方案详情
文
地中海真菌木材降解:生理学、代谢组学和蛋白质组学方法Wood degradation by Fomitiporia mediterranea M. Fischer Physiologic, metabolomic and proteomic approaches
方案详情

地中海真菌木材降解:生理学、代谢组学和蛋白质组学方法Wood degradation by Fomitiporia mediterranea M. Fischer Physiologic, metabolomic and proteomic approachesTYPE Original ResearchfrontiersFrontiers in Plant SciencePUBLISHED 26 September 2022DOI 10.3389/fpls.2022.988709 Schilling et al.10.3389/fpls.2022.988709 Q Check for updates OPEN ACCESS EDIT ED BY G ry Al fre d se n, Norwegian Institu t e of B ioeconom y R e s ea r c h (N IBI O), N o r w ay REVIEWED BY a sectio n o f the j ou r nal REC E I V E D 07 J u ly 2022 ACCEP T E D 16 Augu s t 2022 CI T ATION Sch i lling M, M aia-Gro nd a r d A, B a lt e n weck R, Ro b e r t E, H u gu e ne y P,B ertsch C, Farine S and Gelhaye E (2022)W ood d eg r ada tio n by Fom i tip or ia medite r ranea M. F i sche r: Ph ysio l ogic ,me t abolomic and p rot e omi c a p p roac hes .Fro n t. P la n t Sc i.13:988709.d oi : 10.3389/fp ls .2022.988709 COPYRI G HT ◎ 2022 S chi l l i n g , Ma ia-Gro nd ard,Baltenwe c k , R ob ert, H ug u eney, Be r ts ch,Far i ne and G e lhay e . T his i s a n o pe n -ac ce ss ar t icle dis tri b u ted u nder the ter m s of the Creative Commons Attribu t ion Li cense (CC BY). T he use , d i st r ibutio n or re p rodu c ti o n i n ot he r f or u ms i s permi t t e d , p r ovid e d th e origi na l au th or(s ) an d the copy r ig h t own e r (s) ar e c redi te d and that the o rigi nal p u bl i ca ti on i n t hi s j o ur na l is c i ted, in a c cord an c e wi th acc e pt e d a cad e m i c p ractice. N o u se , d i s tr ibutio n or re produc ti on i s p e rmi t t e d which d oes not comply with these ter m s . 地中海真菌木材降解:生理学、代谢组学和蛋白质组学方法 Wood degradation by Fomitiporia mediterranea M. Fischer: Physiologic,metabolomic and proteomic approaches Marion Schi ll ing**, Alessandra Maia-Grondard²,Raymonde Ba l tenweck², Emilie Robert, Philippe Hugueney2,Christophe Bertsch4, Sib yl le F ar i ne and Er i c Gelhayel* 1Unive r site de Lor r aine,I NRAE, I AM, Nancy, F rance, 2Univers i te de Strasbourg,INRAE, SVQV UMR-A 1131,, Colmar, France, 3U niversite de Lorraine, INRAE , A2F , Nancy, F r ance, L aboratoire Vigne B iotechnologies e t Environnement UPR-3991, Unive r s i te de H aute Alsace, Co l mar , France Fomitiporia mediterranea (Fmed) is one of the main fungal species found in grapevine wood rot , also called "amadou,"one of t h e mo s t typical symptoms of grapevine trunk disease Esca. This fungus is functional l y classified as a white -rot,able to degrade al l wood structure polymers, i .e., hemicelluloses, cellulose, and the most recalc i trant component, lignin. Specific enzymes are secreted by the fungus to degrade those components, namely carbohydrate ac t ive enzymes for hemicelluloses and cel l ulose, which can be highly specific for given polysaccharide,and peroxidases, which enable white-rot to degrade l igni n , with specif i cities relating to lignin composition as well . Furthermore, besides polymers, a highly diverse set of me t abolites often associated with antifungal ac ti vit i es is found in wood, this set differing among the various wood species. Wood decayers possess the ability to detoxify these speci f ic extractives and thi s ability could reflect the adaptation of these fungi to their specific e n vironmen t. Th e aim of this study is to better understand the molecular mechanisms used by Fmed to degrade wood structure, and i n particular i ts potent i al adaptat i on to g r apevine wood. To do so, Fmed was cultivated on sawdust from different origins: grapevine, beech, and spruce. Carbon mineralization rate,mass loss, wood structure polymers content s , targ e ted m etabolites (e xtract i ves)and secreted proteins were measured. We used the well-known white-rot model Trametes versicolor for comparison. Whereas no signif i cant degradat i on was observed with spruce , a higher mass loss was measured on Fmed grapevine culture compared to beech cultur e . Moreover, on both substrates, a si m ult a neous degrada t ion pattern was demonstrated,a n d proteomic analysis identified a relat i ve overproduct i on of oxidoreductases involved i n l i gnin a n d ext r act i ve degradation on grapevine cultures, and only few differences in carbohydrate active enzymes.These results could explain at least pa r t i al l y the adaptat i on of Fmed to grapevine wood s t ructural composition compared to other wood species, and suggest that other biot i c and abiotic factors should be considered to fully understand t h e potential adaptation of Fmed to i ts ecological niche . Proteomics data are available via ProteomeXchange with i denti f ier PXD036889. Esca, wh i te rot, Fomitiporia mediterranea, grapevine wood, adaptation Grapevine, Vitis v i nifera L ., i s one of the most economical l y impor t ant culture s in the world: i t i s produced worldwide and its production value was estimated to more than $73bi l lion s in 2018(Food a n d Ag ri c ul t ur e O rga ni zat i o n of t he U n it ed Na t i o ns St a t i s t i c s (F AOSTATS), 2021). This high valued production is however threatened by many vineyard diseases. Among them,Grapevine Trunk Diseases (GTDs) are considered as the most cha l lenging one (R ei s et al., 2019). In 2012 and 2013, economic losses of , respectively, 11 and 13% of the French vineyard were attributed to GTDs (Dou b l et et a l ., 2014). No sa t isfying treatment exists yet, even i f promising solutions developments are ongoing (G ram a j e et al ., 2018; M o n d e l lo e t a l., 2018). Esca i s one of the main GTDs, caus i ng alone 3% of French viney a rd e conomical losses i n 2013 (D ou blet e t a l , 2014). Its importance tends t o increase over the last years in France and other European countries (B r u ez et a l ., 2013; G ue r in-D ub r a n a e t a l ., 2019). Esca affects mainly mature vines and is considered as a complex of diseases, associated with various fungal species and symptoms (Mugn a i e t al ., 1999; S u r i c o et a l ., 2008; Leco m t e et a l .,2012; B r u e z e t al ., 2014). Esca symptoms ar e usuall y grouped i nto f i ve syndromes descr i bed as : (i ) brown wood streaking, affecting rooted cutt i ngs and, associated with internal brown wood necrosis, (i i ) Petri disease, a ffec t ing young vines and associated with wood and crown symptoms but also foliar symptoms such as l e af chlorosis and y i e l d or vigor loss, (iii) Young Esca or Grapevine Leaf Stripe Disease (GLSD), associated with wood and crown symptoms as w e ll as foliar symptoms such as the typical “ti g er-striped"leaves or even apoplexy of the vine, (iv) Esca, affecting mainly mature vin e s , charact e rized by typic a l white -rot in wood and also called “amadou,and (v) Esca proper, a combination of GLSD a n d Esca syndromes (S urico , 2009; B e rtsch et al., 2013).Among the GTD-assoc i ated fungi, Phaeomoniella chlamydospora and Phaeoacremonium minimum were the main identi f ied species in wood necrosis observed in the f irst three desc r ibed syndromes (brown wood streaking, Petri diseas e and young Esca; Sur i co,2009). In wood affected by Petr i disease, P . chlamydospora is the main i de n ti fi ed fungal s pecies but up to 36 other species wer e also isolated, inc l uding P. minimum and Cadophora luteo -oli v acea as the mos t prevalent (Pil ar Ma r t in e z-D i z e t a l., 2021).Othe r species not direct l y associated with Esca such as Eutypa lata or Pleurostomophora richardsiae could also be present i n wood necrosis, and the role of e a ch assoc i ated fungus is s t ill not ful l y understood (W h ite et al ., 2011; C ana l e e t a l ., 2019). In white-rot associ a ted with Esca and Esca proper syndromes, the main identified f ungi are Basidiomycota and the main genus is Fomit i por i a. In Europe , the most represent e d speci e s is Fomi t iporia mediterranea (Fmed) M. Fischer r (Hymenochaetaceae,Hymeno c haetales , Basidiomycota; Fis c h e r , 2002; Su r ico et al .,2008; B r u ez et al ., 2020; M ore tt i e t al ., 2021). Grapevine trunk di s eases (GTDs)-associ a ted fungi colonize grapevine wood but not the leaves. The i r role in foliar symptoms st i ll need further research (F o nt a i n e e t a l ., 2016; D e l F r a r i et al., 2021). More particul a rly for Fmed, ev i dence for i ts pathogenicity stricto sensu is st i ll lack i ng. In v i tro, Fmed indeed induce s necrosis on detached grapev i ne wood (M a r ka k is et al., 2017) and inhibits growth of grapevine protoblasts (B ru n o an d Spa r a p a n o , 2006a ).Afte r inoculation, Fmed is able to induce white-rot only on mature vines, but without typica l Esca f oliar symptoms (Mo r etti et a l .,2021). St i ll, curettage of Esca-affected vines which consists of removing white -rot zone, where Fmed is found in majority,improves vine resilience and reduces Esca symptoms the following years (C ho l et e t al., 2021; Pacett i e t a l., 2021). M oreover, Fmed was found i n higher abundancy i n wood showing foliar symptoms (D e l Fra r i et al ., 2021). One hypoth e sis of th e se observati o ns i s that Esca leaf symptoms might require the presence i n wood of both F m ed, or other Hymen o chaet a les, and t racheomycotic f ungi (D e l F r ar i e t a l .,2021). F med could be functionally classi f i e d as a white -rot fungus (WRF ), as i t is able to mineralize al l wood structure components,inc l uding l i gnin, the mos t recalc i trant one (Fl o ud as e t al ., 2012;S a h u et a l ., 2021). Due to grapevine wood spec i f ic it i es , Fmed might be spec i fically adapted to grow on this wood species (Sch il l ing et a l ., 2021). As with other WRF, genomic studies revealed that Fmed ha s a l arge var i ety of enzymes which could be involved in the extracellular d e gradation of lignin, and in part i cular class II peroxidases (Class-II PODs ) a n d l a ccases.Class-I I PODs are classified upon their structure and func t ion as Manganese Peroxidases (MnPs), Lignin Peroxidases (LiPs),Versatile Peroxidases (VPs) and Generic Peroxidases (GPs ). T he Fmed genome harbors 16 gene encoding MnPs (including 3 short MnPs, 11 long MnPs and 2 atypical MnPs ), and 1 gene encoding a GP (Fl ou d as et a l ., 2012). I n contras t with other fungi such as Trametes versicolor (Tver; L.) Llo y d, 1921 (Polypor a ceae ,Polyporales, and Basidiomycota), a WRF found on a l arge range of fores t hardwoods and some softwo o ds, n o genes encodi n g LiPs or VPs were found. LiPs cleave l ignin B-1 l inkage (non-aromatic part ) at th e ir typica l cat a lytic tryptophan site, by oxidation and in the presence of hydrogen peroxide as electron acceptor (Fa l a d e e t a l., 2017), while MnPs possess a manganese (II ) oxidation site and use chelated manganese (II I) ions as e l ectron donor , to oxidize lignin phenol i c structures (Hof r i ch t e r , 2002). Concerning VPs,they exhibit both a catalytic t ryptophan site, like LiPs, and a manganese (II ) oxidation s i te, l ike MnPs, whereas GPs show none of t hem. The l atter s probably ac t by oxidizing their substrate at thei r main heme channel (F l o u d as e t al., 2012). The func t ional diffe r ences be t ween LiPs and MnPs were suggested to be involved in specific WRF lignin degrad a tion capacity, depend i ng on l ignin compo s ition (Su z uki et a l ., 2012). Two main d e gradat i on pat t erns were described for WRF : (i ) s i multaneous (or nonselective), when lignin, cellulose and hemice l luloses are simultan e ously degraded,or (ii) select i ve (or preferent i al), when l i gnin is preferentially degraded (B la nc h e t te,1991). H o wever, it was not shown i f LiPs or MnPs could favor one of the two patterns. For example, two WRF ,Tver and Phanerocha e te chrysosporium strain BKM F -1767, both possessing LiPs and MnPs, were shown to cause, respectively,simultaneous and selec t ive degradation (B l a nc h e t t e et a l., 1989). Laccases a re multicopper oxida s es ac t ing on a l a rge range of substrates, including l ignin and l ignin residues, reducing Oz to H,O (R e i ss et a l ., 2013; M a n a va l a n et a l ., 2015). However , their role in lignin degradation remains unclear. These enzymes are able to ox i dize phenolic compounds sugges t ing also a role in detoxif i c a tion of p o tentia l toxic compounds found in wood and in part i cular i n grapev i ne wood. Fmed genome anal y sis revealed the presence of ten genes encoding laccases (sensu stricto) and o ne Fet3 ferroxidase (F l o u da s e t a l ., 2012). Recent investigation of laccases and MnPs activit i es in Fmed culture media confirmed their importance for Fmed growth on wood (P ac e tt i e t a l ., 2022). E n zymes i nvolv ed in polysaccharide degradation, i n par t icula r hemicel l uloses and cellulose , belong to Carbohydrate-Act i ve enZYme s (CAZYs). Th e y c a n be highly spec i fic relat i ve to sugar units or l inkage types (Ki n g e t a l ., 2011; H a g e a nd Ross o , 2021). Among them, Glycoside Hydrolases (GHs )catalyze polysaccharide hydrolysis with high c a rbohydrate and sugar conformation speci f i c i ties (C o i n e s et a l .,,22019).Depending on their molecular mechanisms they are classified into 173 fami l ies (A F M B, 2022b ). The Fmed genome exhibits 50 GHs from nine families. Carbohydrate Esterases (CEs)cataly z e O-or N -deacetylation of heteropolysaccharides, i .e.,hemicel l uloses and pectins, and are further classif i ed into 20families (AFM B,2022a ). They are key enzymes to enable further polysaccharid e d e polymeriz a tion by GHs .Twelve gen e s encoding CEs from four families were detected in the Fmed genome (F l ou d as et a l ., 2012). Auxiliary activity enzymes a re redox enzymes acting conjointly with CAZYs. Firstly classif i ed as GH61 family, Lytic Polysacchari d es Mono -Oxygenases (LPMOs) are now classified as Auxi l iary Activ i ty enzymes family 9 (AA9), since eviden c e for their oxidative activ i t y involved i n polysaccharides degradation was obtained (Ran i S ing hani a et al., 2021). Thirte e n genes encoding L PMOs w e re identified in the Fmed genome (Fl o u d a s e t al ., 2012). Wood not only contai n s s tructural components, but al s o extractives, which are small molecules or metabol i tes easily extrac ti ble with solvent , often associated w i th antimicrobial or antifungal properties and involved in wood d u rability (V a l et t e et al ., 2017; P er r ot e t a l., 2020). Grapevine wood extractives belong mainly to the stilbene family or to other phenolics such as flavonoids or phenolic acids . Many of them are known for their ant i fungal and antioxidant activi ti es (Je a n d e t e t a l ., 2002).Antifunga l activities against Fmed were demonstrated in vitro for six stilbenes((:resveratrol,pter o st il bene, miyabenol C ,isohopeaphenol, and vitis i n A and B; B r u n o an d Sp a r a p an o ,2006b; La mbe r t , 2011; L a m b e r t et al., 2012). However, as shown with other wood-decaying fungi, Fmed has developed both intrac e llular and extrace ll ul a r d e toxi f i cation process e s to de a l w i th such extrac t ives. Intrace l lular detoxif i cation by wood-decaying fungi i nvolv e enzymes const it ut i n g the f ungal xenome, which is mainly represented by cytochrome P450s and glutathione trans fe rases (More l et a l., 2013; M orel -Ro uh i er, 2021). The Fmed genomes showed, respectively, 130 and 38 genes encoding for those tw o enzymes f a mi l ies (Flou d as et a l ., 2012; M o r el et al ., 2013). In paral l el, Fmed extracellular detoxif i cation was suggested,but mechanisms remain not fully understood . It was shown that the presence of resveratrol in Fmed cultures induced laccase activity and a decrease o f resveratrol concentration, concomitantly to t he produc t ion of t wo non-i dentified by-products (Bruno a n d S pa ra p a n o , 2006b). Moreover , a 60kDa laccase isolated from Fmed secre t ome was shown to oxidize resveratrol , and fo u r non-ident i f ied products (mass to charge ratios (m/z) of 471[M-H]-and 499 [M-H]-for two of them) were detected by mass spectrometry (Abo u -Mansour e t a l ., 2009). Oxida t ion and polymerization of resveratrol into stilbenes d i mers by Botrytis c i nerea Pers ., an Ascomycot a and grapev i ne fruit s pathogen, hav e been described (C i c h e wi c z e t al ., 2000). Here should be noticed th a t s t ilbenes oligomers are usually assoc i ated with antifungal activities (L a m b e r t e t a l ., 2012; St em p ie n e t a l., 2017). Thus,dimeri z ation was s ugg e st e d to be a fi rst step i n f ungal resveratrol metabolization (Cic he w ic z et al., 2000). In thi s st u dy , we aimed to investigate wheth er or n ot Fmed is better adapted to degrade grapev i ne wood compared to other wood species, possibl y due to speci f ic degradat i on or detoxif i cation mechanisms. Tver, well studied for fungal wood degradation mechanisms, was used as a model. CO2 prod u ction rate was measured during 3-month cultures of Fmed and Tver on grapev i ne cv .“Gewurztramine r , beech (Fagus sylvat i ca) or spruce (Picea abies ) wo o d sawdust , to follow carbon mineraliz a tion continuously. Global mass loss and wood f i ber chemistry (cellulose , hemice l luloses, l ignin and soluble compounds contents), as well as targeted metabolomic (for primary metabolites and extractive s) analy s is were conducted to compare wood degrad a tion upon substrates for the two WRF, and proteomic analysis at l and 3 months of culture a imed to correlate those observations with the funga l secretome. This const i tutes a f i rs t step for a c o mpreh e nsive approach of Fmed coloniz a tion mechanisms on wood . Materials and methods Fungal strains Fomitiporia mediterranea (Fmed) strain phco36 was prov i ded by Laboratoire Vigne Biotechnologies et Envir o nnement (LVBE)of University of Haute-Alsace in Colmar and i solated from Vitis vinifera L. cv. Ugni blanc ' in 1996 in Saint-Preui l (Charente ,France). Trametes versicolor (Tver ) strain BRFM1218 was obtained from the Center International de Ressources Microbiennes (CIRM) catalog and is olated from Quercus sp. wood i n France. All strains w e re cultivat e d and regul a rly regenerat e d on m odif i ed Pachlewski solid culture media (P25), composed of 20g/l agar,20g/l glucose, 1.0g/l pot a ssium phosphate (KH,PO4), 0.5g/l magnesium sulfatee (MgSO4),3.0g/l 1:ammonium tartrate (CH02(NH,)),5.0g/l maltos e , 0.4 g/l thiamine, 10 mg /lMnCl,12mg/l FeSO4, 7H O, 4.6 mg/l ZnSO4, 7HO, 1.2 mg/l CuCl ,17 mg/l H,BO,, and 1.4mg/l NaMoO ,2H,O. 成熟的琼瑶浆(Gewurztraminer)葡萄树的树干 Mature trunks of Viti s vinifera cv .“Gewurztraminer”were cut in the Soultzmatt vineyard (Haut -Rhin,France ) in February 2021with the kind agreement of Valent i n Zusslin Estate (Orschw i hr,Haut -Rhin, France), and stored a t room temperature before be i ng sawn. Only white parts of the vine trunks, considered as healthy,were selected, sawn into 1 cm’pieces and further r e duced into sawdust by liquid-nitrogen assisted cold ball grinding (Cryomill .Retsch GmBH). Bark, necrosis and black or brown parts were discarded during sawing. Beech (Fagus sylvatica) wood sawdust was provid e d by the B EF laboratory (Biogeochimi e des Ecosystemes Fores t iers , INRAE) from Champenoux forest (Meurthe -et -Mose l le, France). Spruce (Picea abies Karst ) wood sawdust was provided by the NIBIO laboratory (Norwegian In st i tute of Bioeconomy Research) from Norwegi a n forest s ta nds.Al l sawdusts were sifted between 0.5 and 2mm and dried at 105°C before i noculations. Fungal cultures on sawdust The experimental plan is summarized in F i g ur e 1. Wood sawdust was dried for 48 h at 105°C and 2.0g per i ndividual were added in 120 ml gl a s s f la s ks and steri l ized for 20 min at 120°℃. To rich substrate moi s ture saturation, 5.0ml of liquid modi f ied P25culture medi a , without carbon source (1.0g/l K H PO4, 0.5g/l MgSO 4,2.2g/l ) ammonium sul f ate [SO2(N H4), 0.4 g/l t hi a mine,10 mg/l MnCl , 12 mg/l FeSO4, 7HO, 4.6mg/l ZnSO4, 7HO,1.2 mg/l CuCl, 17 mg/l H ,BO, and 1.4mg/l NaMoO4, 2H O],were added und e r sterile condit i ons . One cm inoculum from 2-week-old fungal cultures grown on solid P25 media at 27°℃ was added to each flask. For control f lasks, a similar amount of soli d P25 media was ad d ed. Within 2 h afte r inoculation, three flasks of each test combination (9 combinations of wood (×3) and fungi (×2) or control , see F ig u re 1), further ident i fied as t ime 0, were freeze-dried, lyophilized a n d ground i nto powder by liquid-nitrogen assisted cold bal l grinding (CryomillRetsch GmBH) for methanolic extraction and wood fibers analyses . All other inoculated f l asks were closed hermetically with septum caps and stored in the dark at 27°C until sampl i ng (F i gu re 1). After 1, 2,and/or 3 months of culture (F igu r e 1), three fla s ks per test were freeze-dr i ed and lyophilized for mass l oss and ground into powder for methanolic extractions and wood fibers analyses. At 1 and 3 months for grapevine and beech sawdust cultures , three other flasks per tes t were extracted for proteomic a nalysi s . Measurement of carbon mineralization Carbon mineralization was calculated by measuring the CO pressure in each f l a sk every 1-4 day s . CO content in each f lask was measured in %v/v (%Coz) with a Lambda CZ s.r.o.carbometer by f lushing air contained i n the flask with a KNF Laboport N 86 kt.18 pump and s teri l e needles connected to Ssilicone pipes through 0.2 um filter. Oxygen pressure was renewed after each measurement at atmospheric pressure. %CO2 was converted into COz amoun t (nCO,) i n mol/L according to ideal ga s l a w : where P (CO2)=%CO multiplied by atmospher i c pressure (101,325Pa) V=150 ml corresponding to the flask (120ml ) a n d pipes (30ml ) volume,R =8,314J mol-K-and T the temperature of the room i n Ke l v i n dur in g measurement . Final l y , nCOz w a s converted in mass of mineralized carbon (mg C) in mg and the mineralization r ate was calculat e d i n mg C/day at each measurement point . Maximal mineralization rate corresponds to the mean of all highest mineralization rat e s (i .e., al l rates which were not significantly different to the highest mineralization rate,accordi n g to Tukey test ). Mass lOss Global mass loss per fl a sk was measured the di f ference be t ween cultures dry weight afte r l yophilization and control mean dry weight and i s expressed in percentages of control mean dry weight . Control samples dry we i ght at initial time and afte r each month of culture show e d no di f ference. St a tistical analysis was performed both between each mass loss and it s respective control ,a n d between each releva n t ma ss losses w i th Student -N ewman-Keuls test. Wood fibers analyses Methanolic extraction As internal s ta ndard, 5mg±0.1mg of lyophilized and powdered sample were extracted with 250 pl of LC-MS grade met h anol previously supplemented with 5 pg/ml chloramphenicol (Sigma-Aldrich ). The samples were sonicated in an ultrasonic bath (FB15050, Fisher Sc i enti f ic, Hampton, U n ited St a tes ) for 10 min and cent r i f ugated at 12,000g for 15 min. T h e supernatant Wasanalyzeedd using by y ultra -high -performance l iq uid chromatography coupled to high resolution mass spectrometry (UHPLC-HRMS). Targeted metabolomic analysis Metabolomic analyses were performed using a Dionex Ultimate 3,000 U H PLC system (Thermo Fisher Sc i enti f ic,Waltham, MA, United States ). Liquid chromatography -mass spectrometry (LC-MS) grade methanol and acetonitrile were purchased from Roth Sochiel (France); wat e r was provi d ed by a Millipore water purification system. The chromatographic separations w e re performed on a Nucleodur C18 HTec column (150mmx2mm, 1.8-um particle size; Macherey-Nagel, Diiren,Germany) maintain e d at 30℃. The mobile phase consisted of acetonitrile /formic acid (0.1%, v/v, eluant A) and water/formic acid (0.1%, v/v, eluant B) at a f low rate of 0.3 ml/min . The gradient e lution 1Was programmed as f follows S ::0-1mi n 1,,95% B;1-6 min,95-0% B; 6-8 min, 0% B. The sample volume injected was 1 ul. The UHPLC system was coupl e d to an E x active Orbitrap mass spectrome t er (Thermo Fisher Scienti f ic ), equipped with an el e ctrospray ionization (ESI ) source operating in po s itive a n d negative mode. Parameters were set at 350°C for the ion transfer capil l ary temperature a nd 2,500V for the needle voltag e s.Nebulization with nitrogen sheath gas and auxiliary gas was maint a ined at 40 and 5 arbitrary units, respec ti vely . The spectra were acquired within the mass-to-charge ratio (m/z ) ranging from 75 to 1,500 atomic mass unit, using a resolution of 50,000 a t m/z 200 atomic mass unit. T he system was calibrated internally in positive mode using dibutyl-phthalate as the lock mass at m/z 279.1591, giving a mass accuracy lower t han 1ppm. T he instruments were controlled using the Xcal i bur software (Thermo Fisher Scientific ). Metabol i tes were sought based on the calculated m/z of the corresponding pseudo-molecular ion [M+H ]t in posi ti ve mo d e and [M -H]- in neg a tive mo d e from a li s t of me t abolites of interest using a suspect screening approach (K r a u ss e t a l ., 2010; Flam i n i et a l ., 2013). Putative metabolite ident i fications were proposed based on expert i zed analy s is of the correspondi n g mass spec t ra and comparison with published literature. Further information was retrieved from the Kyoto Encyclopedia of Genes and Genomes (KEGG1) and PubChem2 databases. The identification of some metabol i tes was confirmed with the corresponding standard for: amino-acids (arginine,asparag i ne, glutamine, g glutamate,aspartate,valine,proline e ,,1leucine,tyrosine e ,,isoleucine,phenylalanine, tryptophane, and valine), phenol i c acid s (gal l ic acid and caffeic acid), flavonoids (catechin, epigallocatechin gallate, and naringenin) and st i lbenoids (trans-resveratrol,pterost i lbene, and e -viniferin). All standards were provided by Sigma-A ldr i ch (France). St i lbene oligomers for which no standard was available were put a tively ident i f i ed as s ti l bene dimer M471,stilbene dimer M 473, stilbene trime r M679, stilbene trimer M681,st i lbene tetramer M907, where MXXX stands for the m/z of the corresponding ion in positive mode. Relat i ve quantification of the select e d met a bol i tes was performed using the Xcal i bur sof t ware. Differential me t abolomic an a lyses were performed afte r log10d ata transform a tion, us i ng Tukey 's honest signi fi c a nt di ff erence method followed by false discovery rate (FDR) correction using the Benjamini -Hochberg procedure. Metabolit e s of interes t w e re considered differentially accumulated when the false discovery r a te was below 5%(FDR <0.05). Proteomic samples preparation At 1 and 3 months of culture, for beech and grapevine cultures , extracellular proteins were extracted from three flasks per c u l t ure condi t ion. Each f lask was agitated with 30 ml of 30 mM potassium-ace t ate buffer at pH 4.5 for 2 h at 60rpm at room temperature. Extracts were centr i fuged 20 min at 4,000 rpm at 4°C and supernatant s were precipitated overnight at -20°C in cold 80% acetone. Prec i p i tated prote i ns w e re d r ie d after centrifugation 20 min at 4,000 rpm at 4°C and diluted in 40 ul of concentrat e d Laemml i blue (2-mercaptoethano l 24%(w/v ), bromophenol blue 17.3 M, glycerol 20% (v/v ), sodium dodecyl sulfate 10% (w/v) a n d Tr i s -base 0,4M). Samples w e re heated for10 minutes at 95℃ and 15 ul of each sample were deposited o n 1 mm 15% S D S-PAGE gels for s hort -m i gration.Gels were revealed with Coomassie blue coloration and gel pieces were sent for proteomic analyses . Proteomic analysis Proteomic analyses were achieved by PAPPSO platform,INRAE, Jouy-en-Josas. T he analyses of peptides were obt a ined using an Ul t iMateTM 3,000 RSLCnan o System (Thermo Fisher Sc i enti f ic, San Jose , CA, United States) coupled to an Orbitrap FusionTM LumosTM Tribrid T M mass spectrometer (Thermo Fisher Scientific ). Samples were digested with trypsin and resuspended in 2% ACN and 0.1% TFA buff e r. Four ul w e re injected on an Acclaim PepMap C18 precolumn (5umx300umx5mm) at 20ul/min and separat e d on a n Acclaim PepMap RSLC nano Viperr(C18 column (2um×75 um×500mm , Th e rmo Fisher Scienti f ic ). Mobile phase was composed of A: 98% HO in 0.1% formic acid (HCOOH) and B: 80% AC N in 0.1% HCOOH. A gradient from 1.0% B during 2 min, to 30% B for 48 min and to 40% B for 5 min, followed by regeneration step at 98% B in 2 min for 5 min and equi li bration at 1.0% B i n 0.5 m in for 4.5 min, was applied at 300 n l/min. Peptide ions were anal y sed u s ing Xcalibur 4.1,Tune 3.0. with the following parameters: MS scan m/z 400-1,500 and resolution 120,000; MS/MS collision energy 30%, resolution 30,000, a n d dynamic exclusion set at 100 s.RAW d ata were converted to mzXML data with proteowizard software (C hamber s e t a l, 2012). Proteins annotations was performed using the X!T andemPipeline (Lang el l a et al ., 2017)and d 1https://mycocosm.jgi.doe.go v/Fomme1/Fomme 1.home.html, https://mycocosm .jg i.doe.g o v/Travel/Trave l .home.html and an internal contam i nants database. Only proteins with at le a st two di s tinc t peptides were considered. Proteins relative abundancies were compared using protein abundance index (PAI ) and exponent ia lly modif i ed prot e in abunda n ce index (emPAI ; Is hi ham a et a l ., 2005). Proteins with emPAI higher than 10 w e re selected for comparative analy s is and were functionally annotated according to Joint Genome Institute (JGI) online database . Statistical analyses D ata analysis an d graphs were done using Rstudio, PBC software version 2022.02.3. Shapiro-Wilk test was used for data norm a l i ty check when re l evant . Kruskal -Wall i s tests with Conover-Iman multiple comparisons test were used for nonparame t ric tests a n d Stud e nt Newman -Keuls (SNK-test )for parametr i c tests. All significant d i fferences are indicated by *** f or p -v a lu e s <0.001, ** for p-values <0.005 and * for p-values <0.05. Results Fmed and Tver wer e grow n on sawdus t from three different wood: grapevine cv . Gewurztraminer , beech, and spruce, during 3 months at 27°℃ in 120ml flasks (Figur e 2). Flasks were hermetical l y closed , and oxygen was renewed every 3-4days . At each oxygen renewal , the amount of produced and accumulated CO, was measured, to follow fungal growth and wood degrad a tion in a non-destructive way . Spruce sawdust cultures were stopped after 2 months because no more carbon mineral i zation was measured before the end of the 2-months spruce cul t ures. A s s hown in Fig ure 1, for each test condition, at l e as t 12 replicat e s or flasks were inoculated at the same time. Among them, three replicates wer e sampl e d at t i me 0, 1 month, 2 months (fo r spruce and grapev i ne) and 3 months (for grapevine and beech) for mass loss, methanolic extraction (for metabolomic a nalys e s ) a nd w o od fibers analyses. Three other repl i cates were sampled at 1 and 3 months of culture for prot e omic analyses, for grapevine and beech only as only low carbon mineralization and no mass loss were obs e rved on spruce. Both Fmed and Tver sh o wed distinct morphologie s , as shown in F ig u r e 2. On grapev i ne and beech sawdust , Fmed had the same morphology: white mycelium at the beginning ,turning ye l low while col o nizing the whole volume of the f lask.On the contrary , the Tver mycel i um remains white and at the bottom of the f l as k, on the sawdu s t . On spruce sawdust , a l i ght and white mycel i um was observed with Fmed , as well as a slight change in saw d ust color (darkening). No mycelium was observed in the spruce Tver culture . A l i ght white fruiting bodies were observed in any of the cultures. Carbon mineralization rate Carbon mineralization rates were obtained by measuring the CO, content (%v/v ) and expressed in mgC/day (Fi gur e 3A ). CO content was renewed to atmospheric content after each measurement . Carbon mineralization rates of Fmed cultures differed depending on the substrate. On grapevine, a rapid increase was observed (before day 15th), followed by a stabilizat i on unti l the end of the culture (84days ). On beech sawdust , t hree phases of the mineralization rate were observed: an increase (before day 22nd),a s t a bilization a nd a decrease (afte r day 63rd). Increase phase was longer (or s lower) compared to that observed on grapevine. On spruce sawdust , the Fme d miner a lization rate increased before day 22nd and decreased after day 36th. Th e maximal min e rali z ation rate corresponds to the mean mineralization rate during the stabilization phase. For the latter,no differences w e r e observ e d between Fmed grapevine or be e ch cultures (2.55 and 2.46 mgC/day respect i vely ). A lower maximal mi n eraliz a tion rate was observed for Fmed spruce culture (1.27mgC/day). In Tver cultures, with the thre e substrat e s , three phases of t he mineralization rate were successively observed: an increase, a stabi li zation and a decrease (F i gu r e 3B ). On grapevine and spruce, the increase phase occurred before day 7th w h ereas i t occurred be f ore day 22nd on beech. On grapevine and beech, t he decrease phase started after 43 incubation days while i t started after 15incubation days on spruce . Contrary to Fmed cultures, a higher maximal respiration r ate was observed i n Tver beech culture than in Tver grapevine culture. For both substra t es, this rate was significant l y higher compared to Fmed cultures (p-values <0.001). T ver and Fmed maximal mineralization rates were similar on spruce . Mass lOss Globa l mass los s was obtained from cultures tota l dry weight at 0, 1, 2 or 3 months of culture (F igu re 4). Afte r 1 m onth of fungal exposure to Fmed, no signif i cant mas s loss was observed for any of the three sawdust . After 2 months, F med culture on spruce caused no significant mass loss , whereas 15.7% of mass loss was observed on grapev i ne. After 3 months, a higher mass loss (23.6%) on grapev i ne than on beech (13.8%) was observed. Thus, consistently with mineralization rates, Fmed did not significantly degrade spruce wood and degraded more grapevine wood than beech wood. A f ter 1 and 2 months of incubation in the presence of Tver, mass losses measured in grapevine wood cultures (13.3 and 19.0%respectively ) were higher than with Fmed. As for Fmed cultures, no signif i cant ma s s losses were observed on spruce after 2 months of exposure to Tver. Unl i ke the two firs t months, a similar degrada t ion was observed with both f ungi after 3 months of grapevin e culture (21.7% with Tver ). A s i milar mass loss was observed on beech cul t ures after 3 months of exposure to Tv e r (20.3%). Thus, after 3 months, Fmed and Tver degraded an equivalent amount of grap e v i ne w ood a nd Fmed degraded l e ss beech wood th a n Tv er . Wood fibers analyses Soluble compounds , hemicelluloses, cellulose, and l ignin contents were obtained at 0, 1, 2 and/or 3 m onths of each culture FIGURE 2 Control , Fmed (F mediterranea phco36) and Tver (T . versicolor BRFM1218) cul t ures o n g ra pevine, beec h and spruce at the end of the c u ltures.Grapev i ne and be e ch cultur e s were stopped at 3 mont h s , a n d spruce cultur e s were stopped at 2 months . A ye l low and wol l y m ycelium was observed f or Fmed whereas Tver mycelium remains whi t e and at the bottom of the f lask. On spruce, a sl i ght coloratio n of sawdust and white mycelium are observe d wit h Fmed, and no myc e lum is observed wit h Tver . according to Van Soes t method (V a n Soest an d Mc Q u ee n , 1973).Composition of control wood sawdust was the same at each month of culture, includ i ng ti me 0. Mean compos i tion of control sawdust and sawdust exposed to fungal culture during 2 or 3 months are shown in Fi g u r e 5A for grapevine, Fi g ur e 5B for spruce and F ig ur e 5C for beech. Grapevine init i al l y contained more lignin (19%) than beech (13%) and less than spruce (25%);less hemicel l uloses (28%) than beech (31%) and more than spruce (19%); less cellulose (35%) than both beech (46%) and spruce (43%); and the highest soluble compound s con t ent (18%)compared to beech (10%) and spruce (13%). After 2 and 3 months of fungal e xposure to both Fmed and Tv e r, no signi fi cant variat i ons regarding c e llulose, hemicelluloses and lignin were observ e d for all substrates. Concomitant l y, after 3 months of funga l culture, soluble compounds content increased in beech sawdust exposed to Fmed and t o Tver (+6%). I n grapevine sawdust exposed to Fmed dur i ng 2-and 3-months, this content remained equivalent , whereas i t decreased after 3 months exposed to Tver (-3%). Targeted metabolomic analysis Fo urteen st i lbenes and four flavonoids were detected with grapevine cul t ures (F ig u r e 6A ), three f lavonoids with beech cultures, a n d no wood e xtractives on spruce cultures (Fig u r e 6C),whereas in total twelve amino acid s (AA) and five other phenolic compounds were d iff e renti a l l y detected (Fi g u re 6). Sti l bene oligomers, for which no standards were available, were putatively ide n ti f ied as st i lbene dimers, trimers and tetramers as i nd icat e d .Heatmaps of relative mean peak areas of detected metabol i tes for each wood are given in Su pp lemen t ary Fi gur e S 1. The l att e r shows p-values<0.001, ** f or p-values<0.005 and * fo r p-val u es<0.05. that no ti m e eff e ct was observed, a s no quant i tative variations were observed between 0-,1-,2-, and 3-months control cultures. Signi f icant variations in the relative amount s of the selected metabolites were observed between the control and the culture in a ti me-dependant manner, as shown in F i gur e 6. During Fmed grapevine and beech cul t ures, the content of all selected flavonoids and stilbenes decreased. Epigallocatechin gallate,catechin, trans - and cis -resveratrol, trans - and cis -piceid, trans-piceatannol, pteros ti lbene and two st i lbene dimers (M471 and M473) contents decreased from the f i rst month of culture.Relative amount of naringenin, cis-piceatannol,e-viniferin,one st i lbene trimer and on e st i lbene tetramer (M681 and M907)decreased from 2 months of culture , and one stilbene trimer (M679) co n tent d e cre a sed after 3 months o f cul t ure. Epicatechin content decreased at 1 month and reverted to control value at 2and 3 months. A very slight natural degradation of this compound i n control sample s (Su pp l e m e n t ar y Fi g u re S1), or an increase of epicatechin content during the second month in presence of Fmed . In beech cultures , catechin , epicatechin and naringenin contents al s o decreased after the fi r st month of culture. Thus, most of the selected extract i ves were degraded by Fmed after different culture times. Not al l extrac ti ve content s in Tve r grapevine culture decreased to the same extent , some of them decreasing less intensely or slower than in Fmed culture. Three stilbenes, ci s-piceatannol and the two st i lbene trimers (M679 and M681), were not degraded. A l l other grapev i ne extractives, except c a techin, epicat e chi n , cis -piceid and trans-piceat a nnol were less or more slowly degraded compared to Fmed cultures. B esides extractives, primary metabolites contents in Fmed cultures evolved different i al l y depending on the wood spec i es.With grapev i ne, an increase in eight A A levels was observed (isoleuc i ne, aspartate , glutamate and glutamine afte r l month and l eucine, tyrosine, phenylalanine and tryptophane after 2 months).In beech and in spruce cultures, only three of them (glutamine ,glutamic acid, and aspartate) increased. Prol i ne and arginine levels decreased i n grap e v i ne cultures after 1 and 3 months, respectively.Interestingly, in spruce cul t ures an i ncrease of arginine and proline content was observed. When growing Tver on grapevine sawdus t , differences between fungal cultures and control have been observed for only three AA: glutamine leve l i ncreased after 1 month, proline level decrea s ed af ter 2 months, and valine l e vel d e crea s ed after 1 month .Valine level decreased in beech cultures exposed to Tver for 1 month, and not when exposed to Fmed. AA level s variations were simi l ar in beech and spruce cultures exposed to both Fmed and Tver. Proteomic analysis P roteomic analyses were conducted on 1- and 3-months grapevine and beech cul t ures of Fmed and Tver. Proteins with emPAI above 10 in at least o ne experimental con d ition were selected for comparative analysis. Thus, respectively 113 and 122prot e ins were compared in Fmed and Tve r secretomes (Ta b le 1).As expected, mainly extracellular proteins were detected, except for two P450s i n Fmed cult u res (S u ppl e m ent ar y T able S 1). As Fmed and Tver c u l t ures mass l os s on spruce at 1 and 2 months, gra p ev i ne at 1, 2 and 3 mont h s, a nd beec h at 1 a n d 3 mont h s. Sig n ificant diffe r enc e s obtained w i th Conover-I man test of multiple comparisons a r e i n dicated by *** for p-values<0.001, ** for p-values<0.005 a n d * for p-values<0.05. ■Solubles H emicelluloses Cellulose w Lignin 2m 3m FIGURE 5 Proportions in wood componen t s i n control, Fmed and Tver cultures on (A) grapevine, (B) spruce and (C) beech at 1 month (1m), 2 months (2m) or 3 months (3m) of cu l t u re. Signi f icant di f ferences are given compared to t h e percentage in control accordi ng to Student-Newman-Keuls tes t , and are i ndicated by *** f or p-values<0.001, ** for p-values<0.005 and * for p-values<0.05. shown in T able 1, a simi l ar amount of secreted proteins were found i n Fmed grapevine (101 proteins) and beech cultures (93prote i ns). MnPs were the only class-I I peroxidases detected.Among the 16 MnPs identified in Fmed genome (F lo udas e t a l .,2012), 11 were found on grapev i ne wood whereas e i ght were found on beech. Three and two lacca s es were found on the two substrates, respective l y, among the ten ident i fied in the Fmed genome (Floudas e t al., 2012). Few differences regardi n g th e number of CAZYs detected i n the Fmed secretome on grapevine or beech were observed (50 on grapevi n e a nd 49 on beech). A lso,more peptidases were found with grapev i ne wood (18) compared to beech wood (11). Relative abundances for secreted proteins in Fmed and Tver grapevine and beech cultures are shown in Fi gur e 7. More oxidoreductases w e re found w i th grapev i ne (33%)rather than with beech (18%). Among them, mainly MnPs were found (respectively 24 and 13% of total proteins content ). More laccases were found with grapevine (7%) than with beech (1%).Protease abundancy was al s o higher with grapev i ne (14%) than wi t h beech (10%). Thus, more MnPs , l a ccase s and peptidases were observed i n the Fmed secretome on grapev i ne compared to beech sawdust, both regarding prote i n diversity and abundancy. Tot a l numbers and abundancies of CAZYs were similar between the two substrates , and few di f ferences in CAZYs composition were observed (Su pp l e m e n t ar y T a b l e S 1). I n the Tver genome , eight LiPs and six MnPs were id e ntif i ed,among, respect i vely, 10 and 13 identi f ied in the Tver genome (F l o u das et al., 2012), whereas no VPs nor GPs w e re d e t e ct e d e i ther on grapevine or on beech (T a ble 1). On grapevine and on beech, eight and s e v e n LiPs and f ive and three MnPs, re s pectively ,were i dentified In the Tver secretome. Four laccases on the seven ident i fied i n the Tver genome (F lou d as et al., 2012) were found on grapevine and one on beech. Howeve r, as shown in F i g u r e 7,oxidoreductas e s were less abundant on grapevine than on beech Moreover, co ntrary t the Fmed secretome (Sup p l e me n t a ry Fi g ur e S 2A ), CAZYs abundancies inTver secretome showed substrate specificities for some of these proteins (S up p l ementa ry F i gu re S2B ). Indeed, for the latter,proteins from GH15 and GH43 fami l ies were detected with higher relative abundancies on grapev i ne compared to beech cul t ures . One alph a -amyl a se from GH13 f a mi l y subfamily 32, one GH17 and one glucosidase from GH31 family were more represented on gr a pev i ne than on beech cul t ures. On the contrary in the Fmed secretome, GHs from those families were either not foun d (no G H 13 i d entified) or found on both grapevine a nd beech. Some GH families were ident i fied only in Fmed or in Tver s ecretome (S up pleme n t a ry Table S2), such as GH 1 or GH18 only detected in Fmed secretome. Discussion Important mechanisms i nvolved i n Fmed wood degrad a tion and detoxi f ication were described . A simultaneous degradation pattern, i .e., the concomitant degradation of all wood s t ructure components , was observed w i th the two hardwood species, while no signif i cant mass loss was observed with spruce. In paral l el ,speci f icities of Fmed mechanisms on grapevine wood were highlighted, namely regarding carbon mineralization rate, mass loss, metabolomic a nalyse s and secr e ted proteins analyses . Wood initial compositions of the three substrates were diff e rent: grapevine showed low ce l lulose content s and high soluble compounds contents. Its l ignin content was lower compar e d to spruce and higher compared to beech. A decrease of soluble compounds was observed in grapevine culture s while their co n tent increas e d i n beech cultures. Soluble compounds i n clude extractives and other non-structura l wood compounds, such as soluble nutriments, sugars or nitrogen res e rves . D egradat i on or consumption of the latter could lead to soluble compounds decrease, whereas accumulation of fungal wo o d degradation products or so l uble compounds from myce l ium could l ead to an Fmed/C FIGURE 6 T argeted me t abolomic analysi s of t he wood d e gradation p r oc e ss. Log2 o f significant metabol i te fold chang e s for i nd i cated pairw i se comp a riso n s are g i ven by shades o f red o r blue colors acco r ding to the scale bar fo r (A) grapevine, (B) beech and (C) spruce c u ltures. Data r epresent the mean values of three biological replicates for e ach condi t io n and t i me po i nt . Stat is tical ana l yses were performed by T ukey's honest signi fi can t di ff erence method followed by false discove r y rate (FDR) co r rection, with FDR<0.05. For F D R≥0.05, Log2 fold changes were set to 0. AA: amino acids ; AL:aldehydes; F: flavonoid ; PAc : p he n olic ac i ds; PAl : phenolic alcohols; St1 to St4: St i lbene monome r s, dim er s, trimers and tet r amers ; MXXX: m/z i n pos i tive mode. C: control. increase. Further experiments such as more precise determination of the nature of soluble compounds would giv e more information about fungal grow t h mechanisms. In parallel, the absence of variations in proportions of the three main structur a l components suggests a s i mult a neous WRF degradation pattern (B l an c he t t e et a l ., 1985). Thi s was obs e rved on both grapevi n e and beech wood . This is consistent with one previous study based on wood f i bers analy s i s (P a c et t i et a l ., 2022). Ini t ial lignin and soluble contents i n grapevine wood (respectively, 19 and 18%) are higher than previousl y reported for the same cv . (15.7 and 14.6%), while measured hemicelluloses content is lower than previously report e d (respectively, 28 and 35.7%; P a cetti et a l ., 2022). I n anothe r study with grapevine cv. Sangiovese, higher l ignin and lower hemicellulose content s were observed in brown streaking wood (17.2%) and brown red wood (29.9%) compared to asymptomat i c wood (12.1%; Ag rell i et a l ., 2009). Previous f ung a l degrad a tion, for example by t he two Ascomycot a P. chlamydospora and P. minimum associated with brown necrosis, could explain a higher lignin content (Agr elli et al., 2009). Several other parameters could affect l ignin content in wood, such as env i ronmental stresses (B lan c hette, 1991). For this study , only white parts of wood , considered as healthy, were selected. Fmed ability to degrade healthy grapevi n e woo d is consistent wit h previous studies showing that the l a tte r does not require previous degrad a tion, namely by the two Esca-associated Ascomycota P. chlamydospora and P . minimum (S p a r a pan o e t al ., 2001; B r o w n et a l ., 2020). However , among other factors, di f f er ent structural compositions were shown to induce di f ferent fungal wood degrad a tion patterns (B l an ch e t t e, 1991). F or example the WRF Pleurotus ostreatus shows simultaneous degradation with beech and lignin pref e renti al degradation w i th oak wood (B a r i e t a l ., 2020). Our results show simultaneous degradation pattern on beech and grapev i ne wood for both F med and Tver. This i s consistent with in vitro and in vivo studies showing a simultaneous degradation for Tver on beech , spruce, oak or hornbeam (Karim e t a l ., 2017; Bari et al ., 2020). Fmed wood degradation patterns mi g ht be dif f erent wi t h other substrat e s, such as other wood species or previously degraded wood, or in di f ferent growth co n ditions. Fmed was identif i ed on d ifferent wood species, such as Actinidia sp., Citrus sp. or olive trees (M o retti e t al., 2021) and strains specif i c i t i es were shown for Fmed depending on the wood species from which they were i s olated (M a r k akis e t a l ., 2017).Thus, w o od d egradation pattern might be more spec i fic upon other wood spec i es and investigation with more Fmed strain s and more wood species could highlight more Fmed speci f ic i t i es . Contrary to wood degradation pattern, other parameters suggest a spec i fic ac t iv i ty f or Fmed on grapev i ne cultures compared to the othe r substrates: a l o nger stabi l ization phase for maximal mineralization rate, a higher mineralization rate (compared to spruce only), higher mass loss, more secreted laccases and MnPs and elevated AA levels. Indeed, carbon mineralization rate on grapevine cultures reached a pl a teau and maintained it unti l the end of the 3-month cultures. On the contrary with beech and spruce cul t ures, mineraliza t ion rates decreased before the end of the cultures. CO production or carbon mineralization result s f rom metabolic processes inc l uding funga l respiration. With wood as the only carbon source,carbon mineraliz a tion rate c a n b e considered as an indicator of wood degradation . The firs t increase of carbon mineralization rate f ol l owed by a plateau was already observed i n fungal cultures w i th 18 white or brown rot species isolated from forest softwoods with diff e rent microc l imatic pref e rences (Ca r lsson et al ., 2017). The Fmed Tver Genome Grapevine wood Beech wood Genome 片 Grapevine wood Beech wood All 1 month 3 months All 1 month 3 months All 1 month 3 months All 1 month 3 months Oxidoreductases MnP 16 11 11 10 7 8 8 4 13 6 5 5 0 3 2 2 LiP 0 0 0 0 0 0 0 0 10 8 8 8 1 7 7 0 VP 0 0 0 0 0 0 0 0 3 0 0 0 0 0 0 0 GP 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 Total Class II-POD 17 11 11 10 7 8 8 4 26 14 13 13 1 10 9 2 DyP 3 0 0 0 0 0 0 0 2 1 1 1 1 1 1 1 HTP 4 0 0 0 0 0 0 0 3 0 0 0 0 0 0 0 Laccases“sensu stricto” 10 3 3 3 2 2 2 0 7 4 3 3 1 1 0 1 Ferroxidases 1 0 0 0 0 0 0 0 2 0 0 0 0 0 0 0 Ferroxidase/laccase 0 0 0 0 0 0 0 0 1 0 0 0 0 0 0 0 Glyoxal oxidase 0 1 1 0 1 1 0 1 5 4 2 1 2 4 4 4 Copper radical oxidase 4 0 0 0 0 0 0 0 4 0 0 0 0 0 0 0 CDH 1 0 0 0 0 0 0 0 1 1 1 1 0 0 0 0 Other GMC oxidase 4 4 1 1 1 4 4 3 9 0 0 0 0 0 0 0 Total oxidoreductase 44 19 16 14 11 15 14 8 60 24 20 19 5 16 14 8 CAZYs GH 50 43 40 21 38 38 27 33 47 45 42 35 36 34 30 30 AA9 13 2 2 2 2 2 2 2 18 5 4 4 1 3 3 3 CE 12 8 7 3 6 8 7 8 14 8 6 5 6 8 5 8 PL ? 1 1 1 1 1 1 0 ? 1 1 0 1 0 0 0 Total CAZY 75 54 50 28 47 49 37 43 79 59 53 44 44 45 38 41 incl. CBM ? 8 8 6 7 7 5 5 ? 13 11 11 10 11 9 10 Others Peptidases/proteases 18 15 10 14 11 7 11 14 12 10 9 8 8 6 Others or unknown 22 20 7 18 18 13 16 25 20 14 14 16 8 16 TOTAL 一 113 101 58 90 93 71 78 一 122 105 87 72 85 68 71 Gen o m i c da t a were fo un d in Flo u das e t al.(2012). P r o t e ins f o r w hich e m PAI r e l ati v e abu n d ancy w as higher t ha n ten in at l e as t one of the culture condit i ons w ere s e le cted. M nP: m anganese per o x i d a se, L i P: lignin per o xid a se, VP: v e r s atil e p er o xidase, GP: g eneric p erox i d ase , C l as s -I I -PO D : c l ass -I I p eroxi d as e ,D yP: d ye -d e c o lo r izing per o x i d as e, HTP: h eme-t hi o l a te p er o x i da s e , C DH : c el lobio s e deh y d r ogen a se , GM C: g l ucos e-methan o l -c h olin e oxi d or ed u c t as e, G H : gly c os id e h ydr ol a s e , AA9: aux ili ary e n z ym e family 9 (s yn. LP M O: l y tic polysa c c ha ride mono o xygena s e ),CE : c arb oh yd r a te estera s e, PL: poly sac c h ar i d e s ly a s e , CAZY : c a r b o hy d rat e a c tiv e enzyme , incl . CBM: in c luding cellu lo se bi n ding mo d ule . Relative abundance of main classes of protein s i n Fmed and Tver secretome on grapevine and beec h cultures. Pept.: peptidases, GHs : glycoside hydrolases , PLs : polysa c charides lyas e s, CEs : carbo h ydrate es t erases, Pect .: pectinases,AA9=au x i lia r y en zy m es f a mily 9 (or LPMOs ), MnPs :manganese peroxidases, Lacc .: l accases L i P s : L ignin perox i dases,Oth. Ox.: other oxid o r eductases. Classification was performed according to JGI database and perce n tages are given a c co r ding to PAl relat i ve abundanc i es for al l protei n s w i t h emPAl high er t h a n ten i n at least o n e of the cultur e conditions . authors suggest e d that fungi first grow exponent i all y while colonizing the whole substrate, and t hen reach a subs t rate-species equil i brium. Thus , a longer equil i brium phase suggests a bett e r fungal growth on this substrate. Moreover, t he speed of colonization pha s e and the max i mal mineralization rat e are spec i fic to fungal species, and can be related to different fungal colo n ization strategies (Bo d dy an d H e i lmann -C l ausen , 2008;C a r lss o n et a l., 2017). Here should be noticed that respiration might not only result from wood degradation but also from f ungal storage compounds metaboliza t ion or self -recycling processes (His c ox e t a l ., 2015). Moreover, MnPs act i vities were also shown to produce Co (H o f r i c h t er et al., 1999). Thus, carbon mineralization rates are not only related to fungal growth but also to fungal metabolic act i vity . The l a tter is higher for Fmed with the two hardwoods species compared to spruce wood, and a longer equilibrium phase i s observed with grapevine wood compared to beech. As suggest e d by c a rbon mineralization rate , higher mass loss on grapevine cultures compared to beech and spruce confirm a higher wood degradation rat e for Fmed on grapevine. T his degradation rate is higher th a n the one reported by a previous study (10% after 3-month s) using the same fungal strain and grapev i ne c v. (P acet t i et al., 2022). In the l att e r st u dy grapevine wood block s (0.5×5×2.5cm) were used and, among other parameters, the three-dimensional s tructure of wood ini t ial wood compos it ion might have limited f ungal access to wood polymers (B l a n c h e t t e,1991). On spruce wood, mass losses are lower than on th e two hardwoods for both fungi. T hus, spruce wood appears as more recalc i trant to fungal degradat i on, which is consistent with previous studie s (B ari et a l ., 2020). This can be explained by the higher lignin content observed i n spruce, or by softwood l ignin composition mainl y cont a ining guaiacyl units (B l a n c he tt e et al, 1988). Wood degradation rates between beech a nd grapev i ne for Tver cultures were similar, contrary to Fmed culture s suggesting tha t this la t ter is less efficient to degrade beech wood than grapev i ne wood. Thi s could be at l e a st parti a lly explained by the nature of Class-I I PODs secreted by the two fungi . I ndeed, consi s tently with genomic data (F lou d as et al ., 2012),MnPs were the only c l ass -II PODs d e t e cted in t he Fmed secretome , whereas both LiPs and MnPs were detected in the Tver secretome. Moreover, more MnPs and l accases were secreted by Fmed on grapev i ne than on beech. Enhancement of MnPs a n d laccases gene expression in my c elium exposed to wood compared to control cultures were previously desc r ibed (Pacet t i et al., 2022). The highest wood degradat i on rate wi t h Tver on beech cultures compared to Fmed could be related to LiPs capacity to degrade non-aromatic parts of lignin, additionally to aromatic parts, contrary to MnPs (W o ng, 2009). Our results show a posit i ve correlation be t ween wood degradation rate by Fmed and MnPs relative abundancies. This observation is consistent with the role of those enzymes i n wood d egradation. However, this is not the case in Tver cul t ures. A similar degradation rate with both subs t rates by Tver is associated with more oxidoreductases on beech than on grapevine, suggesting a more i mportant role of CAZYs i n grapev i ne wood degradation by Tver . H i gher abundancies of some GHs families, such as GH15 or GH43, could be involved in spec i fic degrada t ion. Inde e d,speci fi c carbohydrate degra d ation act i vit i es upon the woody substrate were shown for the white-rot species Dichomitus squalens: a high e r x y l anoly t ic activity on hardwood (birch) than on softwood (spruce), which are containing,respec t ively, more x y lan an d m ore mannan (D a ly e t al., 2018).Xylose was reported a s the main sugar found i n hemicelluloses of grapev i ne pruning wood (Ni t so s e t a l., 2016), suggest i ng a majority of xylan and/or xyloglucan. This is also the case for beech wood,consistently with common hardwood hemice l lulose composition (La i n e , 2014; I b r a h im a nd K r u s e, 2020). On the contrary, no clear differences were observed between the two substrates for CAZYs composi t ion in Fmed secretome upon the substrate. Thus, Fmed modulates i ts oxidoreductases secretion upon i t s substrate but not the nature of GHs. Changes i n secretome linked to environmental factors have been we l l desc ri bed, although regulation mechanisms remain not fully understood (McCo t ter e t a l ., 2016). Several factors such as hemicel l ulose or lignin composition, pH or wood extract i ves might hav e induced changes in the two WRF secretomes. Tver secretome modulations were associated to similar wood degr a dation r a t e s , wh e reas Fmed d e graded grapev i ne wood more efficiently than beech wood . Thus , Fmed might be more specifically ad a pt e d to grapevin e wood compared to other wood species. Targeted metabolomic analysi s aimed a t follow i ng wood extractives during exposure to Fmed or Tve r and eventually highligh ti ng fungal metabolic ac t iv i ty through primary metabolite de t ection. All selected extractives, stilbenes and flavonoids in grapevine wood and f lavonoids in beech wood,decreased during Fmed cultures. St il benes are known for their biological activities and for their accumul a tion in grapevine wood in response to a pathog e n attack (Gala r nea u et a l ., 2021).Their presence and levels in wood depend on several factors,incl u di n ggrapevine cult i var (Gabas ton e t al.,22019).Gewurztraminer and Pinot noir were reported as particularly rich in stilbenes compar e d to other cultivars, with total st i lbenes content ranging from 4,628 to 5,830 mg/kg DW (V e r gara e t al.,2012; L a m b e rt et a l ., 2013; Guer r e r o,2016). In our wood material e-viniferin, the most abundant, trans-resveratro l and piceatannol were i d e nti f i ed, which is i n accord a nce w i th the reported st i lbene composition of cv . Gewurztraminer (V ergara et a l ., 2012; L a m be r t e t al ., 2013; Gue r r ero, 2016). Six grapev i ne s t i lbenes (re s veratrol,pter o stilbene , miyabenol C, vitisin A and B and i sohopeaphenol )were shown to part i ally i nhibit Fmed growth (B r u n o an d Spar a pan o , 2006b ; L a mbe r t e t a l ., 2012). Fmed resveratrol detoxification activity was demonstrated in vitro (Brun o an d Sp a rap an o , 2006b), and the decrease of resveratrol content in Fmed cultures is consistent with t his observation. More ef f icient detoxif i cation processes might enhance wood degradation.Indeed, grapevine extractives were degrad e d faster and to a higher extent i n presence of Fmed than of Tver. Interestingly,mor e laccases were d e tected in F med secretome in 1-month cultures compared to 3-months cultures. In addition, almost all extractives were already degraded in 1-month cultures. Thi s is cons i stent with known potent i al detoxification activi t i es of laccases (J o nsson e t a l ., 1998; L eko u n o u go u et al., 2008; V alet t e e t a l ., 2017). Our results suggest a fungal-substrate adaptation , as sev e ral extrac t ive contents i n grapev in e wood (cis -piceata n nol,st i lbene dimers, trimers and tetramer , including e-viniferin, and naringeni n ) decreased faster and to lower levels i n Fmed cultures compared to Tver cultures, and the opposite was observed for catechin in beech cultures. Variations of amino acids levels have been observed depending on the used fungus and subst r ate. AA are found as nitrogen reserves i n grapevine, and arginine was reported as the most represented (Z apa t a e t a l ., 2004). In grapevine cultures exposed to Fmed or to Tver , arginine and proline levels decreases might be due to fungal degradation or metabolizat i on. In previous studies AA levels variations dur i ng grapevine -fungi interac ti ons were a t t ri buted to grapevine reac t ion , a s experiments were conducted on livi n g w o od (Labo i s et al ., 2021). In our conditions, they can be rather at t ributed to fungal metabol i c activity . AA are essent ia l for various fungal metab o lic pathways,and their level s increase i s consi s tent with the presence of proteases observed in both Fmed and Tver secretomes. They might be due to degradation products from wood proteins or to funga l autolys i s proc e sses . Diff e r e nces observ e d i n t he nature and the levels variations of AA, depending on the cultures, suggest fungal -substrate speci f ic activi ti es . To conclude, Fmed was able to degrade two hardwood s peci e s namely grapevine, a l iana on which it i s i nvolved in wood disease , and beech, a forest tree. Simultaneous degradation patterns were observed on both subs t rates, like a majority of WRF including Tver , the comparison mod e l used in this study.Fmed speci f ic traits on grapevine were highl i ghted: a higher degradation rate than with beech , more laccases and MnPs secreted, and a more e f fic i ent extract i ve detoxification compared to beech and compared to Tv e r. Thus, Fmed wood degradation mechanisms seem to be more adapted to grapevine wood than to other wo od s such as beech or spruce. However , the ubiquitous WRF Tver was able to modulate i ts secretome and to degrade grapev i ne wood as e fficiently as Fmed, although i t ha s never been observed on this species. Thus, beside its abil i ty to degrade grapev i ne wood , the widespread of Fmed in vineyards may r e ly on other factors. For example, adaptation to higher extractive contents in l iv i ng grapev i ne wood, or to other biot i c and ab i ot i c factors might also be relevant to explain the growing importance of Fmed in vine y ards. Data availability statement The mass spectrometry proteom i c s data have been deposi t ed to the ProteomeXchange Consort i um via the PRIDE (Pe re z-R i v erol e t a l ., 2022) par t ner repository with the dataset identif i er PXD036889. A u thor contributions EG , SF, CB, and MS contr i buted to conception and d e sign of the study. MS (all data), ER (wood fibers data), AM-G, and RB (metabolomic dat a ) cont ri buted to obtain the experiment a l d a t a .MS and EG (all data), ER (wood fibers data), AM-G, and RB and PH (met a bolomic analysis) contr i but e d to dat a analyses. MS wrote the first draft of the manuscript. Al l authors contributed to the article and approv e d the submitt e d version. Funding UMR I AM i s supported by the French Agency through the Laboratory of Excellence A rbre (A NR-11-LABX-0002-01),the region Grand Est and the Fonds Europeen de Developpement Regiona l (project VitEST ). This work benefited from the ASIA pl a tform (Universite de Lorraine-INRAE; http s://a2f.univ -lorraine.fr/en/asia-2/). Proteomics analyses were performed on the PAPPSO plat f orm (http://pa p pso.inra.fr) which i s supported by INRAE (htt p ://www.inrae.f r ), the I l e-de-France regional counc i l (https://www.i l edefrance.fr/education-recherche),IBiSA (h ttp s://www.ibisa.net ) and CNRS (http://www.cnrs.fr ). Ac k nowled gm ents The authors would like to thank Francis Grof f and the Valentin Zussl i n Estate for prov i ding grapevine wood, Gry Alfredsen (N IBIO) for spruce sawdus t , Bernd Zeller from (BEF,INRAE) for beech sawdust. All author s are grateful to Jean-Pierre J a cquot for constructive cr i ticism of the manuscript . The authors specially thank Celine Henry (PAPPSO platform) for the proteomic analyse s and data deposit References Abou-Mansour, E., Polier, J., Pezet, R., and Tabacchi , R. (2009). Purification and partial characterisation of a 60 KDa laccase from. Phytopathol. Mediterr. 48,447-453 AFMB, Archi t ecture et Fonc t ion des Macromolecules Biologiques UMR7257-CNRS ·Universite dAix -Marseille (2022a). Carbohydrate Esterase Family Classification. CAZy.o rg . Available at: h t t p://www.cazy.o r g/Ca r bo h ydrate-Es t erases.h t m l (Accessed May 7,2022) AFMB, Architec t ure et Fonc ti on de s Macromolecules Biologiques UMR7257-ologiue n ase CNRS · Universite dAix-Marseille (2022b). Glycoside Hydrolase Family Classi f ication. CAZy.org. Available at: h t t p ://www.c azy.or g /Glycos i de-Hyd r o l ases h t m l (Accessed May 7,2022). Agrelli, D., Amalfitano, C., Conte, P, and Mugnai , L. (2009). Chemical and spec t roscopic characteristics of t he wood of Viti s vinifera cv. Sangiovese af f ected by esca disease. J . Agric. Food Chem. 57, 11469-11475.doi : 10.1021/jf903561x Conflic t of i n terest The a uthors declare that the research was conducted in the absence of any commerc i al or f inancial relationships that could be construed as a potential conflic t of interest . Publisher's note Al l cl ai ms expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, th e editors and the reviewers. Any product that may be evaluated i n this article , or claim t hat may be made by i t s manuf a c t urer, i s not guaranteed or endorsed by the publisher . Suppleme n tary material The Supplementary mater i al for thi s article can be found online at : https://www.frontiersin.org/artic l es/10.3389/fpls.2022.988709/ful l #su pp lementary-material SUPPLEMENTARY FIGURE S1 H eatma p of global m e tab o l it e prof i le s dur i ng the wood degr a dat i on process . Log10 mean peak area o f the indicated metabo l ites are given by shades of red, ye l low, or blue colors accordin g to th e sc a le b ar for (A) grapevi n e, (B) beech and (C) spruce cultures. Data r epresen t the mean val u es of t h r ee biologica l replicat e s f or each co n dition a n d ti me poin t.AA: amino acids; A L : aldehydes; F: flavono i d; PAc: phenolic acids ; PAI:phenol i c alco h o l s; St1-S t 4: St il bene mo no mers, dimers, t ri mers a nd tetramers ; MXXX: m/z i n positive mode.C: control . SUPPLEMENTARY FIGURE S2Relat i ve abundance expressed as emPAl for all selected glycoside hydro l as e s i n (A) Fmed and (B) T ver sec r etome o n grapevin e and b e e ch 1- and 3-month cultures. Proteins for which emPAl relative abunda n cv was higher t h an ten i n at least o n e of t h e cul tu re condit i ons were selected. RA: relative abundancy. SUPPLEMENTARY TABLE S 1 Relat i ve abundancies expressed as emPAl for selected prote i ns i n Fmed and T ver sec r etome on gr ap evine and beech cu lt ures . The grou p i n g of prote i ns into protein s u bgroups was done using t he groupi n g algorithm i ncluded i n X !T andemPipe li ne (L a n gel l a et al., 2017). On l y proteins fo r whic h emPAI relat i ve abunda n cy was h i gher tha n ten i n at least one of the culture cond i tions were se l ected. Bulks a r e mix from al l samples a n d were used as quali t y contro l du r ing batc h analys is . Protein I Ds, annotation s and classi f ication a r e g i ve n according to JG l and Gene Ontology (GO) databases . Bari, E., Daniel, G., Yilgor, N., Kim, J. S., Tajick -Ghanbary, M. A., Singh, A. P.,et al . (2020). Comparison of the decay behavior of two white-rot fungi in relation to wood type and exposure conditions . Microorganisms 8:1931. doi : 10.3390/microorganisms8121931 Bertsch, C., Ramirez -Suero, M., Magnin -Robert, M., Larignon, P, Chong,J,Abou-Mansour,E.,e t al .(2013). Grapevine trunk diseases: complex and stil l poorly understood : grapevine trunk diseases . Plant Pathol. 62, 243-265. doi: 10.1111/i.1365-3059.2012.02674.x Blanche t te, R. A. (1991). Delignif i cation by wood-decay fungi. Annu. Rev.Phytopathol. 29,381-403. doi: 10.1146/annurev.py.29.090191.002121 Blanchette, R. A., Abad , A. R., Farrell, R. L., and Leathers , T .D. (1989). Detection of lignin peroxidase and xylanase by immunocytochemical labeling in wood decayed by Basidiomyce t es. Appl. Environ. Microbiol. 55, 1457-1465. doi : 10.1128/aem.55.6.1457-1465.1989 Blanchette, R. A., Obst, J. R., Hedges, J . I ., and Weliky, K. (1988). Resistance of h a rdwood vessels t o degradation by white rot B a sidiomyce t es. Can. J. Bot . 66,1841-1847.doi: 10.1139/b88-251 Blanchette, R. A., Ot j en, L., Effland , M. J ., and Eslyn, W. E. (1985). Changes in struc t ural and chemical components of wood delignified by fungi. Wood Sci.Technol. 19,35-46. doi : 10.1007/BF00354751 Boddy, L., and Heilmann-Clausen, J . (2008).“Basidiomycete community development i n temperate angiosperm wood, in Ecology of Saprotrophic Basidiomycetes British Mycological Soc i ety Symposia Series. eds. L. Boddy, J. C.Frankland a nd P . van Wes t (Netherlands : Academic Press, Elsevier),211-237. Brown, A. A., Lawrence, D. P., and Baumgartner,K. (2020). Role of basidiomycete fungi in the grapevine trunk disease esca. Plant Pathol. 69, 205-220. doi: 10.1111/ppa.13116 Bruez, E., Lecomte, P, Grosman,J ., Doublet, B., Bertsch, C., Ugaglia, A., e t al.(2013).Overv i ew of grapevine trunk diseases in France in the 2000s. Phytopathol .Mediterr .22,5189-5206. d o i : 10.1111/1462-2920.15180 Bruez,E., Vallance,J,Gautier , A., Lava l , V., Compant, S.,Maurer , W., e t al (2020).Major changes in grapev i ne wood microbiota are associated with the onse t of esca, a devastating trunk disease. Environ. Microbiol. 22,5189-5206. doi: 10.1111/1462-2920.15180 Bruez, E., Vallance,J.,Gerbore,J .,Lecomte, P., Da Costa, J.-P., Guer i n-Dubrana, L.,et al. (2014). Analyses of t he t emporal dynamics of fungal communities colonizing T t he heal t hy wood t i s s ues of esca l eaf-symptomat i c and asymptomatic v i nes. PLoS One 9:e95928. doi : 10.1371/journal .pone.0095928 Bruno, G., and Sparapano, L. (2006a). Effects of three esca -associated fungi on Vi t i s vinifera L.: I . characterization of secondary metabolites in culture media and hos t responses to t he pathogens i n call i. Physiol . Mol. Plant Pathol . 69,209-223. doi:10.1016/j .pmpp.2007.04.008 Bruno, G., and Sparapano,L .(2006b). Effect s of three esca-assoc i ated f ungi on Vi t is vini f era L .:I I I . Enzymes produced by the pathogens and t heir role i n fungus -to-plant or in fungus -to-fungus interactions. Physiol. Mol. Plant Pathol. 69, 182-194.doi: 10.1016/j.pmpp.2007.04.006 Canale, M. C.,Nunes Nesi, C., Falkenbach, B. R., Hunhoff Da Silva, C. A., and Brugnara,E.C. (2019). Pleurostomophora richardsiae assoc i ated with ol i ve tree and grapevine decline in southern Brazil. Phytopathol. Mediter r . 58, 201-205. doi:10.13128/Phytopathol _Me d iterr -23357 Carlsson, F, Edman, M., and Jonsson, B. G. (2017). Increased CO2 evolution caused by heat treatment in wood-decaying fungi. Mycol . Prog. 16,513-519. doi:10.1007/s11557-017-1281-5 Chambers, M. C ., Maclean, B., Burke, R ., Amodei , D., Ruderman, D. L.Neumann, S., e t al. (2012). A cross-plat f orm toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 30,918-920. doi: 10.1038/nbt.2377 Cholet , C., Bruez, E., Lecomte , P, Barsacq, A., Mar t ignon, T., Giudic i , M., et al (2021). Plant resilience and physiological modifications induced by curettage of Esca-diseased grapevines. OENO One 55, 153-169. doi: 10.20870/oeno-one.2021.55.1.4478 Cichewicz , R. H., Kouz i , S. A., and Hamann, M. T. (2000). Dimerization of resve r atrol by t he grapevine pathogen Botry t is cinerea. J. Nat . Prod. 63, 29-33. doi:10.1021/np990266n Coines, J, Raich, L., and Rov i ra, C. (2019). Modeling catalytic reac t ion mechanisms in glycoside hydrol a ses. Cur r. Opin. Chem. Biol. 53, 183-191. doi :10.1016/j.cbpa.2019.09.007 Daly, P, Lopez, S. C., Peng, M., Lancefield, C . S., Purvine, S. O., Kim, Y., et al.(2018). Dichomitus squalens par ti al l y t ailors its molecular responses to the composi t ion of solid wood. Environ. Microbiol . 20, 4141-4156. doi : 10.1111/1462-2920.14416 Del Frari, G., Oliveira, H., and Boavida Ferreira, R. (2021). White rot fungi (Hymenochaetales) and Esca of grapevine: i nsights f rom recent microbiome studies.J . Fungi 7:770. doi: 10.3390/jof7090770 Doublet, B., Grosman,J ., Lecomte, P, and Bruez,E .(2014).Observatoire national des maladies d u bois. Paris : INRAE. Falade , A. O., Nwodo, U. U., Iweriebor, B. C., Green, E ., Mabinya, L. V.,and Okoh, A. I. (2017). Lignin peroxidase functionalities and prospective applications.Microbiology Open 6:e00394. doi: 10.1002/mbo3.394 Fischer, M. (2002). A new wood-decaying basidiomycete species associated with esca of grapevine: Fomitiporia mediterranea (Hymenochaetales). Mycol . Prog. 1,315-324. doi: 10.1007/s11557-006-0029-4 Flamini, R., De Rosso, M., De Marchi, F, Dalla Vedova, A., Panighel, A.,G a rdiman, M., et al . (2013). An innovative approach to grape metabolomic s :stilbene profiling by suspect screening analysis. Metabolomics 9, 1243-1253. doi:10.1007/s11306-013-0530-0 Floudas, D., Binder , M., Riley, R., Barry, K., Blanche t te, R. A ., Henrissat , B.,et al. (2012). The paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336, 1715-1719. doi : 10.1126/science.1221748 Fontaine, F, Pinto, C., Valle t , J ., Clement, C., Gomes, A. C., and Spagnolo, A.(2016). The e ff ects of grapev i ne trunk diseases (GTDs) on vine phys i ology . Eur . J .Plant Pathol. 144,707-721. doi : 10.1007/s10658-015-0770-0 Food and Agr i culture Organization of the United Nations Statistics (FAOSTATS)(2021). Data. Available at: h t t p s://www.f ao.o r g/faost a t /e n /#d a ta (Accessed April 4,2022). Gabaston, J ., Leborgne, C., Waffo-Teguo, P., Valls, J , Palos Pinto, A ., Richard ,T,e t al . (2019). Wood an VVOI d roots of major grapevine cultivars and rootstocks: a comparative analysis of stilbenes by UHPLC-DAD-MS/MS and NMR. Phytochem.Anal . 30,320-331. doi : 10.1002/pca.2815 Galarneau, E. R. A., Lawrence , D. P, Wallis, C. M., and Baumgartner, K. (2021).A compar i son of the me t abolomic response of grapevine to infection with ascomycete wood-infec ti ng fungi. Physiol. Mol. Plant Pathol . 113:101596. doi:10.1016/j.pmpp.2020.101596 Gramaje, D., Urbez-Torres, J. R., and Sosnowski, M. R. (2018). Managing grapevine trunk diseases with respect to etiology and epidemiology: current strategies and future prospects. Plant Dis. 102, 12-39. doi : 10.1094/PDIS-04-17-0512-FE Guerin-Dubrana , L., Fontaine, E, and Mugnai, L. (2019). Grapevine trunk disease in European and Mediterranean vineyards : occurrence, distribution and associated disease-affecting cultural factors. Phytopathol. Mediterr. 58, 49-71. doi: 10.14601/Phy t opathol _Medi t err -25153 Guerrero, R. F. (2016). Grapevine cane’s waste is a source of bioactive st i lbenes.Ind. Crop. Prod. 94,884-892. doi: 10.1016/j .indcrop.2016.09.0550926-6690 Hage, H., and Rosso, M.-N. (2021). Evolution of fungal carbohydrate-active enzyme portfolios and adaptation to plant cell -wall polymers. J . Fungi 7:185. doi :10.3390/jof7030185 Hiscox, J, Savoury, M., Vaughan, I . P, Muller, C. T., and Boddy, L. (2015).Antagonistic fungal interac t ions i nfluence carbon dioxide evolution from decomposing wood. Fungal Ecol. 14, 24-32. doi: 10.1016/j.f uneco.2014.11.001 Hofrichter, M. (2002). Review: l i gnin conversion by manganese peroxidase (MnP). Enzym. Microb. Technol.30, 454-466. doi: 10.1016/S0141-0229(01)00528-2 Hof ri chter , M., Scheibner, K., Bublitz, F, Schneegaf, I ., Ziegenhagen, D.,Mar t ens, R., et al. (1999). Depolymer i zation of straw l ignin by manganese peroxidase from Nematoloma frowardii is accompanied by release of carbon dioxide.Holzforschung 53,161-166.doi: 10.1515/HF.1999.027 Ibrahim, Q., and Kruse, A. (2020). Prehydrolysi s and organosolv delignification process for the recovery of hemicellul o se and lignin from beech wood. Bioresour.Technol . Rep. 11:100506. doi : 10.1016/j.biteb.2020.100506 Ishihama, Y., Oda, Y ., Tabata, T., Sato, T., Nagasu, T., Rappsilber, J., et al. (2005).Exponent i ally modi f ied protein abundance index (em PAI) for est i mation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell . Proteomics 4, 1265-1272. doi: 10.1074/mcp.M500061-MCP200 Jeandet, P, Douillet-Breuil, A.-C., Bessis, R., Debord, S., Sbaghi, M., and Adrian, M. (2002). Phytoalex i ns f rom the Vitaceae : biosynthesis , phytoalexin gene expression in transgenic plant s , antifungal act i vity, and metabolism. J . Agric. Food Chem. 50, 2731-2741.doi: 10.1021/jf011429s Jonsson, L. J ., Palmqvist,E., Nilvebrant, N.-O., and Hahn-Hagerdal, B. (1998).De t oxi fi cation of wood hydrol ys ates with laccase and peroxidase f rom t he white-rot fungu s Tr a me t es ve r sicolor. Appl. Microbiol. Biotechnol. 49, 691-697. doi : 10.1007/s002530051233 Karim, M., Daryaei , M. G., Torkaman, J ., Oladi , R., Ghanbary, M. A . T, Bari, E.,e t al . (2017). Natura l decomposit i on of hornbeam wood decayed by t he white rot fungus Trametes versicolor. An. Acad. Bras . Cienc. 89, 2647-2655. doi : 10.1590/0001-3765201720160714 King, B. C ., Waxman, K. D., Nenni, N. V, Wa l ker, L. P, Bergstrom, G . C., and Gibson, D. M. (2011). Arsenal of plant ce l l wall degrading enzymes reflects host preference among plant pathogenic fungi . Biot e chnol. Biofuels 4:4. doi : 10.1186/1754-6834-4-4 Krauss , M., Singer , H., and Hollend e r, J . (2010). LC-high resolution MS in env i ronment al analysis: from t a rget screening to the ident if ication of unknowns.Anal . Bioanal. Chem. 397.943-951. doi: 10.1007/s00216-010-3608-9 Labois , C., Stempien, E., Schneider, J., Schae f fer-Reiss, C., Bertsch, C.,Goddard, M.-L., et al. (2021). Comparative study of secreted proteins, enzyma ti c ac t iv i ties of wood degradation and stilbene me ta bolization in grapevine botryosphaeria dieback fungi. J. Fungi 7:568. doi : 10.3390/j of7070568 Laine, C. (2014). Structures of hemicelluloses and pectins i n wood and pulp.[PhD Thesis]. Espoo (FI ): Helsinky University of Technology Lambert, C. (2011). Etude du role des s t i lbenes dans les defenses de la vigne contre les maladies du bois. [PhD Thes i s]. Bordeaux (FR): University of Bordeaux 2. Lamber t , C., Bisson, J, Waffo-Teguo, P, Papastamoulis, Y., Richard, T,Corio-Costet, M.-F , et al . (2012). Phenolics and their antifunga l role in grapevine wood decay: focus on the Botryosphaeriaceae family. J . Agric. Food Chem. 60,11859-11868. doi : 10.1021/jf303290g Lambert, C ., Richard, T, Renouf, E., Bisson, J ., Waffo-Teguo, P, Bordenave, L.,et al. (2013). Comparative analyses of st i lbenoids in canes of major Vitis vinifera L.cultivars. J. Agr i c . Food Chem. 61,11392-11399. doi: 10.1021/jf403716y Langella, O., Valot, B., Balliau, T., Blein -Nicolas, M., Bonhomme, L., and Zivy , M.(2017). X!TandemPipeline: A De tool to manage sequence redundancy for protein i nference and phosphosite identi f ication. J . Proteome Res. 16, 494-503. doi : 10.1021/acs.jproteome .6b00632 Lecomte, P., Darrieutort , G., L i minana , J .-M., Comont, G.,Muruamendiaraz, A.Legorburu, F -J., e t al. (2012). New insights into Esca of grapevine: the development of foliar symptoms and their association with xylem discoloration. Plant Dis. 96,924-934. doi: 10.1094/PDIS-09-11-0776-RE Lekounougou, S., Mounguengui, S., Dumarcay, S., Rose, C., Courty, P. E.,Garbaye, J ., et al. (2008). Init i al stages of Fagus sylvatica wood colonization by the white-rot basidiomycete Trametes versicolor: enzymatic characterization. Int.Biodeterior. Biodegrad. 61, 287-293. doi : 10.1016/j.ibiod.2007.06.013 Manaval a n, T, Manavalan, A., and Heese, K. (2015). Characterization of lignocellulolytic enzymes from white -rot fungi . Curr. Microbiol . 70, 485-498. doi:10.1007/s00284-014-0743-0 Markakis, E. A., Kavroulakis,N.,Ntougias,S ., Koubouris, G. C., Sergentani, C. K.,and Ligoxigakis, E. K. (2017). Characterization of fungi associated with wood decay of tree species and grapevine in Greece. Plant Dis . 101, 1929-1940. doi : 10.1094/PDIS-12-16-1761-RE McCotter , S. W., Horianopoulos, L. C., and Kronstad ,J. W. (2016). Regulation of the funga l secretome. Curr. Genet. 62, 533-545. doi : 10.1007/s00294-016-0578-2 Mondello, V., Songy A., Batt i ston, E., Pinto, C., Coppin, C., Trotel -Aziz, P , et al.(2018). Grapevine t runk diseases: A review of f ifteen years of t ri a l s for their control with chemicals and biocontrol agents. Pl a nt Dis . 102, 1189-1217. doi : 10.1094/PDIS -08-17-1181-FE Morel, M., Meux, E., Mathieu, Y., Thuillier, A., Chibani, K., Harvengt , L., et al (2013). Xenomic networks var i ability and adaptat i on traits in wood decaying fungi:fungal xenomic networks . Microb. Biotechnol. 6, 248-263. doi : 10.1111/1751-7915.12015 Morel-Rouhier , M. (2021). “Wood as a hostil e habitat for ligninolytic fungi ,"i n Wood Degradation and Ligninolytic Fungi Ad v ances in Botanical Research. eds. M.Morel-Rouhier and R. Sormani (London, UK: Academic Press), 115-149. Moret ti , S., Pacett i , A., Pierron, R ., Kassemeyer , H.-H., Fischer, M., Peros, J.-P.,et al . (2021). Fomit i poria medi t erranea M. Fisch., t he hi s torical Esca agent: a comprehensive review on the main grapevine wood rot agent i n Europe Phytopathol. Mediterr. 60, 351-379. doi: 10.36253/phyto-13021 Mugnai, L., Graniti , A., and Surico, G.(1999). Esca (black meas l es) and brown wood-streaking: two old and elus i ve disease s of grapevines. Plan t Di s . 83, 404-418.doi : 10.1094/PDIS.1999.83.5.404 Nitsos, C. K., Choli-Papadopoulou, T ., Mat i s, K. A ., and Triantafyllidis, K. S.(2016). Optimization of hydrothermal pretreatment of hardwood and softwood lignocellulosi c residues for selective hemicellulose recovery and i mproved cel l ulose enzymatic hydrolysis. ACS Sustain. Chem. Eng. 4, 4529-4544. doi: 10.1021/acssuschemeng.6b00535 Pacett i , A., Moret ti , S., Perrin, C., Gelhaye, E., Bieler, E., Kassemeyer, H.-H., et al.(2022). Grapevine wood-degrading activity of Fomitiporia mediterranea M. Fisch.:a focus on the enzymatic pathway regulation. Front . Microbi o l . 13:844264. doi:10.3389/fmicb.2022.844264 Pacetti, A., Moretti, S ., Pinto, C., Compant, S., Farine, S., Bertsch, C., et al (2021). Trunk surgery as a tool to reduce foliar symptoms in diseases of the esca complex and its influence on vine wood microbiota. J. Fungi 7:521. doi : 10.3390/iof7070521 Perez -Riverol, Y., Bai, J ., Bandla, C., Garcia -Seisdedos, D., Hewapathirana, S.,Kamatchinathan, S., e t al. (2022). The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomic s ev i dences. Nuc l eic A cids Res. 50, D543-D552.doi : 10.1093/nar/gkab1038 Perrot , T ., Salzet, G., Amusant , N., Beauchene, J., Gerardin, P, Dumarcay , S., et al .(2020). A reverse chemical ecology approach to explore wood natural durability.Microb. Biotechnol. 13, 1673-1677. doi: 10.1111/1751-7915.13540 Pi l ar Martinez-Diz, M., Diaz-Losada, E., Andres-Sodupe, M., Bujanda , R.,Maldonado-Gonzalez , M. M.,Ojeda, S., e t al . (2021). Field evaluation of biocontrol agents against black-foot and petr i diseases of grapev i ne. Pest Manag. Sc i . 77,697-708. doi : 10.1002/ps.6064 Rani Singhania, R., Dix i t, P, Kumar Patel, A., Shekher Giri, B., Kuo, C.-H.,Chen, C.-W., et al. (2021). Role and significance of lytic polysaccharide monooxygenases (LPMOs) in lignocellulose deconstruction. Bioresour. Technol.335:125261. doi: 10.1016/j.biortech .2021.12526 Reis, P, Pier r on, R., Larignon, P, Lecomte, P, Abou-Mansour , E., Farine, S ., et al .(2019). Vitis methods to understand and develop strategies for diagnosis and sustainable control of grapevine t runk diseases. Phytopathology 109, 916-931. doi:10.1094/PHYTO-09-18-0349-RVW Reiss, R., Ihssen, J , Richter, M., Eichhorn, E ., Schil l ing, B., and Thony-Meyer,L.(2013). Laccase versus l accase-l i ke mult i -copper oxidase: a compara t ive study of similar enzymes wi t h diverse substrat e spectra. PLoS One 8:e65633. doi: 10.1371/journal .pone.0065633 Sahu, N., Merenyi, Z., Balint, B., Kiss, B., Sipos, G., Owens, R. A., et al.(2021).Hallmarks of Basidiomycete soft- and white-rot in wood-decay -omics data of two Armillaria species. Microorganisms 9:149. doi : 10.3390/microorganisms9010149 Schilling, M., Farine, S., Peros, J.-P., Bertsch, C., and Gelhaye,E. (2021).“Wood degradation in grapevine diseases, i n Wood Degradation and L i gninolytic Fungi Advances in Botanical Research. eds . M. Morel -Rouhier and R. Sormani (London,UK: Academic Press), 175-207. Sparapano, L., Bruno, G., and Graniti, A. (2001). Three-year observation of grapevines cross-inoculated with esca-associated f ungi. Phytopathol. Mediterr. 40,S376-S386. doi: 10.14601/Phytopathol _Mediterr-1643 Stempien,E.,Goddard , M.-L., Wilhelm, K., Tarnus, C., Bertsch, C., and Chong,J (2017). Grapevine Botryosphaeria dieback f ungi have specific aggressiveness f ac t or reper t ory i nvolved in wood decay and st il bene metabol i zation. PLoS One 12:e0188766.doi: 10.1371/journal.pone.0188766 Surico, G. (2009). Towards a redefini t ion of t he disease s within the esca complex of grapevine. Phytopathol . Mediterr . 48, 05-10. doi: 10.14601/Phytopathol _Mediterr-2870 Suri 1C c O o,, G., Mugnai, L ., and Marchi, G. (2008).“The Esca disease complex , in Integrate d Management of Disease s Caused by Fungi, Phytoplasma and Bacteria Integrated Management of Plant Pests and Diseases . eds. A. Ciancio and K. G.Muker j i (Bar i , IT,New Delhi, IN: Spr i nger ), 119-136. Suzuki, H., MacDonald,J., Syed, K., Salamov, A., Hori , C., Aerts, A., e t al .(2012).Comparative genomics of the white-rot fungi , Phanerochaete carnosa and P chr y sospor i um, t o elucidate the genetic basis of t he dis t inct wood types they colonize.BMC Genom. 13:444. doi: 10.1186/1471-2164-13-444 Valet t e, N., Perrot, T., Sormani, R., Gelhaye, E., and Morel-Rouhier, M. (2017).Anti f ungal ac t ivities of wood extract i ves. Fungal Biol. Rev . 31, 113-123. doi :10.1016/j .fbr.2017.01.002 Van Soest, P. J., and McQueen, R . W. (1973). The chemistry and est i mation of fibre. Proc . Nutr. Soc. 32,123-130. doi: 10.1079/PNS19730029 Vergara, C ., Damm, A., Macke, S., Gorena, T ., and Winterhalter, P. (2012). Stilbene l evels i n grape cane of different cultivars i n southern Chile: determination by HPLC-DAD-MS/MS me t hod. J Agric . Food Chem. 60,929-933. doi: 10.1021/jf204482c White, C.-L., Halleen, F , and Mostert, L. (2011). Characterisation of the fungi associated with esca diseased grapevines in South Africa. Phytopathol . Mediterr . 50,204-223. doi : 10.14601/Phytopathol _Mediterr -8983 Wong, D. W. S. (2009). Structure and action mechanism of ligninolytic enzymes.Appl . Biochem. Biotechnol . 157, 174-209. doi: 10.1007/sl2010-008-8279-Z Zapata, C., Deleens , E., Chaillou, S., and Magne, C. (2004). Partitioning and mobilization of starch and N reserves in grapevine (Vitis vinif e ra L .). J . Plant Physiol.161,1031-1040.doi : 10.1016/j .j plph .2003.11.009
确定




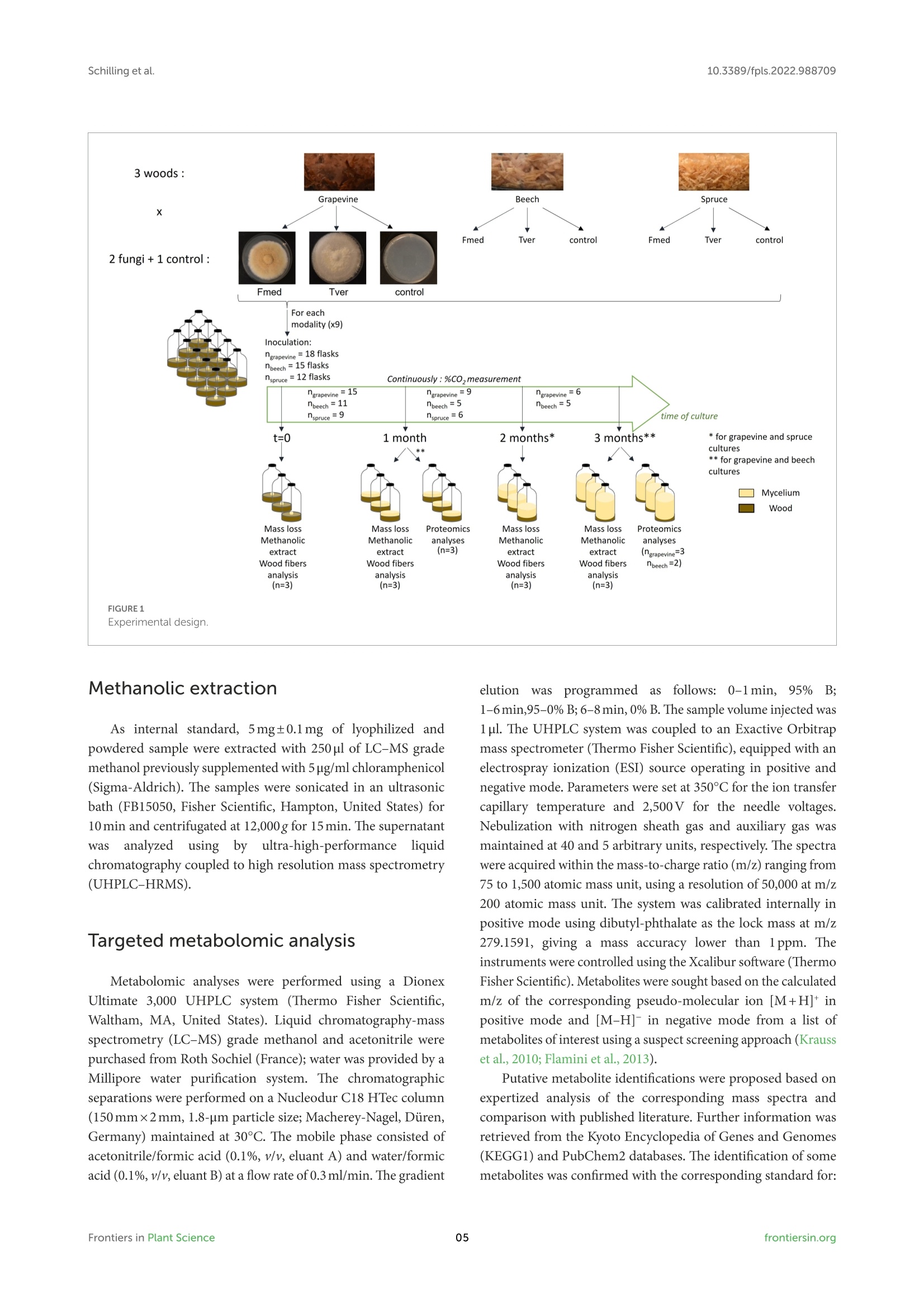


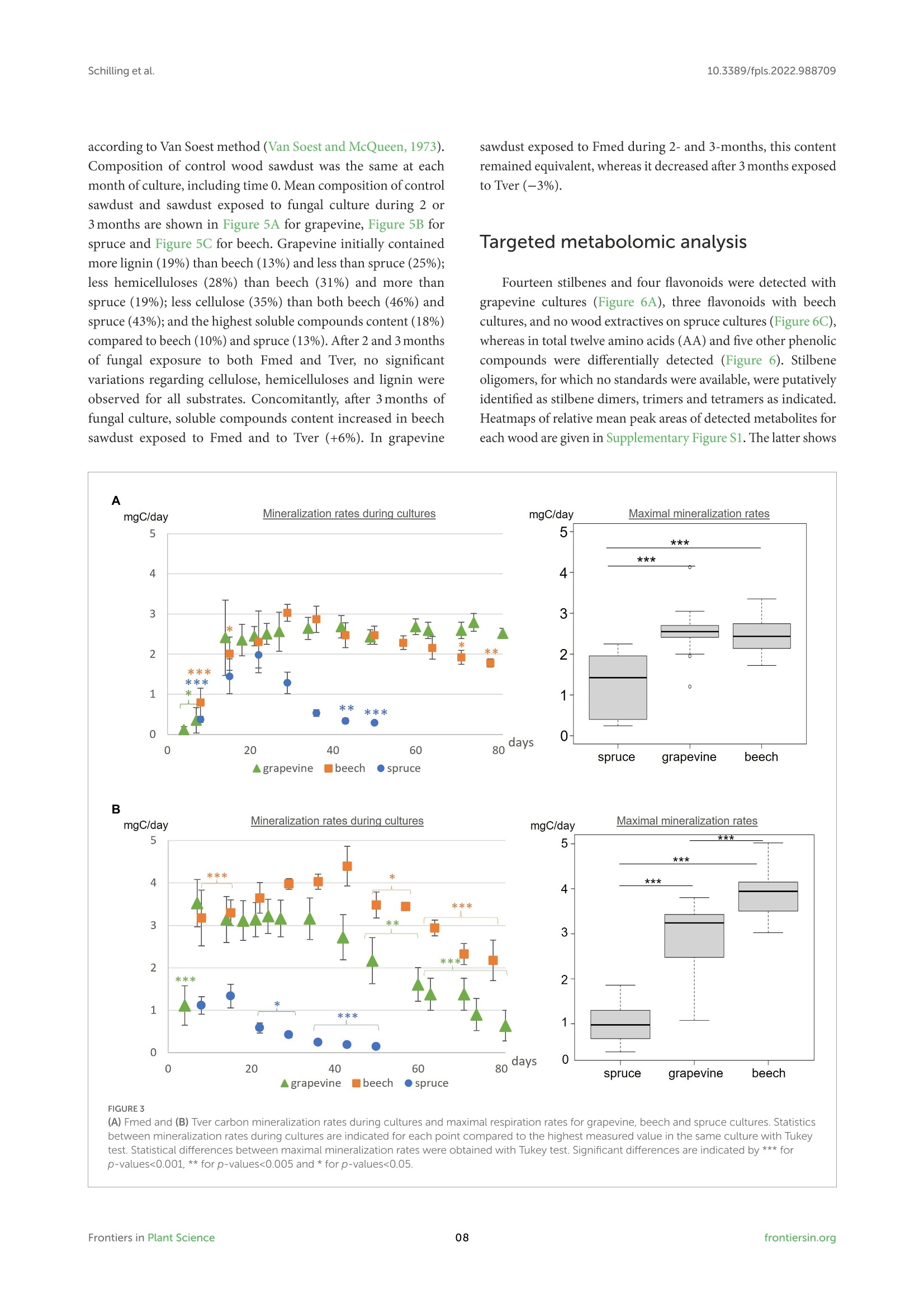
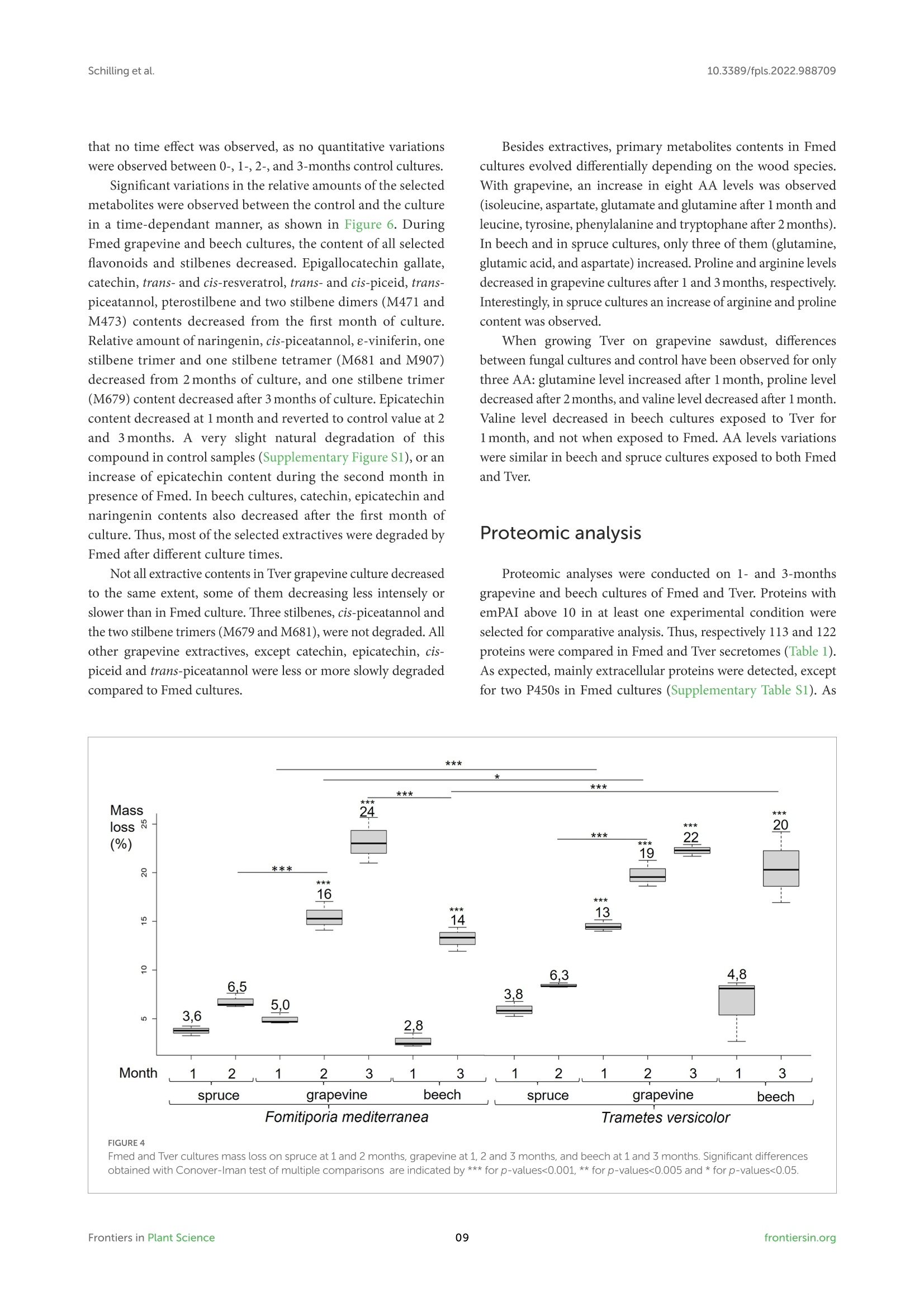
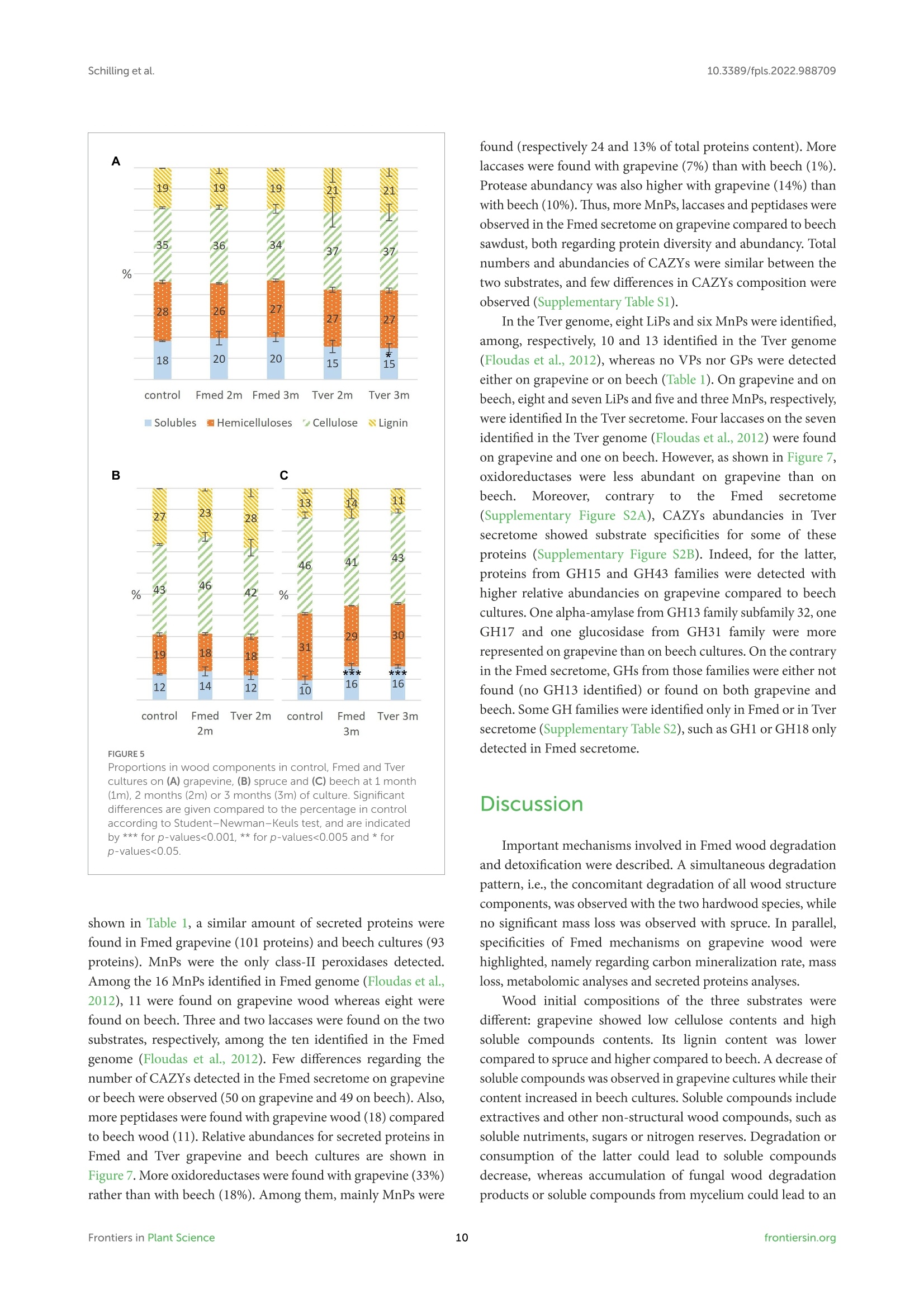

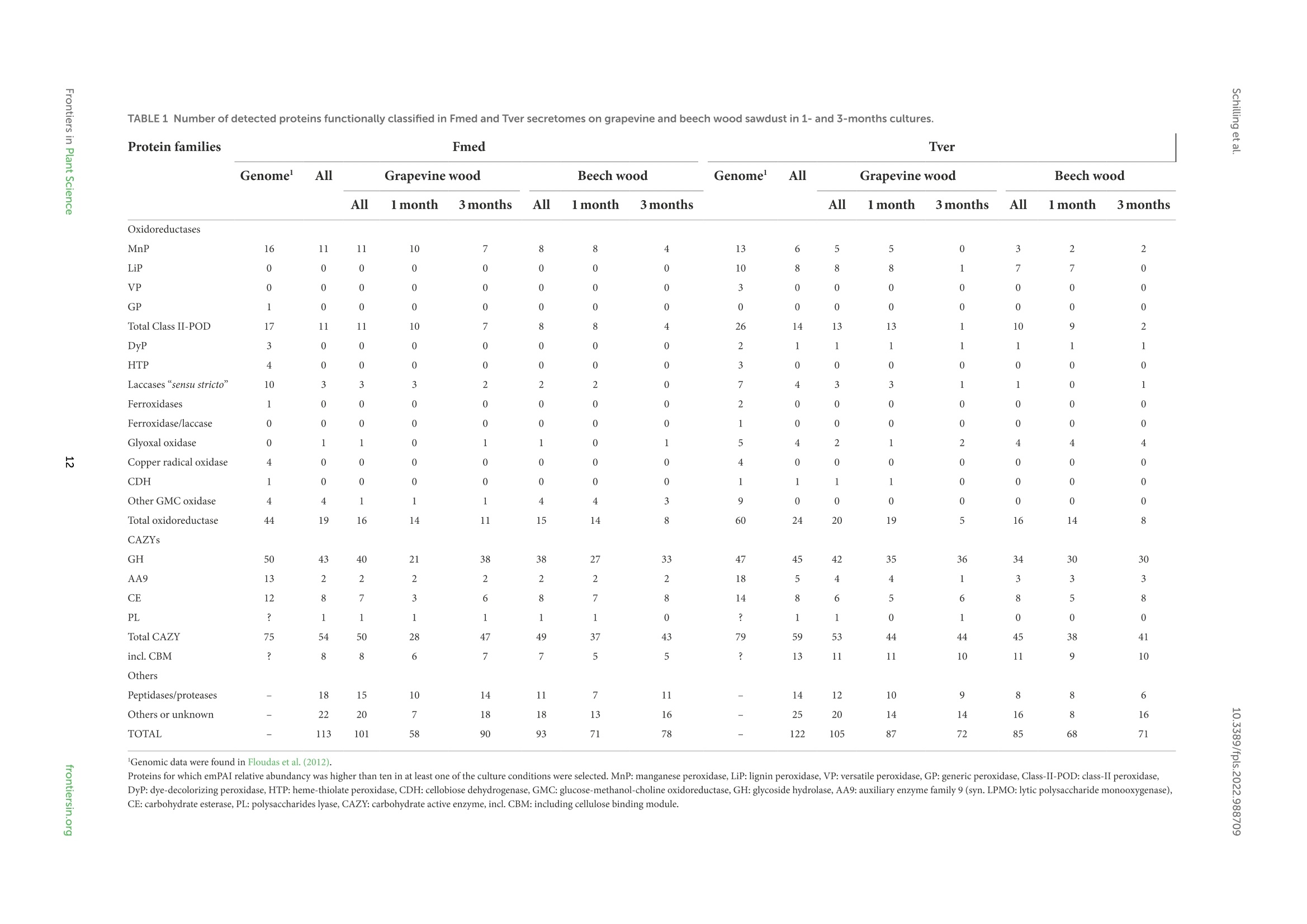
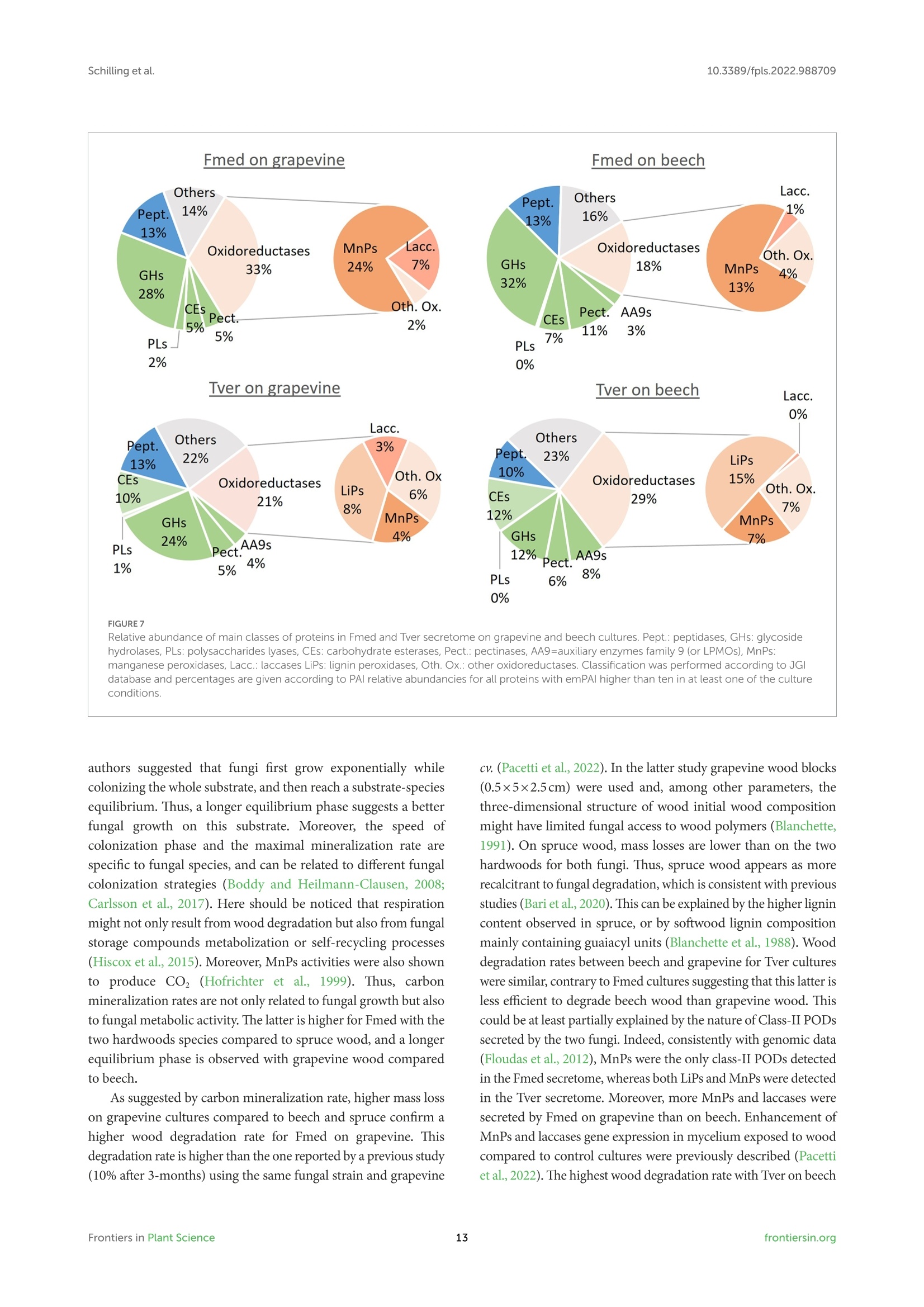




还剩15页未读,是否继续阅读?
中国格哈特为您提供《成熟的琼瑶浆(Gewurztraminer)葡萄树树干中木质素、纤维素、半纤维素含量的检测》,该方案主要用于林产品中理化分析检测,参考标准--,《成熟的琼瑶浆(Gewurztraminer)葡萄树树干中木质素、纤维素、半纤维素含量的检测》用到的仪器有格哈特全自动型纤维分析仪FT12、格哈特快速纤维测定器FBS6、德国移液器MM
推荐专场
相关方案
更多
该厂商其他方案
更多

















