
方案详情
文
舱底水一般含有来自船上活动的燃料、液压油、润滑油、挥发性有机化合物、金属、表面活性剂、脱脂剂和其他化学品(US EPA, 2008)。哥德堡大学与瑞典环境科学研究院团队为了调查在瑞典水域航行的大型客轮渡轮的舱底水对海洋环境的毒性。使用海洋细菌(Vibrio fischeri)的Microtox 法,对处理前后七艘渡轮(A-G)舱底水进行了毒性测试(SS-EN-ISO 11348-3:2008)。
方案详情

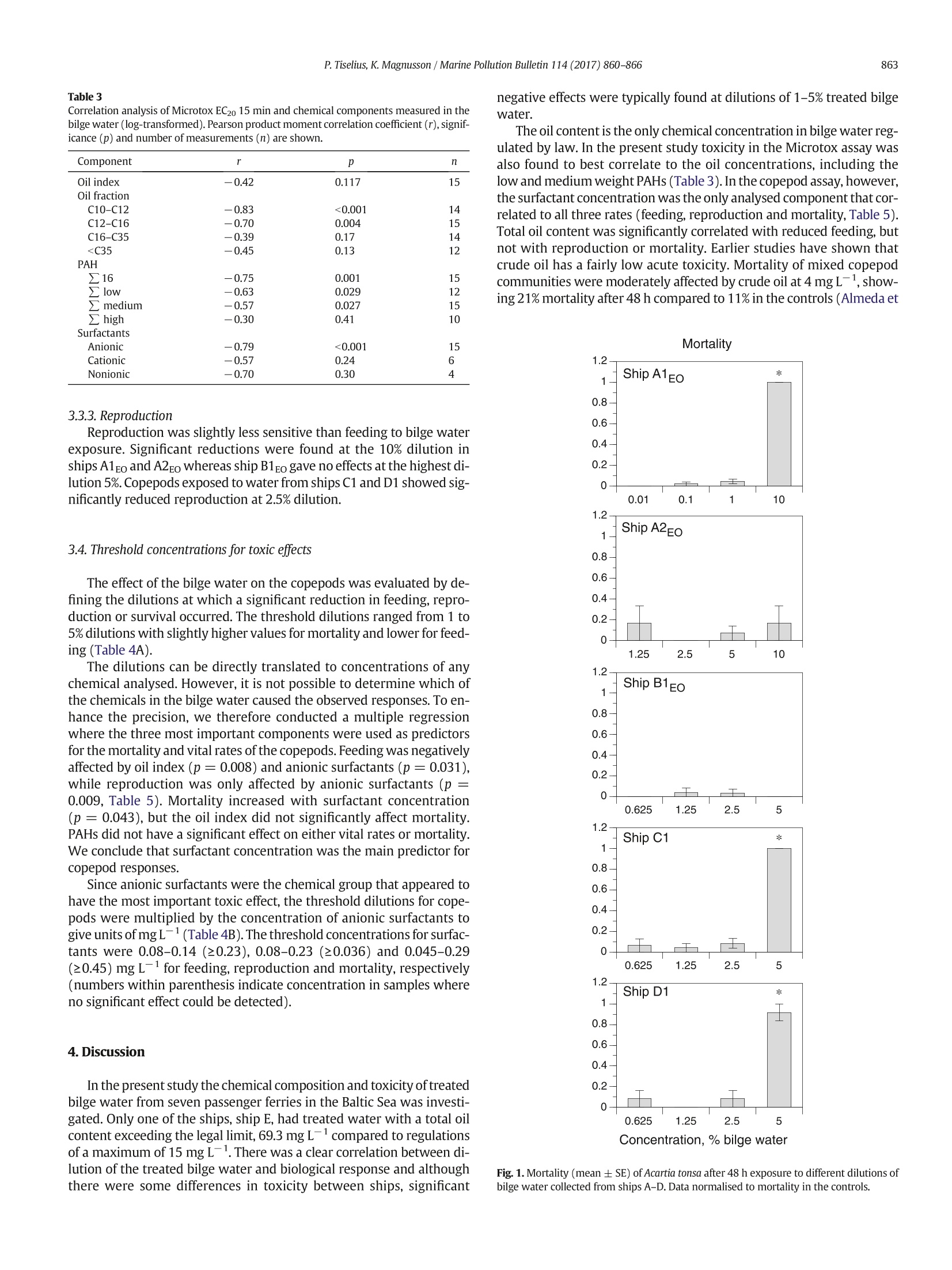
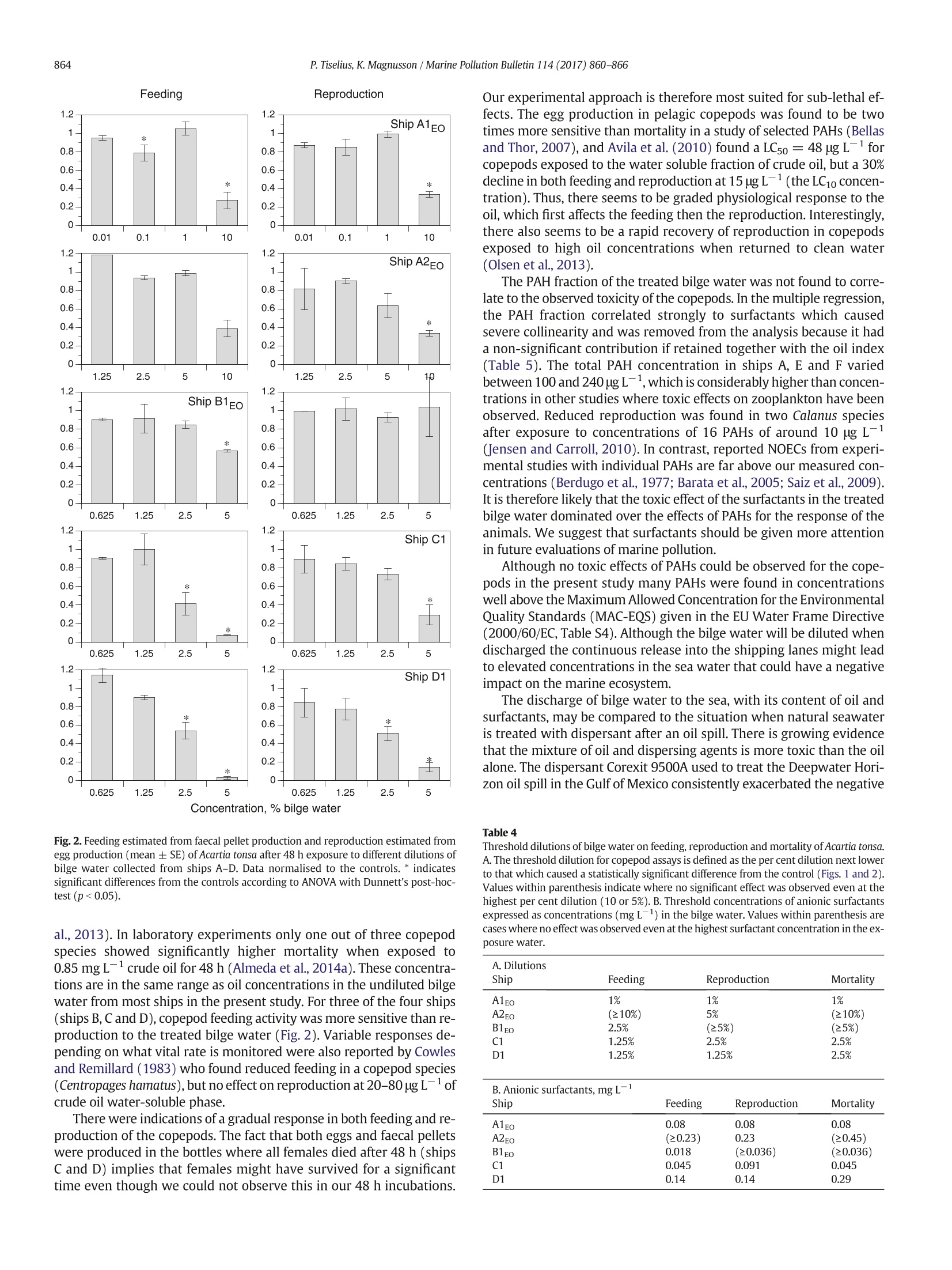
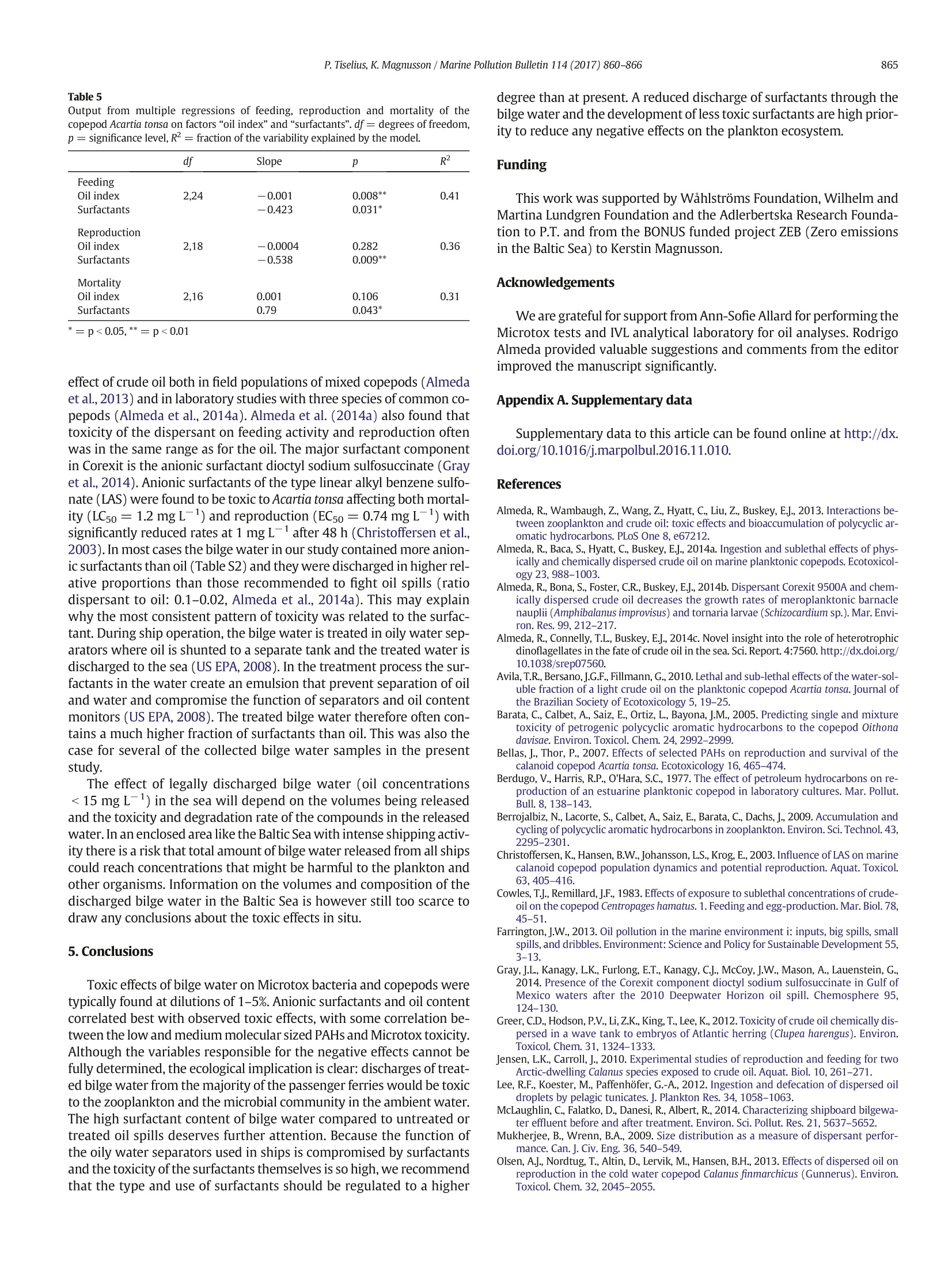
Marine Pollution Bulletin 114 (2017)860-866Contents lists available at ScienceDirect P. Tiselius, K.Magnusson / Marine Pollution Bulletin 114 (2017)860-866861 Marine Pollution Bulletin journal homepage:www.elsevier.com/locate/marpolbul Toxicity of treated bilge water: The need for revised regulatory controlPeter Tiselius a*, Kerstin Magnussonb University of Gothenburg, Department of Biological and Environmental Sciences -Kristineberg, SE-45178 Fiskebackskil, Sweden"IVL Swedish Environmental Research Institute, Kristineberg, SE-45178 Fiskebackskil, Sweden ARTICLEEINFO A BSTRACT Article history: 3 SeiReceived 18 September 2016Received in revised form 8 November 2016Accepted 9 November 2016Available online 14 November 2016 Keywords: Toxicity Water accumulating in the bottom of ships (bilge water), contains a mixture of oil, detergents and other com-pounds from on board activities. To evaluate ecological effects of released bilge water the chemical compositionand toxicity of treated bilge water from seven passenger ships was analysed. The oil content was below15 mg L, the threshold for legal discharge, in all but one ship.Still, significant reductions in feeding and repro-duction of Acartia tonsa were found after 48 h exposure in dilutions with 2.5-5% of bilge water. Mortality was sig-nificant at dilutions of 5-10% in 4 of the 5 bilge water samples. Surfactants were the most significant contributorto the toxicity on copepod vital rates and survival. Toxicity was also tested with Microtox where an ECso wasfound at dilutions between 4.3% and 52%. The results show that ecological effects might occur also in diluted sus-pensions of bilge water. Bilge waterOil pollutionSurfactantsCopepods O 2016 Elsevier Ltd. All rights reserved. Release of oil from various shipping activities has over the past halfcentury caused enormous problems to aquatic ecosystems. Most atten-tion has been given to oil spills where marine biota initially are exposedto high oil concentrations which gradually are reduced throughe.g.evaporation, photo-oxidation and bacterial break-down of the oil com-ponents. Less attention is given to the effects of the legally accepted re-lease of smaller volumes of oil to the sea through activities likedischarge of bilge water, ballast water and cleaning of tanks. Recent es-timates indicate that the total chronic release of oil worldwide to theocean averaged 270,000 tons per year over the period 1990-1999,equal to the largest single oil spills from an oil tanker accident, the At-lantic Empress in 1979, or about one third of the total release of oilfrom the Deepwater Horizon Macondo well in 2010 (Farrington,2013). The input of oil from ship operations is continuous, so eventhough most oil fractions have a fairly rapid half-life they may causepermanently increased oil concentrations in areas with intense ship-ping. Marine biota in these areas hence run the risk of being chronicallyexposed to low but still elevated concentrations of oil. Bilge water is the water that accumulates in the bottom of the shipand it is generated from machinery leakage and wash-down of freshwater. It may contain fuel, hydraulic oils, lubricant oils, volatile organiccompounds, metals, detergents, degreasers and other chemicals derivedfrom activities on board a ship (US EPA, 2008). ( * C orresponding author. ) ( E-mail addresses: p et e r.t i s el ius @ bi o env . gu . se ( P. Tiselius), ke r stin .magnu s so n@iv l . se (K. Magnusson). ) ( h t t p:/ / d x.do i .org/ 1 0 .1 0 16 / j . marp o lbul.20 1 6.11.01 0 0 025-326X/O2016Elsevier Ltd. All ri g h ts reserved. ) The International Maritime Organization (IMO) regulates handlingof bilge water. The focus for regulation is set on the oil content of thedischarged bilge water since this is generally considered to be themost important toxic component. According to the International Con-vention for the Prevention of Pollution from Ships, (MARPOL 73/78)no water may be discharged into the sea if it contains ≥15 mg L-1ofoil. To meet the IMO regulations the bilge water is either treated enroute, in an oil separation system before being discharged to the seaor deposited at reception facilities on land. The treatment is complicateddue to its mixed content of chemicals in the water. The most problem-atic is the mixture of oil and surfactants derived from cleaning, whichprohibits the water from separating into two distinct phases. Despitethis, a recent study indicates that oily water separators mounted onthree container and bulk carriers significantly reduced most substancesfor which there are regulated concentration limits (McLaughlin et al.,2014) The chemical composition of bilge water varies both between ves-sels and also from day to day within a vessel. Cruise ships and passengerferries produce significantly more bilge water than ships of other cate-gories due to their complicated constructions and support for many pas-sengers (US EPA, 2008). In a survey by Det Norske Veritas (DNV) themedian production of bilge water was estimated to be 7500 L day-from passenger ships, 360 L day- from offshore ships, and50 L dayfrom tankers and cargo vessels (Sjofartsdirektoratet,2009). The corresponding amount of oil being released from a passen-ger ship, assuming a maximum allowed oil content of 15 mg L-,would be 112 g oil day-, from an offshore ship 6 g oil dayandfrom tankers and cargo vessels 1 g day- ship. Large cruise shipswith gross tonnage from 20,000 to 78,000 operating off Alaska produced 5-20 mof bilge water per day equal to 75-300 g oil day- in legal dis-charges (US EPA, 2008). There is to our knowledge no data on the vol-umes of other components discharged with the bilge water. An important group of chemicals present in bilge water are thesurfactants. Many surfactants are known to be toxic in themselves andmixtures of oil and surfactants may be more toxic than each of the indi-vidual components, either caused by synergistic effects of the actualtoxicity the two components or as a result of an increased dissolutionof the crude oil by the dispersant making it more bioavailable for the ex-posed organisms (Greer et al.,2012; Wu et al., 2012; Almeda et al.,2014b). In general, toxicity tests of crude oil on planktonic species have beencarried out with just the water-soluble fraction of the oil since this oftenhas been considered to be the only bioavailable fraction (e.g. Berdugo etal., 1977; Berrojalbiz et al., 2009).However, oil discharged to sea wateralso occurs as droplets either formed by natural factors (wind, waves) orby dispersants applied after oil spills (Mukherjee and Wrenn, 2009). Ithas been shown that suspended oil droplets in the size range of foodparticles can be ingested by several species of zooplankton and henceadd to the uptake of oil (Lee et al., 2012; Almeda et al., 2014a, 2014b,2014c). Surfactants in bilge water are also likely to contribute to the for-mation of oil droplets, which might hence affect the toxicity of the oilfraction. Since the bilge water is released in the water column,plankton-ic species in areas with intense shipping are prime targets for environ-mental effects. Zooplankton, larvae and phytoplankton are particularlyat risk since they have limited capabilities to avoid areas of oil-contam-inated water. The aim of the present study was to investigate the toxicity on themarine environment of treated bilge water from large passenger ferriessailing in Swedish waters. No effort has been made to rank differenttreatment technologies. The toxicity of the bilge water from sevenferries collected before and after treatment was tested with Microtox,a screening test with marine bacteria. Since zooplankton is the primegroup affected by a continuous release of contaminated water to thesea, further experiments were conducted using treated bilge waterfrom four of the ferries recording the survival and sub-lethal effects onthe marine copepod Acartia tonsa. 2. Material and methods 2.1. Bilge water sampling Treated bilge water, after passage through an oily water separator,was collected between May 2015 and February 2016 from seven Table 1 ( T reated bilge water samples collected f r om ships A-G. Samples from ships A1, A2, B1 andB2 were taken both at beginning of the bilge water treatment, EO (=Early Operation) andat the end of the treatment, LO ( =L ate Operation). ) Sampling date Ship ID Chemical Test with Test with analyses Microtox Acartia tonsa 7 May 2015 A1Eo X X X 8 May 2015 A1Lo X X 2 June 2015 A2Eo X X X 3 June 2015 A2Lo X X 26 May 2015 B1Eo X X X 27 May 2015 B1Lo X X 15 June 2015 B2Eo X X 15 June 2015 B2Lo X X 17 Nov 2015 B3 X X 1 June 2015 C1 X X X 11 Nov 2015 C2 X X 11 June 2015 D1 X X X 15 June 2015 D2 X X 2 Dec 2015 D3 X X 26 Nov 2015 E X X 27 Nov 2015 F X X 10 July 2015 passenger ships (ships A-G) ranging iin size from 119,700-52,000 gross ton. Sampling was done between one and three timesfrom each ship (Table 1). The oil separating equipment differed be-tween the ships. Water from ships A and B was pumped from a storagetank to the treatment system without any mixing. The chemical compo-sition of the bilge water may therefore depend on the level of the stor-age tank and on what time in the treatment process the sampling wascarried out. Samples were therefore taken both at early operation (EO,Table 1) and late operation (LO). The bilge water in ships C to G wasmixed during treatment and samples were taken only once at each sam-pling occasion. All sampling was done when the ships were in the portand not during operation at sea. The ship-mounted measuring instru-ments always reported the oil content to be <15 mg L, which is theupper limit for bilge water to be released into the sea. The collectedbilge water was stored in dark 1 L glass bottles at 4°℃ in the dark untilchemical analysis and toxicity tests. 2.2. Chemical analyses All sampled bilge water was analysed for the total oil index and car-bon fractions (>C10-C12,>C12-C16,>C16-C35 and>C35 -C16-C35, whereas the water from ship A was dominated by thesmallest defined fraction, >C12-C16. The concentrations of 16PAHs, which are a part of the reported total oil content, showedlarge differences between the ships. The highest concentrations,100-240 ug L, were found in the treated bilge water from ships A, Eand Fand the lowest from ship B, 0.067-0.53 ug L-1. The relative contri-bution of PAHs to the total oil index was highest for ship A, where the16 PAHs made up between 9 and 18%. Naphthalene, which has alow number of carbon atoms (part of PAH-L), was the dominatingPAH in bilge water of all ships except ship B, where pyrene (part ofPAH-M) was found at the highest concentration (Table S2, Table S4).Concentrations of anionic surfactants varied between 450 and14,000 ug L-1, often exceeding the oil index (Table S2). Cationic andnonionic surfactants were only analysed in samples from ships B3 andships C-F and occurred in concentrations similar to or lower thanthose of the anionic surfactants. The ratio between total oil and anionicsurfactants varied considerably with values ranging between 0.01 and29.7 (median 0.28). Metal concentrations varied between ships and also between sam-pling occasions from the same ship (Table S3). Bilge water from ship Bgenerally had the highest concentrations. The concentrations of Zn(filtered<0.45 um) in bilge water from ship B and sample D2 were 10to 20 times higher than the threshold level of 8 ug Zn·L-1in the aquaticenvironment which has been suggested by the Swedish EPA (SwedishEPA, 2008). Also Cu (filtered <0.45 um) was elevated in bilge waterfrom ship B at the first two sampling occasions (11-65 ug L-) com-pared to the suggested threshold value, 4 ug L(Swedish EPA,2008). 3.2. Microtox The most toxic water derived from ship E where EC5o was achievedwhen the exposure water contained 4.3% of bilge water (Table 2). Theleast toxic were the samples from ship B where 50% reduction in biolu-minescence was not reached even in the highest test concentrations (EC5o of≥90% and EC20 between 29 and 39%). Samples from ships Aand B were collected during both the early and late part of the bilgewater treatment process, but only at sampling occasion A1 was thetreated water more toxic during early operation (EO) compared to thelate operation (LO). No such difference was found in the other sam-plings, indicating that the treated water is fairly similar throughoutthe treatment operation. Correlation analyses were done between toxicity to the marine bac-teria in the Microtox test and the chemical components analysed in thetreated bilge water samples (Table 3). Since EC5o values could not be de-termined for ship B (values> 90%), the EC2o values after 15 min expo-sure were used for the correlation analyses. The negative correlationswere strongest for the oil fractions and PAHs with low and mediumnumber of carbon atoms and for the anionic surfactants. An exceptionwas the water from ship D at the third sampling occasion, which hada low toxicity to the Microtox bacteria in spite of relatively high oil con-centrations (ship D3, Table 2). No correlations were found betweenmetal concentrations and toxicity. 3.3. Copepod responses 3.3.1. Mortality Mortality was low (<8%) in the controls and all treatments, exceptthe test waters with 5 and 10% bilge water, where most animals died(Fig.1). From ship A bilge water was sampled at two occasions (A1and A2), which gave slightly different results.All animals died whenthe exposure water contained 10% of the A1Eo bilge water, but only 2out of 12 animals died in 10% of the A2Eo water. Bilge water from shipB1Eo did not affect copepod survival, but all animals died in the 5% dilu-tion from ship C1 and 11 out of 12 animals in ship D2. 3.3.2. Feeding The highest test concentration of bilge water was 10% dilution andthis was only from ship A. At the first sampling occasion (A1) feedingwas significantly reduced to 28% of the controls whereas no significanteffect on feeding was found at the second sampling occasion (Fig. 2).For ship B1Eo, significantly reduced feeding was observed at 5% dilution,while bilge water from ships C1 and D1 reduced feeding significantly al-ready at a dilution with 2.5% bilge water. The strongest negative effectwas found in ships C1 and D1 where almost no feeding was recordedat 5%dilutions. Table 2 Toxicity of the treated bilge water from ships A-G measured with Microtox. Total oil andsurfactant concentrations corresponding to the EC5o values are shown. Samples markedwith * were also used in the tests with Acartia tonsa. Microtox Ship ID EC20 15 min% EC50 15 min% Total oil. Anionic surfactants. mgL- mgL A1EO 2 6.4 0.10 0.52 A1Lo 5.6 26 0.32 4.21 A2EO° 11 38 0.22 1.73 A2Lo 16 51 0.37 2.44 B1Eo 34 >90 0.13 0.64 B1Lo 29 >90 0.14 0.71 B2Eo 36 >90 0.63 0.41 B2Lo 39 >90 0.44 0.53 B3 38 90 2.30 1.61 C1* 2.3 9.8 0.93 0.35 C2 3.6 18 0.29 0.41 D1* 2.6 14 0.01 1.32 D2 3.4 9.8 0.05 1.13 D3 16 52 3.89 4.02 E 1.5 4.3 2.98 0.10 F 3.3 14 0.70 1.96 G 6.3 27 0.13 Correlation analysis of Microtox EC20 15 min and chemical components measured in thebilge water (log-transformed).Pearson product moment correlation coefficient (r), signif-icance (p) and number of measurements (n) are shown. Component r n Oil index -0.42 0.117 15 Oil fraction C10-C12 -0.83 <0.001 14 C12-C16 -0.70 0.004 15 C16-C35 -0.39 0.17 14 Microtox 生物毒性测试技术在舱底污水毒性研究中的应用——哥德堡大学与瑞典环境科学研究院团队基于Microtox对舱底污水进行生物毒性研究Modern Water Microtox 生物毒性检测技术具有快速、简单、廉价等优点,已成为毒性测试领域研究的热点。在环境污染事件监测、饮用水安全保护和应急响应等领域的已成为常规应用,并已成为多个国家认可的官方标准,Microtox 技术在废水出水毒性检测和钻井液检测领域也有着广泛的应用。舱底水一般含有来自船上活动的燃料、液压油、润滑油、挥发性有机化合物、金属、表面活性剂、脱脂剂和其他化学品(US EPA, 2008)。哥德堡大学与瑞典环境科学研究院团队为了调查在瑞典水域航行的大型客轮渡轮的舱底水对海洋环境的毒性。使用海洋细菌(Vibrio fischeri)的Microtox 法,对处理前后七艘渡轮(A-G)舱底水进行了毒性测试(SS-EN-ISO 11348-3:2008)。结果表明,将发光细菌暴露于2.5-5%的舱底水稀释液中48小时发光抑制程度最大;在4个舱底水样品中,稀释度为5-10%时,死亡率较显著;EC50处于4.3%至52%的稀释度之间(Table 2)。在Microtox测试中对海洋细菌的毒性与舱底水样品中的化学成分之间进行了相关性分析(Table 3)。表明具有低和中等碳原子的油馏分、PAHs和阴离子表面活性剂与毒性强弱的负相关性最强;而金属浓度与毒性之间未观测到有明显相关性;阴离子表面活性剂和油含量与毒性作用相关性较强。该研究结果具有重要的生态学意义,表明减少表面活性剂向舱底水的排放以及开发毒性较低的表面活性剂是降低舱底水的生物毒性的主要途径。相关研究结果《Toxicity of treated bilge water: The need for revised regulatory control》已于近期发表在《Marine Pollution Bulletin》上。Microtox 技术也广泛应用于废水处理厂的出水毒性检测。在澳大利亚新南威尔士州环保署(NSWEPA)颁发的环境保护许可中,每个废水出水排放监测点必须定期取样并使用 Microtox 技术进行急性毒性分析。与使用其他生物(网纹蚤、仔鱼)的系统相比,使用费氏弧菌的 Microtox 技术检测时间更短,结果精确度和灵敏性更高,成本更低,是一种理想的废水整体毒性测试方案。
确定



还剩5页未读,是否继续阅读?
Modern Water (英国现代水务)为您提供《舱底污水中生物毒性检测方案(水质毒性分析)》,该方案主要用于废水中综合检测,参考标准--,《舱底污水中生物毒性检测方案(水质毒性分析)》用到的仪器有生物毒性分析仪 Microtox LX、Modern Water 便携毒性监测仪 Microtox® FX
推荐专场
水质毒性分析仪/水质生物毒性分析仪
相关方案
更多
该厂商其他方案
更多
















