Legal regulations demand that organic impurities in pharmaceuticals must be identified and characterized. The isolation of impurities from a pharmaceutical product can be done by preparative-scale liquid chromatography (LC). This Application Note demonstrates the purification of acetaminophen and six known impurities using an Agilent 1290 Infinity II Preparative LC/MSD System. For high throughput and sample load, a column of 50 mm inside diameter (id) is operated at a solvent flow of 118 mL/min. Mass-based fraction collection is combined with UV detection for highest selectivity. The performance of the fraction collection is assessed by determining the purity of collected fractions and the recovery of the active pharmaceutical ingredient (API).
方案详情

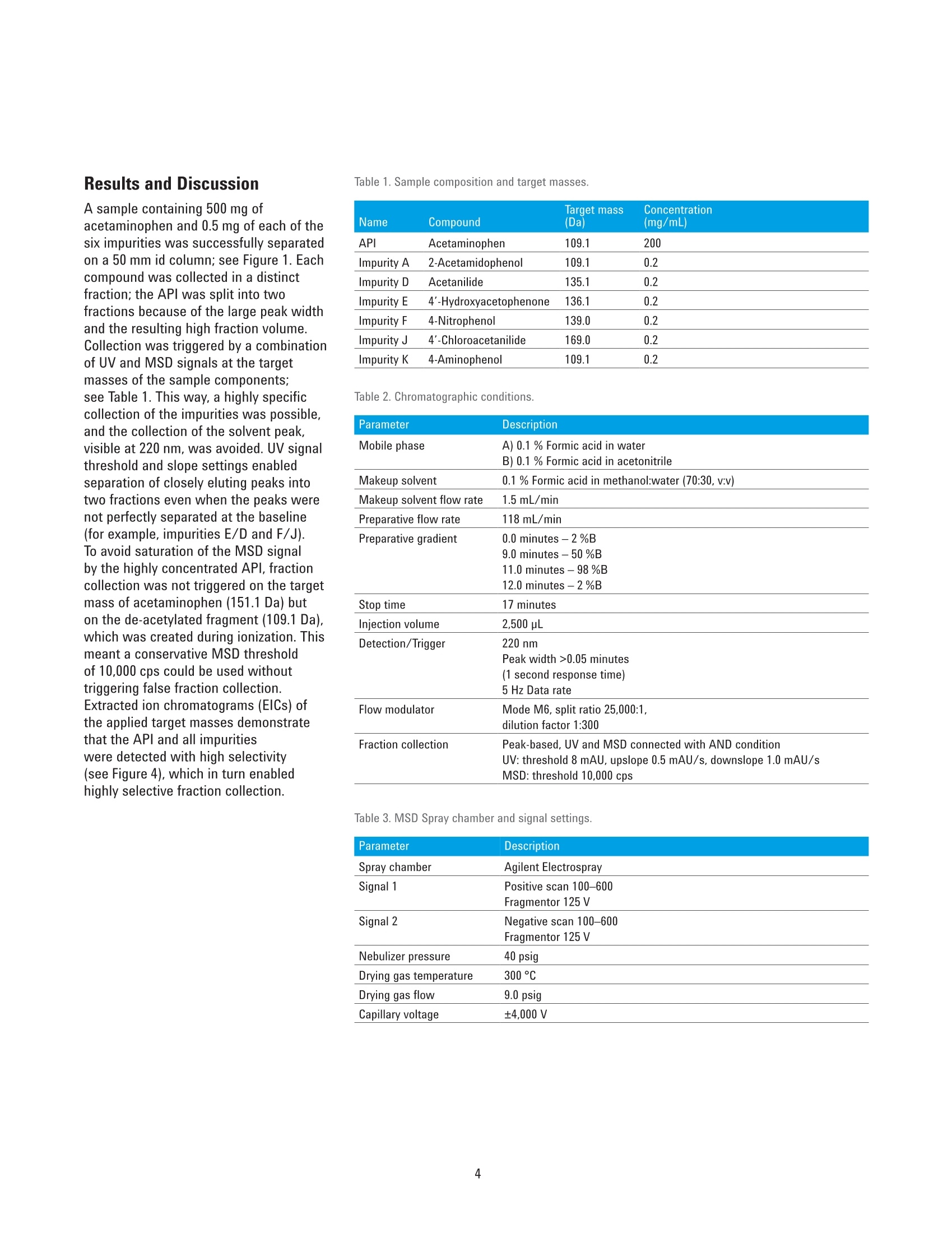
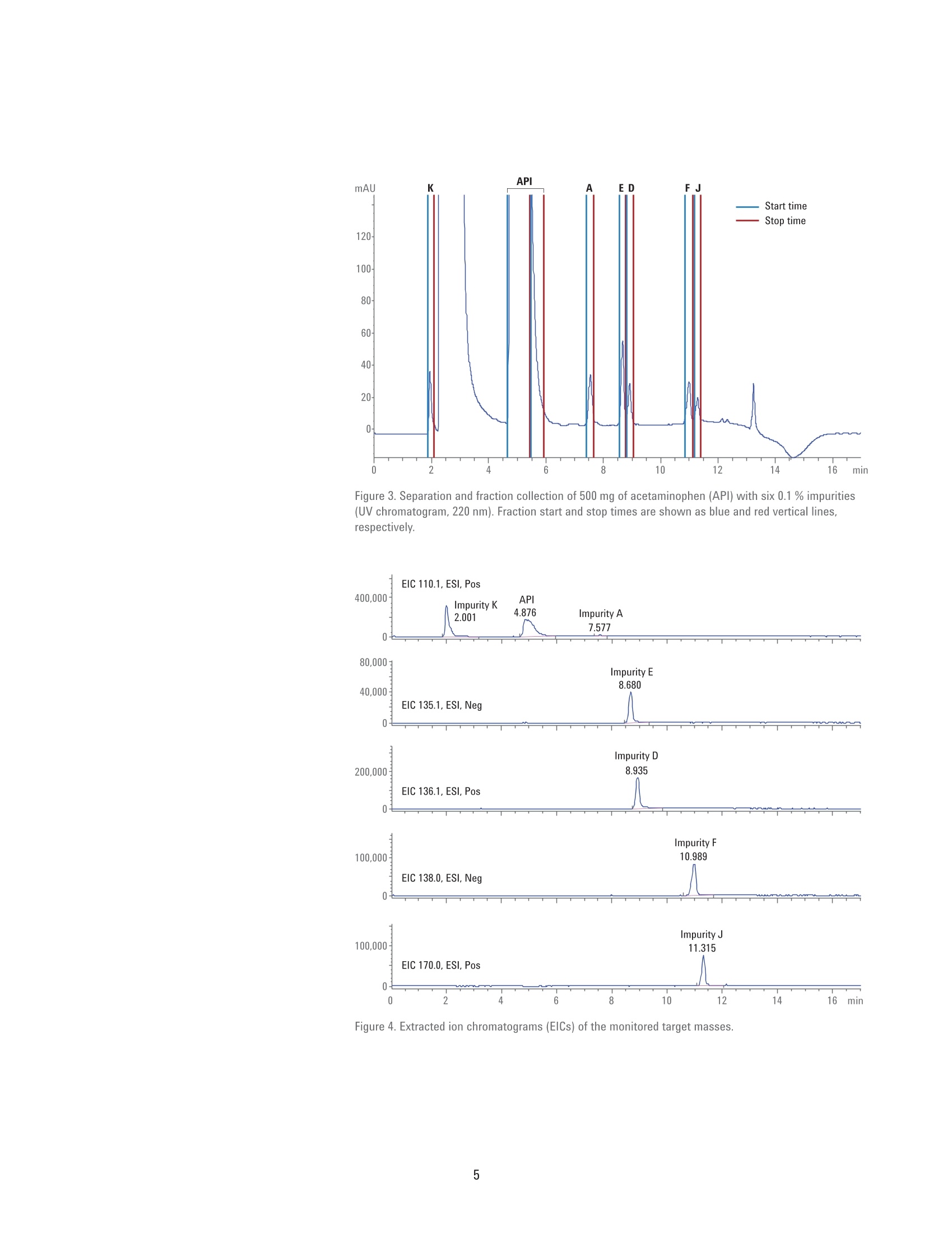
High-Throughput LC/MS Purificationof Pharmaceutical Impurities Application Note Small Molecule Pharmaceuticals Author Abstract Florian RieckAgilent Technologies, Inc. Waldbronn,Germany Legal regulations demand that organic impurities in pharmaceuticals must beidentified and characterized. The isolation of impurities from a pharmaceuticalproduct can be done by preparative-scale liquid chromatography (LC). ThisApplication Note demonstrates the purification of acetaminophen and six knownimpurities using an Agilent 1290 Infinity Il Preparative LC/MSD System. For highthroughput and sample load, a column of 50 mm inside diameter (id) is operatedat a solvent flow of 118 mL/min. Mass-based fraction collection is combined withUV detection for highest selectivity. The performance of the fraction collection isassessed by determining the purity of collected fractions and the recovery of theactive pharmaceutical ingredient (API). Agilent Technologies Introduction Organic impurities in drug manufacturingcan present a major challenge forpharmaceutical companies. Byproductsand intermediates originating from thechemical synthesis can be present insignificant amounts, even in an optimizedproduction process. Regulations suchas the ICH Guideline Q3A(R2) demandidentification and monitoring of allimpurities exceeding a given threshold .The isolation of the different impuritiesfrom the active pharmaceutical ingredient(API) for characterization can be doneby preparative-scale LC. With preciouspharmaceuticals, however, it is key tocollect not only the impurities, but torecover as much of the APl as possible. This Application Note demonstratesthe separation and purification ofacetaminophen (also known asparacetamol) and six common impuritieslisted in the European and United StatesPharmacopoeias23. A total amount of500 mg of the APl and 0.5 mg of eachimpurity (equaling 0.1 %) were separatedon an Agilent Load & Lock column of50 mm inside diameter (id). For thispurpose, an Agilent 1290 Infinity IIPreparative LC/MSD System was used.This system can handle the high flowrates necessary to operate a column ofthese dimensions, and enables fractioncollection triggered by UV and MSDsignals for high selectivity. This systemis one of the Agilent InfinityLab LCPurification Solutions", featuring modulesthat ensure safe and easy operationwhile improving the purification outcome.The Agilent 1260 Infinity II PreparativeAutosampler delivers fast injection cycletimes with lower carryover and injectionsfrom 2- and 5-mL vials. It supportsinjection volumes from microliters up to3.6 mL, and has an excellent injectionlinearity (Figure 1). Another module is the Agilent 1290Infinity I MS Flow Modulator. Thismodule actively splits part of themain flow to the MSD, and integratesseamlessly into Agilent InfinityLabLC Purification Systems, both from asoftware and hardware point of view.Split ratio and dilution, as well as startand stop times are set and saved directlywithin the LC method. Depending onthe makeup and main solvent flow rate,split ratios are calculated on-the-fly,and can be set between 100:1 and500,000:1 (Figure 2). Delay coils, which are necessary to retard the flow tothe fraction collector during MSD dataprocessing, are stored in a dedicatedDelay Coil Organizer. This modulefeatures a closed compartment with aninlet/outlet interface and a leak detector,designed to have the delay coils wellarranged, and shut down the system incase of a leak. An additional externalleak sensor can be connected to thesystem and placed at critical positionson or beneath the table. The externalleak sensor was placed on the floorunderneath the column. Method and sample details Sample Caffeine in water/acetonitrile (ACN) 98/2 (v/v), 20 pg/mLColumn Agilent 5 Prep-C18,21.2×50mm,5 pm (p/n 446905-102)Eluent Water/ACN (85/15) isocratic, degassedFlow rate 30.0 mL/minDraw speed 900 pL/min Eject speed 3,000 pL/min Figure 1. Injection linearity of the Agilent 1260 Infinity Il Preparative Autosampler withneedle seat extension (p/n G7157-68711) between 500 and 2,500 pL. Figure 2. Method settings of the Agilent 1290 Infinity II MS Flow Modulator, displaying the different split ratios calculatedbased on the main and makeup flow. Experimental Instrumentation The Agilent 1290 Infinity II PreparativeLC/MSD System consisted of thefollowing modules: Agilent 1290 Infinity ll PreparativePump (G7161B) Agilent 1260 Infinity II PreparativeAutosampler (G7157A) Agilent 1260 Infinity Il Diode ArrayDetector WR (G7115A) Agilent 1290 Infinity Il PreparativeOpen-Bed Fraction Collector(G7159B) Agilent 1290 Infinity II MS FlowModulator (G7170B) Agilent 1260 Infinity II Delay CoilOrganizer (G9324A) Agilent 1260 Infinity Il IsocraticPump (G7110B) Agilent InfinityLab LC/MSD(G6125B) Agilent External Leak SensorAssembly (p/n 5067-6149) Column Agilent Load & Lock 4002 column,50×500 mm (p/n PCG93LL500X50),packed with Agilent Prep C18, 10 pm bulkmedia (p/n 420910-902), compressed to abed length of 243 mm Software Agilent OpenLAB CDS ChemStationEdition for LC and LC/MS Systems,version C.01.07 SR3 [465] Solvents and samples All solvents used were LC grade. Freshultrapure water was obtained from aMilli-Q Integral system equipped witha 0.22-um membrane point-of-usecartridge (Millipak). Sample componentsand dimethyl sulfoxide (DMSO)were purchased from Sigma-Aldrich,Taufkirchen, Germany. The sample wasprepared in DMSO at a concentration asdescribed in Table 1. Results and Discussion A sample containing 500 mg ofacetaminophen and 0.5 mg of each of thesix impurities was successfully separatedon a 50 mm id column; see Figure 1. Eachcompound was collected in a distintfraction; the API was split into twofractions because of the large peak widthand the resulting high fraction volume.Collection was triggered by a combinationof UV and MSD signals at the targetmasses of the sample components;see Table 1. This way, a highly specificcollection of the impurities was possible,and the collection of the solvent peak,visible at 220 nm, was avoided. UV signalthreshold and slope settings enabledseparation of closely eluting peaks intotwo fractions even when the peaks werenot perfectly separated at the baseline(for example, impurities E/D and F/J).To avoid saturation of the MSD signalby the highly concentrated APl, fractioncollection was not triggered on the targetmass of acetaminophen (151.1 Da) buton the de-acetylated fragment (109.1 Da),which was created during ionization. Thismeant a conservative MSD thresholdof 10,000 cps could be used withouttriggering false fraction collection.Extracted ion chromatograms (EICs) ofthe applied target masses demonstratethat the APl and all impuritieswere detected with high selectivity(see Figure 4), which in turn enabledhighly selective fraction collection. Target mass Concentration Name Compound (Da) (mg/mL) API Acetaminophen 109.1 200 Impurity A 2-Acetamidophenol 109.1 0.2 Impurity D Acetanilide 135.1 0.2 Impurity E 4'-Hydroxyacetophenone 136.1 0.2 Impurity F 4-Nitrophenol 139.0 0.2 Impurity J 4'-Chloroacetanilide 169.0 0.2 Impurity K 4-Aminophenol 109.1 0.2 Table 2. Chromatographic conditions. Parameter Description Mobile phase A) 0.1 % Formic acid in water B) 0.1 % Formic acid in acetonitrile Makeup solvent 0.1 % Formic acid in methanol:water (70:30, v:v) Makeup solvent flow rate 1.5mL/min Preparative flow rate 118 mL/min Preparative gradient 0.0 minutes -2%B 9.0 minutes -50 %B 11.0 minutes -98 %B 12.0 minutes -2 %B Stop time 17 minutes Injection volume 2,500 pL Detection/Trigger 220 nm Peak width >0.05 minutes (1 second response time) 5 Hz Data rate Flow modulator Mode M6, split ratio 25,000:1, dilution factor 1:300 Fraction collection Peak-based, UV and MSD connected with AND condition UV: threshold 8 mAU, upslope 0.5 mAU/s, downslope 1.0 mAU/s Table 3. MSD Spray chamber and signal settings. Parameter Description Spray chamber Agilent Electrospray Signal 1 Positive scan 100-600 Fragmentor 125 V Signal 2 Negative scan 100-600 Fragmentor 125V Nebulizer pressure 40 psig Drying gas temperature 300°C Drying gas flow 9.0 psig Capillary voltage ±4,000 V API Figure 3. Separation and fraction collection of 500 mg of acetaminophen (API) with six 0.1 % impurities(UV chromatogram, 220 nm). Fraction start and stop times are shown as blue and red vertical lines,respectively. Figure 4. Extracted ion chromatograms (EICs) of the monitored target masses. The purity of all collected fractionswas determined by re-analysis on anAgilent 1260 Infinity II LC System.Withthe exception of impurity D, the purityof each fraction was 99 % or higher;see Table 4. This demonstrates the highselectivity of the fraction collection whentriggered by a combination of the UV withthe MSD signal. To determine the amountof APl collected, the two fractions ofthe APl were combined,and the solventwas evaporated. Over a series of threecollections, the average amount of theAPl in the fractions was found to be 99 % of the injected amount. Conclusion The Agilent 1290 Infinity II PreparativeLC/MSD System enabled the separationand purification of a pharmaceuticalsample. A 500 mg amount of activepharmaceutical ingredient with impuritiesat 0.1 % was injected and successfullyseparated on a 50 mm id Agilent Load& Lock column. Fraction collectionwas triggered by a combination of UVand MSD signals,which enabled ahighly specific collection of all samplecomponents by their respective targetmass. The purity of the collected fractionswas generally high (99% or greater),with the exception of one compound.The fraction of the active pharmaceuticalingredient was dried and weighed todetermine the recovery, which was foundto be 99 % of the injected amount. Table 4. Purity of collected fractions. Peak name Compound larget mass (Da) Purity Impurity K 4-Aminophenol 109.1 >99% API Acetaminophen 109.1 >99% Impurity A 2-Acetamidophenol 109.1 >99% Impurity E 4'-Hydroxyacetophenone 136.1 >99% Impurity D Acetanilide 135.1 81% Impurity F 4-Nitrophenol 139.0 99% Impurity J 4'-Chloroacetanilide 169.0 99% References 1. ICH Guideline Q3A(R2): Impurities inNew Drug Substances, 2006. 2. Paracetamol, PharmacopoeiaEuropaea 9.0, 2017. 3. Acetaminophen, United StatesPharmacopoeia [USP 39], 2016. 4. Agilent InfinityLab LC PurificationSolutions, Agilent TechnologiesBrochure, publication number5991-8009EN,2017. www.agilent.com/chem For Research Use Onlv. Not for use in diagnostic procedures. This information is subject to change without notice. C Agilent Technologies, Inc., 2017 Published in the USA, September 1, 20175991-8415EN Agilent Technologies Legal regulations demand that organic impurities in pharmaceuticals must be identified and characterized. The isolation of impurities from a pharmaceutical product can be done by preparative-scale liquid chromatography (LC). This Application Note demonstrates the purification of acetaminophen and six known impurities using an Agilent 1290 Infinity II Preparative LC/MSD System. For high throughput and sample load, a column of 50 mm inside diameter (id) is operated at a solvent flow of 118 mL/min. Mass-based fraction collection is combined with UV detection for highest selectivity. The performance of the fraction collection is assessed by determining the purity of collected fractions and the recovery of the active pharmaceutical ingredient (API).The Agilent 1290 Infinity II Preparative LC/MSD System enabled the separation and purification of a pharmaceutical sample. A 500 mg amount of active pharmaceutical ingredient with impurities at 0.1 % was injected and successfully separated on a 50 mm id Agilent Load& Lock column. Fraction collection was triggered by a combination of UV and MSD signals, which enabled a highly specific collection of all sample components by their respective target mass. The purity of the collected fractions was generally high (99 % or greater), with the exception of one compound. The fraction of the active pharmaceutical ingredient was dried and weighed to determine the recovery, which was found to be 99 % of the injected amount.
确定




还剩4页未读,是否继续阅读?
安捷伦科技(中国)有限公司为您提供《镇痛药中药物杂质检测方案(制备液相色谱)》,该方案主要用于化药制剂中药物杂质检测,参考标准--,《镇痛药中药物杂质检测方案(制备液相色谱)》用到的仪器有Agilent 1290 Infinity II 制备型液相色谱、Agilent 6470 三重四极杆液质联用系统、Agilent 1290 Infinity II Multisampler
推荐专场
相关方案
更多
该厂商其他方案
更多
























