方案详情
文
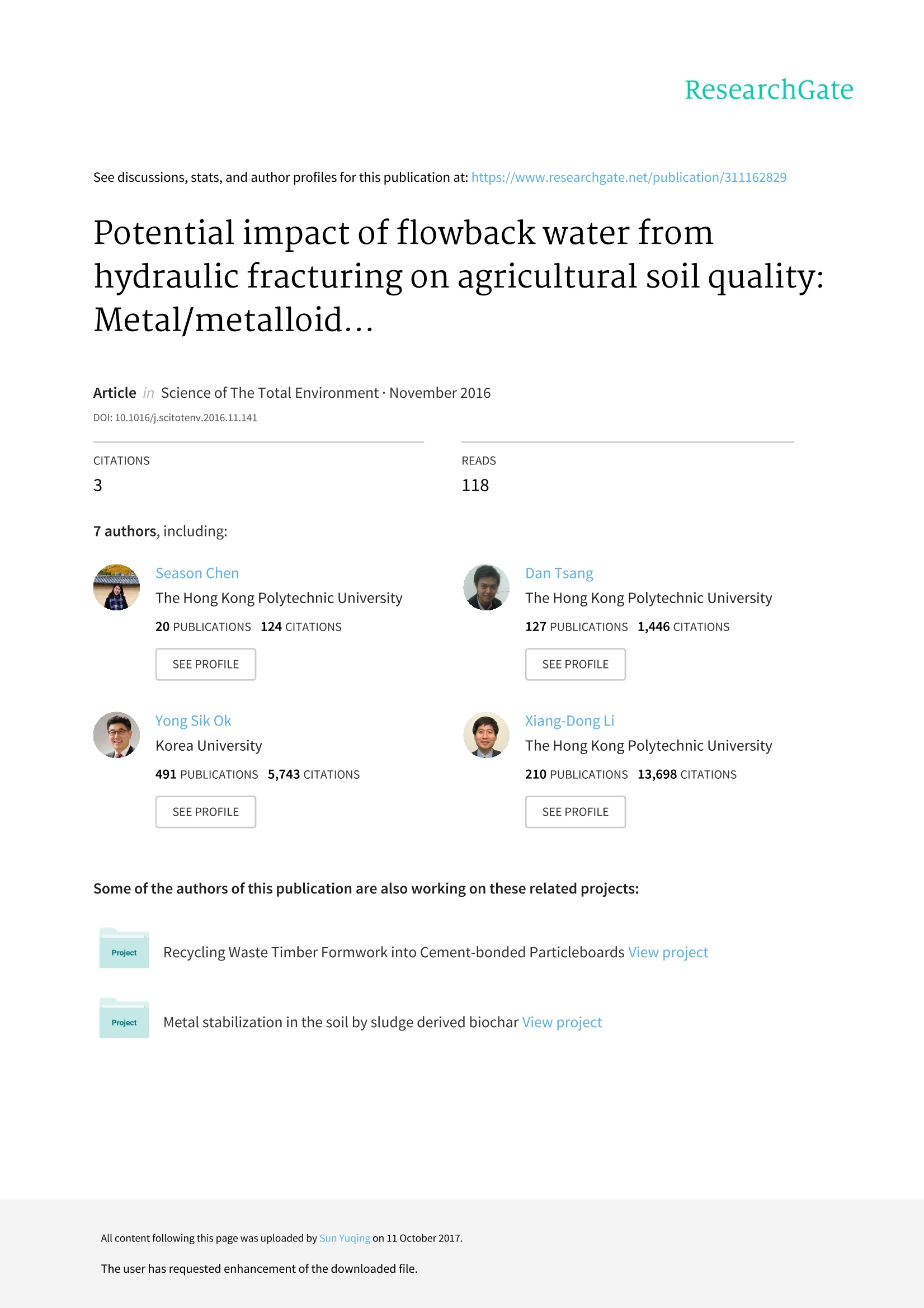
水力压裂技术促进了页岩气开采的发展,而由于含盐量高,金属/准金属(As,Se,Fe和Sr)以及有机添加剂等原因,无意溢出的回流水可能会对周围环境造成危害。本研究对东北地区4个代表性页岩气开采区域,采用Microtox生物测定法(费氏弧菌)和酶活性测试,对回流水溶液对土壤生态系统的影响进行评估。
方案详情

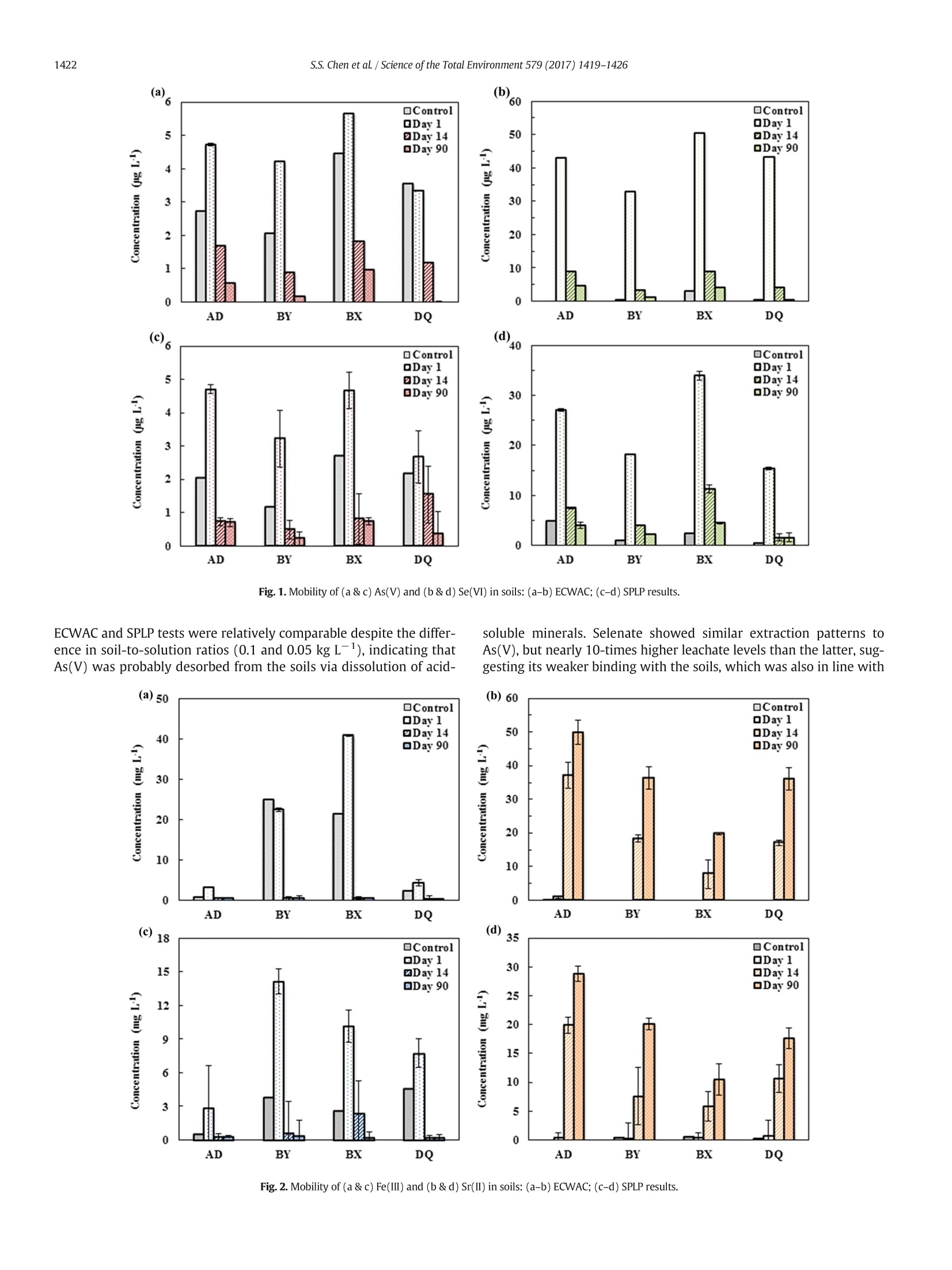
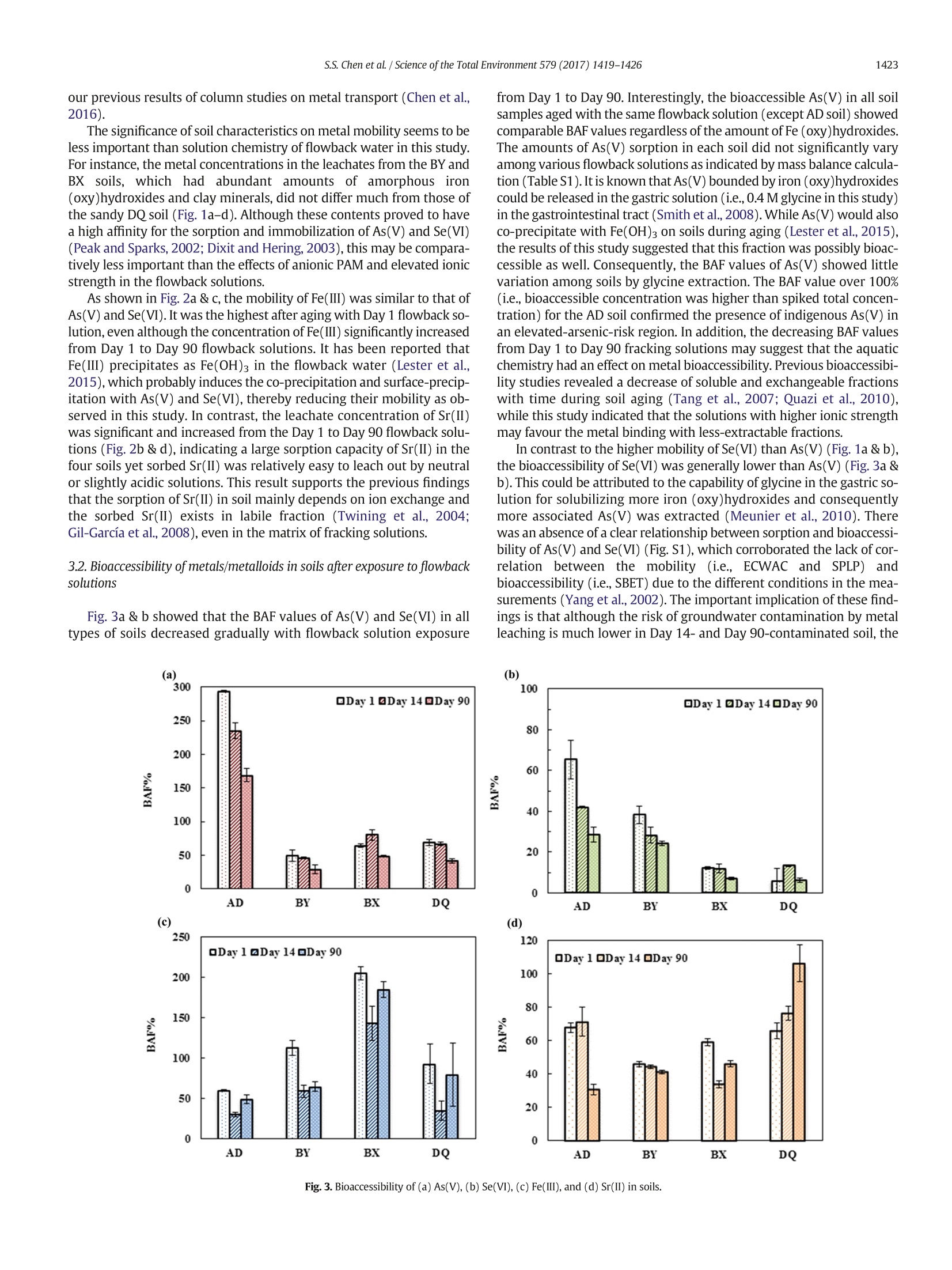
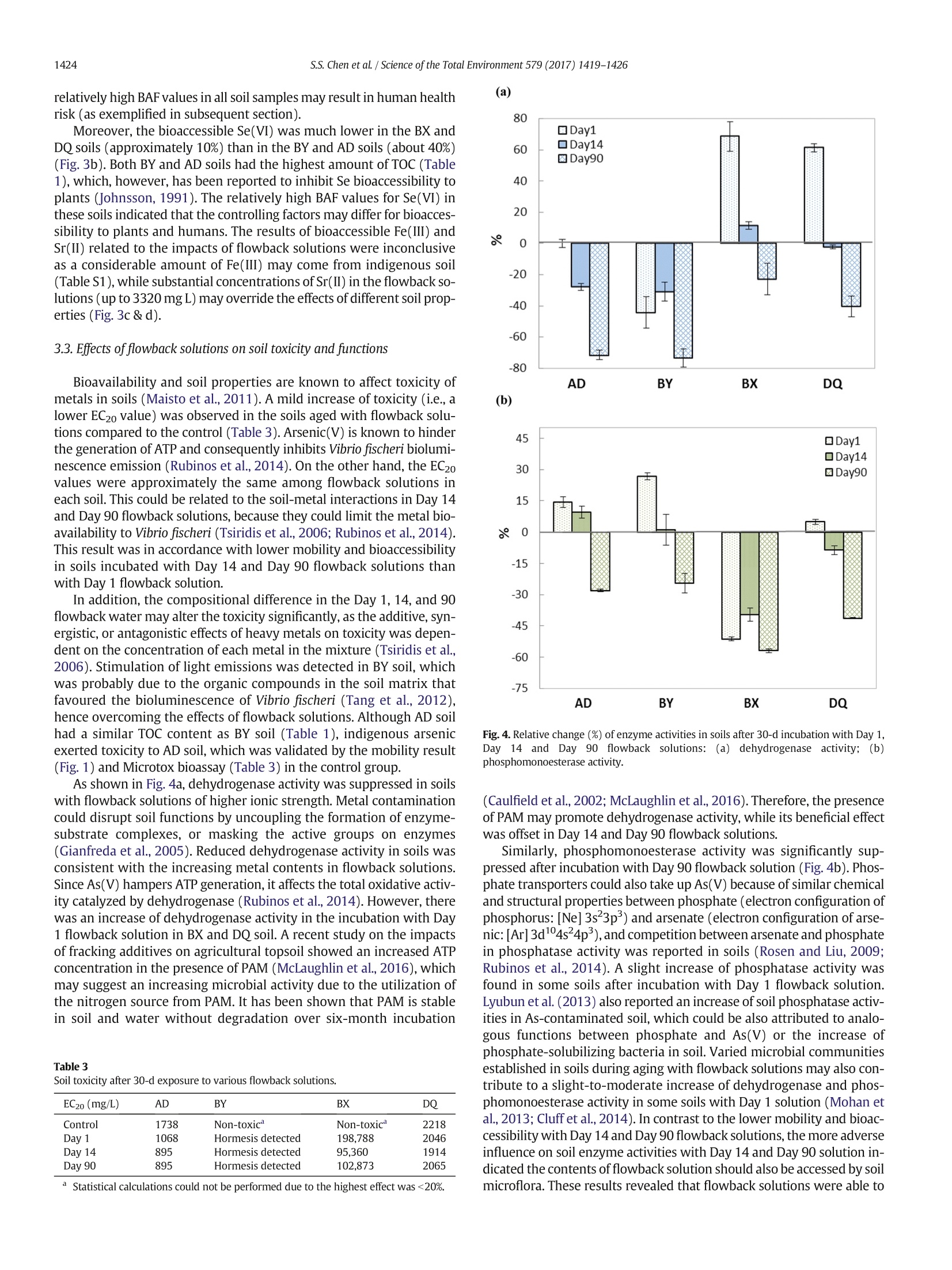
ResearchGateSee discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/311162829 Science of the Total Environment 579 (2017) 1419-1426 Some of the authors of this publication are also working on these related projects: Recycling Waste Timber Formwork into Cement-bonded Particleboards View project Metal stabilization in the soil by sludge derived biochar View project Potential impact of flowback water fromhydraulic fracturing on agricultural soil quality:Metal/metalloid... Article in Science of The TotalEnvironment·November 2016 Contents lists available at ScienceDirect Science of the Total Environment journal homepage: www.elsevier.com/locate/scitotenv Potential impact of flowback water from hydraulic fracturing onagricultural soil quality: Metal/metalloid bioaccessibility, Microtoxbioassay, and enzyme activities Season S. Chen, Yuqing Suna, Daniel C.W. Tsang a.*, Nigel J.D. Graham , Yong Sik Ok,Yujie Feng *, Xiang-Dong Li Department of Civil and Environmental Engineering, Hong Kong Polytechnic University, Hung Hom,Kowloon, Hong Kong, China ” State Key Laboratory ofUrban Water Resource and Environment, Harbin Institute of Technology, Harbin 150090, ChinaEnvironmental and Water Resources Engineering, Department of Civil and Environmental Engineering, Imperial College London, South Kensington, London SW7 2AZ, UK School of Natural Resources and Environmental Science, Korea Biochar Research Center, Kangwon National University, Chuncheon 24341,Republic of Korea · High ionic strength of flowback waterreduced mobility of metalloids. · Relatively high bioaccessibility of met-alloids in spite of low mobility · Soil toxicity moderately increased after1-month aging with flowback water. ·Soil dehydrogenase activity was affect-ed by PAM in flowback water. Article history: Received 9 September 2016Received in revised form 16 November 2016Accepted 20 November 2016 Available online 29 November 2016 Editor: Simon Pollard Keywords:Fracturing fluidsMetal mobility A BSTRACT Hydraulic fracturing has advanced the development of shale gas extraction, while inadvertent spills of flowbackwater may pose a risk to the surrounding environment due to its high salt content, metals/metalloids (As, Se, Feand Sr), and organic additives. This study investigated the potential impact of flowback water on four represen-tative soils from shale gas regions in Northeast China using synthetic flowback solutions. The compositions of thesolutions were representative of flowback water arising at different stages after fracturing well establishment.The effects of solution composition of flowback water on soil ecosystem were assessed in terms of metal mobilityand bioaccessibility, as well as biological endpoints using Microtox bioassay (Vibrio fischeri) and enzyme activitytests. After one-month artificial aging of the soils with various flowback solutions, the mobility and bioaccessibi-lity of As(V) and Se(VI) decreased as the ionic strength of the flowback solutions increased. The results inferred astronger binding affinity of As(V) and Se(VI) with the soils. Nevertheless, the soil toxicity to Vibrio fischeri onlypresented a moderate increase after aging, while dehydrogenase and phosphomonoesterase activities were sig-nificantly suppressed with increasing ionic strength of flowback solutions. On the contrary,polyacrylamide in the ( * Corresponding authors. ) ( E-mail a ddresses: d a n. t sang@poly u .e d u . hk ( D . C.W. Tsang), yuji ef@hit.e d u . c n (Y. Feng). ) flowback solutions led to higher dehydrogenase activity. These results indicated that soil enzyme activities weresensitive to the composition of flowback solutions. A preliminary human health risk assessment related to As(V)suggested a low level of cancer risk through exposure via ingestion, while holistic assessment of environmentalimplications is required. O 2016 Elsevier B.V. All rights reserved. The technology breakthrough of hydraulic fracturing (fracking) hasexpanded the natural gas extraction from shale reserves, of which theglobal volume is estimated to be over 200 trillion m(Kargbo et al.,2010; Kuuskraa et al., 2013). China plans to produce 300 billion mofshale gas by 2020 (China's State Council, 2016). In spite of the huge eco-nomic benefit it may bring, fracking has raised substantial concerns onhuman health and environmental impact, such as air pollution (Bunchet al., 2014; Moore et al., 2014) and water contamination (Warner etal., 2013; Vengosh et al., 2014). Depending on the geological conditionsof the shale formation, the fracking process requires varying amount offracking fluid from 8000 to 19,000 m’per well, which is composed ofabout 90% water, 6-9% proppant and 0.5-2% chemical additives suchas gelling agents, friction reducers, and surfactants (Gregory et al.,2011; Vidic et al., 2013; Vandecasteele et al.,2015). About 10-70% of the injected fluid returning to the surface prior togas recovery is known as flowback water,while the produced water isgenerated during gas production (Gregory et al., 2011; Vidic et al.,2013; Vengosh et al., 2014). These wastewaters contain elevated con-centration of dissolved organic carbon (up to 590 mg L-1) from thechemical additives in fracking fluids. Naturally present substancessuch as radioactive elements, metals/metalloids, and brine salts fromthe shale formations also result in highly concentrated total dissolvedsolids (up to 350,000 mg L-) (Barbot et al., 2013; Kondash et al.,2013; Lester et al., 2015). The release of fracking chemicals into the en-vironment could happen at any stage during shale gas developmentfrom drilling to waste disposal due to accidental spills or operationalfailures (Ziemkiewicz et al., 2014;Vandecasteele et al., 2015). In partic-ular, more than three quarters of the spills may involve flowback andproduced water as revealed by the latest study (McLaughlin et al.,2016), and the associated risks to water resources have aroused exten-sive interests (Ferrar et al., 2013; Warner et al., 2013; Vengosh et al.,2014). Recent studies have reported the positive relationships betweenthe composition in fracking wastewaters and the occurrence of disinfec-tion by-products (Hladik et al., 2014).Elevated concentrations of arse-nic, selenium, and strontium were also found in surrounding streamsof shale gas formations (Fontenot et al., 2013; Abualfaraj et al., 2014).However, contamination in soil environment exposed to the spills offlowback and produced water is poorly understood in the existing liter-ature (Annevelink et al., 2016). In view of the elevated salt contents and organic additives in thefracking wastewater, the physico-chemical properties of soil such as vis-cosity and pH may be altered (Stringfellow et al., 2014). Our recentwork has demonstrated that the leaching of flowback solutions mayalter the transport channels in soil (Chen et al., 2016). Besides, thechemical interactions between fracking wastewater and soil environ-ment may lead to eco-toxicological risks (Stringfellow et al., 2014;Annevelink et al., 2016). It has been shown that the biocide glutaralde-hyde is largely sorbed in soil and it forms cross-linking with polyacryl-amide (PAM), which is a friction reducer used in fracking fluids thatcan remain stable in soil over six months (Caulfield et al., 2002;McLaughlin et al., 2016). The presence of salts in the flowback wateralso prevents the biodegradation of polyethylene glycol, which is a sur-factant employed in fracking fluid (McLaughlin et al., 2016). The com-plexity of the flowback water composition as well as the increasingreports of accidental spills have aggravated the potential risks to our soil environment and human health (Ziemkiewicz et al., 2014). Acci-dental spills of fracking fluids affect colloid mobilization in the unsatu-rated zone (Sang et al., 2014), while fracking activity often occurs nearthe agricultural area and poses a higher risk to agricultural soil(McLaughlin et al., 2016). However, the implications of chemical toxic-ity of flowback water on soil organisms and functionalities of soil micro-bial communities such as enzyme activities of dehydrogenase andphosphomonoesterase still remain uncertain. Therefore, this study investigates the metal mobility/bioaccessibilityand soil health by simulating spills of flowback water on representativesurface soils collected in the vicinity of shale wells. Under acidic and ox-idizing conditions in the flowback solution, As(V) and Se(ⅥI) would bethe predominant form and susceptible to mobilization (Phan et al.,2015; Parnell et al., 2016). Soil quality was assessed in terms of biolog-ical endpoints of the Microtox bioassay and soil enzyme activities,which are rapid and reliable approaches to assessing the chemical tox-icity to organisms and the microbial activity in the soil ecosystem(Pandey and Singh, 2006; Maisto et al., 2011; Lyubun et al., 2013). Po-tential human health risk of accidental spills to neighbouring farmersis also estimated in this study. 2. Materials and methods 2.1. Soil sampling and synthetic flowback solutions The variations of soil properties on resultant metal mobilization andsoil quality were investigated with four types of surface soils sampledfrom shale gas regions, namely Anda, Bayan, Binxian and Daqing, inSongliao Basin, China. The shale formation also overlapped with an ele-vated-arsenic-risk region (Rodriguez-Lado et al., 2013), which may in-crease the risk of the mobility and bioaccessibility of arsenic whenexposed to the spills of flowback solutions. The soil samples wereground and passed through 2mm sieve. The four soils were sampledfrom agricultural land, where the pH ranged from 8.1 to 9.3, reflectingthe prevalence of alkaline soils in this area. The content of total organiccarbon (TOC) and amorphous Fe were measured, as they are known toprovide major adsorption sites for arsenate (As(V)) and selenate(Se(ⅥI)), and may influence the metal mobilization (Goldberg andJohnston, 2001; Peak and Sparks, 2002; Feng et al.,2013). Anda (AD)and Bayan (BY) soil contained similar level of TOC (25.3 mg g-and24.8 mg g, respectively), while the amorphous Fe in BY soil (45.0mgg) was much higher than that in AD soil (21.0mgg). Binxian(BX) and Daqing (DQ) soil contained a relatively low amount of TOC(10.2 mggand 12.4mg g,respectively), and the amorphous Fecontent was comparatively high in BX soil (38.7mg g) but muchless in DQsoil (9.36mg g)(Table 1). Table 1Selected physico-chemical properties of the four surface soils in the shale region. AD BY BX DQ Bulk density (g cm) 1.70 1.65 1.62 1.65 pH 8.8 9.3 8.1 8.3 ToC(mg g-) 25.3 24.8 10.2 12.4 Fe(ⅢII) (mgg 21.0 45.0 38.7 9.36 Measured by dissolving 1 g of soil in 5 mL of deionized water. Extraction by ammonium oxalate at pH 3. ” Total organic carbon measured by TOC Analyzer (SSM-5000A).C 1 The synthetic solutions were prepared according to the characteris-tics of Day 1 and Day 14 (flowback water) as well as Day 90 (producedwater) effluent waters from the comprehensive report(Hayes,2009). Inthis study, they were referred to synthetic flowback solutions. As sum-marized in Table 2, they represented significant temporal variation inionic strength, iron content, and scaling (precipitation) in the field. Poly-acrylamide (0.088% v/v) and ethylene glycol (0.043%v/v) were addedas commonly used fracturing additives (information obtained fromFracFocus database; www.FracFocus.org). The presence of As(V) andSe(VI) has been widely reported in fracking fluids and aroused environ-mental concerns (Balaba and Smart, 2012; Fontenot et al., 2013;Abualfaraj et al., 2014), therefore, 100 pg L concentrations of assurancegrade As(V) and Se(VI) (SPEX CertiPrep, USA) were spiked intoflowback solutions (Sun et al., 2017). The presence of Sr(II) and Fe(III)in flowback solutions was also studied for their potential environmentalimpact. The soil quality and the associated risks of metals/metalloids ofconcern (i.e.,As(V), Se(ⅥI), Sr(II), and Fe(ⅢI)) were accordingly investi-gated in the case of spill and leakage of flowback water near the groundsurface. 2.2.Metals/metalloids leaching and human health risks assessment As the soil samples were obtained from the agricultural land ofNortheast China Plain, where rice is one of the dominating crops(USDA, 2014) and flooded environment is prevalent in rice paddy fields,the fate of chemicals under saturated condition was investigated in thisstudy. According to a sudden spill scenario where spill volume is1000 gal (3785.4 L) over an impacted area of 0.1 acre (404.7 m-)(Gradient, 2012), the solid-to-liquid ratio was calculated to rangefrom 7 to 28 g L- assuming the bulk density of soil of 1.3 ×10kgm-3and the topsoil thickness of 5 to 20 cm. Therefore, the mediansolid-to-liquid ratio (17.5g L1) was rounded up to 20 g L-andadopted in this study. Each type of soil was mixed with Day 1, Day 14,and Day 90 flowback solutions (400 mL), respectively, in 500 mL poly-propylene centrifuge bottles at a soil-to-solution ratio of 20 g L-1. Thesamples were shaken by an end-over-end shaker at 20 rpm for 24 h,and then incubated for 30 days at room temperature with daily shakingby hand for 1 min. At the end of 30 days, the artificially aged sampleswere separated by centrifugation at 4000 rpm. The supernatant solu-tions were acidified with concentrated hydrochloric acid to pH<2 andstored at 4C. The concentrations of As(V) and Se(ⅥI) were determinedby atomic absorption spectrometry with vapour generation accessory(VGA-AAS, Agilent VGA77, limit of detection1 ug L), while Sr(II)and Fe(Ⅲ) were measured by AAS (limit of detection 1 mgL). Theseparated soils were air-dried (for subsequent biological analyses) inan incubator at room temperature with a relative humidity of 20%.The control soils were aged at the same conditions with deionizedwater instead of flowback solutions. Portions of the dried soils was Table 2Composition and characteristics of the synthetic flowback solutions. Flowback solutions Day 1 Day 14 Day 90 Unit Calcium 676 11,050 18,450 mg/L Barium 387 1835 2135 mg/L Magnesium 121 938 1700 mg/L Sodium 6015 31,750 43,700 mg/L Chloride 8410 79,000 123,500 mg/L Bromide 87.9 708.5 1175 mg/L Iron(III) 19.5 74.9 117 mg/L Strontium(II) 156.5 3320 3140 mg/L Boron 8.1 16.4 17.8 mg/L Polyacrylamide 0.088% 0.088% 0.088% v/v Ethylene glycol 0.043% 0.043% 0.043% V/V Arsenic(V) 100 100 100 Hg/L Selenium(VI) 100 100 100 Hg/L pH 7.2 6.5 5.9 Ionic strength 0.35 2.49 4.10 M assessed by Synthetic Precipitation Leaching Procedure (SPLP, US EPA,1994) and European Council Waste Acceptance Criteria (ECWAC,prCEN/TS 12457-3)tests, simulating the mobility under leaching ofacidic rainwater and unbuffered deionized water, respectively (Tsanget al.,2013a,2013b). In regard of human health risks in the vicinity of sensitive regions,the bioaccessibility of As(V), Se(ⅥI), Sr(II), and Fe(ⅢI) from the controland flowback-water-contaminated soils for human receptors wasassessed by an in vitro simplified physiologically based extraction test(SBET) for estimating the bioaccessible metals in a simulated gastric en-vironment (Drexler and Brattin, 2007; Beiyuan et al., 2016). The non-. carcinogenic and carcinogenic risks of As(V), Se(Ⅵ), and Sr(II) werecharacterized using the SBET results and US EPA standard methodsThe hazard quotients (HQ) of a specific metal element from the expo-sure routes via accidental soil ingestion were added to determine a haz-ard index (HI) (US EPA, 1997; US EPA, 2001). The human risks wereadjusted by the bioaccessible fraction (BAF) rather than the totalmetal concentration, and the corresponding hazard index (HIB) was de-rived (Luo et al., 2012; Beiyuan et al.,2016). Three major exposure path-ways of heavy metals in soils were considered in this study: (a)ingestion; (b) inhalation; and (c) dermal absorption (De Miguel et al.,2007),while only (a) and (c) were of concern for As(V),Se(V), andSr(II) in flowback water contamination. Detailed calculations areshown in the Supplementary Information. 2.3. Ecological impact on soil quality The Microtox bioassay and selected soil enzyme activity tests werecarried out on the soils after one-month exposure to flowback solutions.The soil toxicity was examined by the Microtox@ Basic Solid-Phase Test(AZUR, Model M500 Analyzer), which detects changes to specific lumi-nescent bacteria (Vibrio fischeri). The EC20 was adopted to represent thesoil concentration at 20% effects on Vibrio fischeri bioluminescence upon30-min exposure, which provides more sensitive endpoints (Fulladosaet al.,2005). Dehydrogenases and phosphomonoesterase activitieswere examined as a relatively rapid and easy procedure to assess soilhealth, which reflect the total metabolic activity of soil microorganismsand regulated phosphorus metabolism in soils, respectively (Lyubun etal., 2013; Beiyuan et al., 2017). Dehydrogenases activity was deter-mined by standard assay that reduced 2,3,5-triphenyltetrazolium chlo-ride to triphenylformazan, of which the intensity at 485 nm wasdetected by UV/vis spectrophotometer (Biochrom,Libra S35). Since allthe soils used in this study were alkaline soils, the predominant alkalinephosphomonoesterase activities were determined by measuring the p-nitrophenol released from p-nitrophenyl phosphate using UV/visiblespectrophotometer at 410 nm. 3. Results and discussion 3.1. Mobility of metals/metalloids in soils after exposure to flowbacksolutions The mobility of As(V) and Se(VI) in the soils incubated with Day 1flowback solution was more than 50% and 5 times higher than thosein the control soils when subject to DI water leaching and acidic rainfall,respectively (Fig. 1a-d). The mobility of both metalloids drastically de-creased with increasing ionic strength of flowback solutions (Table 2).The concentrations of extracted As(V) from the soils incubated withDay 14 and Day 90 flowback solutions were even lower than thosefrom the control (Fig. 1a &c). This suggested an unexpected suppressionof metal mobility by the chemical composition of the flowback solu-tions. The results may be explained by possible change of bindingphases in soil or formation of precipitates with elevated cation concen-trations in the flowback solutions, particularly for Ba(II), Ca(II), andFe(III) as shown in previous studies on arsenate (Zhang and Selim,2005; Zhu et al., 2005). Besides, the concentrations of As(V) in the Fig. 1. Mobility of (a &c) As(V) and (b & d) Se(VI) in soils: (a-b) ECWAC; (c-d) SPLP results. ECWAC and SPLP tests were relatively comparable despite the differ-ence in soil-to-solution ratios (0.1 and 0.05 kg L), indicating thatAs(V) was probably desorbed from the soils via dissolution of acid- soluble minerals. Selenate showed similar extraction patterns toAs(V), but nearly 10-times higher leachate levels than the latter, sug-gesting its weaker binding with the soils, which was also in line with Fig.2. Mobility of (a &c) Fe(III) and (b & d) Sr(II) in soils: (a-b) ECWAC; (c-d) SPLP results. The significance of soil characteristics on metal mobility seems to beless important than solution chemistry of flowback water in this study.For instance, the metal concentrations in the leachates from the BY andBX soils, which had abundant amounts of amorphous iron(oxy)hydroxides and clay minerals, did not differ much from those ofthe sandy DQ soil (Fig.1a-d). Although these contents proved to havea high affinity for the sorption and immobilization of As(V) and Se(VI)(Peak and Sparks, 2002; Dixit and Hering, 2003), this may be compara-tively less important than the effects of anionic PAM and elevated ionicstrength in the flowback solutions. As shown in Fig. 2a & c, the mobility of Fe(ⅢI) was similar to that ofAs(V)and Se(ⅥI). It was the highest after aging with Day 1 flowback so-lution, even although the concentration of Fe(III) significantly increasedfrom Day 1 to Day 90 flowback solutions. It has been reported thatFe(III) precipitates as Fe(OH)3 in the flowback water (Lester et al.,2015), which probably induces the co-precipitation and surface-precip-itation with As(V)and Se(VI), thereby reducing their mobility as ob-served in this study. In contrast, the leachate concentration of Sr(I)was significant and increased from the Day 1 to Day 90 flowback solu-tions (Fig.2b &d), indicating a large sorption capacity of Sr(II) in thefour soils yet sorbed Sr(II) was relatively easy to leach out by neutralor slightly acidic solutions. This result supports the previous findingsthat the sorption of Sr(II) in soil mainly depends on ion exchange andthe sorbed Sr(II) exists in labile fraction (Twining et al., 2004;Gil-Garcia et al., 2008), even in the matrix of fracking solutions. 3.2.Bioaccessibility of metals/metalloids in soils after exposure to flowbacksolutions Fig. 3a &b showed that the BAF values of As(V) and Se(ⅥI) in alltypes of soils decreased gradually with flowback solution exposure from Day 1 to Day 90.Interestingly, the bioaccessible As(V) in all soilsamples aged with the same flowback solution (except AD soil) showedcomparable BAF values regardless of the amount of Fe (oxy)hydroxides.The amounts of As(V) sorption in each soil did not significantly varyamong various flowback solutions as indicated by mass balance calcula-tion (Table S1). It is known that As(V) bounded by iron (oxy)hydroxidescould be released in the gastric solution (i.e., 0.4 M glycine in this study)in the gastrointestinal tract (Smith et al.,2008). While As(V) would alsoco-precipitate with Fe(OH)3 on soils during aging (Lester et al., 2015),the results of this study suggested that this fraction was possibly bioac-cessible as well. Consequently, the BAF values of As(V) showed littlevariation among soils by glycine extraction. The BAF value over 100%(i.e., bioaccessible concentration was higher than spiked total concen-tration) for the AD soil confirmed the presence of indigenous As(V) inan elevated-arsenic-risk region. In addition, the decreasing BAF valuesfrom Day 1 to Day 90 fracking solutions may suggest that the aquaticchemistry had an effect on metal bioaccessibility. Previous bioaccessibi-lity studies revealed a decrease of soluble and exchangeable fractionswith time during soil aging (Tang et al., 2007; Quazi et al., 2010),while this study indicated that the solutions with higher ionic strengthmay favour the metal binding with less-extractable fractions. In contrast to the higher mobility of Se(ⅥI) than As(V) (Fig. 1a & b),the bioaccessibility of Se(ⅥI) was generally lower than As(V) (Fig.3a &b). This could be attributed to the capability of glycine in the gastric so-lution for solubilizing more iron (oxy)hydroxides and consequentlymore associated As(V) was extracted (Meunier et al., 2010). Therewas an absence of a clear relationship between sorption and bioaccessi-bility of As(V) and Se(ⅥI) (Fig. S1), which corroborated the lack of cor-relation between the mobility (i.e., ECWAC and SPLP) andbioaccessibility (i.e.,SBET) due to the different conditions in the mea-surements (Yang et al., 2002). The important implication of these find-ings is that although the risk of groundwater contamination by metalleaching is much lower in Day 14- and Day 90-contaminated soil, the Fig. 3. Bioaccessibility of (a) As(V), (b) Se(VI), (c) Fe(III), and (d) Sr(II) in soils. relatively high BAF values in all soil samples may result in human healthrisk (as exemplified in subsequent section). Moreover, the bioaccessible Se(VI) was much lower in the BX andDQ soils (approximately 10%) than in the BY and AD soils (about 40%)(Fig.3b). Both BY and AD soils had the highest amount of TOC (Table1), which, however, has been reported to inhibit Se bioaccessibility toplants (Johnsson, 1991). The relatively high BAF values for Se(ⅥI) inthese soils indicated that the controlling factors may differ for bioacces-sibility to plants and humans. The results of bioaccessible Fe(III) andSr(II) related to the impacts of flowback solutions were inconclusiveas a considerable amount of Fe(II) may come from indigenous soil(Table S1), while substantial concentrations of Sr(II) in the flowback so-lutions (up to3320 mg L) may override the effects of different soil prop-erties (Fig.3c & d). 3.3. Effects of flowback solutions on soil toxicity and functions Bioavailability and soil properties are known to affect toxicity ofmetals in soils (Maisto et al., 2011). A mild increase of toxicity (i.e., alower EC20 value) was observed in the soils aged with flowback solu-tions compared to the control (Table 3). Arsenic(V) is known to hinderthe generation of ATP and consequently inhibits Vibrio fischeri biolumi-nescence emission (Rubinos et al.,2014). On the other hand, the EC20values were approximately the same among flowback solutions ineach soil.This could be related to the soil-metal interactions in Day 14and Day 90 flowback solutions, because they could limit the metal bio-availability to Vibrio fischeri (Tsiridis et al., 2006; Rubinos et al., 2014).This result was in accordance with lower mobility and bioaccessibilityin soils incubated with Day 14 and Day 90 flowback solutions thanwith Day 1 flowback solution. In addition, the compositional difference in the Day 1, 14,and 90flowback water may alter the toxicity significantly, as the additive, syn-ergistic, or antagonistic effects of heavy metals on toxicity was depen-dent on the concentration of each metal in the mixture (Tsiridis et al.,2006). Stimulation of light emissions was detected in BY soil, whichwas probably due to the organic compounds in the soil matrix thatfavoured the bioluminescence of Vibrio fischeri (Tang et al., 2012),hence overcoming the effects of flowback solutions. Although AD soilhad a similar TOC content as BY soil (Table 1), indigenous arsenicexerted toxicity to AD soil, which was validated by the mobility result(Fig. 1) and Microtox bioassay (Table 3) in the control group. As shown in Fig. 4a, dehydrogenase activity was suppressed in soilswith flowback solutions of higher ionic strength. Metal contaminationcould disrupt soil functions by uncoupling the formation of enzyme-substrate complexes, or masking the active groups on enzymes(Gianfreda et al., 2005). Reduced dehydrogenase activity in soils wasconsistent with the increasing metal contents in flowback solutions.Since As(V) hampers ATP generation, it affects the total oxidative activ-ity catalyzed by dehydrogenase (Rubinos et al., 2014). However, therewas an increase of dehydrogenase activity in the incubation with Day1 flowback solution in BX and DQ soil. A recent study on the impactsof fracking additives on agricultural topsoil showed an increased ATPconcentration in the presence of PAM (McLaughlin et al., 2016), whichmay suggest an increasing microbial activity due to the utilization ofthe nitrogen source from PAM. It has been shown that PAM is stablein soil and water without degradation over six-month incubation Table 3Soil toxicity after 30-d exposure to various flowback solutions. EC20 (mg/L) AD BY BX DQ Control 1738 Non-toxic° Non-toxic 2218 Day 1 1068 Hormesis detected 198,788 2046 Day 14 895 Hormesis detected 95,360 1914 Day 90 895 Hormesis detected 102,873 2065 Statistical calculations could not be performed due to the highest effect was <20%. Fig. 4. Relative change (%) of enzyme activities in soils after 30-d incubation with Day 1,Day 14 and Day 90 flowback solutions: (a) (dehydrogenase activity; (b)phosphomonoesterase activity. (Caulfield et al., 2002;McLaughlin et al., 2016). Therefore, the presenceof PAM may promote dehydrogenase activity, while its beneficial effectwas offset in Day 14 and Day 90 flowback solutions. Similarly, phosphomonoesterase activity was significantly sup-pressed after incubation with Day 90 flowback solution (Fig. 4b). Phos-phate transporters could also take up As(V) because of similar chemicaland structural properties between phosphate (electron configuration ofphosphorus: [Ne]3s3p) and arsenate (electron configuration of arse-nic:[Ar]3d4s 4p),and competition between arsenate and phosphatein phosphatase activity was reported in soils (Rosen and Liu, 2009;Rubinos et al., 2014). A slight increase of phosphatase activity wasfound in some soils after incubation with Day 1 flowback solution.Lyubun et al. (2013) also reported an increase of soil phosphatase activ-ities in As-contaminated soil,which could be also attributed to analo-gous functions between phosphate and As(V) or the increase ofphosphate-solubilizing bacteria in soil. Varied microbial communitiesestablished in soils during aging with flowback solutions may also con-tribute to a slight-to-moderate increase of dehydrogenase and phos-phomonoesterase activity in some soils with Day 1 solution (Mohan etal., 2013; Cluffet al., 2014). In contrast to the lower mobility and bioac-cessibility with Day 14 and Day 90 flowback solutions, the more adverseinfluence on soil enzyme activities with Day 14 and Day 90 solution in-dicated the contents of flowback solution should also be accessed by soilmicroflora. These results revealed that flowback solutions were able to Table 4 Chemical and biological impact of flowback solutions on soil environment. Impact on soil Implications environment Mobility of Decreasing mobility of As(V),Se(ⅥI), and Fe(III) but metals/metalloids increasing Sr(II) with increasing ionic strength of flowback solutions. Bioaccessibility of Decreasing bioaccessibility of As(V), Se(VI) but metals/metalloids increasing Sr(II) with increasing ionic strength of flowback solutions. Microtox bioassay Moderate increase of soil toxicity to Vibrio fischeri, but not sensitive to the change of ionic strength of flowback solutions Enzyme activities Reduced enzyme activities with increasing ionic strength of flowback solutions alter the soil microbial functions and the influence varied with soil type,while only a gentle increase of toxicity was determined by Microtox bio-assay. Table 4 provides a summary of the chemical and biological impactof various flowback solutions on the soil environment. The carcinogenic risk assessment was based on the As(V) concentra-tion in soils after one-month aging with flowback solutions. Among thefour concerned metals in this study, As(V) is classified as a knownhuman carcinogen by the Integrated Risk Information System of USEPA. The results revealed that the adjusted carcinogenic risk posed byAs(V) ranged from 4.56× 10-7to 2.09×10-6, of which more than85% was attributed to exposure via ingestion (Table S2). According tothe US EPA (2001), the cancer risks derived in this study would be con-sidered acceptable (10-4-10-6), so that remediation might not beneeded immediately. As for non-carcinogenic risks related to the metalspresent in this study (As(V),Se(ⅥI) and Sr(II)), ingestion was also thedominant pathway followed by dermal contact and inhalation. Thenon-cancer hazard index representing the aggregated non-carcinogeniceffects of metals was smaller than 1 in this study, which meant that con-cern about an increase of health hazard was less likely (US EPA, 2001).Although this study has provided an approximate assessment ofhuman health risks, the evaluation needs to be validated by site-specificexposure pathways. Potential synergistic or antagonistic effects associ-ated with the complex matrices and other components in the flowbackwater should be taken into account in order to provide a more compre-hensive evaluation of the environmental risks. 4. Conclusions During hydraulic fracturing, inadvertent spills of flowback waterhave been reported. Here, we investigated the impact of contaminatedflowback waters on environmental quality,soil ecological health, andhuman health risk exposed to the surrounding soils of representativeshale gas areas in China. Both the mobility and bioaccessibility ofAs(V) and Se(ⅥI) decreased as the ionic strength in flowback solutionincreased, indicating a change of metal-binding phases with varying so-lution pH and composition. The results revealed a mild increase of soiltoxicity (Microtox bioassay) compared to the control after one-monthaging with flowback solutions, while the dehydrogenase activity andphosphomonoesterase activity were significantly decreased with in-creasing ionic strength in flowback solutions. A preliminary humanhealth risk assessment implied no immediate need for remediationdue to As(V) and Se(V). This study illustrates that the temporal changeof flowback water compositions leads to different environmental impli-cations, which deserve more comprehensive evaluation for a holisticunderstanding of the environmental impact of fracking process. Acknowledgements The authors appreciate the financial support from the National Nat-ural Science Foundation of China (21407121), Hong Kong Research Grants Council (PolyU 538613 and 15222115), and State Key Laboratoryof Urban Water Resource and Environment (HCK201209) for this study. Appendix A. Supplementary data Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.scitotenv.2016.11.141. References Abualfaraj, N., Gurian, P.L.,Olson, M.S., 2014. Characterization of Marcellus Shale flowbackwater. Environ. Eng. Sci. 31, 514-524. Annevelink, M.P.J.A., Meesters, J.A.J., Hendriks, A.J., 2016. Environmental contaminationdue to shale gas development. Sci. Total Environ. 550, 431-438. Balaba, R.S., Smart, R.B., 2012. Total arsenic and selenium analysis in Marcellus shale,high-salinity water, and hydrofracture flowback wastewater. Chemosphere 89,1437-1442. Barbot, E., Vidic, N.S., Gregory, K.B., Vidic, R.D., 2013. Spatial and temporal correlation ofwater quality parameters of produced waters from Devonian-age shale following hy-draulic fracturing. Environ. Sci. Technol. 47, 2562-2569. Beiyuan,J., Tsang, D.C.W., Ok, Y.S., Zhang, W., Yang, X., Baek, K., Li, X.D., 2016. IntegratingEDDS-enhanced washing with low-cost stabilization of metal-contaminated soil froman e-waste recycling site. Chemosphere 159, 426-432. Beiyuan,J., Tsang, D.C.W., Valix, M., Zhang, W., Yang, X., Ok,Y.S., Li, X.D., 2017. Selectivedissolution followed by EDDS washing of an e-waste contaminated soil: extractionefficiency, fate of residual metals, and impact on soil quality. Chemosphere 166,489-496. Bunch, A., Perry, C., Abraham, L., Wikoff, D., Tachovsky, J., Hixon,J., Urban, J., Harris, M.,Haws, L., 2014. Evaluation of impact of shale gas operations in the Barnett Shale re-gion on volatile organic compounds in air and potential human health risks. Sci.Total Environ. 468,832-842. Caulfield, M.J., Qiao, G.G., Solomon, D.H., 2002. Some aspects of the properties and degra-dation of polyacrylamides. Chem. Rev. 102, 3067-3083. Chen, S.S., Sun,Y., Tsang, D.C.W., Graham, N.J.D., Ok, Y.S., Feng, Y., Li, X.D., 2016. InsightsInto the subsurface transport of As(V) and Se(ⅥI) in flowback water from hydraulicfracturing.Environ. Pollut. (under review). China's State Council, 2016. National People's Congress 13th Five-Year Plan. http://www.china.org.cn/china/NPC_CPPCC_2016/node_7234656.htm. Cluff, M.A., Hartsock, A., MacRae, J.D., Carter, K., Mouser, P.J., 2014. Temporal changes inmicrobial ecology and geochemistry in produced water from hydraulically fracturedMarcellus Shale gas wells. Environ. Sci. Technol. 48, 6508-6517. De Miguel, E., Iribarren, I.,Chacon, E.,Ordonez, A., Charlesworth, S.,2007.Risk-based eval-uation of the exposure of children to trace elements in playgrounds in Madrid(Spain). Chemosphere 66,505-513. Dixit, S., Hering,J.G., 2003.Comparison of arsenic (V) and arsenic (ⅢII) sorption onto ironoxide minerals: implications for arsenic mobility. Environ. Sci. Technol. 37,4182-4189. Drexler, J.W., Brattin, W.J., 2007. An in vitro procedure for estimation of lead relative bio-availability: with validation. Hum. Ecol.Risk. Assess. 13, 383-401. Feng, Q.,Zhang, Z., Chen, Y., Liu, L., Zhang, Z., Chen, C., 2013.Adsorption and desorptioncharacteristics of arsenic on soils: kinetics, equilibrium, and effect of Fe(OH)3 colloid,HzSiOs colloid and phosphate. Procedia Environ.Sci. 18, 26-36. Ferrar, K.J., Michanowicz, D.R., Christen, C.L., Mulcahy, N.,Malone, S.L., Sharma, R.K., 2013.Assessment of effluent contaminants from three facilities discharging Marcellus Shalewastewater to surface waters in Pennsylvania. Environ. Sci. Technol. 47, 3472-3481. Fontenot,B.E., Hunt, L.R., Hildenbrand, Z.L., Carlton Jr., D.D., Oka, H., Walton, J.L., Hopkins, D., Osorio,A., Bjorndal, B., Hu,Q.H., Schug, K.A., 2013. An evaluation of water qualityin private drinking water wells near natural gas extraction sites in the Barnett Shaleformation. Environ. Sci. Technol. 47, 10032-10040. Fulladosa, E., Murat, J.c., Martinez, M., Villaescusa, I., 2005. Patterns of metals and arsenicpoisoning in Vibrio fischeri bacteria. Chemosphere 60, 43-48. Gianfreda,L., Rao, M.A., Piotrowska,A., Palumbo, G., Colombo, C., 2005. Soil enzyme activ-ities as affected by anthropogenic alterations: intensive agricultural practices and or-ganic pollution. Sci. Total Environ. 341, 265-279. Gil-Garcia, C.J.,Rigol, A., Rauret, G., Vidal, M., 2008. Radionuclide sorption-desorption pat-tern in soils from Spain. Appl. Radiat. Isot. 66, 126-138. Goldberg,S.,Johnston, C.T., 2001. Mechanisms of arsenic adsorption on amorphous oxidesevaluated using macroscopic measurements, vibrational spectroscopy, and surfacecomplexation modelling. J.Colloid Interface Sci. 234, 204-216. Gradient, 2012. Human Health Risk Evaluation for Hydraulic Fracturing Fluid Additives.https://yosemite.epa.gov/sab/sabproduct.nsf/3D73316595F846C185257C2400686964/$File/Gradient+Human+Health+Risk+Evaluation.pdf. Gregory,K.B., Vidic, R.D., Dzombak, D.A., 2011. Water management challenges associatedwith the production of shale gas by hydraulic fracturing. Elements 7,18-186. Hayes, T., 2009. Sampling and Analysis of Water Streams Associated with the Develop- ment of Marcellus Shale Gas. Final Report Prepared for Marcellus Shale CoalitionGasTechnology Institute, Des Plaines, IL, p. 60018. Hladik,M.L., Focazio,M.J.,Engle, M., 2014. Discharges of produced waters from oil and gasextraction via wastewater treatment plants are sources of disinfection by-products toreceiving streams. Sci. Total Environ. 466,1085-1093. Johnsson, L., 1991. Selenium uptake by plants as a function of soil type, organic mattercontent and pH. Plant Soil 13,57-64. Kargbo,D.M., Wilhelm, R.G., Campbell,D.J.,2010. Natural gas plays in the Marcellus Shale:challenges and potential opportunities. Environ. Sci. Technol. 44,5679-5684. Kondash, A.J., Warner, N.R., Lahav, O., Vengosh, A., 2013. Radium and barium removalthrough blending hydraulic fracturing fluids with acid mine drainage. Environ. Sci.Technol. 48,1334-1342. Kuuskraa, V., Stevens, S.H., Moodhe, K.D., 2013. Technically Recoverable Shale Oil andShale Gas Resources: An Assessment of 137 Shale Formations in 41 Countries Outsidethe United States. Lester, Y., Ferrer, I., Thurman, E.M., Sitterley, K.A., Korak, J.A., Aiken, G., Linden, K.G., 2015.Characterization of hydraulic fracturing flowback water in Colorado: implications forwater treatment. Sci. Total Environ. 512, 637-644. Luo, X.S.,Ding,J., Xu, B., Wang, Y.J., Li, H.B., Yu,S.,2012. Incorporating bio-accessibility intohuman health risk assessments of heavy metals in urban park soils.Sci. Total Environ.424,88-96. Lyubun, Y.V.,Pleshakova, E.V.,Mkandawire,M., Turkovskaya, O.V., 2013. Diverse effects ofarsenic on selected enzyme activities in soil-plant-microbe interactions. J. Hazard.Mater. 262, 685-690. Maisto, G., Manzo, S., De Nicola, F., Carotenuto, R., Rocco, A., Alfani, A., 2011. Assessment ofthe effects of Cr, Cu, Ni and Pb soil contamination by ecotoxicological tests. J. Environ.Monit. 13, 3049-3056. McLaughlin, M.C., Borch, T., Blotevogel,j., 2016. Spills of hydraulic fracturing chemicals onagricultural topsoil: biodegradation, sorption, and co-contaminant interactions.Envi-ron. Sci. Technol. 50,6071-6078. Meunier, L., Wragg,J., Koch, I., Reimer, K.J., 2010. Method variables affecting the bioacces-sibility of arsenic in soil. J. Environ. Sci. Health A 45, 517-526. Mohan, A.M.,Hartsock, A., Bibby, K.J., Hammack, R.W., Vidic, R.D., Gregory, K.B., 2013. Mi-crobial community changes in hydraulic fracturing fluids and produced water fromshale gas extraction. Environ. Sci.Technol. 47, 13141-13150. Moore, C.W., Zielinska, B., Petron, G.,Jackson, R.B., 2014. Air impacts of increased naturalgas acquisition, processing, and use: a critical review. Environ. Sci. Technol. 48,8349-8359. Pandey,S., Singh, D.K., 2006. Soil dehydrogenase, phosphomonoesterase and arginine de-aminase acticities in an insecticide treated groundnut (Arachis hypogaea L.) field.Chemosphere 63, 869-880. Parnell, J., Brolly, C., Spinks,S., Bowden, S., 2016. Selenium enrichment in carboniferousshales, Britain and Ireland: problem or opportunity for shale gas extraction? Appl.Geochem. 66, 82-87. Peak, D., Sparks, L., 2002. Mechanisms of selenite adsorption on iron oxides and hydrox-ides. Environ. Sci.Technol. 36,1460-1466. Phan, T.T., Capo, R.C., Stewart, B.W., Graney, J.R., Johnson, J.D., Sharma,S., Toro, J., 2015.Trace metal distribution and mobility in drill cuttings and produced waters fromMarcellus Shale gas extraction: uranium, arsenic, barium. Appl. Geochem. 60,89-103. Quazi, S., Sarkar,D., Datta, R., 2010. Effect of soil aging on arsenic fractionation and bio-ac-cessibility in inorganic arsenic pesticide contaminated soils. Appl. Geochem.25,1422-1430. Rodriguez-Lado, L., Sun,G., Berg, M., Zhang, Q., Xue, H.B., Zheng, Q.M., Johnson, C.A., 2013.Groundwater arsenic contamination throughout China. Science 341, 866-868. Rosen, B.P., Liu, Z., 2009. Transport pathways for arsenic and selenium: a minireview. En-viron. Int. 35, 512-515. Rubinos, D.A., Calvo, V.,Iglesias,L., Barral, M.T., 2014.Acute toxicity of arsenic to Alivibriofischeri (MicrotoxQ bioassay) as influenced by potential competitive-protectiveagents. Environ. Sci. Pollut. Res. 21, 8631-8644. Sang, W., Stoof, C.R., Zhang, W., Morales, V.L., Gao, B., Kay, R.W., Liu, L., Zhang, Y.,Steenhuis, T.S., 2014. Effect of hydrofracking fluid on colloid transport in the unsatu-rated zone. Environ. Sci. Technol. 48,8266-8274. Smith, E., Naidu, R., Weber,J., Juhasz, A.L., 2008. The impact of sequestration on the bio-accessibility of arsenic in long-term contaminated soils. Chemosphere 71,773-780. Stringfellow, W.T., Domen, J.K., Camarillo, M.K., Sandelin, W.L, Borglin, S., 2014. Physical,chemical, and biological characteristics of compounds used in hydraulic fracturing.J. Hazard. Mater. 275, 37-54. Sun, Y., Chen, S.S., Tsang, D.C.W., Graham, N.J.D., Ok, Y.S., Feng, Y.,Li, X.D., 2017. Zero-valent iron for the abatement of arsenate and selenate from flowback water of hy-draulic fracturing. Chemosphere 167,163-170. Tang, X.Y., Zhu, Y.G., Shan, X.Q., McLaren, R., Duan,J., 2007.The ageing effect on the bio-accessibility and fractionation of arsenic in soils from China. Chemosphere 66,1183-1190. Tang, J.Y.M., Glenn,E, Thoen, H., Escher, B.I., 2012. In vitro bioassay for reactive toxicitytowards proteins implemented for water quality monitoring. J. Environ. Monit. 14,1073-1081. Tsang, D.C.W., Olds, W.E., Weber, P.A., Yip, A.C.K., 2013a. Soil stabilisation using AMDsludge, compost and lignite: TCLP leachability and continuous acid leaching.Chemosphere 93,2839-2847. Tsang, D.C.W., Olds, W.E., Weber, P.A., 2013b. Residual leachability of CCA-contaminatedsoil after treatment with biodegradable chelating agents and lignite-derived humic.r c.substances. J.Soils Sediments 13, 895-905. Tsiridis, V., Petala, M., Samaras, P., Hadjispyrou, S., Sakellaropoulos, G., Kungolos,A., 2006.Interactive toxic effects of heavy metals and humic acids on Vibrio fischeri. Ecotoxicol.Environ. Saf. 63, 158-167. Twining, J.R., Payne, T.E., Itakura, T., 2004. Soil-water distribution coefficients and planttransfer factors for 134Cs, 8sr and 65Zn under field conditions in tropical Australia.J.Environ. Radioact. 71, 71-87. US EPA, 1994. Method 1312: Synthetic Precipitation Leaching Procedure.US EPA, 1997. Exposure Factors Handbook (EPA/600/P-95/002Fa) (Update to Exposure Factors Handbook (EPA/600/8-89/043)) (Washington,D.C., USA). US EPA, 2001. Risk Assessment Guidance for Superfund: Volume I - Part A, Process forConducting Probabilistic Risk Assessment (Washington,DC). USDA, 2014. Northeast China: Prospects for U.S. Agricultural Exports. http://www.fas.usda.gov/data/northeast-china-prospects-us-agricultural-exports. Vandecasteele, I.,Mari Rivero, I.,Sala, S., Baranzelli, C., Barranco, R., Batelaan, O., Lavalle, C.,2015. Impact of shale gas development on water resources: a case study in northernPoland. Environ. Manag. 55, 1285-1299. Vengosh, A., Jackson, R.B., Warner, N., Darrah, T.H., Kondash, A., 2014. A critical review ofthe risks to water resources from unconventional shale gas development and hydrau- lic fracturing in the United States. Environ. Sci. Technol. 48, 8334-8348. Vidic, R., Brantley,S., Vandenbossche,J.,Yoxtheimer, D., Abad,J., 2013. Impact of shale gasdevelopment on regional water quality.Science 340, 12350091-12350099. Warner, N.R., Christie, C.A.,Jackson, R.B., Vengosh,A., 2013. Impacts of shale gas wastewa-ter disposal on water quality in Western Pennsylvania. Environ. Sci. Technol. 47,11849-11857. Yang, J.K., Barnett, M.O., Jardine, P.M., Basta, N.T., Casteel, S.W., 2002. Adsorption, seques-tration, and bio-accessibility of As(V) in soils. Environ. Sci. Technol. 36, 4562-4569. Zhang, H.,Selim, H.M., 2005. Kinetics of arsenate adsorption-desorption in soils. Environ.Sci. Technol. 39,6101-6108. Zhu, Y.,Zhang, X., Xie, Q., Chen, Y., Wang, D., Liang, Y., Lu,J., 2005. Solubility and stabilityof barium arsenate and barium hydrogen arsenate at 25℃. J. Hazard. Mater. 120,37-44. Ziemkiewicz, P.F., Quaranta, J.D., Darnell, A.D., Wise, R., 2014. Exposure pathways relatedto shale gas development and procedures for reducing environmental and public risk.J. Nat. Gas Sci. Eng. 16,77-84. http://dx.doi.org/j.scitotenv. View publication stats Microtox 生物毒性测试技术在页岩气开采过程中的应用——香港理工大学、哈尔滨工业大学、伦敦帝国理工学院与韩国江原大学团队基于Microtox对页岩气开采过程中周边的土壤生态系统进行了毒性评价 Modern Water Microtox 生物毒性检测技术具有快速、简单、廉价等优点,已成为多个国家认可的官方标准,并在废水出水毒性检测、钻井液检测、船舱水检测领域也有着广泛的应用。水力压裂技术促进了页岩气开采的发展,而由于含盐量高,金属/准金属(As,Se,Fe和Sr)以及有机添加剂等原因,无意溢出的回流水可能会对周围环境造成危害。本研究对东北地区4个代表性页岩气开采区域,采用Microtox生物测定法(费氏弧菌)和酶活性测试,对回流水溶液对土壤生态系统的影响进行评估。结果显示,在回流溶液影响的老化土壤中观察到毒性的轻微增加(即,较低的EC20值)(Table 3)。已知砷(V)阻碍ATP的产生并因此抑制费氏弧菌生物发光发射(Rubinos等,2014)。另一方面,每种土壤中回流溶液的EC20值几乎相同,这可能与第14天和第90天回流溶液中的土壤-金属相互作用有关,因为它们可能会限制费氏弧菌对金属的生物利用度(Tsiridis等,2006; Rubinos等,2014)。在BY土壤中检测到了光辐射的刺激,这可能是由于土壤基质中的有机化合物有利于费氏弧菌的生物发光过程(Tang et al,2012)。结果表明,受影响的土壤对Vibrio fischeri的毒性仅在老化后呈现适度增加,而脱氢酶和磷酸单酯酶活性随着回流水溶液离子强度的增加而受到显着抑制。相反,回流溶液中的聚丙烯酰胺导致更高的脱氢酶活性,即土壤酶活性对回流溶液的组成非常敏感。Microtox 技术也广泛应用于废水处理厂的出水毒性检测。与使用其他生物(网纹蚤、仔鱼)的系统相比,使用费氏弧菌的 Microtox 技术检测时间更短,结果精确度和灵敏性更高,成本更低,是一种理想的废水整体毒性测试方案。Microtox® LX 分析仪是一款用于实验室的毒性测试仪,带有温控和自动色度校正功能,用于急性毒性的分析。Microtox® LX 采用生物发光检测技术,可对事故或人为导致的饮用水及废水污染紧急事件进行快速毒性检测。Microtox FX 是一款简单快捷且灵敏度极高的便携式水质检测仪,专门为筛查急性毒性及三磷酸腺苷(ATP)而设计。Microtox FX 使用生物荧光技术,对饮用水污染及化学品进入水体等造成的紧急事件进行快速毒性检测。Microtox FX 是使用 Microtox® 技术进行毒性测定的便携仪器。
确定





还剩7页未读,是否继续阅读?
Modern Water (英国现代水务)为您提供《水力压裂回流水中生物毒性检测方案(水质毒性分析)》,该方案主要用于废水中综合检测,参考标准--,《水力压裂回流水中生物毒性检测方案(水质毒性分析)》用到的仪器有生物毒性分析仪 Microtox LX、Modern Water 便携毒性监测仪 Microtox® FX
推荐专场
水质毒性分析仪/水质生物毒性分析仪
相关方案
更多
该厂商其他方案
更多

















