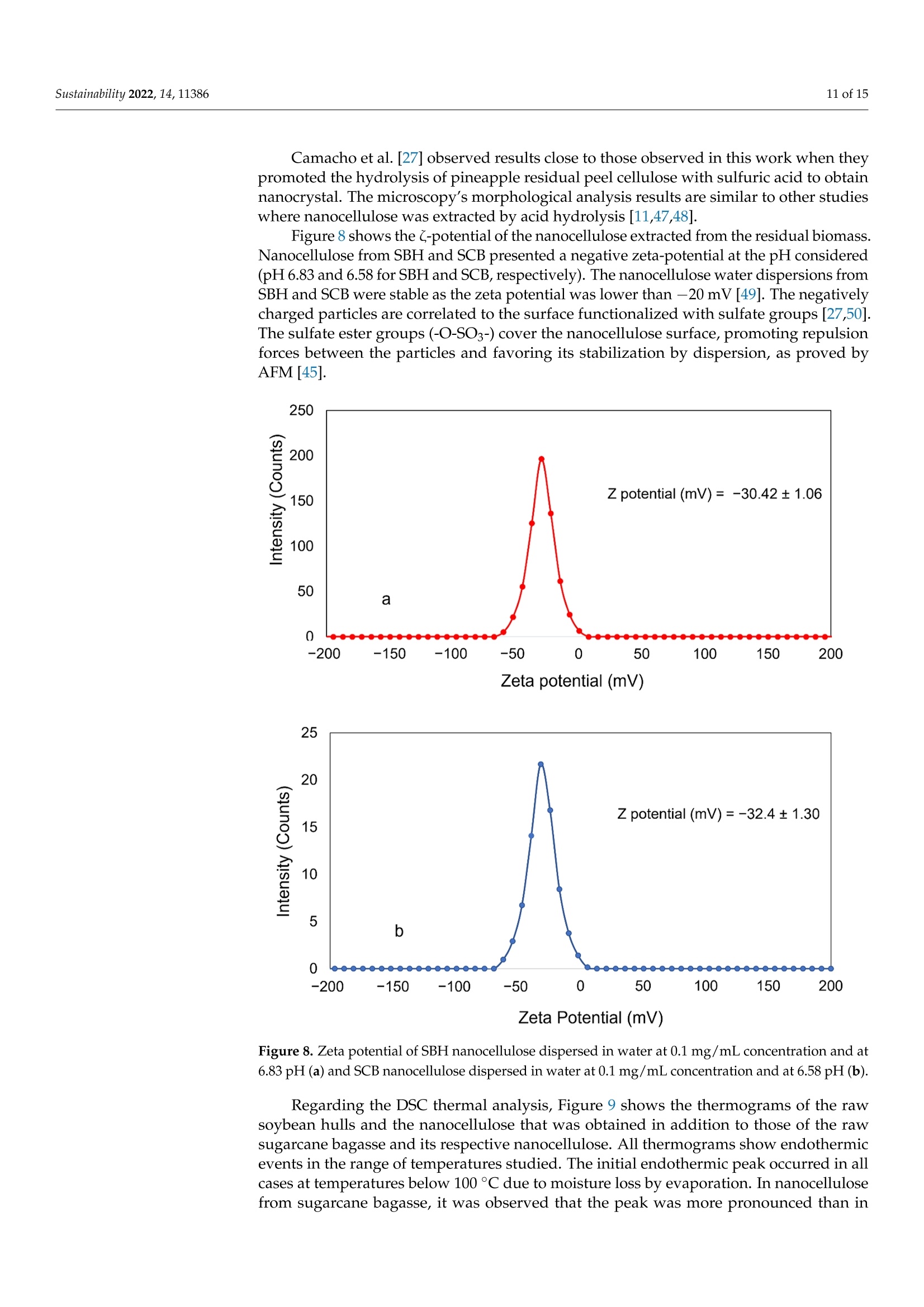
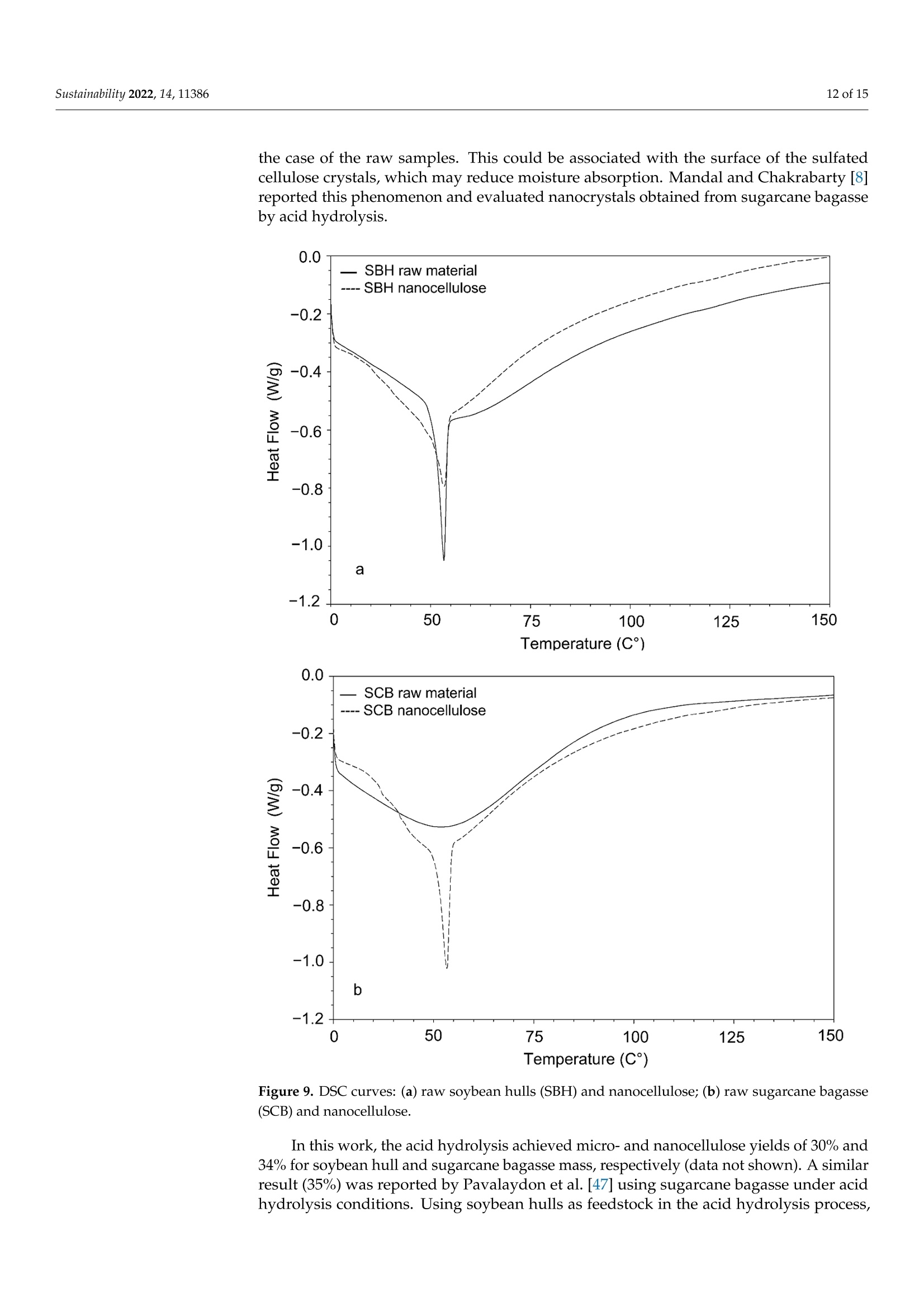
从巴拉圭剩余农业工业生物质中提取的纳米纤维素:提取过程、理化和形态学表征Nanocellulose Extracted from Paraguayan Residual Agro-Industrial Biomass Extraction Process, Physicochemical and Morphological CharacterizationMDPIsustainability 2 of 15Sustainability 2022,14,11386 Citation: Velazquez, M.E.; Ferreiro,O.B.;Menezes, D.B.; Corrales-Urefia, Y.; Vega-Baudrit, J.R.; Rivaldi,J.D.Nanocellulose Extracted fromParaguayan Residual Agro-IndustrialBiomass: Extraction Process, Physicochemical and MorphologicalCharacterization. Sustainability 2022,14,11386. https://doi.org/10.3390/su141811386 Academic Editors: Maria CristinaArea and Maria Evangelina Vallejos Received: 7 July 2022 Accepted: 21 August 2022 Published: 10 September 2022 Publisher's Note: MDPI stays neutralwith regard to jurisdictional claims inpublished maps and institutional affil-iations. Copyright: @ 2022 by the authors.Licensee MDPI, Basel, Switzerland.This article is an open access articledistributed under the terms andconditions of the Creative CommonsAttribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/) 2 Facultad de Ciencias Quimicas, Universidad Nacional de Asuncion, San Lorenzo 111421, Paraguay 3 Laboratorio Nacional de Nanotecnologia LANOTEC, CENAT, Pavas, San Jose 10109, Costa Rica 4 Faculty of Production Engineering, University of Bremen, Am Fallturm 1, 28359 Bremen, Germany 5 Laboratorio de Polimeros, Escuela de Quimica, Universidad Nacional, Heredia 40104, Costa Rica 2 Correspondence: danielrivaldi@gmail.com Abstract: Residual biomasses from agro-industries in Paraguay, including soybean hulls (SBHs) andsugarcane bagasse (SCB), were studied as a source for nanocellulose extraction for the first time. Forthat purpose, both biomasses were delignified in a semi-pilot stainless-steel reactor, and the cellulosepulp was subjected to a bleaching process with NaClO (2.5%,w/o). The nanocellulose (CNC) wasobtained after two-step acid hydrolysis. Firstly, the bleached cellulose was hydrolyzed with HCl (17%,w/w) for two hours at 60 °C to obtain microcrystals by removing most of the amorphous fraction.The celluloses were then treated with H2SO4 (65%,w/w) at 45C for 45 min to obtain nanocellulose.Physicochemical and morphological properties were analyzed using attenuated total reflectanceFourier transform infrared spectroscopy (ATR-FTIR), thermogravimetric analysis (TGA), scanningelectron microscopy (SEM), atomic force microscopy (AFM), and X-ray diffraction analysis (XRD).The SBHs nanocellulose had a whisker-like form with a 230 ± 42 nm diameter and a 12±2nmheight, and the SCB nanocellulose had a fibril-like form with a 103 ±30 nm diameter and a heightof 6 ±3 nm. The nanocellulose from SBHs and SCB had good thermal stability as its degradationtemperature started at 250 C. Furthermore, the nanocellulose obtained was negatively charged andformed stable dispersion in water at 0.1 mg/mL concentration and a pH of around 6.5. Keywords: residual biomass; soybean hulls; sugarcane bagasse; acid hydrolysis; nanocellulose 1. Introduction The Paraguayan economy is highly dependent on the agricultural and forestry exportsector [1]. The processing of agricultural raw materials leads to the generation of largevolumes of residual biomass, including sugarcane bagasse, soybean hulls, rice husks, andcorn residues [2]. These residues are generally used for animal feed or cover soils, providingorganic matter and protection against erosion. The current major challenge in the world is to develop technologies that allow thevalorization of such residues to increase production efficiency by obtaining high-value-added products, such as micro- and nanocellulose [3]. The SBH and SCB biomasses containcellulose, hemicellulose, and lignin. Cellulose is a linear polysaccharide with more than4000 monomeric glucose units linked by B-1,4-glycosidic bonds, representing between30 and 50% of the biomass composition, with highly crystalline structural fractions andamorphous fractions [4-7]. The interest in the extraction and use of micro-and nanocellulose from waste biomasshas shown remarkable growth in recent years due to the renewable nature, availability, and, mainly, the physicochemical and mechanical properties of this type of material [8-10].CNC consists of rod-shaped cellulose crystals or fibrils, with widths and lengths of 5-70 nmand between 100 nm and several micrometers, respectively [11-13]. Nanocellulose extraction requires the treatment of the biomass by chemical and/orphysical methods, such as ultrasonic technique, microwave radiation, enzymatic hydrol-ysis, and alkaline and acid hydrolysis [14,15], to obtain pure cellulose, followed by thetransformation to cellulose nanocrystals [16]. Usually, alkaline hydrolysis followed by acidhydrolysis of residual biomass using sulfuric acid and or hydrochloric acid allows rapidremoval of amorphous cellulose to obtain highly crystalline nanofibers [5]. The utilizationof sulfuric acid allows the stabilizing of the colloidal system formed by the CNC due to theesterification of the hydroxyl groups by sulfate ions, giving rise to a sulfate group (-O-SO3-)on the surface. When hydrochloric acid is used instead of sulfuric acid, the tendency is toform aggregates of CNC [17-20]. Wang et al [20] demonstrated that the combination ofsulfuric and hydrochloric acids allows better thermal stability of the nanocellulose thanwhen using pure sulfuric acid. Besides the traditional inorganic acids cited, nitric acid, phosphoric acid, oxalic acid,maleic acid, citric acid,p-toluenesulfonic acid, and mixtures of organic and inorganic acidshave also been used for micro- and nanocellulose extraction [21-25]. The size of nanocellu-lose fibers or CNCs varies depending on the acid concentration, hydrolysis temperature,crystallinity degree, and the starting cellulose polymerization degree [4,10,26,27]. Nanocellulose exhibits high stiffness and mechanical strength, with elastic modulusbetween 150 and 250 GPa, flexural strength in the range of 13-24 GPa, high specific surfacearea, and properties that enable its use for reinforcing and increasing the strength of othermaterials [13]. This material finds essential applications in the food industry as an emulsionstabilizer and thickener, reinforcing filler for polymers used for packaging, and as anexcipient in the pharmaceutical and cosmetic industries [10,28]3].. The availability of soybean hulls and sugarcane bagasse residues in Paraguay makesthis country a potential nanocellulose supplier. These residual biomasses are characterizedby a high cellulose content relative to the lignin content (less than 22% w/w), in additionto their abundance and spatial distribution in the Paraguayan territory (Figure 1). Theseconsiderations highlight the potential of these residues for obtaining micro- and nanocellu-lose. In this respect, considering the residue per product rate (RPR), corresponding to 0.06 tsoybean hulls/t soybean and 0.290 t bagasse/t sugarcane, and the average annual process-ing volume of these agriculture feedstocks for the period 2015-2020, an average amount ofsugarcane bagasse and soybean hulls in Paraguay of 1,950,000 t/year and 211,000 t/year,respectively, was estimated [2,29]. In the first instance, it could indicate that cost is the limiting factor for obtaining cellu-lose and micro- and nanocellulose from these biomasses. In Paraguay, the cost of soybeanhulls exceeds USD 120/t, while sugarcane bagasse costs USD 24/t [30,31]. However, the es-timated annual volume of cellulose nanomaterials required (for manufacturing packaging,paper coating, and fabrics) is about 33 million metric tons for the global market. Its marketis expected to grow from USD 346 million in 2021 to USD 963 million by 2026 [32]. Theestimated prices for cellulose nanomaterials range from USD 1100/ton to USD 4400/ton [9].This way, the added value that could be given to both residual biomasses could reacharound 40 times if we assume that the residue values are those mentioned above. This work describes nanocellulose extraction from SBH and SCB using bleaching,alkaline, and acid hydrolysis processes in Paraguay for the first time. Characterization tech-niques such as scanning electron microscopy (SEM), atomic force microscopy (AFM),attenu-ated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR), X-ray diffractionanalysis (XRD), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC),and zeta potential analysis were used to analyze the extracted materials. Figure 1. Geographical distribution of soybean and sugarcane processing industries in Paraguay 2. Material and Methods 2.1. Materials Soybean hulls (Figure 2a) were provided by Cargill S.A (Minga Guazu, Paraguay),and sugarcane bagasse (Figure 2b) was obtained from Destileria Luis Mussi (Piribebuy,Paraguay). The residual biomasses were dried at 105°C for 24 h, finely ground in a hammermill, and sieved with 400 and 300 um mesh sieves. The cellulose, hemicellulose, and lignincontent of the finely separated material was characterized. 2.2. Residual Biomass Characterization Crude fiber, neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid de-tergent lignin (ADL) were determined in an automated fiber analyzer, Fibretherm FT 12(C. Gerhardt GmbH & Co. KG Konigswinter, Germany), using the FibreBag method. Theanalysis complies with the standard methods specified by Weender and van Soest. Thesamples were digested with H2SO4 and NaOH in a filter bag for the fiber content determi-nation. Cellulose and hemicellulose content was calculated by subtracting the ADL andADF value, respectively, from NDF. Crude ash content was determined according to thestandard method ISO 5984:2002. 2.3. Alkaline Hydrolysis The raw materials were subjected to alkaline hydrolysis in a 10 L stainless-steel reactor(Ingest S.A, Buenos Aires, Argentina) in a 2-stage process to remove lignin, accordingto Camacho et al. [27]. In both stages, the ratio of solid to liquid used was 1:10. In thefirst stage, hydrolysis was carried out using NaOH (20%, w/v) for 1.5 h at 70 °℃ andwashing with distilled water. Then, the material was hydrolyzed again to increase thelignin removal using NaOH (12%, w/v) for 1.0 h at the same temperature. The materialwas filtered on polypropylene mesh and washed with deionized water to neutral pH. Theresulting cellulose was kept wet for the bleaching process. Figure 2. Soybean hulls (a) and sugarcane bagasse (b) used as feedstock for nanocellulose extraction. 2.4. Bleaching Process The delignified cellulose was subjected to a bleaching process with NaClO (2.5%,w/v)at 60 °C for 2 h. The bleached cellulose pulp was separated by filtration and washed withdeionized water several times to remove the bleaching agent. 2.5. Nanocellulose Preparation The applied protocol was based on the process reported by Camacho et al. [27] fornanocellulose extraction from pineapple residues. The bleached cellulose was subjectedto acid hydrolysis in two stages. Firstly, a partial hydrolysis was performed using an HClsolution (17%w/w) for 2 h at 60 °C to obtain microcellulose, followed by hydrolysis withH2SO4 (65%, w/w) at 45°C under constant stirring for 45 min to prepare nanocrystallinecellulose. This step was carried out on a smaller scale. At the end of the process, the solidmaterial was filtered and washed with deionized water repeatedly until neutral pH wasachieved. The suspension containing nanocellulose was sonicated in an ultrasonic bath for15 min in order to form a stable suspension and then dialyzed on a regenerated cellulosemembrane for 24 h to remove excess acid. Finally, the samples were frozen and lyophilizedfor preservation [27]. 2.6. Physicochemical and Structural Characterization of Materials 2.6.1. ATR-FTIR Analysis The previously pulverized and dried samples were analyzed, and the spectra wererecorded on a Thermo ScientificTM Nicolet 6700 spectrometer (Thermo Scientific, Waltham,MA, USA), with a spectral resolution of 4 cm-l and a spectral range of 500-4000 cm-1. Theresults were analyzed with OMNIC 8.1 software (OMNIC Series 8.1.10, Thermo FischerScientific, Waltham,MA,USA) [27]. 2.6.2. Superficial Morphology The material morphology was analyzed using a scanning electron microscope (SEM),JEOL JSM-6390LV (Jeol USA Inc., Peabody, MA, USA), at an accelerating voltage of 10 kVwith secondary electrons (SEI) and a spot size of 40. The hydrolyzed and raw samples werepreviously coated with a 10 um gold film. 2.6.3. Topographic Analysis The topography of the nanocellulose samples was analyzed using an atomic forcemicroscope (AFM), Asylum Research (Oxford Instruments, Santa Barbara, CA, USA),operated in air tapping mode. The silicon AFM (Tap150AL-G) probe with the back sideof the cantilever covered with Al (Aluminum) was operated at a resonant frequency of150 kHz and a constant force of 5 N/mn. 2.6.4. Thermogravimetric Analysis Thermogravimetric analysis (TGA) was performed using a TGA-Q500 thermogravi-metric analyzer (TA Instruments, New Castle,DE,USA), equipped with Universal Analysis2000 software version 4.5A (TA Instruments, New Castle, DE,USA). Samples of approxi-mately 5.8 mg were placed in a pre-weighed standard platinum tray and the analysis wasconducted with a nitrogen purge flow rate of 10 mL/min for the balance and a purge flowrate of 90 mL/min for the sample. Initially, the equipment was kept at equilibrium at 25°℃for 1 min. Subsequently, heating was performed at 10 C/min, and changes in mass wererecorded in a temperature range from 25 to 1000 °℃. 2.6.5. Crystallinity Analysis Samples were analyzed using an X'Pert3 Powder Diffractometer (Malvern Panalytical,Malvern, UK) using a nickel-filtered CuK& radiation at 45 kV and 40 mA, 20 in the rangeof 10-40° with 0.03° and 1.5 s spacing. The degree of crystallinity was calculated using thefollowing equation (Equation (1)): where Ioo2 = is the maximum intensity at 20=22.3°; Iam = corresponds to the intensity of the minimum. 2.6.6. Differential Calorimetric Analysis The thermal behavior of the samples was studied in a DSC Q200 differential scanningcalorimeter (TA Instruments, New Castle, DE, USA). The approximately 2.3 mg samplewas heated from 20 to 150°C at a heating rate of 5°℃/min. Thermograms were used todetermine thermal events related to initial melting and crystallization temperatures. 2.6.7. Zeta Potential Measurements Zeta potential (() of aliquots of the aqueous suspension of CNC was measured usinga Zetasizer Nano S90 (Malvern Panalytical, Malvern, UK) at 入1=628 nm and 入2=523 nm,without adjusting ionic strength. The <-potential was determined by electrophoretic mo-bility of the particles of SBH and SCB in solution (0.1 mg/mL) at a pH of 6.83 and 6.58,respectively. Five measurements were conducted for each suspension, and the mean andstandard deviation were reported. 2.7. Nanocellulose Extraction Efficiency The efficiency of micro- and nanocellulose extraction from soybean hulls and sugarcanebagasse was calculated according to Equation (2): where: Po = mass of feedstock submitted to hydrolysis,g P1 = mass of micro- and nanocellulose from the acid hydrolysis, g; Xo = cellulose mass fraction in the feedstock. 3. Results and Discussion Table 1 shows the soybean hull and sugarcane bagasse raw material composition. Ascan be seen, both residues exhibit a high content of cellulose relative to the lignin content.This allows the use of a less aggressive process to carry out cellulose extraction. Table 1. Composition of agro-industrial residual biomass for nanocellulose extraction. Parameter Soybean Hull Sugarcane Bagasse Method Crude fiber,% 41.32 47.48 FibreBag Neutral detergent fiber,% 62.38 76.66 FibreBag Acid detergent fiber,% 49.79 58.79 FibreBag Acid det1e1rgent lignin,% Cellulose,% 2.66 14.64 FibreBag 47.13 44.5 Calculated Hemicellulose,% 12.59 17.87 Calculated Ash,% 4.30 8.45 ISO 5984:2002 Figure 3 shows the ATR-FTIR spectra of sugarcane and soybean bagasse raw materialand of samples after alkaline hydrolysis. Although peaks from the hemicellulose and ligninoverlap with the ones from the cellulose, the spectra of the biomass subjected to chemicaltreatment presented peaks characteristic of cellulose. Samples of biomasses subjected toalkaline treatment were analyzed for the presence of aryl groups, characteristic of lignin.Structurally, lignin is a three-dimensional macromolecule of aromatic nature composedof aryl ethers connected by various bonds that form a complex and amorphous branchedstructure [33]. The peak related to the vibration of distinct C-O aryl groups is observedin the range of 1250-1255 cm-1 (Figure 3a-c), present in both sugarcane bagasse and rawsoybean hulls [4,34]. However, this peak is not observed in samples chemically treated. The peak at 1730 cm-in the FTIR spectra of raw SCB and raw SBHs is predominantlyattributed to the C=O vibration of the acetyl and uronic ester groups of hemicellulose orthe ester bond of the carboxylic group of ferulic and p-coumaric acids of lignin [8,35]. Forthe alkaline-treated samples, no comparable peaks were observed in the spectra of treatedbiomass, indicating that most of the hemicellulose and lignin was removed. For the raw soybean hulls, the peaks around 1300-1400 cm-1 could be attributed tothe presence of hemicellulose and lignin, which are not presented in the spectra of thesample treated by alkaline hydrolysis [36]. The raw materials and alkaline chemically treated spectra present a broad band in theregion of 3600-3100 cm-1, characteristic of a stretching vibration of O-H groups in cellulosemolecules [26]. Moreover, the spectra showed peaks corresponding to the vibration of C-Hbonds close to 2890 cm-1 and those corresponding to C-H and C-O bonds of polysacchariderings at 1330-1360 cm-1 [27]. The peak at 1060 cm- in all samples is associated withcellulose, which has a higher intensity in the alkaline-treated samples [37]. In this sense, the SBH spectra showed that the alkaline hydrolysis affected the cellu-lose structure as can be seen from the changes observed in the peaks at 2900 cm-l and1640 cm-1. In the nanocellulose spectra of sugarcane bagasse and soybean hulls, a bandat 900-910 cm- is distinguishable and associated with B-glycosidic bonds of crystallinecellulose molecules [8,38]. Wavenumber (cm-1) Wavenumber (cm-) Figure 3. ATR-FTIR spectroscopy of alkali-treated soybean hulls (a), raw soybean hulls (b), alkali-treated sugarcane bagasse (c), and raw sugarcane bagasse (d) for wavelengths in the range of4000-500 cm-- Figure 4 shows the X-ray spectra of the product obtained after the acid treatment ofthe biomasses. The extracted cellulose from SBH (Figure 4a) and SCB (Figure 4b) displayeda typical cellulose XRD pattern, with a predominance of Cellulose type I. Cellulose I isthe most abundant form of cellulose obtained from plants, bacteria, fungi, and algae.Typically, this cellulose consists of two phases, a one-chain triclinic structure correspondingto cellulose Ix and a cellulose Ip with a two-chain monoclinic structure [4,5,39]. Diffracted peaks observed in both samples of nanocellulose at 20 around 22° (plane200) and 34°(plane 004) represent the lattice planes of this cellulose structure that ischaracteristic of native cellulose [36]. The peak observed around 12° (plane 101) in bothdiffractograms could be attributed to cellulose type II. Additionally, in Figure 4a, a charac-teristic peak of cellulose II at 20° (plane 101) is observed. According to Tao et al. [15], thechemical treatment conditions affect the hydrogen bond and van der Waals force whichsignificantly influences the cellulose's crystallinity and crystal lattice. Sulfuric acid couldsolubilize the cellulose type I, which is re-precipitated as cellulose type II, and this effectis more pronounced with a long hydrolysis time [11]. In Figure 4b, the diffracted peakobserved around 16° is presented in lignocellulosic materials [37]. This result suggests thatthe SCB has a higher resistance to the alkaline treatment than the soybean hulls. The crystallinity index was calculated from Equation (1), obtaining values between 60and 63%. This indicates that acid hydrolysis was efficient for obtaining cellulose crystalsfrom SBHs and SCB. The degree of crystallinity of the cellulose obtained was similar to thosereported in the literature for microcrystals of various lignocellulosic biomasses, confirmingthat the method used is feasible, mainly because of its ease and low cost [15,40-43]. 20 (degree) Figure 4. X-ray spectrum of cellulose extracted by acid hydrolysis from soybean hulls (a) andsugarcane bagasse (b). The thermograms of nanocrystalline cellulose from SBHs and SCB are presented inFigure 5. Water loss was observed at around 100 C, represented by the slightly negativeslope at the beginning of the curve for all samples analyzed. For the nanocrystallinecellulose samples (Figure 5a,b), a more pronounced mass reduction w20CWas observed00°C and 350 °C wh betweene n c o m p a re d t o t h e th e rm o gr a v VS8imetric curve of the raw samples. It ispossible to observe a difference in the decomposition temperature of the starting biomassand nanocellulose, which correlates with the rapid reduction in the molecular weight ofcellulose in the biomass during acid hydrolysis. Temperature (C°) Figure 5. TGA thermograms of soybean hull (SBH) nanocellulose and raw soybean hulls (a), sugar-cane bagasse (SCB) nanocellulose and raw sugarcane bagasse (b). The morphology of the biomasses and nanofibers was analyzed by scanning electronmicroscopy. Figure 6 shows SEM images of nanofibers obtained from soybean hulls andthe starting material. Figure 6a shows the surface morphology of the raw soybean hulls,which is characterized by an irregular flat surface, with no fibrous components identifiedin the structure. In contrast, Figure 6b shows the presence of fibers with a diameter of lessthan 1 um. The morphology analysis was made difficult because a freeze-dried sample ofthe suspension containing nanoparticles was used, resulting in the superposition of layers. 10kV X2,5004110pm 000010 40 SEI10kV X4,500 5pm 000001140 SEI (a) (b) Figure 6. Scanning electron microscopy (SEM) of raw soybean hulls (a) and alkali-treated soybeanhulls (b) Numerous reports indicate that acid treatment of previously delignified celluloseremoves the amorphous fraction and that nanoscale diameter cellulose fibers are sub-sequently exposed [8,27,36,44]. Figure 7 shows the AFM images of cellulose nanofibersthat were obtained. Figure 7c,d shows a cross-section of the nanocellulose. In both cases,nanoparticles were obtained. The morphology of the SBH was not fibrillar but whisker-like.On the other hand, the SCB nanoparticles exhibited a fibril-like form. The whisker-likeform of the SBH nanocellulose obtained was 230±42 nm in diameter and 12 ±2nm inheight, and the fibril-like form of the SCB nanocellulose was 103 ± 30 nm in diameter and6 ±3 nm in height. Figure 7. AFM images from SBH (a) and SCB (b-d). Cross-section line profiles highlighted in (a,b),respectively Camacho et al. [27] observed results close to those observed in this work when theypromoted the hydrolysis of pineapple residual peel cellulose with sulfuric acid to obtainnanocrystal. The microscopy’s morphological analysis results are similar to other studieswhere nanocellulose was extracted by acid hydrolysis [11,47,48]. Figure 8 shows the <-potential of the nanocellulose extracted from the residual biomass.Nanocellulose from SBH and SCB presented a negative zeta-potential at the pH considered(pH 6.83 and 6.58 for SBH and SCB, respectively). The nanocellulose water dispersions fromSBH and SCB were stable as the zeta potential was lower than -20 mV [49]. The negativelycharged particles are correlated to the surface functionalized with sulfate groups [27,50].The sulfate ester groups (-O-SO3-) cover the nanocellulose surface, promoting repulsionforces between the particles and favoring its stabilization by dispersion, as proved byAFM [45]. Figure 8. Zeta potential of SBH nanocellulose dispersed in water at 0.1 mg/mL concentration and at6.83 pH (a) and SCB nanocellulose dispersed in water at 0.1 mg/mL concentration and at 6.58 pH(b). Regarding the DSC thermal analysis, Figure 9 shows the thermograms of the rawsoybean hulls and the nanocellulose that was obtained in addition to those of the rawsugarcane bagasse and its respective nanocellulose. All thermograms show endothermicevents in the range of temperatures studied. The initial endothermic peak occurred in allcases at temperatures below 100 °℃ due to moisture loss by evaporation. In nanocellulosefrom sugarcane bagasse, it was observed that the peak was more pronounced than in the case of the raw samples. This could be associated with the surface of the sulfatedcellulose crystals, which may reduce moisture absorption. Mandal and Chakrabarty [8]reported this phenomenon and evaluated nanocrystals obtained from sugarcane bagasseby acid hydrolysis. —SCB raw material ---- SCB nanocellulose -0.2 -0.4 -0.6 -0.8 -1.0 b -1.2 Temperature (C°) Figure 9. DSC curves: (a) raw soybean hulls (SBH) and nanocellulose; (b) raw sugarcane bagasse(SCB) and nanocellulose. In this work, the acid hydrolysis achieved micro- and nanocellulose yields of 30% and34% for soybean hull and sugarcane bagasse mass, respectively (data not shown). A similarresult (35%) was reported by Pavalaydon et al. [47] using sugarcane bagasse under acidhydrolysis conditions. Using soybean hulls as feedstock in the acid hydrolysis process, Flauzino Neto et al. [11] achieved nanocellulose yields of 8% and 20% after 30 and 40 minof acid hydrolysis, respectively. Katakojwala et al. [51] reported cellulose and nanocelluloseextraction yields of 34% and 15% from soybean hulls and sugarcane bagasse, respectively.In another study, cellulose nanocrystals from royal palm tree agro-industrial waste wereobtained by strong acid hydrolysis synthesis at different times and temperatures, achievingyields in the range of 7.8-48.8% [51]. 4. Conclusions In this study, alkaline treatment and consecutive hydrolysis with sulfuric acid andhydrochloric acid was effective for obtaining CNC from soybean hulls and sugarcanebagasse residues, as evidenced by AFM. The products obtained exhibited the forms offibers and whiskers with a diameter of less than 230 nm and maximum height of 20 nm. Ahigh crystallinity (63%) was determined by XRD analysis; this indicates potential for theirapplication to reinforce other types of materials such as films or polymeric membranes. Thenanocellulose from SBH and SCB demonstrated good thermal stability as its degradationtemperature started at 250°C, which indicates its potential for reinforcement of differentmaterials. Furthermore, the nanocrystals were negatively charged and formed stabledispersion in water. Therefore, the present study has significant importance as it indicatesan alternative process to produce value-added products, such as CNC, from residualbiomass in Paraguay. The data presented can contribute to the implementation of futurepolicies on biomass utilization for production and the installation of biorefinery facilitiesin Paraguay. Author Contributions: Conceptualization, M.E.V. and O.B.F.; methodology, M.E.V., O.B.F., J.D.R.,D.B.M. and Y.C.-U.; formal analysis, M.E.V.,O.B.F., J.D.R. and J.R.V.-B.; investigation, M.E.V., O.B.F.,J.D.R. and D.B.M.; writing-original draft preparation, J.D.R. and O.B.F; writing-review and editing,J.D.R., O.B.F., J.R.V.-B. and Y.C.-U. All authors have read and agreed to the published version ofthe manuscript. Funding: Financial support was received from Consejo Nacional de Ciencia y Tecnologia (CONACYT,Paraguay) and the Fondo para la Excelencia de la Educación y la Investigacion (FEEI) providedthrough the project identification PINV18-128. Institutional Review Board Statement: Not applicable for studies not involving humans or animalsInformed Consent Statement: Not applicable. Acknowledgments: The authors would like to thank Hyun Ho Shin for his invaluable assistance. Conflicts of Interest: The authors declare that they have no known competing financial interest orpersonal relationships that could have appeared to influence the work reported in this paper. Abbreviations CNC cellulose nanocrystal SBHs soybean hulls SCB sugarcane bagasse ATR-FTIR attenuated total reflectance Fourier transform infrared spectroscopy TGA thermogravimetric analysis SEM scanning electron microscopy AFM atomic force microscopy XRD X-ray diffraction analysis RPR residue per product rate NDF neutral detergent fiber ADF acid detergent fiber ADL acid detergent lignir H extraction efficiency mass of micro- and nanocellulose from the acid hydrolysis mass of feedstock submitted to hydrolysis cellulose mass fraction in the feedstock 2 maximum intensity at 20 =22.3° References 1. SSEE. Subsecretaria de Estado de Economia 2020. Ministerio de Hacienda. Perfil Economico y Comercial-Paraguay—Junio 2020.Available online: https://economia.gov.py/application/files/1115/9231/4944/Perfil_Economico_y_Comercial_de_Paraguay.pdf(accessed on 20 December 2021). 2. MAG-2022. Ministerio de Agricultura y Ganaderia. Sintesis Estadisticas. Available online: http://www.mag.gov.py/index.php/institucion/dependencias/sintesis-estadistica (accessed on 20 December 2021). 3. Torgbo, S.; Quan, V.M.; Sukyai, P. Cellulosic value-added products from sugarcane bagasse. Cellulose 2021, 28,5219-5240.ICrossRef 4 Plermjai, K.; Boonyarattanakalin, K.; Mekprasart, W.; Pavasupree, S.; Phoohinkong, W.; Pecharapa, W. Extraction and charac-terization of nanocellulose from sugarcane bagasse by ball-milling-assisted acid hydrolysis. AIP Conf. Proc. 2018, 2010,020005.[CrossRefl 5. Liu, H.M.; Li, H.Y. Application and conversion of soybean hulls. In Soybean-The Basis of Yield, Biomass and Productivity;IntechOpen: London, UK, 2017.[CrossRef] 6. Haldar, D.; Purkait, M.K. Micro and nanocrystalline cellulose derivatives of lignocellulosic biomass: A review on synthesis,applications and advancements. Carbohydr. Polym. 2020, 250,116937. [CrossRef] [PubMed] 7. Ho, N.W.Y.; Ladisch, M.R.; Sedlak, M.; Mosier, N.; Casey, E. Biofuels from Cellulosic Feedstocks. In Comprehensive Biotechnology;Elsevier: Amsterdam, The Netherlands, 2011; pp.51-62. [CrossRef] 8. Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr.Polym. 2011, 86, 1291-1299.[CrossRef] 9 Cowie, J; Bilek, E.T.; Wegner, T.H.; Shatkin, J.A. Market projections of cellulose nanomaterial-enabled products-Part 2:Volume estimates. TAPPI J. 2014,13,57-69. Available online: https://www.fs.usda.gov/treesearch/pubs/46175 (accessed on4 April 2022). [CrossRef] 10. Thakur, V.; Guleria, A.; Kumar, S.; Sharma, S.; Singh, K. Recent advances in nanocellulose processing, functionalization andapplications: A review. Mater. Adv. 2021,2,1872-1895. [CrossRef] 11. Flauzino Neto, W.P.; Silverio, H.A.; Dantas,N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals fromagro-industrial residue-Soy hulls. Ind. Crop. Prod. 2013, 42,480-488.[CrossRef] 13. Kafy, A.; Kim, H.C.; Zhai, L.; Kim, J.W.; Hai, L.V; Kang, T.J. Cellulose long fibers fabricated from cellulose nanofibers and itsstrong and tough characteristics. Sci. Rep. 2017, 7, 17683. [CrossRef] 14. Pang, Z.; Wang, P.;Dong, C. Ultrasonic pretreatment of cellulose in ionic liquid for efficient preparation of cellulose nanocrystals.Cellulose 2018,25,7053-7064. [CrossReff] 15. Tao, P.;Zhang, Y.; Wu, Z.; Liao, X.; Nie, S. Enzymatic pretreatment for cellulose nanofibrils isolation from bagasse pulp: Transitionof cellulose crystal structure. Carbohydr. Polym. 2019,214,1-7. [CrossRef] [PubMed] 16. Trache, D.; Tarchoun, A.F; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From fundamen-tals to advanced applications. Front. Chem. 2020, 8,392. [CrossRef] 17. Huang, S.; Liu, X.; Chang, C.; Wang, Y. Recent developments and prospective food-related applications of cellulose nanocrystals:A review. Cellulose 2020,27,2991-3011. [CrossRef] 18. Phanthong, P.;Reubroycharoen, P;Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. CarbonResour. Convers 2018,1,32-43. [CrossRef] 19. Rojas, J. Current Trends in the Production of Cellulose Nanoparticles and Nanocomposites for Biomedical Applications. InCellulose: Fundamental Aspects and Current Trends; Bedoya, M., Ed.; IntechOpen: London, UK, 2015; pp. 193-228. 20. Wang,N.;Ding, E.; Cheng, R. Preparation and liquid crystalline properties of spherical cellulose nanocrystals. Langmuir 2008, 24,5-8. [CrossRef] [PubMed] 21. Li, D.; Henschen, J; Ek, M. Esterification and hydrolysis of cellulose using oxalic acid dihydrate in a solvent-free reaction suitablefor preparation of surface-functionalised cellulose nanocrystals with high yield. Green Chem. 2017,19,5564-5567.[CrossRef] 22. Bian, H.; Luo, J.; Wang, R.; Zhou, X.;Ni, S.; Shi, R.;Dai, H. Recyclable and reusable maleic acid for efficient production of cellulosenanofibrils with stable performance. ACS Sustain. Chem. Eng. 2019,7,20022-20031. [CrossRef] 23. Yu, H.; Abdalkarim, S.Y.H.; Zhang, H.; Wang, C.; Tam, K.C. Simple process to produce high-yield cellulose nanocrystals usingrecyclable citric/hydrochloric acids. ACS Sustain. Chem. Eng. 2019,7,4912-4923.[CrossRef] 25. Cheng, M.; Qin, Z.; Hu, J.; Liu, Q.; Wei, T.; Li, W. Facile and rapid one-step extraction of carboxylated cellulose nanocrystals byH2SO4/HNO3 mixed acid hydrolysis. Carbohyd. Polym. 2020, 231,115701. [CrossRef] 26. Jiang, F.; Hsieh, Y.L. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr. Polym.2013,95,32-40. [CrossRef][PubMed] 27. Camacho, M.; Urena, Y.R.; Lopretti, M.; Carballo, L.B.; Moreno, G.; Alfaro, B.; Baudrit, J.R. Synthesis and characterization ofnanocrystalline cellulose derived from pineapple peel residues. J. Renew. Mater. 2017,5,271-279.[CrossRef] 28. Fotie, G.; Limbo, S.; Piergiovanni, L. Manufacturing of food packaging based on nanocellulose: Current advances and challenges.Nanomaterials 2020,10,1726.[CrossRef] [PubMed] 29. Koopmans, A.; Koppejan, J. Agricultural and Forest Residues Generation, Utilization, and Availability. In Proceedings ofthe Regional on Modern Applications of Biomass Energy, Kuala Lumpur, Malaysia, 6-10 January 1997. Available online:http://www.fao.org/3/ad576e/ad576e00.pdf (accessed on 20 May 2022). 30. Rodas, R.I.; Cano, V.E.; Frutos, M.L. Analisis de la cadena de valor de la soja en el Paraguay: Chain value analysis of soybean andits manufactures in Paraguay. S. Fla. J. Dev. 2021,2,7412-7429. [CrossRef] 31. Fleck, J.C. Estudio de Factibilidad Economica del Uso del Bagazo de Cana de Azúcar para la Obtencion de Papel de Impresion yEscritura en el Paraguay. Master’s Thesis, Universidad Nacional de Misiones, Garupa, Argentina, 2009. 32. Research and Market. 2022. Available online: https://www.researchandmarkets.com/reports/5009171/global-nanocellulose-market-by-type-mfc-and-nfc#rela4-5305030 (accessed on 20 May 2022). 33. Dorrestijn, E.; Laarhoven, L.J.J; Arends,I.W.C.E.; Mulder, P. The occurrence and reactivity of phenoxyl linkages in lignin and lowrank coal. J. Anal. Appl. Pyrolysis 2000,54,153-192. [CrossRef] 34. Merci, A.; Urbano, A.; Grossmann, M.V.E.; Tischer, C.A.; Mali, S. Properties of microcrystalline cellulose extracted from soybeanhulls by reactive extrusion. Food Res. Int. 2015, 73,38-43.[CrossRef] 35. Huang, S.; Zhou, L.; Li, M.C.; Wu, Q.; Zhou, D. Cellulose nanocrystals (CNCs) from corn stalk: Activation energy analysis.Materials 2017, 10,80. [CrossRef] 36. Nang An, V.;Nhan, C.; Thuc, H.; Tap, T.D.; Van, T.T.T.; Van Viet, P.; Van Hieu, L. Extraction of high crystalline nanocellulose frombiorenewable sources of Vietnamese agricultural wastes. J. Polym. Environ. 2020,28,1465-1474.[CrossRef] 37. Debiagi,F.; Faria-Tischer, P.; Mali, S. Nanofibrillated cellulose obtained from soybean hull using simple and eco-friendly processesbased on reactive extrusion. Cellulose 2020,27,1975-1988.[CrossRef] 38. Pappas,C.; Tarantilis,P.A.;Daliani, I.; Mavromoustakos, T.;Polissiou, M. Comparison of classical and ultrasound-assisted isolationprocedures of cellulose from kenaf (Hibiscus cannabinus L.) and eucalyptus (Eucalyptus rodustrus Sm.). Ultrason. Sonochemistry2002,9,19-23. [CrossRef] 39. Sugiyama, J.; Vuong, R.; Chanzy, H. Electron diffraction study on the two crystalline phases occurring in native cellulose from analgal cell wall. Macromolecules 1991,24,4168-4175. [CrossRef] 40. Frone, A.N.;Chiulan, I.;Panaitescu,D.M.;Nicolae, C.A.; Ghiurea, M.; Galan, A.M. Isolation of cellulose nanocrystals from plumseed shells, structural and morphological characterization. Mater. Lett. 2017, 194,160-163. [CrossRef] 41..CCoelho, C.C.; Michelin, M.; Cerqueira, M.A.; Goncalves, C.; Tonon, R.V.; Pastrana, L.M.; Freitas-Silva, O.; Vicente, A.A.;Cabral, L.M.; Teixeira, J.A. Cellulose nanocrystals from grape pomace: Production, properties and cytotoxicity assessment.Carbohydr. Polym. 2018,192,327-336. [CrossRef] [PubMed] 42. Sainorudin, M.H.; Abdullah, N.A.;Rani, M.S.;Mohammad,M.; Abd Kadir, N.H.; Razali,H.; Asim, N.; Yaakob, Z. Investigation ofthe structural, thermal and orphological properties of nanocellulose synthesised from pineapple leaves and sugarcane bagasse.Curr. Nanosci. 2022,18,68-77.[CrossRef] 43. Hernandez, J.A.; Soni, B.; Iglesias, M.C.; Vega-Erramuspe, I.B.; Frazier, C.E.; Peresin, M.S. Soybean hull pectin and nanocellulose:Tack properties in aqueous pMDI dispersions. J. Mater. Sci. 2022,57,5022-5035. [CrossRef] 44. Kim, D.Y.; Lee, B.M.; Koo, D.H.;Kang, P.H.; Jeun, J.P. Preparation of nanocellulose from a kenaf core using E-beam irradiationand acid hydrolysis. Cellulose 2016,23,3039-3049. [CrossRef] 45. Lin, N.; Dufresne, A. Surface chemistry, morphological analysis and properties of cellulose nanocrystals with gradiented sulfationdegrees. Nanoscale 2014, 6,5384-5393. [CrossRef] 46. Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterialcellulose. Biomacromolecules 2004, 5,1671-1677. [CrossRef] 47. Pavalaydon,K.; Ramasawmy, H.; Surroop,D. Comparative evaluation of cellulose nanocrystals from bagasse and coir agro-wastesfor reinforcing PVA-based composites. Environ. Dev. Sustain. 2022, 24, 9963-9984. [CrossRef] 48. Gond, R.K.; Gupta, M.K.; Jawaid, M. Extraction of nanocellulose from sugarcane bagasse and its characterization for potentialapplications. Polym. Compos. 2021, 42,5400-5412.[CrossRef] 49. Andrade, F.K.; Morais, J.P.S.; Muniz, C.R.; Nascimento, J.H.O.; Vieira, R.S.; Gama, F.M.P.; Rosa, M.F. Stable microfluidizedbacterial cellulose suspension. Cellulose 2019,26,5851-5864. [CrossRef] 50. Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of lignocellulosic biomass to nanocellulose: Structure and chemical process. Sci.World J. 2014,2014,631013.[CrossRef] [PubMed] 51. Katakojwala, R.;Mohan, S.V.Multi-product biorefinery with sugarcane bagasse: Process development for nanocellulose, ligninand biohydrogen production and lifecycle analysis. Chem. Eng. J. 2022,446,137233. [CrossRef]
确定







还剩13页未读,是否继续阅读?
中国格哈特为您提供《用于生产纳米纤维素的大豆皮、甘蔗渣中粗纤维、中性洗涤纤维(NDF)、酸性洗涤纤维(ADF)、酸性洗涤木质素(ADL)含量检测》,该方案主要用于纳米材料中粗纤维、中性洗涤纤维(NDF)、酸性洗涤纤维(ADF)、酸性洗涤木质素(ADL)检测,参考标准--,《用于生产纳米纤维素的大豆皮、甘蔗渣中粗纤维、中性洗涤纤维(NDF)、酸性洗涤纤维(ADF)、酸性洗涤木质素(ADL)含量检测》用到的仪器有格哈特全自动型纤维分析仪FT12
推荐专场
相关方案
更多
该厂商其他方案
更多















