方案详情
文
饲粮中添加DL-蛋氨酸铬可提高非洲鲶鱼的生长性能Dietary Supplementation with Chromium DL-Methionine Enhances Growth Performance of African Catfish (Clarias gariepinus)
方案详情

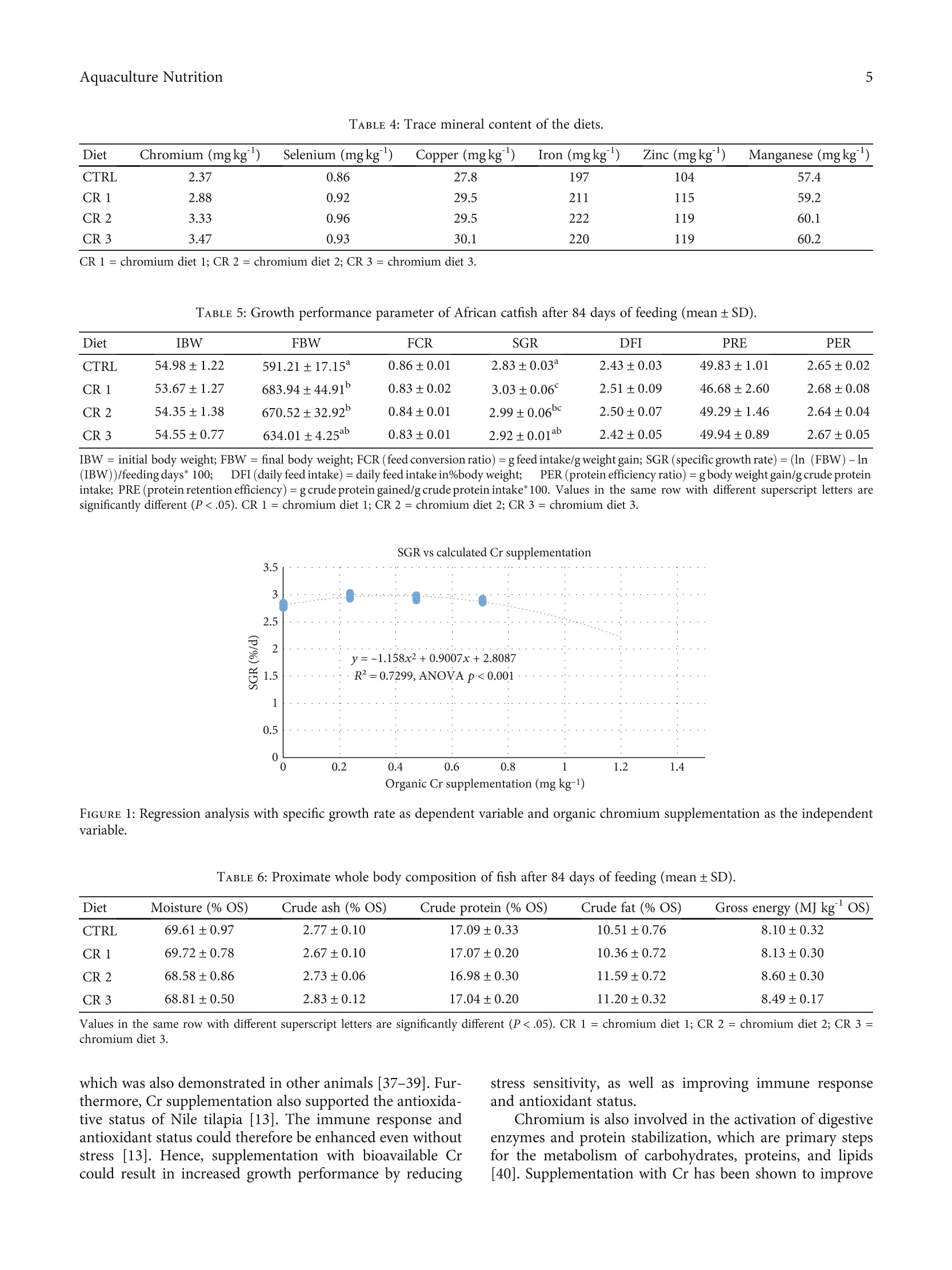
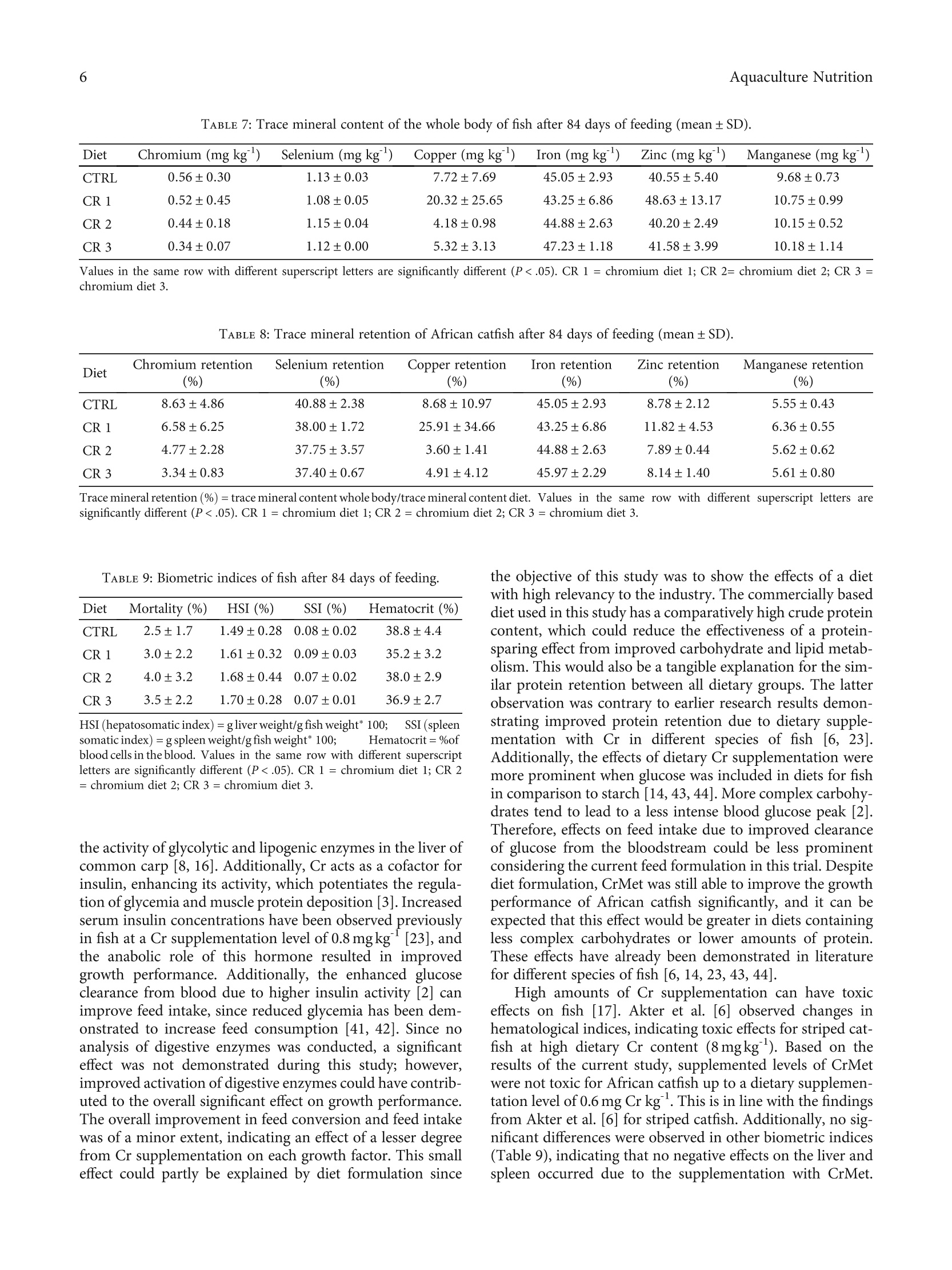
饲粮中添加DL-蛋氨酸铬可提高非洲鲶鱼的生长性能Dietary Supplementation with Chromium DL-Methionine Enhances Growth Performance of African Catfish (Clarias gariepinus)HindawiWILEYQHindawiAquaculture NutritionVolume 2023,Article ID 7092657,8 pageshttps://doi.org/10.1155/2023/7092657 Aquaculture Nutrition2 饲粮中添加DL-蛋氨酸铬可提高非洲鲶鱼的生长性能 Research Article Dietary Supplementation with Chromium DL-MethionineEnhances Growth Performance of African Catfish(Clarias gariepinus) Frederik KaiserD,,2 Michael SchlachteriDD,Carsten SchulzziiD1,2and Claudia Figueiredo-Silva D3 Institute of Animal Breeding and Husbandry, Department of Marine Aquaculture, Christian-Albrechts-University Kiel, Olshausenstrafe 40, 24098 Kiel, Germany Fraunhofer Research Institution for Individualized and Cell-Based Medical Engineering, Aquaculture und Aquatic Resources,Hafentorn 3, 25761 Biisum, Germany Zinpro Corporation, 10400 Viking Drive, Eden Prairie, MN 55344, USA Correspondence should be addressed to Frederik Kaiser; fkaiser@tierzucht.uni-kiel.de Received 4 November 2022; Revised 15 December 2022; Accepted 7 January 2023; Published 24 January 2023 Academic Editor: Mahmoud Dawood Copyright @ 2023 Frederik Kaiser et al. This is an open access article distributed under the Creative Commons AttributionLicense, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work isproperly cited. Sustainable aqua feeds have become an urgent necessity for future-oriented aquaculture sector development, and especiallymineral supply could be limited when diets are being prepared with low amounts of animal-based sources. Since knowledgeabout the efficiency of organic trace mineral supplementation in different species of fish is limited, the effects of chromiumDL-methionine in African catfish nutrition were evaluated. Four commercially based diets with increasing chromium DL-methionine supplementation (0, 0.2, 0.4, and 0.6 mg Cr kg) in the form of Availa-Cr 1000 were fed to African catfish (Clariasgariepinus B., 1822) in quadruplicate groups for 84 days. Growth performance parameters (final body weight, feed conversionratio, specific growth rate, daily feed intake, protein efficiency ratio, and protein retention efficiency), biometric indices(mortality, hepatosomatic index, spleen somatic index, and hematocrit), and mineral retention efficiency were assessed at theend of the feeding trial. The specific growth rate was significantly increased in fish-fed diets with 0.2 mg Cr kg and 0.4 mg Crkg supplementation in comparison with control and based on the second-degree polynomial regression analysis;supplementation with 0.33 mg Cr kgwas optimal in commercially based diets for African catfish. Chromium retentionefficiency was reduced with increasing supplementation levels; however, the chromium content of the whole body wascomparable to literature. The results suggest that organic chromium supplementation is a viable and safe supplement for dietsto increase the growth performance of African catfish. 1. Introduction Feed is one of the highest cost factors in fish farming, and ithas become evident that the utilization of diets for aquaticspecies has to be as efficient as possible to reduce pollutionof the environment [1]. Decade-long research has demon-strated that supplementation of aqua feeds with differentnutrients, vitamins, or minerals can be beneficial for fish health, growth, and overall feed efficiency, especially for dietslow in fish meal [2]. Chromium is an essential mineral for humans and certainanimals [3], although an essentiality could not be demon-strated in fish based on the definition of an essential trace ele-ment [4]. However, dietary supplementation with Cr in fishdiets resulted in enhanced growth performance as well asimproved immune response and stress sensitivity in numer- ous species of fish [5-15]. Especially trivalent chromium(Crt) can support the metabolism of carbohydrates, lipids,and proteins by elevating the activity of digestive enzymes[16] and potentiating the action of insulin [3]. These mecha-nisms can lead to increased energy and protein utilizationand subsequently improved growth performance of fish [8].However, high amounts of Cr in diets can lead to toxic effectslike interrupting cellular integrity and altering several hemato-logical indices [6, 17], and the health status of fish should bemonitored when including additional sources of Cr in fishdiets. Generally, organic chromium sources are more bioavail-able in comparison with inorganic chromium [3] and weretherefore preferentially included in aqua feeds. Among differ-ent organic sources of chromium, dietary chromium methio-nine (CrMet) supplementation was recently demonstrated tobe nontoxic for fish even at high doses (2 mgkg) and to resultin improved growth rate and feed efficiency in comparisonwith chromium oxide and chromium picolinate [18]. Effects on growth performance and health status offish varied depending on the form and dose of Cr, as wellas on experimental duration and fish species [6]. The aimof this study was therefore to investigate the impact ofCrMet supplementation in the form of Availa-Cr 1000, acommercially available product containing a chromiumDL-methionine complex, on growthperformance andhealth status of African catfish after 84 days of feeding.To enable the highest level of relevance towards a practicalapplication, a commercially based diet with low fish mealcontent was supplemented with gradually increasing levelsof the product under review. African catfish were selectedfor this study as they are of great importance to the globalaquaculture sector [19]. 2. Materials and Methods 2.1. Ethical Considerations. The research site adhered to theguidelines set out in the German Animal Welfare Act andwas supervised by an animal welfare officer. The registrationnumber of the trial at the ministry was V242 -32390/2021. 2.2. Experimental Setup. 800 mixed-sex African catfish(54.4±1.2g, Aquaculture ID, Nederweert, Netherlands)were adapted to the recirculating system of the FraunhoferResearch Institution for Individualized and Cell-Based Med-ical Engineering, Buisum, Germany, for 7 days. During adap-tation, fish were fed a commercial catfish diet daily at 2% oftheir body weight (Aller CLARIA FLOAT 4.5 mm; 42g100g crude protein, 12g 100g crude fat, 29.5 100gNfE, 5.6 100gash, 2.9 100gfiber, and 20.2 MJkg grossenergy; Aller Aqua A/S, Christiansfeld, Denmark) beforebeing starved for two days before the experimental start.Subsequently, quadruplicate groups of catfish consisting of50 individuals were assigned to 16 aquaria (60l) in a semi-randomized manner to avoid potential tank effects. The ini-tial stocking density was set to 50kg m. After 6 weeks, thetank volume was doubled by pulling a bulkhead. The aquaria were embedded in a recirculating system(15 m, water turnover rate 4h) including a drum filter(mesh size 20um), moving bed biofilter (6m), and UV treatment (3×100 W). Water parameters were measureddaily to ensure optimal rearing conditions during the growthtrial. Oxygen saturation was at 103.9±6.1% (Handy Polaris;Oxy-Guard International A/S, Birkerod, Denmark), the tem-perature at 27.4±0.9·C, and pH at 7.4±0.3 (GMH 5550,Digital pH-/mV-/Thermometer, Greisinger Electronic, D).Total ammonia nitrogen remained below 0.4 mgland totalnitrate nitrogen below 1 mgl(Microquant test kit for NH4and NO ; Merck KgaA, Darmstadt, Germany). The photo-period was set to 24 h of darkness. Red light headlamps wereused to check the mortalities and health status of fish duringthe experiment. African catfish were fed twice daily (9:00 am and 3:00pm) to apparent satiation for 84 days. Additionally, a fixedrate of 0.5% of body weight was fed during night hours byautomated belt feeders to reduce aggression among fish. Tocalculate the exact feed intake, an average pellet weight wasmeasured, and excess pellets were collected after the feedingevents. 2.3. Experimental Diets. Four diets were prepared for thefeeding trial (CRTL = control, CR 1 = chromium diet 1, CR2= chromium diet 2, and CR 3 = chromium diet 3). A dietreflecting typical commercial catfish feeds in ingredient andnutrient composition served as control (Tables 1 and 2), whilethe remaining diets were copies of the control, supplementedwith increasing levels of Availa-Cr 1000 (200, 400, and600 mgkg,Table 3). Extruded floating pellets were producedby SPAROS, Olhao, Portugal. The analyzed chromium con-tent of the control diet was 2.37 mgkg , and the content ofsupplemented diets exceeded expectations (Tables 3 and 4).However, the analyzed chromium content of diets was stillwithin relevant doses, and a continuous increase in supple-mentation level persisted among experimental diets. The eval-uated product was chromium DL-methionine (Availa-Cr1000) with batch number HPA19122, provided by ZinproCorporation in a ready-to-use powder form. The content ofchromium methionine in the product was 2g 100g. Theana-lyzed chromium content of the product was 1180 mg Crkg. 2.4. Sampling. The experiment and fish sampling were con-ducted according to the German Animal Welfare Act asamended 2016 and according to the German Animal Wel-fare Laboratory Animal Regulation. The trial was registeredas number V242-32390/2021 at“Ministerium fur Energie-wende, Landwirtschaft, Umwelt, Natur und Digitalisierung". All fish were starved for 48 hours before sampling. Afterthe initial adaptation phase as well as after 84 days of feed-ing, three fish per tank were sampled for determination ofwhole body composition, trace mineral content, hepatoso-matic index (HSI), spleen somatic index (SSI), and hemato-crit. Fish were anesthetized with a blunt hit on the centralnervous system and subsequently euthanized by piercingthe heart. Whole body samples were stored at -20℃ untilbeing homogenized and freeze-dried (Alpha 1-2 LD plus,Christ, Osterode, Germany) before analysis. For the calcula-tion of hematocrit, blood was sampled with syringes at thecaudal vein and subsequently centrifuged (Haematokrit210, Andreas Hettich GmbH & Co. KG, Tuttli n TABLE 1: Feed ingredients of control diet. Ingredient (g 100g) Fish meal 60 7.50 Poultry meal 7.50 Soy protein concentrate 10.00 Wheat gluten 4.00 Corn gluten meal 15.00 Soybean meal 22.50 Sunflower meal 40 5.00 Wheat meal 10.45 Wheat bran 5.00 Vitamin premix 1% 1.00 Mineral premix ZINPRO 2% 2.00 Vitamin E50 0.05 Antioxidant 0.20 Monoammonium phosphate 0.70 L-lysine HCL 99% 0.60 Fish oil 2.00 Rapeseed oil 6.50 Vitamin premix (IU/mgkgdiet, PREMIX Lda., Neiva, Portugal): DL-alphatocopherol acetate, 100 mg; sodium menadione bisulfate, 25 mg; retinylacetate, 20,000 IU; DL-cholecalciferol, 2,000 IU; thiamin, 30 mg; riboflavin,30 mg; pyridoxine, 20 mg; cyanocobalamin, 0.1 mg; nicotinic acid, 200 mg;folic acid, 15 mg; ascorbic acid, 500 mg; inositol, 500 mg; biotin, 3mg;calcium pantothenate, 100 mg; choline chloride, 1,000 mg, betaine, 500 mg;excipient wheat middlings. Mineral premix (mgkg diet, PREMIX Lda.,Neiva, Portugal): zinc from Availa@Zn 100, 80; selenium from Availa@Se2000, 0.2; manganese from Availa"Mn 80, 30; iron from Availa@Fe 90, 100;copper from AvailaCu 100, 10; excipient wheat. Germany). Values for hematocrit were determined opticallywith the scale attached to the centrifuge. Growth performance parameters were calculated includ-ing feed conversion ratio (FCR = gfeed intake/g weight gain), specific growth rate (SGR= (ln (FBW)-In (IBW))/feeding days* 100), daily feed intake (DFI = FCR*SGR),pCrotein efficiency ratio (PER = g body weight gain/g crudeprotein intake), and protein retention efficiency (PRE= gcrude protein gained/g crude protein intake*100). 2.5. Analysis of Nutrients and Trace Minerals. Analysis ofmacronutrients in diets and the whole body was carriedout according to the European Commission Regulation(EC) No. 152/2009 [20]. Analysis of diets and homogenizedwhole bodies was carried out in duplicates. For the determi-nation of crude lipid content in the whole body, methodsaccording to the Soxhlet protocol (Soxtherm, C. GerhardtGmbH & Co. KG, Konigswinter, Germany) were applied.Crude protein content was determined using standard Kjel-dahl methods. Before the determination of ash content via acombustion oven (P300, Nabertherm, Lilienthal, Germany)at 550℃ for 12 hours, the dry matter content of sampleswas ascertained by drying (ED 53, Binder GmbH, Tuttlin-gen, Germany) for 4 hours at 103℃. Nitrogen-free extractsplus crude fiber content were defined as the remaining por-tion of macronutrients in diets and the whole body. Trace minerals (Cr, Cu, Fe, Zn, Mn, and Se) in diets andwhole body were analyzed by an external lab (AgrolabLUFA, Kiel, Germany) following the standards for tracemineral analysis [21, 22]. 2.6. StatisticalAnalysis.The statisticall analysesVwereperformed using SPSS 21 for Windows (SPSS Inc., Chicago,U.S.). Data are presented as mean ± standard deviation (SD)for each treatment and comparisons between treatments.Before the application of one-way analysis of variances(ANOVA), Kolmogorov-Smirnov and Levene tests wereapplied to determine normal distribution and homogeneityof variances. Tukey’s HSD test was used for multiple com-parisons when differences among groups were identified.The aggregate type I error was defined at 5% (P<.05) foreach set of comparisons to determine statistical significance. Additionally, regression analysis between the calculatedorganic Cr supplementation level as the independent vari-able and SGR as the dependent variable was calculated bypolynomial regressions. The optimum dosage was calculatedfor slope =0. 3. Results 3.1. Growth Performance. After 84 days of feeding, growthperformance was significantly affected by CrMet supplemen-tation. Final body weight and SGRwere significantlyincreased in dietary groups CR 1 (683.94±44.91 and 3.03±0.06) and CR 2 (670.52±32.92 and 2.99±0.06) com-pared to control (591.21±17.15 and 2.83±0.03; Table 5).The remaining growth performance parameters were at asimilar level among dietary treatments. The second-degree polynomial regression of dietaryorganic chromium supplementation and SGR was highlysignificant (P<0.001, Figure 1), which allowed a calculationof the optimal chromium content by performing the firstderivation of the equation and calculating x for f(x)=0.The resulting optimal dietary organic chromium supple-mentation level was 0.39mgkg(0.33 mg Cr kg fromCrMet). 3.2. Body Composition. No significant differences betweendietary groups were detected in proximate whole body com-position (Table 6), trace mineral content of whole body(Table 7), or trace mineral retention (Table 8) after 84 daysof feeding. 3.3.BBiometric Indices. No significant differences wereobserved in any of the biometric indices investigated in thisstudy (Table 9). 4. Discussion In the present day, sustainable aqua feeds appear to be aninevitable necessity, and feed additives could contributesignificantly by improving the effective utilization of feedingredients. The present study demonstrated that the sup-plementation of diets with CrMet could improve the growthperformance of African catfish. This effect could have multi-ple rationales. Firstly, organic minerals are more readily TABLE 2: Crude nutrient and gross energy content of diets (mean±SD). Diet Dry matter (%) Ash (% DM) Crude protein (%DM) Crude fat (% DM) NfE (%DM) Energy (MJ/kg DM) CTRL 92.43±0.07 6.35±0.05 48.02±0.03 13.59±0.05 32.05 22.12±0.04 CR 1 92.90±0.03 6.31±0.02 47.77±0.02 13.31±0.01 32.61 22.11±0.01 CR 2 92.46±0.04 6.33±0.03 47.81±0.01 13.39±0.07 32.47 22.17±0.03 CR 3 92.72±0.00 6.35±0.01 47.94±0.13 13.66±0.04 32.05 22.14±0.02 NfE (nitrogen -free extract)+ crude fiber = 100-(crude protein + crude lipid + crude ash). CR 1= chromium diet 1; CR 2= chromium diet 2; CR 3 =chromium diet 3. TABLE3:Supplementation1 of diets with Availa-Cr 1000:supplemented and calculated levels of inclusion. Diet Availa-Cr 1000 inclusion in feed (mgkg) Supplementation levels (mg Cr kg ) Calculated organic Cr levels in diet (mgkg) CTRL 0 0 CR 1 200 0.2 0.24 CR 2 400 0.47 CR 3 600 0.6 0.71 Based on the analyzed amount of Cr in the product. CR 1= chromium diet1; CR 2 = chromium diet 2; CR 3= chromium diet 3. available for fish compared to their inorganic counterparts[3, 9]. Secondly, dietary chromium supplementation hasbeen demonstrated to support the immune response [13]and reduce the stress level of fish [8, 12]. Lastly, diets withchromium addition can improve the energy metabolism offish by activating various digestive enzymes and enhancingthe activity of insulin [2, 3, 8, 10, 18]. According to the results of the current study, dietaryorganic chromium supplementation appears to have aminor positive effect on both feed conversion and feedintake, which in turn resulted in a significantly improvedgrowth rate of catfish. Multiple earlier studies demon-strated a positive effect on growth rates of various speciesof fish including hybrid tilapia (Oreochromis niloticus L.,1758 x Oreochromis aureus S., 1864;[14]; form of chro-mium supplemented: CrCl,6H,O, Na,CrO 4H,0, andCr,O), Nile tilapia (Oreochromis niloticus; [11]; Cr picoli-nate, 0.6-1.8 mgkg ), grass carp (Ctenopharyngodon idellaV., 1844; [23]; Cr picolinate, 0.2-3.2 mgkg ), commoncarp (Cyprinus carpio L., 1758; [8, 18]; Cr methionine,0.31-3.64 mgkg; Cr oxide, Cr picolinate, and Cr methio-nine, 2 mg kg), mirror carp (Cyprinus carpio; [5]; Cr chlo-ride, Cr picolinate, and Cr yeast, 0.5-2mgkg), large158yellow croaker (Larimichthys crocea R., 1846; [15]; Crpolynicotinate, 5-80 mgkg), blunt snout bream (Megalo-brama amblycephala Y., 1955; [24]; Cr picolinate, 0.2-12 mgkg), snakehead (Channa argus C., 1842; [10]; Cryeast, 200mgkg), and striped catfish (Pangasianodonhypophthalmus S., 1878; [6]; Cr, 2-8mgkg ). However,other studies could not show any response from differentspecies of fish to dietary Cr supplementation ([25]; Crpicolinate, 0.8-1.2mgkg; [26]; Cr yeast, 0.8 mgkg ; [27];Cr picolinate, 2mgkg ; [28]; Cr picolinate, 1.6mgkg). These contradictory results can most likely be explainedby different factors influencing the effects of dietary Cr,including form and dose of Cr, duration of experiment,and behavior of concerned species [6]. The low bioavailability of inorganic chromium is causedby a multitude of factors including the formation of nonso-luble Cr oxides, binding to natural chelate-forming com-pounds in feeds, and interference with ion forms of otherminerals [3]. Results suggest that supplementation withorganic CrMet is beneficial for the nutritious value of com-mercially based diets for African catfish. This positive effectis in accordance with Cui et al. [18], who demonstrated thatdietary CrMet supplementation is superior in comparisonwith inorganic Cr and Cr picolinate in common carp dueto increased absorption efficiency. Dietary CrMet supple-mentation also improved the growth performance of com-mon carp in earlier research [8] and of different crustaceanspecies [29, 30]. Additionally, organic-chelated mineralscould provide a complex that is more stable in the upperdigestive tract in comparison with mineral salts, therebyincreasing the bioavailability of the minerals [31]. Accordingto Pechova and Pavlata [3], the absorption efficiency of inor-ganic Cr is inversely proportional to the dietary level. Asimilar trend was also observed in the present study withorganic Cr supplementation, somewhat contradicting obser-vations from Cui et al. [18]. However, the analyzed contentof Cr in diets differed from the expected values from organicsupplementation (Tables 3 and 4), which was most likelycaused by contamination with inorganic Cr during feed pro-duction. These elevated levels of inorganic Cr could explainthe reduced absorption efficiency of Cr. Despite a trendtoward reduced absorption of Cr at higher supplementationlevels, the content of Cr in the whole body is still comparableto the results of other literature [5]. Due to handling, fish might have been exposed to shortperiods of stress during the experiment. Stress can increasethe demand for Cr in humans and animals [3]. The stress-related secretion of cortisol, which acts as an antagonist forinsulin, elevates the blood glucose concentration. Latter ele-vation results in the mobilization and subsequent excretionof Cr [32]. Multiple studies have shown a reduced sensitivityto different stressors due to dietary supplementation with Crin various animals including fish [7, 12, 33-35]. Thisreduced sensitivity to stress can in turn elevate growth per-formance by enhancing energy utilization, absorption, andallocation [36]. Additionally, Risha et al. [13] demonstratedthat Cr supports the nonspecific immunity in Nile tilapia, TABLE 4: Trace mineral content of the diets. Diet Chromium (mgkg) Selenium (mgkg) Copper (mgkg) Iron (mgkg ) Zinc (mgkg ) Manganese (mgkg) CTRL 2.37 0.86 27.8 197 104 57.4 CR 1 2.88 0.92 29.5 211 115 59.2 CR 2 3.33 0.96 29.5 222 119 60.1 CR 3 3.47 0.93 30.1 220 119 60.2 CR 1 = chromium diet l; CR 2 = chromium diet 2; CR 3= chromium diet 3. TABLE 5: Growth performance parameter of African catfish after 84 days of feeding (mean ±SD). Diet IBW FBW FCR SGR DFI PRE PER CTRL 54.98±1.22 591.21±17.15 0.86±0.01 2.83±0.03° 2.43±0.03 49.83±1.01 2.65±0.02 CR 1 53.67±1.27 683.94±44.91° 0.83±0.02 3.03±0.06 2.51±0.09 46.68±2.60 2.68±0.08 CR 2 54.35±1.38 670.52±32.92° 0.84±0.01 2.99±0.06c 2.50±0.07 49.29±1.46 2.64±0.04 CR 3 54.55±0.77 634.01±4.25D 0.83±0.01 2.92±0.01ab 2.42±0.05 49.94±0.89 2.67±0.05 IBW = initial body weight; FBW= final body weight; FCR (feed conversion ratio) = g feed intake/g weight gain; SGR (specific growth rate)=(ln (FBW)-ln(IBW))/feeding days* 100; DFI (daily feed intake)= daily feed intake in%body weight;PER (protein efficiency ratio) = g body weight gain/g crude proteinintake; PRE (protein retention efficiency) = g crude protein gained/g crude protein intake*100. Values in the same row with different superscript letters aresignificantly different (P<.05). CR1 = chromium diet 1; CR 2= chromium diet 2; CR 3 = chromium diet 3. Organic Cr supplementation (mg kg-1) FIGURE 1: Regression analysis with specific growth rate as dependent variable and organic chromium supplementation as the independentvariable. TABLE 6: Proximate whole body composition of fish after 84 days of feeding (mean±SD). Diet Moisture (% OS) Crude ash (% OS) Crude protein (%OS) Crude fat (% OS) Gross energy (MJkgOS) CTRL 69.61±0.97 2.77±0.10 17.09±0.33 10.51±0.76 8.10±0.32 CR 1 69.72±0.78 2.67±0.10 17.07±0.20 10.36±0.72 8.13±0.30 CR 2 68.58±0.86 2.73±0.06 16.98±0.30 11.59±0.72 8.60±0.30 CR 3 68.81±0.50 2.83±0.12 17.04±0.20 11.20±0.32 8.49±0.17 Values in the same row with different superscript letters are significantly different (P<.05). CR 1 = chromium diet 1; CR 2= chromium diet 2; CR 3 =chromium diet 3. which was also demonstrated in other animals [37-39]. Fur-thermore, Cr supplementation also supported the antioxida-tive status of Nile tilapia [13]. The immune response andantioxidant status could therefore be enhanced even withoutstress [13]. Hence, supplementation with bioavailable Crcould result in increased growth performance by reducing stress sensitivity, as well as improving immune responseand antioxidant status. Chromium is also involved in the activation of digestiveenzymes and protein stabilization, which are primary stepsfor the metabolism of carbohydrates, proteins, and lipids[40]. Supplementation with Cr has been shown to improve TABLE 7: Trace mineral content of the whole body of fish after 84 days of feeding (mean±SD). Diet Chromium (mg kg) Selenium (mg kg) Copper (mg kg) Iron (mg kg) Zinc (mg kg) Manganese (mg kg) CTRL 0.56±0.30 1.13±0.03 7.72±7.69 45.05±2.93 40.55±5.40 9.68±0.73 CR 1 0.52±0.45 1.08±0.05 20.32±25.65 43.25±6.86 48.63±13.17 10.75±0.99 CR 2 0.44±0.18 1.15±0.04 4.18±0.98 44.88±2.63 40.20±2.49 10.15±0.52 CR 3 0.34±0.07 1.12±0.00 5.32±3.13 47.23±1.18 41.58±3.99 10.18±1.14 Values in the same row with different superscript letters are significantly different (P<.05). CR 1 = chromium diet 1; CR 2= chromium diet 2; CR 3=chromium diet 3. TABLE 8: Trace mineral retention of African catfish after 84 days of feeding (mean±SD). Diet Chromium retention (%) Selenium retention (%) Copper retention (%) Iron retention (%) Zinc retention (%) Manganese retention (%) CTRL 8.63±4.86 40.88±2.38 8.68±10.97 45.05±2.93 8.78±2.12 5.55±0.43 CR 1 6.58±6.25 38.00±1.72 25.91±34.66 43.25±6.86 11.82±4.53 6.36±0.55 CR2 4.77±2.28 37.75±3.57 3.60±1.41 44.88±2.63 7.89±0.44 5.62±0.62 CR 3 3.34±0.83 37.40±0.67 4.91±4.12 45.97±2.29 8.14±1.40 5.61±0.80 Trace mineral retention (%)= trace mineral content whole body/trace mineral content diet. Values in the same row with different superscript letters aresignificantly different (P<.05). CR 1=chromium diet 1; CR 2 = chromium diet 2; CR 3= chromium diet 3. Diet Mortality (%) HSI (%) SSI (%) Hematocrit (%) CTRL 2.5±1.7 1.49±0.28 0.08±0.02 38.8±4.4 CR 1 3.0±2.2 1.61±0.32 0.09±0.03 35.2±3.2 CR 2 4.0±3.2 1.68±0.44 0.07±0.02 38.0±2.9 CR 3 3.5±2.2 1.70±0.28 0.07±0.01 36.9±2.7 HSI (hepatosomatic index) = g liver weight/g fish weight* 100; SSI (spleensomatic index) = g spleen weight/g fish weight* 100; Hematocrit=%ofblood cells in the blood. Values in the same row with different superscriptletters are significantly different (P<.05). CR 1 = chromium diet 1; CR 2= chromium diet 2; CR 3= chromium diet 3. the activity of glycolytic and lipogenic enzymes in the liver ofcommon carp [8, 16]. Additionally, Cr acts as a cofactor forinsulin, enhancing its activity, which potentiates the regula-tion of glycemia and muscle protein deposition [3]. Increasedserum insulin concentrations have been observed previouslyin fish at a Cr supplementation level of 0.8 mgkg [23],andthe anabolic role of this hormone resulted in improvedgrowth performance. Additionally, the enhanced glucoseclearance from blood due to higher insulin activity [2] canimprove feed intake, since reduced glycemia has been dem-onstrated to increase feed consumption [41, 42]. Since noanalysis of digestive enzymes was conducted, a significanteffect was not demonstrated during this study; however,improved activation of digestive enzymes could have contrib-uted to the overall significant effect on growth performance.The overall improvement in feed conversion and feed intakewas of a minor extent, indicating an effect of a lesser degreefrom Cr supplementation on each growth factor. This smalleffect could partly be explained by diet formulation since the objective of this study was to show the effects of a dietwith high relevancy to the industry. The commercially baseddiet used in this study has a comparatively high crude proteincontent, which could reduce the effectiveness of a protein-sparing effect from improved carbohydrate and lipid metab-olism. This would also be a tangible explanation for the sim-ilar protein retention between all dietary groups. The latterobservation was contrary to earlier research results demon-strating improved protein retention due to dietary supple-mentation with Cr in different species of fish [6, 23].Additionally, the effects of dietary Cr supplementation weremore prominent when glucose was included in diets for fishin comparison to starch [14, 43, 44]. More complex carbohy-drates tend to lead to a less intense blood glucose peak [2].Therefore, effects on feed intake due to improved clearanceof glucose from the bloodstream could be less prominentconsidering the current feed formulation in this trial. Despitediet formulation, CrMet was still able to improve the growthperformance of African catfish significantly, and it can beexpected that this effect would be greater in diets containingless complex carbohydrates or lower amounts of protein.These effects have already been demonstrated in literaturefor different species of fish [6, 14,23,43,44]. High amounts of Cr supplementation can have toxiceffects on fish [17]. Akter et al. [6] observed changes inhematological indices, indicating toxic effects for striped cat-fish at high dietary Cr content (8mgkg ). Based on theresults of the current study, supplemented levels of CrMetwere not toxic for African catfish up to a dietary supplemen-tation level of 0.6 mg Cr kg. This is in line with the findingsfrom Akter et al.[6] for striped catfish. Additionally, no sig-nificant differences were observed in other biometric indices(Table 9), indicating that no negative effects on the liver andspleen occurred due to the supplementation with CrMet. However, it should be noted that the analysis of healthparameters was not the main focus of this study and addi-tional conformation for the safe application of CrMet shouldbe collected in future trials with a more comprehensiveamount of analyzed health parameters. Our results demonstrated that supplementing a com-mercially based diet containing 2.37 mg Cr kg-1with330 mgkg CrMet (0.39 mg Cr kg) optimizes the growthperformance of African catfish. A similar value has beendetermined for striped catfish (2.82 mg Cr kgtotal dietarysupply; [6]), Nile tilapia (3.49 mg Cr kg total dietary sup-ply; [45]), and grass carp (0.8 mg Cr picolinate kg supple- 5. Conclusion Supplementing commercially based diets of African catfishwith CrMet significantly improved growth performance atdietary organic Cr supplementation levels of 0.22and0.4 mg kg. Based on regression analysis, 0.33 mg Cr kg fromCrMet supplementation in commercially based diets for Afri-can catfish was optimal. Data Availability Data will be made available upon reasonable request. Conflicts of Interest The authors declare that they have no known competingfinancial interests or personal relationships that could haveappeared to influence the work reported in this paper. Acknowledgments This study was funded by the Zinpro Corporation, 10400Viking Drive, Eden Prairie, MN 55344, United States, andOpen Access funding enabled and organized by ProjektDEAL. We acknowledge the financial support by the LandSchleswig-Holsteinwithin the funding program OpenAccess Publications funds. References [1] FAO, The State of World Fisheries and Aquaculture. Towardsblue transformation, Food and Agriculture Organization ofthe United Nations, 2022. [2] National Research Council, Nutrient Requirements of Fish andShrimp, National academies press, 2011. [3] A. Pechova and L. Pavlata,“Chromium as an essential nutri-ent: a review," Veterinary Medicine, vol. 52, no. l, pp. 1-18,2007. [4] S. P. Lall and S. J. Kaushik, “Nutrition and metabolism of min-erals in fish,” Animals, vol. 11, no. 9, p. 2711, 2021. 5A. R. Ahmed, A. N. Jha, and S. J. Davies,“The efficacy of chro-mium as a growth enhancer for mirror carp (Cyprinus carpioL): an integrated study using biochemical, genetic, and histo-logicalresponses,”BiologicalTTraceeElement Research,vol. 148, no. 2, pp. 187-197,2012. [6] S. Akter, N. Jahan, M. F. Rohani, Y. Akter, and M. Shahjahan,“Chromium supplementation in diet enhances growth andfeed utilization of striped catfish (Pangasianodon hypophthal-mus),” Biological Trace Element Research, vol. 199, no. 12,pp. 4811-4819,2021. [7] M. P. Castro, G. S. Claudiano, T. R. Petrillo et al., “Acuteaerocystitis in Nile tilapia bred in net cages and supplementedwith chromium carbochelate and Saccharomyces cerevisiae,”Fish e Shellfish Immunology, vol. 36, no. 1, pp. 284-290,2014. [8] P. Cui, S. Yin, Z. Cheng, X. Qiao, and Q. Zhou, “Effects of dietary chromium methionine on growth performance, hemato-logical characteristics and carbohydrate metabolic enzymeactivities of common carp (Cyprinus carpio),” Israeli Journalof Aquaculture - Bamidgeh, vol. 70, 2018. [9] P. P. Gatta, K. D. Thompson, R. Smullen, A. Piva, S. Testi, andA. Adams,“Dietary organic chromium supplementation andits effect on the immune response of rainbow trout (Oncorhyn-chus mykiss)," Fishe Shellfish Immunology, vol. 11, no. 5,pp. 371-382,2001. [10] Y. Hou, Y. Hou, L. Yao, S. Chen, J. Fan, and L. Qian, “Effects ofchromium yeast, tributyrin and bile acid on growth perfor-mance, digestion and metabolism of Channa argus," Aquacul-ture Research, vol. 50, no.3, pp.836-846,2019. [11] H. Li, X. Meng, W. Wan et al., “Effects of chromium picolinatesupplementation on growth, body composition, and biochem-ical parameters in Nile tilapia Oreochromis niloticus,”FishPhysiology and Biochemistry, vol. 44, no. 5, pp. 1265-1274,2018. [12] H. Liang, X. Ge, D. Xia, M. Ren, H. Mi, and L. Pan,“The role ofdietary chromium supplementation in relieving heat stress ofjuvenile blunt snout bream Megalobrama amblycephala,”FishShellfish Immunology, vol. 120, pp. 23-30, 2022. [13] E. Risha, F. Ahmed, A. A. Khaled, F. M. A. Hossain,N. Akhtar,and E. Zahran,“Interactive effects of dietary betaine and chro-mium picolinate on the immunomodulation, antioxidativeresponse and disease resistance of Nile tilapia (Oreochromisniloticus),”Aquaculture Research, vol. 53, no. 9, pp. 3464-3477,2022. [14] S.Y. Shiau and M. J. Chen, "Carbohydrate utilization by tilapia(Oreochromis niloticusx O. aureus) as influenced by differentchromium sources," The Journal of Nutrition, vol. 123,no. 10, pp. 1747-1753,1993. [15] J. Wang, Q. Ai, K. Mai, H. Xu, and R. Zuo,“Dietary chromiumpolynicotinate enhanced growth performance, feed utilization,and resistance to Cryptocaryon irritans in juvenile large yellowcroaker (Larmichthys crocea)," Aquaculture, vol. 432, pp. 321-326,2014. [16] A. R. Ahmad, A. N. Jha, and S. J. Davies, “The effect of dietaryorganic chromium on specific growth rate, tissue chromiumconcentrations, enzyme activities and histology in commoncarp, Cyprinus carpio L,” Biological Trace Element Research,vol. 149, no. 3, pp. 362-370, 2012. [17] S. Aslam and A. M. Yousafzai,“Chromium toxicity in fish: areview article," Journal of Entomology and Zoology Studies,vol. 5, no. 3, pp. 1483-1488,2017. [18] P. Cui, Z. Cheng, and J. Sun, “Effects of different chromiumsources on growth performance, serum biochemical, hepato-pancreas glycometabolism enzymes activities, IR, GLUT2and SGLT1 gene expression of common carp (Cyprinus car-pio),” Aquaculture Research, vol. 53, no. 4, pp. 1573-1581,2022. [19] M. K. Zakaria, Z. A. Kari, H. Van Doan et al.,“Fermented soy-bean meal (FSBM) in African catfish (Clarias gariepinus) diets:effects on growth performance, fish gut microbiota analysis,blood haematology, and liver morphology,” Life, vol. 12,no. 11, p. 1851,2022. [20] European Commission,“Commission Regulation (EC) No152/2009 of 27 January 2009 laying down the methods of sam-pling and analysis for the official control of feed," Official Jour-nal of the European Union, vol. 54, pp. 1060-1130, 2009. [21] DIN EN 15621, Futtermittel - Probenahme- und Untersu-chungsverfahren -Bestimmung von Calcium, Natrium, Phos-phor, Magnesium, Kalium, Schwefel, Eisen, Zink, Kupfer,Mangan und Cobalt nach Druckaufschluss mittels ICP-AES,Beuth Verlag, Berlin, 2017. [22] DIN EN 17053, Futtermittel - Probenahme- und Untersu-chungsverfahren - Bestimmung von Spurenelementen, Schwer-metallen und anderen Elementen in Futtermitteln mittelsICP-MS (Multimethode), Beuth Verlag, Berlin, 2018. [23] T. Liu, H. Wen, M. Jiang et al., “Effect of dietary chromiumpicolinate on growth performance and blood parameters ingrass carp fingerling, Ctenopharyngodon idellus,"Fish Physiol-ogy and Biochemistry, vol. 36, no. 3, pp. 565-572,2010. [24]1M. Ren, A. Mokrani, H. Liang et al., “Dietary chromium pico-linate supplementation affects growth, whole-body composi-tion, and gene expression related to glucose metabolism andlipogenesis in juvenile blunt snout bream, Megalobramaamblycephala,”Biological Trace Element Research, vol. 185,no. l, pp. 205-215, 2018. [25] E. H. El-Sayed, E. I. Hassanein, M. H. Soliman, and N. R. El-Khatib,“The effect of dietary chromium picolinate on growthperformance, blood parameters and immune status in Niletilapia, Oreochromis niloticus,"in Proceedings of the 3rd GlobalFisheries and Aquaculture Research Conference, Foreign Agri-cultural Relations (FAR), pp. 51-63, Egypt, 2010. [26]IP. P. Gatta, A. Piva, M. Paolini et al.,“Effects of dietary organicchromium on gilthead seabream (Sparus aurata L.) perfor-mances and liver microsomal metabolism,” AquacultureResearch, vol. 32, pp. 60-69, 2001. [27] Q. Pan, S. Liu, Y. G. Tan, and Y. Z. Bi,“The effect of chromiumpicolinate on growth and carbohydrate utilization in tilapia,Oreochromis niloticus x Oreochromis aureus,” Aquaculture,vol. 225, no. 1-4, pp. 421-429, 2003. [28] Z. Selcuk, S. U. Tiril, F. Alagil et al, “Effects of dietary L-carnitine and chromium picolinate supplementations on per-formance and some serum parameters in rainbow trout (Onco-rhynchus mykiss),”Aquaculture International, vol. 18, no. 2,pp. 213-221,2010. [29] B. Shi, X. Tao, M. B. Betancor et al.,“Dietary chromium mod-ulates glucose homeostasis and induces oxidative stress inPacific white shrimp (Litopenaeus vannamei),”Aquatic Toxi-cology, vol. 240, article 105967, 2021. [30] Y. Zhang, J. Luo, T. Zhu et al., “Dietary chromium couldimprove growth, antioxidant capacity, chromium accumula-tion in tissues and expression of genes involved into glucoseand lipid metabolism in juvenile mud crab Scylla paramamo-sain,”Aquaculture Reports, vol. 23, article 101088,2022. [31] R. W. Hardy and S. J. Kaushik, Eds., Fish Nutrition, Academicpress, 2021. [32] J. S. Borel, T. C. Majerus, M. M. Polansky, P. B. Moser, andR. A. Anderson, “Chromium intake and urinary chromiumexcretion of trauma patients,” BiologicalTrace ElementResearch, vol. 6, no. 4, pp. 317-326, 1984. [33] X. Chang and D. N. Mowat, “Supplemental chromium forstressed and growing feeder calves," Journal of Animal Science,vol. 70, no. 2, pp. 559-565, 1992. [34] S. Moonsie-Shageer and D. N. Mowat, “Effect of level of sup-plemental chromium on performance, serum constituents,and immune status of stressed feeder calves,” Journal of Ani-mal Science, vol. 71, no. 1, pp. 232-238, 1993. [35] A. Pechova,L. Pavlata, and J. Illek,“Metabolic effects of chro-mium administration to dairy cows in the period of stress,”Czech Journal of Animal Science-UZPI (Czech Republic),vol. 47, no. 1, pp. 1-7,2002. [36] B. Sadoul and M. M. Vijayan,“Stress and growth," Fish Phys-iology, vol. 35, pp. 167-205,2016. [37] S. K. Jain, J. L. Rains, and J. L. Croad,“Effect of chromiumniacinate and chromium picolinate supplementation on lipidVCaperoxidation, TNF-c, IL-6, CRP, glycated hemoglobin, triglyc-erides, and cholesterol levels in blood of streptozotocin-treateddiabetic rats,” Free Radical Biologye Medicine, vol. 43, no. 8,pp. 1124-1131, 2007. [38] A. I. Mehrim, “Physiological, biochemical and histometricresponses of Nile tilapia (Oreochromis niloticus L.) by dietaryorganic chromium (chromium picolinate) supplementation,”Journal of Advanced Research, vol. 5, no. 3, pp. 303-310,2014. [39] Y.Y. Tian, L. Y. Zhang, B. Dong, J. Cao, J. X. Xue, and L. M.Gong,“Effects of chromium methionine supplementation ongrowth performance, serum metabolites, endocrine parame-ters, antioxidant status, and immune traits in growing pigs,”Biological Trace Element Research, vol. 162, no. 1-3, pp. 134-141,2014. [40] S. Lashkari, M. Habibian, and S. K. Jensen,“A review on therole of chromium supplementation in ruminant nutrition-ef-fects on productive performance, blood metabolites, antioxi-dant status, and immunocompetence," Biological TraceElement Research, vol. 186, no. 2, pp. 305-321,2018. [41] J. L. Soengas, J. M. Cerda-Reverter, and M. J. Delgado,“Centralregulation of food intake in fish: an evolutionary perspective,”Journal of Molecular Endocrinology, vol. 60, no. 4, pp. R171-R199,2018. [42] A. Tran-Duy, B. Smit, A. A. van Dam, and J. W. Schrama,“Effects of dietary starch and energy levels on maximum feedintake, growth and metabolism of Nile tilapia, Oreochromisniloticus,” Aquaculture, vol. 277, no. 3-4, pp. 213-219,2008. [43] S. Y. Shiau and H. S. Liang, “Carbohydrate utilization anddigestibility by tilapia, Oreochromis niloticus xO. aureus, areaffected by chromic oxide inclusion in the diet,” The Journalof Nutrition, vol. 125, no. 4, pp. 976-982,1995. [44] S. Y. Shiau and S. F. Lin, “Effect of supplemental dietary chro-mium and vanadium on the utilization of different carbohy-drates in tilapia, Oreochromis niloticusxCO. aureusS,,Aquaculture, vol. 110, no. 3-4, pp. 321-330,1993. [45] Y. Yuliana, S. Subandiyono, and S. Hastuti,“The effect of die-tary chromium on growth and survival rate of tilapia (Oreo-chromis niloticus),” Omni-Akuatika, vol. 17, no. 2, pp. 118-126,2022.
确定





还剩6页未读,是否继续阅读?
中国格哈特为您提供《非洲鲶鱼日粮中蛋白质和总脂肪含量的检测》,该方案主要用于饲料中营养成分检测,参考标准--,《非洲鲶鱼日粮中蛋白质和总脂肪含量的检测》用到的仪器有格哈特全自动超级总脂肪测定系统HT6+SOX416、格哈特凯氏消化系统KT8S、格哈特维克松废气实验室废物处理系统涤气VS、格哈特带自动进样器凯氏定氮仪VAP500C、德国加液器MM、滤纸筒
相关方案
更多
该厂商其他方案
更多