Abstract: Scutellarin, a flavone glycoside, popularly applied for the treatment of cardiopathy, has been purified in two-step purification by high-speed counter-current chromatography (HSCCC) from Erigeron breviscapus (vant.) Hand. Mazz. (Deng-zhan-hua in Chinese), a well-known traditional Chinese medicinal plant for heart disease. Two solvent systems, n-hexane–ethyl acetate–methanol–acetic acid–water (1:6:1.5:1:4, v/v/v/v/v) and ethyl acetate–n-butanol–acetonitrile–0.1% HCl (5:2:5:10, v/v/v/v) were used for the two-step purification. The purity of the collected fraction of
scutellarin was 95.6%. This study supplies a new alternative method for purification of scutellarin.
方案详情

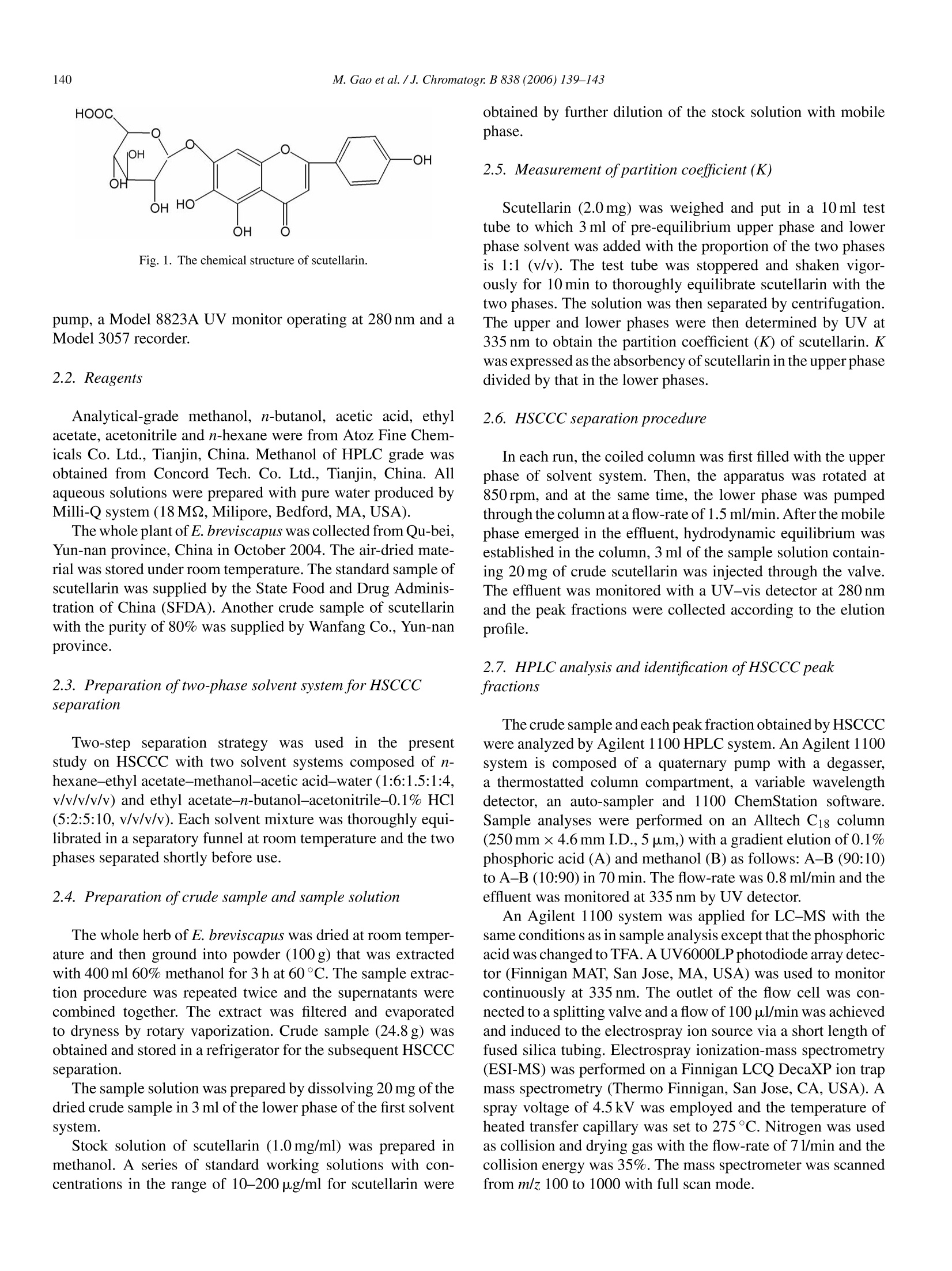
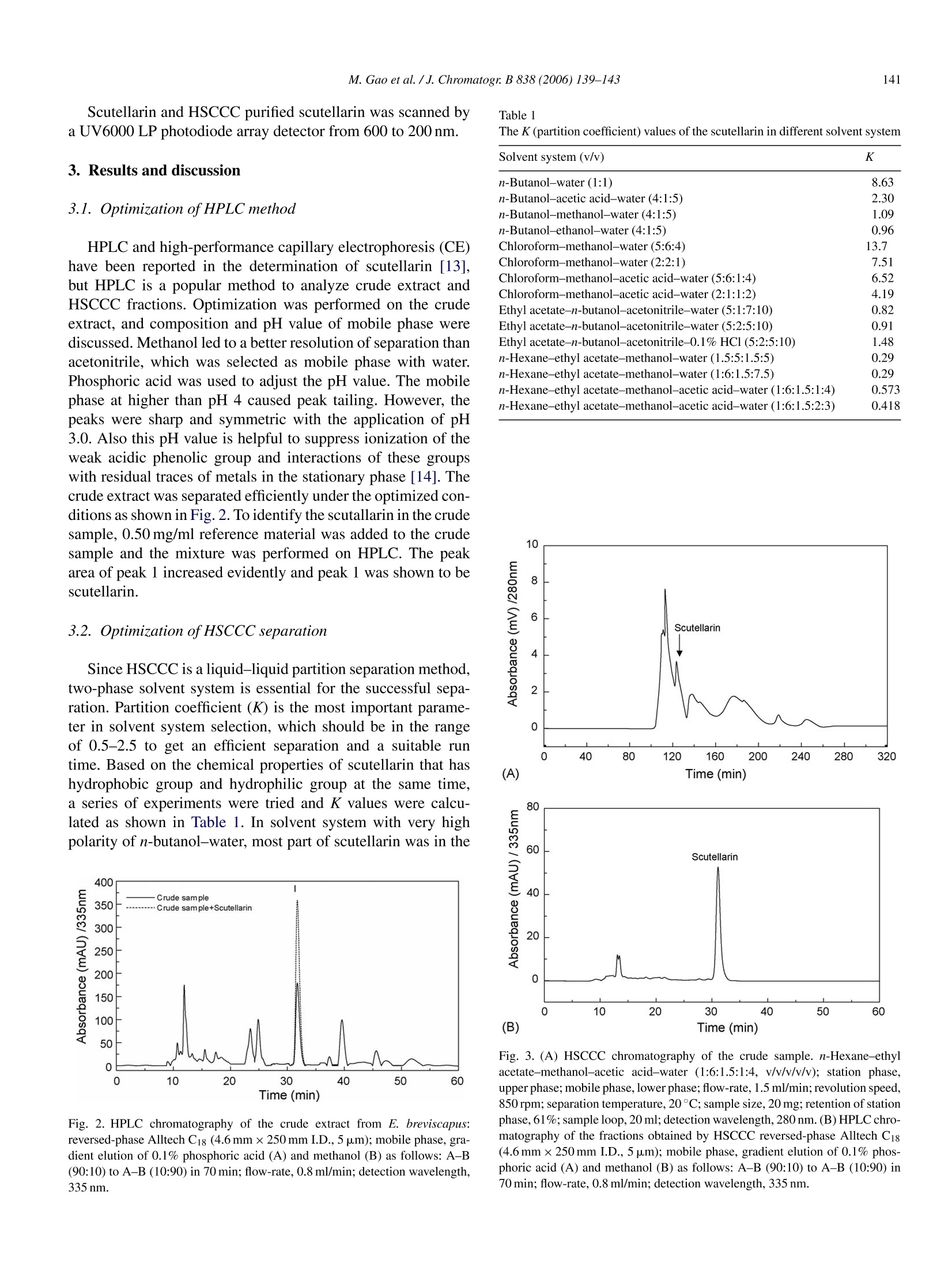
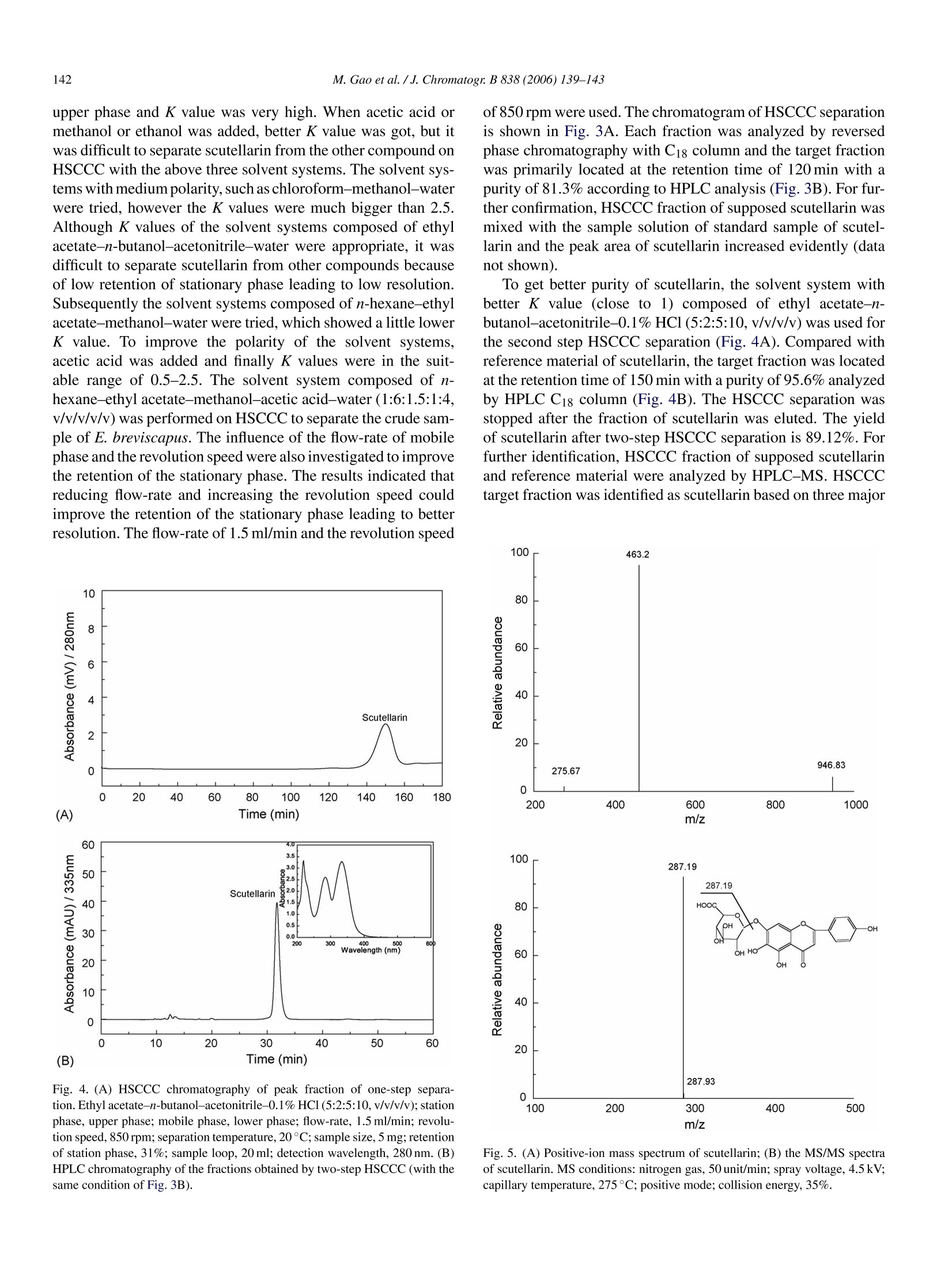
Available online at www.sciencedirect.comJOURNAL OFCHROMATOGRAPHY BJournal of Chromatography B, 838 (2006) 139-143www.elsevier.com/locate/chrombShort communication 140M. Gao et al./J. Chromatogr. B 838 (2006) 139-143 1570-0232/$-see front matter @ 2006 Elsevier B.V. All rights reserved.doi:10.1016/j.jchromb.2006.04.030 Two-step purification of scutellarin from Erigeron breviscapus (vant.)Hand. Mazz. by high-speed counter-current chromatography Min Gao a.b, Ming Gu, Chun-zhao Liu a,b,* a Phytochemical Engineering Group, National Key Laboratory of Biochemical Engineering, Institute ofProcess Engineering,Chinese Academy of Sciences, Beijing 100080, PR China Graduate School of the Chinese Academy of Sciences, Beijing 100049, PR China Received 12 December 2005; accepted 25 April 2006Available online 21 June 2006 Abstract Scutellarin, a flavone glycoside, popularly applied for the treatment of cardiopathy, has been purified in two-step purification by high-speedcounter-current chromatography (HSCCC) from Erigeron breviscapus (vant.) Hand. Mazz. (Deng-zhan-hua in Chinese), a well-known traditionalChinese medicinal plant for heart disease. Two solvent systems, n-hexane-ethyl acetate-methanol-acetic acid-water (1:6:1.5:1:4,v/v/v/v/v) andethyl acetate-n-butanol-acetonitrile-0.1% HCl (5:2:5:10, v/v/v/v) were used for the two-step purification. The purity of the collected fraction ofscutellarin was 95.6%. This study supplies a new alternative method for purification of scutellarin. O 2006 Elsevier B.V. All rights reserved. Keywords: Scutellarin; Erigeron breviscapus (vant.) Hand. Mazz.; Flavone glycoside; Polyphenol; Counter-current chromatography 1. Introduction Erigeron breviscapus (vant.) Hand. Mazz. is one of the mostimportant traditional Chinese medicinal plants for cardiopathy[1], and is also used for expelling the cold, relieving exteriorsyndrome, removing stagnancy of indigested food and reliev-ing pain [2-4]. Scutellarin, the major active component of E.breviscapus, is a flavone glucuronide whose structure is shownin Fig. 1. Scutellarin is drawing particular interests because itsignificantly dilates blood vessel, improves microcirculation,increases cerebral blood flow and inhibits platelet aggregationactivity [5,6]. Normally, the separation and purification of scutellarin fromthe extract of E. breviscapus are performed by polyamide chro-matography and macroporous resin adsorption chromatography[7,8]. It is pretty easy to enlarge the scale of separation by thesetwo methods. However, it is impossible to eliminate the irre-versible adsorptive loss of samples onto the solid support matrix,and column deterioration is a popular problem for gel chro-matography, even for preparative reversed phase chromatogra- ( * Corresponding a uthor. Tel.: +8 6 10 82622280; fa x :+86 10 82622280. E-mail address: c zliu@ ho me.i p e . a c .cn (C.-z. Li u). ) phy [9]. High-speed counter-current chromatography (HSCCC)is a unique liquid-liquid partition chromatography without anysolid matrix, which eliminates the irreversible adsorption ofsamples on solid support leading to no column deterioration forHSCCC. HSCCC has been applied to the separation of differ-ent kinds of natural products, including flavonoids and flavoneglycosides [10-12]. By now, purification of scutellarin from E. breviscapus by HSCCC has not been reported. The present studydescribes successful semi-preparative separation and purifica-tion of scutellarin from the crude extract of E. breviscapus byHSCCC. 2. Experimental 2.1. Apparatus HSCCC (TBE-300) is from Tauto Biotech, Shanghai, China,with three preparative coils connected in series (diameter of2.6 mm, total volume 300 ml) and a 20 ml sample loop. Therevolution radius or the distance between the holder axis andcentral axis of the centrifuge (R) was 5 cm, and the p value var-ied from 0.5 at internal terminal to 0.8 at the external terminal(β=r/R, where r is the distance from the coil to the holder shaft)The HSCCC systems are equipped with a Model S constant-flow Fig. 1. The chemical structure of scutellarin. pump, a Model 8823A UV monitor operating at 280 nm and aModel 3057 recorder. 2.2. Reagents Analytical-grade methanol, n-butanol, acetic acid, ethylacetate, acetonitrile and n-hexane were from Atoz Fine Chem-icals Co. Ltd., Tianjin, China. Methanol of HPLC grade wasobtained from Concord Tech. Co. Ltd., Tianjin, China. Allaqueous solutions were prepared with pure water produced byMilli-Q system (18MQ, Milipore, Bedford,MA, USA). The whole plant of E. breviscapus was collected from Qu-bei,Yun-nan province, China in October 2004. The air-dried mate-rial was stored under room temperature. The standard sample ofscutellarin was supplied by the State Food and Drug Adminis-tration of China (SFDA). Another crude sample of scutellarinwith the purity of 80% was supplied by Wanfang Co., Yun-nanprovince. 2.3. Preparation of two-phase solvent system for HSCCCseparation Two-step separation strategy was used in the presentstudy on HSCCC with two solvent systems composed of n-hexane-ethyl acetate-methanol-acetic acid-water (1:6:1.5:1:4,v/v/v/v/v) and ethyl acetate-n-butanol-acetonitrile-0.1% HCl(5:2:5:10,v/v/v/v). Each solvent mixture was thoroughly equi-librated in a separatory funnel at room temperature and the twophases separated shortly before use. 2.4. Preparation of crude sample and sample solution The whole herb of E. breviscapus was dried at room temper-ature and then ground into powder (100 g) that was extractedwith 400 ml 60% methanol for 3 h at 60°C. The sample extrac-tion procedure was repeated twice and the supernatants werecombined together. The extract was filtered and evaporatedto dryness by rotary vaporization. Crude sample (24.8g) wasobtained and stored in a refrigerator for the subsequent HSCCCseparation. The sample solution was prepared by dissolving 20 mg of thedried crude sample in 3 ml of the lower phase of the first solventsystem. Stock solution of scutellarin (1.0mg/m) was prepared inmethanol. A series of standard working solutions with con-centrations in the range of 10-200 pg/ml for scutellarin were obtained by further dilution of the stock solution with mobilephase. 2.5. Measurement of partition coefficient (K) Scutellarin (2.0 mg) was weighed and put in a 10 ml testtube to which 3 ml of pre-equilibrium upper phase and lowerphase solvent was added with the proportion of the two phasesis 1:1 (v/v). The test tube was stoppered and shaken vigor-ously for 10 min to thoroughly equilibrate scutellarin with thetwo phases. The solution was then separated by centrifugation.The upper and lower phases were then determined by UV at335 nm to obtain the partition coefficient (K) of scutellarin. Kwas expressed as the absorbency of scutellarin in the upper phasedivided by that in the lower phases. 2.6. HSCCC separation procedure In each run, the coiled column was first filled with the upperphase of solvent system. Then, the apparatus was rotated at850rpm, and at the same time, the lower phase was pumpedthrough the column at a flow-rate of 1.5 ml/min. After the mobilephase emerged in the effluent, hydrodynamic equilibrium wasestablished in the column, 3 ml of the sample solution contain-ing 20 mg of crude scutellarin was injected through the valve.The effluent was monitored with a UV-vis detector at 280 nmand the peak fractions were collected according to the elutionprofile. 2.7. HPLC analysis and identification of HSCCC peakfractions The crude sample and each peak fraction obtained by HSCCCwere analyzed by Agilent 1100 HPLC system. An Agilent 1100system is composed of a quaternary pump with a degasser,a thermostatted column compartment,a variable wavelengthdetector, an auto-sampler and 1100 ChemStation software.Sample analyses were performed on an Alltech C18 column(250mm x4.6 mm I.D., 5 pm,) with a gradient elution of 0.1%phosphoric acid (A) and methanol (B) as follows: A-B (90:10)to A-B (10:90) in 70 min. The flow-rate was 0.8 ml/min and theeffluent was monitored at 335 nm by UV detector. An Agilent 1100 system was applied for LC-MS with thesame conditions as in sample analysis except that the phosphoricacid was changed to TFA. A UV6000LP photodiode array detec-tor (Finnigan MAT, San Jose, MA, USA) was used to monitorcontinuously at 335 nm. The outlet of the flow cell was con-nected to a splitting valve and a flow of 100 pl/min was achievedand induced to the electrospray ion source via a short length offused silica tubing. Electrospray ionization-mass spectrometry(ESI-MS) was performed on a Finnigan LCQ DecaXP ion trapmass spectrometry (Thermo Finnigan, San Jose,CA, USA). Aspray voltage of 4.5 kV was employed and the temperature ofheated transfer capillary was set to 275°C. Nitrogen was usedas collision and drying gas with the flow-rate of 71/min and thecollision energy was 35%. The mass spectrometer was scannedfrom m/z 100 to 1000 with full scan mode. Scutellarin and HSCCC purified scutellarin was scanned bya UV6000 LP photodiode array detector from 600 to 200 nm. 3. Results and discussion 3.1. Optimization of HPLC method HPLC and high-performance capillary electrophoresis (CE)have been reported in the determination of scutellarin [13],but HPLC is a popular method to analyze crude extract andHSCCC fractions. Optimization was performed on the crudeextract, and composition and pH value of mobile phase werediscussed. Methanol led to a better resolution of separation thanacetonitrile, which was selected as mobile phase with water.Phosphoric acid was used to adjust the pH value. The mobilephase at higher than pH 4 caused peak tailing. However, thepeaks were sharp and symmetric with the application of pH3.0. Also this pH value is helpful to suppress ionization of theweak acidic phenolic group and interactions of these groupswith residual traces of metals in the stationary phase [14]. Thecrude extract was separated efficiently under the optimized con-ditions as shown in Fig. 2. To identify the scutallarin in the crudesample, 0.50 mg/ml reference material was added to the crudesample and the mixture was performed on HPLC. The peakarea of peak 1 increased evidently and peak 1 was shown to bescutellarin. 3.2. Optimization of HSCCC separation Since HSCCC is a liquid-liquid partition separation method,two-phase solvent system is essential for the successful sepa-ration. Partition coefficient (K) is the most important parame-ter in solvent system selection, which should be in the rangeof 0.5-2.5 to get an efficient separation and a suitable runtime. Based on the chemical properties of scutellarin that hashydrophobic group and hydrophilic group at the same time,a series of experiments were tried and K values were calcu-lated as shown in Table 1. In solvent system with very highpolarity of n-butanol-water, most part of scutellarin was in the Fig. 2. HPLC chromatography of the crude extract from E. breviscapus:reversed-phase Alltech C18 (4.6mmx250 mm I.D., 5 pm); mobile phase, gra-dient elution of 0.1% phosphoric acid (A) and methanol (B) as follows: A-B(90:10) to A-B (10:90) in 70 min; flow-rate, 0.8 ml/min; detection wavelength,335 nm. The K (partition coefficient) values of the scutellarin in different solvent system Solvent system (v/v) K n-Butanol-water (1:1) 8.63 n-Butanol-acetic acid-water (4:1:5) 2.30 n-Butanol-methanol-water (4:1:5) 1.09 n-Butanol-ethanol-water (4:1:5) 0.96 Chloroform-methanol-water (5:6:4) 13.7 Chloroform-methanol-water(2:2:1) 7.51 Chloroform-methanol-acetic acid-water (5:6:1:4) 6.52 Chloroform-methanol-acetic acid-water (2:1:1:2) 4.19 Ethyl acetate-n-butanol-acetonitrile-water(5:1:7:10) 0.82 Ethyl acetate-n-butanol-acetonitrile-water (5:2:5:10) 0.91 Ethyl acetate-n-butanol-acetonitrile-0.1% HCl (5:2:5:10) 1.48 n-Hexane-ethyl acetate-methanol-water (1.5:5:1.5:5) 0.29 n-Hexane-ethyl acetate-methanol-water(1:6:1.5:7.5) 0.29 n-Hexane-ethyl acetate-methanol-acetic acid-water (1:6:1.5:1:4) 0.573 n-Hexane-ethyl acetate-methanol-acetic acid-water (1:6:1.5:2:3) 0.418 Fig. 3. (A) HSCCC chromatography of the crude sample. n-Hexane-ethylacetate-methanol-acetic acid-water (1:6:1.5:1:4, v/v/v/v/v); station phase,upper phase; mobile phase, lower phase; flow-rate, 1.5 ml/min; revolution speed,850 rpm; separation temperature, 20°C; sample size, 20 mg; retention of stationphase, 61%;sample loop, 20 ml; detection wavelength,280 nm.(B)HPLC chro-matography of the fractions obtained by HSCCC reversed-phase Alltech Ci8(4.6mm×250mm I.D., 5 pm); mobile phase, gradient elution of 0.1% phos-phoric acid (A) and methanol (B) as follows: A-B (90:10) to A-B (10:90) in70 min; flow-rate, 0.8 ml/min; detection wavelength, 335 nm. of 850 rpm were used. The chromatogram of HSCCC separationis shown in Fig. 3A. Each fraction was analyzed by reversedphase chromatography with C18 column and the target fractionwas primarily located at the retention time of 120 min with apurity of 81.3% according to HPLC analysis (Fig.3B). For fur-ther confirmation, HSCCC fraction of supposed scutellarin wasmixed with the sample solution of standard sample of scutel-larin and the peak area of scutellarin increased evidently (datanot shown). To get better purity of scutellarin, the solvent system withbetter K value (close to 1) composed of ethyl acetate-n-butanol-acetonitrile-0.1% HC1 (5:2:5:10,v/v/v/v) was used forthe second step HSCCC separation (Fig. 4A). Compared withreference material of scutellarin, the target fraction was locatedat the retention time of 150 min with a purity of 95.6% analyzedby HPLC C18 column (Fig. 4B). The HSCCC separation wasstopped after the fraction of scutellarin was eluted. The yieldof scutellarin after two-step HSCCC separation is 89.12%. Forfurther identification, HSCCC fraction of supposed scutellarinand reference material were analyzed by HPLC-MS. HSCCCtarget fraction was identified as scutellarin based on three major (A) Fig. 4. (A) HSCCC chromatography of peak fraction of one-step separa-tion. Ethyl acetate-n-butanol-acetonitrile-0.1%HC1 (5:2:5:10,v/v/v/v); stationphase, upper phase; mobile phase, lower phase; flow-rate, 1.5 ml/min; revolu-tion speed, 850 rpm; separation temperature, 20°C; sample size, 5 mg; retentionof station phase, 31%; sample loop, 20 ml; detection wavelength, 280 nm. (B)HPLC chromatography of the fractions obtained by two-step HSCCC (with thesame condition of Fig. 3B). Fig. 5. (A) Positive-ion mass spectrum of scutellarin; (B) the MS/MS spectraof scutellarin. MS conditions: nitrogen gas, 50 unit/min; spray voltage, 4.5 kV;capillary temperature, 275°C; positive mode; collision energy, 35%. parameters were included as follows: retention time of HPLCanalysis, UV spectrum and MS spectrum. HPLC-MS showedan [M+1]* peak at m/z 463.2 corresponding to the molecularformula C21H18O12 (Fig.5A). The MS/MS spectrum of scutel-larin showed a fragmentation pattern at m/z 287.2 correspondingto the aglycone of scutellarin (Fig.5B). 4. Conclusions Atwo-step separation method was developed for thesemi-preparative purification of scutellarin from E. brevisca-pus on HSCCC with two solvent systems, n-hexane-ethylacetate-methanol-acetic acid-water (1:6:1.5:1:4, v/v/v/v/v)and ethyl acetate-n-butanol-acetonitrile-0.1% HCl (5:2:5:10,v/v/v/v). The purity of the scutellarin fraction was 95.6%, whichserved an alternative method to yield high-purity of scutellarin. Acknowledgment The authors acknowledge the financial support from the“Hundred Talents Program”of the Chinese Academy ofSciences. ( [1 ] J . Qu, Y.M. Wang, G .A. Lu o , J. C h r omatogr. A 9 1 9 (2001) 43 7 . ) ( [2] D.F. Zhong, B.H. Yang, X .Y. C hen, K . Li, J .H. Xu, J. Chromatogr. B 796 ( 2003) 4 39. ) ( [ 3 ] J. Qu, Y.M. W ang, G . A. L u o, Z.P. W u , J. C h romatogr. A 9 28 (20 0 1)155. ) ( [4] H. H ong, G.Q. L i u, Life Sci. 7 4 ( 2004) 2959. ) ( [ 5 ] B. H . Z hu, Y . Y . Guan, H . He, M. J . Li n , Li f e Sc i . 65 (1 9 99) 155 3 . ) ( [6] S. Nagashima, M . Hi r otani, T. Y o shikawa, Phytochemistry 53 (200 0 )533. ) [7] X.F. Ma, P.F. Tu, Y.J. Chen, T.Y. Zhang, Y. Wei, Y. Ito, J. Chromatogr.A 992 (2003)193. [8] X.F. Ma, P.F. Tu, Y.J. Chen, T.Y. Zhang, Y. Wei, Y. Ito, J. Chromatogr.A 1023 (2004)311. [9] J.C. Delaunay, C. Castagnino, C. Cheze, J. Vercauteren, J. Chromatogr.A 964 (2002) 123. [10] X. Wang, F.W. Li, H.X. Zhang, Y.L. Geng, J.P. Yuan, T. Jiang, J. Chro-matogr. A 1090 (2005) 188. [11] H.T. Lu, Y. Jiang, F. Chen, J. Chromatogr. A 1026 (2004) 185. [12] R.M. Liu, A.F. Li, A.L. Sun, J.C. Cui, L.Y. Kong, J. Chromatogr. A1064 (2005) 53. [13] Q.C. Chu, T. Wu, L. Fu, J.N. Ye, J. Pharm. Biomed. Anal. 37 (2005)535. [14] D.F. Zhang, B.H. Yang, X.Y. Chen, K. Li, J.H. Xu, J. Chromatogr. B796 (2003) 439.
确定


还剩3页未读,是否继续阅读?
上海同田生物技术有限公司-高速逆流色谱仪HSCCC为您提供《黄芩素苷中分离纯化检测方案(高速逆流色谱)》,该方案主要用于植物油脂和提取物中含量测定检测,参考标准--,《黄芩素苷中分离纯化检测方案(高速逆流色谱)》用到的仪器有TBE-200V 高速逆流色谱仪、TBE300B+AKTA高速逆流色谱仪/离心分配色谱/萃取仪/制备色谱仪、TBE-1000A制备色谱仪/萃取仪/快速分离制备色谱仪
推荐专场
相关方案
更多
该厂商其他方案
更多


















