
营养不平衡,例如水果中的钙缺乏,通常是苹果采后失调的诱因。叶面施用钙作为氯化钙是目前提高苹果钙浓度的方法,尽管这种方法的效果往往不令人满意。在这项研究中,我们测试了钙与精选的生物刺激剂联合应用对改善苹果品质和降低贮藏障碍发生率的功效。这项实验是在两个“乔纳森”苹果园进行的,这两个苹果园的管理制度和特点不同。树冠单独喷洒氯化钙,并与含有锌和硅的商业产品或海藻提取物结合使用。海藻提取物提高了苹果的红颜色(+32%的颜色指数)和提高了果皮的最终花青素浓度,从而提高了苹果的品质。两种生物刺激剂在贮藏160天后显著降低了(20%)这种被称为“乔纳森斑点”的生理紊乱的发生率。探讨了施用生物刺激剂后苹果果皮中营养物质(Ca、Zn和Mn)浓度的增加,以及贮藏过程中酚类物质的变化,可能是苹果果实对采后病害易感性降低的可能原因。
方案详情

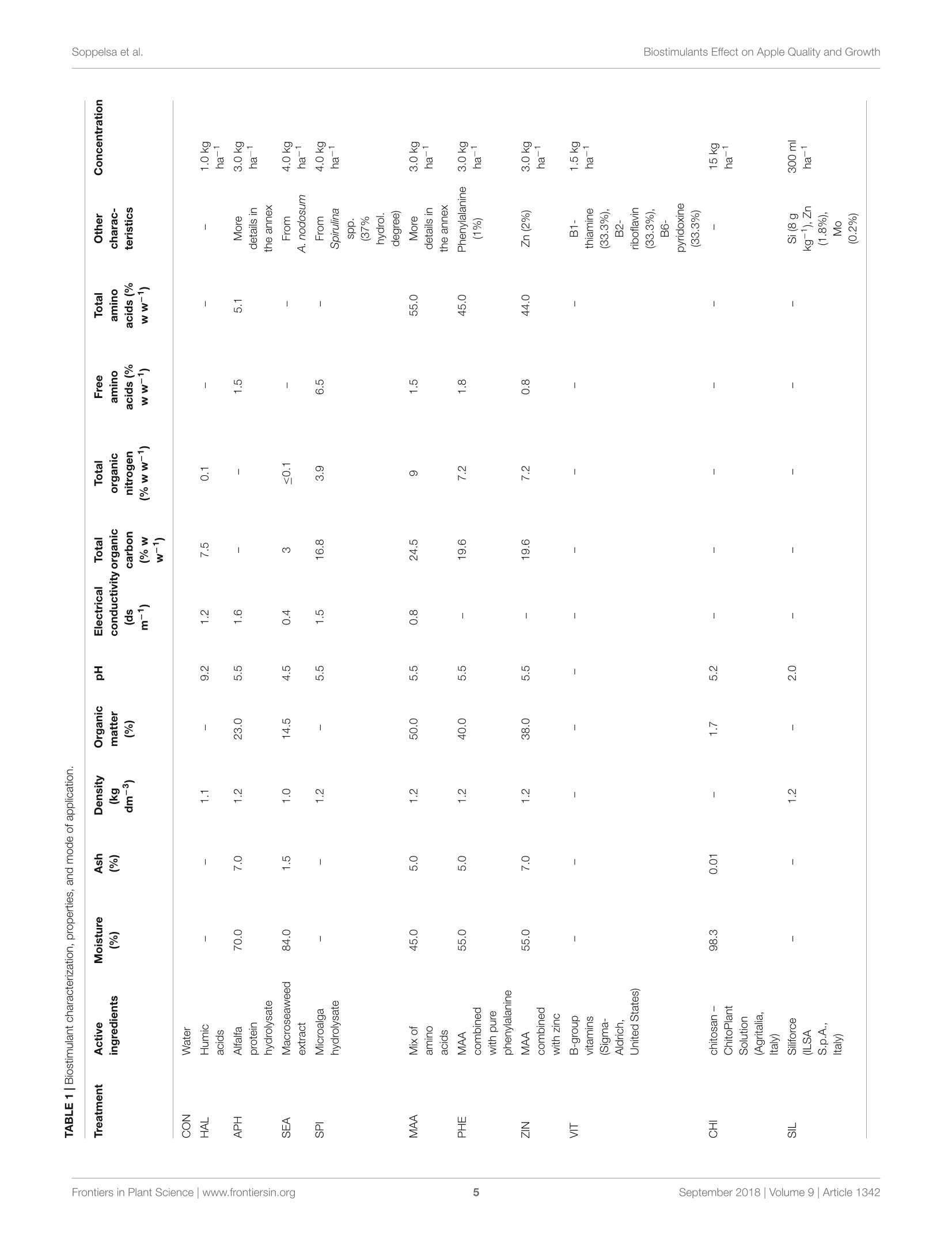
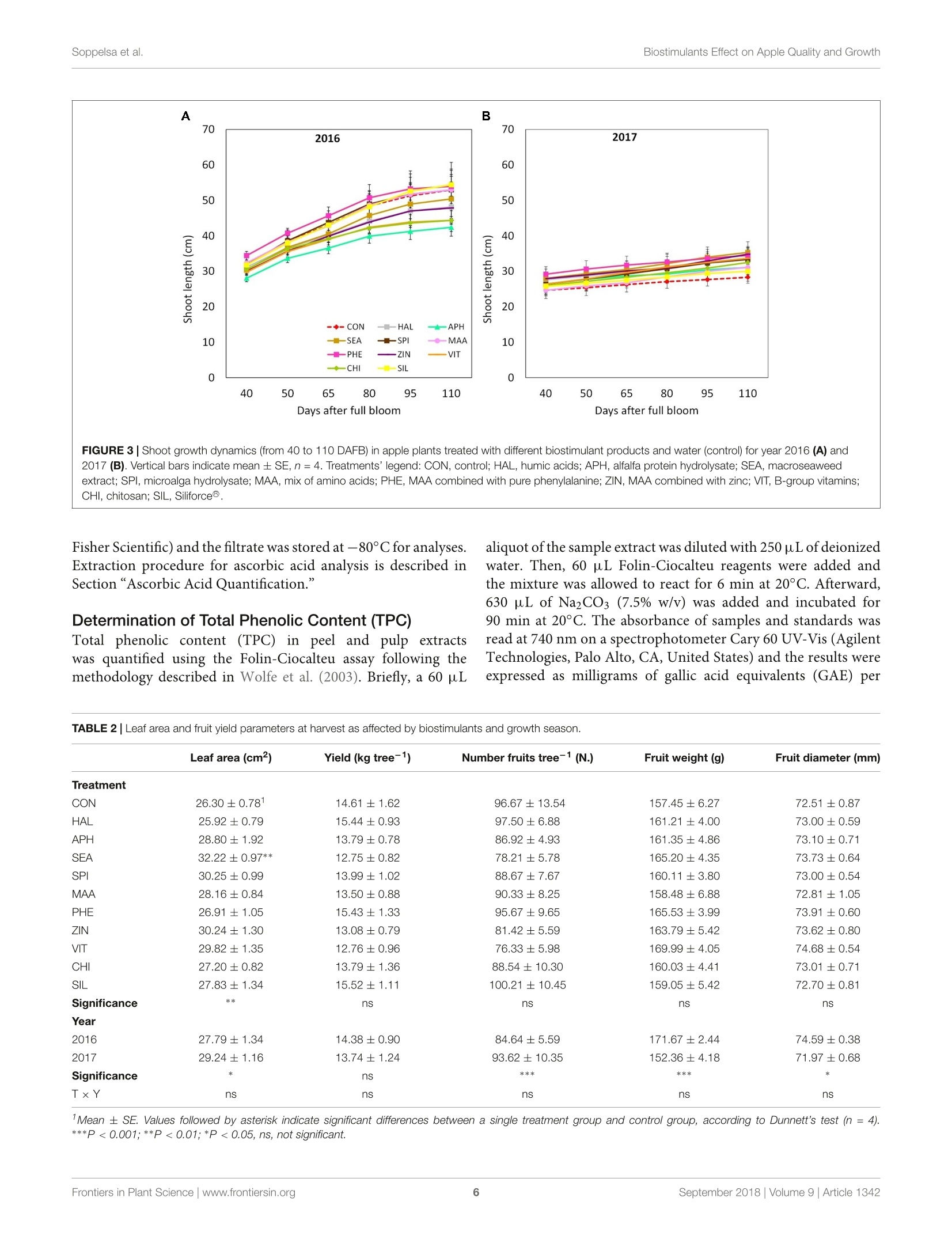
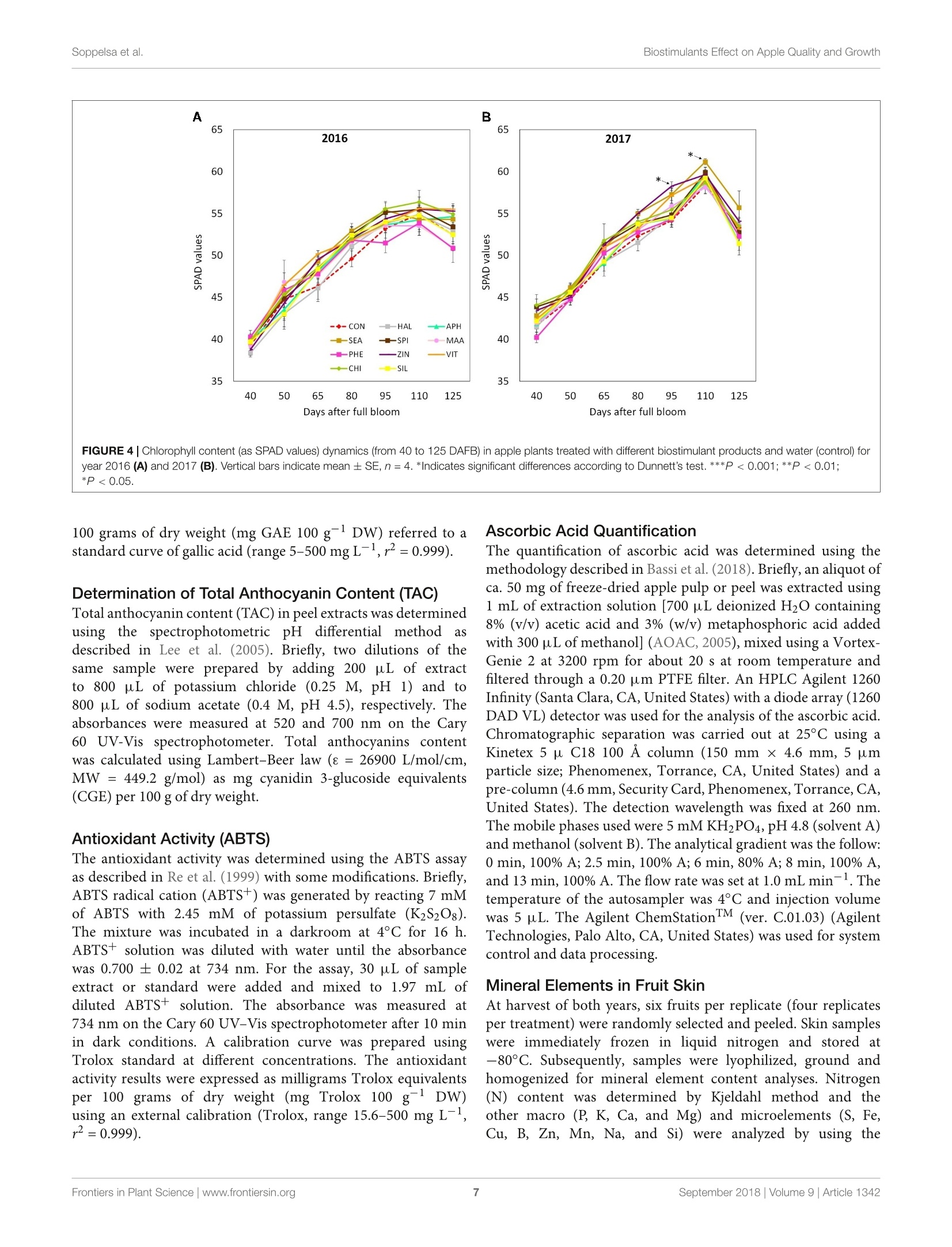
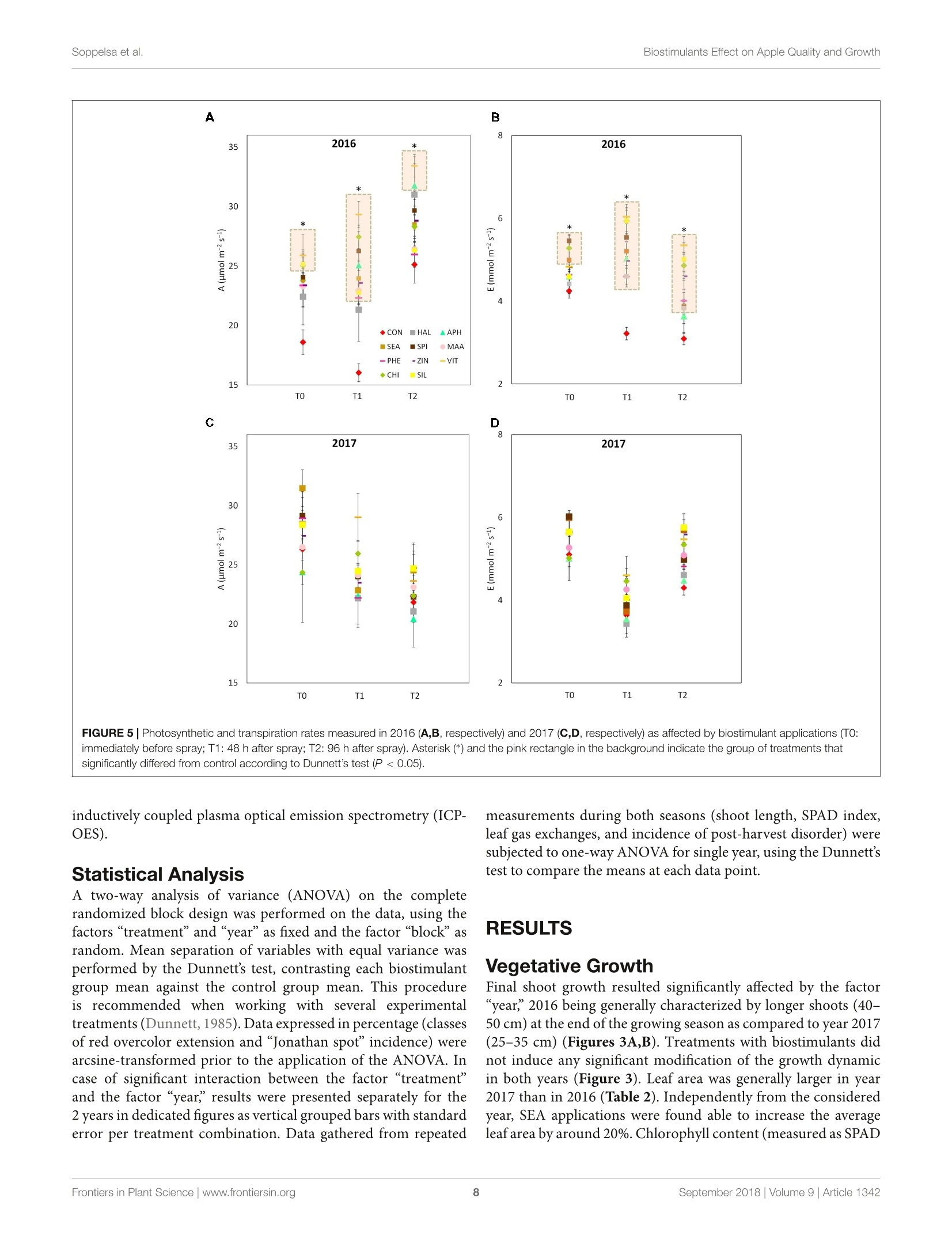
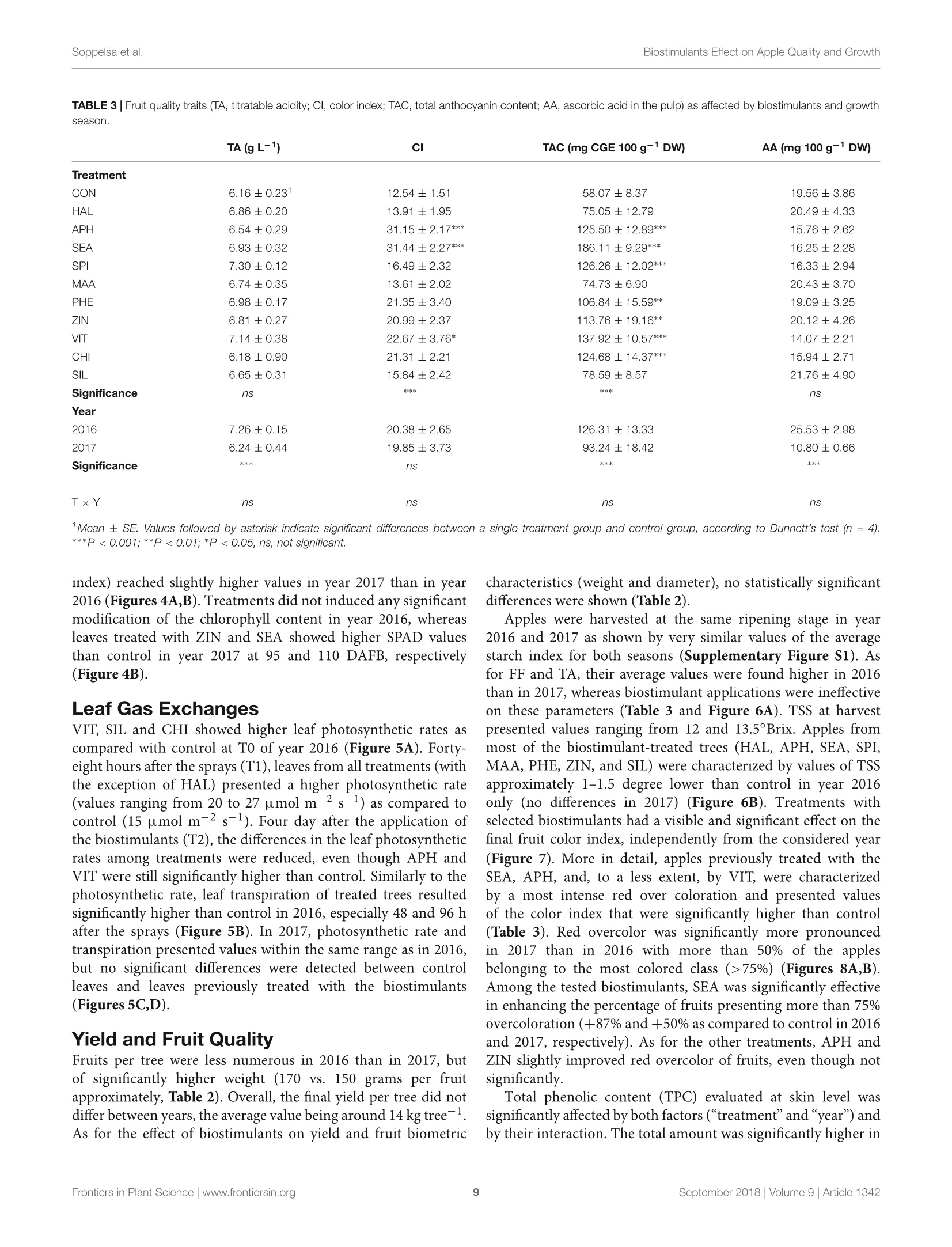
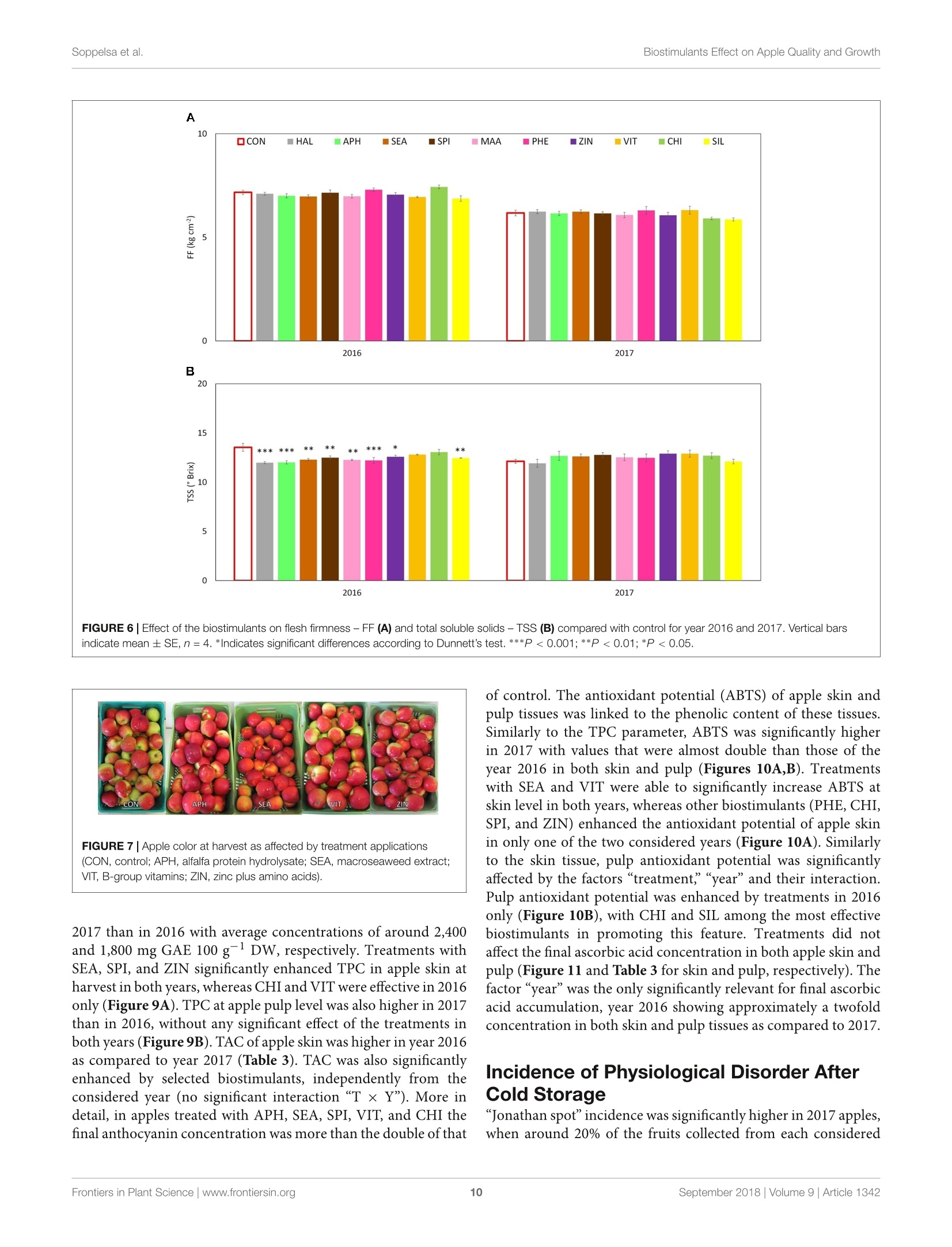
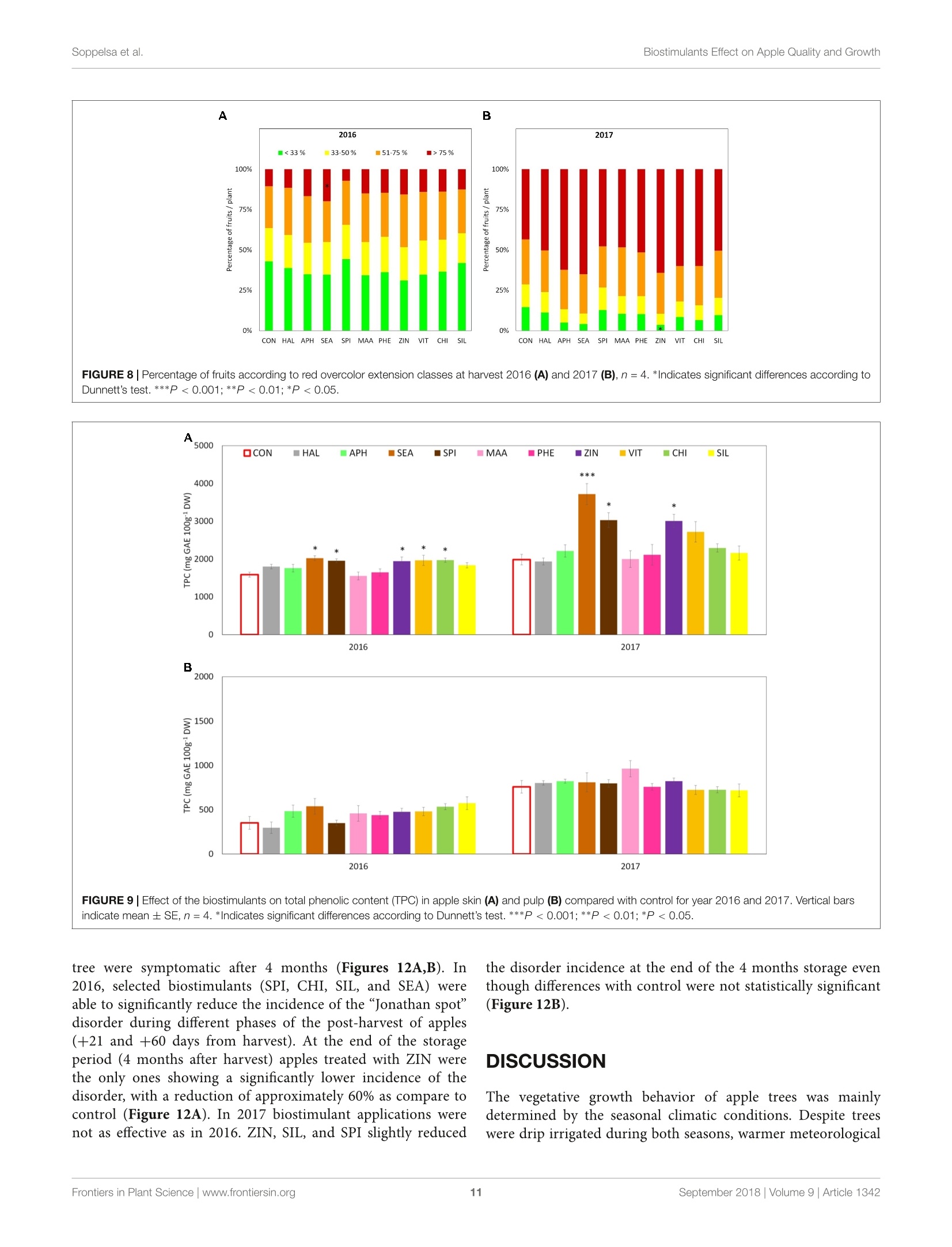
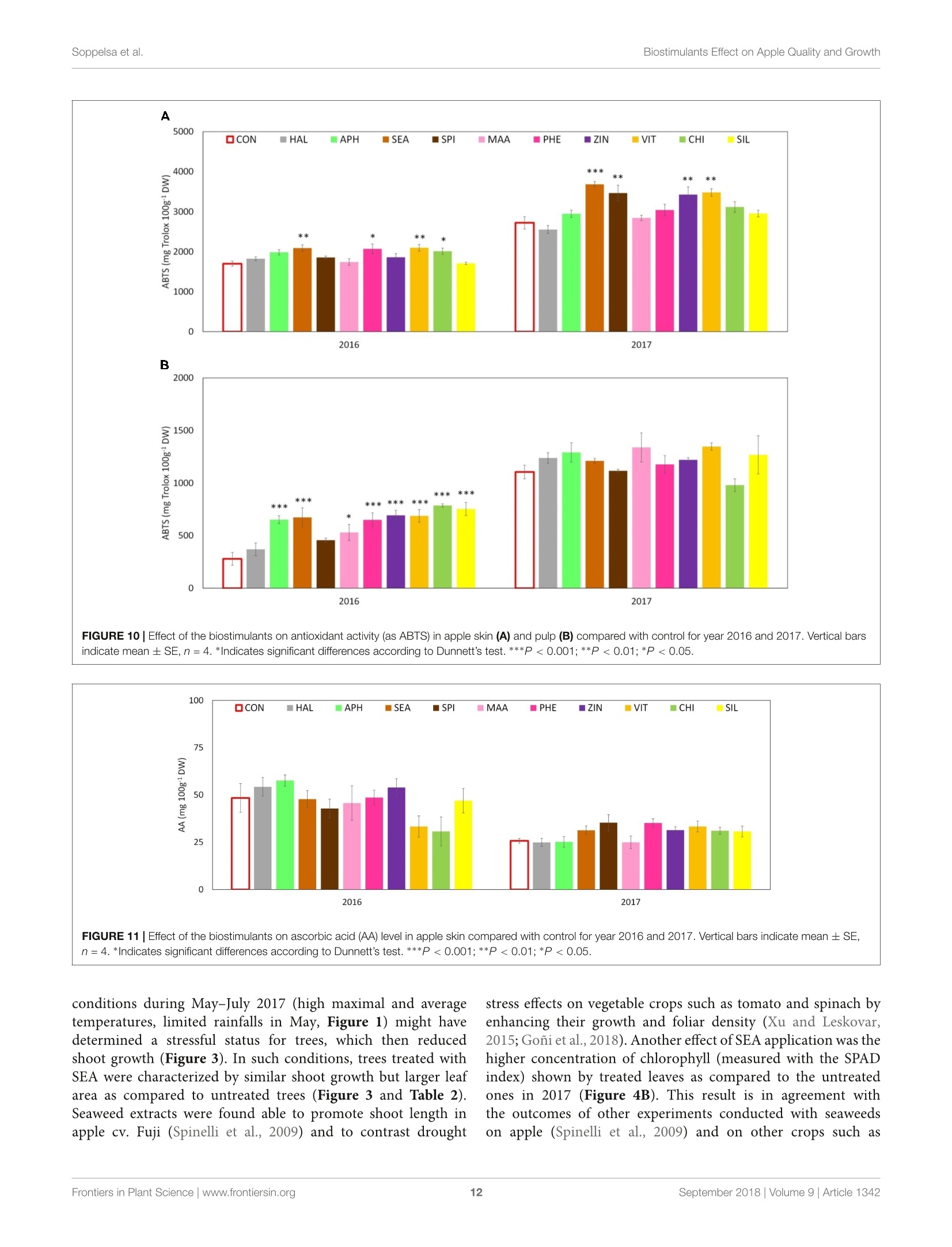
frontiersin Plant ScienceORIGINAL RESEARCHpublished: 20 September 2018doi: 10.3389/fpls.2018.01342 Biostimulants Effect on Apple Quality and GrowthSoppelsa et al. OPEN ACCESS Edited by: Youssef Rouphael,Universita degli Studi di NapoliFederico ll, Italy Reviewed by: Riccardo Lo Bianco,Universita degli Studi di Palermo, ItalySophie Colombie,Institut National de la RechercheAgronomique (INRA), FranceLechaudel Mathieu,Centre de Cooperation Internationaleen Recherche Agronomique pour leDeveloppement (CIRAD), France *Correspondence: Carlo Andreotticarlo.andreotti@unibz.it Specialty section: This article was submitted toCrop and Product Physiology,a section of the journalFrontiers in Plant Science Received: 30 May 2018 Accepted: 24 August 2018Published: 20 September 2018 Citation: Soppelsa S, Kelderer M, Casera C,Bassi M, Robatscher P andAndreotti C (2018)Use of Biostimulants for OrganicApple Production: Effects on TreeGrowth, Yield, and Fruit Qualityat Harvest and During Storage.Front. Plant Sci. 9:1342.doi: 10.3389/fpls.2018.01342 Use of Biostimulants for OrganicApple Production: Effects on TreeGrowth, Yield, and Fruit Quality atHarvest and During Storage Sebastian Soppelsa1, Markus Kelderer2, Claudio Casera2, Michele Bassi2,Peter Robatscher?and Carlo Andreotti1* Faculty of Science and Technology, Free University of Bozen-Bolzano, Bolzano, Italy, Laimburg Research Centre, Vadena,Italy The experiment was conducted during two consecutive seasons (years 2016 and 2017)in an organic apple orchard of the cultivar Jonathan. Several biostimulants were tested(10 in total), including humic acids, macro and micro seaweed extracts, alfalfa proteinhydrolysate, amino acids alone or in combination with zinc, B-group vitamins, chitosanand a commercial product containing silicon. Treatments were performed at weeklyintervals, starting from the end of May until mid-August. The macroseaweed extractwas effective in stimulate tree growth potential in both years, as shown by a significantlylarger leaf area (+20% as compared to control) and by an higher chlorophyll contentand leaf photosynthetic rate in year 2016. As for the yield performances and applesquality traits at harvest (average fruit weight, soluble solids content, titratable acidity,and flesh firmness), they were generally affected by the different climatic conditionsthat characterized the two growing seasons (year 2017 being characterized by highermaximal and average temperatures and by limited rainfalls at the beginning of theseason). Treatments with macroseaweed extract, B-group vitamins and alfalfa proteinhydrolysate were able to significantly improve the intensity and extension of the redcoloration of apples at harvest. Correspondingly, the anthocyanin content in the skinof apples treated with the same biostimulants resulted significantly higher than control,highlighting the potential influence of these substances on the synthesis of secondarymetabolites in apple. The incidence of physiological disorders was also monitored duringapple storage period. Amino acids plus zinc application was effective in reducing (morethan 50%) the incidence of the“Jonathan spot,” the main post-harvest disorder for thiscultivar. Keywords: Malus x domestica, seaweed extract, photosynthesis, phenolic compounds, anthocyanins,physiological disorders, organic production INTRODUCTION Organic farming, including organic apple production, is generally characterized by lower cropyieldas compared with conventional production systems mainly because of the limitation imposed onfertilization (no use of chemical fertilizers) and on plant defense (no use of pesticides) (Amaranteet al., 2008; de Ponti et al., 2012; Seufert et al., 2012; Orsini et al., 2016). In order to reduce this gap of productivity, the organic agriculture sector is thereforeconstantly seeking for new agroecological practices to integratein the management of the cultivation systems. Biostimulants areconsidered among the most innovative and promising solutionsto improve sustainability and profitability of organic agriculture(Povero et al.,2016). Biostimulants are defined as “any substanceor microorganism applied to plants with the aim to enhancenutrition efficiency, abiotic stress tolerance and/or crop qualitytraits, regardless of its nutrients content" (du Jardin, 2015).The main categories of plant biostimulants include naturalsubstances such as humic and fulvic acids, protein hydrolysates,seaweed extracts (Battacharyya et al., 2015; Canellas et al., 2015;Colla et al, 2017), beneficial fungi (e.g., arbuscular mycorrhizalfungi and Trichoderma spp.) (Rouphael et al., 2015) and plantgrowth promoting rhizobacteria (Ruzzi and Aroca, 2015). Othersubstances (e.g., vitamins, chitosan and other biopolymers,inorganic compounds) can have biostimulant properties, buttheir classification within the group of biostimulants is still underconsideration. Organic farming can benefit from the use of biostimulantsbecause these substances can enhance plant resilience tothe nutrient limitation ttypical of thisSIproduction:system,therefore reducing the gap between organic and conventionalyieldss (De Pascale et al, 2017). The increase of nutrientuptake and assimilation by biostimulant substances can followdifferent mechanisms. Biostimulants such as humic substancesand protein hydrolysates can enhance nutrient availability bychanging the physico-chemical properties of soils (i.e., increasingthe cation exchange capacity of sandy soils) and by formingcomplexes with micronutrients more available to plants (Canellaset al., 2015; Colla et al., 2017). Moreover, the use of biostimulants(e.g., humic acids, protein hydrolysates, and seaweed extracts)can promote root growth and development, allowing a bettersoil exploration and consequently nutrient uptake (Battacharyyaet al., 2015; Hernandez-Herrera et al., 2016; Scaglia et al., 2016;Colla et al., 2017). The nutrient assimilation process can be alsopositively affected by biostimulants as shown by the increasedactivity of key enzymes (e.g., nitrate reductase) following theapplication of bioactive substances (protein hydrolysates andseaweed extracts) to roots and leaves (Schiavon et al., 2008;Ertani et al., 2009; Zhang et al., 2010). Despite the large andincreasing number of publications dealing with biostimulants(Colla and Rouphael, 2015), scientific-based information on theiroptimal use, crop specificity, and interaction with the growingconditions is anyway still incomplete. Studies on the effectof biostimulants on the growth and yield potential of plantshave been conducted primarily on vegetable crops. Rouphaelet al. (2017) and Ertani et al. (2015) on tomato and pepper,respectively, demonstrated how protein hydrolysates were ableto increase plant productivity, probably because of a stimulationof the plant primary metabolism trigged by signaling molecules(peptides, oligopeptides, and free amino acids) contained in thehydrolysate. This fostering effect on the primary metabolism isparticularly significant when plants are under stress conditions,as demonstrated for tomato and spinach suffering of droughtand treated with seaweed extracts (Xu and Leskovar, 2015; Goniet al., 2018). Similar studies are more complex when conducted on woody plants, also due to the role played by nutrient reservesstored in the permanent woody structure of the tree for its growthmetabolism. This could be one of the reasons explaining thecurrent lack of solid evidences connecting the use of biostimulantcompounds with the final growth and yield of fruit crops. Biostimulants have been found active in promoting finalcrop quality and, more in detail, researches had highlighted therelevance of biostimulant applications for selected functionalquality traits. The concentration of secondary metabolites suchas phenols, flavonoids, and ascorbic acid were enhanced afterthe application of protein hydrolysates or seaweed extract intomato (Rouphael et al., 2017), pepper (Ertani et al., 2015),onion, and potato (Lola-Luz et al., 2014). Giving the highantioxidant activity of phenolic compounds, the nutraceuticalvalue of those vegetables was also improved. The physiologicalmechanism behind these results is the up-regulation of genesresponsible for the secondary metabolism in plants treatedwith the biostimulants as demonstrated with the microarraystechnique on tomato (Ertani et al., 2017). Color is another cropquality trait considered as very relevant because of its tightconnection with the consumer-choice behavioral mechanism. Inthe case of apple fruits, color intensity and extension is largelyrelated to the anthocyanin biosynthesis and accumulation in skintissue (Liu et al., 2013). In order to promote apple final colorationat harvest, several cultivation techniques have been tested suchas defoliation, reflective mulches in the inter-row space prior toharvest, overhead sprinkler irrigation, and fruit bagging (Whaleet al., 2008; Blanke and Kunz, 2016). In addition, also the useof synthetic growth regulators (ethephon) to stimulate pigmentsbiosynthesis can be an option, even though not allowed by theorganic production system (Ubi, 2004). Selected biostimulantshave been found able to improve final coloration of different fruitcrops (Roussos et al., 2009; Portu et al., 2015; Blanke and Kunz,2016) probably thanks to their capacity to modulate the activityof endogenous plant hormones (Wally et al., 2013),leading to aninduction of the anthocyanin biosynthetic pathway at fruit skinlevel. Biostimulants couldalso be considered for theirimplementation in the post-harvest management of fruits.Biostimulants containing mineral nutrients such as zinc andsilicon might contribute with calcium to the strengthening ofcell wall structure (Ferguson et al., 1999), therefore allowing thepreservation of fruit quality attributes for longer period. This isof particular interest for the organic apple production systemthat is presently lacking of any useful means to manage applephysiological disorders during storage. Our field experiment was conducted in an organic appleorchardlocatediiinthe Alto-Adige/SouthTTyrolProvince,northeast of Italy. This area is the major apple-growing districtof Italy, accounting for approximately 65% of the total nationaland 10% of EU production (FAOSTAT, 2017, ISTAT, 2017).Pushed by the concern of the public opinion about the intensiveuse of pesticides in this area and by the favorable marketconditions, the organic apple production sector has gainedrelevance constantly during the last years. Today around 10% ofthe apple orchard surface in Alto-Adige/South Tyrol is cultivatedaccording to the organic protocol and about one third of all organic apples in Europe are harvested in this province (Alto-Adige/South Tyrol Province, 2017). To sustain further the growthand profitability of the organic apple sector, the implementationof new agroecological means, such as the biostimulant products,in the management of the organic horticultural systems is highlyrequested by the growers. The use of these new tools must followanyway information deriving from scientifically sound researchabout their effects on plant physiological and biochemicalresponses. With this goal, this work aimed to investigate theeffects of biostimulant applications on the growth, yield, and fruitquality of organically cultivated apple trees, belonging to the cv.Jonathan. Some of the selected substances were tested for thefirst time on apple crop and, to the best of our knowledge, thiswas the first study where the efficacy of several biostimulants wasevaluated simultaneously and during two consecutive growingseasons. In addition, their effect was considered also during thestorage period of fruits by measuring the incidence of the mainpost-harvest physiological disorder of“Jonathan"apples. MATERIALS AND METHODS Experimental Site and BiostimulantApplications The experiment was conducted over two growing seasons (years2016 and 2017) in an experimental apple orchard located atthe Laimburg Research Centre, in the municipality of Ora/Auer(46°22North; 11°17' East; 237 m a.s.l.) in Alto Adige/SouthTyrol, Italy. Meteorological conditions during the growingseasons (from April to August 2016 and 2017) are reportedin Figure 1. The 8-year-old “Jonathan"apple trees (Malusxdomestica Borkh.“Red Jonathan") were grafted on M.9 rootstock,spaced 1.0 m x 3.0 m (3,333 trees ha-), and trained to spindlesystem. The orchard received standard horticultural cares inaccordance with the regulation governing organic production. The experiment set up was organized as a completelyrandomized block design with four replications per treatmentand five trees per replicate. To avoid any contaminationbetween treatments, replicates on the same row were separatedby an interval of 10 untreated trees, whereas a buffer rowwas used to separate plots on adjacent rows. Trees wereselected according to their uniformity as for flowering andgrowth, by estimating the number of flowers per tree andmeasuring trunk circumference at 20 cm from the ground,respectively. The same set of trees:s was selected for theexperiment in both the considered growing seasons. Biostimulantapplications to the tree canopy started40o(days after fullbloom (DAFB) at the end of May and were performed atweekly intervals until the end of August(1(1 week beforeharvest). Details on the names, abbreviations, and physico-chemicalcharacteristics of the utilized biostimulants, including the wayof application are reported in Table 1 and SupplementaryTable S1. All treatments were performed with a total volume of1,500 L ha-l; each tree was sprayed until the run-off point usinga pulled sprayer under favorable weather forecast (no rainfallsexpected in the following 24h). Vegetative Growth and Leaf GasExchanges Two shoots per plant were selected at aasimilar canopyheight and position and tagged in order to measure theshoot elongation at 2-week interval,from May until growthcessation. Two fully expanded leaves from the tagged shootswere used for the indirect evaluation of the chlorophyllcontent with a SPAD-502 Chlorophyll Meter (Konica Minolta,Tokyo, Japan) and the measurements conducted at 2-weekinterval. At mid-July of both years two leaves per plant,chosen at an intermediate position along the same tagged appleshoots, were collected and leaf area was determined with aLI-COR 3000 Leaf Area Meter (LI-COR Inc., Lincoln, NE,United States). In mid-summer (28th, 30th of July and 1stof August of both years), when trees had already receivednine applications of all the treatments, net assimilation (A,umol m-2s-l) and transpiration (E, mmol m-2s-l) rates ofleaves were evaluated using a portable gas exchange analyzer(LCpro ADC, Hoddesdon Bioscientific,Ltd., United Kingdom).The gas exchange evaluations were conducted at time 0 (TO,immediately before the tenth application of biostimulants),time 1 (T1, 48 h), and time 2 (T2, 96 h) after application.Measurements were performed on a young, fully expanded leafof five randomly selected shoots per treatment and were takenunder saturating light conditions (1,800 umol photons m-2s-l),around midday (11:00-13:00 h) using a broad leaf gas chamberwith a window size of 6.25 cm² and a flow rate of 400 mlmin-1 Yield and Fruit Quality Apples were harvested on the 1st of September of both years,approximately 140 DAFB, based on the starch-iodine maturitytest. Starting from the 10th of August (approximately 20 daysprior the expected time of harvest), 10 apples per replicate(40 per treatments) were randomly sampled and evaluated fortheir starch index (Supplementary Figure S1). The harvest wasperformed when the apples reached uniformly a value of thestarch index around 2.5, which is considered the optimal pickingtime for the cv. Jonathan. Yield per tree was determined bycollecting all fruits from three out of the five trees per replicate(those located in the central position of each replicate). Fruitsfrom each tree were placed in tagged boxes in order to keeptrack of the tree they come from, and then transported inthe laboratory where they were automatically sorted with asorting machine (Aweta, Nootdorp, Netherlands). This devicedelivers the following parameters: number of fruits and totalyield per tree, average fruit weight and red overcolor extensionin percentage of apple fruit surface (four classes: <33%, 33-50%,51-75%, and >75%). The colorimetric coordinates (L*,a*, and b*) were determined with a colorimeter (Minolta,model CR-400, Tokyo, Japan) by measuring 10 fruits randomlyselected among those belonging to the same replicate at fivedifferent positions around the equatorial side of each fruit.Values are presented as color index [CI= (1000*a)/(L*b)],with higher CI value indicating a more intense red color inthe fruit (Tessmer et al., 2016). The total soluble solids (TSS FIGURE1|Meteorological conditions during the growing season 2016 and 2017 at the Laimburg Research Centre in Ora (Italy). Average temperature (dotted lines)and cumulated monthly rainfalls (columns). as Brix), titratable acidity (TA as g L-1 of malic acid) andflesh firmness (FF as kg cm-2) of 10 fruits per replicate (40per treatment) were determined at harvest with the automaticmeasuring device“Pimprenelle”(Satop Giraud Technologie,Cavillon, France). Physiological Disorder After Storage The tagged boxes containing around one hundred apples per tree(around 300 per replicate) were kept in cold room (2°C and RH85-90%) and sampled regularly (every 2 months) to monitorthe post-harvest ripening process. Moreover, the incidence ofthe physiological disorder “Jonathan spot" was evaluated duringstorage period by counting the number of symptomatic fruits perplant. “Jonathan spot"symptoms are characterized by irregularsmall black spots on the skin of apples as shown in Figure 2.Number of spots on each fruit (severity) can be very variableand, differently from those of the more studied apple bitter pit,the necrosis rarely involve cells of the inner pulp tissue of theapple. Biochemical Analysis of Apple FruitsChemicals Ethanol (96%) was obtained from J.T. Baker (Center Valley,PA, United States). Acetic acid (96%), potassium chloride, andhydrochloric acid (36%) were from Merck (Kenilworth, NJ,United States) and Fisher Chemical (Thermo Fisher Scientific,Waltham, MA, United States), respectively. Phosphoricacid (≥99%), Folin-Ciocalteu’s phenol reagent, sodiumcarbonate, 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonicacid) diammonium salt (ABTS), sodium fluoride, ascorbic acid(99%) and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were purchased from Sigma-Aldrich (St. Louis,MO, United States). Sodium acetate (anhydrous) was purchasedfrom Fisher Chemical (Thermo Fisher Scientific, Waltham,MA, United States). Potassium persulfate (K2S2Os) and gallicacid (≥99%) were purchased from Carl Roth (Karlsruhe,Germany). Methanol(HPLC-grade)waspurchased fromVR Chemicals (Milan, Italy), and meta-phosphoric acid(≥99%) and monopotassium phosphate (≥99%) were fromThermo Fisher Scientific Inc. (Waltham, MA, United States).The ultrapurewater wasprepared withhaaMilli-Q-waterpurification system (EMD Millipore Corporation, Billerica, MA,United States). Sample Preparation and Extraction Procedure Six fruits per replicate (24 per treatment) were randomly collectedat harvest. Apples were peeled and pulp and skin samples weresampled, immediately frozen in liquid nitrogen and stored at-80°C. Extraction was conducted using 25 mg of lyophilizedsample which were homogenized and extracted in 1.8 mL ofextraction solution (80% methanol acidified with H3PO4, pH1.0)and in 30 pL of 0.1 M NaF solution for 15 min at 5°C. Theextract was then filtered with PTFE filters (0.45 um, Thermo FIGURE 3|Shoot growth dynamics (from 40 to 110 DAFB) in apple plants treated with different biostimulant products and water (control) for year 2016 (A) and2017 (B). Vertical bars indicate mean ± SE, n=4. Treatments’legend: CON, control; HAL, humic acids; APH, alfalfa protein hydrolysate; SEA, macroseaweedextract; SPI, microalga hydrolysate; MAA, mix of amino acids; PHE, MAA combined with pure phenylalanine; ZIN, MAA combined with zinc; VIT, B-group vitamins;CHI, chitosan; SIL, Siliforce@. Fisher Scientific) and the filtrate was stored at -80°C for analyses.Extraction procedure for ascorbic acid analysis is described inSection“Ascorbic Acid Quantification.” Determination of Total Phenolic Content (TPC) Total phenolic content (TPC) in peel and pulp extractswas quantified using the Folin-Ciocalteu assay following themethodology described in Wolfe et al. (2003). Briefly, a 60 uL aliquot ofthe sample extract was diluted with 250 uL ofdeionizedwater. Then, 60 uL Folin-Ciocalteu reagents were added andthe mixture was allowed to react for 6 min at 20°C. Afterward,630 uL of Na2CO3 (7.5% w/v) was added and incubated for90 min at 20°C. The absorbance of samples and standards wasread at 740 nm on a spectrophotometer Cary 60 UV-Vis (AgilentTechnologies, Palo Alto, CA, United States) and the results wereexpressed as milligrams of gallic acid equivalents (GAE) per TABLE 2|Leaf area and fruit yield parameters at harvest as affected by biostimulants and growth season. TABLE 2|Leaf area and fruit yield parameters at harvest as affected by biostimulants and growth season. Leaf area (cm2) Yield (kg tree-1) Number fruits tree-1 (N.) Fruit weight (g) Fruit diameter (mm) Treatment CON 26.30±0.78 14.61±1.62 96.67十13.54 157.45±6.27 72.51±0.87 HAL 25.92±0.79 15.44±0.93 97.50±6.88 161.21±4.00 73.00±0.59 APH 28.80±1.92 13.79±0.78 86.92±4.93 161.35±4.86 73.10±0.71 SEA 32.22±0.97** 12.75±0.82 78.21±5.78 165.20±4.35 73.73±0.64 SPI 30.25±0.99 13.99±1.02 88.67±7.67 160.11±3.80 73.00±0.54 MAA 28.16±0.84 13.50±0.88 90.33±8.25 158.48±6.88 72.81±1.05 PHE 26.91 ±1.05 15.43±1.33 95.67±9.65 165.53±3.99 73.91±0.60 ZIN 30.24±1.30 13.08±0.79 81.42±5.59 163.79±5.42 73.62±0.80 VIT 29.82±1.35 12.76±0.96 76.33±5.98 169.99±4.05 74.68±0.54 CHI 27.20±0.82 13.79±1.36 88.54±10.30 160.03±4.41 73.01±0.71 SIL 27.83±1.34 15.52±1.11 100.21±10.45 159.05±5.42 72.70±0.81 Significance ** ns ns ns ns Year 2016 27.79±1.34 14.38±0.90 84.64±5.59 171.67±2.44 74.59±0.38 2017 29.24±1.16 13.74±1.24 93.62±10.35 152.36±4.18 71.97±0.68 Significance * ns *** *** * TxY ns ns ns ns ns 'Mean ± SE. Values followed by asterisk indicate significant differences between a single treatment group and control group, according to Dunnett’s test (n =4).***P<0.001;**P<0.01; *P <0.05, ns, not significant. FIGURE 4|Chlorophyll content (as SPAD values) dynamics (from 40 to 125 DAFB) in apple plants treated with different biostimulant products and water (control) foryear 2016 (A) and 2017 (B). Vertical bars indicate mean ± SE, n=4. *Indicates significant differences according to Dunnett's test. ***P<0.001; **P<0.01; *P<0.05. 100 grams of dry weight (mg GAE 100 g-lDW) referred to astandard curve of gallic acid (range 5-500 mg L-l,r²=0.999). Determination of Total Anthocyanin Content (TAC) Total anthocyanin content (TAC) in peel extracts was determinedusing the spectrophotometric pH differential methodasdescribed in Lee et al. (2005). Briefly, two dilutions of thesame sample were prepared by adding 200 pL of extractto 800 uL of potassium chloride (0.25 M, pH 1) and to800 uL of sodium acetate (0.4 M, pH 4.5), respectively. Theabsorbances were measured at 520 and 700 nm on the Cary60 UV-Vis spectrophotometer. Total anthocyanins contentwas calculated using Lambert-Beer law (8 = 26900 L/mol/cm,MW = 449.2 g/mol) as mg cyanidin 3-glucoside equivalents(CGE) per 100 g of dry weight. Antioxidant Activity (ABTS) The antioxidant activity was determined using the ABTS assayas described in Re et al. (1999) with some modifications. Briefly,ABTS radical cation (ABTS+) was generated by reacting 7 mMof ABTS with 2.45 mM of potassium persulfate (K2S2O8).The mixture was incubated in a darkroom at 4°C for 16 h.ABTS+ solution was diluted with water until the absorbancewas 0.700 ±0.02 at 734 nm. For the assay, 30 uL of sampleextract or standard were added and mixed to 1.97 mL ofdiluted ABTS+ solution. The absorbance was measured at734 nm on the Cary 60 UV-Vis spectrophotometer after 10 minin dark conditions. A calibration curve was prepared usingTrolox standard at different concentrations. The antioxidantactivity results were expressed as milligrams Trolox equivalentsper 100 grams of dry weight (mg Trolox 100 g DW)using an external calibration (Trolox, range 15.6-500 mg L-,r2=0.999). Ascorbic Acid Quantification The quantification of ascorbic acid was determined using themethodology described in Bassi et al. (2018). Briefly, an aliquot ofca. 50 mg of freeze-dried apple pulp or peel was extracted using1 mL of extraction solution [700 pL deionized H2O containing8% (v/v) acetic acid and 3%(w/v) metaphosphoric acid addedwith 300 uL of methanol](AOAC, 2005), mixed using a Vortex-Genie 2 at 3200 rpm for about 20 s at room temperature andfiltered through a 0.20 pm PTFE filter. An HPLC Agilent 1260Infinity (Santa Clara, CA, United States) with a diode array (1260DAD VL) detector was used for the analysis of the ascorbic acid.Chromatographic separation was carried out at 25°C using aKinetex 5 p C18 100 A column (150 mm × 4.6 mm, 5 umparticle size; Phenomenex, Torrance, CA, United States) and apre-column (4.6 mm, Security Card, Phenomenex, Torrance, CA,United States). The detection wavelength was fixed at 260 nm.The mobile phases used were 5 mM KH2PO4, pH 4.8 (solvent A)and methanol (solvent B). The analytical gradient was the follow:0 min, 100% A; 2.5 min, 100% A; 6 min, 80% A; 8 min, 100% A,and 13 min, 100% A. The flow rate was set at 1.0 mL min-. Thetemperature of the autosampler was 4°C and injection volumewas 5 uL. The Agilent ChemStationTM (ver. C.01.03) (AgilentTechnologies, Palo Alto, CA, United States) was used for systemcontrol and data processing. Mineral Elements in Fruit Skin At harvest of both years, six fruits per replicate (four replicatesper treatment) were randomly selected and peeled. Skin sampleswere immediately frozen in liquid nitrogen and stored at-80°C. Subsequently, samples were lyophilized, ground andhomogenized for mineral element content analyses. Nitrogen(N) content was determined by Kjeldahl method and theother macro (P, K, Ca, and Mg) and microelements (S, Fe,Cu, B, Zn, Mn Na, and Si).) were analyzed by using the FIGURE 5| Photosynthetic and transpiration rates measured in 2016 (A,B, respectively) and 2017 (C,D, respectively)as affected by biostimulant applications (TO:immediately before spray; T1: 48 h after spray; T2: 96 h after spray). Asterisk (*) and the pink rectangle in the background indicate the group of treatments thatsignificantly differed from control according to Dunnett’s test (P <0.05). inductively coupled plasma optical emission spectrometry (ICP-OES). Statistical Analysis A two-way analysis of variance (ANOVA) on the completerandomized block design was performed on the data, using thefactors “treatment”and“yearas fixed and the factor“block”asrandom. Mean separation of variables with equal variance wasperformed by the Dunnett's test, contrasting each biostimulantgroup mean against the control group mean. This procedureis recommended when working with several experimentaltreatments (Dunnett, 1985). Data expressed in percentage (classesof red overcolor extension and "Jonathan spot"incidence) werearcsine-transformed prior to the application of the ANOVA. Incase of significant interaction between the factor “treatment”and the factor “year, results were presented separately for the2 years in dedicated figures as vertical grouped bars with standarderror per treatment combination. Data gathered from repeated measurements during both seasons (shoot length, SPAD index,leaf gas exchanges, and incidence of post-harvest disorder) weresubjected to one-way ANOVA for single year, using the Dunnett’stest to compare the means at each data point. RESULTS Vegetative Growth Final shoot growth resulted significantly affected by the factor“year,2016 being generally characterized by longer shoots (40-50 cm) at the end of the growing season as compared to year 2017(25-35 cm) (Figures 3A,B). Treatments with biostimulants didnot induce any significant modification of the growth dynamicin both years (Figure 3). Leaf area was generally larger in year2017 than in 2016 (Table 2). Independently from the consideredyear, SEA applications were found able to increase the averageleafarea by around 20%.Chlorophyll content (measured as SPAD TABLE 3|Fruit quality traits (TA, titratable acidity; Cl, color index; TAC, total anthocyanin content; AA, ascorbic acid in the pulp) as affected by biostimulants and growthseason. TA(gL-1) Cl TAC (mg CGE 100 g-1 DW) AA (mg 100 g-1 DW) Treatment CON 6.16±0.23 12.54±1.51 58.07±8.37 19.56±3.86 HAL 6.86±0.20 13.91±1.95 75.05±12.79 20.49±4.33 APH 6.54±0.29 31.15±2.17*** 125.50±12.89*** 15.76±2.62 SEA 6.93±0.32 31.44±2.27*** 186.11±9.29*** 16.25±2.28 SPI 7.30±0.12 16.49±2.32 126.26±12.02*** 16.33±2.94 MAA 6.74±0.35 13.61±2.02 74.73±6.90 20.43±3.70 PHE 6.98±0.17 21.35±3.40 106.84±15.59** 19.09±3.25 ZIN 6.81±0.27 20.99±2.37 113.76±19.16** 20.12±4.26 VIT 7.14±0.38 22.67±3.76* 137.92±10.57*** 14.07±2.21 CHI 6.18±0.90 21.31±2.21 124.68±14.37*** 15.94±2.71 SIL 6.65±0.31 15.84±2.42 78.59±8.57 21.76±4.90 Significance ns *** *** ns Year 2016 7.26±0.15 20.38±2.65 126.31±13.33 25.53±2.98 2017 6.24±0.44 19.85±3.73 93.24±18.42 10.80±0.66 Significance *** ns *** *** TxY ns ns ns ns IMean ± SE. Values followed by asterisk indicate significant differences between a single treatment group and control group, according to Dunnett’s test (n=4).***P <0.001;**P<0.01; *P<0.05, ns, not significant. index) reached slightly higher values in year 2017 than in year2016 (Figures 4A,B). Treatments did not induced any significantmodification of the chlorophyll content in year 2016, whereasleaves treated with ZIN and SEA showed higher SPAD valuesthan control in year 2017 at 95 and 110 DAFB, respectively(Figure 4B). Leaf Gas Exchanges VIT, SIL and CHI showed higher leaf photosynthetic rates ascompared with control at T0 of year 2016 (Figure 5A). Forty-eight hours after the sprays (T1), leaves from all treatments (withthe exception of HAL) presented a higher photosynthetic rate(values ranging from 20 to 27 pmol m-2s-l) as compared tocontrol (15 umol m-s-1). Four day after the application ofthe biostimulants (T2), the differences in the leaf photosyntheticrates among treatments were reduced, even though APH andVIT were still significantly higher than control. Similarly to thephotosynthetic rate, leaf transpiration of treated trees resultedsignificantly higher than control in 2016, especially 48 and 96 hafter the sprays (Figure 5B). In 2017, photosynthetic rate andtranspiration presented values within the same range as in 2016,but no significant differences were detected between controlleaves and leaves previously treated with the biostimulants(Figures 5C,D). Yield and Fruit Quality Fruits per tree were less numerous in 2016 than in 2017, butof significantly higher weight (170 vs. 150 grams per fruitapproximately, Table 2). Overall, the final yield per tree did notdiffer between years, the average value being around14 kg tree.As for the effect of biostimulants on yield and fruit biometric characteristics (weight and diameter), no statistically significantdifferences were shown (Table 2). Apples were harvested at the same ripening stage in year2016 and 2017 as shown by very similar values of the averagestarch index for both seasons (Supplementary Figure S1). Asfor FF and TA, their average values were found higher in 2016than in 2017, whereas biostimulant applications were ineffectiveon these parameters (Table 3 and Figure 6A). TSS at harvestpresented values ranging from 12 and 13.5°Brix. Apples frommost of the biostimulant-treated trees (HAL, APH, SEA, SPI,MAA, PHE, ZIN, and SIL) were characterized by values of TSSapproximately 1-1.5 degree lower than control in year 2016only (no differences in 2017) (Figure 6B). Treatments withselected biostimulants had a visible and significant effect on thefinal fruit color index, independently from the considered year(Figure 7). More in detail, apples previously treated with theSEA, APH, and, to a less extent, by VIT, were characterizedby a most intense red over coloration and presented valuesof the color index that were significantly higher than control(Table 3). Red overcolor was significantly more pronouncedin 2017 than in 2016 with more than 50% of the applesbelonging to the most colored class (>75%) (Figures 8A,B).Among the tested biostimulants, SEA was significantly effectivein enhancing the percentage of fruits presenting more than 75%overcoloration (+87% and +50% as compared to control in 2016and 2017,respectively). As for the other treatments, APH andZIN slightly improved red overcolor of fruits, even though notsignificantly. Total phenolic content (TPC) evaluated at skin level wassignificantly affected by both factors ("treatment’and"year") andby their interaction. The total amount was significantly higher in FIGURE 6|Effect of the biostimulants on flesh firmness- FF (A) and total soluble solids- TSS (B) compared with control for year 2016 and 2017. Vertical barsindicate mean ± SE, n =4. *Indicates significant differences according to Dunnett's test. ***P <0.001; **P<0.01;*P<0.05. FIGURE 7|Apple color at harvest as affected by treatment applications (CON, control; APH, alfalfa protein hydrolysate; SEA, macroseaweed extract; VIT, B-group vitamins; ZIN, zinc plus amino acids). 2017 than in 2016 with average concentrations of around 2,400and 1,800 mg GAE 100 g-DW, respectively. Treatments withSEA, SPI, and ZIN significantly enhanced TPC in apple skin atharvest in both years, whereas CHI and VIT were effective in 2016only (Figure 9A). TPC at apple pulp level was also higher in 2017than in 2016, without any significant effect of the treatments inboth years (Figure 9B). TAC of apple skin was higher in year 2016as compared to year 2017 (Table 3). TAC was also significantlyenhanced by selected biostimulants, independently from theconsidered year (no significant interaction “T×Y"). More indetail, in apples treated with APH, SEA, SPI, VIT, and CHI thefinal anthocyanin concentration was more than the double ofthat of control. The antioxidant potential (ABTS) of apple skin andpulp tissues was linked to the phenolic content of these tissues.Similarly to the TPC parameter, ABTS was significantly higherin 2017 with values that were almost double than those of theyear 2016 in both skin and pulp (Figures 10A,B). Treatmentswith SEA and VIT were able to significantly increase ABTS atskin level in both years, whereas other biostimulants (PHE, CHI,SPI, and ZIN) enhanced the antioxidant potential of apple skinin only one of the two considered years (Figure 10A). Similarlyto the skin tissue, pulp antioxidant potential was significantlyaffected by the factors “treatment,“year"and their interaction.Pulp antioxidant potential was enhanced by treatments in 2016only (Figure 10B), with CHI and SIL among the most effectivebiostimulants in promoting this feature. Treatments did notaffect the final ascorbic acid concentration in both apple skin andpulp (Figure 11 and Table 3 for skin and pulp, respectively). Thefactor“year"was the only significantly relevant for final ascorbicacid accumulation, year 2016 showing approximately a twofoldconcentration in both skin and pulp tissues as compared to 2017. Incidence of Physiological Disorder AfterCold Storage “Jonathan spotincidence was significantly higher in 2017 apples,when around 20% of the fruits collected from each considered A FIGURE 8|Percentage of fruits according to red overcolor extension classes at harvest 2016 (A) and 2017 (B), n=4. *Indicates significant differences according toDunnett's test. ***P <0.001; **P<0.01;*P<0.05. A5000 FIGURE 9 |Effect of the biostimulants on total phenolic content (TPC) in apple skin (A) and pulp (B) compared with control for year 2016 and 2017. Vertical barsindicate mean ± SE, n=4.*Indicates significant differences according to Dunnett's test. ***P<0.001;**P<0.01; *P<0.05. tree were symptomatic after 4 months (Figures 12A,B). In2016, selected biostimulants (SPI, CHI, SIL, and SEA) wereable to significantly reduce the incidence of the “Jonathan spot"disorder during different phases of the post-harvest of apples(+21 and +60 days from harvest). At the end of the storageperiod (4 months after harvest) apples treated with ZIN werethe only ones showing a significantly lower incidence of thedisorder, with a reduction of approximately 60% as compare tocontrol (Figure 12A). In 2017 biostimulant applications werenot as effective as in 2016. ZIN, SIL, and SPI slightly reduced the disorder incidence at the end of the 4 months storage eventhough differences with control were not statistically significant(Figure 12B). DISCUSSION The vegetative growth behavior of apple trees was mainlydetermined by the seasonal climatic conditions. Despite treeswere drip irrigated during both seasons, warmer meteorological A FIGURE 10|Effect of the biostimulants on antioxidant activity (as ABTS) in apple skin (A) and pulp (B) compared with control for year 2016 and 2017. Vertical barsindicate mean ± SE, n=4. *Indicates significant differences according to Dunnett’s test. ***P<0.001;**P<0.01; *P<0.05. FIGURE 11|Effect of the biostimulants on ascorbic acid (AA) level in apple skin compared with control for year 2016 and 2017. Vertical bars indicate mean ± SE,n =4. *Indicates significant differences according to Dunnett's test. ***P<0.001;**P<0.01;*P<0.05. conditions during May-July 2017 (high maximal and averagetemperatures, limited rainfalls in May, Figure 1) might havedetermined a stressful status for trees, which then reducedshoot growth (Figure 3). In such conditions, trees treated withSEA were characterized by similar shoot growth but larger leafarea as compared to untreated trees (Figure 3 and Table 2).Seaweed extracts were found able to promote shoot length inapple cv. Fuji (Spinelli et al., 2009) and to contrast drought stress effects on vegetable crops such as tomato and spinach byenhancing their growth and foliar density (Xu and Leskovar,2015; Goni et al., 2018). Another effect of SEA application was thehigher concentration of chlorophyll (measured with the SPADindex) shown by treated leaves as compared to the untreatedones in 2017 (Figure 4B). This result is in agreement withthe outcomes of other experiments conducted with seaweedson apple (Spinelli et al., 2009) and on other crops such as FIGURE 12|“Jonathan spot" incidence during apple storage in 2016 (A) and 2017 (B), n = 4. *Indicates significant differences according to Dunnett's test.***P<0.001;**P<0.01;*P<0.05. grapevine (Sabir et al., 2014), cabbage (Rengasamy et al., 2016),and tomato (Goni et al, 2018). This represents therefore afurther evidence of a possible role of seaweed extracts inthe reduction of chlorophyll degradation and in delaying leafsenescence (Battacharyya et al., 2015). As for the gas exchangesat leaf level, the first measurement was conducted when treeshad already received nine biostimulant applications (every weekstarting from 40 DAFB). This might explain the higher A and Erates shown by the biostimulant-treated leaves before (T0) andafter (T1 and T2) the 10th spray in year 2016 (Figures 5A,B).Photosynthetic and transpiration rates did not differ betweenbiostimulants and control in 2017 (Figures 5C,D). Moreover,despite the higher values of chlorophyll concentration detectedin SEA-treated leaves at the end of the 2017 season, theirphotosynthetic activity was not enhanced on that year. Theseresults are partially different from those reported by Spinelliet al. (2009) on “Fuji”apple trees, where a consistent increaseof chlorophyll content and photosynthetic activity was detectedafter the application of a commercial seaweed extract. Beside thedifferent conditions that could have characterized the plants atthe time of the measurements (leaf temperature, leaf water status,stomatal conductance, etc.), also the different methodology usedfor the analysis of the gas exchanges (punctual measurements atleaf level vs. continuous measurements at whole canopy level)might explain this partial inconsistency. In addition, accordingto values reported in the literature (Jakopic et al.,2007), thechlorophyll content was above the sufficiency threshold forapple leaves, leaving the photosynthetic rate more dependentfrom other environmental or endogenous factors. The onlybiostimulant showing a rather consistent effect on photosyntheticrate was the B-group vitamins, which was able to increasethe photosynthetic potential of treated leaves in both years(Figures 5A,C). The use of single vitamin B1 (thiamine) wasfound ineffective on photosynthetic rate of rice leaves (Bahugunaet al., 2012); comparison with the outcome of the present research is anyway difficult giving that a complex mix of vitamins(including B1, B2, and B6) and not the single thiamine was usedin our study. At harvest, no difference was found on tree productivity inboth years (Table 2). This outcome partially differs from anotherresearch performed on apple (Spinelli et al., 2009) where theuse of a similar seaweed extract (from Ascophyllum nodosum)was found able to induce a higher final yield in a year of lowcrop load. The protein hydrolysate from alfalfa resulted alsoineffective on final yield (Table 2). Differently, other studiesconducted on vegetable crops (Rouphael et al., 2017 and Poloand Mata, 2018 on tomato; Ertani et al., 2015 on hot pepper)showed an increment of plant productivity, probably as the resultof a stimulation mechanism of the plant primary metabolismtriggered by signaling molecules (peptides, oligopeptides, andfree amino acids) contained in the hydrolysate. The effect of the biostimulants on primary apple qualitytraits (FF, TSS, and TA) was limited (Figure 6 and Table3).Only in 2016, the fruit TSS was found lower in treated apples(Figure 6B). According to the available literature, biostimulantscan have different effect on final sugar accumulation in fruits.Protein hydrolysate-based substances were found able to enhancefinal sugar content in hot pepper and tomato (Ertani et al.,2015; Rouphael et al., 2017). Differently, seaweed extracts didnot changed or slightly reduced final Brix value in strawberryfruits (Roussos et al., 2009) and grapevine berries (Frioni et al.,2018). In our conditions, the lower TSS could be the result ofan internal trade-off at fruit level for carbon skeleton betweensugars and secondary metabolites (i.e., phenolic compounds) thatmight had occurred in year 2016, as also suggested by the worksof Lux-Endrich et al. (2000) and Ruhmann et al. (2002) on apple. One of the major effect of treatments application was thesignificant change in final apple coloration obtained with SEA,APH, and VIT. This outcome was confirmed by the colorimetriccoordinates (color index), by the total anthocyanin concentration measured on fruit samples taken from different replications andby the evaluation of the red overcolor extension performed onall fruits harvested from all the considered trees under evaluation(Table 3 and Figures 7, 8). These results confirm those describedby Malaguti et al. (2002) on apple “Gala"and those by Frioniet al. (2018) on red grapevine cultivars evaluated in differentcultivation areas. The boosted final red over color of applesmight be ascribed to a modulation of the metabolism of plantendogenous growth regulators (mainly cytochins and abscisicacid) obtained with the application ofthe biostimulant substances(i.e., A. nodosum; Wally et al., 2013), leading to an induction ofanthocyanin biosynthesis and accumulation in fruit skin prior toharvest (Table 3). Another relevant effect of the biostimulant applications wasthe higher concentration of phenolic compounds detected in theskin tissue of apples treated with SEA, SPI, and ZIN in the 2 years(Figure 9A). Since no difference was found for the ascorbic acidconcentration, this increase in phenolic compounds was likelyresponsible for the higher ABTS values showed by skin samplesof fruits previously treated with the biostimulants mentionedabove (Figure 10A). Phenolic compounds are biologically activemetabolites showing antioxidant potential (Rice-Evans et al.,1997) and therefore highly considered as health-promotingsubstances in fruits (Slavin and Lloyd, 2012). Similar health-promoting responses were found in onion and potato afterthe application of A. nodosum (Lola-Luz et al., 2014). Proteinhydrolysates of different origin (legume and alfalfa) promotedthe antioxidant capacity of tomato (Rouphael et al., 2017) andgreen or red pepper (Ertani et al., 2015) similarly to what wasdetected for the apple pulp in our study (Figure 10B). It has beenshown that the protein hydrolysate mode of action involves theup-regulation of a number of genes responsible for the secondarymetabolism of plants leading to the synthesis and accumulationof phenolics and terpenes which are responsible for the enhancedantioxidant activity and for the increased tolerance to biotic andabiotic stresses (Ertani et al., 2017). Moreover apple treated withAPH were also characterized by a higher total anthocyanin and alower nitrogen content at skin level (Table 3 and SupplementaryTable S2). A negative correlation between the concentrationof nitrogen and the concentration of anthocyanin and totalflavonoids was often found when measured in apple skin tissue(Awad and de Jager, 2002). Low nutrients concentration at skinlevel are often positive for the final coloration of fruits, probablyas a result of the internal trade-off between the synthesis ofsecondary substances and the growth primary metabolism as alsodescribed by Veberic (2016). Higher phenolic concentration andantioxidant activity were also detected in apple skin after B-groupvitamins application (Figures 9A, 10A). Similar results wereobtained with vitamin B1 (thiamine) on grapevine. It was foundthat thiamine was able to elicit different genes belonging to thephenylpropanoid pathway (including phenylalanine ammonialyase, chalcone synthase, and stilbene synthase) leading to ahigher accumulation of secondary metabolites and antioxidantactivity with positive effect on tolerance to downy mildew ingrapevine (Boubakri et al., 2013). Zinc applications also inducedphenolic compounds accumulation and antioxidant potential inapple (Figures 9A, 10A,B). This result partially conflicts with those of Aglar et al. (2016) which described a negative effectof the application of zinc on both phenols and antioxidantpotential in apple. Studies on the role of zinc on the applephenolic metabolism are currently not available in the literature.An interpretation of this conflicting result could consider thedifferent product formulation used in the present study (a mixof zinc and amino acids), whereas it was zinc sulfate alone in thecase of Aglar et al.(2016). Moreover, differences in the cultivarsstudied and in the experimental conditions could also have playeda role on the outcomes of the experiments. Anyhow, the roleof genotype in plant response to zinc treatments seems to be ofrelevance, considering that the phenolic accumulation after zincsulfate application was found enhanced in grapevine (Song et al.,2015) and in aromatic herbs extracts such as dill and anise, butreduced in other species such as fennel (Majdoub et al., 2017). Nutrient concentration at the skin tissue level has oftenbeen linked to the incidence of physiological disorders in apple(Amarante et al., 2013; Baugher et al., 2017). In our experiment,the application of zinc (in combination with a mix of aminoacids) was found effective in lowering the incidence of thephysiological disorder of apple during storage (Figure 12). Thiseffect was consistent in both years, even though incidencepercentage was significantly higher in 2017 probably because ofthe higher rainfalls that characterized the month of July andAugust of that year. Deficit in nutrient concentration in fruitskin (calcium in particular) as well as adverse meteorologicalconditions are commonly associated with post-harvest disorders(Ferguson et al., 1999). The role of other mineral nutrients(such as zinc and silicon) for fruit storability is anyway stillunclear. Pais and Petho (1970) found that foliar applicationsof calcium plus zinc retarded the development of post-harvestdisorder in apple cv. Jonathan. Zinc applications at 10-dayintervals from 8 weeks after full bloom to harvest were foundto reduce loss of mechanical properties of apples after storage(Johnson and Dover, 2002). The authors explained this result asa possible indirect effect of zinc applications on the enhancedcalcium concentration of fruits, which likely had a positiveconsequence on apple storability. In our study, apples treatedwith zinc in the form of zinc + amino acids were foundsignificantly richer in zinc and showed a tendency to highercalcium concentration (Supplementary Tables S2, S3). Boththese elements may have contributed to strengthen the middlelamella and primary cell wall structure (Henriques et al., 2012;Guerriero et al., 2016), therefore contrasting the development ofthe post-harvest disorder “Jonathan spot"during storage period. CONCLUSION This study is the first comprehensive investigation of the use ofdifferent classes of biostimulants on organic apple productionover a period of two consecutive years. The results of thestudy indicate that products based on alfalfa protein hydrolysate,seaweed extracts and B-group vitamins improved final redcoloration of apple“Jonathan"in both years, therefore enhancingtheir market potential. Moreover, the same biostimulants hada positive effect on fruit functional traits as shown by the higher phenolic compounds concentration, total anthocyaninand antioxidant potential of treated apples. Taken together, thesefindings suggest a role for selected biostimulants in promotingthe secondary metabolism of treated plants, leading to animprovement of fruit quality,appearance, and nutritional value.The research has also shown that biostimulants containingzinc are effective in reducing the incidence of physiologicaldisorders in cold stored apples, strengthening the idea of apositive role of this element on the structure and resistanceof cell walls and membranes at fruit level. Giving the currentlack of effective means for the post-harvest management oforganic fruits, this finding might have significant implicationon the practices presently used for organic apple conservationand commercialization. As for the fine tuning of the useof biostimulants under orchard conditions, further researchneeds to be done to deepen our understanding on the way ofapplication of the available products (number of applications,period, and concentrations) for the different apple cultivars onthe market. AUTHOR CONTRIBUTIONS SS contributed in the set-up of the experimental protocol,performed the agronomic study, processed the fruit samples,analyzed the data, and participated to the writing of the paper.MK contributed to the set-up of the experiment and to theinterpretation of the results. CC contributed to the agronomicimplementation of the study. MB contributed to the analysis ofphenols, anthocyanins, antioxidant potential, and ascorbic acid. REFERENCES ( Aglar, E., Yildiz , K. , Ozkan, Y., Ozturk, B., and Erdem, H . (2016). T he e f fects of a minoethoxyvinylglycine an d foliar zi n c treatments on pre - harvest dropsand fruit q uality attributes of jersey m a c ap p les. Sc i . Ho r tic. 21 3 , 173 - 178. doi: 10.1016/j.scienta.2016.10.026 ) ( Alto-Adige/South Tyrol Province (2017) . WWW Document. Ava i lable at : http://www.provincia.bz.it/agricoltura-foreste/agricoltura/relazione-agraria- forestale.asp [accessed August 03.2018]. ) ( Amarante, C . V . T. , do St e ffens, C . A. , M a f ra, AL . , an d Alb u querque, J. A . (2008). Yield and f ruit qualit y of apple from conventional and o rganicproduction s ystems. P esqui. Agropecudria B ras 4 3, 3 33-340. doi: 1 0 .1590/S0100-204X2008000300007 ) ( Amarante, C. V. T. , Silveira, J. P. G., Steffens, C. A., Paes, F . N. , and Argenta,L. C. (2013). Tissue s ampling method and mine r al attributes to predict bitterpit occurrence i n a p ple fr u it: a multivariate ap p roach. Acta Hort i c. 1012 , 1 133-113 9 . doi: 10.17660/ActaHortic.2013.1012.153 ) ( AOAC (2005).“V i tamin C i n Jui c es and Vitamin Pre p arations, in Pro c eedings ofthe 18th AOAC Official Methods of Analysis (Gaithersburg, MD: Association ofOfficial Analytical Chemists). ) ( Awad, M . A., and de J ager, A. (20 0 2). Rel a tionships between fruit nutr i ents and 1c c oncentrations o f f l avonoids and chlorogenic c a cid in Elstar'appl e s kin. Sci. Hortic . 9 2,265-276 doi: 1 10.1016/S0304-4238(01)00 290-4 ) ( Bahuguna,R. N.,Joshi, R., Shukla,A., Pandey, M., and Kumar,J.(2012). Thiamine primed defense provides r e liable alternative to systemic fungicide carbendazim against sheath b light disease in rice (Oryza sativa L.). Plant Physiol . Biochem.57, 159- 1 67. doi: 1 0.1016/j.plaphy.2012.05.003 ) ( B assi, M ., Lubes, G., Bi a nchi, F., Agnolet, S., C iesa, F., Brunner, K., et al. (2018 ) .Ascorbic acid content in apple pulp, peel, and monovarietal cl o udy juices of 64 ) PR contributed to the set-up of the analytical work related to thequality indexes ofthe fruits. CA coordinated the research, workedon the statistical analysis, and contributed to the writing of severalsections of the manuscript. FUNDING The research was co-founded by the Free University of Bozen-Bolzano (Project BIO_TOOL TN 1B07), Laimburg ResearchCentre, and ILSA S.p.A. Laimburg Research Centre was fundedby the Autonomous Province of Bolzano, financial supportfor the “Technology Park” (Decision no. 1472, 07.10.2013) isgratefully acknowledged. This work was also supported by theOpen Access Publishing Fund provided by the Free Universityof Bozen-Bolzano. SS was funded by a Ph.D. grant from the FreeUniversity of Bozen-Bolzano. ACKNOWLEDGMENTS The authors would like to thank Dr. Aldo Matteazzi for technicalassistance. SUPPLEMENTARY MATERIAL The Supplementary Material for this article can be found onlineat: https://www.frontiersin.org/articles/10.3389/fpls.2018.01342/full#supplementary-material different cultivars. Int. J. Food Prop. 20, S2626-S2634. doi: 10.1080/10942912.2017.1381705 Battacharyya, D., Babgohari, M. Z., Rathor, P., and Prithiviraj, B. (2015). Seaweedextracts as biostimulants in horticulture. Sci. Hortic. 196,39-48. doi: 10.1016/j.scienta.2015.09.012 Baugher, T. A., Marini, R., Schupp, J. R., and Watkins, C. B. (2017). Prediction ofbitter pit in Honeycrisp apples and best management implications. HortScience52,1368-1374.doi: 10.21273/HORTSCI12266-17 Blanke, M.. M., and Kunz, A..((2016). Alternatives to phosphonates forfruit colouration. Sci. Hortic. 198, 434-437. doi: 10.1016/j.scienta.2015.12.019 Boubakri, H., Poutaraud, A., Wahab, M. A., Clayeux, C., Baltenweck-Guyot, R., Steyer, D., et al. (2013). Thiamine modulates metabolism ofthe phenylpropanoid pathway leading to enhanced resistance to Plasmoparaviticola in grapevine. BMC Plant Biol. 13:31. doi: 10.1186/1471-2229-13-31 Canellas, L. P., Olivares, F. L., Aguiar, N. O.,Jones, D. L., Nebbioso, A., Mazzei, P.,et al. (2015). Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic.196, 15-27. doi: 10.1016/j.scienta.2015.09.013 ( Colla, G ., H oagland, L. , Ruzzi, M. , Cardarelli, M., Bonini, P., Canaguier, R., e t al.(2017). Biostimulant action of protein h y drolysates: unraveling their effects onplant p hysiology and m i crobiome. Front. Pl a nt Sci.8:2 2 02. doi : 10. 3 389/fpls.2017.02202 ) Colla, G., and Rouphael, Y. (2015). Biostimulants in horticulture. Sci. Hortic. 196,1-2. doi: 10.1016/j.scienta.2015.10.044 ( De Pascale, S., Rouphael, Y., and Colla, G. (2017). Plant biostimulants: innovativetool for enhancing plant nutrition i n o rganic farming. Eur. J . H ortic. S ci. 82, 277-285. doi: 10.17660/eJHS.2017/82.6.2 ) de Ponti, T., Rijk, B., and van Ittersum,M. K. (2012). The crop yield gap betweenorganic and conventional agriculture. Agric. Syst. 108, 1-9. doi: 10.1016/j.agsy.2011.12.004 ( du Jardin, P . (2015). Plant biostimulants: definition, concept, m ain categories andregulation. S ci. Hortic. 196, 3-14 . doi: 1 0.1016/j.scienta.2015.09.021 ) ( Dunnett, C . W. ( 1985). “ Multiple Comparisons between s e veral t reatments anda specified treatment, i n L i near St a tistical I n ference, eds T. C a linski and W. Kloneck i (New Y o rk, N Y : Springer), 39-47. ) ( Ertani, A ., Cavani, L. , P izzeghello, D . , B r andellero, E. , Altissimo, A. , Ci a vatta, C. , et al.(2009). Biostimulant activity of two p r otein hydrolyzates in t he growth andnitrogen m etabolism of maize se e dlings. J. Plant N u tr. Soil Sci. 172, 237- 2 44. doi: 10.1002/jpln.200800174 ) ( Ertani, A., Sambo, P . , Nicoletto, C., S antagata, S., Schiavon, M., and Na r di, S . (2015). The use of organic biostimulants in hot pepper plants to help low input sustainable agriculture. Chem. B i ol. Technol. A g ric. 2:11. doi: 1 0.1186/s40538- 015-0039-z ) ( E rtani, A., Schiavon, M., and Nardi, S. ( 2017). Transcriptome-wide identification of differentiall y expresse d genes in Solanum lycopersicon L . i n response t o an alfalfa-protein hydrolysate u sing microarrays. F r ont. P l ant Sci. 8 : 1159. d o i: 10.3389/fpls.2017.01159 ) ( FAOSTAT. (2017). WWW Document. Available at:http://www.fao.org/faostat/en/#data/QC [ a ccessed August 03 2018]. ) ( Ferguson, I , V olz, R ., and W o olf, A . (1 9 99). P r e harvest f a ctors a f fecting physiological disorders of f ruit. Postharvest Biol . Technol. 15, 2 55-262. doi: 10.1016/S0925-5214(98)00089-1 ) ( Frioni, T ., S abbatini, P., Tombesi, S., No r rie, J, P on i, S., G att i , M., e t al . (2018). Effects o f a biostimulant derived from the brown seaweed Ascophyllum nodosum on ripening dynamics and fruit quality of grapevines. Sci. Hortic. 232, 97- 1 06. doi: 1 0.1016/j.scienta.2017.12.054 ) ( Goni, O ., Q uille, P ., and O 'Connell, S. ( 2 018). A s cophyllum no d osum extr a ctbiostimulants and t heir r ole in enhancing t o lerance t o drought s t ress i ntomato plants.P l ant P h ysiol. Biochem. 1 2 6, 63-7 3 . doi: 1 0 .1016/j.plaphy.2018.02.024 ) ( Guerriero, G., Hausman, J . -F., and L egay, S . ( 2016). S ilicon and t he plant extracellular matrix. Front. P lant Sci 7:463. doi: 10.3389/fpls.2016.00463 ) ( Henriques, A. R . , Chalfun-Junior, A . , and A a rts, M. (2012). Strategies to increase zinc deficiency tolerance and homeostasis in p l an t s. Braz . J. Plant Physiol. 24, 3-8. doi: 10.3389/fpls.2013.00144 ) ( Hernandez-Herrera, R. M ., S antacruz- R uvalcaba, F ., Zanudo-Hernandez, J ., and H ernandez-Carmona, G . (2 0 16). Activity o f s e aweed e x tracts a ndpolysaccharide-enriched e xtracts from U lva lactuca and P a dina gymnosporaas growth p romoters o f tomato a n d mung bean plants. J. Appl. P h ycol. 2 8 , 2549- 2 560. doi: 10.1007/s108 1 1-015-0781-4 ) ( ISTAT. ( 2017). WWW Docu m ent. Avail a ble at: h t tps : //www.istat.it (ac c essed August03.2018) ) ( Jakopic,J., Veberic, R ., Zupancic, K., and Stampar,F. (2007). Influence of nitrogen on t he contents of carbohydrates and organic acids in a pples ( Malus domestica Borkh.) cv.Golden Deli c ious’. Eur. J. Hortic. Sci. 72:66. ) ( J ohnson, D. S . , a nd Dover, C . J. (2002). T h e effect o f calcium and z i nc s p rays on t he t exture of “Cox’s Orange Pippin”apples i n c ontrolled atmospherestorage. Acta H orticulturae 5 94, 4 27-433. d o i: 1 0 .17660/ActaHortic.2002.594.55 ) ( Lee,J., Durst,R . W.,and Wrolstad,R. E. (2005). Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, andwines by t h e p H di f ferential m e thod: collaborative study. J. AO A C Int. 88,1269-1278. ) ( Liu, Y ., Che, F ., Wang, L . , M eng, R., Zhang, X., an d Zhao, Z. (2013). Frui t coloration and a nthocyanin b i osynthesis a f ter bag removal in non-red and red apples (Malus x d omestica Borkh.). Molecules 18, 1549-1563. doi: 1 0 .3390/ molecules18021549 ) ( Lola- L uz, T., Hennequart, F., and Gaffney, M. (2014). Effe c t on health promotingphytochemicals foll o wing s e aweed ap p lication, in potato an d oni o n cropsgrown u n der a lo w in p ut agricultural system. Sci. H o rtic. 170, 2 2 4-2 2 7. d oi: 1 0.1016/j.scienta.2014.03.022 ) ( Lux-Endrich, A . , T r eutter, D., and F e ucht, W. (20 0 0). Influence of n utrientsand carbohydrate supply on t he p henol c omposition of apple shoot cultures. Plant Cell Tissue Organ Cult. 60, 15-21. doi: 10.1023/A:100640652 7242 ) ( M ajdoub, N., e l -Guendouz, S., Re z gui, M. , Carlier, J. , Costa, C . , K a ab, L . B . B . , et al . (2017). Growth, photosynthetic pigments, phenolic content and biological activities of Foeniculum v ulgare M ill., Anethum g r aveolens L . a n d Pimpinella ) ( anisum L.( A piaceae) i n response t o zinc. Ind. Crop Prod 1 0 9, 627-636. doi: 1 0.1016/j.indcrop.2017.09.012 ) ( Malaguti, D., Rombola, A . D., Gerin, M. , Simoni, G., Tag l iavini, M. , andMarangoni, B. ( 2 002). E ffect of seaweed e xtracts-based leaf sprays on themineral status, yield and fruit quality of apple. Acta H orticu l turae 594, 357-359.doi: 10.17660/ActaH o rtic.2002.594.44 ) ( Orsini, F., Maggio, A. , Rouphael, Y ., and D e P a scale, S. (2016).“Physiological quality" o f o rganically grown vegetables. Sci. Hortic. 208,131-139. doi: 10.1016/ i .scienta.2016.01.033 ) ( Pais, L., and Petho, F . (1970).Investigations on causes of J onathan spot. I . Szolo Gyumolcstermesztes 6, 31-42. ) ( Polo, J ., and M ata, P. (2018). E v aluation of a bi o stimulant (pe p ton) based in enzymatic hydrolyzed a n imal protein in comparison to s e aweed extracts on root development, vegetative g r owth, flowering, and yield of gold cherry tomatoes grown u n der low stress a m bient f i eld conditions. F ront. Plant Sci 8:2261 doi: 10.3389/fpls.2017.02261 ) ( Portu, J ., Lopez-Alfaro, I ., Gomez-Alonso, S. , L o pez, R. , and Ga r de-Cerdan, T. (2015). Changes o n grape phenolic composition indu c ed by gr a pevine foliar applications of phenylalanine and urea. Food Chem. 180,171-180. doi: 10.1016/ j .foodchem.2015.02.042 ) ( P overo, G., Mejia, J. F ., Di T ommaso, D., Piaggesi, A. , an d Warrior, P. ( 2 016). A systematic approach t o discover and c h aracterize natural plant biostimulants.Front. P lant Sci 7:435. doi: 10.3389/fpls.2016.00435 ) ( Re, R., Pellegrini , N. , Proteggente , A., Pannala , A., Yang, M. , and Rice-Evans, C. (1999). Antioxidant activit y applying an improved A BTS ra d ical c a tion decolorization assay. Free Radic. B iol. Med. 2 6, 1 231- 1 237. d o i: 1 0 .1016/S0891- 5849(98)00315-3 ) ( Rengasamy, K. R. R., K ulkarni, M. G., Pa p enfus, H. B., and Van Staden, J. (2016). Quantification of p lant growth b i ostimulants, phloroglucinol and eckol, in fo u r commercial seaweed liq u id fer t ilizers a n d some by-products. Alga l Res. 20, 5 7-60. doi: 10.1016/j.algal.2016.09.017 ) ( Rice-Evans, C ., Miller, N., and P aganga, G . (1997). Antioxidant p r operties o f phenolic c ompounds. T r ends Pl a nt Sc i . 2 , 1 52 -159. do i : 10.1016/S1360- 1385(97)01018 - 2 ) ( R ouphael, Y., C olla, G. , Giordano, M .,El-Nakhel, C., K y riacou, M. C. , and De P ascale, S. (2017). Foliar applications of a legume-derived p r otein hydrolysate elicit dose-dependent increases of growth, leaf mineral co m position, yield and f ruit quality in two greenhouse tomato cultivars. Sci. H o rtic. 2 2 6, 3 5 3-360. doi:10.1016/j.scienta.2017 . 09.007 ) ( Rouphael, Y ., F ranken, P ., S chneider, C . , Schwarz, D ., G iovannetti, M., Agnolucci,M., et al. (2015). Arbuscular mycorrhizal fungi act as biostimulants i n h orticultural c rops. S ci. Hortic. 1 96, 9 1- 1 08. d o i: 1 0 .1016/j.scienta.2015. 09.002 ) ( Roussos, P. A., Denaxa, N.-K., and Damvakaris,T.(2 0 09). Strawberry fruit quality attributes after application of plant growth stimulating compounds. Sci. H o rtic. 119, 138-1 4 6. doi: 10.1016/j.scienta.2008.07.021 ) ( Ruhmann,S., Leser,C., Bannert, M., and Treutter,D. (2002). Relationship between growth, seconda r y metabolism, an d res i stance of apple. Pl a nt Biol. 4, 137-143. doi: 10.1007/s00203-016 - 1207-7 ) ( Ruzzi, M., and A roca, R . ( 2015). Plant growth-promoting rhizobacteria act as biostimulants i n h o rticulture. S c i. H orti c . 1 9 6, 1 2 4-134. do i : 10.1016/j.scienta.2015.08.042 ) ( Sabir, A ., Yazar, K ., Sabir, F., Kara, Z ., Yazici, M. A ., and Goksu, N. (2014). Vine growth, yield, b erry quality attributes a nd l e af nutrient content of grapevinesas i nfluenced by seaweed e xtract (Ascophyllum nodosum) a nd n a nosize f ertilizer pulverizations. Sci. H ortic. 1 7 5,1-8 . doi : 10.1 0 16/j.scienta.2014.05.021 ) ( Scaglia, B., Nunes, R. R., Rezende, M . O. O., Tambone, F., and Ad a ni, F. (2016). Investigating organic molecules r esponsible o f auxin- l ike activity of humic a cid f raction extracted from v ermicompost. Sci. T o tal En v iron. 562, 2 8 9-295. doi:10. 1 016/j.scitotenv.2016.03.212 ) ( Schiavon, M ., E r tani, A . , a n d Na r di, S. ( 20 0 8). Effec t s of an a l fa l fa prot e inhydrolysate on the gene expression and activity of enzymes of t he tricarboxylicacid (TCA) cycle and nitrogen metabolism in Zea mays L. J. Agric. Food Chem. 5 6,11800-1 1 808.doi: 10.1021/jf802362g ) ( Seufert, V., Ramankutty, N ., a nd Foley, J. A. ( 2012). Comparing the yields o f organic a nd c o nventional ag r iculture. N a ture 48 5 , 22 9 -232. doi : 10.1 0 38/nature11069 ) Slavin,J. L., and Lloyd, B. (2012). Health benefits of fruits and vegetables. Adv. Nutr.3,506-516.doi: 10.3945/an.112.002154 ( S ong,J.,Du, L. , L i , L., Kalt, W., Palmer, L . C., Fillmore, S.,et al . (2015). Quantitativechanges in proteins r esponsible f or flavonoid and anthocyanin biosynthesis instrawberry fruit at different ripening stages: a targeted quantitative proteomicinvestigation employing multiple reaction m onitoring.J. Proteomics 1 2 2, 1 - 10. d oi: 1 0. 1 016/j.jprot.2015.03.017 ) ( Spinelli, F., Fiori , G. , Noferini, M., Sprocatti, M. , and Costa,G. (2009). Perspectiveson the use of a s eaweed extract t o moderate the negative effects of alternate b earing i n apple trees. J . H ortic. S ci. Biotechnol. 84, 1 31-13 7 . d o i: 1 0. 10 8 0/ 14620316.2009.11512610 ) ( T essmer, M. A.,Appezzato-da-Gloria, B., and Antoniolli, L . R. (2016). I nfluence of g rowing sites a n d physicochemical fea t ures on the i nci d ence of le n ti c el breakdown inGala and Galaxy apples. Sci. Hortic. 205, 1 19- 1 26. doi: 10.1016/ j . scienta.2016.04.027 ) Ubi, B. E.(2004). External stimulation of anthocyanin biosynthesis in apple fruit.J. Food Agric. Environ.2, 65-70. ( Veberic, R. (2016). The impact o f p roduction t echnology on p l ant p h enolics. Horticulturae 2:8. doi: 10.3390/horticulturae2030008 ) ( W ally, O . S. D.,Critc h ley, A. T., H i l t z, D., C r ai g ie, J. S., Han, X . , Za h aria,L. I., et a l . (2013). Erratum to: regulation of phytohormone biosynthesis and accumulation in arabidopsis following treatment with commercial e x tract from t h e m a rinemacroalga A scophyllum n odosum. J. P l ant G r owth Re g ul. 3 2, 3 4 0 -341.doi: 10.1007/s00344-012-9311-7 ) Whale, S. K., Singh, Z., Behboudian, M. H., Janes, J.,and Dhaliwal, S. S. (2008).Fruit quality in Cripp’s Pink apple, especially colour, as affected by preharvest sprays of aminoethoxyvinylglycine and ethephon. Sci. Hortic. 115, 342-351.doi: 10.1016/j.scienta.2007.10.015 Wolfe,K., Wu,X., and Liu,R. H. (2003). Antioxidant activity of apple peels. J. Agric.Food Chem. 51,609-614. doi: 10.1021/jf020782a Xu, C., and Leskovar, D. I. (2015). Effects of A. nodosum seaweed extracts onspinach growth, physiology and nutrition value under drought stress. Sci.Hortic. 183, 39-47. doi: 10.1016/j.scienta.2014.12.004 Zhang, X., Wang, K., and Ervin, E. H. (2010). Optimizing dosages of seaweedextract-based cytokinins and zeatin riboside for improving creeping bentgrassheat tolerance. Crop Sci. 50:316. doi: 10.2135/cropsci2009.02.0090 Conflict of Interest Statement: ILSA S.p.A. provided part of the biostimulantsused for the experiment. Collection of data, analysis and interpretation, as well asarticle writing, was carried out by the authors independently. The authors declare that the research was conducted in the absence of anycommercial or financial relationships that could be construed as a potential conflictof interest. Copyright C 2018 Soppelsa, Kelderer, Casera, Bassi, Robatscher and Andreotti. Thisis an open-access article distributed under the terms of the Creative CommonsAttribution License (CC BY). The use, distribution or reproduction in other forumsis permitted, provided the original author(s) and the copyright owner(s) are creditedand that the original publication in this journal is cited, in accordance with acceptedacademic practice. No use, distribution or reproduction is permitted which does notcomply with these terms. eptember Volume Article rontiers in Plant Sciencewww.frontiersin.org Frontiers in Plant Sciencewww.frontiersin.orgSeptember Volume | Article 营养不平衡,例如水果中的钙缺乏,通常是苹果采后失调的诱因。叶面施用钙作为氯化钙是目前提高苹果钙浓度的方法,尽管这种方法的效果往往不令人满意。在这项研究中,我们测试了钙与精选的生物刺激剂联合应用对改善苹果品质和降低贮藏障碍发生率的功效。这项实验是在两个“乔纳森”苹果园进行的,这两个苹果园的管理制度和特点不同。树冠单独喷洒氯化钙,并与含有锌和硅的商业产品或海藻提取物结合使用。海藻提取物提高了苹果的红颜色(+32%的颜色指数)和提高了果皮的最终花青素浓度,从而提高了苹果的品质。两种生物刺激剂在贮藏160天后显著降低了(20%)这种被称为“乔纳森斑点”的生理紊乱的发生率。探讨了施用生物刺激剂后苹果果皮中营养物质(Ca、Zn和Mn)浓度的增加,以及贮藏过程中酚类物质的变化,可能是苹果果实对采后病害易感性降低的可能原因。
确定




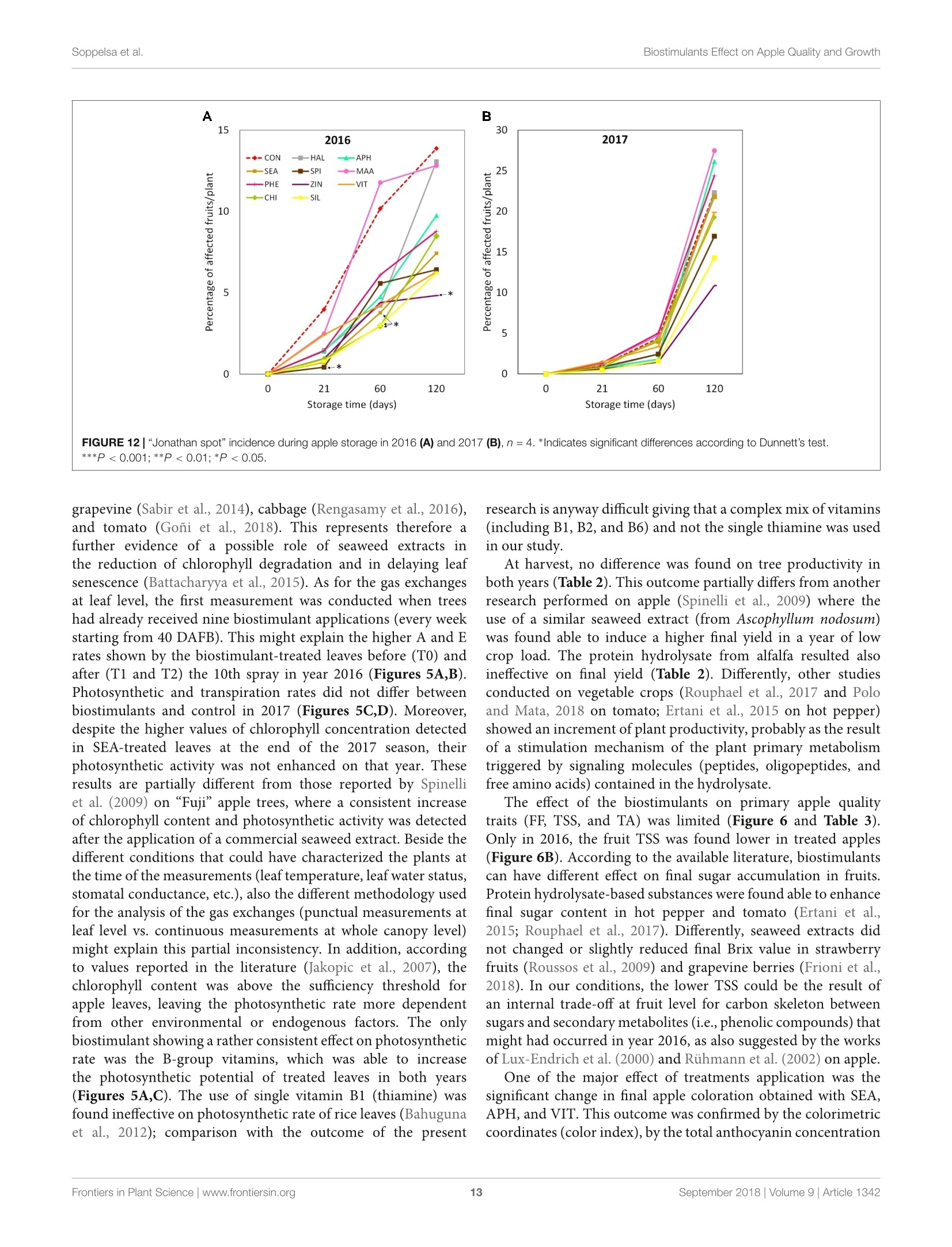




还剩15页未读,是否继续阅读?
图拉扬科技有限公司为您提供《苹果中糖度、酸度检测方案(作物无损检测)》,该方案主要用于蔬菜中营养成分检测,参考标准--,《苹果中糖度、酸度检测方案(作物无损检测)》用到的仪器有Pimprenelle全自动水果质量分选系统
推荐专场
相关方案
更多
该厂商其他方案
更多















