小叶霉诱导云杉预降解阶段对真菌和细菌群落的影响Impact of Norway spruce pre-degradation stages induced by Gloeophyllum trabeum on fungal and bacterial communities
方案详情

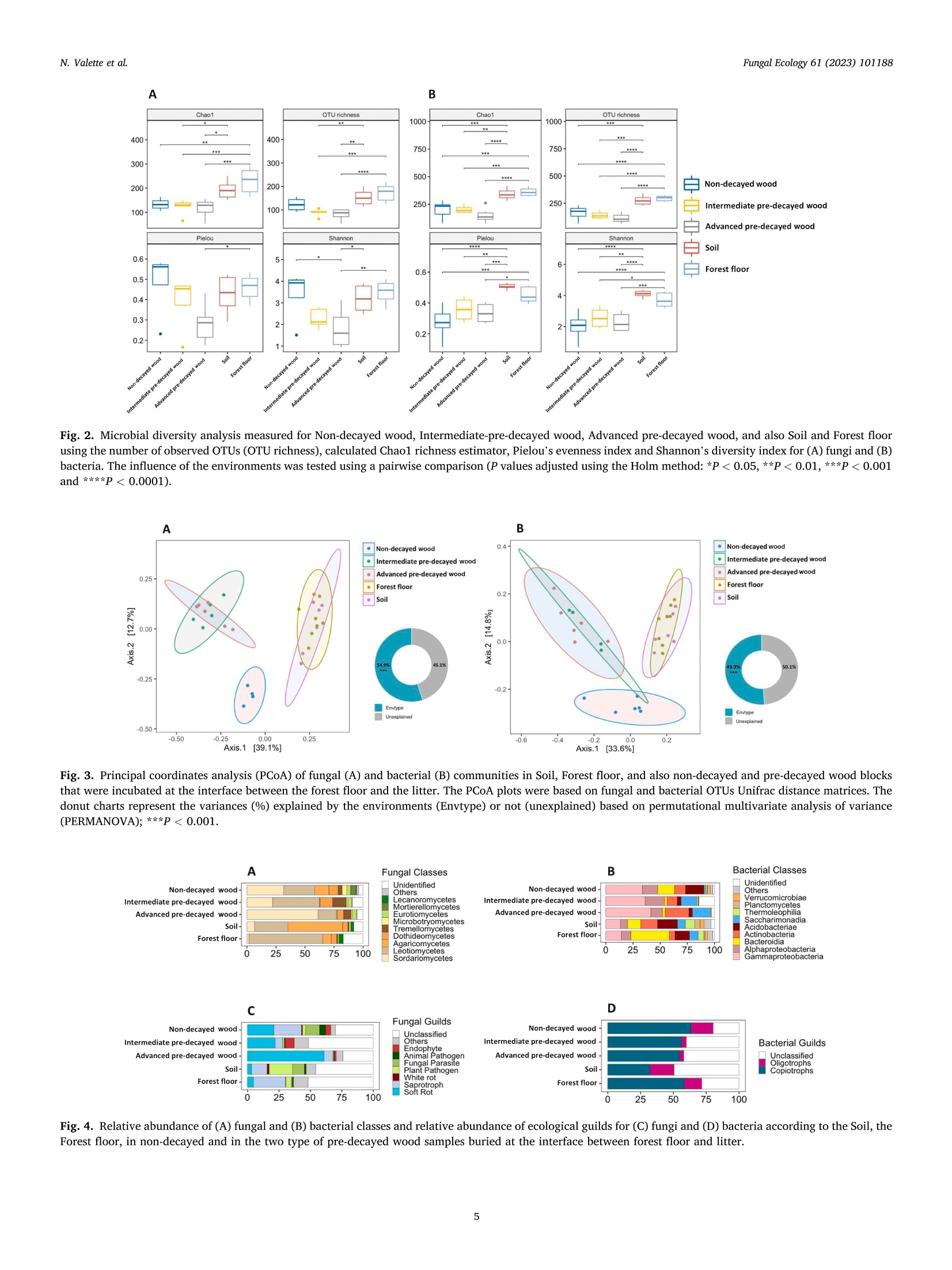
小叶霉诱导云杉预降解阶段对真菌和细菌群落的影响Impact of Norway spruce pre-degradation stages induced by Gloeophyllum trabeum on fungal and bacterial communitiesFungal Ecology 61 (2023)101188Contents lists available at ScienceDirect N. Valette et al.Fungal Ecology 61 (2023) 101188 Fungal Ecology ELSEVIER journal homepage: www.elsevier.com/locate/funeco 小叶霉诱导云杉预降解阶段对真菌和细菌群落的影响 Impact of Norway spruce pre-degradation stages induced by Gloeophyllum trabeum on fungal and bacterial communities Nicolas Valette a b ,*, Arnaud Legout , Barry Goodell, Gry Alfredsen , Lucas Auer,Eric Gelhaye , Delphine Derrien b 5.*** aUniversite de Lorraine, INRAE , IAM, F-54000 Nancy, France INRAE, BEF, F -54000 Nancy, France Goodell Laboratory, Department of Microbiology, University of Massachusetts, Amherst, MA, United States d Norwegian Institute of Bioeconomy Research (NIBIO), Division of Forests and Forest Resources , Wood Technology, NO-1431, As, Norway ARTICLEINFO ABSTRACT Corresponding Editor: Yu Fukasawa Keywords:Fungi Bacteria Wood Decay Microbial colonization In forest ecosystems, fungi are the key actors in wood decay. They have the capability to degrade l ignified substrates and the woody biomass of coniferous forests, with brown rot fungi being common colonizers. Brown rots are typically involved in the ear l iest phase of l i gnocellulose breakdown, which therefore influences colo-nization by other microorganisms. However, few studies have focused on the impact of introducing decayed wood into forest environments to gauge successiona l colonization by natural bacteria l and fungal communi t ies following partial decay. This study aimed to address this i ssue by invest i gat i ng the bacterial and fungal colo-nization of Norway spruce (Picea abies) wood, after intermediate and advanced laboratory-based, pre-decay, by the brown rot fungus Gloeophyllum trabeum. Using Illumina metabarcoding, the i n situ colonization of the wood blocks was monitored 70 days after the blocks were placed on the forest floor and covered with litter. We observed significant changes in the bacterial and fungal communities associated with the pre-decayed stage.Further, the wood substrate condi t ion acted as a gatekeeper by reducing richness for both microbia l communi t ies and diversity of funga l communities. Our data also suggest that the growth of some fungal and bacterial species was driven by similar environmental conditions. 1. Introduction Forests cover ~30% of the global terrestrial area, but forest soils account for more than 50% of the total soil organic carbon stocks (C ar v al h a i s e t a l., 2014). In forest ecosystems, coarse woody debris is the primary source of biomass for soil organic matter (SOM) formation. I t is composed pr i marily of polysaccharides and lignin at 65-80% and 20-35% (M a o e t a l ., 2019), respectively. The Basidiomycota fungi play a central role in the decomposition of l igni f ied substrates, such as wood.They are the major players able to remove or circumvent the lignin barrier that hinders access to plant polysaccharides, which are the major plant components that can support microbial growth (A r a nt es a nd G o odel l , 2014). Although i t is recognized that intergradation between different types of wood decay can occur (R il e y et al ., 2014), the decay fungi are classically categorized into two distinct, but related functional groups of Basidiomycota: the brown rot and white rot fungi (Fl o u das e t a l ., 2012). Indeed, the emergence of the brown rot l ineage is reported to be the resul t of multiple evolutionary events which occurred i n white rot ancestors with the contraction of gene families involved in lignin decomposition (Ea s t wo od et al., 2011). White rot fungi are sub-divided into species able to decompose cellulose, hemicelluloses and lignin s i multaneously, and species which selectively depolymerize lignin before polysaccharides, primarily using enzymatic systems. Brown rot fungi employ a different biodegradative strategy and they can ren carbohydrates from plant tissues by disrupt i ng and then leaving a res-idue of modified lignin. The brown rot fungi generate hydroxyl radicals via what has been described as a chelator-mediated Fenton (CMF) re-action (Good el l e t a l ., 2017). These hydroxy l radicals depolymerize l i gnin, which then allows polysaccharides to di f fuse out o f the cel l wal l where fungal enzymes would be located around the fungal hyphae (Go o de ll et al., 2017; Zhu et a l., 2020). CMF reactions may also provide fungal enzymes greater accessibi l ity to the wood ce l l wall depending on * Corresponding author . Universite de Lorraine, INRAE, IAM, F-54000 Nancy, France. ** Corresponding author. INRAE, BEF, F-54000 Nancy, France. E-mail addresses: val ette .n i colas 2@gmai l .c om (N. Valette), de lp hin e.d er rien@in ra e .f r (D. Derrien). Received 18 January 2022; Received in revised form 19 July 2022; Accepted 21 July 2022Available online 27 September 2022 the type/species of wood and fungi that are involved (Ara n te s a nd G o ode l l , 2014; Good e ll e t al., 2017; P r e s ley e t a l ., 2018). A third type of wood decay known as soft rot is produced primari l y by wood-rotting Ascomycota species (B la nc h et t e 2000). Soft rot is typically localized on the outer few millimeters of the wood surface and the fungi responsible for this wood decay are categorized as either (i ) fungi forming cavities in the secondary cell wall (Type-I) or (i i ) fungi eroding primarily the surface of the secondary cell wal l (Type-II) (G o odell 2020;B la nchette 2000). They possess all the enzymatic machineries required for carbohydrate and lignin degradation (K am e s hw ar e t a l ., 2016). The order and timing for species colonization of wood residues during community assembly can affect species abundance and functional di-versity, affecting wood decay rates (Fukami , 2015; Z h a ng e t a l., 2016,2019). Relative to the decay of wood in soils, the ability of the microbial community to establ i sh as they are impacted by the intrinsic properties of the ecosystem is also very important relative to the successional establishment of primary and secondary microbial species (D i ck i e e t a l .,2012; Zhalnina e t al ., 2015; L o pez -M o nde ja r et a l ., 2015; H i sc ox e t a l.,2016). In living trees, dead wood is usually already colonized by fungi before it fal l s to the forest floor (Bo d d y, 2001). In contrast, J ohnsto n et al. (2016) suggested that deadwood bacteria are not endophytes but establish primarily from soil contact, and would therefore potentially be selected in association with a soil fungal colonizer or by fungi that have already colonized living trees. The importance of the primary fungal colonizer is underlined by several field experiments where the presence of different decay fungi in wood both filtered and regulated the estab-l i shment of secondary microbial communities (H i sc ox et a l ., 2018;Hi scox e t a l., 2016; J oh ns to n e t al., 2019; Ch risto fides et al .,2019). Recently, V i o tt i e t a l. (2021) highlighted the different bacterial and fungal community structure dynamics occurring between oak sapwood and heartwood, suggesting that wood physiochemical properties are important i n the microbial assemblage history. Indeed, wood decay rates were correlated with the chemical composition of wood, and decay was strongly reduced both by increased lignin and extractives content (Kah l et al ., 2017). Heartwood extractives are known to have a composi t ion and level of antifungal activity that varies widely with tree species (V a le t t e et a l ., 2017). This variabi l ity influences the colonization of wood by fungi that must detox i fy many of these heartwood chemicals (Pe r r o t e t a l ., 2020). Consequently, wood physiochemical properties are important drivers of microbial wood colonization, and they especially influence the establishment of the pioneer organisms in succession.Thus, during wood decay processes, both fungi and bacteria modify wood physiochemical properties, and particularly in heartwood, t hey must first employ specialized secreted metabolites to cope with extrac-tives, while concurrently enabling cellular machinery to deconstruct the major wood cell wal l polymers. While some studies have already addressed the influence of pr i mary colonizing fungi on bacterial or fungal colonization strategies, little attention has been paid to examining joint fungal-bacterial colonization and succession. Most studies have focused on the primary colonizing organisms and not on the chemical proper t ies of the decaying wood substrate (Fo lm a n e t a l ., 2008; Her v e e t a l ., 2014; H i s co x e t a l., 2016;Jo hn st o n et a l ., 2019; C h ri s t of id e s e t a l ., 2019). To help fi l l this knowledge gap, we carried out a natural soi l incubation experiment using Norway spruce (Picea abies) blocks that had previously been incubated with the brown rot fungus Gloeophyllum trabeum (pre--decomposition) to produce spruce blocks decayed to different degrees.The aim of this work was to examine the succession of organisms found in coniferous wood i nit i ally colonized by brown rot fungi, when sub-sequently exposed to the microbia l communities of a coniferous forest soil. After pre-decay by G. t rabeum, the spruce blocks were placed on forest soil and covered by forest litter in a Norway spruce forest in NE France, followed by i ncubation for 70 days. We hypothesized that both fungal and bacterial secondary colonizers would attack the wood differently depending on the extent of brown rot pre-decay. To assess this, fungal and bacterial communities in the blocks were monitored over time using high-throughput amplicon sequencing. We also inves-tigated whether the secondary decomposers originated from the forest floor or from the l i tter , and observed putative fungi/bacteria i n-teractions using coincident data pattern assessment. 2. Materials and methods 2.1. Laboratory-based pre-degradation of Norway spruce blocks by G. trabeum Norway spruce blocks (Picea abies) (50x25×15 mm) originating from Norwegian forest stands were placed in petri dishes containing malt agar medium and four 7 mm i noculum plugs of Gloeophyllum t ra-RAM beum (BAM Ebw 109, permanent culture from CIRAD, France) for decay according to a similar method of the European standard EN 113-2(2020). This standard method i s commonly used to determine the nat-ural durabil i ty of t imber against wood-destroying Basidiomycota species cultured on a malt extract agar medium. Cultures were incubated at 25°C for 50, 80, 105,130,150 and 165 d (pure culture pre-degradation)and the surface mycelium was then removed from the blocks with a razor blade under sterile conditions. All wood samples were then dried (60°C,12h), weighed to determine mass loss (3 replicate samples), and al l samples were then placed individually in porous polyamide bags to exclude macrofauna but that would allow colonization by fungal hyphae (nylon fabric mesh size of 1 mm, Buisine Society). Samples were stored at -20 °C before in situ forest incubation. We anticipated that most G.trabeum mycelium in the wood blocks would be killed during the drying phase prior to installation at the forest site and our objective was not to introduce large amounts of G. trabeum to the forest site by introducing blocks with live fungus. We could not exclude that l i mited amounts of G.trabeum mycelia or spores (specifically chlamydospores) could have survived within the samples, but G. trabeum is naturally widespread in French forests. 2.2. Wood biochemistry of Norway spruce samples A modified Van Soest protocol with “Fibrebag”technology (C. Ger-hardt GmbH & Coo..,, GGeerrmmaannyy)) (S o e st a n d Wi n e , 1967; Va n S c McQue en , 1973) was used i n the chemical analysis of the wood blocks,with the fibrebag used to f i lter solubilized wood polymers during each sequential solvent treatment stage. Briefly summarized, these stages were: (1) Removal of extractives through solubi l ization using a neutral detergent f iber solution (sodium dodecyl sulfate, sodium EDTA, sodium phosphate monobasic, sodium tetraborate decahydrate and triethylene glycol, wi t h pH ranging between 6.9 and 7.1). (2) Hemicel l ulose was then solubil i zed using an acid detergent f iber solution (CTAB dissolved in 11 of sulfuric acid 0.5 mol/l). (3) Finally, an acid detergent treatment (sulfuric acid, 72%) was used to solubi l ize cellulose,leaving lignin as the final product. Each solubilized fraction was collected and dried over-night at 105 °C before weighing to determine mass loss as compared to undecayed reference samples. 2.3. Field experiment Wood samples were installed in Spring 2019 at the Breui l -Chenue experimental forest site, in the Morvan area of France (47°18'N,4°5'E). The forest i s located on a plateau at 640 m, the annua l rainfall is 1280 mm and the average temperature is 9 °C. The soil is an alocrisol that developed over ‘La Pierre qui Vire'leucogranite with a topsoil pH ranging between 3.8 and 4.5 (Ba i ze an d G i r ard, 1988; Le vr e l a nd R a n ger , 2006) (T ab l e S5). In 1976, a part of the native forest was clear-cut and the area was planted with several mono-speci f ic planta-t ions (1000 m" for each). Among these, one plot that was planted with Norway spruce was selected for this study. We selected a 90×45 cm area and carefully removed the forest floor for placement of the bagged wood block samples in a block design on the topsoil as described below. Six replicates were used for each treatment i ncluding the non-decayed control. Treatments included “non-decayed wood controls”(0% labo-ratory mass loss),“intermediate” decayed samples (13.36±0.17%laboratory mass loss), and“advanced" decayed samples (31.71±1.07%laboratory mass loss). These are termed “pre-decayed"samples in the remainder of this paper to distinguish the decay which occurred because of G. trabeum pure-culture exposure in the laboratory, from any decay which may have occurred with subsequent exposure of the same blocks at the forest site. The bagged blocks were uniformly distributed throughout the soi l surface of the selected forest site area, covered with the original forest floor, and then after 70 d these samples were collected for analysis. Eight forest f loor samples, and eight soil cores from the 0-5cm mineral horizon were also collected (F i gs . S 4 a n d S5) for DNA extraction and analysis. All samples were transported to the laboratory on ice, and then were stored at -80 °C. Before DNA extraction, wood samples were cut in half under sterile conditions. One half was used to extract Genomic DNA, with the other half used to determine if addi -tional mass loss had occurred during forest exposure beyond that pro-duced by G. trabeum in the l aboratory pre-degradation stage. Samples of soi l , l i tter l ayer or wood were comminuted using a cryomil l (Retsch GmbH, Germany) following the manufacturer's instructions. 2.4. DNA extraction, PCR amplification and sequencing Genomic DNA was extracted from three replicate 700 mg commi-nuted subsamples (soil, forest floor or wood) using a RNeasy PowerSoil DNA elution kit following the manufacturer’s instructions (Qiagen GmbH, Hilden, Germany). As the colonization of“non-decayed”samples was very low, we selectively extracted genomic DNA (three replicate),W from the outer surfaces of the blocks where we expected any first col-onizers to be present. For this, a few millimeters of external wood were scraped from the block surface using a sterile razor blade and DNA was extracted with a success rate of 75% with at least 2 successful DNA extractions for each non-decayed wood block. Amplification of the fungal ITS2 region was then performed using forward primer ITS86f (5-GTGAATCATCGAATCTTTGAA-3) and reverse e primer ITS4r(5'-TCCTCCGCTTATTGATAGTC-3) (Op D e B e e ck e t al ., 2014). For bacte-rial communities, the 16S rRNA V4 region was amplified with forward primer 515f (5'-GTGCCAGCMGCCGCGGTAA-3) and reverse primer 806r (5'-GGACTACHVGGTWTCTAAT-3) (Ca por aso et al., 2011). For each DNA extraction, three independent PCR reactions were carried out in a final volume of 50 pl with Herculase II fusion DNA polymerase (Agilent), the replicate samples were then pooled and confirmed on gel electrophoresis for both fungi and bacteria. The PCR conditions used were 95 °C for 3min, 33 cycles of 45 s at 95°C (denaturation), 55°C for 30 s (anneal i ng) and 72 °C for 30 s (extension), followed by 10 min at 72 °C. The multiplexing and the I llumina MiSeq sequencing were per-formed by Genoscreen Society (Lille, France, h ttp s://w ww.gen os cree n .f r /fr /). The raw MiSeq sequences were submitted to the Sequence Read Archive (SRA) and are available under the BioProject ID:PRJNA775075. 2.5. Miseq sequence processing and bioinformatic analyses Sequences were demultiplexed with CASAVA v1.0 software (script PERL ConfigureBclToFastq.pl) and merged with a FLASH tool config-ured to suppress the PCR primer (100% of identity), to trim extremities with a score ≤Q30, and to maintain a minima l length of 30 nucleotides with an i dentity yield of 97% (M ago c and Sa l z be r g , 2011). The paired sequences (read 1 and read 2 merged) were then processed with FROGS pipeline (Galaxy Solutions from Genotoul Bioinformatic platform h tt p://fr o gs .to ul o u s e.inr a .f r ) (E sc ud i é et a l ., 2018). Briefly, dereplicated sequences were clustered with Swarm (aggregation distance set to 1:M a h é et a l ., 2014), chimera detection and deletion was based on VSEARCH using the de novo UCHIME method (Ed g ar , 2010; R og ne s et al., 2016), and OTUs below 0.005% abundance were considered noise and filtered (B o kulic h et al.,2013). Sequence taxonomic annotation was performed using a basic local alignment search tool (BLAST) on s i lva138_ pintail100 16S database for bacterial sequences and Uni -te _Fungi _8.2_20200204 database for fungal sequences. A sequence ag-gregation step was then performed using 98% of identity and a coverage of 98%. Analysis of the bacteria colonizing the pre-decayed wood blocks based on 31 samples was successfully sequenced and provided 1,204,722 high quality reads (554,534 sequences for the incubated wood blocks, 323,776 sequences for the forest floor, and 288,031 sequences for the forest soil). Colonizing fungi analysis was based on 29 samples,which provided 1,201,401 high quality reads (575,581 sequences for the incubated wood blocks, 356,480 sequences for the forest floor and 269,340 sequences for the forest soil). For funga l data, G. trabeum sequences were removed and then normalized (lowest sequencing depth among the samples) by random sampling to 2886 distributed into 526 OTUs (T a bl e S6, F i g .S3). For bacteria, Mitochondria sequences were removed and the number of sequences per sample was normalized (lowest sequencing depth among the samples) by random sampling at 3488representing 582 OTUs (T abl e S6). Bacterial OTUs belonging to several Proteobacteria classes (alpha, beta and gamma) or the Bacteroidetes phylum were assigned as copiotrophs, whereas Deltaproteobacteria and Acidobacteria were categorized as oligotrophic organisms (Fierer e t a l.,2007). The trophic mode of f ungal OTUs was assigned using FUNGuild (Ngu y en et al., 2016). Fungi were parsed between saprotroph, patho-genic and symbiotroph, and these were then respectively sub-divided into either (a) soft rot, brown rot and white rot fungi; (b) in planta or anima l pathogens and funga l parasites; (c) endophytic, ericoid mycor-rhizal, or (d) ectomycorrhizal fungi. Another guild was created to regroup all OTUs potentially corresponding to “yeast” (V io tt i et al .,2021). 2.6. Statistical analyses Statistical analyses and data visualization were performed with R using the phyloseq and ggplot2 packages (McM urd i e and H o lme s,2013). Alpha microbial diversity was assessed using the OTU richness (defined as the tota l number of OTUs within each sample), the estimated number of OTUs, taking i nto account the rare OTUs (Chaol estimator),the distribution of individual species (Shannon’s diversity index (H))and the equal distribution (Pielou’s evenness index) based on the ratio between Hmax (theoretical maximum of Shannon) and H’(O k sanen et a l., 2019). OTU richness, Chaol estimator and Shannon’s index were determined using a Phyloseq package (htt p s://r d rr .i o/bio c/ph y lo seq /m an /phyl oseq -p a c kage .html ). Following this, pairwise comparisons us i ng the Wilcoxon rank sum test were performed to determine di f fer-ences between environments (Holm method). Differences in bacterial and fungal OTU composition were visualized with principal coordinates analyses (PCoA) on Unirac distance matrices using the plot _ordination function in the Phyloseq package. PCoA was used rather than principal components analysis to better reflect the 2-D distance between samples.Permutational multivariate analysis of variance (PERMANOVA) was performed on Unifrac matrices using the Adonis function of the vegan package in order to determine the effect of wood on the bacterial and fungal community structures as well as measuring the impact of degradation when pre-decayed wood was exposed to the forest soil environment. Bacterial and fungal co-occurrence was analyzed using the Corrplot package in R by selec t ing the Spearman correlation and recognizing only significant values (P value <0.001). 3. Results 3.1. Chemical composition of Norway spruce pre-decayed by G. trabeum prior in situ incubation biochemistry (Tabl e S1; F ig . 1). Mass loss increased with pre-decay durat i on but was quite variable (F ig . 1A). For example, 8.1±1.9,15.2±8.4 and 24.4±4.7% of the initial wood mass was lost after 40,105 and 165 d of pre-decay, respectively. Pre-decay by G. trabeum also resulted in a relative decrease in polysaccharides, with a relative in-crease in both soluble carbohydrates and in lignin; which is in accor-dance with the mechanisms employed by brown rot f ungi (G r e e n a n d Hig h l ey , 1997; Bader et al ., 2012; A rant es an d Go o de ll, 2014). Wood blocks were grouped according to thei r percent mass loss (independent of their preincubation ti me) as either: non-decayed wood (0%,n =6),intermediate decayed wood (13.36±0.17%,n = 6) or advanced decayed wood (31.71± 1.07%, n = 6) (Tab le S1; F ig . 1B). In the following sections, the term“pre-decay” i s used when referring to wood that was decayed in t he l aboratory by pure cultures of G. trabeum before the samples were exposed to diverse microbial activity in the forest litter/soil. 3.2. Norway spruce pre-decay i mpacted richness and microbial diversity Although wood substrates (non-decayed controls or pre-decayed blocks) significantly reduced bacteria l and fungal richness in compari-son to the diversity of organisms present i n the soil and forest floor, the pre-decay of Norway spruce did not i mpact microbial richness compared to non-decayed wood (Fi g. 2). For fungi, Shannon diversity and Pielou’s evenness indices were not significantly di f ferent for non-decayed wood exposed in the forest, as compared to the forest floor and soi l samples analyzed. Although these two indices decreased as pre-decay increased, diversity and evenness were only significantly r educed in t he advanced pre-decayed wood that was then exposed i n the forest (F i g.2A). Moreover, the sequencing data highlighted that G. trabeum genomic DNA was retrieved in greater proportion in advanced pre-decayed wood (Fi g . S3). Therefore, fungi with low abundance might not be sequenced and f unga l diversity might be underestimated within the advanced pre-decayed samples. Relative to the bacter i al community, diversity and evenness were reduced i n wood samples at all pre-decay stages compared to forest floor and soi l samples, while no significant differences were observed in wood samples whatever their pre-decay stage (Fig. 2B). 3.3. Norway spruce pre-decay influenced the composition of microbial communities Bacterial and fungal community y composition Was strongly influenced by the stage of pre-decay present in the samples that were subsequently incubated in the forest floor environment (F ig.3). Indeed,PCoA and PERMANOVA showed that the degree of pre-decay (grouped into categories ) in the blocks explained 51.8 or 54.9% of the variance in the OTU composition, respectively, for fungi (Fi gs . 3A) and 48.4 or 49.9% for bacteria (F ig . 3B). Although pre-decay of Norway spruce modified the composition of bacterial and fungal communities in com-parison to non-decayed control wood, forest floor and soil, no differ-ences were highlighted between intermediate and advanced pre-decayed wood (Fig . 3A and B). Wood substrates (at any pre-decay stage) significantly impacted bacterial and fungal communities compared to the diversity of microorganisms found in soil and forest floor samples (Tab le S2). Moreover, bacterial and fungal communities i n non-decayed wood represented an intermediate state on axis 1 of the PCoA between soil/forest floor and intermediate/advanced decayed wood, but were distinct from all other environments on axis 2 of the PCoA (Fig . 3A and B). PERMANOVA analysis confirmed the result s ob-tained with PCoA and showed that, in comparison to non-decayed wood,pre-decay explained approximately 37% and 32% of the variance in OTU composi t ion for bacteria and fungi, respectively (F i g. 3 and Tab l e S2). Moreover, no signi f icant di f ferences were found between intermediate and advanced pre-decayed wood with PERMANOVA (T a bl e S2). Two other factors, the drying phase at 60 °C before incu-bation in the field and, perhaps more i mportantly, the dry weather conditions over the 70 d of field exposure (F ig. S6), may have also influenced the colonization of wood blocks by wood-decay fungi.Moreover, nylon mesh may prevent wicking of water, therefore i t i s less l i kely the blocks would have absorbed water by contact with the soi l. At the end of the field experiment, it should be noted that no additional mass loss was observed (data not shown) in any of the three wood groups beyond the mass loss observed during the l aboratory pre-decay period.We hypothesize that the period of two months in the field was too short to produce detectable mass loss due to a low decay rate of the spruce and the dry condi t ions discussed above (Alban et al., 2014). 3.4. Bacterial and fungal taxonomic composition in pre-decayed Norway spruce and forest l itter differed from that in the forest soil Differences in fungal and bacterial taxonomic composition high-lighted the influence of the wood pre-decay stage on the relative mi-crobial abundance observed after in situ forest incubation (F i g . 4,T ab l e S3). Ascomycota dominated in non-decayed wood, and in both pre-decay stages after samples were exposed on the forest f loor. Fig. 2. Microbia l diversity analysis measured for Non-decayed wood, Intermediate-pre-decayed wood, Advanced pre-decayed wood, and also Soi l and Forest floor using the number of observed OTUs (OTU richness), calculated Chaol richness estimator, Pielou’s evenness index and Shannon’s diversity i ndex for (A) fungi and (B)bacteria. The i nfluence of the environments was tested using a pairwise compar i son (P values adjusted using the Holm method: *P < 0.05, **P <0.01,***P <0.001and ****P <0.0001). Fig. 3. Principal coordinates analysis (PCoA) of f ungal (A) and bacter i al (B) communit i es i n Soil, Forest f loor, and also non -decayed and pre-decayed wood blocks that were incubated at the interface between the forest floor and the l itter. The PCoA plots were based on fungal and bacterial OTUs Unifrac distance matrices. The donut charts represent the var i ances (%) explained by the environments (Envtype) or not (unexplained) based on permutational multivariate analysis of variance (PERMANOVA); ***P <0.001. Fig. 4. Relative abundance of (A) fungal and (B) bacterial c l asses and re l ative abundance of ecological guilds for (C) fungi and (D) bacteria according to the Soil, the Forest floor, in non-decayed and i n the two type of pre-decayed wood samples buried at the interface between forest floor and litter. Conversely, Ascomycota and Basidiomycota were almost equally dist r ibuted i n the soi l . Interest i ngly, more Ascomycota were present i n the non-decayed wood control and the intermediate pre-decayed wood compared to soil and forest li t ter, and Ascomycota increased further stil l in the advanced pre-decayed wood (F i g. 4A, Tabl e S3). The changes were also observed at the microbial class/family level: the forest floor was dominated by Leotiomycetes (64%), the soil colonized primarily by Agaricomycetes (47%) and Leotiomycetes (29%), whereas wood samples were colonized by Sordariomycetes (40%) and Leotiomycetes (29%)(Fi g . 4A, Ta bl e S3). Interestingly, the pre-decay stage influenced Basi-diomycota classes since a potential shift was observed between Agar-icomycetes in non-decayed wood to Tremellomycetes in both i ntermediate and advanced pre-decayed wood (Fi g . 4A). Moreover, the lack of wood pre-decay (non-decayed wood controls) favored colonization by Mor-tierellomycetes (F ig . 4A). At the genus level, pre-decay favored several genera such as Penicillium and Trichoderma compared to soil and forest floor samples. Non-decayed wood also favored the presence of Penicil-lium genera compared to the soil and forest floor samples (T a bl e S3). The relative abundance of bacterial classes was dramatically different when comparing colonization in the non-decayed, intermedi-ate- and advanced pre-decayed samples to the soil and forest floor samples (Fig . 4B, Tabl e S 3). The forest floor was significantly enriched by the Bacteroidia class (35%) whereas t he major organism classes in soil were Acidobacteriae (18%), Actinobacteria (15%), Gammaproteobacteria (13%), and Bacteroidia (12%). Wood samples, independent of pre-decay stage, were populated by a higher proportion of Gammaproteobacteria (37%) than the forest floor and soil. Moreover, the influence of pre-decay stage was also observed with less abundant microbial classes,resulting in a decrease in the relative abundance of Verrucomicrobiae and Acidobacteriae in pre-decayed samples relative to non-decayed wood (F ig. 4B, T able S 3). The influence of pre-decay stage was also observed at the family level (Table S3). The forest floor contained the greatest amount of Sphingobacteriaceae (27%), with the soi l , and also pre-decayed wood samples, containing less of this family. Compared to the forest floor and the soil samples, wood samples that were pre-decayed favored the establishment of families such as Burkholder-iaceae, Acetobacteraceae, Microbacteriaceae but proportionally decreased numbers of Acidobacteriaceae, Chthoniobacteraceae, Rhodanobacteraceae families (Table S 3). The Burkholderiaceae family was over-represented in the intermediate (34%) and advanced (39%) pre-decayed wood samples compared to the control non-decayed wood (9%). Microbacteriaceae and Acetobacteraceae families also increased in the intermediate (7 and 12%,respectively) and advanced (19 and 6%) pre-decayed wood compared to non-decayed wood control (1 and 1.6%, respectively). Conversely, the Acidobacteriaceae family was strongly counter-selected by pre-decay with a total abundance of 3%, 3% and 16% i n the intermediate,advanced and non-decayed wood control, respectively. No significant differences were observed for these families between intermediate and advanced pre-decayed wood (T ab l e S 3). 3.5. Bacterial and fungal guild composition Up to 50% of the OTUs were unassigned in the soi l, forest floor and wood samples. However , analysis of the changes in fungal guild pop-ulations with changes i n pre-decayed stages highlights the difference in microbial compositions initially present in both soi l and forest litter environments (F ig.4C). Among the assigned OTUs, t he forest f loor was dominated by saprotrophs (25%), the soil community consisted pri-marily of plant pathogens (19%) and saprotrophs (17%). All wood samples were represented to a greater extent by saprotrophs at 44%,28% and 68% in the non-decayed, intermediate, and advanced pre-decayed samples respectively . The fungal guilds in wood samples were dominated by soft rot at 21%, 22% and 61% for non-decayed, inter-mediate, and advanced pre-decayed wood, respectively (Fi g. 4C). All wood samples showed a greater proportion of fungal soft rot species when compared to soil and forest floor samples, with the advanced pre- decayed samples most intensively colonized by this fungal guild. This phenomenon corresponded to an increase in the relative abundance of Sordariomycetes (F ig. 4A). An i mportant variation was also observed for saprotrophic organisms, with their presence being reduced in the in-termediate (6%), and advanced (7%) pre-decayed blocks in comparison to non-decayed wood samples (22%). Endophyte f ungi were only observed in wood samples and confirmed the impact of the wood sub-strate, regardless of pre-decay stage, on fungal guild composition (F ig . 4C). Although many OTUs could not be assigned to any guild, a higher proportion of copiotroph bacteria compared to oligotroph bacteria were observed in samples from all pre-decay stages (F i g. 4D). Although the percentage of copiotroph bacteria was equivalent (around 54-64%)when compared to the forest f loor and the three wood pre-decay sample types, soil samples had a lower proportion of copiotrophs with only 32%(F i g . 4D). The increased number of copiotrophic bacteria corresponded with a reduced relative abundance of Alphaproteobacteria, Gammapro-teobacteria and Bacteroidia in soil than in the forest litter or wood sam-ples (Fi g . 4B). Moreover, the relative abundance of oligotrophic bacteria in the intermediate and advanced pre-decayed wood (around 3%) was reduced compared to the non-decayed wood controls, but also as compared to the forest floor and soil (which ranged from 13 to 18%)(F ig. 4D). We attribute this reduction primarily to a significant decrease in Acidobacteriae in the pre-decayed samples (Fig. 4B). The level of pre-decay in wood samples appeared to have a major influence on bacterial guild composition with intermediate and advanced pre-decayed wood decreasingly colonized by oligotrophic bacteria compared to soil sam-ples with strongly reduced copiotroph bacteria populations. 3.6. Abundant fungi and bacteria in wood samples originated from both the soil and forest floor, with co-occurrence of some microbial species irrespective of pre-decay stage Analysis of the most abundant OTUs from the forest floor litter, soil,and wood samples showed that 116 bacterial and 127 fungal OTUs appeared with at least 1% abundance in at l east one sample (F i g s. S 1 a n d S 2). Al l OTUs found in the non-decayed intermediate and advanced pre-decayed wood were also present in both soil and forest floor samples.Consequently, it is not possible to determine the specific origin for a specific OTU. Conduct i ng a Spearman rank correlation with 11 fungal and 30 bacterial OTUs, selected because of their abundance in inter-mediate and advanced pre-decayed wood samples, positive correlations (p < 0.001) were observed between bacterial and fungal species. Asso-ciations were noted between the bacteria Burkholderiaceae, Micro-bacteriaceae and Acetobacteraceae, and several fungal species including Trichoderma spirale, Penicillium aethiopicum, Saitozyma podzolica and Scleroconidioma sphagnicola (T a b l e S 4). 4. Discussion We found that l aboratory pre-decay of wood by G. trabeum promoted the establishment of bacterial and fungal communi t ies following burial of the samples at the interface between the forest floor and minera l soil.Indeed, we observed that specific microbia l communities were s i gnifi-cantly enhanced in the pre-decayed wood compared to forest floor, soil and the non-decayed control wood. 4.1. Pre-decay of Norway spruce by G. trabeum enhanced selection of competitive fungal species Norway spruce decayed wood containing dead mycelium from G.trabeum provided a habitat similar to that colonized by living decay fungi. In decaying wood, actively metabolizing fungi are typically found only at the decay front , with dead mycelia (fungal necromass) found in areas of wood that have already been fully or partially degraded (P a rk 1968; W atki ns o n e t a l ., 2006; J o h ns t o n et al ., 2016). In these areas, which we produced via Norway spruce pre-decay, the establishment of soft-rot fungi was significantly favored compared to the other environ-ments in our analyses (F i g. 4A and Tab le S3). This ecologica l guild was mainly represented by the Trichoderma genus, which contains fast growing species, and their colonization was likely favored by the in-crease i n soluble oligosaccharides (i nduced by the laboratory pre-decay)and the residual G. trabeum hyphal necromass in pre-decayed wood (F i g. 1 and F ig . S3). Trichoderma spp. is well known for its ability to secrete a variety of CAZymes involved in wood polysaccharide degra-dation, and for their use of dead mycelia as thei r carbon-nutrient source (K rog h et a l ., 2004; Str a ko ws ka e t a l ., 2014; Bla sz c z yk et al ., 2016; I si -dorov et al ., 2016; H i s co x et a l.,2018; G o mez-Br an don et al .,2020). The colonization of pre-decayed wood, mainly by Trichoderma ssp., may imply a slow down of wood carbon recycling, s i nce sof t rot fungi generally cause a slower decay compared to Basidiomycota species and are able to outcompete other microorganisms through the secretion of antifunga l compounds (Sch ust er an d S c h m ol l , 2010; S us i et al ., 2011;Go od e l l 2020). Moreover, pre-decay also favored the establishment of Trem-ellomycetes species in comparison to other environments. Although these species possess genes i nvolved in decomposition of wood poly-saccharides, they are described primarily as colonizers of fungal nec-romass and are mycoparasites, and they cycle carbon from either dead or live mycelia of other species (K urtzman a n d B o ek h o ut , 2017; Ki m e t a l., 2018; Mai l l ar d e t a l., 2021). One other dominant genera found in pre-decayed wood, i.e. Penicillium, produces CAZymes involved in some types of cellulose decomposition and possesses antagonistic properties against fungi (Kr o gh e t a l ., 2004; Ni col e tt i an d Stefa n o, 2004; Ko uko l a n d K o v a r o v a , 2007). Consequently, our data showed that the pre-decayed blocks provided a unique forest habitat which play an important role in the establ i sh-ment of broader microbial communities . The first colonizers found in this study have the ability to decompose wood components, especially polysaccharides, and to outcompete other f ungal species by antagonistic mechanisms. Hence, we hypothesize that these species may outcompete the colonization of wood by Basidiomycota species and therefore slow wood carbon recycling. Other recent studies, however, have determined that early colonization by fungal species that metabolize necromass can enhance establishment of Basidiomycota wood decomposing fungi in later stages, and this synergy promotes rapid wood carbon cycling (Mai l l a rd e t al., 2020). 4.2. Potential roles of bacteria selected by fungal pre-decay of Norway spruce Bacterial colonization of the i ntermediate and advanced pre-decayed wood samples resulted in a decrease in oligotrophic bacteria which corresponded to a reduction in Acidobacteria. This led to an increase in the Proteobacteria:Acidobacteria ratio suggesting that the depolymerized wood with higher free oligosaccharide and sugar content in the pre-decayed samples aided in this selection (K ie l ak e t al ., 2016). More-over, the impact of fungi on the ambient pH is also an important parameter that influences the composition of the bacterial community (J oh ns to n e t a l .,2016). Norway spruce pre-decay also significantly increased colonization by the Burkholderiaceae family compared to all other environments.Moreover, it was the most important family in intermediate and advanced pre-decayed wood . Although this family possesses many genes involved in lignocellulosic degradation, Jo h n s t on et al. (2016) reported numerous associations of Burkholderiaceae with fungi suggesting another role for this family in wood decay. Indeed, many species are known to be N2 fixing bacteria and can provide nitrogen essential for fungi (Gom e z-B r a n do n et a l ., 2020). Moreover, they could also be involved in carbon cycling of lignin breakdown products from fungal activity. They are known to degrade mono-, poly- and heterocyclic ar-omatic compounds (Perez-Pa n t o ja e t al., 2012; M o r ya e t al., 2020;Jun g et al ., 2016). Norway spruce pre-decay significantly favored the establishment of the Microbacteriaceae f amily belonging to the Actinobacteria compared to non-decayed control wood, soil and the forest floor. Interestingly, V iot t i e t al . (2021) suggested that this type of bacteria could be involved in carbon recycl i ng from dead mycelia which would be consistent with our resul t s. However, many of these bacteria are saprotrophic and chemo-heterotrophic, which allows them to use a wide variety of nutritional sources ranging from polysaccharides to l i gnin-related aromatic com-pounds and polymeric lignin, potentially suggesting direct decomposi-tion of lignocellulosic material (Zim m e r mann , 1990; S c hel lenberg er e t a l ., 2009; Ba rka e t al ., 2016; Wan g e t a l ., 2016). Other Actinobacteria have been described as endophytic diazotrophs i n Norway spruce logs and could potentially provide nitrogen for fungal activity (P u r i e t al.,2018). In addition, pre-decay also favored the establishment of the-Acetobacteraceae family which are also known for their capacity to fix nitrogen as wel l as their abi l ity to solubilize phosphorous and zinc (Suman e t a l., 2001; Sar a v a nan et a l ., 2007). Overall, the predominant bacteria found in the intermediate and advanced pre-decayed wood were those that could: (a) metabolize ol i-gosaccharides and sugars tha t are more abundant i n brown rot degraded wood, (b) utilize carbon from dead mycelium, and (c) fix important nutrients such as nitrogen. We hypothesize that some of these bacteria were active in lignocellulose decomposition, while others were involved in promot i ng symbiotic fungal growth and carbon recycling from dead mycelium. 4.3. Bacteria and fungi coexist in environments influenced by the pre-decay of wood by G. trabeum The abundance of several bacterial families (Burkholderiaceaea,Microbacteriaceae and Acetobacteraceae) was posi t ively correlated with the presence of two fungal genera (Penicillium and Trichoderma) in all environments. Based on these data, we cannot define specifically the nature of the interactions that may occur between these bacteria and fungi. However, bacteria and fungi are favored by similar conditions promoted by G. trabeum pre-decay. In this context, we hypothesize that bacteria could take up carbon from fungal hypha exudates or lignocel-lulose decomposition products, while at the same time also supplying the fungi with essential nutrients such as nitrogen, phosphorus or zinc (S a r a v a na n e t al., 2007; Schel l enberger et a l., 2009; Kirker e t al., 2017;Z h a ng e t a l ., 2020; Ze l i n k a et a l ., 2021). Moreover, some bacteria can detoxify ant i fungal compounds and therefore they may also help to synergistically protect fungal species in the same environment (N a z i r et a l ., 2014). 4.4. Understanding how recalcitrant brown rotted lignin affects forest soil,litter, and wood microbial communities Wood carbon recycling i s strongly i nfluenced by fungal colonization and especial l y by the mechanisms of wood decomposing fungi to deconstruct the wood cell wall. Indeed, brown rot fungi promote the depolymerization of complex polysaccharides, while modifying lignin to produce a recalcitrant aromatic residue that can persist in soils for many years (Ye l le et al . 2008, 2011; A r ant es a n d Go o de l l 2014; S i e r r a et a l.,2018). A significant proportion of lignin-derived carbon produced by the brown rot f ungi cons i sts of aromatic carboxyls and polyaromatic groups that have a strong affinity for i ron and aluminum hydroxyoxides (K ai s er a nd G ug g e n b e r g er 2000; F i l l e y e t a l ., 2002). Formation of this recalci-trant modified carbon by brown rot fungi and the binding of i ron and aluminum hydroxides, and clays, by this modified carbon is a key mechanism for increasing wood carbon retention time in soil (Kle ber e t al ., 2015). In our experimental set-up, G. trabeum metabolized only a portion of the wood polysaccharides during laboratory pre-decay and much of the crystalline cellulose remained, particularly in the intermediate pre- decayed material (F i g . 1). In future research it may be useful to conduct additional laboratory pre-decay experiments to obtain wood blocks containing almost entirely modified lignin residues. This could help reveal the ecological role of microbial communi t ies involved in decomposition of the brown rot-modified l ignin to also develop a better understanding of the long-term persistence of this unique material as a component of soil organic matter (Ye l le e t al., 2008, 2011; A r a n t es a n d Go od e l l 2014). 5. Conclusion Pre-decay of wood by the brown rot fungus G. trabeum provided a unique habita t that favored the establishment of bacteria and fungi able to cycle carbon from dead mycelium and metabolize polysaccharide compounds from the decay process. Microbial communities found i n non-decayed control wood, soil and the forest floor were different . This study is unique because both wood composi t ion and microbia l coloni-zation data were assessed to provide a better understanding of: (i ) the impact of brown rot fungal mechanisms on subsequent microbial colo-nization, (ii ) carbon recycling of modified l ignin produced by brown rot fungi, and (iii) the prolonged wood carbon retention time in soil after pre-decay by brown rot fungi. Moreover, based on this study, in the future these important ecological questions may include other organ-isms such as white rot fungi or brown rot from diverse phylogenetic lineages. Conflict of Interest the content and the authors The content and the authorship of the submitted manuscript has been approved by all authors, i . e, Nicolas Valette, Arnaud Legout, Barry Goodell, Gry Alfredsen, Lucas Auer, Eric Gelhaye and Delphine Derrien.Moreover, all prevailing local, national and international regulations and conventions, and normal scient i fic ethical practices, have been respected. Acknowledgements This work was supported by the Laboratory of Excellence Arbre (ANR-11-LABEX-0002-01; BRAWO project). The UMR1136 and the UR1138 are supported by the French Agency through the Laboratory of Excellence Arbre (ANR-11-LABX-0002-01). Goodell was supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture project number S1075 -MAS00503, the Center for Agri-culture, Food and t he Environment , and the Microbiology department at University of Massachusetts Amherst. Alfredsen was supported by the NIBIO strategic project 11077. The contents are solely the responsibility of the authors and do not necessar i ly represent t he official views of the USDA or NIFA. The Breui l -Chenue site belongs to French national research infrastructure ANAEE-France (ANR-11-INBS-0001). We grate-fully acknowledge the f inancial support received successively from GIP ECOFOR, AllEnvi, ANAEE France, the LTSER Zone Atelier Bassin Moselle and INRAE (DISC, ECODIV). We would also li ke to thank POf-fice National des Forets, the Parc Naturel Regional du Morvan and Serge Didier for his contribution to the Breui l -Chenue site management. Appendix A. Supplementary data Supplementary data to this article can be found online at h t t ps ://do i .org /10.1016/j .funec o .2022.101188. References Arantes, V ., Goodell , B., 2014. Cur r ent understanding of brown-rot fungal biodegradation mechanisms: a Review. In: Schultz, T.P., Goodell, B., Nicholas,D.D.(Eds.), ACS Symposium Series. Amer i can Chemical Society, Washington, DC,pp. 3-21. ht tps://d o i .org /10.1021/b k -2014-1158.c h 001. Bader, T.K., Hofstetter , K., Al f redsen, G ., Bollmus , S., 2012. Changes in microstructure and s t i f fness of Scots pine (Pinus sylves t ris L) sapwood degraded by Gloeophyllum trabeum and Trametes versicolor - Part I I: anisotropic sti f fness properties.Holzforschung 66. htt ps://d o i .or g/10.1515/H F.2011.153. Baize , D ., Gira r d , M.C ., 1988. R efe r en t i el Pe d o l ogi qu e Francais. Barka, E.A., Vatsa, P., Sanchez, L., Gaveau-Vaillant, N., Jacquard, C., Klenk, H.-P.,Clement, C., Ouhdouch, Y., van Weze l, G.P., 2016. Taxonomy, physiology, and natural products of Actinobacteria . Microbiol. Mol . Biol . Rev. 80, 1-43. h t tp s://d oi .or g/10.1128/MMB R .00019-15. Blanchette, R.A., 2000. A review of microbia l deterioration found i n archaeological wood from different environments. Int. Biodeterior. Biodegrad. 46, 189-204. h t tps://d o i .or g /10.1016/S0964-8305(00)00077-9. Blaszczyk, L., Strakowska, J ., Chelkowski , J., Gabka-Buszek, A., Kaczmarek, J., 2016.Tr i choderma species occurring on wood with decay symptoms in mountain forests in Central Europe: genetic and enzymatic characterization. J . Appl. Genet. 57,397-407. htt p s://doi .org/10.1007/s13353-015-0326-1. Boddy, L., 2001. Fungal community ecology and wood decompos i tion processes in angiosperms: from standing tree to complete decay of coarse woody debris. Ecol .Bull. 43-56. h t tp s://www.j s t or .or g/s table/20113263. Bokulich , N.A., Subramanian, S., Faith, J .J ., Gevers, D., Gordon, J.I ., Knight , R., Mi l ls, D.A., Caporaso, J.G., 2013. Quality -fi l tering vastly improves diversity estimates from I l lumina amplicon sequencing. Nat. Methods 10, 57-59. ht tps://d o i .o r g/10.1038/n m et h .2276. Caporaso, J .G., Lauber, C.L., Walters, W.A., Berg-Lyons, D., Lozupone, C.A.,Turnbaugh, P.J., Fierer, N., Knight, R., 2011. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl . Acad. Sci . USA 108,4516-4522. htt p s ://d oi .or g/10.1073/p n a s .1000080107. Carvalhais, N., Forkel, M., Khomik, M., Be l larby , J., Jung, M., Migl i avacca, M., Mu, M.,Saatchi, S., Santoro, M., Thurner, M., Weber, U., Ahrens, B., Beer , C., Cescatti , A.,Randerson, J .T., Reichstein, M., 2014. Global covariation of carbon turnover times with climate i n terrestrial ecosystems. Nature 514, 213-217. htt ps ://doi.or g /10.1038/na tu re13731. Christofides, S.R., Hiscox, J., Savoury, M., Boddy, L., Weightman, A.J., 2019. Fungal control of early-stage bacterial community development in decomposing wood.Funga l Ecol. 42, 100868 h t tps ://doi .or g /10.1016/j .f un e co.2019.100868. Dickie, I.A., Fukami, T., Wilkie, J.P., Allen, R.B., Buchanan, P.K., 2012. Do assembly history effects attenuate from species to ecosystem properties? A field test with wood-inhabi ti ng f ungi : assembly history ef f ects. Ecol . Lett . 15, 133-141. h t tp s://d o i .o r g/10.1111/j.1461-0248.2011.01722.x . Eastwood, D.C., Floudas , D., Binder, M., Majcherczyk, A., Schneider, P., Aerts , A.,Asiegbu, F.O., Baker, S.E., Barry, K., Bendiksby, M., Blumentri tt , M., Coutinho, P.M.,Cullen, D., de Vries, R.P., Gathman, A., Goodell, B., Henrissat , B., Ihrmark, K.,Kauserud , H., Kohler , A., LaBut t i , K., Lapidus, A., Lavin, J .L., Lee, Y .-H.,Lindquist, E., Lilly, W., Lucas, S., Morin, E., Murat, C., Oguiza, J.A., Park, J.,Pisabarro, A.G., Riley, R., Rosl i ng, A., Salamov, A., Schmidt,O., Schmutz, J.,Skrede, I., Stenlid, J ., Wiebenga, A., Xie, X., Kues, U ., Hibbet t, D.S., Hoffmeister , D.,Hogberg, N., Martin, F., Grigoriev, I.V., Watkinson, S.C., 2011. The plant cell wal l -decomposing machinery underlies the functional diversity of forest fungi. Sc i ence 333, 762-765. h tt ps://d o i.o r g/10.1126/s ci e nc e .1205411. Edgar, R.C ., 2010. Search and clustering orders of magnitude faster than BLAST.Bioinformat i cs 26, 2460-2461. h t tps://d o i.or g/10.1093/bioi nfor m at i cs/b tq 461. Escudie, F., Auer, L., Bernard, M., Mariadassou, M., Cauqui l , L., Vidal , K., Maman, S.,Hernandez-Raquet , G., Combes, S., Pascal, G., 2018. FROGS: f i nd, rapidly , OTUs with galaxy solution. Bioinformat i cs 34, 1287-1294. htt ps ://do i .o r g/10.1093/b ioi nf or mat i cs /b t x791. Fierer, N., Bradford, M.A., Jackson, R.B., 2007. Toward an ecological classification of soil bacteria . Ecology 88, 1354-1364. htt ps://d oi .o r g/10.1890/05-1839. Fi l ley, T.R., Cody, G.D., Goode l l, B., Jel l ison, J., Noser, C., Ostrofsky, A., 2002. L i gnin demethy l ation and polysaccharide decomposition in spruce sapwood degraded by brown r ot f ungi. Org. Geochem. 33, 111-124. h t t p s://do i .org /10.1016/S 0146-6380(01)00144-9. Floudas, D ., Binder , M., Riley, R., Barry, K., Bl a nchette, R.A., Henrissat, B., Mart i nez, A.T., Oti l lar, R., Spatafora, J.W., Yadav, J.S., Aerts, A., Benoi t , I ., Boyd , A., Carlson, A.,Copeland, A., Coutinho, P.M., de Vries, R.P., Ferreira, P., Findley, K., Foster, B.,Gaskell , J., Glotzer , D., Gorecki, P., Heitman, J ., Hesse, C., Hori, C., Igarashi , K.,Jurgens, J.A., Kallen, N., Kersten, P., Kohler, A., Kiies, U., Kumar, T.K.A., Kuo, A.,LaButti, K., Larrondo, L.F., Lindquist, E., Ling, A., Lombard, V., Lucas, S., Lu n dell, T 3110.,Martin, R., McLaughlin, D.J., Morgenstern, I., Morin, E., Murat, C., Nagy , L.G .,Nolan, M., Ohm, R.A., Patyshakuliyeva, A., Rokas, A., Ruiz-Duenas, F .J ., Sabat, G.,Salamov , A., Samejima, M., Schmutz, J ., Slot , J.C., St. John, F., Stenlid, J., S un , H.,Sun, S., Syed, K., Tsang, A., Wiebenga, A., Young, D., Pisabarro, A., Eastwood, D.C.,Martin, F., Cul l en, D., Grigoriev, I.V., Hibbet t, D.S., 2012. The paleozoic origin of enzymatic l ignin decomposition reconstructed from 31 fungal genomes. Science 336,1715-1719. htt ps://d o i.o r g/10.1126/sc i ence.1221748. Folman , L.B., Klein Gunnewiek, P.J.A., Boddy, L., De Boer, W., 2008. Impact of white-rot fungi on numbers and community compos i tion of bacteria colonizing beech wood from forest soil: impact of white-rot fungi on bacteria colonizing wood. FEMS (Fed .Eur . Microbiol. Soc.) Microbiol. Ecol. 63, 181-191. h t t p s://do i.org/10.1111/j.1574-6941.2007.00425.x . Fukami, T., 2015. Historical cont i ngency in community assembly: i ntegrating niches,species pools, and priority effec t s. Annu. Rev . Ecol. Evol . Syst . 46,1-23. h t tp s://d oi.org /10.1146/a nn urev -ecolsys-110411-160340. Gomez-Brandon, M., Probst , M., Siles, J.A., Peintner, U ., Bardel li , T ., Egli , M., Insam, H.,Ascher-Jenull, J., 2020. Funga l communities and their association with nitrogen-fixing bacteria affect ear ly decomposition of Norway spruce deadwood . Sci. Rep. 10,8025. h t t p s ://doi.org /10.1038/s 41598-020-64808-5. Goodel l, B., 2020. Fungi i nvolved in the biodeter i oration and bioconversion of lignocellulose substrates, Chap. 15. In: Benz, J.P., Schipper, K. (Eds.), The Mycota.Genetics and Biotechnology. Springer International Publishing, Cham, pp. 369-397.h t t ps ://d o i.org /10.1007/978-3-030-49924-2_15. 8/1Goodell , B., Zhu, Y., Kim, S., Kafle, K., Eastwood, D., Danie l , G ., Jel l ison, J., Yoshida, M.,Groom, L., Pingali, S.V., O'Neill, H., 2017. Modification of the nanostructure of lignocellulose cel l wal l s via a non-enzymati c l ignocellulose deconstruction system in brown rot wood-decay fungi . Biotechnol . Biofuels 10, 179. h t tp s ://doi .o r g/10.1186/s13068-017-0865-2. Green, F ., Highley, T.L., 1997. Mechanism of brown-rot decay: paradigm or paradox. Int.Biodeterior. Biodegrad. 39,113-124. h t t p s://d o i .o r g/10.1016/S 0964-8305(96)00063-7. Herve, V., Le Roux, X., Uroz, S., Gelhaye, E., Frey-Klett, P., 2014. Diversity and structure of bacterial communities associated with Phanerochaete chrysosporium during wood decay: bac t eria of the white-rot mycosphere. Environ. Microbiol. 16, 2238-2252.h t tps://do i .org/10.1111/1462-2920.12347. Hiscox, J., Clarkson, G ., Savoury, M., Powell, G ., Savva , I., Lloyd, M., Shipcott, J.,Choimes, A., Amargant Cumbriu, X., Boddy , L., 2016a. Effects of pre -colonisation and temperature on i nterspeci f ic fungal interactions i n wood. Fungal Ecol. 21,32-42. ht tps ://d oi.or g/10.1016/j .f un e co.2016.01.011. Hiscox, J., O'Leary, J., Boddy, L., 2018. Fungus wars: basidiomycete battles in wood decay. Stud. Mycol . 89, 117-124. htt ps ://doi.org /10.1016/j.s i myco .2018.02.003. Hiscox, J., Savoury, M., Johnston, S.R., Parfi tt , D., Muller, C.T., Rogers, H.J., Boddy, L.,2016b. Location, location, location: pr i or i ty e f fects in wood decay communities may vary between sites: location and priority effects i n fungi. Environ. Microbiol. 18,1954-1969. h t tp s://d oi.o r g/10.1111/1462-2920.13141. Isidorov, V., Tyszkiewicz, Z., Piroznikow, E., 2016. Fungal succession in relation to volat i le organic compounds emissions from Scots pine and Norway spruce leaf l i tter-decomposing fungi. Atmos. Environ. 131, 301-306. ht tps ://d o i.o r g /10.1016/j .a t mosen v .2016.02.015. Johnston, S.R., Boddy, L ., Weightman, A.J ., 2016. Bacteria in decomposing wood and their interactions with wood-decay fungi. FEMS (Fed. Eur. Microbiol. Soc.)Microbiol. Ecol. 92, fiw179. h t tps://doi .o rg/10.1093/fe m s ec /fi w179. Johnston, S.R., Hiscox, J., Savoury, M., Boddy , L., Weightman, A.J., 2019. Highly compet i tive fungi manipulate bacterial communi t ies in decomposing beech wood (Fagus sylvatica). FEMS (Fed . Eur. Microbiol. Soc.) Microbiol. Ecol. 95 h t tp s://doi .org/10.1093/fems ec /f i y 225. Jung, D.-H., Kim, E.-J., Jung, E., Kazlauskas, R.J., Choi, K.-Y., Kim, B.-G., 2016.Product i on of p-hydroxybenzoic acid from p-coumaric acid by Burkholderia glumae BGR1: production of p-Hydroxybenzoate by B. glumae. Biotechnol. Bioeng. 113,1493-1503. h t tp s://doi .org/10.1002/b i t.25908. Kahl , T., Arnstadt , T., Baber, K., Bassler, C., Bauhus, J ., Borken, W., Buscot , F., Floren, A.,Heibl, C., Hessenmoller, D., Hofrichter, M., Hoppe, B., Kellner, H., Kr i iger , D.,Linsenmair, K.E., Matzner, E., Otto, P., Purahong, W., Sei l winder , C., Schulze, E.-D.,Wende, B., Weisser, W.W., Gossner, M.M., 2017. Wood decay rates of 13 temperate tree species in relation to wood pro TO p P erties, enzyme activities and organismic diversities. For. Ecol. Manag. 391, 86-95. h t tp s://do i .or g/10.1016/j.for e co .2017.02.012. Kaiser, K., Guggenberger, G., 2000. The role of DOM sorption to mineral surfaces in the preservation of organic matter in soi l s. Org. Geochem. 31,711-725. ht tp s://doi .org /10.1016/S0146-6380(00)00046-2. Kameshwar, A., Kumar, S., Qin, W., 2016. Recent developments in using advanced sequencing technologies for the genomic studies o f l ignin and cellulose degrading microorganisms . Int. J. Biol. Sci . 12, 156-171. h t t ps://doi .o r g/10.7150/i jbs.13537. Kielak, A.M., Barreto, C.C., Kowalchuk, G.A., van Veen, J.A., Kuramae, E.E., 2016. The ecology of Acidobacteria: moving beyond genes and genomes. Front. Microbiol. 7h t tps://d o i .org /10.3389/fm i c b .2016.00744. Kim, J.-Y., Jang, J.H., Park, J.-H., Jung, H.-Y., Park, J .-S., Cho, S.-J., Lee, H.B.,L i mtong, S., Subramani , G., Sung, G.-H., Kim, M.K., 2018. Cellulose degrading basidiomycetes yeast isolated from the gut of grasshopper in korea. Korean J.Microbiol . 54, 362-368. htt p s://doi.org /10.7845/KJM.2018.8079. Ki r ker, G., Zel i nka, S., Gleber, S.-C., Vine, D., Finney, L., Chen , S., Hong, Y.P., Uyarte, O.,Vogt, S., Jellison, J., Goodell, B., Jakes, J.E., 2017. Synchrotron-based X -ray fluorescence microscopy enables multiscale spatia l v i sualization of ions involved in fungal l ignocel l ulose deconstruction. Sci. Rep. 7, 41798 h tt ps ://d o i .org /10.1038/sre p 41798. Kleber, M., Eusterhues, K., Keiluwei t , M., Mikutta, C., Mikutta, R., Nico, P.S., 2015.Mineral-organic associations: formation, properties, and relevance in soil environments. In: Advances in Agronomy. Elsevier, pp. 1-140. htt ps ://d o i.o rg /10.1016/bs .ag r on .2014.10.005. Koukol, O., Kovarova, M., 2007. Autecology of Scleroconidioma sphagnicola particularly in sumava national park (Czech republic). Czech Mycol. 59, 111-123. h t t ps://d o i .o r g /10.33585/c m y .59113. Krogh, K.B., Morkeberg, A., Jorgensen, H., Fr i svad, J.C., Olsson, L., 2004. Screening genus Penicillium for producers of cellulolytic and xylanolytic enzymes. In:Breckenridge, C.O. (Ed.), Proceedings of the Twenty -Fifth Symposium on Biotechnology for Fuels and Chemicals Held May 4-7,2003. Humana Press, Totowa,NJ, pp. 389-401. h t tps ://d oi .org/10.1385/ABAB :114:1-3:389. Kurtzman, C.P., Boekhout, T., 2017. Yeast s as dist i nct l ife forms of f ungi. In: Buzzini, P.,Lachance, M.-A., Yurkov , A. (Eds.), Yeasts i n Natural Ecosystems: Ecology . Springer Internationa l Publi s hing, Cham, pp . 1-37. h t tps ://do i .org /10.1007/978-3-319-61575-21. Levre l , G ., Ra n ger, J ., 2006. Effe t de s s u bst i tut i ons d'essenc e s f ores tie res et des a me ndemen t s su r l e s pr op ri é t es phys i q u es d 'u n a loc ri s o l . Si t e ex p ér i men ta l d e l a foret de Br eu i l -C h en ue, Mo r van , Fr ance. E t u d e G est i on S o l s 13, 71-88. Lopez-Mondejar, R., Voriskova, J., Vetrovsky, T., Baldrian, P., 2015. The bacterial community i nhabit i ng temperate deciduous forests is vertical l y strat i f i ed and undergoes seasonal dynamics. Soil Biol. Biochem. 87, 43-50. h tt ps://d o i .o r g /10.1016/j .so i l bi o .2015.04.008. Magoc, T ., Salzberg, S.L ., 2011. FLASH: f ast l ength adjustment of short reads to improve genome assembl i es. Bioinformatics 27, 2957-2963. h t t p s://do i .org/10.1093/b i o i nfo rm at ic s/b t r 507. Mahe, F ., Rognes, T ., Quince, C., de Vargas, C., Dunthorn, M., 2014. Swarm: robust and fast clustering method for amplicon-based studies. PeerJ 2, e593. htt p s://doi .org/10.7717/peerj .593. Mai l lard, F., Schi l l i ng, J., Andrews, E., Schreiner, K.M., Kennedy , P., 2020. Functional convergence in the decomposition of fungal necromass i n soil and wood. FEMS (Fed.Eur . Microbiol. Soc .) Microbiol. Ecol . 96, fiz209. h t tp s://d oi.org/10.1093/femsec /fiz209. Maillard, F., Kennedy, P.G., Adamczyk, B., Heinonsalo, J., Buee, M., 2021. Root presence modifies the long-term decomposition dynamics of fungal necromass and the associated microbia l communities in a boreal forest. Mol. Ecol. 30, 1921-1935.h t tps://do i.org/10.1111/me c .15828. Mao, Z., Derrien, D., Didion, M., Liski , J., Eglin, T., Nicolas, M., Jonard, M., Saint-Andre, L ., 2019. Model i ng soi l organic carbon dynamics in tempe r ate forests with Yasso07. Biogeosciences 16, 1955-1973. h t tps ://d o i .org/10.5194/b g -16-1955-2019. McMurdie, P.J., Holmes, S., 2013. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8, e61217. h t tp s://d oi.org /10.1371/j ou rn a l .p o n e .0061217. Morya, R., Salvachúa, D., Thakur, I.S., 2020. Burkholderia : an untapped but promising bacterial genus for the conversion of aromatic compounds. Trend s Biotechnol. 38,963-975. htt ps://do i .org /10.1016/j .t i bt ech.2020.02.008. Nazir , R., Tazetdinova, D.I ., van Elsas, J.D., 2014. Burkholderia terrae BS001 migrates proficiently with diverse fungal hosts through soil and provides protection from antifungal agents. Front. Microbiol. 5 https ://doi .org /10.3389/fmicb.2014.00598. Nguyen, N.H., Song, Z., Bates, S.T., Branco, S., Tedersoo, L., Menke, J ., Schilling, J.S.,Kennedy , P.G., 2016. FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 20, 241-248. h t tp s://d oi.o r g /10.1016/j.f une co .2015.06.006. Ni colett i , R., Stefano, M.D., n.d. Penicillium restrictum as an antagonist of plant pathogenic fungi. Dyn. Biochem. Process Biotechnol. Mol. Biol. 6(2), 61-69. Oksanen, J., Blanchet , F.G., Friendly, M., Kindt , R., Legendre, P., McGlinn, D., et al.,2019. Vegan: Community Ecology Package R Package Version 2.5-6. https ://C R A N.R-p r oject .org /p ac k age =vega n , 2019. Op De Beeck, M., Lievens, B., Busschaert, P., Declerck , S., Vangronsveld, J ., Colpaert, J .V., 2014. Comparison and va l idat i on of some ITS primer pairs useful for fungal metabarcoding studies. PLoS One 9, e97629. h t tps ://d o i .or g/10.1371/j ou r na l .p o ne.0097629. Park, D.,1968. The Ecology of Ter r estrial Fungi . The Fungal Population. Academic press,pp. 5-39. h t tp s ://d o i.org /10.1016/B 978-1-4832-2744-3.50007-9, 9781483227443. Perez-Pantoja, D., Donoso, R., Agull6, L ., Cordova, M., Seeger, M., Pieper, D.H.,Gonzalez, B., 2012. Genomic analysis of the potential for aromatic compounds biodegradation in Burkholderiales: degradation of aromatic compounds in Burkholderiales. Environ. Microbiol. 14, 1091-1117. h t t ps://d o i .o r g /10.1111/i.1462-2920.2011.02613.x . Perrot, T., Salzet, G., Amusant, N., Beauchene, J., Gerardin, P., Dumarcay, S.,Sormani, R., Morel-Rouhier, M., Gelh 1.aye, E., 2020. A reverse chemical ecology 一十 approach to explore wood natural durabi l ity. Microb. Biotechnol . 13, 1673-1677.h t tps ://d o i .org/10.1111/1751-7915.13540. Presley, G.N., Panisko, E., Purvine, S.O., Schill i ng, J.S., 2018. Coupling secretomics with enzyme ac t ivities to compare the tempora l processes of wood metabolism among white and brown rot fungi . Appl. Environ. Mic r obiol. 84 https://do i.o rg /10.1128/AEM.00159-18. Puri , A., Padda, K.P., Chanway, C.P., 2018. Ev i dence of endophytic diazotrophic bacteria Glan w ay,:in lodgepole pine and hybrid white spruce trees growing in soils wi t h di f ferent nutrient statuses in the West Chilcotin region of Bri ti sh Columbia, Canada. For. Ecol.Manag. 430, 558-565. htt p s ://doi .org/10.1016/j .f oreco .2018.08.049. Riley , R., Salamov, A.A., Brown, D.W., Nagy, L.G., Floudas, D., Held , B.W., Levasseur, A.,Lombard, V., Morin, E., Ot il l ar , R., L i ndquist, E.A., Sun , H., LaButti , K.M.,Schmutz , J., Jabbour, D., Luo, H., Baker, S.E., Pisabarro, A.G., Walton, J .D.,Blanchette, R.A., Henrissat, B., Martin, F., Cullen, D., Hibbett , D.S., Grigoriev, I.V.,2014. Extensive sampling of basidiomycete genomes demonstrates inadequacy of the whi t e-rot /brown-rot paradigm for wood d e cay fungi. Proc. Natl. Acad. Sci. U.S.A.111,9923-9928. https ://do i.or g /10.1073/p nas .1400592111. Rognes, T., Flouri, T., Nichols, B., Quince, C., Mahe, F., 2016. VSEARCH: a versat i le open source tool for metagenomics. PeerJ 4, e2584. h t tps://d o i .org /10.7717/p ee r j .2584. Saravanan, V.S., Madhaiyan, M., Thangaraju, M., 2007. Solubilization of zinc compounds by the diazotrophic, plant growth promoting bacterium Gluconacetobacter diazotrophicus. Chemosphere 66,1794-1798. htt ps://d oi.o r g /10.1016/j chemosp h ere.2006.07.067.C h Schellenbe r ger, S ., Kolb, S., Drake, H.L ., 2009. Metabolic responses of novel ce l lulolytic and saccharolytic agricultural soil Bacteria to oxygen: metabolic response of soil cellulose degraders. Env i ron. Microbiol . 12, 845-861. https ://do i.org/10.1111/ i.1462-2920.2009.02128.x . Schuster, A., Schmoll, M., 2010. Biology and biotechnology of Trichoderma. Appl.Microbiol . Biotechnol. 87, 787-799. htt ps://d o i.or g/10.1007/s00253-010-2632-1. Sierra, C.A., Hoyt, A.M., He, Y., Trumbore, S.E., 2018. Soil organic matter persistence as a stochastic process: age and transit time distributions of carbon in soils. Global Biogeochem. Cycles 32, 1574-1588. htt ps ://doi.or g /10.1029/2018G B 005950. Soest, P.J.V., Wine, R.H., 1967. Use of detergents in the analysis of fibrous feeds. iv.determination of plant cell -wall constituents. J . AOAC Int. 50, 50-55. h t tps ://doi .org/10.1093/j aoa c /50.1.50. St r akowska, J ., Blaszczyk, L., Chelkowski, J., 2014. The s i gni f icance of cellulolytic enzymes produced by Trichoderma i n opportunistic l i festyle of this fungus: cellulases produced by Trichoderma. J. Basic Microbiol. 54, S2-S13. h t tp s://doi.org /10.1002/io b m.201300821. Suman, A., Shasany, A.K., Singh, M., Shahi , H.N., Gaur, A., Khanuja, S.P.S., n.d.Molecular assessment of diversity among endophytic diazotrophs isolated from subtropical Indian sugarcane . World J. Microbiol. Biotechnol. 17,39-45. ht tps ://d o i.or g/10.1023/A :1016624701517. Susi , P ., Aktuganov, G ., Himanen, J., Korpela, T ., 2011. Biological control of wood decay against fungal infect i on. J. Environ. Manag. 92, 1681-1689. htt p s://doi .org /10.1016/j.je n vma n .2011.03.004. Valette, N., Perrot, T ., Sormani , R., Gelhaye, E., Morel-Rouhier, M., 2017. Ant i fungal activities of wood extractives. Fungal Biol . Rev. 31, 113-123. h t t ps://d o i .o r g /10.1016/j .fb r .2017.01.002. Van Soest , P.J., McQueen, R.W., 1973. T he chemistry and est i mation of f ibre. Proc. Nutr.Soc. 32, 123-130. h t tps ://d o i .org /10.1079/P N S 19730029. Viott i , C., Bach, C., Maillard , F., Ziegler-Devin , I., Mieszkin, S., Buee, M., 2021. Sapwood and heartwood affect differentially bacterial and fungal community structure and successional dynamics during Quercus petraea decomposi t ion. Environ. Microbiol.1462-2920,15522 https ://d o i .o r g /10.1111/1462-2920.15522. Wang, C., Dong, D., Wang, Haoshu, Muiller, K., Qin, Y., Wang, Hailong, Wu, W., 2016.Metagenomic analysis of microbial consortia enriched from compost : new insightsTO CO into the role of Act i nobacteria in lignocellulose decomposition. Biotechnol. Biofuels 9, 22. h t tps://d o i .o r g /10.1186/s13068-016-0440-2. Watkinson, S., Bebber , D., Darrah, P., Fricker, M., Tlalka, M., Boddy, L., 2006. The role of wood decay fungi in the carbon and ni t rogen dynamics of the forest floor. In:Gadd, G.M. (Ed.), Fungi in Biogeochemical Cycles. Cambridge University Press,Cambridge, pp. 151-181.ht tps ://d o i .or g/10.1017/CBO9780511550522.008. Yelle, D.J., Ralph, J ., Lu, F., Hammel, K.E., 2008. Evidence for cleavage of l ignin by a brown rot basidiomycete. Environ. Microbiol. 10, 1844-1849. h t t ps://d o i.o r g /10.1111/j .1462-2920.2008.01605.x . Yelle, D.J ., Wei, D., Ralph, J ., Hammel, K.E., 2011. Multidimensional NMR analysis reveals tr uncated l ignin structures in wood decayed by the brown rot basidiomycete Postia placenta: ligninolysis during funga l brown rot of wood. Environ. Microbiol.13,1091-1100. htt ps://d oi.o r g/10.1111/j .1462-2920.2010.02417.x . Zelinka , S.L., Jakes, J.E., Kirker, G.T., Bishel l , A.B., Boardman, C.R., Lai , B.,Sterbinsky, G.E., Jel l ison, J ., Goodell , B., 2021.Oxidation states of i ron and manganese in l i gnocellulose alte r ed by the brown rot fungus Gloeophyllum trabeum measured in-situ using X-ray absorption near edge spectroscopy (XANES). Int.Biodeterior. Biodegrad. 158, 105162 h t tps ://do i .or g /10.1016/j.i b io d.2020.105162. Zhalnina, K., Dias, R., de Quadros, P.D., Davis -Richardson, A., Camargo, F.A.O., Clark, I.M., McGrath, S.P., Hirsch, P.R., Triplett, E.W., 2015. Soil pH determines microbial diversity and composi t ion i n t he park grass experiment. Microb. Ecol . 69, 395-406.h t tps://do i .o rg /10.1007/s00248-014-0530-2. Zhang, J ., Presley, G.N., Hammel, K.E., Ryu, J.-S., Menke, J.R., F i gueroa, M., Hu, D.,Orr, G., Schill i ng, J.S., 2016. Localizing gene regulation reveals a staggered wood decay mechanism f or the brown rot f ungus Post i a placenta. Proc. Natl . Acad . Sci. U .S.A. 113, 10968-10973. h t t p s ://doi.org /10.1073/p nas .1608454113. Zhang, J ., Silverstein, K.A.T ., Castano, J.D., Figueroa , M., Schi l l ing, J.S., 2019. Gene Regulat i on Shi ft s Shed Light on Fungal Adaption in Plant Biomass Decomposers, vol .10. mBio. htt p s ://d o i .o r g/10.1128/mB i o.02176-19. Zhang, Y ., Hao, X., Garcia-Lemos, A.M., Nunes, I., Nicolaisen, M.H., Nybroe, O.,2020.Di f ferent effects of soi l fertilization on bacteria l community composition in t he Penicillium canescens hyphosphere and in bulk soil. Appl. Environ. Microbiol. 86h t t p s ://doi .o rg /10.1128/AEM.02969-19. Zhu, Y., P l aza, N ., Koj i ma, Y., Yoshida, M., Zhang, J., Je l l i son, J., Pingal i, S.V.,O'Neil l , H., Goode l l, B., 2020. Nanostructural analysis of enzymatic and non-enzymatic brown rot fungal deconstruc t ion of the lignocellulose cel l wal l . Front.Microbiol. 11, 1389. ht tps ://doi.o r g /10.3389/f mic b.2020.01389. Zimmermann, W., 1990. Degradation of lignin by bacteria. J . Biotechnol. 13, 119-130.https ://d o i .org /10.1016/0168-1656(90)90098-V .
确定








还剩8页未读,是否继续阅读?
中国格哈特为您提供《未腐朽云杉、中期腐朽云杉、高度腐朽云杉中木质素含量的检测》,该方案主要用于林产品中理化分析检测,参考标准--,《未腐朽云杉、中期腐朽云杉、高度腐朽云杉中木质素含量的检测》用到的仪器有格哈特全自动型纤维分析仪FT12、格哈特全自动快速索氏提取SOXTHERM、格哈特快速纤维测定器FBS6、德国加液器MM、滤纸筒
相关方案
更多
该厂商其他方案
更多