方案详情
文
细胞外囊泡(EVs)或外泌体在传染病和癌症的病理生理学研究中有重要意义。猿类免疫缺陷病毒(SIV)和人类免疫缺陷病毒(HIV)编码的负调节因子(Nef)在获得性免疫缺陷综合征(AIDS)的致病过程中起非常重要的作用,严重损害胞内运输体系。HIV-1的负调节因子Nef是否会被分泌到细胞外囊泡中,这个问题一直存在争议。人们对SIV负调节因子Nef与细胞外囊泡之间的联系,也一无所知。但是在来源于所培养细胞、经亲和层析纯化所得到的细胞外囊泡和来源于已感染SIV的猕猴的细胞外囊泡中,研究者都发现了SIV和HIV-1负调节因子蛋白。而且,含有Nef的细胞外囊泡是有生物学功能的,也就是说,这类细胞外囊泡能参与膜融合过程,将它们的内含物释放到受体细胞中。所以,这些细胞外囊泡能将负调节因子Nef传递到受体细胞,这意味着负调节因子Nef很容易进入外泌体的生物发生途径,HIV病毒体可以细胞质膜处完成组装。这揭示了慢病毒影响未感染细胞和不可感染细胞(即不含CD4的细胞)的一个新机制。
方案详情

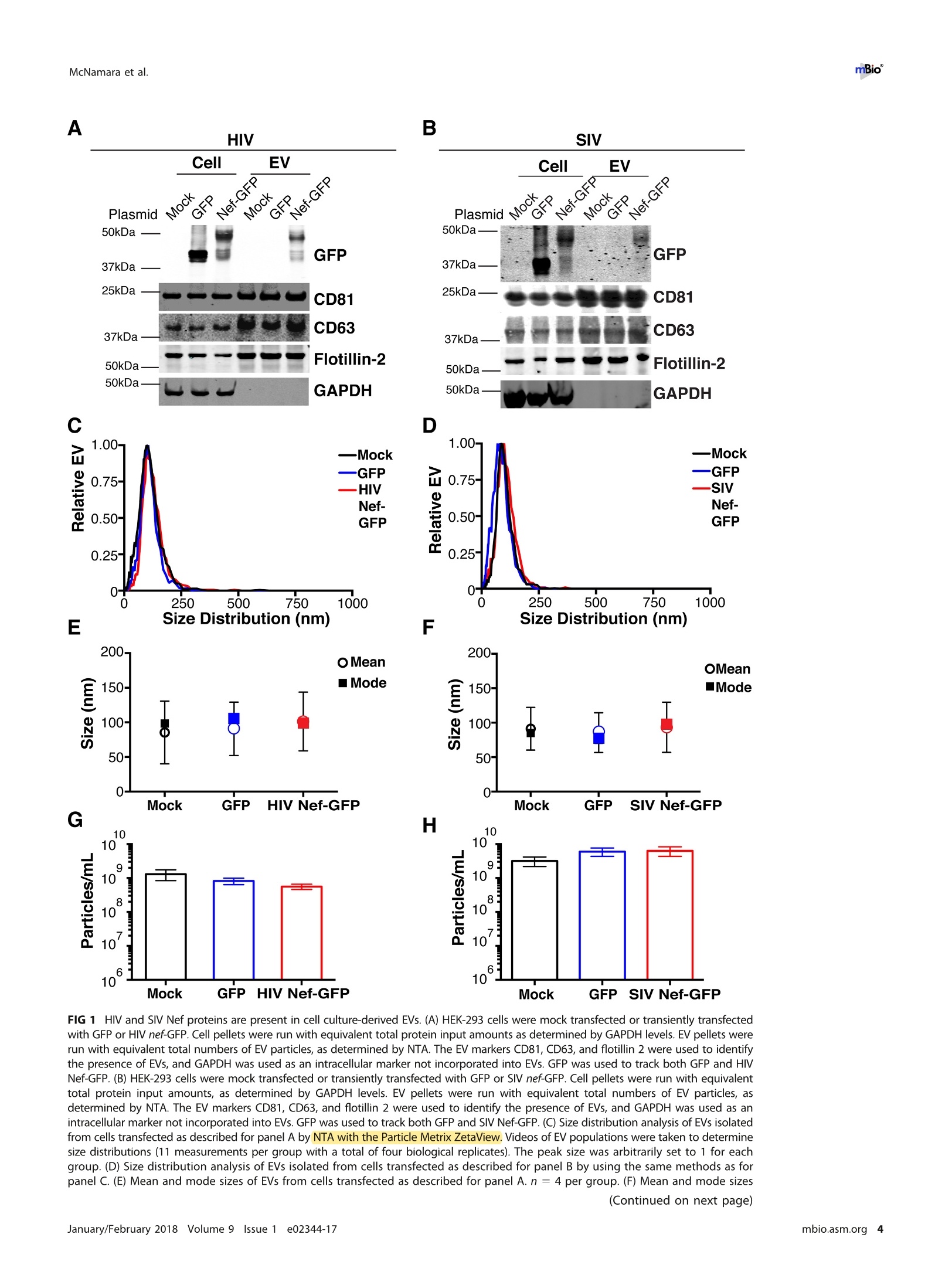
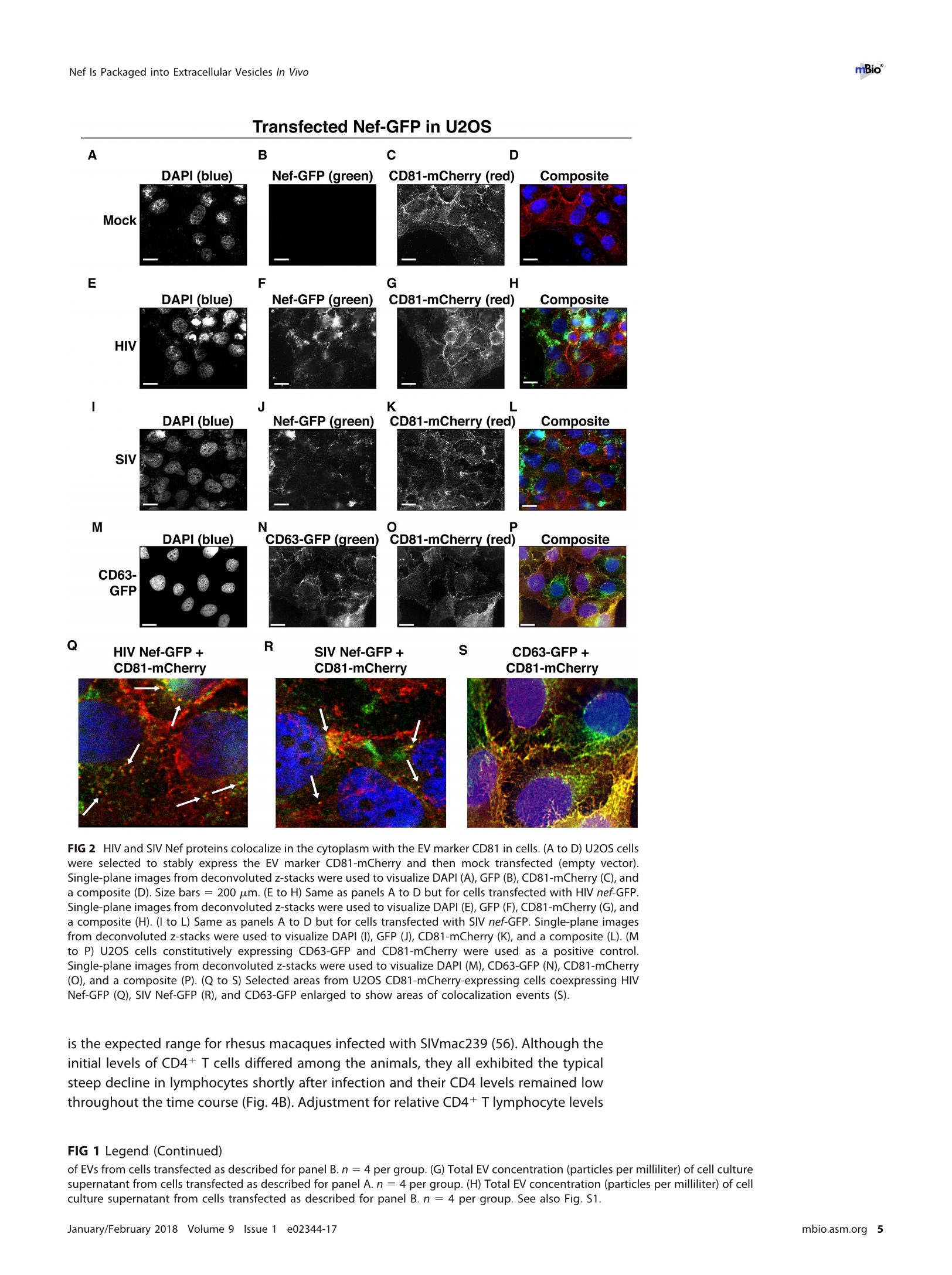
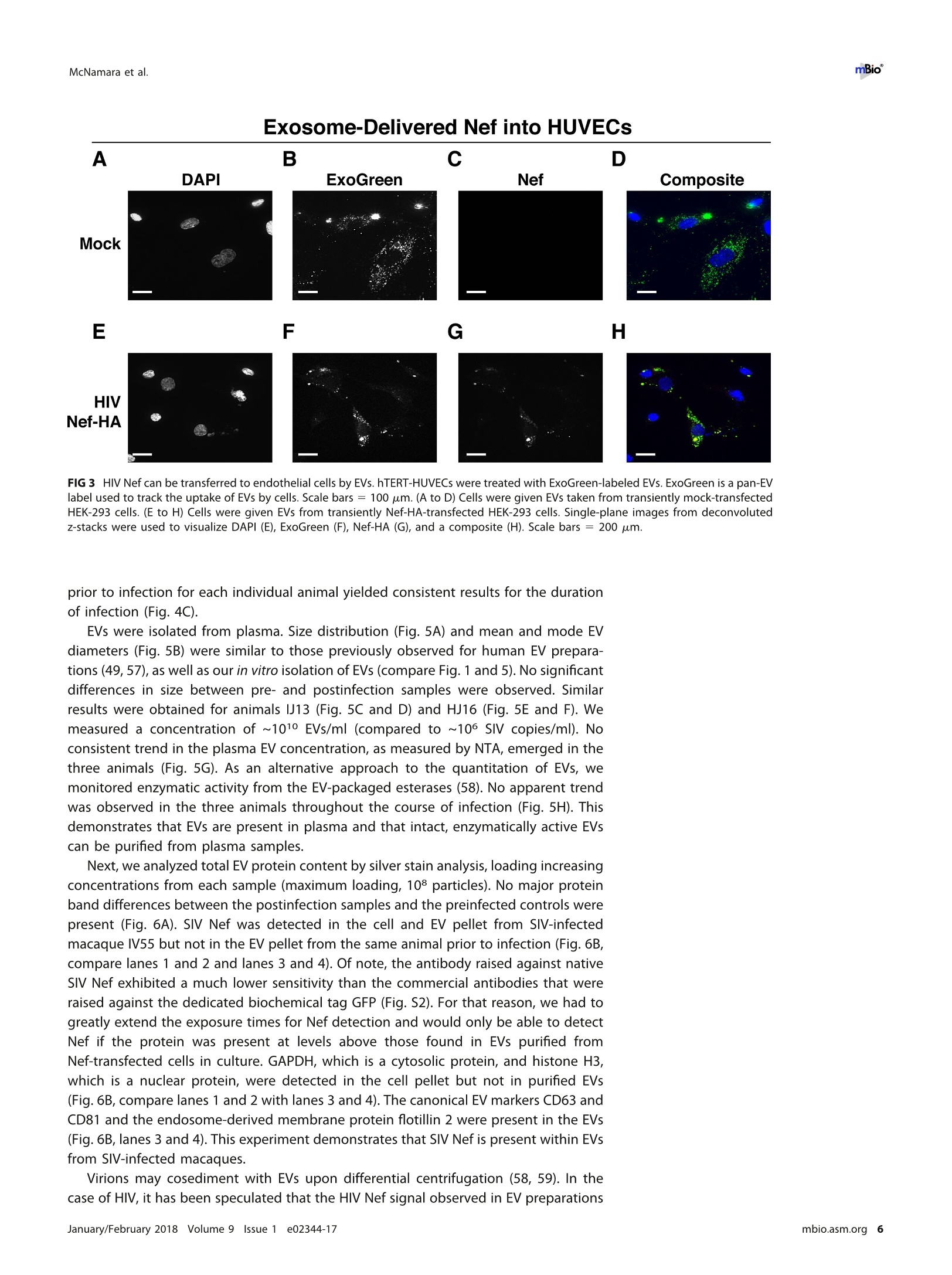
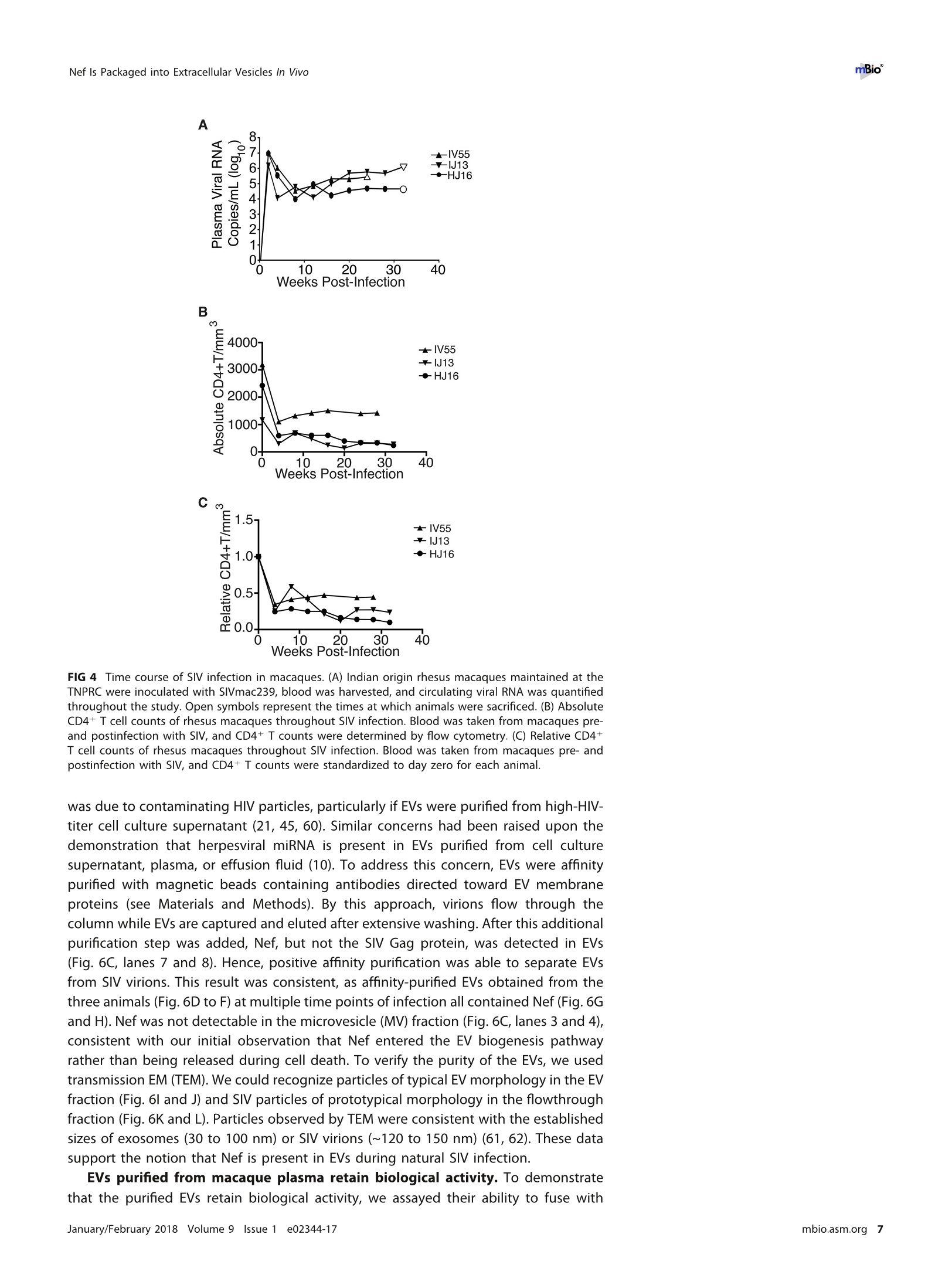
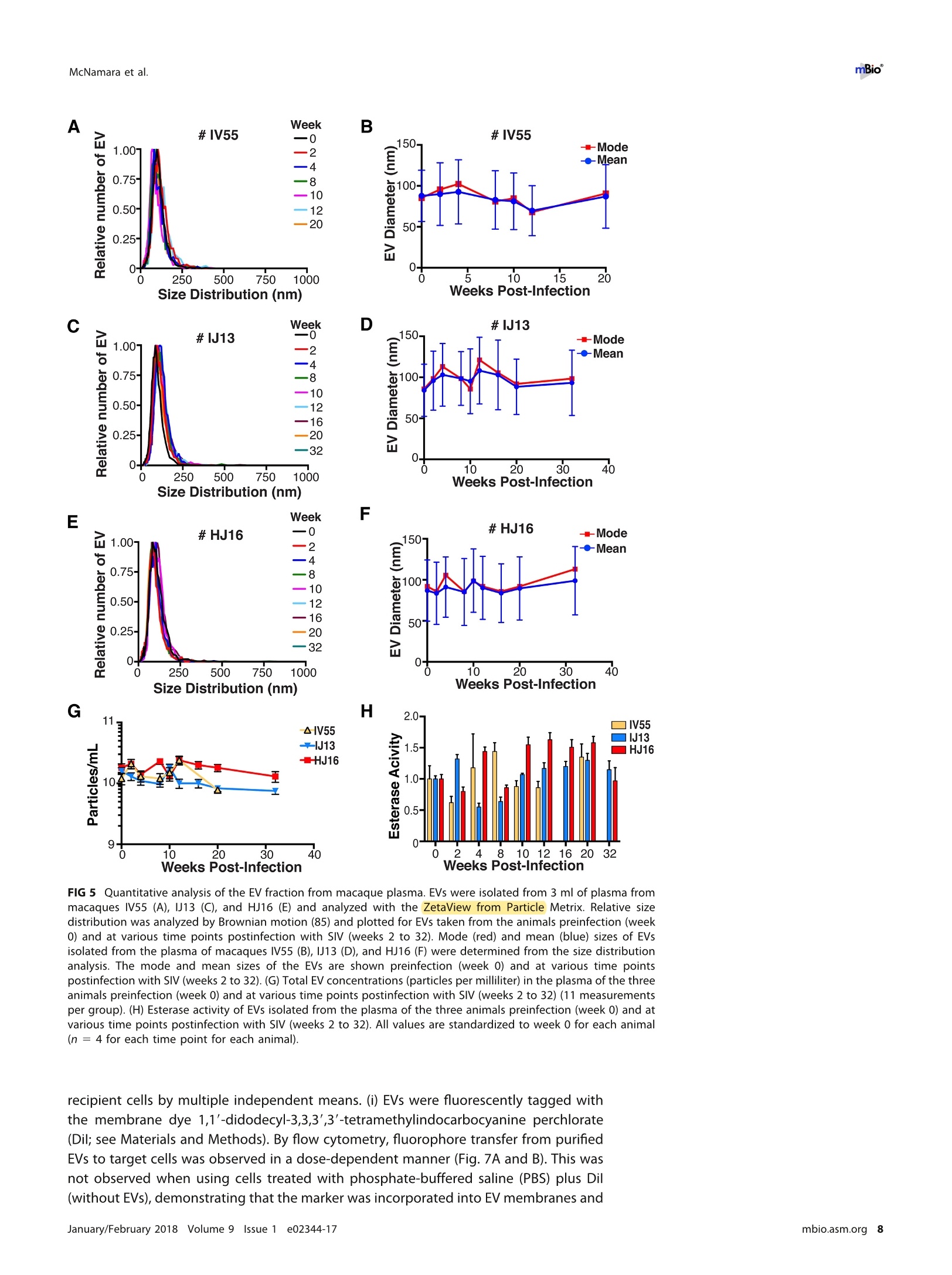
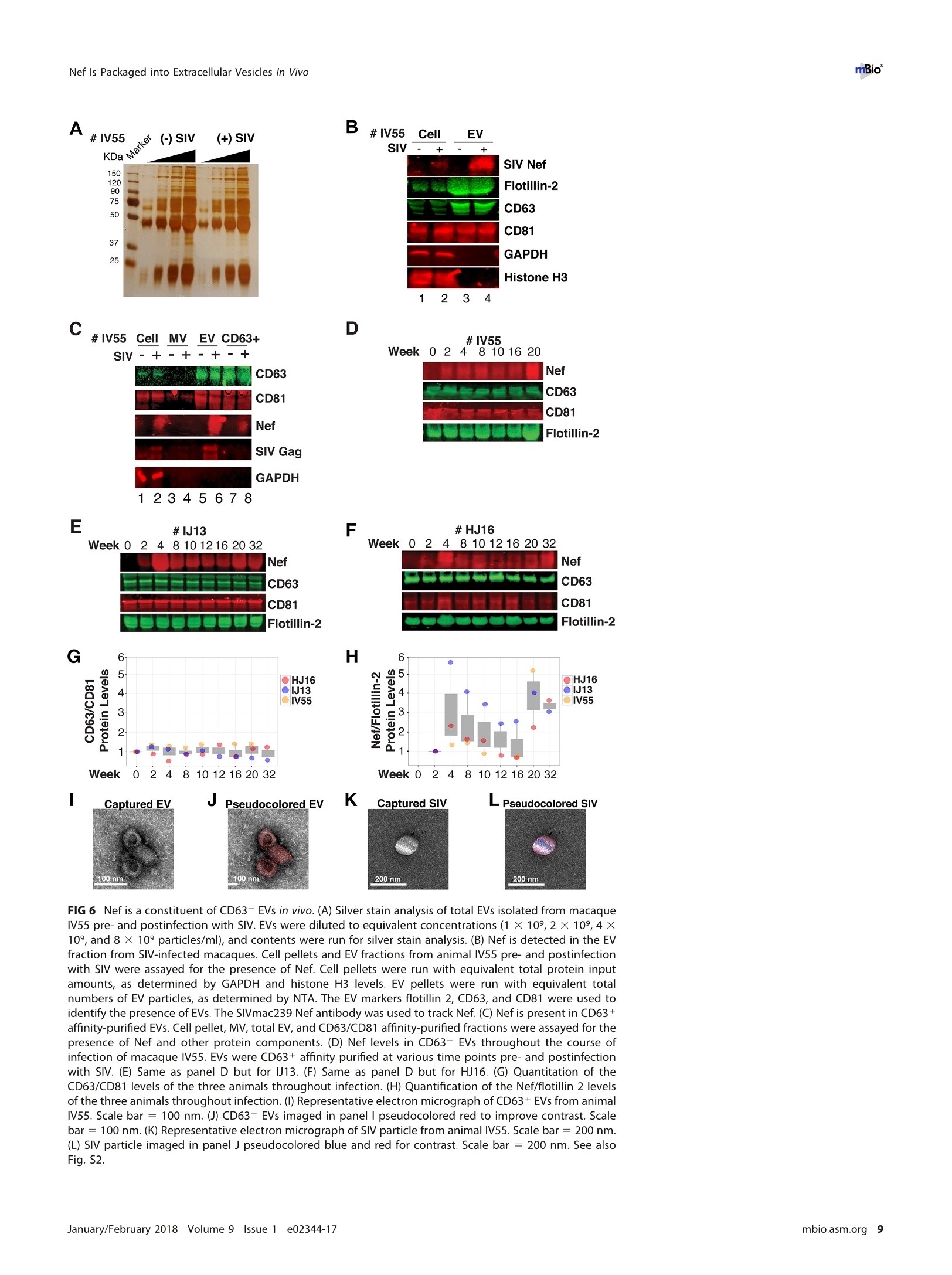
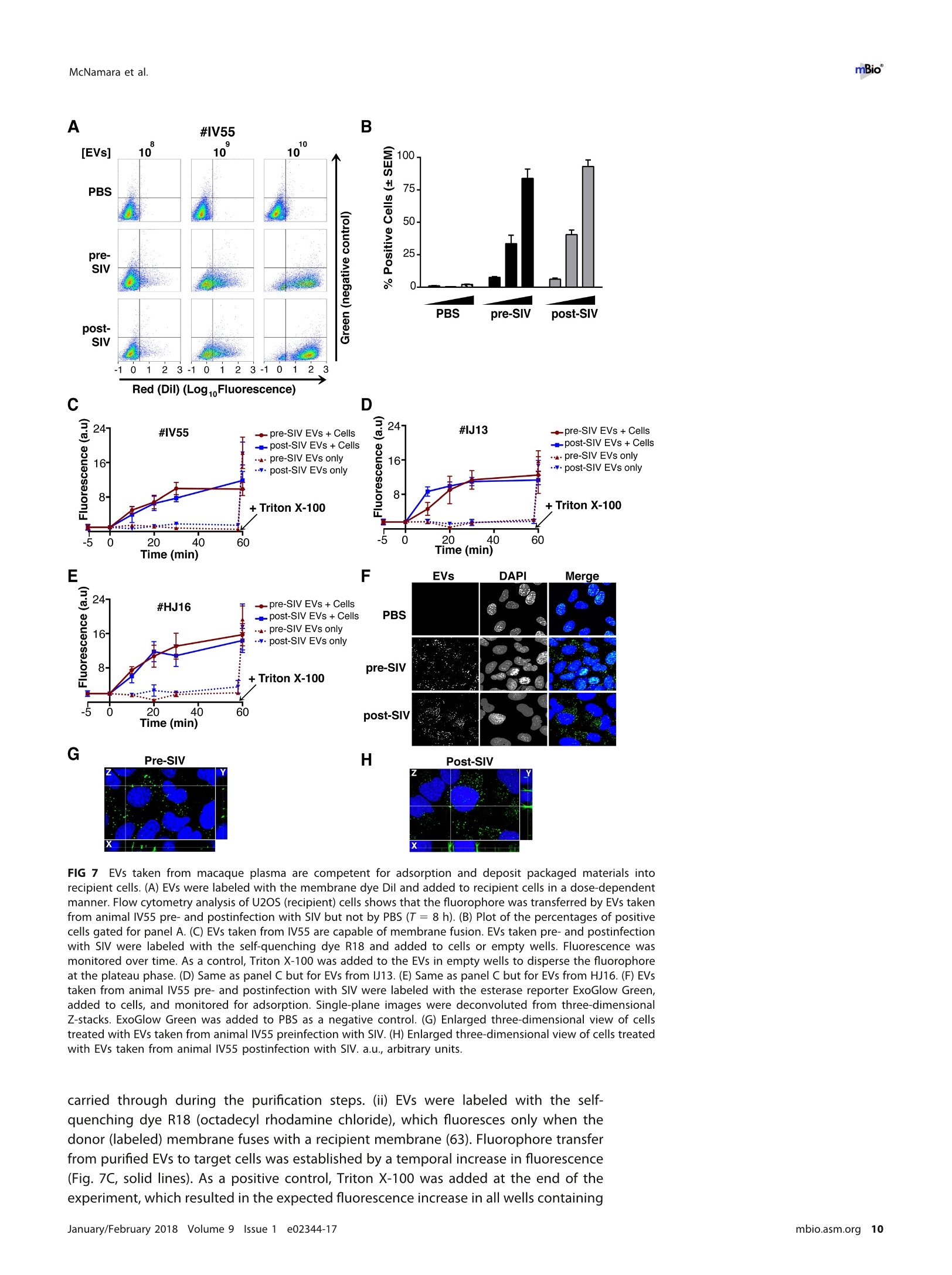
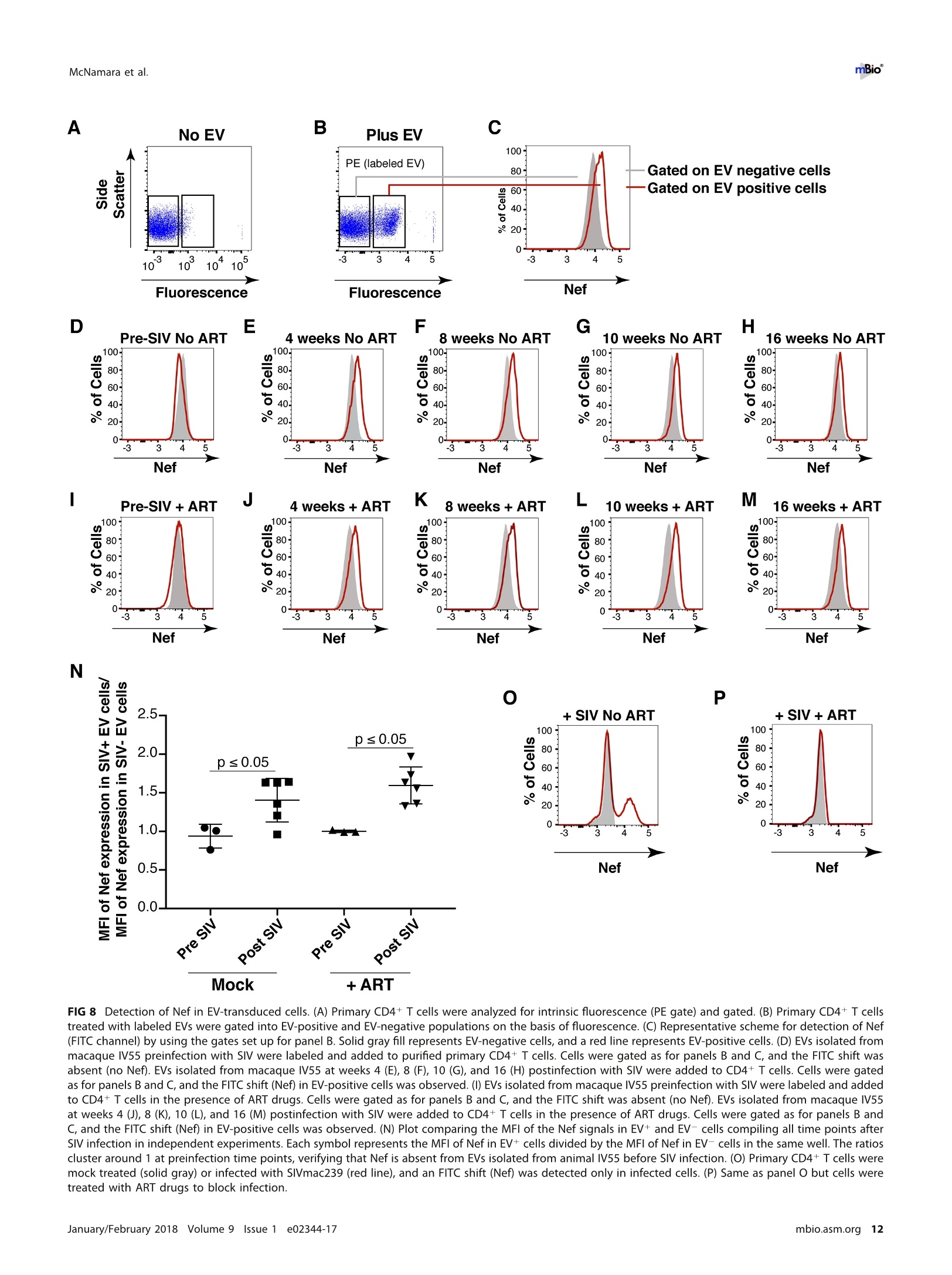
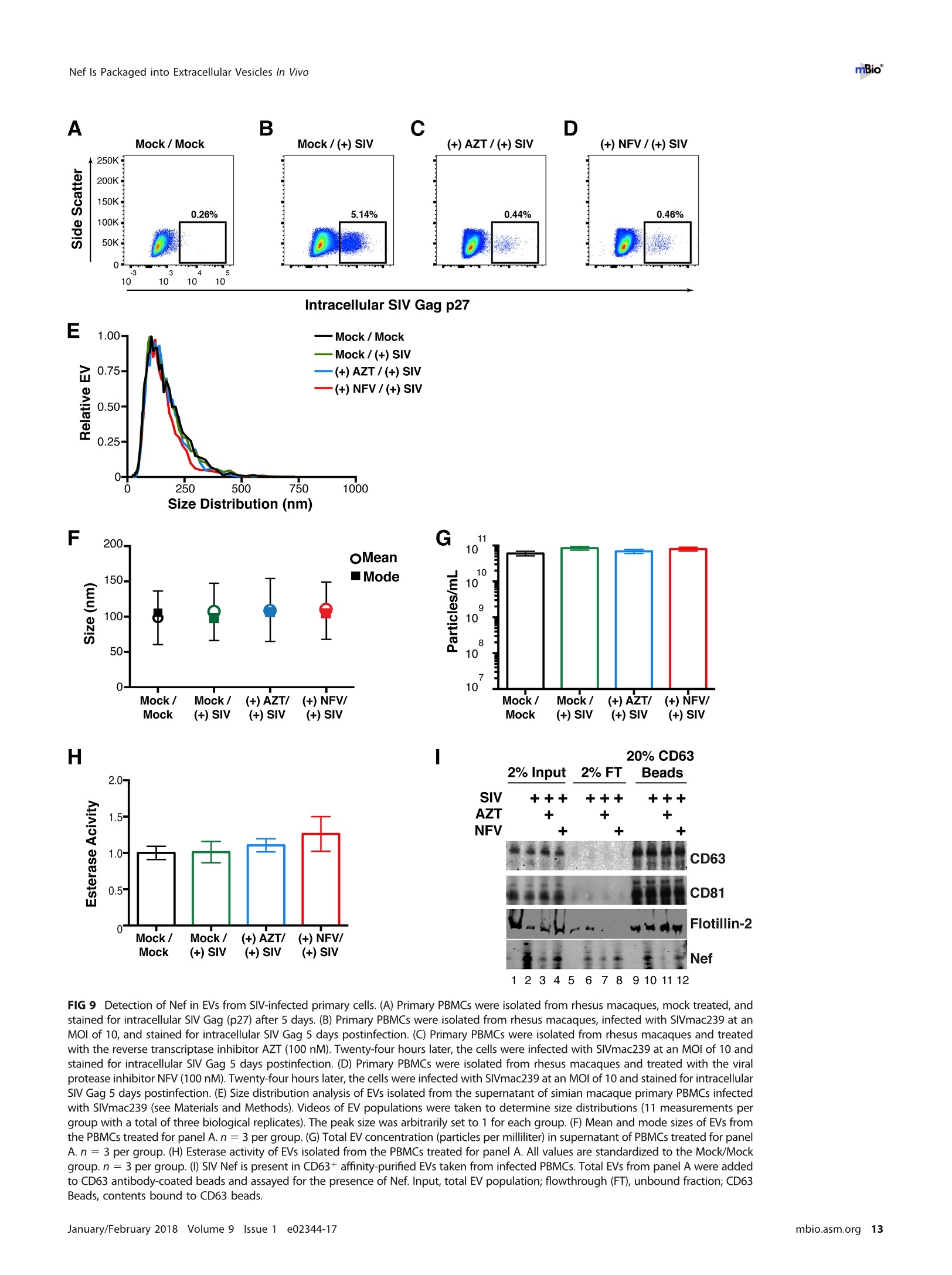
RESEARCH ARTICLE mBio°McNamara et al. ABSTRACTExtracellular vesicles (EVs) or exosomes have been implicated in thepathophysiology of infections and cancer. The negative regulatory factor (Nef) en-coded by simian immunodeficiency virus (SIV) and human immunodeficiency virus(HIV) plays a critical role in the progression to AIDS and impairs endosomal traffick-ing. Whether HIV-1 Nef can be loaded into EVs has been the subject of controversy,and nothing is known about the connection between SIV Nef and EVs. We find thatboth SIV and HIV-1 Nef proteins are present in affinity-purified EVs derived from cul-tured cells, as well as in EVs from SIV-infected macaques. Nef-positive EVs were func-tional, i.e., capable of membrane fusion and depositing their content into recipientcells. The EVs were able to transfer Nef into recipient cells. This suggests that Nefreadily enters the exosome biogenesis pathway, whereas HIV virions are assembledat the plasma membrane. It suggests a novel mechanism by which lentiviruses caninfluence uninfected and uninfectable, i.e., CD4-negative, cells. IMPORTANCE Extracellular vesicles (EVs) transfer biologically active materials fromone cell to another, either within the adjacent microenvironment or further re-moved. EVs also package viral RNAs, microRNAs, and proteins, which contributes tothe pathophysiology of infection. In this report, we show that both human immuno-deficiency virus (HIV) and simian immunodeficiency virus (SIV) incorporate the virus-encoded Nef protein into EVs, including EVs circulating in the blood of SIV-infectedmacaques and that this presents a novel mechanism of Nef transfer to naive andeven otherwise non-infectable cells. Nef is dispensable for viral replication but essen-tial for AIDS progression in vivo. Demonstrating that Nef incorporation into EVs isconserved across species implicates EVs as novel mediators of the pathophysiologyof HIV. It could help explain the biological effects that HIV has on CD4-negative cellsand EVs could become biomarkers of disease progression. KEYWORDS HIV, Nef, SlV, exosomes, extracellular vesicles, microvesicles xtracellular vesicles (EVs) or exosomes have come to be appreciated as a novel andbiologically important means of cell-to-cell communications in development, viraland bacterial infections, and cancer (reviewed in reference 1). We use the term EV hereto refer to vesicles <100 nm in diameter, containing one or more tetraspanin mole-cules, and with a characteristic shape observable by electron microscopy (EM). EVspackage biologically active materials, such as enzymes, mRNAs, long non-coding RNAs,microRNAs (miRNAs), small-molecule metabolites, etc., and deliver them to recipientcells (2-9). Herpesviruses such as herpes simplex virus 1, Epstein-Barr virus, and Kaposi'ssarcoma-associated herpesvirus incorporate virus-encoded miRNA into EVs (2,3, 10,11). Received 18 December 2017 Accepted 10January 2018 Published 6 February 2018Citation McNamara RP, Costantini LM, MyersTA, Schouest B, Maness NJ,Griffith JD,DamaniaBA, MacLean AG, Dittmer DP. 2018.Nef. secretion into extracellular vesicles orexosomes is conserved across human and simian immunodeficiency viruses. mBio 9:e02344-17.https://doi.org/10.1128/mBio.02344-17Editor Vincent R.Racaniello, ColumbiaUniversity College of Physicians & SurgeonsCopyright 2018 McNamara et al. This is anopen-access article distributed under the termsof the Creative Commons Attribution 4.0International license.Address correspondence to Andrew GMacLean, amaclean@tulane.edu, or Dirk PDittmer, ddittmer@med.unc.edu.This article is a direct contribution from a Fellow of the American Academy of Microbiology. Solicited external reviewers: Charles Wood, University of Nebraska-Lincoln; Cheryl Stoddart, UCSF. Hepatitis A virus incorporates its entire capsid into EVs (5, 12-14), furthering internalspread, while external transmission is mediated by a membraneless virion. Human immunodeficiency virus type 1 (HIV-1) and HIV-2 entered the humanpopulation in the early 20th century from the ancestral simian immunodeficiency virus(SIV) (15, 16). Similar to HIV, SIV establishes chronic infection of the host, ultimatelyprogressing to simian AIDS (sAIDS) in rhesus macaques (Macaca mulatta). SIV and HIVbelong to the Retroviridae family of viruses (genus Lentivirus) and infect macrophagesand CD4+T cells through defined receptor-coreceptor pairs that are expressed only onthese specialized cells; yet, they cause phenotypes dependent on a much larger rangeof cell types. For instance, SIV and HIV induce a gradual reduction in the level ofcirculating CD4+ lymphocytes over time (15-18). Here, a substantial amount of CD4+T cell death occurs independently of successful viral infection (19, 20). Distinct patho-physiological changes, such as HIV-associated neurocognitive disorders, persist inlatently infected individuals or in individuals on successful antiretroviral therapy (ART),implying an indirect mechanism of pathogenesis. HIV and SIV encode a total of nine genes. A set of early transcripts are fully splicedand encode the accessory proteins Tat, Rev, and Nef. Tat is a potent transcriptionalactivator that drives the elongation of paused RNA polymerase ll, Rev is an RNA-bindingprotein that facilitates the export of unspliced viral RNAs to the cytoplasm, and Nef isa modulator of the endosomal trafficking network in infected cells (21-26). Nef is asmall (~27-kDa) protein that localizes to the cytosol and undergoes several posttrans-lational modifications, such as myristoylation, that lead to membrane association (18,27-29). Nef mediates the degradation of the viral receptor CD4 (21, 23,30-32), and thatmay be its primary function in productively infected cells. The importance of Nef becomes visible only within the context of the host. While Tatand Rev are indispensable for viral propagation in cultured T cells, the gene for Nef canbe removed entirely or replaced with the gene for green fluorescent protein (GFP) (orother genes) and the resulting recombinant virus retains full replication potential inculture. However, SIV recombinants lacking functional Nef are highly attenuated in vivo(21, 33-35) and SIV strains containing point mutations in the nef open reading framerapidly adapt to restore wild-type Nef function upon in vivo infection (18, 36, 37). Nefmutations in HIV-infected human patients are overrepresented among natural long-term nonprogressors (38, 39). Nef has been found in the plasma of infected primatesand humans (18, 40-45), though not all earlier reports were consistent (35, 43, 44, 46,47). This suggests that Nef's role in pathogenesis is not limited to infected cells, but thatit could contribute to the more systemic and long-term sequelae of HIV/SIV infection.At that point, a possible interaction between SIV Nef and EVs had not been reported. We asked if Nef of both HIV and SIV could be detected in secreted EVs. This wouldestablish the conservation of this phenotype and further substantiate the role of the SIVmacaque model in HIV research. We were able to demonstrate that (i) the SIV and HIVNef proteins are consistently present in EVs from transiently transfected cells, (ii) SIV Nefcan be detected in systemically circulating EVs of macaques after infection, and (iii) SIVNef can be transferred to uninfected cells via EVs. Key to our argument for the presenceof Nef in EVs was adding a positive affinity purification step that separated EVs fromvirions, as we had previously validated for EVs and herpesvirus virions (10). Thesefindings support the model in which EVs provide a mechanism for Nef to influence thephysiology of uninfected and uninfectable (CD4-negative) cells. The most likely recip-ients are endothelial cells lining the vascular and lymphatic systems, e.g., of theblood-brain barrier, as these are constantly exposed to EVs that circulate at a concen-tration as high as 1011 particles/ml (48). RESULTS HlV and SIV Nef proteins are present in EVs released from transfected cells. Totest the hypothesis that Nef could be incorporated into EVs independently of other viralcomponents, we transiently expressed the HIV and SIV Nef proteins in human embry-onic kidney (HEK-293) cells. We used Nef tagged with GFP to monitor transfection efficiency. As an epitope tag control, we transfected wild-type GFP alone. HIV Nef-GFP,as well as SIV Nef-GFP, but not GFP alone, was detectable in the EV fraction (Fig. 1A andB). See Materials and Methods for the details of the EV purification protocol used, whichis similar to that described in reference 49. We used the tetraspanin markers CD81 andCD63, as well as EV-associated flotillin 2 (50, 51), as markers for EV purity and loadingcontrols and cytosolic glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a con-trol for contamination with cytosolic proteins. We verified the biophysical properties ofEV fractions by nanoparticle tracking analysis (NTA). Through zeta potential and Brown-ian motion, we were able to plot the relative size distribution of the isolated EVs.Regardless of the cell origin, the EV size distribution profiles were highly similar andshowed a canonical curve shape (2, 3, 10, 49, 52, 53) (Fig. 1C and D; see Movies S1 toS6 in the supplemental material). Mean and mode sizes were consistent across allexperiments (Fig. 1E and F). Total EV release did not differ among cells expressing HIVNef-GFP (Fig.1G) or SIV Nef-GFP (Fig. 1H) compared to GFP-or mock-transfected cells.This phenotype was repeatable with cells transfected with hemagglutinin (HA) epitope-tagged HIV Nef, demonstrating that Nef's presence in EVs was not due to being taggedwith a cytoplasmic protein such as GFP (Fig. S1). These experiments demonstrate thatthe HlV and SIV Nef proteins are loaded into EVs in the absence of other viruscomponents. Nef and the EV marker CD81 colocalize. To test the hypothesis that Nef colocal-izes with EV components during EV maturation, we employed fluorescence microscopy.Exosomes are derived from the inward budding of endosomes into the multivesicularbody (MVB), trafficked from there to the plasma membrane, and released (52, 54).Therefore, EV components such as CD63, CD81, CD9, and flotillin 2 can be used to markmaturing late endosomes/MVBs inside the cell. We generated U2OS cells, whichconstitutively express CD81-mCherry (Fig. 2A to D). We then used these cells totransiently express HIV Nef-GFP (Fig. 2E to H) or SIV Nef-GFP (Fig. 2l to L). As a control,we analyzed patterns of colocalization of CD81-mCherry and CD63-GFP (Fig. 2M to P).Diffuse patterns of localization, as well as several regions of punctate colocalizationevents, could be identified involving CD81-mCherry and both HIV Nef (Fig. 2Q) and SIVNef (Fig. 2R) and in our CD63-GFP positive control (Fig.2S). This is consistent with thenotion that Nef enters the EV maturation pathway and eventually resides inside EVs. Nef is transferred to recipient cells by EVs. To test the hypothesis that EV-associated Nef was released and transferred to target cells, we conducted fusion assays.EVs were purified from HEK-293 cells transiently expressing HIV Nef-HA and added tohuman telomerase reverse transcriptase-human umbilical vein endothelial cells (hTERT-HUVECs). HUVECs are a physiologically relevant target because endothelial cells liningthe vasculature are constantly exposed to all EVs released into the circulation. Levels ofEVs circulating in the blood have been estimated at 1010 to 1012/ml (55). Upon theaddition of ExoGreen-labeled EVs, green punctate structures representing EV fusion arereadily apparent (Fig. 3A to D). Only in cells treated with EVs taken from HIV Nef-HA-transfected cells,not in cells treated with EVs taken from mock-transfected cells, was anHA signal also apparent (Fig. 3E to H). The Nef-HA signal (red) was observed only in thepresence of colocalizing ExoGreen (pan-EV stain); while the ExoGreen signal was alsoobserved in areas without Nef. This was expected, as not all EVs also contain Nef, evenwhen overexpressed, and demonstrated that Nef can be transferred into recipient cellsvia EVs. Nef is detectable in EVs purified from SIV-infected macaques. The biochemicalexperiments established that loading into EVs and transfer were conserved in the SIVand HIV Nef proteins. To test the hypothesis, that SIV Nef was systemically circulatingduring natural SIV infection, three rhesus macaques (IV55, IJ13,and HJ16) were infectedwith SIVmac239. The animals were monitored for viral load and disease progression for32weeks. Plasma was obtained from the animals pre-and postinfection, and viral RNAwas quantified by quantitative reverse transcription (qRT)-PCR (Fig. 4A). Chronic-phaseset point (>10 weeks postinfection) SIV titers ranged from 105 to 107 copies/ml, which Transfected Nef-GFP in U2OS A B C D DAPI (blue) Nef-GFP (green)1) CD81-mCherry (red) Composite Q FIG 2 HIV and SIV Nef proteins colocalize in the cytoplasm with the EV marker CD81 in cells. (A to D) U2OS cellswere selected to stably express the EV marker CD81-mCherry and then mock transfected (empty vector).Single-plane images from deconvoluted z-stacks were used to visualize DAPI (A), GFP (B),CD81-mCherry (C), anda composite (D). Size bars =200 um. (E to H) Same as panels A to D but for cells transfected with HIV nef-GFP.Single-plane images from deconvoluted z-stacks were used to visualize DAPI (E), GFP (F), CD81-mCherry (G),anda composite (H). (I to L) Same as panels A to D but for cells transfected with SIV nef-GFP. Single-plane imagesfrom deconvoluted z-stacks were used to visualize DAPI (I), GFP (J), CD81-mCherry (K), and a composite (L). (Mto P) U2OS cells constitutively expressing CD63-GFP and CD81-mCherry were used as a positive control.Single-plane images from deconvoluted z-stacks were used to visualize DAPI (M), CD63-GFP (N), CD81-mCherry(0), and a composite (P). (Q to S) Selected areas from U2OS CD81-mCherry-expressing cells coexpressing HIVNef-GFP (Q), SIV Nef-GFP (R), and CD63-GFP enlarged to show areas of colocalization events (S). is the expected range for rhesus macaques infected with SIVmac239 (56). Although theinitial levels of CD4+ T cells differed among the animals, they all exhibited the typicalsteep decline in lymphocytes shortly after infection and their CD4 levels remained lowthroughout the time course (Fig. 4B). Adjustment for relative CD4+ T lymphocyte levels FIG 1 Legend (Continued) of EVs from cells transfected as described for panel B. n =4 per group. (G) Total EV concentration (particles per milliliter) of cell culturesupernatant from cells transfected as described for panel A. n =4 per group. (H) Total EV concentration (particles per milliliter) of cellculture supernatant from cells transfected as described for panel B. n =4 per group. See also Fig. S1. Exosome-Delivered Nef into HUVECs FIG 3 HIV Nef can be transferred to endothelial cells by EVs. hTERT-HUVECs were treated with ExoGreen-labeled EVs. ExoGreen is a pan-EVlabel used to track the uptake of EVs by cells. Scale bars = 100 um. (A to D) Cells were given EVs taken from transiently mock-transfectedHEK-293 cells. (E to H) Cells were given EVs from transiently Nef-HA-transfected HEK-293 cells. Single-plane images from deconvolutedz-stacks were used to visualize DAPI (E), ExoGreen (F), Nef-HA (G), and a composite (H). Scale bars = 200 um. prior to infection for each individual animal yielded consistent results for the durationof infection (Fig.4C). EVs were isolated from plasma. Size distribution (Fig. 5A) and mean and mode EVdiameters (Fig.5B) were similar to those previously observed for human EV prepara-tions (49, 57), as well as our in vitro isolation of EVs (compare Fig. 1 and 5). No significantdifferences in size between pre- and postinfection samples were observed. Similarresults were obtained for animals IJ13 (Fig.5C and D) and HJ16 (Fig. 5E and F). Wemeasured a concentration of ~1010 EVs/ml (compared to ~106 SIV copies/ml). Noconsistent trend in the plasma EV concentration, as measured by NTA, emerged in thethree animals (Fig. 5G). As an alternative approach to the quantitation of EVs, wemonitored enzymatic activity from the EV-packaged esterases (58). No apparent trendwas observed in the three animals throughout the course of infection (Fig. 5H). Thisdemonstrates that EVs are present in plasma and that intact, enzymatically active EVscan be purified from plasma samples. Next, we analyzed total EV protein content by silver stain analysis, loading increasingconcentrations from each sample (maximum loading, 108 particles). No major proteinband differences between the postinfection samples and the preinfected controls werepresent (Fig. 6A). SIV Nef was detected in the cell and EV pellet from SIV-infectedmacaque IV55 but not in the EV pellet from the same animal prior to infection (Fig. 6B,compare lanes 1 and 2 and lanes 3 and 4). Of note, the antibody raised against nativeSIV Nef exhibited a much lower sensitivity than the commercial antibodies that wereraised against the dedicated biochemical tag GFP (Fig. S2). For that reason, we had togreatly extend the exposure times for Nef detection and would only be able to detectNef if the protein was present at levels above those found in EVs purified fromNef-transfected cells in culture. GAPDH, which is a cytosolic protein, and histone H3,which is a nuclear protein, were detected in the cell pellet but not in purified EVs(Fig.6B, compare lanes 1 and 2 with lanes 3 and 4). The canonical EV markers CD63 andCD81 and the endosome-derived membrane protein flotillin 2 were present in the EVs(Fig. 6B, lanes 3 and 4).This experiment demonstrates that SIV Nef is present within EVsfrom SIV-infected macaques. Virions may cosediment with EVs upon differential centrifugation (58, 59). In thecase of HIV, it has been speculated that the HIV Nef signal observed in EV preparations A --IV55 Weeks Post-Infection FIG 4 Time course of SIV infection in macaques. (A) Indian origin rhesus macaques maintained at theTNPRC were inoculated with SIVmac239, blood was harvested, and circulating viral RNA was quantifiedthroughout the study. Open symbols represent the times at which animals were sacrificed. (B) AbsoluteCD4+ T cell counts of rhesus macaques throughout SIV infection. Blood was taken from macaques pre-and postinfection with SIV, and CD4+ T counts were determined by flow cytometry. (C) Relative CD4+T cell counts of rhesus macaques throughout SIV infection. Blood was taken from macaques pre- andpostinfection with SIV, and CD4+ T counts were standardized to day zero for each animal. was due to contaminating HIV particles, particularly if EVs were purified from high-HIV-titer cell culture supernatant (21, 45, 60). Similar concerns had been raised upon thedemonstration that herpesviral miRNA is present in EVs purified from cell culturesupernatant, plasma, or effusion fluid (10). To address this concern, EVs were affinitypurified with magnetic beads containing antibodies directed toward EV membraneproteins (see Materials and Methods). By this approach, virions flow through thecolumn while EVs are captured and eluted after extensive washing. After this additionalpurification step was added, Nef, but not the SIV Gag protein, was detected in EVs(Fig.6C, lanes 7 and 8). Hence, positive affinity purification was able to separate EVsfrom SIV virions. This result was consistent, as affinity-purified EVs obtained from thethree animals (Fig. 6D to F) at multiple time points of infection all contained Nef (Fig.6Gand H). Nef was not detectable in the microvesicle (MV) fraction (Fig. 6C, lanes 3 and 4),consistent with our initial observation that Nef entered the EV biogenesis pathwayrather than being released during cell death. To verify the purity of the EVs, we usedtransmission EM (TEM). We could recognize particles of typical EV morphology in the EVfraction (Fig. 6l and J) and SIV particles of prototypical morphology in the flowthroughfraction (Fig. 6K and L). Particles observed by TEM were consistent with the establishedsizes of exosomes (30 to 100 nm) or SIV virions (~120 to 150 nm) (61, 62). These datasupport the notion that Nef is present in EVs during natural SIV infection. EVs purified from macaque plasma retain biological activity. To demonstratethat the purified EVs retain biological activity, we assayed their ability to fuse with C Week Week E G F H IJ13 HJ16 FIG 5 Quantitative analysis of the EV fraction from macaque plasma. EVs were isolated from 3 ml of plasma frommacaques IV55 (A), IJ13 (C), and HJ16 (E) and analyzed with the ZetaView from Particle Metrix. Relative sizedistribution was analyzed by Brownian motion (85) and plotted for EVs taken from the animals preinfection (week0) and at various time points postinfection with SIV (weeks 2 to 32). Mode (red) and mean (blue) sizes of EVsisolated from the plasma of macaques IV55 (B), IJ13 (D), and HJ16 (F) were determined from the size distributionanalysis. The mode and mean sizes of the EVs are shown preinfection (week 0) and at various time pointspostinfection with SIV (weeks 2 to 32). (G) Total EV concentrations (particles per milliliter) in the plasma of the threeanimals preinfection (week 0) and at various time points postinfection with SIV (weeks 2 to 32) (11 measurementsper group). (H) Esterase activity of EVs isolated from the plasma of the three animals preinfection (week 0) and atvarious time points postinfection with SlV (weeks 2 to 32). All values are standardized to week 0 for each animal(n = 4 for each time point for each animal). recipient cells by multiple independent means. (i) EVs were fluorescently tagged withthe membrane dye 1,1'-didodecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate(Dil; see Materials and Methods). By flow cytometry, fluorophore transfer from purifiedEVs to target cells was observed in a dose-dependent manner (Fig. 7A and B). This wasnot observed when using cells treated with phosphate-buffered saline (PBS) plus Dil(without EVs), demonstrating that the marker was incorporated into EV membranes and C # IV5555 CellI MV EV CD63+ E #IJ13 G D 4#IV55 Week 0 22 F # HJ16 H FIG 6 Nef is a constituent of CD63+ EVs in vivo. (A) Silver stain analysis of total EVs isolated from macaqueIV55 pre- and postinfection with SIV. EVs were diluted to equivalent concentrations (1× 109,2× 109, 4 ×10°, and 8 × 10° particles/ml), and contents were run for silver stain analysis. (B) Nef is detected in the EVfraction from SIV-infected macaques. Cell pellets and EV fractions from animal IV55 pre- and postinfectionwith SIV were assayed for the presence of Nef. Cell pellets were run with equivalent total protein inputamounts, as determined by GAPDH and histone H3 levels. EV pellets were run with equivalent totalnumbers of EV particles, as determined by NTA. The EV markers flotillin 2, CD63, and CD81 were used toidentify the presence of EVs. The SIVmac239 Nef antibody was used to track Nef. (C) Nef is present in CD63+affinity-purified EVs. Cell pellet, MV, total EV, and CD63/CD81 affinity-purified fractions were assayed for thepresence of Nef and other protein components. (D) Nef levels in CD63+ EVs throughout the course ofinfection of macaque IV55. EVs were CD63+ affinity purified at various time points pre- and postinfectionwith SIV. (E) Same as panel D but for IJ13. (F) Same as panel D but for HJ16. (G) Quantitation of theCD63/CD81 levels of the three animals throughout infection. (H) Quantification of the Nef/flotillin 2 levelsof the three animals throughout infection. (I) Representative electron micrograph of CD63+ EVs from animalIV55.Scale bar = 100 nm. (J) CD63+ EVs imaged in panelI pseudocolored red to improve contrast. Scalebar =100 nm.(K) Representative electron micrograph of SIV particle from animal IV55. Scale bar =200 nm.(L) SIV particle imaged in panel J pseudocolored blue and red for contrast. Scale bar =200 nm. See alsoFig. S2. McNamara et al. A B C D E Time (min) G Pre-SIVH Post-SIV FIG 7 EVs taken from macaque plasma are competent for adsorption and deposit packaged materials intorecipient cells. (A) EVs were labeled with the membrane dye Dil and added to recipient cells in a dose-dependentmanner. Flow cytometry analysis of U2OS (recipient) cells shows that the fluorophore was transferred by EVs takenfrom animal IV55 pre- and postinfection with SIV but not by PBS (T= 8 h). (B) Plot of the percentages of positivecells gated for panel A. (C) EVs taken from IV55 are capable of membrane fusion. EVs taken pre- and postinfectionwith SlV were labeled with the self-quenching dye R18 and added to cells or empty wells. Fluorescence wasmonitored over time. As a control, Triton X-100 was added to the EVs in empty wells to disperse the fluorophoreat the plateau phase. (D) Same as panel C but for EVs from J13. (E) Same as panel C but for EVs from HJ16. (F) EVstaken from animal IV55 pre- and postinfection with SIV were labeled with the esterase reporter ExoGlow Green,added to cells, and monitored for adsorption. Single-plane images were deconvoluted from three-dimensionalZ-stacks. ExoGlow Green was added to PBS as a negative control. (G) Enlarged three-dimensional view ofcellstreated with EVs taken from animal IV55 preinfection with SIV. (H) Enlarged three-dimensional view of cells treatedwith EVs taken from animal IV55 postinfection with SIV. a.u., arbitrary units. carried through during the purification steps. (ii) EVs were labeled with the self-quenching dye R18 (octadecyl rhodamine chloride), which fluoresces only when thedonor (labeled) membrane fuses with a recipient membrane (63). Fluorophore transferfrom purified EVs to target cells was established by a temporal increase in fluorescence(Fig. 7C, solid lines). As a positive control, Triton X-100 was added at the end of theexperiment, which resulted in the expected fluorescence increase in all wells containing EVs (Fig. 7C, dashed lines; see Materials and Methods). No differences were observed inthe fusion rates of EVs pre- and postinfection of the three animals infected with SIV(Fig. 7C to E). (iii) To test if EV contents were taken up by cells, EVs were incubated witha membrane-permeating ExoGreen esterase reporter, which fluoresces green andcovalently links to proteins inside EVs. Addition of labeled EVs, but not our PBS control,to cells showed intracellular uptake of the reporter (Fig. 7F to H). The punctateformation of the proteins delivered by EVs likely represents larger EV aggregates andnot end point compartmentalized proteins per se. This was consistent with deliveredEVs taken from a tissue culture setting. This assay demonstrated that this EV purifiedfrom plasma contained the EV-defining, enzymatically active esterases and remainedcompetent for membrane fusion and transfer of proteins into recipient cells. EVs purified from naturally infected macaques transfer SIV Nef to recipientcells. To test the hypothesis that SIV Nef from infected animals in EVs can be transferredinto recipient cells, we used a flow cytometry-based assay optimized for intracellularNef staining (64). This was a rather difficult experiment that approached the limits ofsensitivity, as Nef was neither overexpressed nor tagged. Treatment of purified primaryCD4+T cells from SIV-naive macaques with fluorescently labeled EVs derived fromuninfected macaques demonstrated successful transfer of the fluorophore [phycoeryth-rin (PE) channel], signifying that EVs adsorbed to the cells at high rates (Fig. 8A and B).We then gated for cells that showed uptake of EVs versus those that did not and usedthe fluorescein isothiocyanate (FITC) channel to detect intracellular Nef (Fig. 8C). Asexpected, no shift in mean FITC fluorescence was detected in cells treated with EVstaken from macaques before SIV infection (Fig.8D). In cells treated with EVs isolatedfrom macaques postinfection with SIV, a shift in the FITC (Nef) channel was observedat multiple time points throughout infection (Fig. 8E to H). The addition of the ARTdrugs zidovudine (AZT; reverse transcriptase inhibitor) and nelfinavir (NFV; proteaseinhibitor) did not antagonize the FITC (Nef) shift observed postinfection with SIV inEV-positive cells relative to EV-negative cells (Fig. 8l to M), demonstrating that the Nefsignal did not originate from new infections. Mean fluorescence intensities wereclustered into pre- or postinfection in the absence (Mock) or presence of ART drugs, andstatistical significance (P < 0.05) was calculated with pairwise t tests (Fig.8N). Thesefindings were in contrast to those obtained with cells directly infected with SIVmac239(Fig. 80), in which Nef detection was abolished when cells were treated with the ARTdrug cocktail (Fig. 8P). Taken together, the results show that ART abolished thedetection of Nef in the context of SIV infection but had no impact on Nef detection inthe context of EV-mediated transfer. This experiment demonstrates that SIV Nef puri-fied from naturally infected animals can be transferred to recipient cells by an EV. PBMCs infected with SIV incorporate Nef into EVs. To assay if we could recapit-ulate our in vivo infections of macaques in an in vitro model, we isolated peripheralblood mononuclear cells (PBMCs) from four SIV-naive macaques (see Materials andMethods). The cells were activated with concanavalin A (ConA) and interleukin-2 (IL-2)for 48 h and then mock treated or treated with the reverse transcriptase inhibitor AZTor the viral protease inhibitor NFV. The cells were then infected with SIVmac239 at amultiplicity of infection (MOI) of 10. Detection of intracellular Gag was done by flowcytometry and showed successful infection of the cells. Importantly, the percentage ofcells that stained positive for SIV Gag p27 was much lower after treatment with AZT andNFV (Fig. 9A to D). This was expected, as virus infection is inhibited in cells treated withAZT and maturation of the virus particle is inhibited in cells treated with NFV. However,full inhibition of p27 detection was not accomplished by these two methods (seebelow). The supernatant of the cells was harvested, and total EVs were isolated. The sizedistribution profiles of the treatment groups were similar (Fig. 9E), and no changes inmean or mode sizes were identified (Fig. 9F). The concentrations of EVs (Fig. 9G) andtheir enzymatic activity were remarkably similar as well (Fig. 9H). We next asked if Nefcould be captured by CD63 antibody-coated beads, similar to the approach used in our A B Plus EV C Fluorescence Fluorescence DPre-SIV No ART E Pre-SIV+ART M O P FIG 8 Detection of Nef in EV-transduced cells. (A) Primary CD4+ T cells were analyzed for intrinsic fluorescence (PE gate) and gated. (B) Primary CD4+ T cellstreated with labeled EVs were gated into EV-positive and EV-negative populations on the basis of fluorescence. (C) Representative scheme for detection of Nef(FITC channel) by using the gates set up for panel B. Solid gray fill represents EV-negative cells, and a red line represents EV-positive cells. (D) EVs isolated frommacaque IV55 preinfection with SIV were labeled and added to purified primary CD4+T cells. Cells were gated as for panels B and C, and the FITC shift wasabsent (no Nef). EVs isolated from macaque IV55 at weeks 4 (E), 8 (F), 10 (G), and 16 (H) postinfection with SIV were added to CD4+ T cells. Cells were gatedas for panels B and C, and the FITC shift (Nef) in EV-positive cells was observed. (I) EVs isolated from macaque IV55 preinfection with SIV were labeled and addedto CD4+ T cells in the presence of ART drugs. Cells were gated as for panels B and C, and the FITC shift was absent (no Nef). EVs isolated from macaque IV55at weeks 4 (J), 8 (K), 10 (L), and 16 (M) postinfection with SIV were added to CD4+ T cells in the presence of ART drugs. Cells were gated as for panels B andC, and the FITC shift (Nef) in EV-positive cells was observed. (N) Plot comparing the MFI of the Nef signals in EV+ and EV- cells compiling all time points afterSIV infection in independent experiments. Each symbol represents the MFI of Nef in EV+ cells divided by the MFI of Nef in EV- cells in the same well. The ratioscluster around 1 at preinfection time points, verifying that Nef is absent from EVs isolated from animal IV55 before SIV infection. (O) Primary CD4+ T cells weremock treated (solid gray) or infected with SIVmac239 (redline), and an FITC shift (Nef) was detected only in infected cells.(P) Same as panel O but cells weretreated with ART drugs to block infection. A B C D Nef 123456 7 89 10 11 12 FIG 9 Detection of Nef in EVs from SIV-infected primary cells. (A) Primary PBMCs were isolated from rhesus macaques, mock treated, andstained for intracellular SIV Gag (p27) after 5 days.(B) Primary PBMCs were isolated from rhesus macaques, infected with SIVmac239 at anMOI of 10, and stained for intracellular SIV Gag 5 days postinfection. (C) Primary PBMCs were isolated from rhesus macaques and treatedwith the reverse transcriptase inhibitor AZT (100 nM). Twenty-four hours later, the cells were infected with SIVmac239 at an MOl of 10 andstained for intracellular SIV Gag 5 days postinfection. (D) Primary PBMCs were isolated from rhesus macaques and treated with the viralprotease inhibitor NFV (100nM). Twenty-four hours later, the cells were infected with SIVmac239 at an MOI of 10 and stained for intracellularSIV Gag 5 days postinfection. (E) Size distribution analysis of EVs isolated from the supernatant of simian macaque primary PBMCs infectedwith SIVmac239 (see Materials and Methods). Videos of EV populations were taken to determine size distributions (11 measurements pergroup with a total of three biological replicates). The peak size was arbitrarily set to 1 for each group.(F) Mean and mode sizes of EVs fromthe PBMCs treated for panel A. n =3 per group. (G) Total EV concentration (particles per milliliter) in supernatant of PBMCs treated for panelA. n =3 per group. (H) Esterase activity of EVs isolated from the PBMCs treated for panel A. All values are standardized to the Mock/Mockgroup. n= 3 per group. (I) SIV Nef is present in CD63+ affinity-purified EVs taken from infected PBMCs. Total EVs from panel A were addedto CD63 antibody-coated beads and assayed for the presence of Nef. Input, total EV population; flowthrough (FT), unbound fraction; CD63Beads, contents bound to CD63 beads. in vivo macaque infections. Tetraspanins such as CD63 and CD81 were readily detectedin the total EV fraction (input) of all of our treatment groups,as was the EV markerflotillin 2 (lanes 1 to 4). Nef was present only in SIV-infected samples, and its presencewas greatly reduced in cells treated with AZT (lane 3) and to a lesser extent in cellstreated with NFV (lane 4). Nef was present in the flowthrough fraction (lanes 6 to 8),indicating that not all Nef is present in CD63+ EVs, consistent with previous reports ofthe protein being present in viral particles (18, 45). We were able to detect Nef in theCD63+ EV fraction of SIV-infected PBMCs (lane 10) and at lower levels in cells treatedwith AZT (lane 11) and NFV (lane 12). The detection of Nef in AZT-treated samples waslikely due to the inability of the drug to completely block infection; detection of Nef inNFV-treated cells was expected,as the drug targets the maturation of the viral particle,which occurs long after Nef production. DISCUSSION The role of EVs, particularly exosomes (EVs ≤100 nm in diameter with a definedmaturation pathway), in virus infection has received considerable attention in recentyears. Exosomes are a class of EVs that originate from the inward budding of endo-somes into the MVB and subsequent release into the supernatant (reviewed in refer-ences 1 and 52). The mechanism of incorporation of Nef into EVs is currently unknown.We suspect that this incorporation occurs within maturing late endosomes and/or theMVB on the basis of our analysis of Nef colocalization with CD81, although a higher-resolution approach is necessary. We therefore refrained from characterizing the puri-fied EVs as "exosomes,"as these vesicles emerge from the cell exclusively from an MVB.Moreover, within "exosomes," subpopulations can be distinguished on the basis of EVmarker protein expression (43, 65,66). These studies show Nef to be present in thetetraspanin CD63- or CD81-positive fraction of EVs. Further investigation of the proteincomposition of HIV and SIV Nef EVs and how Nef traffics inside the cell is indicated. The majority of prior studies on EVs and Nef were conducted by using cell culture-based experimental designs, as in vivo studies remain limited by the availability of largevolumes of body fluids positive for HIV or SIV. It was thus gratifying to be able to detectSIV Nef in routine plasma samples from naturally infected animals. SIV has maintaineda zoonotic transmission cycle in macaques for >32,000 years (67).In contrast, HIVemerged in the human population in the late 19th/early 20th century from SIV (68-71).We speculate that the continuous transmission of the virus over tens of thousands ofyears allowed it ample time to evolve high-affinity interactions with multiple hostsignaling and vesicle maturation pathways,including those that yield and are mediatedby EVs. Characterization of the EV-SIV Nef interaction will complement similar studiesof the EV-HIV Nef interaction and add the experimental accessibility of the SIV nonhu-man primate model. This study was also motivated by the controversy surrounding the detection of HIVNef in EVs. Several groups have reported that HIV Nef is present in EVs (35, 43, 44,72);others have contested these findings (46). It is worth noting that not all groups isolateEVs in the same manner. We chose to isolate them with the crowding agent polyeth-ylene glycol (PEG) 8000 by a method similar to that described in reference 49, asrepeated high-speed ultracentrifugation can disrupt the integrity of small vesicles.Low-speed centrifugation, followed by antibody-mediated affinity purification, retainedEV function, as explored by multiple assays. Some have reported that tetraspaninmolecules, the targets of our affinity purification, can also be associated with HIVparticles, particularly those produced from macrophages (73, 74). We did not detect anyGag carryover after affinity purification, though we cannot rule out the possibility thatminute amounts of virus would be present in EVs purified from plasma. Regardless, Gagis not required for Nef incorporation into EVs, as we observed both HIV and SIV Nefproteins in EVs taken from transiently transfected cells. In the tissue culture setting orto evaluate the EV association of individual proteins, single-step purification of EVs byeither ultracentrifugation or PEG precipitation is convenient and suitable; however, for in vivo samples, a second step is needed to remove coprecipitating particles such ashigh-density lipoproteins and, in the case of virus infection, virion particles. Nef was present in EVs from multiple animals at multiple time points after infection,and no correlation with the CD4 count or SIV load was observed until the very endstage of sAIDS in one animal. The heterogeneous population of circulating vesicularbodies in vivo can make biochemical characterization difficult. Key differences caninclude, but are not limited to, surface markers, incorporated proteins and nucleic acids,and different physical characteristics such as size (75-79). Hence, we used TEM andnanoparticle size distribution to ascertain the physical properties of EVs. This studyadhered to a recommendation from the International Society for Extracellular Vesiclesthat defines minimal requirements for defining EVs (80) by using three or more markersto attribute the presence of Nef to EVs in both tissue culture and in vivo settings. The process by which EV-delivered proteins disseminate after delivery is largelyunknown. We observed that EVs delivered proteins (labeled green with our pan-EVstain ExoGreen) into punctate structures, which are likely intermediates of the endo-somal trafficking network and have been previously observed with Nef localization (81)A well-studied role for Nef in productively infected T cells is the degradation of surfacemolecules such as CD4 and major histocompatibility complex class I (22, 23, 30, 31). Toaccomplish this, Nef must localize to the inner leaflet of the plasma membrane. We didnot observe Nef dispersion throughout the cytoplasm upon delivery by EVs to CD4-negative, Lck-negative HUVECs, which are the most likely target of EV-transferred Nef.This could be due to the small amounts of Nef being delivered via this mechanism,its.diffusion to the point of no longer being detectable by standard fluorescence methods,or the requirement of T lineage-specific cofactors. Identifying the fate of EV-deliveredproteins will increase our understanding of how viruses usurp this pathway to delivervirus-encoded factors to neighboring cells and elicit paracrine phenotypes.Overall, thisstudy supports a larger theme whereby viruses utilize EVs to facilitate long-termpersistence. The list of viruses that utilize EV/exosome biogenesis or signaling consistsof evolutionarily distinct viruses such as herpesviruses, flaviviruses, picornaviruses, andothers (5, 11-14, 78, 82). With this study, we propose that lentiviruses such as SIV andHIV also use this pathway of extracellular communication for pathogenesis. Ethics statement. All of the macaques used in this study were maintained at the Tulane NationalPrimate Research Center (TNPRC), and care was provided by the staff in accordance with all institutionalquidelines and recommendations. slV load assay. SlV titers and genome copy numbers were determined by qRT-PCR as previouslydescribed (83). EV isolation. EVs were isolated from HEK-293 cells, macaque plasma, and PBMC culture supernatantby a method similar to that of Rider et al. (49). In brief, 50 ml of HEK-293 cell supernatant, 3 ml of plasma,or 45 ml of PBMC supernatant was centrifuged at 800 × g at 4℃ for 10 min to remove cells and cellulardebris. The supernatant was then transferred to new tubes and centrifuged at 16,000 ×g at 4℃ for30 min to pellet larger vesicles such as apoptotic bodies and MVs. Supernatant was collected and filteredthrough a 0.22-um filter, and EVs were precipitated out of solution by the addition of PEG 8000 to a finalconcentration of 40 mg/ml (Fisher Scientific) (stock was made at 400 mg/ml in 1× PBS [pH 7.4] andmaintained at 4℃). EVs were allowed to precipitate for >8 h at 4℃ during nutation and pelleted at1,200 × g at 4℃ for 60 min. EV labeling. To label EVs with the membrane dye Dil, the PEG precipitate was incubated with 1 uMVybrant CM Dil (Life Technologies, Inc.) and 100 ug/ml RNase A (Roche) in 500 ul of PBS at 4℃ for 1 h.As a negative control,1 uM Dil and 100 ug/ml RNase A were added to a separate tube containing 500 ulof 1× PBS (EV resuspension volume). While the EV solution was incubating with RNase A and Vybrant CMDil, Sephadex G-75 (GE) columns were equilibrated with 5 volumes of cold 1× PBS. Excess PEG 8000, Dil,RNase A, and non-EV-associated proteins/peptides were removed by adding the EV suspension toequilibrated columns. EVs were eluted from the columns with 1 ml of fresh, cold 1× PBS. EVs were thenquantified (see below), and 100-ul aliquots were either added immediately to target cells or placed at-80℃ for future use. For labeling with the membrane-permeating esterase reporter ExoGlow (SBI), the PEG precipitate wasincubated with 1× (50 ul) ExoGlow and 100 ug/ml RNase A at 37℃ for 10 min. Excess RNase A,nonincorporated dye, and proteins/peptides were removed with Sephadex G-75, and labeled EVs wereeluted as outlined above. EVs were quantifed and added immediately to target cells, or 100-ul aliquotswere placed at-80℃ for future use. For labeling with the self-quenching dye R18 (Thermo Fisher), a similar approach was taken inaccordance with Montecalvo et al.(63). In brief, affinity-purified EVs were incubated with 50 uM dye atroom temperature for 30 min. Excess dye was removed with G-25 spin columns (GE), and EVs werequantified and then added immediately to target cells (see below). EV affinity capture. To affinity purify EVs, ExoCap (JSR) beads, which are coated with antibodiesdirected against CD63 and CD81, were used. One hundred microliters of bead slurry was added to each100-pl aliquot of EVs. Beads were washed three times with washing/dilution buffer and eluted either with40 ul of exosome elution buffer or directly with Laemmli protein loading buffer. EM. SIV capsid was purified from the PEG precipitation step through immunoprecipitation with anSIV p27-specific antibody and EVs by CD63/CD81 bead purification. The final product was allowed toadsorb to glow-charged carbon-coated 400-mesh copper grids for 3 min and then stained with 2%(wt/vol) uranyl acetate in water. TEM images were obtained with a Philips CM12 electron microscope at80 kV and captured on a Gatan Orius camera (2,000 by 2,000 pixels) with Digital Micrograph software(Gatan, Pleasanton, CA). Protein analysis. For total protein analysis, EVs were diluted to equal concentrations and run onpolyacrylamide gels. Bands were visualized by silver staining (Thermo Fisher). For immunoblot analysis,EVs and control fractions were loaded onto a polyacrylamide gel and transferred onto a nitrocellulosemembrane (GE). Membranes were blocked with 7% dry milk in Tris-buffered saline with Tween 20 (TBST)at room temperature for 1 h. The primary antibodies used were diluted in 7% dry milk in TBST to thefollowing final concentrations: GAPDH (Abcam 9485), 200 ng/ml; flotillin 2 (Cell Signaling 3244), 100 ng/ml; histone H3 (Abcam 1791), 200 ng/ml; SIVmac239 p27 monoclonal (4B2) (AIDS Reagent Database2321), 1 ug/ml; SIV Nef monoclonal (clone 17.2) (AIDS Reagent Database 2659), 1 ug/ml; CD63 (H-193)(Santa Cruz Biotech sc-15363), 200 ng/ml; CD81 (Q-14) (Santa Cruz Biotech sc-31234), 200 ng/ml; GFP(ab290), 100 ng/ml. Secondary fluorescent antibodies were also diluted in 7% dry milk in TBST to thefollowing final concentrations: donkey anti-rabbit lgG IRDye 800CW (LiCor P/N 926-32213), 100 ng/ml;donkey anti-goat lgG IRDye 680RD (P/N 926-68074), 100 ng/ml; donkey anti-mouse lgG IRDye 680RD(LiCor P/N 926-68072), 100 ng/ml. Peroxidase-labeled secondary antibodies were diluted in TBST to afinal concentration of 100 ng/ml. Western blot assay images were obtained with the LiCor Odysseysystem or X-ray film. Cell culture, transfection, and EV adsorption. The plasmid encoding HIV Nef-HA was a gift fromWarner Green (Addgene plasmid no. 24162). Human CD81-mCherry was a gift from Michael Davidson(Addgene plasmid no. 55012). Plasmids encoding Nef fused to GFP in the pCG vector were a gift fromFrank Kirchhoff (84). HEK-293 and human osteosarcoma (U2OS) cells were obtained from the AmericanType Culture Collection and grown in Dulbecco's modified Eagle medium (DMEM) supplemented with10% EV-free fetal bovine serum (Sigma) and 1×Pen/Strep (Gibco) at 37℃ at 5% CO . HEK-293 cells were transfected with 5 ug of plasmid by using Lipofectamine 2000 (Invitrogen). Inbrief, 5 ug of plasmid was added to 500 ul of unsupplemented DMEM, and 20 ul of Lipofectamine wasadded to a separate tube containing 500 ul of unsupplemented DMEM. The Lipofectamine mixture wasthen added to the plasmid mixture, mixed thoroughly, and incubated at room temperature for 15 minprior to dropwise addition to the cells. To monitor EV adsorption, 2.5 ×105 cells were seeded into six-well tissue culture plates to a totalvolume of 3 ml in serum-free medium. For cells treated with EVs labeled with Vybrant CM Dil, increasingamounts of labeled EVs (5 ×108 to 5×1010/ml) were added to the cells and allowed to adsorb for 2 h.Cells were then trypsinized and analyzed by flow cytometry. U2OS cells treated with EVs labeled withExoGlow Green were grown on coverslips and treated with 1010 EVs for 8 h in serum-free medium. Cellswere then prepared for fluorescence imaging (see below). For analysis of lipid fusion (R18 fluorescenceassay), cells were grown in 96-well tissue culture plates. Twenty-four hours after cell seeding, R18-labeledEVs were added at a concentration of 8 × 1010 particles/ml.Fluorescence of cells was monitored with theFLUOstar Optima (BMG Labtech). The mean fluorescence intensity (MFI) of four independent wells wasdetermined. Initial baseline fluorescence readings (no EVs added) were taken 5 min prior to the additionof EVs and arbitrarily set to 1. Fold fluorescence was then calculated for each time point after EV addition.For the wells containing only EVs, a similar approach was taken. At the end of the time course, TritonX-100 was added to a final concentration of 0.1% to burst EVs and release quenched R18. Development of stable CD81-mCherry cell lines. U2OS cells were transfected with CD81-mCherry/Neo vector (Addgene plasmid no. 24162) and selected with 500 ug/ml Geneticin (G418 salt;Gibco). After7 days of selection, cells were sorted with the FACSAria Il at the University of North Carolina FlowCytometry Core.Highly fluorescent cells were sorted and maintained under continuous selection withmedium supplemented with 250 ug/ml Geneticin. The same process was used for the dual CD81-mCherry and CD63-enhanced GFP cell line that was used as a positive control. Immunofluorescence assay. To detect EV-transferred Nef-HA, we performed an immunofluores-cence assay. As the primary antibody, we used a mouse anti-HA antibody (ab18181) or normal mouseserum diluted to 1 ug/ml in 5% bovine serum albumin (BSA). For the secondary antibody, we used anAlexa Fluor 647-conjugated goat anti-mouse lgG antibody (ab150115) diluted at 200 ng/ml in 5% BSA.4',6-Diamidino-2-phenylindole (DAPI) was added to a final concentration of 100 ng/ml, and coverslipswere mounted onto slides with Vectashield (Vector Laboratories). Images were taken and processed as indicated below. To detect EV transfer, cells were grown on coverslips, treated with ExoGlow Green EVs(or ExoGlow in PBS filtered through G-75 columns as a negative control), and fixed with 4% parafor-maldehyde at room temperature for 15 min. Cells were then washed twice with 1× PBS, permeabilizedwith 0.5% Triton X-100 at room temperature for 15 min, and again washed twice with 1×PBS. DAPI wasadded to a final concentration of 100 ng/ml, and coverslips were mounted onto slides with Vectashield(Vector Laboratories). Microscopy and three-dimensional reconstruction. Z-stack images were taken with a LeicaDM5500 microscope with a 63× objective and a Retiga-2000RV camera (Qlmaging). Images weredeconvoluted with MetaMorph v 7.8.12.0 (Molecular Devices) and visualized with Imaris v 8.3.1 (Bitplane). Flow cytometry. A total of 5 × 105 U2OS cells grown in a six-well plate were monitored for EVadsorption with the MACSQuant VYB flow cytometry machine. Gates for forward and side scatter wereset with FlowJo v10.1, and the percentage of positive cells was calculated. Detection of Nef by flow cytometry. PBMCs were obtained from blood from SIV-naive TNPRCcolony animals. CD4+ T cells were magnetically isolated from the PBMCs with CD4 microbeads (Miltenyi).These cells were activated with ConA for 2 days and cultured in the presence of 50 U/ml IL-2 (PeproTech).A total of 750,000 CD4+ T cells were incubated with 1.5 × 101° EVs (isolated at time points pre- andpostinfection with SIVmac239) in a final volume of 250 ul in a 48-well plate. After 4 h, the volume wasincreased to 1 ml. Cells were harvested 24 h later, washed, fixed, and then permeabilized, and theanti-Nef monoclonal antibody (clone 17.2) and an FITC-labeled anti-mouse secondary antibody wereadded. Data were collected with a BD LSRII instrument and analyzed with FlowJo v10.0.8. For EV-adsorbed cells, differential gating of EV-positive and EV-negative cells allowed for comparison of Nefexpression in the two gates. In some cultures, two ART drugs, AZT and NFV, were added at 100 nM atthe beginning of the experiment to prevent virion maturation and de novo expression of Nef. Infection of PBMCs with SIV from naive macaques. PBMCs were isolated from 60 ml of blood fromeach of four SIV-naive macaques. CD8-positive cells were removed by magnetic separation (Miltenyi).CD8-negative cells from the four animals were mixed and cultured with ConA for 2 days prior toinfection. ConA was then removed, and the cells were split into four cultures of approximately 90 millioneach in medium containing 15% exosome-depleted serum and 50 U/ml IL-2. Two of the cultures weremaintained with ART drugs; one was maintained with 100 nM AZT, and the other was maintained with100 nM NFV. Twenty-four hours later, the cells were infected with SIVmac239 at an MOl of 10 andmaintained for 5 days. As a control group, one flask was left uninfected. Medium containing the ARTdrugs was replaced every other day. After 5 days,culture supernatant was removed and clarified for EVcharacterization as outlined above. SUPPLEMENTAL MATERIAL Supplemental material for this article may be found at https://doi.org/10.1128/mBio.02344-17. FIG S1, TIF file, 0.8 MB. FIG S2, TIF file, 0.3 MB. MOVIE S1, MOV file, 8.4 MB. MOVIE S2, MoV file, 8.4 MB. MOVIE S3, MOV file, 8.3 MB. MOVIE S4, MOV file, 8.3 MB. MOVIE S5, MOV file, 8.4 MB. MOVIE S6, MOV file, 8.1 MB. ACKNOWLEDGMENTS We thank B. A. Eason,C. Caro-Vegas,and C. J. Sherrod for their constructive feedbackon the preparation of the manuscript, as well as D. A. Hicks for help with microscopyimaging. This work was supported by TNPRC base grant OD11104 (formerly RR00164);MH07544 and R21 MH113517 to A.G.M.; National Institute of General Medical Sciences(NIGMS), National Institutes of Health (NIH), Training, Workforce Development andDiversity division K12GM000678 to L.M.C.; T32AI007151-38 to R.P.M.; and 1R01DA040394 toD.P.D. The University of North Carolina Flow Cytometry Core is supported by the P30CA016086 Cancer Center Core Support Grant to the UNC Lineberger ComprehensiveCancer Center. REFERENCES ( 1 . Raab-Traub N, Dittmer DP. 2017. Viral effects on the content a nd func- t ion o f e xtracellular vesicles. Nat Rev Microbiol 15:559-572. htt p s:// do i.org/ 1 0 .1 038/nrmic r o.2017.60 . ) 2. Meckes DG, Gunawardena HP, Dekroon RM, Heaton PR, Edwards RH, Ozgur S, Griffith JD, Damania B,Raab-Traub N.2013. Modulation of B-cellexosome proteins by gamma herpesvirus infection. Proc Natl Acad SciU S A 110:E2925-E2933. https://doi.org/10.1073/pnas.1303906110. ( 3. Meckes D G, Jr., Shair KHY, Marquitz AR, Kung CP, E dwards R H , R a ab- ) ( T raub N .2 0 10. Human tumor virus util i zes exosomes for i nte r cellular communication. Proc Natl Acad Sci U S A 107:20370-20375.ht tps://doi . o r g/ 1 0 .1 073/pn a s.10141 9 4107. ) ( 4. Wang Z , D eng Z , Dahmane N, T s ai K , Wang P, W i lliams DR, KossenkovAV, Showe LC, Zhang R, Huang Q, C onejo-Garcia JR, Lieberman PM. 2 015. Telomeric repeat-containing RNA (TERRA) constitutes a nucleopro-tein component of extracellular inflammatory exosomes. P roc Natl AcadSci U S A 1 12 :E6293-E6300. h ttps: // doi . org/10.1073/ p na s.1 5 05962112. ) ( 5. Bukong TN, M o men-Heravi F, Ko d ys K, B ala S, Sz a bo G. 20 1 4. Ex o somes from hepatitis C infected p atients t ransmit HCV infection a n d co n tainreplication competent viral RNA in complex with Ago2-miR122-HSP90.PLoSPathog 10:e1004424.ht tps://doi . org/10.1 3 71/jour na l.p pa t . 1004424 . ) ( 6 . Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, ChengHH, Arroyo JD, Meredith E K , G a llichotte E N, P ogosova-Agadjanyan EL,Morrissey C, Stirewalt DL, Hladik F, Yu EY, Higano C S, Tewari M . 2014.Quantitative a nd stoichiometric a nalysis of the m icroRNA content of exosomes. Proc Natl Acad Sci U S A 1 11:14888-14893. h t t ps:/ /do i.org/ 10.1073/pnas.140830 1 11 1 . ) ( 7 . Melo S A , Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vi d al A, Qiu L, Vitkin E, Perelman L T, Melo CA,Lucci A, Ivan C, Calin GA, Kalluri R. 2014. Cancer exosomes perform c ell-independent microRNA b iogenesis andpromote tumorigenesis. C ancer Cell 26:707-721. h t tps:/ / doi . org/10 .1 016/j.ccell . 201 4 . 0 9.005. ) ( 8. L i J, Li u K, Liu Y, Xu Y, Zhan g F, Yan g H, Liu J, Pan T, Chen J, Wu M, Zhou X, Yuan Z . 2 013 . Exosomes mediate th e ce l l-to-cell t ransmission o f IFN-alpha-induced antiviral activity. Nat I mmunol 14 : 793-803. http s :// doi.org/ 1 0. 1 038/ni. 2 647. ) ( 9 . V ojtech L, Woo S, Hughes S, Levy C, Bal l weber L, Sauteraud RP, Strobl J, Westerberg K, Gottardo R,Tewari M, Hladik F. 2014. Exosomes in human semen c a rry a d i stinctive r epertoire of small non-coding RNAs withpotential regulatory functions. Nucleic Acids Res 42:7290-7304.h ttp s :/ /doi.or g /10.1093/nar/gk u 347. ) ( 10. Chugh PE, Sin S H, O zgur S , Henry DH, Menezes P, Gr i ffith J, E ron JJ,Damania B, Dittmer DP . 20 1 3. Systemically circulating viral and tu m or-derived microRNAs i n KSHV-associated m a lignancies. P LoS Pathog9:e1003484.h t tps://doi . org/10. 13 71/jo u rnal . ppat. 1 003484. ) ( 11. Kalamvoki M, D u T, Roizman B. 2014. Cells i n fected with h e rpes simplex virus 1 export to uninfected c ells exosomes c ontaining S TING, v iral mRNAs, and microRNAs. Proc Natl A cad Sci U S A 111:E4991-E4996. https :/ /doi.or g /10.1073/p n as.1 4 19338111 . ) ( 12. Ramakrishnaiah V,Thumann C, Fofana l, H abersetzer F, Pan Q, de Ruiter PE, Willemsen R , Demmers JAA, Stalin R aj VS, Jenster G , Kwekkeboom J,Tilanus HW, Haagmans B L , Baumert T F , van d e r L a an LJW. 2013.Exosome-mediated t r ansmission o f h epatitis C viru s between human hepatoma H u h7.5 c e lls. Proc Natl Acad Sci U S A 110:13109-13113. https://doi.org / 10 . 1073/ p n a s . 1221 8 99110. ) ( 13. Feng Z , Hensley L , McKnight KL, H u F , Madden V, Pi n g L, Jeong SH , Walker C, Lanford RE, Lemon S M. 2 0 13. A p a thogenic p i cornavirusacquires an e nvelope by h i jacking c ellular membranes. Nature 4 9 6:367-371. h ttps:// d oi.org/10.1038/nature1 2 029. ) ( 14. Longatti A , B oyd B , C h isari F V . 2 0 15. V i rion-independent transfer ofreplication-competent hepatitis C virus RNA between permissive cells. JVirol 89:2956-2961.h tt ps: //doi.org / 1 0. 1 128/JVI.02721- 1 4 . ) ( 15. Sharp PM, Hahn BH. 2011. Origins of HIV and the AIDS pandemic. Cold Spring Harb P erspect M ed 1 : a006841. h t tps://doi.org / 10 . 1 1 01 / cshpe r spe c t.a006841 . ) ( 16. Wolfe ND, Switzer WM, Carr JK, Bhullar V B , Shanmugam V, Tamoufe U,Prosser AT, Torimiro JN, Wright A, Mpoudi-Ngole E , McCutchan F E , Birx DL, Folks TM, B urke D S, Heneine W. 20 0 4. Na t urally acquired simian r etrovirus infections in Central African hunters. Lancet 3 63:932-937. https://doi.org / 10.1016/S0140-6 73 6(04) 1 5787- 5 . ) ( 17. Veazey RS, DeMaria M , Chalifoux LV, Shvetz DE , Pa u ley DR, Kn i ght HL, Rosenzweig M , J o hnson RP, Desrosiers RC, Lackner AA. 1998. G a stroin- testinal tract as a major site o f CD4+ T cell d e pletion and vir a l re p lication in SlV i nfection. Science 280:427-431. ht t ps:// d oi.org/10.112 6 /science .2 8 0.5362. 4 27 . ) ( 18. Kirchhoff F, Schindler M, Specht A , Arhel N , Munch J . 2008. R o le o f Nef in primate lentivira l immunopathogenesis. Cell Mol Life Sci 65:2621-2636. h t tps://doi.org/10.1007/s0001 8 - 0 08-8094-2. ) ( 19. Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, H u nt PW, Hatano H, S owinski S , Munoz-Arias l , Greene WC. 2014. Cell death by pyroptosis drives CD4 T-cell d e pletion in HI V -1 infection. Na t ure 505 : 509-514. h t tp s: / /doi.org/10.1038/nat u re1 2 940. ) ( 20. Doitsh G , Greene WC. 2 0 16. D i ssecting how CD 4 T c e l l s are lost d u ring ) HIV infection. Cell Host Microbe 19:280-291. https://doi.org/10.1016/j.chom.2016.02.012. 21. Laguette N, Bregnard C, Benichou S, Basmaciogullari S. 2010. Humanimmunodeficiency virus (HIV) type-1, HIV-2 and simian immunodefi-ciency virus Nef proteins. Mol Aspects Med 31:418-433. https://doi.org/10.1016/j.mam.2010.05.003. ( 22. Roeth JF, Collins KL. 2006. Human i m munodeficiency vir u s type 1 N e f : adapting t o i n tracellular t rafficking pathways. M i crobiol Mol Bi o l Re v 70:548-563. h ttps://doi . org/10 . 1 1 28/MMBR.00042-05. ) 23. Piguet V, Gu F, Foti M, Demaurex N, Gruenberg J, Carpentier JL, Trono D.1999. Nef-induced CD4 degradation: a diacidic-based motif in Nef functionsas a lysosomal targeting signal through the binding of beta-COP in endo-somes. Cell 97:63-73. https://doi.org/10.1016/S0092-8674(00)80715-1. ( 24. S anfridson A, Hester S , Doyle C. 1997. Nef proteins encoded b y humanand s imian immunodeficiency viruses i n duce the accumulation of en- dosomes and l y sosomes i n human T ce l ls. P roc Natl Acad Sci U S A 94:873-878. h t tps:/ / doi.org/10.1073/pn as .94.3.873. ) 25. Mukerji J, Olivieri KC, Misra V, Agopian KA, Gabuzda D. 2012. Proteomicanalysis of HIV-1 Nef cellular binding partners reveals a role for exocystcomplex proteins in mediating enhancement of intercellular nanotubeformation. Retrovirology 9:33. https://doi.org/10.1186/1742-4690-9-33. 26. Kim S, Ikeuchi K, Byrn R, Groopman J, Baltimore D. 1989. Lack of anegative influence on viral growth by the nef gene of human immuno-deficiency virus type 1. Proc Natl Acad Sci U S A 86:9544-9548. https://doi.org/10.1073/pnas.86.23.9544. ( 27. H ill BT, Skowronski J . 2 005. H uman N-myristoyltransferases form stable complexes with le n tiviral N ef and o t her viral and cellular substrate proteins. J Virol 79:1133-1141.ht t ps: / / doi.org/ 10 .1 128 /JVI.7 9 .2.113 3 -114 1 .2 0 05. ) ( 28. Morita D, Igarashi T, Horiike M, M ori N, Sugita M. 2011. C u tting edge: T cells monitor N-myristoylation of the Nef protein in simian immunode- f iciency virus-infected monkeys . J Immunol 1 87:608-612. h ttps: / /doi.org /1 0.4049/jimmunol . 1 1 01216 . ) ( 29. Jager S, Cimermancic P, Gulbahce N, Johnson JR, McGovern K E , Clarke SC, Shales M, Mercenne G, Pache L, Li K, Hernandez H , Jang GM, Roth SL, Akiva E, Marlett J, Stephens M, D'Orso l, Fernandes J, Fahey M, Mahon C, O'Donoghue AJ, Todorovic A, Mo r ris JH , Maltby DA , Alber T, Cagney G, B ushman FD, Young JA, Chanda S K , Sundquist WI, Kortemme T , H ernan-dez R D, C raik C S, B urlingame A, Sali A, Frankel AD, Krogan NJ.2 0 11. Global landscape of HIV-human protein complexes. Nature 481:365-370. h ttps://doi.org/10.1038 / nature10719. ) ( 30. Aiken C, Konner J, Landau NR, Lenburg ME, Trono D . 1994. Nef inducesCD4 endocytosis: r e quirement f o r a cr i tical dileucine motif i n t he m embrane-proximal CD4 cytoplasmic domain. Cell 76:853-864.ht tp s: // doi.org/10.1016/0092-8674(94)90360- 3 . ) ( 31. Lindwasser OW, Chaudhuri R , Bonifacino JS. 2 007. M echanisms o f CD4 downregulation by the Nef and Vpu proteins o f primate immu- nodeficiency v iruses. Curr M ol M e d 7: 1 7 1-184. htt p s://d o i.org/ 1 0 . 21 7 4/ 15 6652407780059177 . ) ( 32. Piguet V , Schwartz O, L e Gall S, Trono D . 1999. The d ownregulation ofCD4 a n d MHC-I by primate lentiviruses: a p a radigm fo r the modulationof c ell surface receptors. Immunol Rev 1 6 8 :51-63. ht t ps://doi.org/10. 1 1 1 1 /j . 1 600-065X. 1 999.tb01282.x. ) ( 33. Kestler HW ⅢI, R ingler D J, M ori K , P anicali DL, Sehgal P K, Daniel MD,Desrosiers RC. 1991. Importance of the nef gene for maintenance of highvirus loads and f or development of AIDS. Cell 6 5 :651-6 6 2. ht t ps:// d oi .org /1 0. 10 16/0092- 8 674(9 1 )90097-I . ) ( 34. Kirchhoff F, G reenough TC, Bre t tler DB, Sullivan JL, Desrosiers RC. 199 5 . Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive H I V-1 i n fection. N Engl J Med 332:228-232. htt ps://d o i . org /1 0 . 1056/NEJM 1 9950126 3 320405. ) ( 35. Raymond A D , D i az P , C hevelon S, A gudelo M , Yndart-Arias A, Ding H, Kaushik A , J ayant RD, Nikkhah-Moshaie R, Ro y U, Pi l akka-Kanthikeel S, N air M P. 2 016. M icroglia-derived HIV Nef+ e xosome impairment of th e blood-brain b arrier is treatable by nanomedicine-based d e livery of Nef p eptides. J N eurovirol 22:12 9 -139. ht t ps:// d oi.org/1 0. 1007/s13365-015 -0397-0 . ) ( 36. C hakrabarti L A , Metzner KJ, Ivanovic T, C heng H, Loui s -Virelizier J, Con- nor R l, Cheng-Mayer C . 2003. A truncated form o f N e f s e lected duringpathogenic r eversion o f s imian i m munodeficiency vi r us SlVmac239Deltanef increases vi r al r e plication. J Virol7 7 :1245-1256. h t tp s:/ / doi . org/ 1 0.1128/JVI.77.2. 1 245-1256.2 0 03. ) ( 37. Whatmore A M , Cook N, Hall GA, Sharpe S, R ud E W, Cranage MP. 1 9 95. R epair and e volution of nef in vivo modulates simian immunodeficiency virus virulence. J Virol 69:5117-5123. ) ( 38. Huang Y, Zhang L , Ho D D . 1995. Characterization of nef sequences inlong-term survivors of human i m munodeficiency virus type 1 infection. J Virol 69:93-100. ) ( 39. Rhodes DI, Ashton L, Solomon A, Carr A , Cooper D, Kaldor J, Deacon N . 2000. Characterization of three nef-defectiv e human immunodeficiency virus type 1 s trains associated w ith long-term nonprogression. Aus t ra-lian Long-Term Nonprogressor St u dy Group. J V i rol 74 : 10581-10588. https://doi.org/10 . 1 1 28/JVI.74.22.10581-10588.2000. ) ( 40. James CO, Huang MB, Khan M, Garcia-Barrio M, Powell MD, Bond VC. 2004. E xtracellular Nef protein t argets C D4(+) T ce l ls f o r apoptosis by interacting with C XCR4 surface receptors. J Virol 78:3099-3109. htt ps : / /doi.org/10.1 12 8/J V I.78.6.3099-3109.20 0 4. ) ( 41. Flaherty MT, Barber SA, Clements JE. 1998.Neurovirulent simian immu-nodeficiency v irus i ncorporates a N e f-associated ki n ase ac t ivity intovirions. AIDS Res Hum Retroviruses 14:163-170. htt ps://doi.o rg /10.1089/ aid. 1 998. 1 4. 1 63. ) ( 42. Asztalos BF, Mujawar Z, Morrow MP, G rant A , Pushkarsky T , Wanke C,Shannon R, Geyer M , K i rchhoff F, S viridov D, Fitzgerald ML, Bukrinsky M,Mansfield K G. 2 010. C irculating Nef induces d yslipidemia in simian i mmunodeficiency v i rus-infected macaques by suppressing c holesterolefflux. J Infect Dis 202:614-623. h ttp s: //doi.org/10.1086/65 4 817. ) ( 43. L ee JH, Schierer S, Blume K, Di n dorf J, Wi t tki S, Xiang W, Ost a lecki C, Koliha N, Wild S, Schuler G, Fackler OT, Saksela K , Harrer T , Baur AS. 2016. H IV-Nefa n d A D AM17-containing pla s ma extracellular ve s icles i n duceand correlate with immune pathogenesis in ch r onic HI V in f ection. E B io-Medicine 6:103-113.ht tps://doi.org/10.101 6 /j . ebiom.2016.03.004. ) ( 44. Lenassi M, Cagney G, Liao M, Vaupotic T , Bartholomeeusen K, Cheng Y, Krogan NJ, P lemenitas A, Peterlin BM. 2 010. H IV Nef i s secreted in exosomes a n d triggers a p optosis i n bystander C D 4(+) T c ells. T r affic 11:110-122. h t t ps ://doi.org/10.1 1 11/j.1600-0854.20 0 9.0 1 0 0 6.x. ) ( 45. P andori M W, Fitch NJS, C r aig H M , Ri c hman DD, Spin a CA, Guatelli JC.1996. Producer-cell modification of human immunodeficiency virus type 1 :Nef is a v irion p rotein. J Virol 70:4283-4290. ) ( 46. Luo X , F an Y, P ark IW, He JJ. 2 015. E xosomes ar e un l ikely involved inintercellula r Nef transfer . PLoS One 1 0 :e0124436. ht t ps://doi. o rg/1 0 . 1371/journa l .pone.0 1 24436. ) ( 47. Garcia J V , Miller AD . 1991 . Serin e phosphorylation-independent down-regulation o f cell-surface CD4 by N ef. N ature 350:508-511. h tt ps:/ / doi . or g/ 1 0.1038/350508a0 . ) ( 48. Caby MP, Lankar D, Vincendeau-Scherrer C, R a poso G, Bonnerot C. 2005.Exosomal-like vesicles are present in human blood plasma.Int Immunol17:879-887. h tt ps ://doi . o r g/10.1093/intimm/dxh267. ) ( 49. Rider MA, Hurwitz SN, Meckes D G, Jr. 2016. E xtraPEG: a polyethyleneglycol-based m ethod for e n richment of ex t racellular v e sicles. Sci Rep6:23978. ht t ps://doi.org/10.1038/sre p 23978 . ) ( 50. Hemler ME. 2014. Tetraspanin p r oteins promote mu l tiple ca n cer st a ges.Nat R ev C ancer 1 4 :49-60. h t tps:/ / doi. o rg /1 0.1038/ n rc3640. ) ( 51. P ols MS, Klumperman J. 200 9 . Tra f ficking and function of the tetraspaninCD63. Exp Cell R es 315:1584-1592. ht tp s :/ /do i .org/10.10 1 6/j.y exc r .20 08 .09.0 2 0. ) ( 52. M eckes DG , Jr. , Raab-Traub N. 201 1 . Microvesicles and v iral infection. J Virol 85:12844-12854. h t tps:/ / doi.org/10.1128/JVI.05853-11. ) ( 53. Ghossoub R, Lembo F, Rubio A, Gai l lard CB, Bouchet J, Vi t ale N, Sl a vik J,Machala M , Z immermann P. 2014. Syntenin-ALIX exosome biogenesis and b udding i n to m u ltivesicular bodies a re controlled by ARF6 and PLD2. Nat Commun 5: 3 477.htt ps :/ /d oi .org/10. 10 38/nc o mms4477. ) ( 54. Antonyak M A , Cerione RA . 20 1 5. Em e rging picture of t he distinct tr a its and f unctions of microvesicles and exosomes. Proc Natl A cad Sci U S A 1 1 2:3589-3590.ht tps://doi.org/ 1 0.1073/pnas.15025901 1 2. ) ( 55. Baranyai T, Herczeg K, Onodi Z, Voszka l , Modos K, Marton N, Nagy G,Mager l, Wood MJ, El Andaloussi S, Palinkas Z, Kumar V, Nagy P, Kittel A, Buzas El, Ferdinandy P, G iricz Z. 2015. Isolation of exosomes from bloodplasma: q ualitative and q uantitative comparison of u l tracentrifugationand s ize exclusion chromatography methods. P L oS One 10:e0145686. https: / /doi.org/10.1371/journal.pone.0145686. ) ( 56. F uchs SP, M artinez-Navio JM, Piatak M , Lifson J D, Gao GP, Desrosiers R C. 2015. A AV-delivered a ntibody m e diates si g nificant p r otective effects against S IVmac239 ch a llenge in the abs e nce of neut r alizing acti v ity. PLoSPathog 11:e1005090. ht tp s:// d oi. o rg/10.13 7 1/journa l .ppat.1005090. ) ( 57. Navakanitworakul R , H ung W T, G unewardena S, D a vis JS, Chotigeat W, Christenson L K. 2016. Characterization and small RNA content of extra- cellular vesicles in follicular fluid of developing bovine antral follicles.SciRep 6 :25486-25486. h t tp s : / / doi.org/10. 1 038/srep2 5 486. ) ( 58. Konadu KA, Huang MB, Ro t h W, Ar m strong W, Powell M, V illinger F, ) ( Bond V. 2 016. I solation of exosomes f rom the plasma o f HIV-1 positiveindividuals. J Vis Exp. h ttp s://do i .or g/ 10.3791/53495. ) ( 59. Cantin R , Diou J , Belanger D, Tremblay AM, Gilbert C. 2008. Discrimina- t ion b etween e x osomes and HIV-1: purification of both ve s icles f r omcell-free supernatants. J Immunol Methods 33 8 :21-30. htt ps : / /d oi.org/ 1 0.1016/j .j im.2008.07.007. ) ( 60. S chiavoni l, T rapp S , Santarcangelo AC, Piacentini V, P ugliese K, Bau r A, Federico M. 2004. HIV- 1 Nef enhances both membrane expression andvirion incorporation of Env products-a model fo r the Nef-dependentincrease o f H IV-1 infectivity. J Bio l Chem 279:22996-23006.http s :// do i .org/ 1 0 .1 074/jbc.M312453200. ) ( 61. Sougrat R, B artesaghi A , Lifson JD, Bennett AE, Bess JW, Z abransky DJ,Subramaniam S. 2007. Electron tomography o f the contact b etween Tcells and S IV/HIV-1: i mplications f or viral e ntry. P LoS Pathog 3: e 63. h t tp s://doi . org / 10 . 137 1 / j ournal . p pa t.0030063. ) ( 62. Grief C, Hockley DJ, Fromholc CE, K itchin PA. 1989. The m orphology of simian immunodeficiency virus as s hown b y negative s t aining electron microscopy. J G en V i rol 7 0 :2215-2219. ht t p s://doi.org/ 1 0. 10 99/0022 - 131 7 -70-8-2215 . ) ( 63. Montecalvo A , Larregina AT, Shufesky WJ, S tolz DB, Sullivan MLG,Karlsson JM, Baty C J, Gibson GA, Erdos G, Wang ZL, Milosevic J,TkachevaOA, D ivito S J, Jordan R , L yons-Weiler J, W atkins SC, Morelli AE. 2012. Mechanism of t r ansfer o f functional microRNAs between mouse den-dritic cell s via exosomes. Blood 119:756-766. h t tps: / /doi . org/1 0 .1182/blood-2011-02-338004. ) ( 64. Weiler AM, Das A, Akinyosoye O, Cui S , O'Connor SL, S c heef EA, R e ed JS, Panganiban AT, Sacha JB, R a kasz EG, Friedrich T C , M a ness NJ . 2015. A cute v i ral e scape s e lectively impairs Nef-mediated ma j orhistocompatibilit y complex clas s I downmodulation and increasessusceptibility t o a ntiviral T c ells. J V irol 9 0:2119-2126. htt p s:// d oi. or g/10. 1 128/JVI.01975 - 15 . ) ( 65. H urwitz SN, Rider MA, B u ndy JL , L iu X , Singh R K , M e ckes DG , Jr. 2016. P roteomic profiling o f NCI-60 e x tracellular vesicles uncovers c ommonprotein cargo a nd cancer t y pe-specific b iomarkers. O ncotarget 7 :86999-87015. ht t p s: //do i .org/10 . 18632/oncotarget.13 5 69. ) ( 66. Hurwitz SN, N kosi D, Conlon M M , Yo r k SB, Li u X, Tremblay DC, Meckes DG, J r. 2017. CD63 regulates Epstein-Barr virus LMP1 exosomal packag- i ng, enhancement of ve s icle production, and noncanonical NF-KB sig-naling. J Virol 91:e02251-16. h ttps: / /doi . org/10.1128/JVI.02251- 16. ) ( 67. W orobey M, Telfer P, Souquiere S, H u nter M, Coleman CA, Met z ger MJ, R eed P, M akuwa M, Hearn G, Ho n arvar S, Roques P, Ap e trei C, Kazanji M, M arx PA. 2010. I sland b iogeography reveals t he d eep h i story of S IV. Science 329:1487-1487.h t tp s: //doi. or g/10.1126/sc i ence . 1193550. ) ( 68. Gao F , B ailes E, Robertson DL, Chen YL, Rodenburg CM, M i chael S F ,Cummins LB, A r thur LO, Peeters M , Shaw GM, S h arp PM, Hahn BH . 19 9 9.Origin o f HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature397:436-441. h tt p s://doi . org/10 . 1038/ 1 7130. ) ( 69. C hen Z , L u ckay A, Sodora DL, Telfer P, R e ed P, Gettie A, K a nu JM , Sa d ek RF, Yee J, Ho DD, Zhang L, Marx PA. 1997. Human immunodeficiency virus type 2 ( HIV-2) seroprevalence and characterization o f a d i stinct H IV-2 g enetic s ubtype from t h e natural r a nge of simian im m unodefi-ciency virus-infected sooty m angabeys. J V irol 7 1 :3953-3960. ) 70. Peeters M, D'Arc M, Delaporte E. 2014. Origin and diversity of humanretroviruses. AIDS Rev 16:23-34. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4289907. 71. Hirsch VM, Olmsted RA, Murphey-Corb M, Purcell RH, Johnson PR. 1989.An African primate lentivirus (SIVsm) closely related to HIV-2. Nature339:389-392. https://doi.org/10.1038/339389a0. ( 72. Shelton MN, H uang M B , Ali SA, Powell MD, B o nd VC. 2012. S e cretionmodification r egion-derived peptide disrupts H IV-1 Nef's interaction with m ortalin and b l ocks virus a n d Ne f exosome release. J Virol 86: 406-419. ht t ps : //do i .org/10. 1 1 2 8/JV I .0 5 720 - 11. ) ( 73. Chertova E, C hertov O , C o ren LV , Ro s er JD, Tru b ey CM, Bess JW, Jr., S owder R C , I I , Barsov E, Hood B L, Fisher RJ, Nagashima K, Conrads TP, V eenstra TD, Lifson JD, Ott DE. 2006. Proteomic and biochemical an a lysisof purified human i mmunodeficiency v i rus type 1 p roduced from in-fected m onocyte-derived macrophages. J Virol 80:9039-9052. h ttp s:// doi.org/10. 1 1 2 8/ J VI.0101 3 - 0 6. ) ( 74. Pelchen-Matthews A, Kramer B, Marsh M. 2003. Infectious HI V -1 as s em- b les in late endosomes in primary macrophages. J Cell Biol 162:443-455. https:/ / do i .org / 10.1083/ j cb.200304008. ) ( 75. Kowal J, Arras G, Colombo M , Jouve M, Morath J P , Primdal-Bengtson B , DingliF, Loew D, Tkach M, Théry C. 2016. Proteomic comparison defines novel m arkers t o characterize h e terogeneous populations of extracellu- ) ( lar vesicle subtypes. Proc N atl A cad S c i U S A 11 3 :E968-E977. http s : // doi.org/10.1073/pnas.1521230113. ) ( 76. Buzas E l, Gyorgy B, Nagy G, Falus A, Gay S. 2014. Emerging role of extracellular vesicles in inflammatory diseases. N at Rev R h eumatol 10: 356-364. h tt p s: / /doi.or g/ 10.1038/n rrh e u m.2014.19. ) ( 77. H eijnen H F , S c hiel A E , F i jnheer R, G e uze HJ, Sixma JJ. 199 9 . Activatedplatelets release two t ypes o f membrane ves i cles: mi c rovesicles bysurface shedding a n d exosomes derived from exocytosis of multivesicu-lar bodies and a lpha-granules. B lood 94:3791-3799. ) ( 78. Schorey JS, Cheng Y , S ingh PP, S mith VL. 2015. Exosomes and ot h erextracellula r vesicl e s i n host-pathogen interaction s . EMBO Rep 1 6:24-43. h ttps://doi . o rg/ 10.1 5 252/e m br.201 4 39363. ) ( 79. B udnik V , R u iz-Canada C, Wendler F. 2 0 16. Extracellular vesicles rou n doff communication in the nervous system. Nat Rev Neurosci 1 7 :160-172. https://doi.org/10.1038/ nrn. 2015.29. ) ( 80. L otvall J, Hil l AF , Hochberg F, Buzas E l, Di Vizio D, Gardiner C , Gho Y S , Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, WaubenMH, Witwer KW, Thery C. 2014. Minimal experimental req u irements fordefinition of e xtracellular vesicles and their functions: a p o sition stat e -ment from the Int e rnational Society for Extracellular Vesicles. J ExtracellVesicles 3:26913. h t tp s://doi.org /1 0.3402/jev.v3.26913. ) 81. Campbell TD, Khan M, Huang MB, Bond VC, Powell MD. 2008. HIV-1 Nefprotein is secreted into vesicles that can fuse with target cells andvirions. Ethn Dis 18(2 Suppl 2):S2-S14. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3418053/. 82. Madison MN, Okeoma CM. 2015.Exosomes: implications in HIV-1 patho-genesis. Viruses 7:4093-4118. https://doi.org/10.3390/v7072810. 83. Monjure CJ, Tatum CD, Panganiban AT, Arainga M, Traina-Dorge V,MarxPA, Jr., Didier ES. 2014. Optimization of PCR for quantification of simianimmunodeficiency virus genomic RNA in plasma of rhesus macaques(Macaca mulatta) using armored RNA. J Med Primatol 43:31-43.https://doi.org/10.1111/jmp.12088. 84. Heigele A, Kmiec D, Regensburger K, Langer S, Peiffer L, Sturzel CM,Sauter D, Peeters M, Pizzato M, Learn GH, Hahn BH, Kirchhoff F. 2016.The potency of Nef-mediated SERINC5 antagonism correlates with theprevalence of primate lentiviruses in the wild. Cell Host Microbe 20:381-391. https://doi.org/10.1016/j.chom.2016.08.004. 85. Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJ, Hole P,Carr B, Redman CW, Harris AL, Dobson PJ, Harrison P, Sargent IL. 2011.Sizing and phenotyping of cellular vesicles using nanoparticle trackinganalysis. Nanomedicine 7:780-788. https://doi.org/10.1016/j.nano.2011.04.003. January/February Volume Issue eBiombio.asm.org 细胞外囊泡(EVs)或外泌体在传染病和癌症的病理生理学研究中有重要意义。猿类免疫缺陷病毒(SIV)和人类免疫缺陷病毒(HIV)编码的负调节因子(Nef)在获得性免疫缺陷综合征(AIDS)的致病过程中起非常重要的作用,严重损害胞内运输体系。HIV-1的负调节因子Nef是否会被分泌到细胞外囊泡中,这个问题一直存在争议。人们对SIV负调节因子Nef与细胞外囊泡之间的联系,也一无所知。但是在来源于所培养细胞、经亲和层析纯化所得到的细胞外囊泡和来源于已感染SIV的猕猴的细胞外囊泡中,研究者都发现了SIV和HIV-1负调节因子蛋白。而且,含有Nef的细胞外囊泡是有生物学功能的,也就是说,这类细胞外囊泡能参与膜融合过程,将它们的内含物释放到受体细胞中。所以,这些细胞外囊泡能将负调节因子Nef传递到受体细胞,这意味着负调节因子Nef很容易进入外泌体的生物发生途径,HIV病毒体可以细胞质膜处完成组装。这揭示了慢病毒影响未感染细胞和不可感染细胞(即不含CD4的细胞)的一个新机制。
确定











还剩18页未读,是否继续阅读?
大昌华嘉科学仪器为您提供《人类和猿类免疫缺陷病毒中负调节因子Nef会被分泌到细胞外囊泡或外泌体检测方案(Zeta电位仪)》,该方案主要用于其他中负调节因子Nef会被分泌到细胞外囊泡或外泌体检测,参考标准--,《人类和猿类免疫缺陷病毒中负调节因子Nef会被分泌到细胞外囊泡或外泌体检测方案(Zeta电位仪)》用到的仪器有Microtrac纳米粒度及Zeta电位分析仪
推荐专场
相关方案
更多
该厂商其他方案
更多