方案详情
文
碱如何帮助绿茶叶片残渣中的蛋白提取:叶片综合生物炼制的基础How Does Alkali Aid Protein Extraction in Green Tea Leaf Residue A Basis for Integrated Biorefinery of Leaves
方案详情

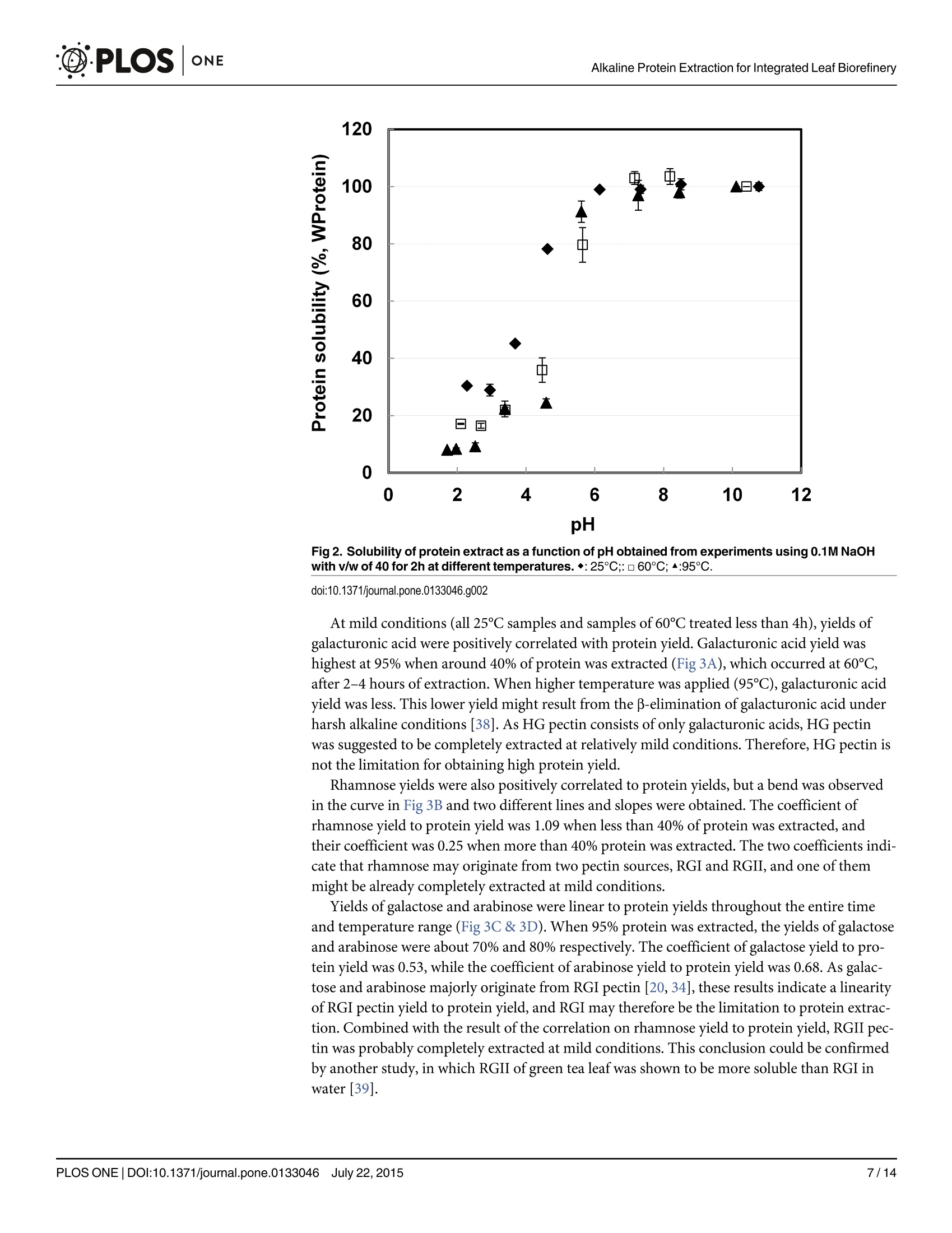
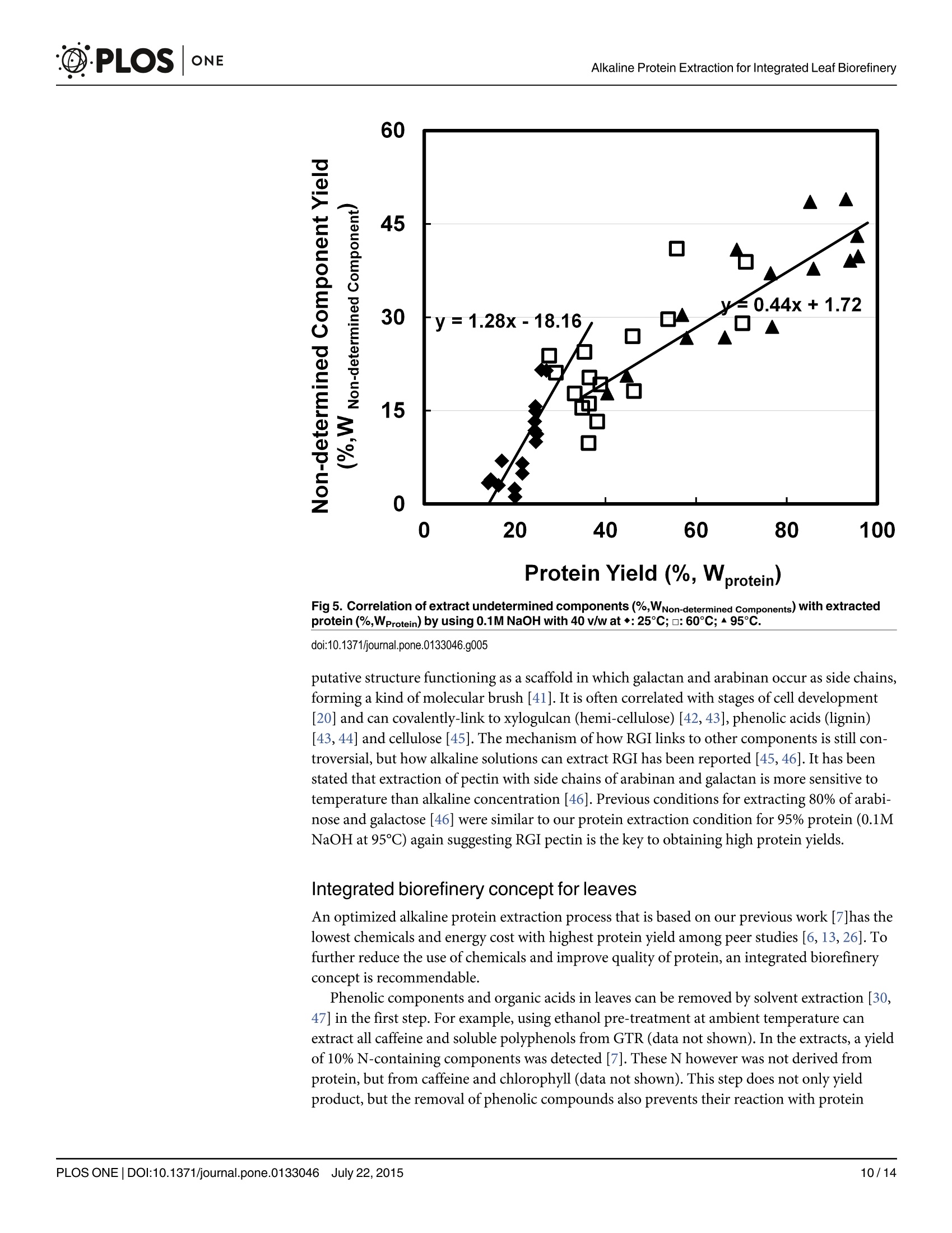
碱如何帮助绿茶叶片残渣中的蛋白提取:叶片综合生物炼制的基础How Does Alkali Aid Protein Extraction in Green Tea Leaf Residue A Basis for Integrated Biorefinery of Leaves@D·PLOSONE碱如何帮助绿茶叶片残渣中的蛋白提取:叶片综合生物炼制的基础 C·PLOSONEAlkaline Protein Extraction for Integrated Leaf Biorefinery 口 OPEN ACCESS Citation: Zhang C, Sanders JPM, Xiao TT, Bruins ME (2015) How Does Alkal i Aid Protein Extraction in Green Tea Leaf Residue: A Basis for Integrated Biorefinery of Leaves. PLoS ONE 10(7): e0133046.doi:10.1371/journal.pone.0133046 Editor: Jingdong Mao, Old Dominion Univ., U NITED STATES Received: March 9,2015Accepted: June 22,2015 Published: July 22, 2015 Copyright: @ 2015 Zhang et al . This is an open access article distributed under the terms of the C reat i ve C ommons A t tr i b u t ion L i c e n se, which permits unrestricted use, distribution, and reproduction in any medium, provided t he original author and source are credited. Data Availability Statement: All relevant data are within the paper. Funding: This research is funded by STW project (Netherlands), and it i s co-funded by DSM (h ttp ://www.d s m.co m /cou n t r ys i t e s/d s m n l /n l _N L/h o m e.h t ml ),Agrifirm (h ttp ://www.a gr i f i rm .co m /) and Mali Biocarburant (h t tp://www.m a l i bio ca rb u r a n t .c om /ma l i b io /). CZ, JS, and MB have received this grant.The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. RESEARCH ARTICLE How Does Alkali Aid Protein Extraction in Green Tea Leaf Residue: A Basis for Integrated Biorefinery of Leaves Chen Zhang1*, Johan P. M. Sanders1.2, Ting T. Xiao, Marieke E. Bruins1.2 1 Biobased Chemistry and Technology Group, AFSG, Bornse Weilanden 9, 6708WG Wageningen,Wageningen UR, the Nether l ands, 2 Food and Biobased Research I nstitute, Bornse Weilanden 9,6708WG Wageningen, Wageningen UR , t he Netherlands,3 Department of Plant Sciences, Laboratory of Molecular Biology, Droevendaalsesteeg 1,6708 PB, Wageningen, Wageningen UR, the Netherlands * chen .zha ng@wu r .n l Abstract Leaf protein can be obtained cost-efficiently by alkaline extraction, but overuse of chemicals and low quality of (denatured) protein limits its application. The research objective was to investigate how alkali aids protein extraction of green tea leaf residue, and use these results for f urther improvements i n alkaline protein biorefinery. Protein extraction yield was studied for correlation to morphology of leaf tissue structure, protein solubility and hydrolysis degree, and yields of non-protein components obtained at various conditions. Alkaline pro-tein extraction was not facilitated by increased solubility or hydrolysis of protein, but posi-tively correlated to leaf tissue disruption. HG pectin, RGII pectin, and organic acids were extracted before protein extraction, which was followed by the extraction of cellulose and hemi-cellulose. RGI pectin and lignin were both linear to protein yield. The yields of these two components were 80% and 25% respectively when 95% protein was extracted, which indicated that RGI pectin is more likely to be the key l imitation to leaf protein extraction. An integrated biorefinery was designed based on these results. Introduction Leaf protein has been considered as an additional protein source since 1960s [1, 2]. These proteins can be used in food [3], animal feed [4], or when hydrolysed to amino acids for N-chemicals bulk chemicals [5]. However, applicat i ons of these proteins are l imited by i ts low cost-efficient produc t ion [6], particularly in extraction processes. This limitation was prelim-inarily solved by using alkaline conditions at higher than 60℃ [7]. Drawbacks of this technique are overuse of chemicals and low quality of (denatured) protein. To overcome these drawbacks and design an integrated process for protein and other products, the basis of alkaline protein extraction in leaf should be better understood. Alkali might aid leaf protein extraction as a result of leaf tissue disruption. Leaf has three major tissue systems: epidermis, mesophyll , and vascular. Vascular tissues are located in Competing I nterests: This research is co-funded by SM (h tt p ://www.d s m.c o m /co u n tr ysit e s/d sm nl /nl /h o me .h t ml), Agrifirm (h t t p ://www .agrifi r m.c om /) and Mali Biocarburant (http://www.ma l i bi o c ar b u r an t .c om/ma l i b io /). T here are no patents, products in development or marketed products to declare. This does not alter the authors' adherence to all the PLOS ONE policies on sharing data and mater i als. mesophyl l tissues covered by epidermis tissue. Those tissues are adhered by lamella layer embracing a large quantity of pectin. Epidermis is a tabular and l ayered sheet of cells on surface of leaf covered by a waxy cuticle functioning as mechanical protection of mesophyl l tissue [8,9]. As most leaf proteins, including lectins, enzymes (Rubisco), storage proteins, cell wall pro-teins and some toxins [10] are located in mesophyll tissues [8, 11], disruption of epidermis and lamella might aid protein release from mesophyll tissues. Alkali might also aid leaf protein extract i on by increasing protein solubility or / and hydro-lysis degree. Most leaf proteins are considered insoluble [12-14]. These protein are hydropho-bic in neutral solution, because they are bound to other compounds, such as polyphenol [15]or polysaccharides (membrane protein) [16]. Solubility of these proteins can be increased with the increase of pH by adding alkali . At high temperature, alkali can even hydrolyse protein into small peptides [17,18], which reduces protein molecular size, and therefore increases protein solubility and accelerates protein diffusion. Finally, alkali might aid in disruption of cell wal l and thus leading to high protein yield. Leaf proteins within cells are well protected by the cell walls, which consist of middle lamella, pri-mary wall , and secondary wall [8]. In cell wall, carbohydrates (including pectin, hemi-cellulose,and cellulose) and lignin are two major components other than protein [8]. Pectin is a family of complex polysaccharides located i n primary plant cell wall and middle lamella [8, 19]. I t can be roughly divided into three types: homogalacturonan (HG), rhamnogalacturonan I (RGI),and rhamnogalacturonan I I (RGII) [20]. Hemi-cellulose and cellulose are mainly found in both primary and secondary plant cell wall , and they have a simpler composition than pectin.In comparison, lignin could be the most complicated component located in secondary plant cell wall [8]. It is a complex phenolic polymer t hat drives out water and strengthens the cell wall [8]. Solubilisation of carbohydrates and lignin performs differently under different alkaline conditions [21-24], therefore correlating yields of these components with protein yield may profile how alkali disrupts cell wall, and of f er a basis for integrated leaf biorefinery. Green tea residue (GTR) is used as a mode l material f or research on leaf biorefinery. It was demonstrated earlier that the concept of protein extraction using GTR as a model material can also be applied in Oolong tea leaf residue, Jatropha leaf , barley straw [7], and even on algae.GTR is the waste of tea leaves after hot water extraction, containing high value components,such as polyphenols (10-15%), proteins (20-30%), and carbohydrates (30-40%) [25, 26]. It is now only used for energy generation through burning. An integrated biorefinery targeting on high value components of GTR wil l increase its value. In this study, GTR was again used as a model material, and protein extracts were obtained at various alkaline extraction condi t ions based on our previous work [7]. Alkaline treated leaf tissues were analysed by microscope; solubility and hydrolysis degree of extracted protein and the composition of extracted carbohydrates was determined; extracted amounts of l ignin were estimated. These results were all correlated to protein yield to analyse how alkali aids protein extraction in GTR. Based on these results, potential of integrated biorefinery of protein and other components for leaves was discussed. Methods and Materials Ethics statement “N/A” No specific permissions were required for these locations/activities because we used local species or species present naturally in our environment. We confirm that the field studies did not involve endangered or protected species. We had the permission for the research on this green tea residue from Damin Company. Materials Green tea residue (GTR) is our main material, which is a gift from Damin Company, Fujian Province, China. This residue f rom tea lemonade production was collected from C. sinensis trees in Zhejiang province, and it was sun-dried after soaking green tea leaves in water at 85℃for 45min. Chemicals used for analysis were purchased from Sigma (USA, analytic grade) if not stated otherwise. Preparation of alkaline extracts Protein extraction was performed by soaking 0.5g GTR i n 20ml 0.1M NaOH at 25℃,60℃,and 95℃ for 2h. After subsequent centrifugation, which was always performed at 15000g for 10min (Sorvall centrifuge, Thermo Fisher Scientific, the USA), the supernatants were then stored at -20℃ for further analysis . The corresponding solid residues were collected and washed with water for 3 times, and then they were i mmersed in water and analysed by micros-copy immediately. For analysing the correlations of carbohydrates or lignin with protein, samples were made by soaking 0.5g GTR in 20ml 0.1M NaOH at 25℃, 60℃, and 95℃ over time (5min-24h).Sam-ples were freeze-dried and stored a t room temperature till further use. Visualization of leaf tissues Solid samples obtained after alkaline treatment for 2h, as well as a control of untreated material and a control treated by 0.05M NaOH, were examined under the microscope (SMZ-U,Nikon,Japan). Pictures were taken with a BCE-C050 Camara (Mightex, US) that was fitted on to the microscope. Determination of protein properties Protein content of extracts. Protein content (g L`) was determined by Kjeldahl method [27], using Kjeldahl equipment from Gerhardt , which consist of digestion unit (Gerhardt Kjel-dahlterm) and rapid dist i llation unit (Gerhardt Vapodest). Kjeldahl measures all nitrogen,including non-protein N-containing components, such as caffeine, chlorophyll , and theobro-mine [28]. However, we assumed that it is all protein and used a conversion factor of 6.25 to calculate protein concentrations. Protein extract i on yield was calculated as extracted protein /total protein * 100%. Protein hydrolysis degree. F Protein hydrolysis degree i s defined as the percentage of cleaved peptides bonds. As the amount of fully hydrolysed protein f rom GTR is t he same for each sample, here we use the difference of-NH2 residue before and after hydrolysis to discuss the hydrolysis degree. The content of-NH residue was determined by a modified o-phthaldial-dehyde (OPA ) method [29]. 1.5ml OPA reagent (OPA 0.88g L,dithiothreitol 0.88g L ’, SDS lgL, and NazB40710H,O 38.1g L ) was mixed with 200ul serine (0.1g L`'), sample, or water (as a blank). After exactly 2 min, absorbance of the mixture was determined spectrophotomet-rically at 340nm. Concentration of-NH2 residue was calculated using serine as a reference. Protein solubility. Alkaline extracts were diluted to a protein concentration of 10gL.Protein solutions pHs were adjusted to 2-11 using 0.5 M HCl , after which the solution was kept stirring at room temperature for 1 h. Samples were subsequently centrifuged at 15000g for 10min at room temperature and the supernatant was collected. The amount of soluble protein in the filtrate was determined by Lowry method (Sigma, Lowry total protein determination kit)using bovine serum albumin as standard. Solubility i s presented as a percentage of total protein in weight. July 22,2015 Water and ash content Samples were pre-weight and dried for 24 hours at 60℃ to evaporate all water. After cooling down in a desiccator, the residual weights of samples were measured. Then, samples were transferred to a 550℃ furnace for 16h to burn off all the organic matter. After cooling in a des-iccator, the crucibles were weighed. Water content and ash content were calculated as weight percentage of the starting material. Polyphenol content Content of tea polyphenols i n tea extracts was determined spectrophotometrically with scaling down reagent usage [30, 31]. Polyphenols content was calculated assuming a concentration of polyphenols of 3.914g L leads to an adsorption of 1 at 540 nm after r eaction. Galacturonic acid determination Galacturonic acid content was determined as anhydro-uronic acid by an automated m-hydro-xydiphenyl assay with an auto-analyzer (Skalar Analytical BV, Breda, The Netherlands) [32].Galacturonic acid (Fluka AG, Buchs, Switzerland) was used as a reference in a concentration range from 12.5 to 200 mg L. Neutral sugar composition Freeze dried alkaline extracts were pre-hydrolysed with 72% (w/w) sulphuric acid at 39℃ for 1 h, followed by hydrolysis in 1 M sulphuric acid at 100℃ for 3 h. Monosaccharides were analysed using GC according to Englyst's method [33]. Inositol was used as internal standard.Response factor was determined using a standard sugar solution of L-(+)-rhamnose,L-(+)-arabinose,D-(+)-xylose,D-(+)-mannose,D-(+)-galactose (97%), D-(+)-glucose (99,5%)with concentrations of1 gL. To quantify carbohydrates, indirect assays are often used. Samples are hydrolysed to mono-sugars, and these sugars are quantified by HPLC or GC [33].HG pectin consists only of galact-uronic acid of which some of the carboxyl groups are methyl esterified [20, 34].HG pectin can be analysed by galacturonic acid content. RGI consists of a backbone of repeating disaccharide of galacturonic acid and rhamnose. A variety of different glycan chains (principally arabinan and galactan) are attached to the rhamnose residues. As RGI pectin is predominated by side chains [20, 34] mainly constituted from arabinose and galactose, it can be analysed by the con-tents of these two mono-sugars. In comparison, RGII has a backbone of HG with complex side chains attached to the galacturonic acid [20, 34]. Therefore RGI I analysis needs information of all sugars contents. Hemi-cellulose’s backbone comprises of xylose and glucose, while cellulose is a linear chain of glucose [23, 24]. All the experiments were performed in duplicate, and all results were plotted in the figures. Results Influence of alkali on leaf tissues As mentioned, leaf consists of three major tissues systems adhered by middle lamella. To study the disruption of leaf tissues under alkaline conditions, alkali treated GTRs were analysed micro-scopically. Although GTR is t he leftover of green tea l eaves treated by hot water, the structure of its tissue is sti l l intact . Shown in F ig 1A , the epidermal layer of untreated GTR was normally attached to the mesophyl l tissue and the lamella was not solubilized, shown as a low transpar-ency of leaf tissue and visible f ragments suspended in the solution. After treatment with 0.1M NaOH, leaf tissues were transparent and solutions became clear (F ig 1C, 1D and 1E ). At 25℃, July 22,2015 C d Fig 1. Morphology of GTR tissues after a 2h treatment with 40 v/w solution (scale bar: 100pm). a. GTR treated with water at 25°C. b. GTR treated with 0.05M NaOH at 95°C. c. GTR treated with 0.1M NaOH at 25C. d. GTR treated with 0.1M NaOH at 60°C. e. GTR treated with 0.1M NaOH at 95°C. doi:10.1371/journal.pone.0133046.g001 the epidermal layer was st i ll attached to the mesophyl l tissue (F i g 1C), but i t started to peel off when higher temperature was applied (F i g 1D a n d 1E ). As protein yield increased with the increase of temperature (23% obtained by 25℃, 38% obtained by 60℃, and 84% obtained by 95℃), these figures indicate a correlation of leaf tissue disruption with protein yield under alka-line conditions. When alkaline concentration was limited to 0.05M, GTR tissues (Fi g 1B ) are similar as the untreated one (Fig 1A ). The protein yield obtained by using 0.05M NaOH at 95℃ was about 40% [7], which was similar to the yield obtained by 0.1M NaOH at 60℃ (38%). However, the July 22,2015 transparency of the tissue treated with 0.05M NaOH was l ower than that treated by 0.1M NaOH. This indicates t hat alkali was used to solubilize substances between cells, while high temperature was related to protein located inside cells or membrane, which is commonly iden-tified as insoluble [16]. To f urther understand how does alkali aids in protein extraction, influ-ence of alkali on protein hydrolysis and solubility was tested, and yields of non-protein components were subsequently analysed and correlated to protein yields. Composition of GTR To calculate yields of all components in alkaline condition, composition of GTR was analysed.Carbohydrate and protein (or more accurate: all N-containing components) are the two major components, accounting for 31% and 27% of GTR. Other components are polyphenol (8%),water (7%), and ash (6%). The residual undetermined part majorly consists of l i pid (wax,organic acids) and lignin [35]. As l ipid and lignin will not be extracted by the pre-treatment with hot water that was done i n the factory, the ratio of lipid to lignin (2:6.5) presented in green tea leaf [35] i s estimated to remain constant in GTR, leading to 5% and 17% of dry GTR respectively. Carbohydrates were quantified by summing all mono-sugars. Glucose and galact-uronic acid are t he major sugar components constituting 13.4% and 7.6% of GTR, followed by galactose, arabinose, and xylose with percentages of 3.4%, 2.8%, and 2.1% of GTR respectively.Other less abundant sugars are mannose with 1.1%, rhamnose with 0.8%, and fucose with 0.4%. Influence of alkali on protein hydrolysis and solubility Generally, 1g native protein already contains 0.342-0.457 mmol-NH2 groups , when protein is fully hydrolysed,-NH2 content will increase to 8.6 mmol depending on amino acid composition [7,29]. To compare hydrolysis degree, protein extracts obtained using 0.1M NaOH with v/w of 40 for 2h at 25℃, 60℃, and 95℃ were tested by OPA method. The protein yields were 23%at 25℃, 38% at 60℃, and 84% at 95℃. In these samples,-NH2 content in 1g protein was 0.43±0.03 mmol , 0.54±0.07 mmol, 0.19 ± 0.02 mmol respectively. Those numbers are close to the value of native protein, suggesting that only very limited hydrolysis occurred. When 95℃ was applied, the-NH2 content was even reduced. This may result from reactions of-NH2with other components, such as polyphenol [36]. Protein hydrolysis degree did not correlate with protein yield. To determine the correlation of protein solubility with protein yield, protein solubil i ty was measured as a function of pH. Samples from three temperatures, as mentioned above, were used and the result is depicted in F i g 2. At pH higher t han 6, protein solubility of al l samples remained at the maximal, added amount of 10g L. This is over t wice the amount of extracts from other research [26, 37]. Based on these results, we conclude that protein solubi l ity was not a limitation to protein yield. Below pH 6, solubility of all three protein extracts decreased.The 95℃ sample had its lowest protein solubility at pH<4.5. In other cases solubil i ty of hydro-lysed protein increased between pH 2-6 when more severe hydrolysis had happened [17,18].This again indicates that protein was not severe ly hydrolysed after alkaline treatment at 95℃.Alkaline protein extraction is therefore not facilitated by hydrolysis or increased solubility of protein. Correlation of carbohydrates yields with protein yields Pectin. As mentioned in introduction, galacturonic acid, rhamnose, galactose, and arabi-nose can be used to analyse pectin yields and types. Yields of galacturonic acid, rhamnose,galactose, and arabinose i n protein extracts were plotted against protein yields in Fig 3. July 22,2015 12 Fig 2. Solubility of protein extract as a function of pH obtained from experiments using 0.1M NaOH with v/w of 40 for 2h at different temperatures.◆:25℃;:□60°C;▲:95℃. doi:10.1371/journal.pone.0133046.g002 At mild conditions (all 25℃ samples and samples of 60℃ treated less than 4h), yields of galacturonic acid were positively correlated with protein yield. Galacturonic acid yield was highest at 95% when around 40% of protein was extracted (Fi g 3A ), which occurred at 60℃,after 2-4 hours of extraction. When higher temperature was applied (95℃), galacturonic acid yield was l ess. This lower yield might result from the p-elimination of galacturonic acid under harsh alkaline conditions [38]. As HG pectin consists of only galacturonic acids, HG pectin was suggested to be completely extracted at relatively mild conditions. Therefore, HG pectin is not the l imitation for obtaining high protein yield. Rhamnose yields were also positively correlated to protein yields, but a bend was observed in the curve in Fi g 3B and two different lines and slopes were obtained. The coefficient of rhamnose yield to protein yield was 1.09 when less than 40% of protein was extracted, and their coefficient was 0.25 when more than 40% protein was extracted. The two coefficients indi-cate that rhamnose may originate from two pectin sources , RGI and RGII, and one of them might be already completely extracted at mild conditions. Yields of galactose and arabinose were linear to protein yields throughout the entire time and temperature range (Fi g 3C & 3D ). When 95% protein was extracted, the yields of galactose and arabinose were about 70% and 80% respectively. The coefficient of galactose yield to pro-tein yield was 0.53, while t he coefficient of arabinose yield to protein yield was 0.68. As galac-tose and arabinose majorly originate from RGI pectin [20, 34], these results indicate a linearity of RGI pectin yield to protein yield, and RGI may therefore be the limitation to protein extrac-tion. Combined with the result of the correlation on rhamnose yield to protein yield, RGII pec-tin was probably completely extracted at mild conditions. This conclusion could be confirmed by another study, in which RGII of green tea leaf was shown to be more soluble than RGI in water [39]. July 22,2015 a C Fig 3. Weight based correlation of extracted pectin related sugars with extracted protein (%,WProtein) by using 0.1M NaOH with 40 v/w at ◆:25;:60°℃;95°C. a. Galacturonic acid (%,WGalacturonic Acid). b. Rhamnose (%,WRhamnose).c. Galactose (%,WGalactose). d.Arabinose (%,WArabinose). doi:10.1371/journal.pone.0133046.g003 Hemi-cellulose& cellulo o s s e e.. To analyse the correlation of the solubil i sed hemi-cellulose and cellulose with protein extraction, yields of xylose and glucose were measured and plotted against protein yields. Generally, yields of xylose and glucose are less than 8% (F i g 4A and 4B )with a highest yield of around 20% only at t he harshest condition applied, when al l protein had already been extracted. The yield coefficients of these two sugars to protein are therefore rela-tively l ow, 0.06 for xylose and 0.05 for glucose. Considering some xylose and glucose may be emanated f rom side chains of RGI and RGII pectin, the correlation slopes of these two sugars with protein are close to zero. Therefore, high protein yield is probably not due to disruption of hemi-cellulose or cellulose. Correlation of non-determined component yields with protein yields To investigate the influence of lignin on protein extraction, yields of non-determined compo-nents (likely to be majorly lignin and lipid) were plotted against yields of protein (Fi g 5). Gener-ally, yields of non-determined components were positively correlated to protein yields, but a bend was observed in the curve of F ig 5 and two correlation slopes were calculated. When pro-tein yield was l ess than 40%, the yield coefficient of non-determined components to protein was 1.28, which was almost 3 t imes higher as when protein yield was above 40%. The two coefficients might indicate that the yields of two major non-determined components, lipid and lignin, have July 22,2015 Fig 4. Correlation of extracted cellulose and hemi-cellulose related sugars with extracted protein (%,WProtein) by using 0.1M NaOH with 40 v/w at ◆:25℃; o :60℃;^95℃.a. Xylose (%,Wxylose). b.Glucose (%,WGlucose). doi:10.1371/journal.pone.0133046.g004 different correlation with yields of protein, and one of them was completely extracted at mild condition. As lipid can be released with mild alkaline while lignin was insoluble in the same con-dition [23, 40], lipid possibly contributed to the higher slope while l ignin contributed to the lower (0.44). The extractability of lignin showed a maximum of only 25% of total lignin at 95%protein extraction. This means that it is either not necessary to completely dissolve lignin to facil-itate protein extraction, or that another component is limiting protein extraction. Discussion How does alkali aid protein extraction in leaves Although t he role of lignin in protein extraction is still unclear, t he correlation of RGI extrac-tion yield with protein extraction yield i s clearly shown. In leaf cell wall , RGI i s rooted in a July 22,2015 c o o 'c o Z Fig 5. Correlation of extract undetermined components (%,WNon-determined Components) with extracted protein (%,WProtein) by using 0.1M NaOH with 40 v/w at◆:25C;o :60℃;^95℃. doi:10.1371/journal.pone.0133046.g005 putative structure functioning as a scaffold in which galactan and arabinan occur as side chains,forming a kind of molecular brush [41]. It is often correlated with stages of cell development [20] and can covalently-link to xylogulcan (hemi-cellulose) [42, 43], phenolic acids (lignin)[43, 44] and cellulose [45]. The mechanism of how RGI links to other components is still con-troversial, but how alkaline solutions can extract RGI has been reported [45, 46]. It has been stated that extraction of pectin with side chains of arabinan and galactan is more sensitive to temperature t han alkaline concentration [46]. Previous conditions for extracting 80% of arabi-nose and galactose [46] were similar to our protein extraction condition for 95% protein (0.1M NaOH at 95℃) again suggesting RGI pectin is the key to obtaining high protein yields. Integrated biorefinery concept for leaves An optimized alkaline protein extraction process that is based on our previous work [7]has the lowest chemicals and energy cost with highest protein yield among peer studies [6, 13,26].To further reduce the use of chemicals and improve quality of protein, an integrated biorefinery concept is recommendable. Phenolic components and organic acids in leaves can be removed by solvent extraction [30,47] in the first step. For example, using ethanol pre-treatment at ambient temperature can extract all caffeine and soluble polyphenols from GTR (data not shown). In the extracts, a yield of 10% N-containing components was detected [7]. These N however was not derived from protein, but from caffeine and chlorophyll (data not shown). This step does not only yield product, but the removal of phenolic compounds also prevents their reaction with protein July 22,2015 Fig 6. Integrated leaf biorefinery concept. Numbers are estimated based on results with green tea residue (GTR). doi :10.1371/journal.pone.0133046.g006 under alkaline conditions, and may thereby also improve the digestibility of the f inal protein product [48]. Using relatively mild alkaline conditions (20℃3.0.c o;2-b 45. Zykwinska AW, Ralet M-CJ, Garnier CD, Thibault J-FJ. Evidence for In Vitro Binding of Pectin Side Chains to Cellulose. Plant Physiol. 2005;139(1):397-407. doi : 10.1104/pp.105.065912 PMID:16126855 46. Zykwinska A, Rondeau-Mouro C, Garnier C, Thibault J-F,Ralet M-C. Alkaline extractability of pectic arabinan and galactan and their mobility in sugar beet and potato cell walls. Carbohydr Polym. 2006;65(4):510-20. doi: h ttp ://dx .d oi.o r g/10.1016/j .c a rb p ol .2006.02.012. 47. Durling NE, Catchpole OJ, Grey JB, Webby RF, Mitchell KA, Foo LY, et al . Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol-water mixtures. Food Chem. 2007;101(4):1417-24. doi: h ttp://d x .d oi .or g/10.1016/j.f oo dc hem.2006.03.050. 48. de Toledo NMV, Rocha LC, da Silva AG, Canniatt i Brazaca SG. Interaction and digestibility of phaseo-lin/polyphenol in the common bean. Food Chem. 2013;138(2-3):776-80. doi: http ://dx.d oi .org/10.1016/j .f oodch e m.2012.11.079. doi :10.1016/j.f ood c h e m.2012.11.079 PMID:23411175 49. Testova L,Nieminen K, Penttila PA, Serimaa R, Potthast A, Sixta H. Cellulose degradation in alkaline media upon acidic pretreatment and stabilisation. Carbohydr Polym. 2014; 100:185-94. doi : h t t p://dx.d oi .o rg /10.1016/j .c a rbp o l .2013.01.093. doi: 10.1016/j .c a rbpo l.2013.01.093 PMID: 24188853 50. Maas RH, Bakker RR, Jansen ML, Visser D, de Jong E, Eggink G, et al. Lactic acid production from lime-treated wheat straw by Bacillus coagulans: neutralization of acid by fed-batch addition of alkaline substrate . Appl Microbiol Biotechnol.2008;78(5):751-8. Epub 2008/02/06. doi: 10.1007/s00253-008-1361-1 PMID: 18247027;PubMed Central PMCID: PMC2270352. July 22,2015
确定











还剩12页未读,是否继续阅读?
中国格哈特为您提供《绿茶渣碱提残留物中蛋白质含量的检测》,该方案主要用于茶叶中营养成分检测,参考标准《GB 5009.5 食品安全国家标准 食品中蛋白质的测定》,《绿茶渣碱提残留物中蛋白质含量的检测》用到的仪器有格哈特带自动进样器凯氏定氮仪VAP500C、格哈特快速干燥仪STL56、格哈特凯氏消化系统KT8S、格哈特维克松废气实验室废物处理系统涤气VS、德国加液器MM、凯氏消化管
该厂商其他方案
更多