
方案详情
文
?This Application Note demonstrates:
• Configuration of the Agilent 1200 Series Rapid Resolution LC (RRLC)analytical scale (AS) purification system.
• Use of the Agilent 1200 Series RRLC AS purification system with anoptimized method for purification of peptide nucleic acids from solidphase synthesis.
方案详情

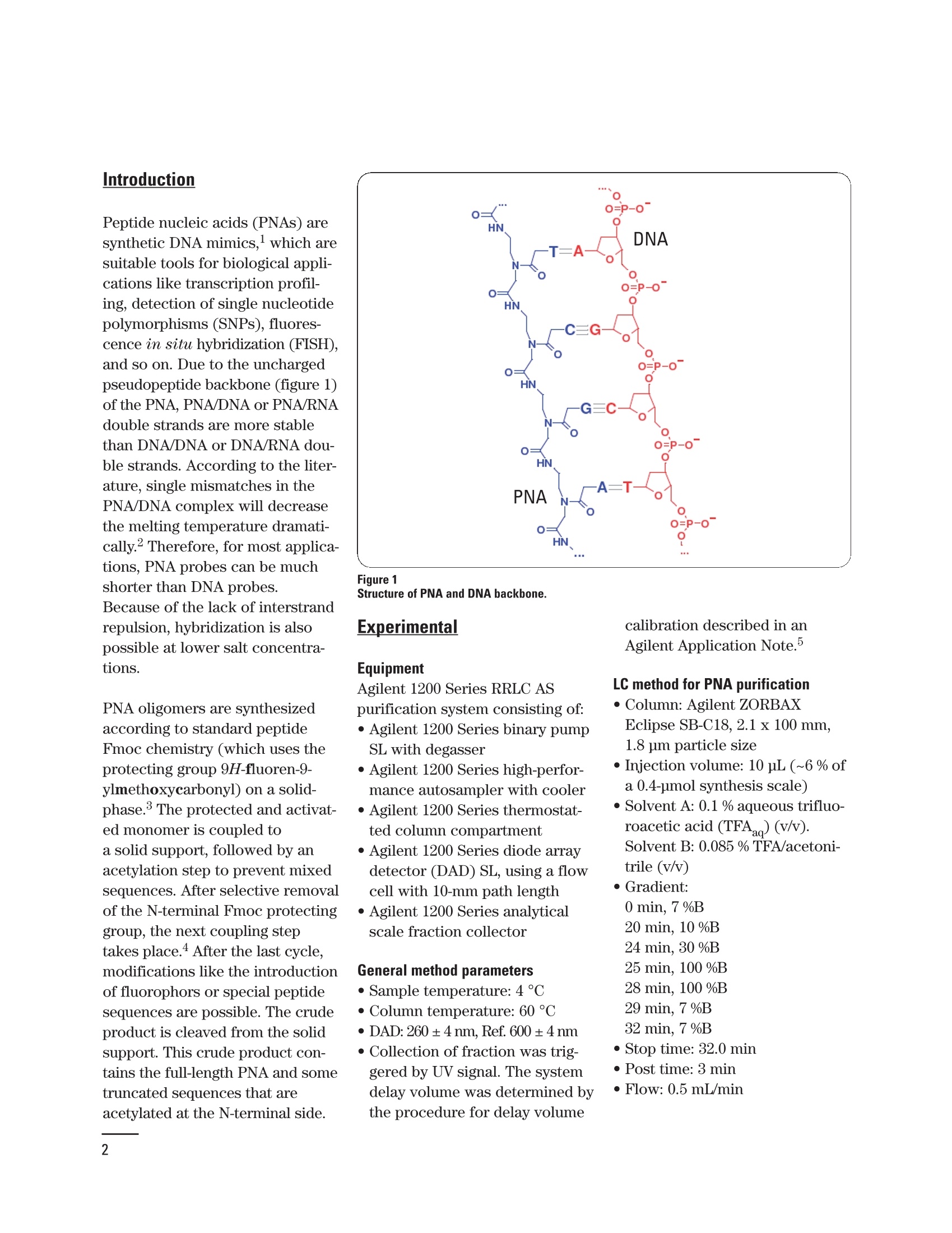
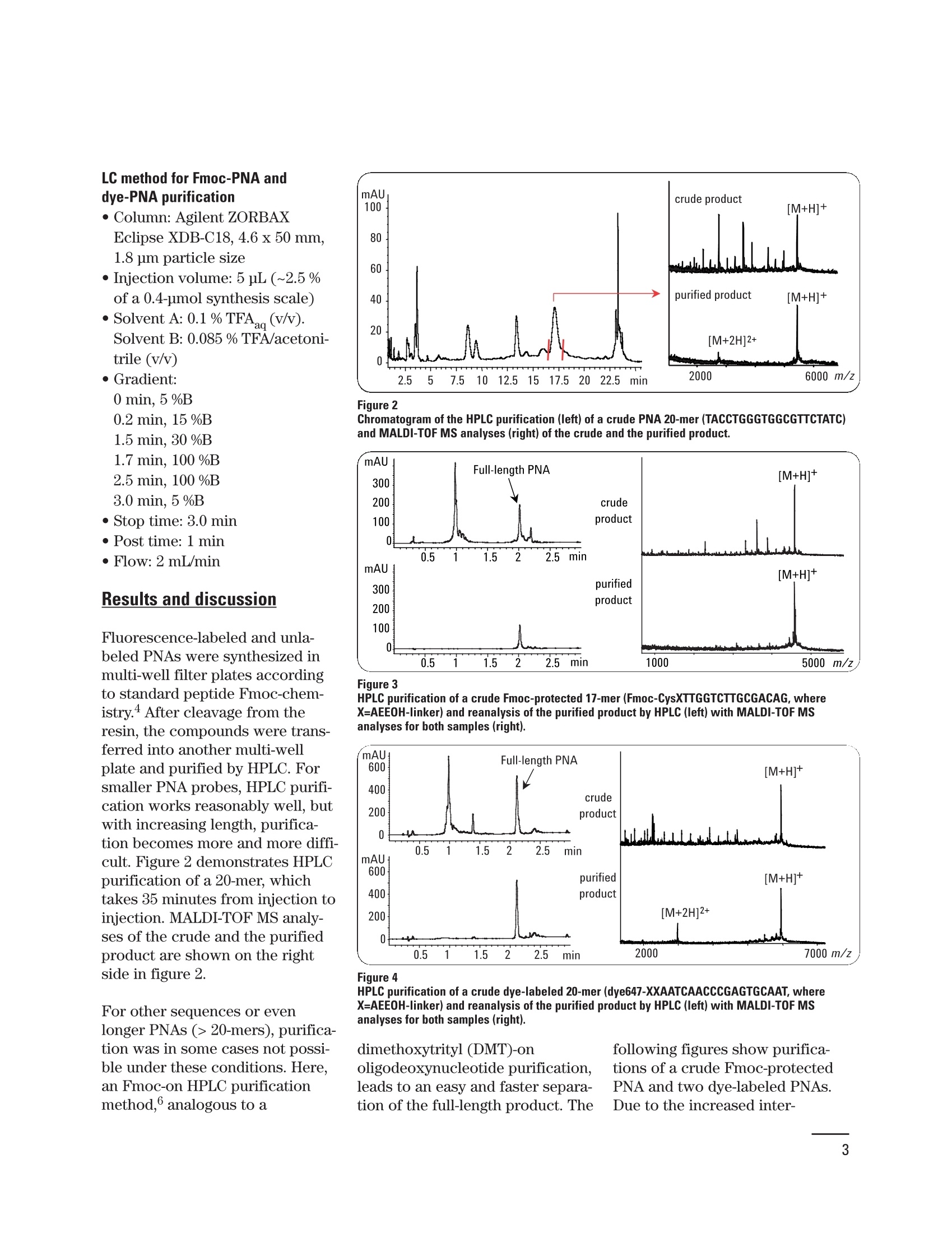
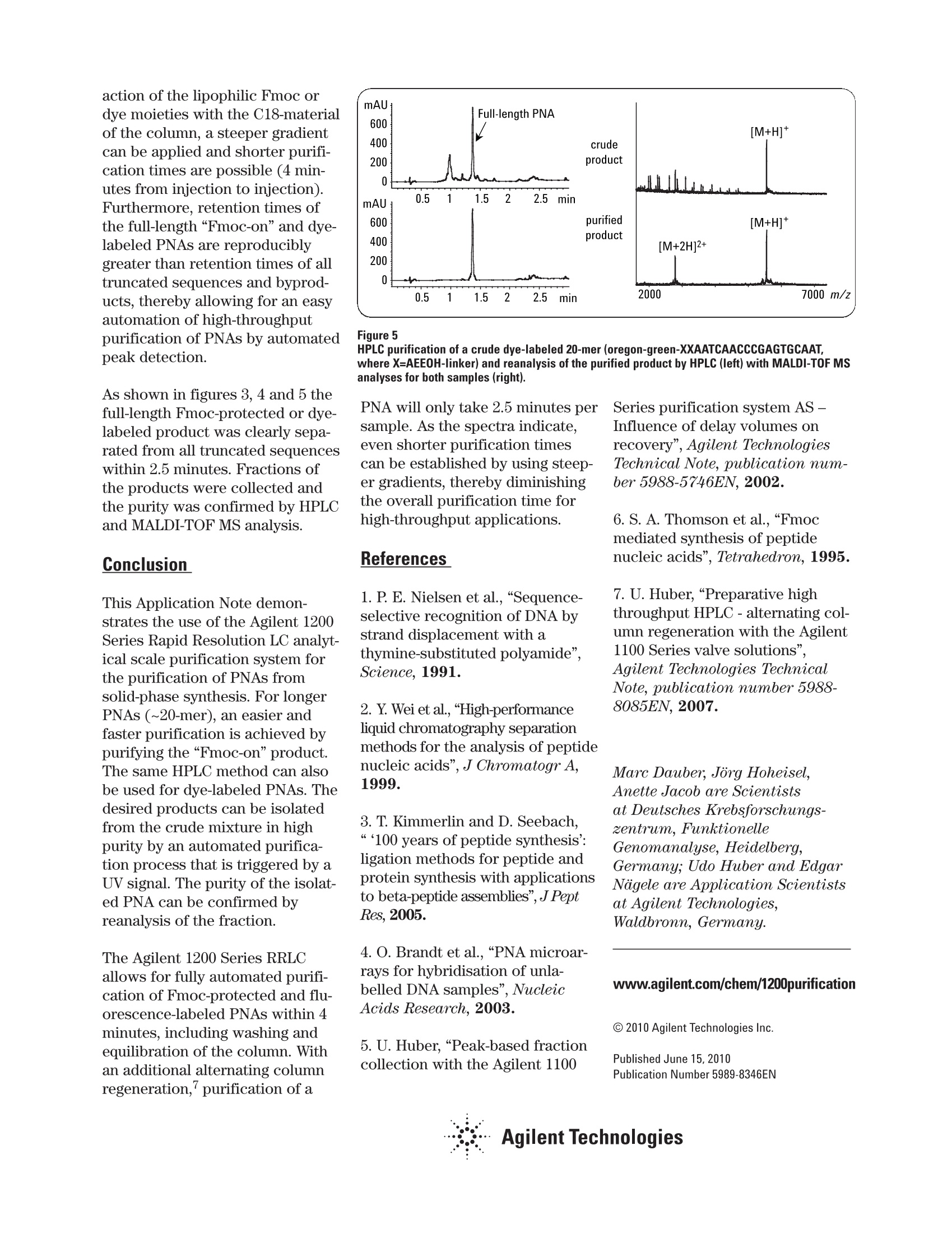
HPLC purification of dye-labeled orFmoc-protected peptide nucleic acids· from solid-phase synthesis Application Note Marc Dauber, Jorg Hoheisel, Anette Jacob Deutsches Krebsforschungszentrum, Funktionelle Genomanalyse, Heidelberg, Germany Udo Huber, Edgar Nagele Agilent Technologies, Waldbronn, Germany Abstract This Application Note demonstrates: ·Configuration of the Agilent 1200 Series Rapid Resolution LC (RRLC)analytical scale (AS) purification system. · Use of the Agilent 1200 Series RRLC AS purification system with anoptimized method for purification of peptide nucleic acids from solid-phase synthesis. Peptide nucleic acids (PNAs) aresynthetic DNA mimics,which aresuitable tools for biological appli-cations like transcription profil-ing, detection of single nucleotidepolymorphisms (SNPs), fluores-cence in situ hybridization (FISH),and so on. Due to the unchargedpseudopeptide backbone (figure 1)of the PNA, PNA/DNA or PNA/RNAdouble strands are more stablethan DNA/DNAor DNA/RNA dou-ble strands. According to the liter-ature, single mismatches in thePNA/DNA complex will decreasethe melting temperature dramati-cally. Therefore, for most applica-tions, PNA probes can be muchshorter than DNA probes.Because of the lack of interstrand repulsion, hybridization is alsopossible at lower salt concentra-tions. PNA oligomers are synthesizedaccording to standard peptideFmoc chemistry (which uses theprotecting group 9H-fluoren-9-ylmethoxycarbonyl)on a solid-phase.The protected and activat-ed monomer is coupled toa solid support, followed by anacetylation step to prevent mixedsequences. After selective removalof the N-terminal Fmoc protectinggroup, the next coupling steptakes place.4 After the last cycle,modifications like the introductionof fluorophors or special peptidesequences are possible. The crudeproduct is cleaved from the solidsupport. This crude product con-tains the full-length PNA and sometruncated sequences that areacetylated at the N-terminal side. Experimental Equipment Agilent 1200 Series RRLC ASpurification system consisting of: ·Agilent 1200 Series binary pumpSL with degasser · Agilent 1200 Series high-perfor-mance autosampler with cooler · Agilent 1200 Series thermostat-ted column compartment · Agilent 1200 Series diode arraydetector (DAD) SL, using a flowcell with 10-mm path length · Agilent 1200 Series analyticalscale fraction collector General method parameters · Sample temperature: 4°C ·Column temperature: 60℃ ·DAD:260±4 nm,Ref. 600 ±4 nm ·Collection of fraction was trig-gered by UV signal. The systemdelay volume was determined bythe procedure for delay volume calibration described in anAgilent Application Note. LC method for PNA purification ·Column: Agilent ZORBAXEclipse SB-C18, 2.1 x 100 mm,1.8 jm particle size · Injection volume: 10 pL (~6 % ofa 0.4-pmol synthesis scale) · Solvent A: 0.1 % aqueous trifluo-roacetic acid (TFAag)(v/v).Solvent B:0.085% TFA/acetoni-trile (v/v) · Gradient: 0 min, 7%B 20 min, 10 %B 24 min, 30 %B 25 min, 100 %B 28 min, 100 %B 29 min, 7 %B 32 min, 7%B ·Stop time: 32.0 min Post time: 3 min · Flow: 0.5 mL/min LC method for Fmoc-PNA anddye-PNA purification ·Column: Agilent ZORBAXEclipse XDB-C18, 4.6 x 50 mm,1.8 pm particle size ·Injection volume: 5 pL (~2.5 %of a 0.4-pmol synthesis scale) ·Solvent A: 0.1% TFA(v/v).Solvent B: 0.085 %TFA/acetoni-trile (v/v) · Gradient: 0 min, 5 %B 0.2 min, 15 %B 1.5 min, 30 %B 1.7 min, 100 %B 2.5 min, 100 %B 3.0 min, 5 %B · Stop time: 3.0 min · Post time: 1min · Flow: 2 mL/min Results and discussion Fluorescence-labeled and unla-beled PNAs were synthesized inmulti-well filter plates accordingto standard peptide Fmoc-chem-istry 4 After cleavage from theresin, the compounds were trans-ferred into another multi-wellplate and purified by HPLC. Forsmaller PNA probes, HPLC purifi-cation works reasonably well, butwith increasing length, purifica-tion becomes more and more diffi-cult. Figure 2 demonstrates HPLCpurification of a 20-mer, whichtakes 35 minutes from injection toinjection. MALDI-TOF MS analy-ses of the crude and the purifiedproduct are shown on the rightside in figure 2. For other sequences or evenlonger PNAs (>20-mers), purifica-tion was in some cases not possi-ble under these conditions. Here,an Fmoc-on HPLC purificationmethod,6 analogous to a Figure 2 Chromatogram of the HPLC purification (left) of a crude PNA 20-mer (TACCTGGGTGGCGTTCTATC)and MALDI-TOF MS analyses (right) of the crude and the purified product. Figure 3 HPLC purification of a crude Fmoc-protected 17-mer (Fmoc-CysXTTGGTCTTGCGACAG, whereX=AEEOH-linker) and reanalysis of the purified product by HPLC (left) with MALDI-TOF MSanalyses for both samples (right). Figure 4 HPLC purification of a crude dye-labeled 20-mer (dye647-XXAATCAACCCGAGTGCAAT, whereX=AEEOH-linker) and reanalysis of the purified product by HPLC (left) with MALDI-TOF MSanalyses for both samples (right). dimethoxytrityl (DMT)-onoligodeoxynucleotide purification,leads to an easy and faster separa-tion of the full-length product. The following figures show purifica-tions of a crude Fmoc-protectedPNA and two dye-labeled PNAs.Due to the increased inter- action of the lipophilic Fmoc ordye moieties with the C18-materialof the column, a steeper gradientcan be applied and shorter purifi-cation times are possible (4 min-utes from injection to injection).Furthermore, retention times ofthe full-length“Fmoc-on”and dye-labeled PNAs are reproduciblygreater than retention times of alltruncated sequences and byprod-ucts, thereby allowing for an easyautomation of high-throughputpurification of PNAs by automatedpeak detection. As shown in figures 3, 4 and 5 thefull-length Fmoc-protected or dye-labeled product was clearly sepa-rated from all truncated sequenceswithin 2.5 minutes. Fractions ofthe products were collected andthe purity was confirmed by HPLCand MALDI-TOF MS analysis. Conclusion This Application Note demon-strates the use of the Agilent 1200Series Rapid Resolution LC analyt-ical scale purification system forthe purification of PNAs fromsolid-phase synthesis. For longerPNAs (~20-mer), an easier andfaster purification is achieved bypurifying the“Fmoc-on”product.The same HPLC method can alsobe used for dye-labeled PNAs. Thedesired products can be isolatedfrom the crude mixture in highpurity by an automated purifica-tion process that is triggered by aUV signal. The purity of the isolat-ed PNA can be confirmed byreanalysis of the fraction. The Agilent 1200 Series RRLCallows for fully automated purifi-cation of Fmoc-protected and flu-orescence-labeled PNAs within 4minutes, including washing andequilibration of the column. Withan additional alternating columnregeneration, purification of a Figure 5 HPLC purification of a crude dye-labeled 20-mer (oregon-green-XXAATCAACCCGAGTGCAAT,where X=AEEOH-linker) and reanalysis of the purified product by HPLC (left) with MALDI-TOF MSanalyses for both samples (right). PNA will only take 2.5 minutes persample. As the spectra indicate,even shorter purification timescan be established by using steep-er gradients, thereby diminishingthe overall purification time forhigh-throughput applications. References 1. P. E. Nielsen et al., “Sequence-selective recognition of DNA bystrand displacement with athymine-substituted polyamide",Science, 1991. 2. Y. Wei et al.,"High-performanceliquid chromatography separationmethods for the analysis of peptidenucleic acids”, J Chromatogr A,1999. 3. T. Kimmerlin and D. Seebach,“‘100 years of peptide synthesis':ligation methods for peptide andprotein synthesis with applicationsto beta-peptide assemblies”, J PeptRes, 2005. 4. O. Brandt et al.,“PNA microar-rays for hybridisation of unla-belled DNA samples",NucleicAcids Research,2003. 5. U. Huber,“Peak-based fractioncollection with the Agilent 1100 Series purification system AS -Influence of delay volumes onrecovery", Agilent TechnologiesTechnical Note, publication num-ber 5988-5746EN,2002. 6. S. A. Thomson et al., “Fmocmediated synthesis of peptidenucleic acids", Tetrahedron, 1995. 7. U.Huber,“Preparative highthroughput HPLC -alternating col-umn regeneration with the Agilent1100 Series valve solutions”.Agilent Technologies TechnicalNote, publication number 5988-8085EN,2007. Marc Dauber, Jorg Hoheisel,Anette Jacob are Scientistsat Deutsches Krebsforschungs-zentrum, FunktionelleGenomanalyse,Heidelberg,Germany; Udo Huber and EdgarNogele are Application Scientistsat Agilent Technologies,Waldbronn, Germany. www.agilent.com/chem/1200purification C 2010 Agilent Technologies Inc. ( Published June 15, 2010Publication Number 5989-8346EN ) Agilent Technologies This Application Note demonstrates:• Configuration of the Agilent 1200 Series Rapid Resolution LC (RRLC)analytical scale (AS) purification system.• Use of the Agilent 1200 Series RRLC AS purification system with anoptimized method for purification of peptide nucleic acids from solidphase synthesis.This Application Note demonstrates the use of the Agilent 1200 Series Rapid Resolution LC analytical scale purification system for the purification of PNAs from solid-phase synthesis. For longer PNAs (~20-mer), an easier and faster purification is achieved by purifying the “Fmoc-on” product. The same HPLC method can also be used for dye-labeled PNAs. The desired products can be isolated from the crude mixture in high purity by an automated purification process that is triggered by a UV signal. The purity of the isolated PNA can be confirmed by reanalysis of the fraction.The Agilent 1200 Series RRLC allows for fully automated purification of Fmoc-protected and fluorescence-labeled PNAs within 4 minutes, including washing and equilibration of the column. With an additional alternating column regeneration, purification of a PNA will only take 2.5 minutes per sample. As the spectra indicate, even shorter purification times can be established by using steeper gradients, thereby diminishing the overall purification time for high-throughput applications.
确定

还剩2页未读,是否继续阅读?
安捷伦科技(中国)有限公司为您提供《氨基酸中肽核酸检测方案(制备液相色谱)》,该方案主要用于其他中肽核酸检测,参考标准--,《氨基酸中肽核酸检测方案(制备液相色谱)》用到的仪器有Agilent 1260 Infinity II 制备型液相色谱、Agilent 1290 Infinity II Multisampler
推荐专场
相关方案
更多
该厂商其他方案
更多
















