注射药物开发和生产中的颗粒一直是一个严重的问题。 在包括蛋白质制剂在内的生物制药中,通过聚集体和颗粒对产品功效,安全性和免疫原性的影响对产生的问题进行总结。 FDA法规强烈建议深入描述蛋白质制剂中颗粒的特性和数量。 颗粒问题在FDA意见书中越来越常见,并且在某些情况下,颗粒问题直接导致药物召回。
方案详情

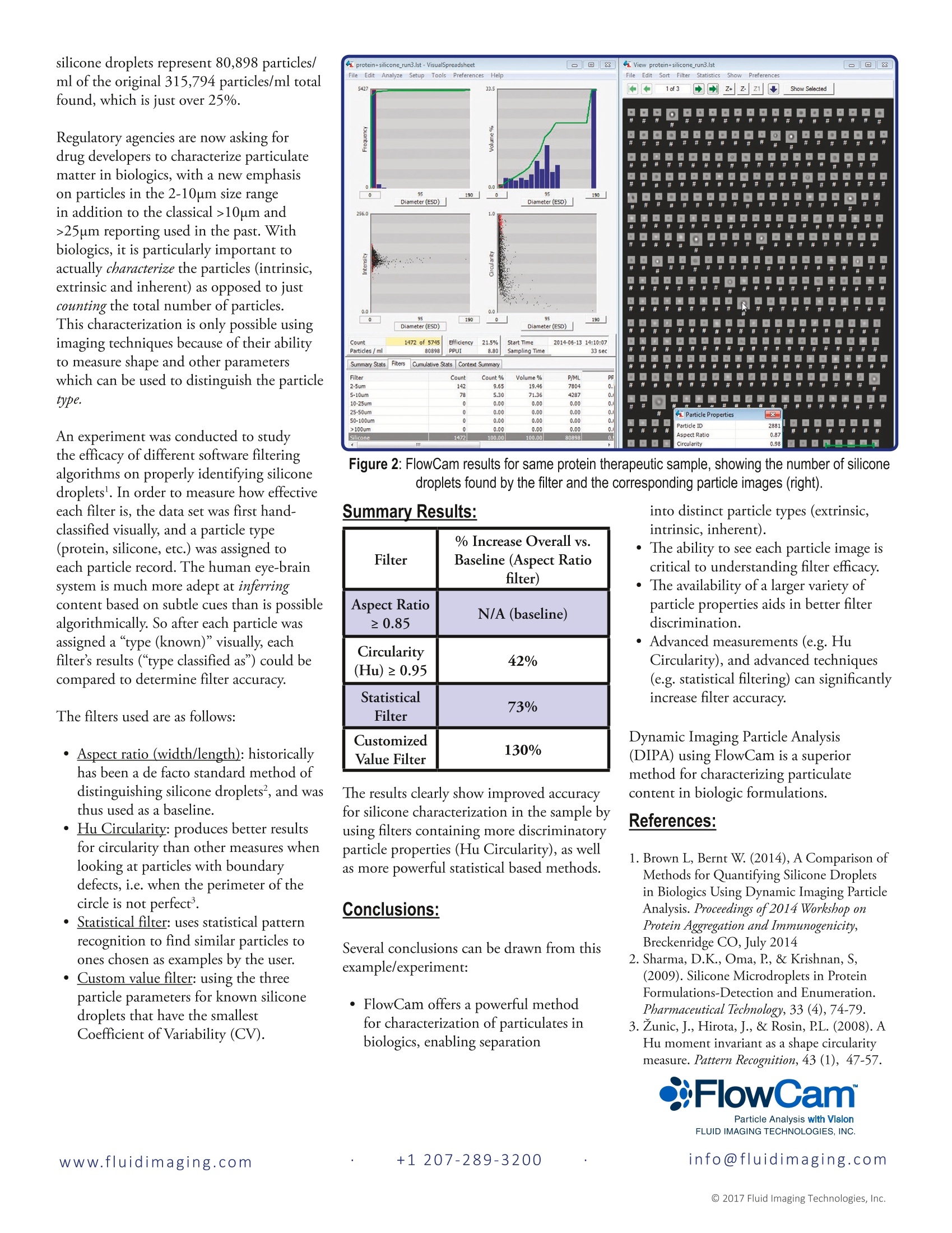
www.fluidimaging.com FlowCam° Application Note Characterization of Protein Aggregates and Other Inherent,Intrinsic, and Extrinsic particles in Biopharmaceuticals Objective Particulates in parenteral drug developmentand production have always been aserious issue. In biopharmaceuticals,including protein therapeutics, the issueis compounded by reported impacts ofaggregates and particles on the product’sefficacy, safety and immunogenicity. FDAregulations strongly recommend in-depthcharacterization of the identity and quantityof particles in protein therapeutics. Particleconcerns are increasingly common in FDAsubmissions, and in some cases, particlematters have resulted in drug recalls. Quantifying particles larger than 10um and25um has been a USP <788> requirementfor drug product release since 1975. Inprotein therapeutics, the addition of“inherent" particles in the form of proteinaggregates, alongside the“intrinsic”particles (such as silicone droplets) and“extrinsic”(foreign) particles usuallyfound in parenterals makes particulatecharacterization more difficult than withsmall molecule formulations. For thisreason, the USP <1787> InformationalChapter states that: “Because multiplepotential sources of particles exist, it isimportant to identify the particles anddetermine whether they are extrinsic,intrinsic, or inherent.” While conventional microscopy can be used,the time required for sample preparationand the lack of statistical significancecaused by small sample sizes are limitingfactors. As a result, other automated particlesizing methods such as light obscuration,electrozone sensing and laser diffractionhave also been used. The problem with thesetechniques is that they assume all particlesare spherical in shape, recording only anEquivalent Spherical Diameter (ESD) foreach particle. As a result, they can not inferinformation on a particle’s identity. Inaddition, light obscuration, one of the mostcommon techniques used to meet USPrequirements, has challenges detecting andproperly sizing particles with a low refractiveindex (such as protein aggregates). As a result of this insensitivity, a batch mightpass USP <788> even though it maycontain unacceptable levels of proteinaggregates which the light obscurationsystem could not detect. Method FlowCam° was developed to addressexisting technology limitations and assistbiopharmaceutical scientists to furtherunderstand their products, andconsequently comply with FDArequirements. The system stores a digitalimage and over 30 different measurementsfor each particle. Scientists can thereforecharacterize particles based on size andshape, enabling the automaticdifferentiation between inherent proteinaggregates, intrinsic particles(such as silicone oil droplets) and extrinsicparticles (such as fibers, plastic, etc.). Sinceall images are saved, the results can bevisually examined to ensure data accuracyand for troubleshooting. Additionally,FlowCam’s VisualSpreadsheet° software hasversions designed to meet 21 CFR Part 11requirements, which is essential in regulatedenvironments. Because of this, FlowCam is ideal for extended particle characterizationduring development and release. The screenshot in Figure 1 shows the resultsof a FlowCam run (AutoImaging mode)with a polyclonal IgG sample containingsilicone droplets. A total particulate load of315,794 particles/ml was found. Sampleimages in the right hand window show thediversity of particles found, including bothprotein aggregates and silicone droplets. In this particular sample, it is apparent thatmany of the particles are, in fact, silicone oildroplets. These particles are intrinsicto this formulation which i delivered in apre-filled syringe containing silicone as alubricant. VisualSpreadsheet allows for theremoval of these silicone droplets and thenrecalculating particulate load without theseintrinsic particles included. The last filter shown in the left windowof Figure 1 is a silicone filter created inVisualSpreadsheet to isolate the siliconedroplets only. Figure 2 (next page) showsthe result of selecting this filter and thesoftware displaying the particle images thatfit the filter (silicone droplets). Note thatthe silicone droplets represent 80,898 particles/ml of the original 315,794 particles/ml totalfound, which is just over 25%. Regulatory agencies are now asking fordrug developers to characterize particulatematter in biologics, with a new emphasison particles in the 2-10um size rangein addition to the classical >10um and>25um reporting used in the past. Withbiologics, it is particularly important toactually characterize the particles (intrinsic,extrinsic and inherent) as opposed to justcounting the total number of particles.This characterization is only possible usingimaging techniques because of their abilityto measure shape and other parameterswhich can be used to distinguish the particletype. An experiment was conducted to studythe efficacy of different software filteringalgorithms on properly identifying siliconedroplets. In order to measure how effectiveeach filter is, the data set was first hand-classified visually, and a particle type(protein, silicone, etc.) was assigned toeach particle record. The human eye-brainsystem is much more adept at inferringcontent based on subtle cues than is possiblealgorithmically. So after each particle wasassigned a “type (known)" visually, eachfilter’s results (“type classified as") could becompared to determine filter accuracy. The filters used are as follows: · Aspect ratio (width/length): historicallyhas been a de facto standard method ofdistinguishing silicone droplets, and wasthus used as a baseline. · Hu Circularity: produces better resultsfor circularity than other measures whenlooking at particles with boundarydefects, i.e. when the perimeter of thecircle is not perfect. Statistical filter: uses statistical patternrecognition to find similar particles toones chosen as examples by the user. Custom value filter: using the threeparticle parameters for known siliconedroplets that have the smallestCoefficient of Variability (CV). Figure 2: FlowCam results for same protein therapeutic sample, showing the number of siliconedroplets found by the filter and the corresponding particle images (right). into distinct particle types (extrinsic,intrinsic, inherent). The ability to see each particle image iscritical to understanding filter efficacy. The availability of a larger variety ofparticle properties aids in better filterdiscrimination. ·Advanced measurements (e.g. HuCircularity), and advanced techniques(e.g. statistical filtering) can significantlyincrease filter accuracy. Dynamic Imaging Particle Analysis(DIPA) using FlowCam is a superiormethod for characterizing particulatecontent in biologic formulations. References: 1. Brown L, Bernt W. (2014), A Comparison ofMethods for Quantifying Silicone Dropletsin Biologics Using Dynamic Imaging ParticleAnalysis. Proceedings of2014 Workshop onProtein Aggregation and Immunogenicity,Breckenridge CO, July 2014 2. Sharma, D.K., Oma, P, & Krishnan, S,(2009). Silicone Microdroplets in ProteinFormulations-Detection and Enumeration.Pharmaceutical Technology,33 (4),74-79. 3. Zunic, J., Hirota, J., & Rosin, PL. (2008). AHu moment invariant as a shape circularitymeasure. Pattern Recognition, 43 (1), 47-57. 注射药物开发和生产中的颗粒一直是一个严重的问题。 在包括蛋白质制剂在内的生物制药中,通过聚集体和颗粒对产品功效,安全性和免疫原性的影响对产生的问题进行总结。 FDA法规强烈建议深入描述蛋白质制剂中颗粒的特性和数量。 颗粒问题在FDA意见书中越来越常见,并且在某些情况下,颗粒问题直接导致药物召回。
确定

还剩1页未读,是否继续阅读?
大昌华嘉科学仪器为您提供《蛋白质聚集体中内在和外在颗粒的表征检测方案(图像粒度粒形)》,该方案主要用于其他中内在和外在颗粒的表征检测,参考标准--,《蛋白质聚集体中内在和外在颗粒的表征检测方案(图像粒度粒形)》用到的仪器有流式颗粒成像分析系统FlowCam®8100、颗粒成像法+光阻法分析系统 FlowCam® + LO、纳米流式颗粒成像分析系统 FlowCam® Nano
推荐专场
相关方案
更多
该厂商其他方案
更多
















