方案详情
文
Peak-based fraction collection triggered by a UV signal threshold leads to satisfactory results in most cases. Some samples and applications, however, require special attention when fraction collection parameters are set. This Technical Overview describes different strategies on how to achieve best collection results using different settings on an Agilent 1260 Infinity II Preparative LC/MSD System. UV and mass-based fraction collection, fraction pooling, as well as recovery collection are discussed in detail to provide knowledge that can be turned into successful and confident fraction collection.
方案详情

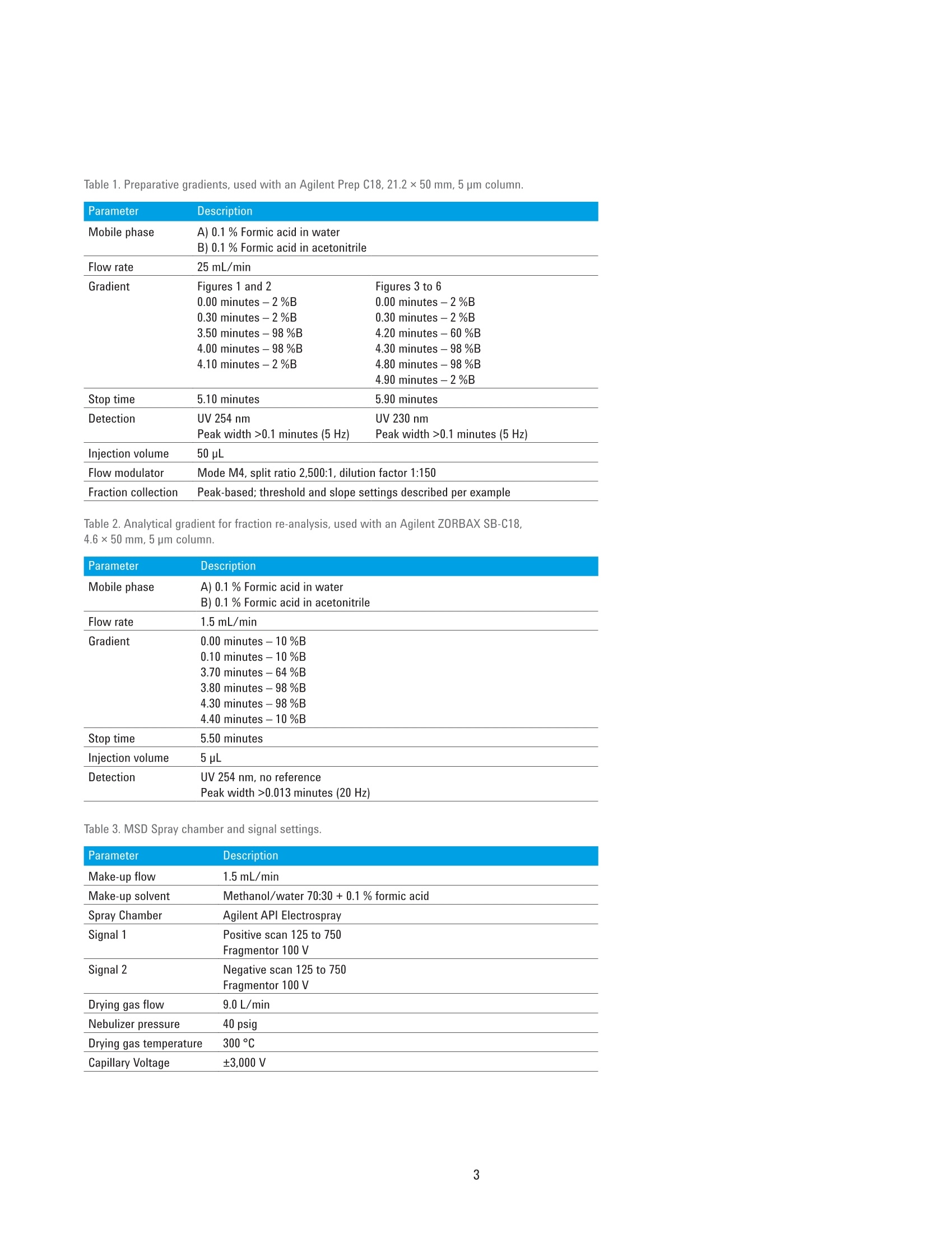
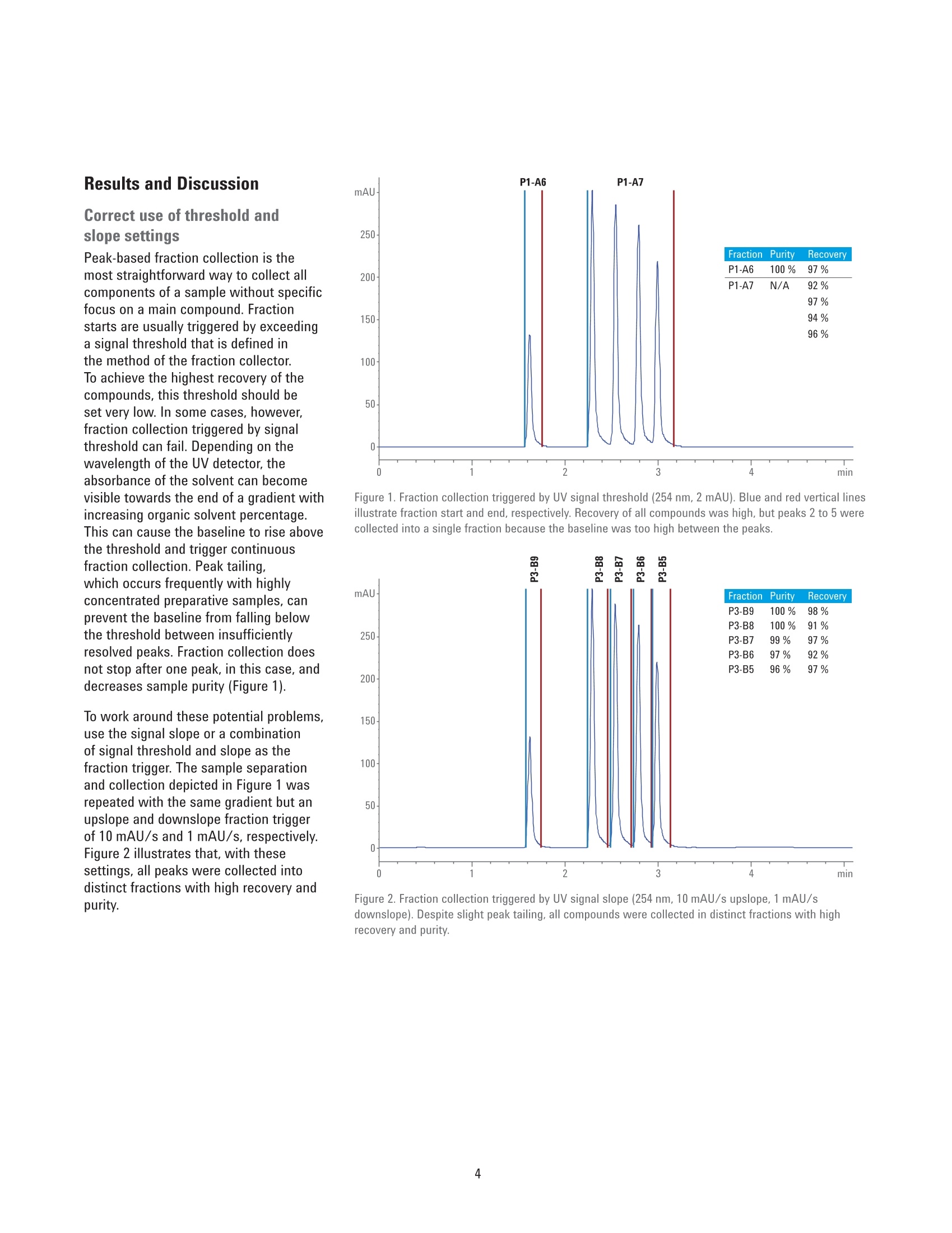
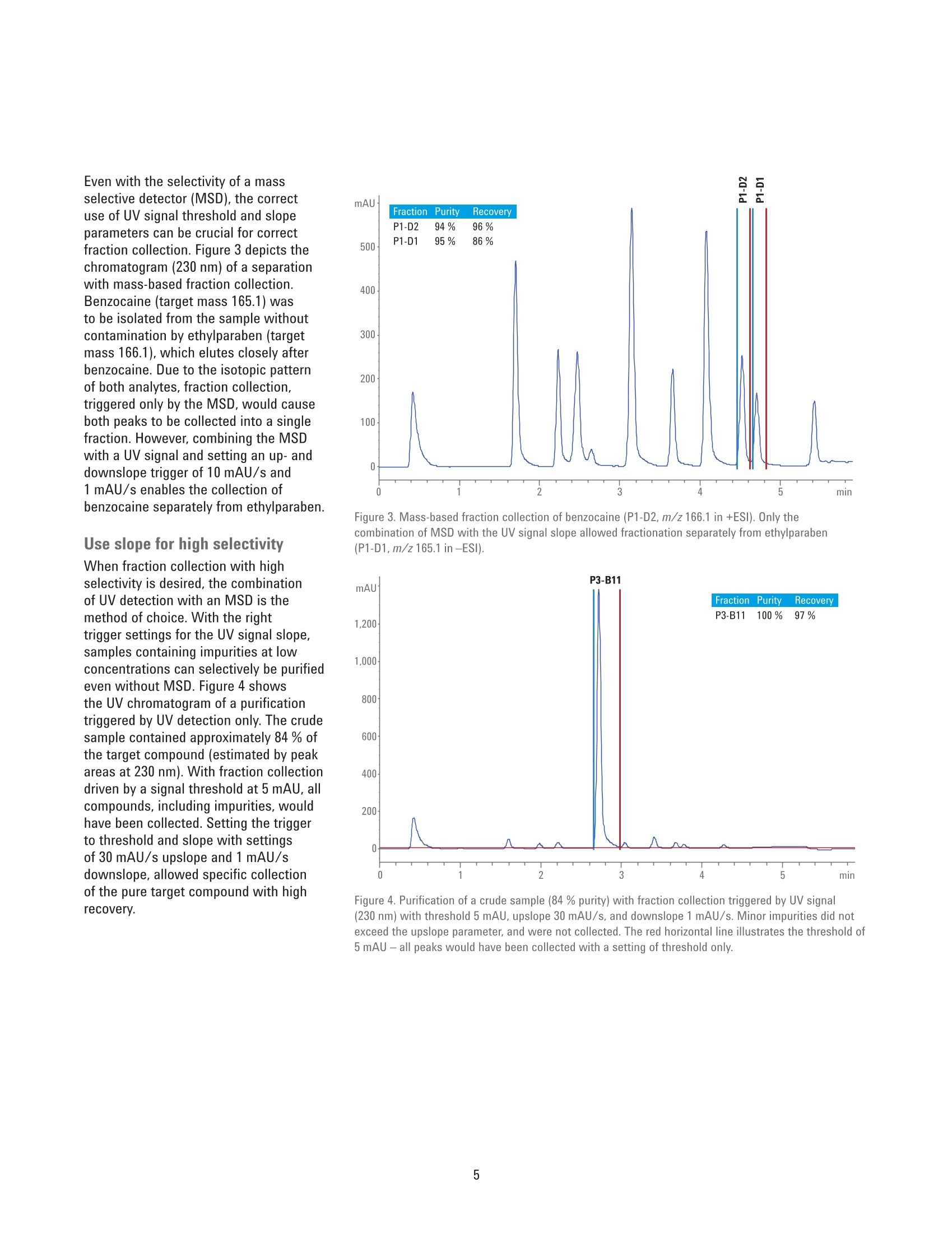
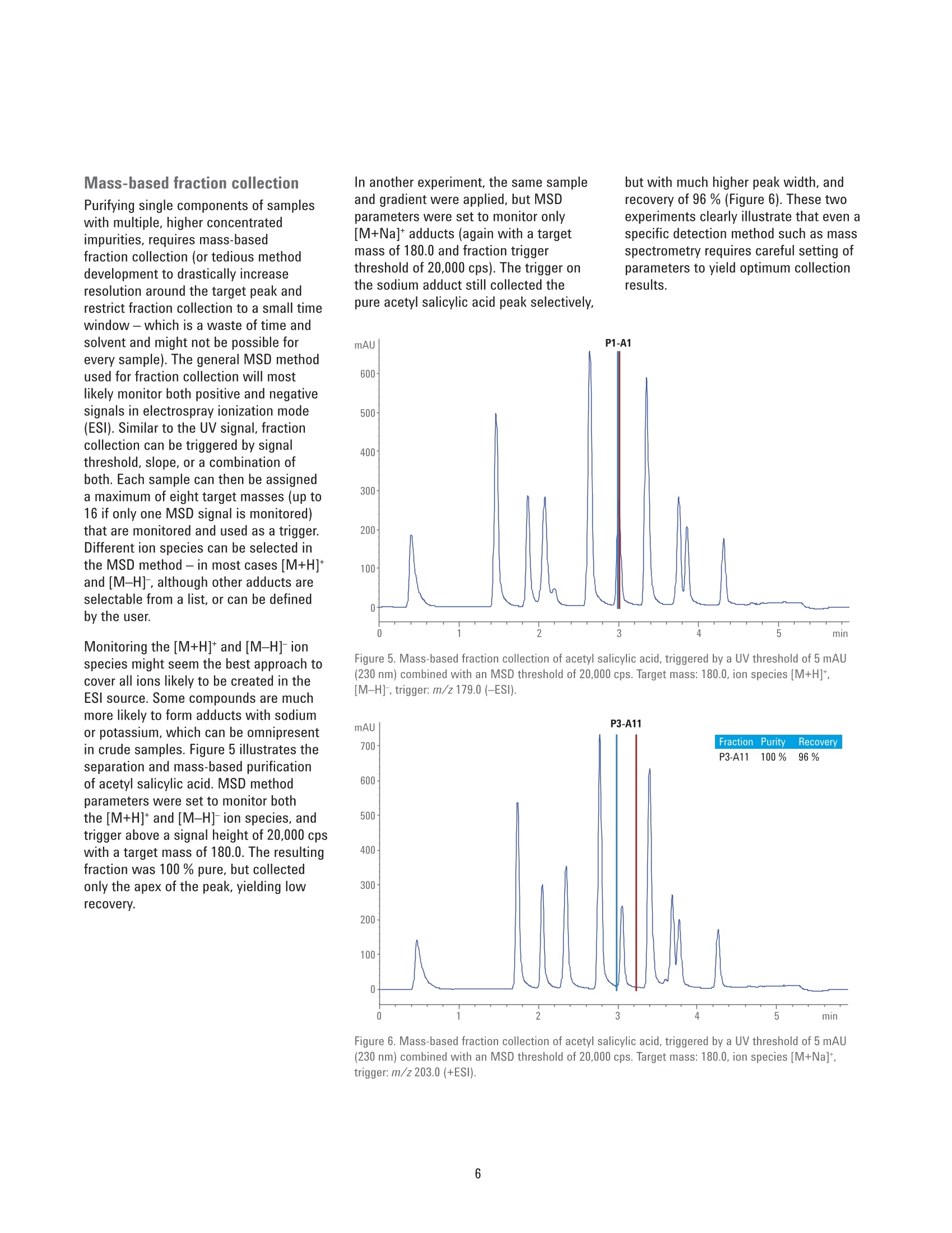
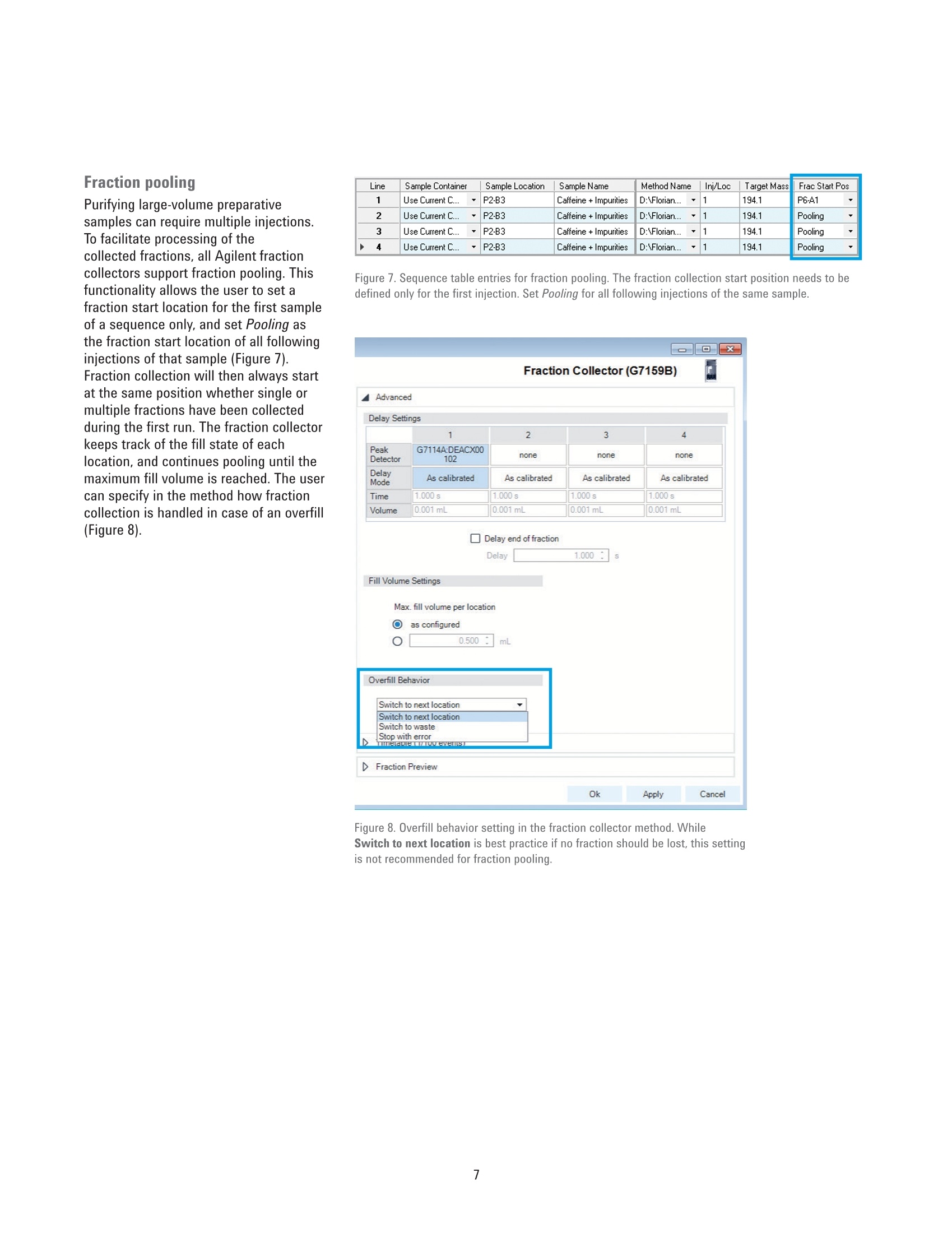
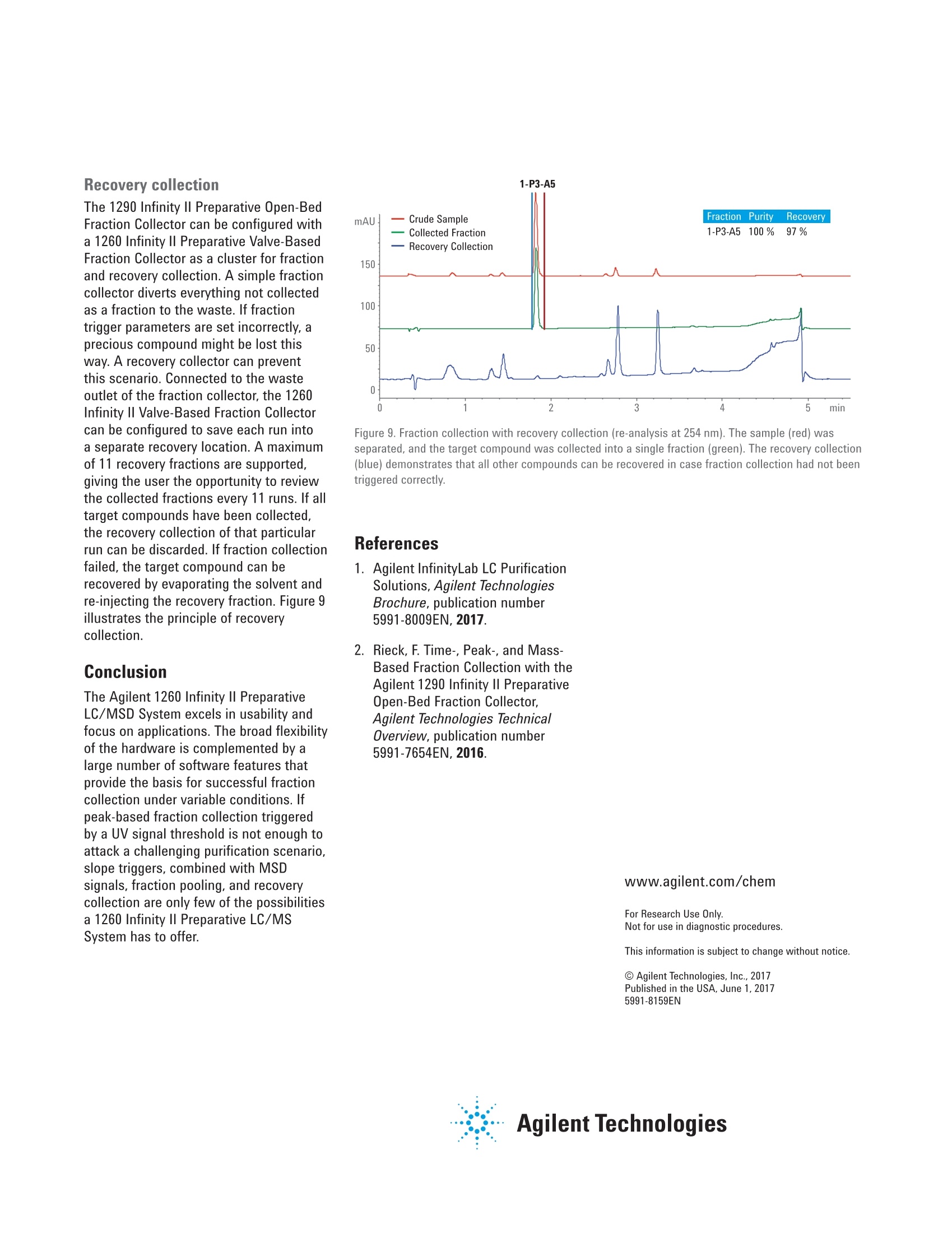
Developing Purification Strategies forthe Agilent 1260 Infinity lI PreparativeLC/MSD System Technical Overview Author Abstract Florian RieckAgilent Technologies,Inc. Waldbronn, Germany Peak-based fraction collection triggered by a UV signal threshold leads tosatisfactory results in most cases. Some samples and applications, however,require special attention when fraction collection parameters are set. ThisTechnical Overview describes different strategies on how to achieve bestcollection results using different settings on an Agilent 1260 Infinity Il PreparativeLC/MSD System. UV and mass-based fraction collection, fraction pooling, as wellas recovery collection are discussed in detail to provide knowledge that can beturned into successful and confident fraction collection. Agilent Technologies Introduction Agilent InfinityLab LC PurificationSolutions mark a giant leap in efficientpurification. The Agilent 1290 Infinity IlPreparative Open-Bed FractionCollector offers the highest flexibilityregarding fraction collection modesand configuration of the fraction bed.The Agilent 1260 Infinity II PreparativeLC/MSD System provides the base forsuccessful fraction collection with a plusin usability. In a compact housing, the Agilll Pent1260 Infinity II Preparative BinaryPump delivers high performance forpreparative separations using columnsfrom 9.4 up to 30 mm inside diameter.Samples are injected much faster withthe Agilent 1260 Infinity II PreparativeAutosampler, while carryover has beenreduced significantly by a redesignedneedle seat and integrated needle wash.With the Agilent extended portfolio ofpreparative fraction collectors, the systemcan be tailored to every need. For extrareliability, add an Agilent 1260 Infinity IlPreparative Valve-Based FractionCollector as a recovery collector, divertingthe fraction collector waste outlet todistinct recovery positions. To get the most out of the 1260 Infinity IlPreparative LC/MSD System, fractioncollection parameters need to be setproperly. Although, in most cases,simple peak-based fraction collectiontriggered by the signal of an ultraviolet(UV) detector will produce satisfactoryresults, some scenarios might requirespecial attention during method setup.This Technical Overview demonstratesdifferent strategies on how to exploit thevast possibilities of the Agilent OpenLABCDS ChemStation software to succeedwith fraction collection even withchallenging samples. Instrumentation The system used for the experimentscomprised the following modules: Agilent 1260 Infinity II PreparativeBinary Pump (G7161A) Agilent 1260 Infinity ll PreparativeAutosampler (G7157A) Agilent 1260 Infinity Il VariableWavelength Detector (G7114A)with a 0.3-mm preparative flow cell(Option #024) Agilent 1290 Infinity II MS FlowModulator (G7170B) Agilent 1260 Infinity Il IsocraticPump (G7110B) Agilent LC/MSD (G6125B) Agilent 1260 Infinity Il ColumnOrganizer (G9328A) Agilent Delay Coil Organizer(G7163 60010) with knitted delay coil5mx1.0 mm (5067-6184) Agilent 1290 Infinity Il PreparativeOpen-Bed Fraction Collector(G7159B) Agilent 1260 Infinity II PreparativeValve-Based Fraction Collector(G7166A), configured as recoverycollector Fraction reanalyses were conducted onan Agilent 1260 Infinity II Binary LC in aconfiguration as follows: Agilent 1260 Infinity II Binary Pump(G7112B) Agilent 1260 Infinity II Vialsampler(G7129A) Agilent 1260 Infinity ll Diode ArrayDetector (G7115A), equipped with a10-mm standard cell (G1315-60022) Preparative column Agilent Prep C18, 21.2×50 mm,5 pmPrepHT cartridge (p/n 446905-102) withPrepHT end fittings (p/n 820400-901) Analytical column Agilent ZORBAX SB-C18,4.6 ×50 mm, 5 pm (p/n 846975-902) Software Agilent OpenLAB CDS ChemStationEdition for LC and LC/MS Systems,version C.01.07 SR2 [263] Solvents and samples All solvents used were LC grade. Freshultrapure water was obtained from aMilli-Q Integral system equipped witha 0.22-pm membrane point-of-usecartridge (Millipak). Acetaminophen,acetanilide, acetylsalicylic acid,benzocaine, benzyl-4-hydroxybenzoate,caffeine, ethyl-4-hydroxybenzoate,methyl-4-hydroxybenzoate, propyl-4-hydroxybenzoate, salicylic acid,sulfamerazine sodium salt, anddimethylsulfoxide were bought fromSigma-Aldrich, Taufkirchen, Germany. Table 1. Preparative gradients, used with an Agilent Prep C18, 21.2×50 mm, 5 pm column. B) 0.1 % Formic acid in acetonitrile Flow rate 25 mL/min Gradient Figures 1 and 2 Figures 3 to 6 0.00 minutes-2%B 0.00 minutes-2%B 0.30 minutes-2%B 0.30 minutes-2%B 3.50 minutes -98 %B 4.20 minutes -60 %B 4.00 minutes-98%B 4.30 minutes-98 %B 4.10 minutes-2%B 4.80 minutes-98 %B 4.90 minutes-2%B Stop time 5.10 minutes 5.90 minutes Detection UV 254 nm UV 230 nm Peak width >0.1 minutes (5 Hz) Peak width >0.1 minutes (5 Hz) Injection volume 50 pL Table 2. Analytical gradient for fraction re-analysis, used with an Agilent ZORBAX SB-C18,4.6×50 mm, 5 um column. Mobile phase A) 0.1 % Formic acid in water B) 0.1 % Formic acid in acetonitrile Flow rate 1.5 mL/min Gradient 0.00 minutes - 10 %B 0.10 minutes -10%B 3.70 minutes -64%B 3.80 minutes-98 %B 4.30 minutes -98 %B 4.40 minutes-10 %B Stop time 5.50 minutes Injection volume 5pL Detection UV 254 nm, no reference Table 3. MSD Spray chamber and signal settings. Parameter Description Make-up flow 1.5 mL/min Make-up solvent Methanol/water 70:30+0.1% formic acid Spray Chamber Agilent API Electrospray Signal 1 Positive scan 125 to 750 Fragmentor 100 V Signal 2 Negative scan 125 to 750 Fragmentor 100 V Drying gas flow 9.0 L/min Nebulizer pressure 40 psig Drying gas temperature 300 °C Capillary Voltage ±3,000 V Correct use of threshold andslope settingsPeak-based fraction collection is themost straightforward way to collect allcomponents of a sample without specificfocus on a main compound. Fractionstarts are usually triggered by exceedinga signal threshold that is defined inthe method of the fraction collectorTo achieve the highest recovery of thecompounds, this threshold should beset very low. In some cases, however,fraction collection triggered by signalthreshold can fail. Depending on thewavelength of the UV detector, theabsorbance of the solvent can becomevisible towards the end of a gradient withincreasing organic solvent percentage.This can cause the baseline to rise abovethe threshold and trigger continuousfraction collection. Peak tailing,which occurs frequently with highlyconcentrated preparative samples, canprevent the baseline from falling belowthe threshold between insufficientlyresolved peaks. Fraction collection doesnot stop after one peak, in this case, anddecreases sample purity (Figure 1). To work around these potential problems,use the signal slope or a combinationof signal threshold and slope as thefraction trigger. The sample separationand collection depicted in Figure 1 wasrepeated with the same gradient but anupslope and downslope fraction triggerof 10 mAU/s and 1 mAU/s, respectively.Figure 2 illustrates that, with thesesettings, all peaks were collected intodistinct fractions with high recovery andpurity. Figure 1. Fraction collection triggered by UV signal threshold (254 nm, 2 mAU). Blue and red vertical linesillustrate fraction start and end, respectively. Recovery of all compounds was high, but peaks 2 to 5 werecollected into a single fraction because the baseline was too high between the peaks. Figure 2. Fraction collection triggered by UV signal slope (254 nm, 10 mAU/s upslope, 1 mAU/sdownslope). Despite slight peak tailing, all compounds were collected in distinct fractions with highrecovery and purity. Even with the selectivity of a massselective detector (MSD), the correctuse of UV signal threshold and slopeparameters can be crucial for correctfraction collection. Figure 3 depicts thechromatogram (230 nm) of a separationwith mass-based fraction collection.Benzocaine (target mass 165.1) wasto be isolated from the sample withoutcontamination by ethylparaben (targetmass 166.1), which elutes closely afterbenzocaine. Due to the isotopic patternof both analytes, fraction collection,triggered only by the MSD, would causeboth peaks to be collected into a singlefraction. However, combining the MSDwith a UV signal and setting an up- anddownslope trigger of 10 mAU/s and1mAU/s enables the collection ofbenzocaine separately from ethylparaben. Use slope for high selectivity When fraction collection with highselectivity is desired, the combinationof UV detection with an MSD is themethod of choice. With the righttrigger settings for the UV signal slope,samples containing impurities at lowconcentrations can selectively be purifiedeven without MSD. Figure 4 showsthe UV chromatogram of a purificationtriggered by UV detection only. The crudesample contained approximately 84 % ofthe target compound (estimated by peakareas at 230 nm). With fraction collectiondriven by a signal threshold at 5 mAU, allcompounds, including impurities, wouldhave been collected. Setting the triggerto threshold and slope with settingsof 30 mAU/s upslope and 1 mAU/sdownslope, allowed specific collectionof the pure target compound with highrecovery. Figure 3. Mass-based fraction collection of benzocaine (P1-D2, m/z 166.1 in +ESl). Only thecombination of MSD with the UV signal slope allowed fractionation separately from ethylparaben(P1-D1,m/z165.1 in-ESl). Figure 4. Purification of a crude sample (84 % purity) with fraction collection triggered by UV signal(230 nm) with threshold 5 mAU, upslope 30 mAU/s, and downslope 1 mAU/s. Minor impurities did notexceed the upslope parameter, and were not collected. The red horizontal line illustrates the threshold of5 mAU - all peaks would have been collected with a setting of threshold only. Mass-based fraction collection Purifying single components of sampleswith multiple, higher concentratedimpurities, requires mass-basedfraction collection (or tedious methoddevelopment to drastically increaseresolution around the target peak andrestrict fraction collection to a small timewindow -which is a waste of time andsolvent and might not be possible forevery sample). The general MSD methodused for fraction collection will mostlikely monitor both positive and negativesignals in electrospray ionization mode(ESl). Similar to the UV signal, fractioncollection can be triggered by signalthreshold, slope, or a combination ofboth. Each sample can then be assigneda maximum of eight target masses (up to16 if only one MSD signal is monitored)that are monitored and used as a trigger.Different ion species can be selected inthe MSD method - in most cases [M+H]*and [M-H]-, although other adducts areselectable from a list, or can be definedby the user. Monitoring the [M+H]+ and [M-H]-ionspecies might seem the best approach tocover all ions likely to be created in theESl source. Some compounds are muchmore likely to form adducts with sodiumor potassium, which can be omnipresentin crude samples. Figure 5 illustrates theseparation and mass-based purificationof acetyl salicylic acid. MSD methodparameters were set to monitor boththe [M+H]+ and [M-H]-ion species, andtrigger above a signal height of 20,000 cpswith a target mass of 180.0. The resultingfraction was 100 % pure, but collectedonly the apex of the peak, yielding lowrecovery. In another experiment, the same sampleand gradient were applied, but MSDparameters were set to monitor only[M+Na]t adducts (again with a targetmass of 180.0 and fraction triggerthreshold of 20,000 cps). The trigger onthe sodium adduct still collected thepure acetyl salicylic acid peak selectively but with much higher peak width,andrecovery of 96 % (Figure 6). These twoexperiments clearly illustrate that even aspecific detection method such as massspectrometry requires careful setting ofparameters to yield optimum collectionresults. Figure 5. Mass-based fraction collection of acetyl salicylic acid, triggered by a UV threshold of 5 mAU(230 nm) combined with an MSD threshold of 20,000 cps. Target mass: 180.0, ion species [M+H]*,[M-H]-, trigger: m/z 179.0 (-ESI). Figure 6. Mass-based fraction collection of acetyl salicylic acid, triggered by a UV threshold of 5 mAU(230 nm) combined with an MSD threshold of 20,000 cps. Target mass: 180.0, ion species [M+Na]*,trigger: m/z 203.0 (+ESl). Fraction pooling Purifying large-volume preparativesamples can require multiple injections.To facilitate processing of thecollected fractions, all Agilent fractioncollectors support fraction pooling. Thisfunctionality allows the user to set afraction start location for the first sampleof a sequence only,and set Pooling asthe fraction start location of all followinginjections of that sample (Figure 7).Fraction collection will then always startat the same position whether single ormultiple fractions have been collectedduring the first run. The fraction collectorkeeps track of the fill state of eachlocation, and continues pooling until themaximum fill volume is reached. The usercan specify in the method how fractioncollection is handled in case of an overfill(Figure 8). Line Sample Container Sample Location Sample Name Method Name Inj/Loc Target Mass Frac Start Pos 1 Use Current C... P2-B3 Caffeine +Impurities D:AFlorian... 1 194.1 P6-A1 2 Use Current C. P2-B3 Caffeine+Impurities D:AFlorian... 1 194.1 Pooling 3 Use Current C... P2-B3 Caffeine+Impurities D:\Florian... 1 194.1 Pooling 4 Use Current C... P2-B3 Caffeine+Impurities D:\Florian... 1 194.1 Pooling Figure 7. Sequence table entries for fraction pooling. The fraction collection start position needs to bedefined only for the first injection. Set Pooling for all following injections of the same sample. Figure 8. Overfill behavior setting in the fraction collector method. WhileSwitch to next location is best practice if no fraction should be lost, this settingis not recommended for fraction pooling. Recovery collection 1-P3-A5 The 1290 Infinity II Preparative Open-BedFraction Collector can be configured witha 1260 Infinity II Preparative Valve-BasedFraction Collector as a cluster for fractionand recovery collection. A simple fractioncollector diverts everything not collectedas a fraction to the waste. If fractiontrigger parameters are set incorrectly, aprecious compound might be lost thisway.A recovery collector can preventthis scenario. Connected to the wasteoutlet of the fraction collector, the 1260Infinity Il Valve-Based Fraction Collectorcan be configured to save each run intoa separate recovery location. A maximumof 11 recovery fractions are supported,giving the user the opportunity to reviewthe collected fractions every 11 runs. If alltarget compounds have been collected,the recovery collection of that particularrun can be discarded. If fraction collectionfailed, the target compound can berecovered by evaporating the solvent andre-injecting the recovery fraction. Figure 9illustrates the principle of recoverycollection. Figure 9. Fraction collection with recovery collection (re-analysis at 254 nm). The sample (red) wasseparated, and the target compound was collected into a single fraction (green). The recovery collection(blue) demonstrates that all other compounds can be recovered in case fraction collection had not beentriggered correctly. References 1. Agilent InfinityLab LC PurificationSolutions, Agilent TechnologiesBrochure,publication number5991-8009EN, 2017. Conclusion The Agilent 1260 Infinity II PreparativeLC/MSD System excels in usability andfocus on applications. The broad flexibilityof the hardware is complemented by alarge number of software features thatprovide the basis for successful fractioncollection under variable conditions. Ifpeak-based fraction collection triggeredby a UV signal threshold is not enough toattack a challenging purification scenario,slope triggers, combined with MSDsignals, fraction pooling, and recoverycollection are only few of the possibilitiesa 1260 Infinity II Preparative LC/MSSystem has to offer. 2. Rieck, F. Time-, Peak-, and Mass-Based Fraction Collection with theAgilent 1290 Infinity II PreparativeOpen-Bed Fraction Collector,Agilent Technologies TechnicalOverview,publication number5991-7654EN,2016. www.agilent.com/chem For Research Use Only. Not for use in diagnostic procedures. This information is subject to change without notice. @ Agilent Technologies, Inc., 2017 Published in the USA, June 1. 2017 5991-8159EN Agilent Technologies Peak-based fraction collection triggered by a UV signal threshold leads to satisfactory results in most cases. Some samples and applications, however, require special attention when fraction collection parameters are set. This Technical Overview describes different strategies on how to achieve best collection results using different settings on an Agilent 1260 Infinity II Preparative LC/MSD System. UV and mass-based fraction collection, fraction pooling, as well as recovery collection are discussed in detail to provide knowledge that can be turned into successful and confident fraction collection.The Agilent 1260 Infinity II Preparative LC/MSD System excels in usability and focus on applications. The broad flexibility of the hardware is complemented by a large number of software features that provide the basis for successful fraction collection under variable conditions. If peak-based fraction collection triggered by a UV signal threshold is not enough to attack a challenging purification scenario, slope triggers, combined with MSD signals, fraction pooling, and recovery collection are only few of the possibilities a 1260 Infinity II Preparative LC/MS System has to offer.
确定


还剩6页未读,是否继续阅读?
安捷伦科技(中国)有限公司为您提供《药物中安非他酮检测方案(制备液相色谱)》,该方案主要用于化药制剂中含量测定检测,参考标准--,《药物中安非他酮检测方案(制备液相色谱)》用到的仪器有Agilent 1260 Infinity II 制备型液相色谱、Agilent 6545 Q-TOF 液质联用系统、Agilent 1290 Infinity II Multisampler、OpenLAB 软件
推荐专场
相关方案
更多
该厂商其他方案
更多

























