方案详情
文
Abstract: A preparative high-speed counter-current chromatography (HSCCC) was used to isolate and separate curculigoside and curculigoside B from the famous traditional Chinese medicinal herb Curculigo orchioides. Some parameters including two-phase solvent system, separation temperature,flow rate of the mobile phase and revolution speed of the apparatus were all investigated, and a successfully isolation and purification was achieved at the following conditions: the two-phase solvent system composed of ethyl acetate-ethanol-water at a volume ratio of 5:1:5(v/v/v) was selected and the lower phase of the system was used as the mobile phase at the flow rate of 2.0ml/min, and the isolation temperature and revolution speed were set at 30◦C and 800rpm.The isolation produced a total of 14.5 mg curculigoside B and 72.8 mg curculigoside with purities of 96.5% and 99.4% determined by high performance liquid chromatography (HPLC) from 300 mg crude extract after cleaning-up by D101 macroporous resin,which was necessary for the excellent purification. The recoveries of the two compounds were 91.6% and 92.5%, respectively, and the chemical structure identification was carried out by UV, MS and NMR. This separation method was more effective than some conventional techniques.
方案详情

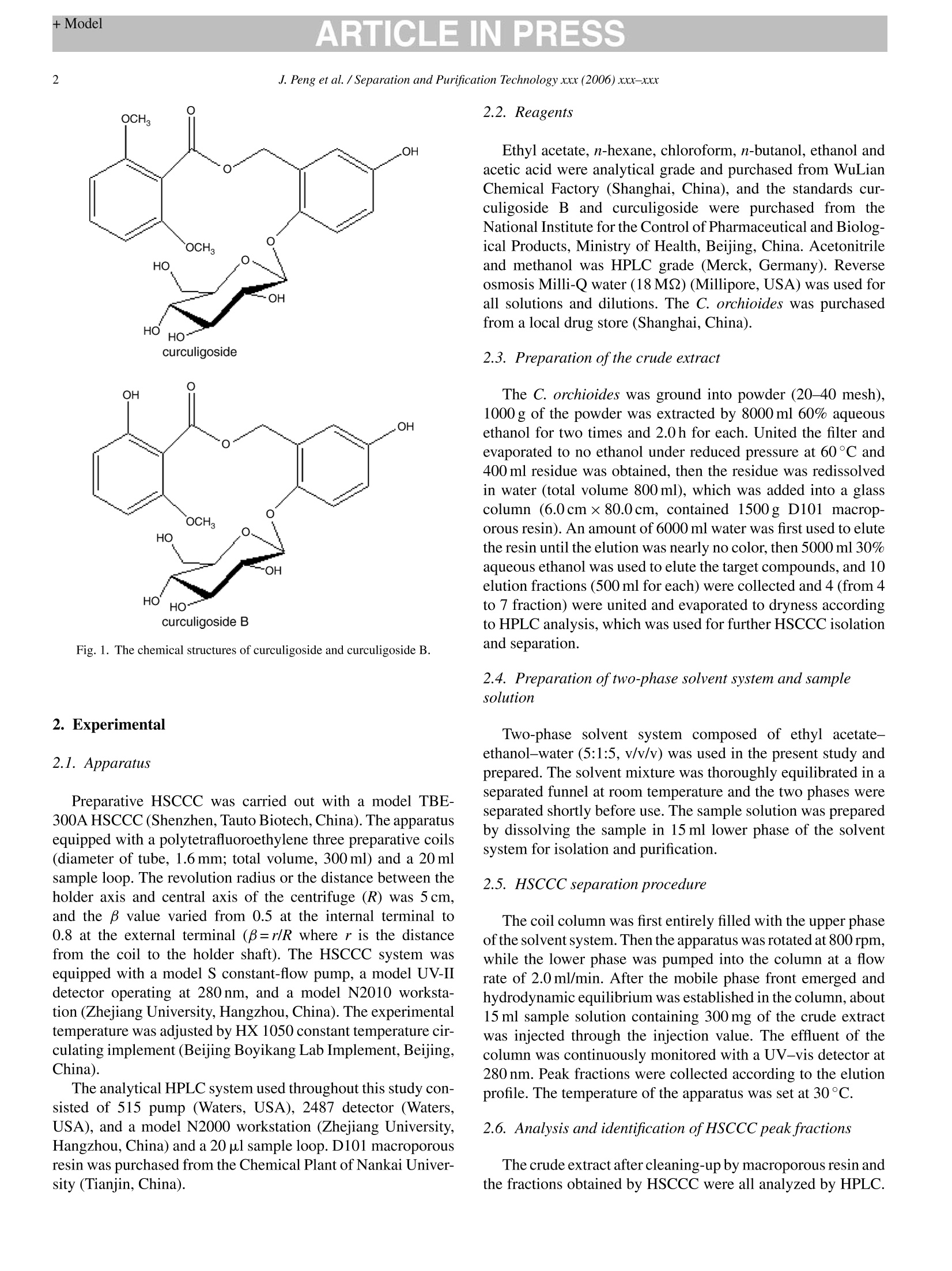
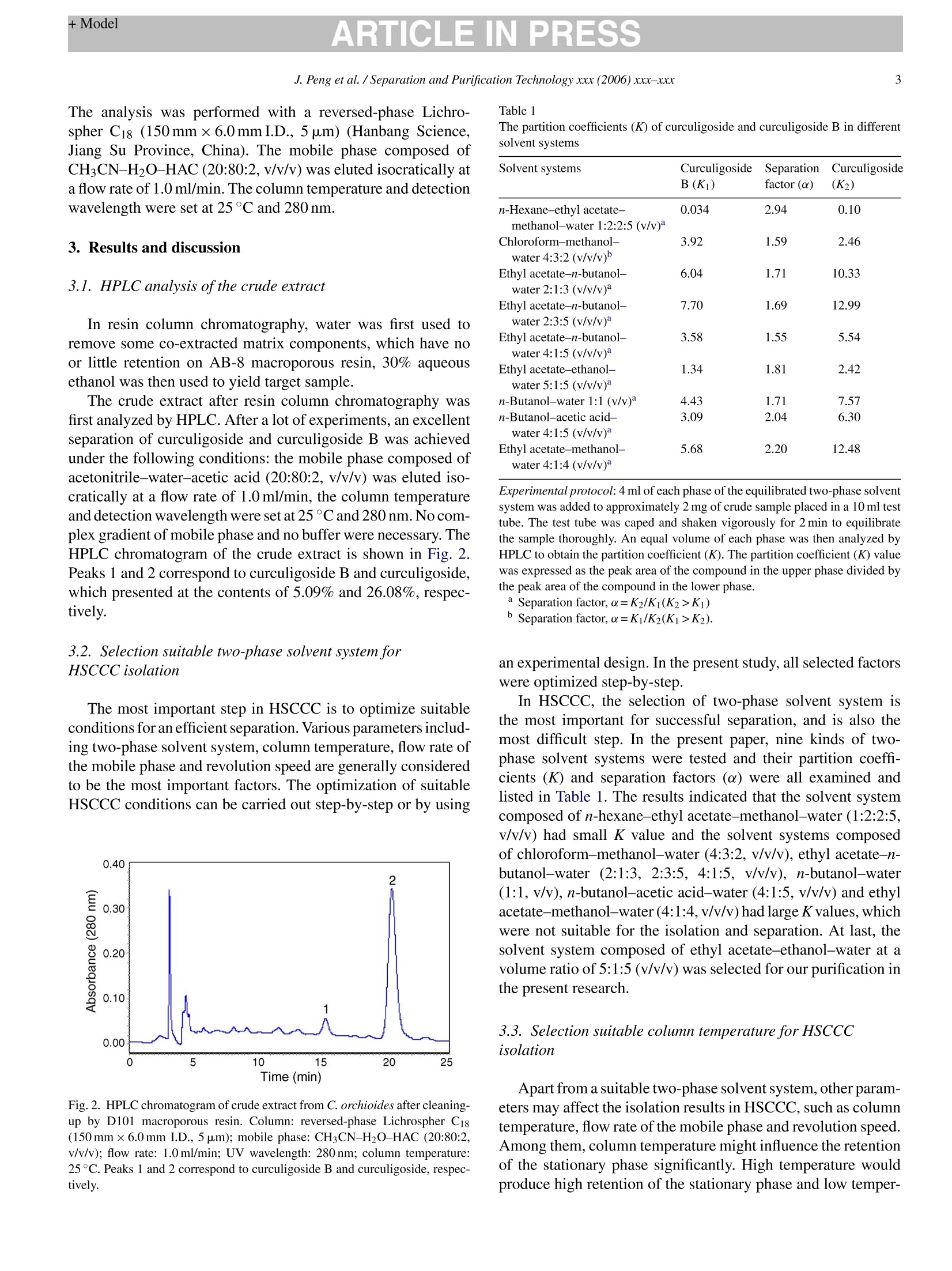
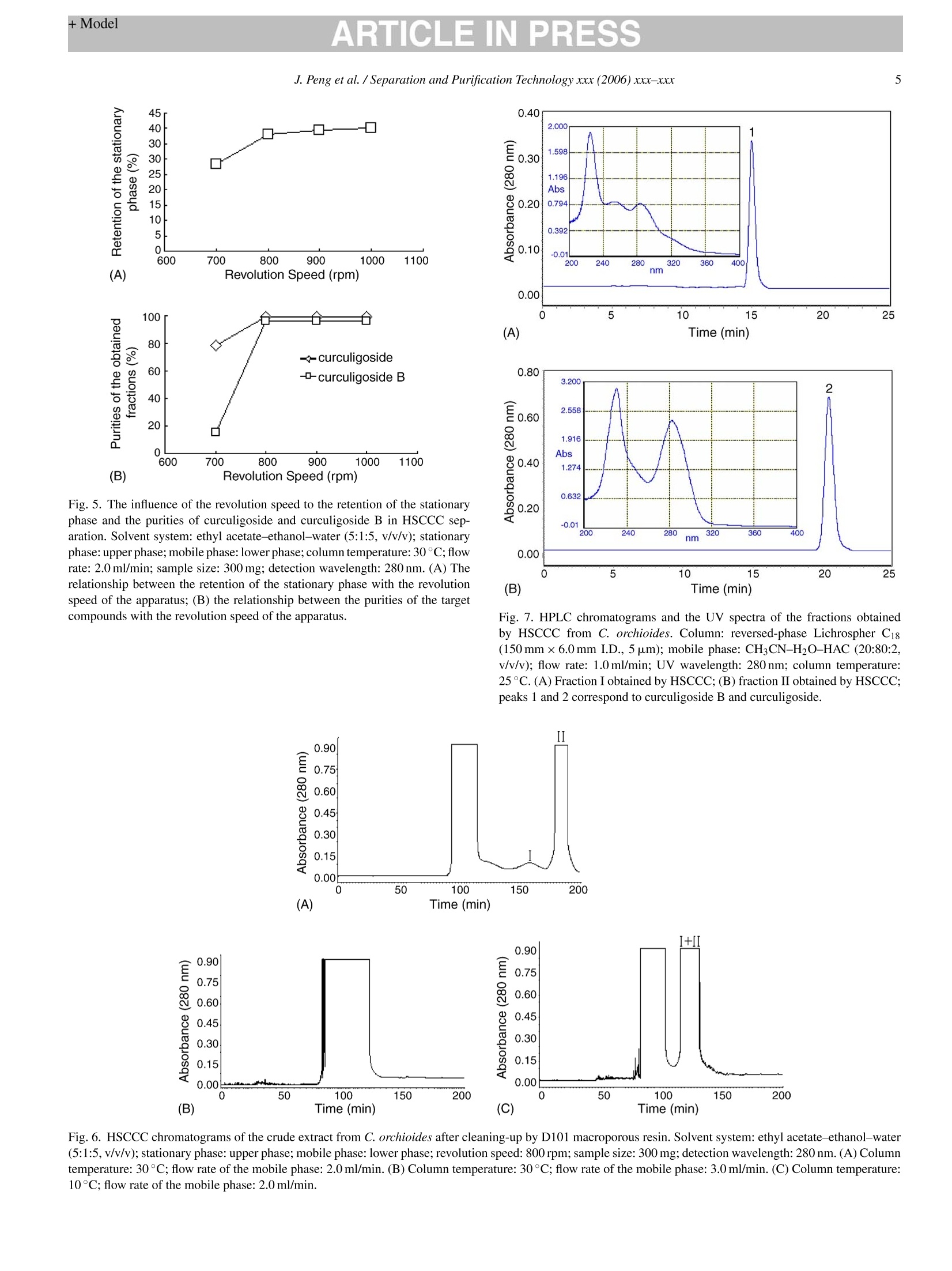
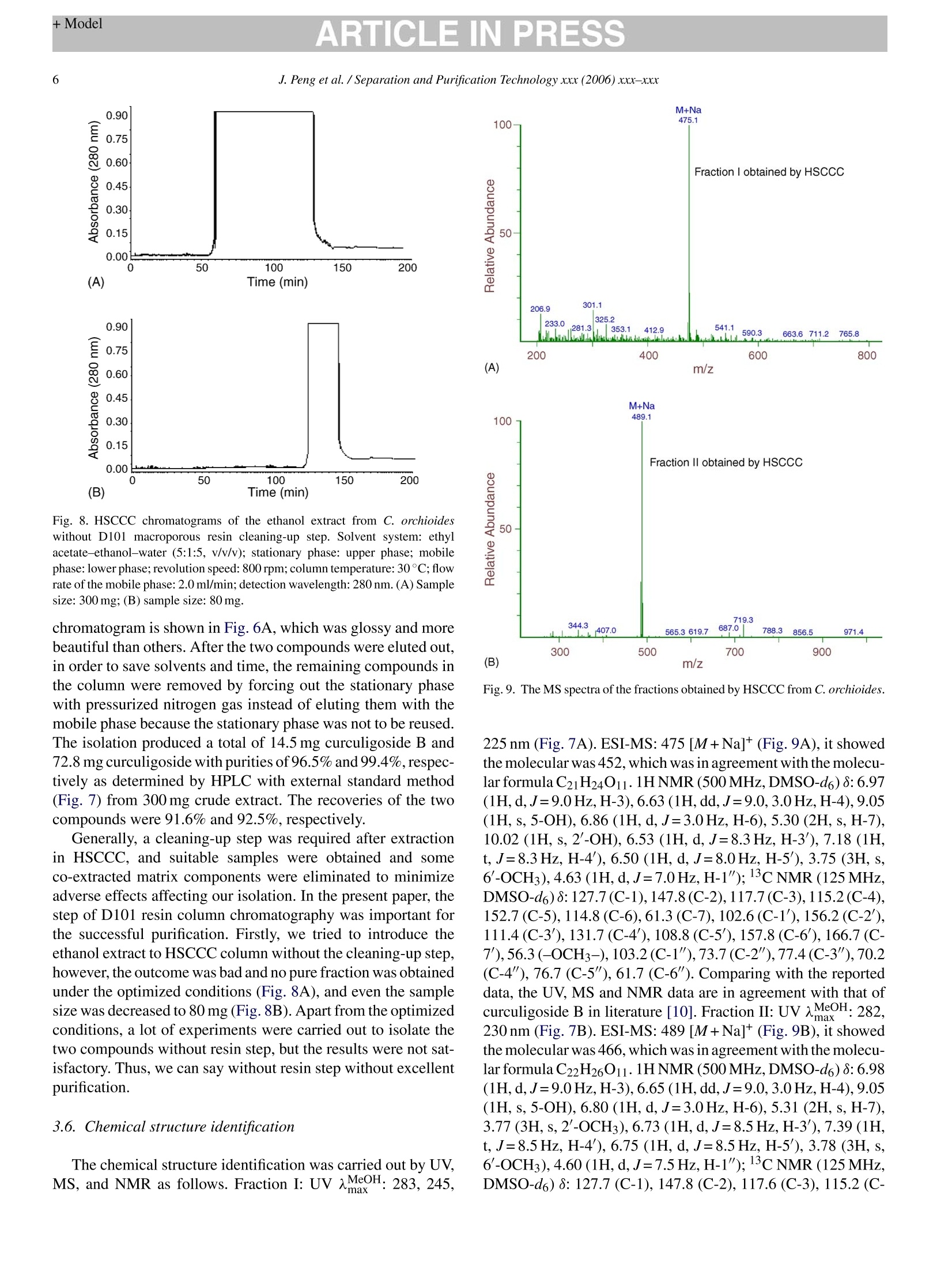
ARTICLE IN PRESSAvailable online at www.sciencedirect.comSeparation and Purification Technology xxx (2006) xxx-xxx +ModelARTICLE IN PRESS2J. Peng et al. / Separation and Purification Technology xxx (2006) xxx-xxx SEPPUR-8553; No. of Pages 7 Separation高PurificationTechnology ELSEVIER www.elsevier.com/locate/seppur Optimization suitable conditions for preparative isolation and separationof curculigoside and curculigoside B from Curculigo orchioides byhigh-speed counter-current chromatography Jinyong Peng , Yunyun Jiang , Guorong Fan a*, Bin Chen ,Qiaoyan Zhang b, Yifeng Chai, Yutian Wua Shanghai Key Laboratory for Pharmaceutical Metabolite Research,School of Pharmacy,Second Military Medical University, No.325 Guohe Road, Shanghai 200433, China ' Department of Pharmacognosy, School of Pharmacy, Second Military Medical University, No.325 Guohe Road, Shanghai 200433, China Received 6 September 2005; received in revised form 6 March 2006; accepted 14 March 2006 Abstract A preparative high-speedcounter-current chromatography (HSCCC) was used to isolate and separate curculigoside and curculigoside B from thefamous traditional Chinese medicinal herb Curculigo orchioides. Some parameters including two-phase solvent system, separation temperature,flow rate of the mobile phase and revolution speed of the apparatus were all investigated, and a successfully isolation and purification was achievedat the following conditions: the two-phase solvent system composed of ethyl acetate-ethanol-water at a volume ratio of 5:1:5 (v/v/v) was selectedand the lower phase of the system was used as the mobile phase at the flow rate of 2.0 ml/min, and the isolation temperature and revolution speedwere set at 30℃ and 800 rpm. The isolation produced a total of 14.5 mg curculigoside B and 72.8 mg curculigoside with purities of 96.5% and99.4% determined by high performance liquid chromatography (HPLC) from 300 mg crude extract after cleaning-up by D101 macroporous resin,which was necessary for the excellent purification. The recoveries of the two compounds were 91.6% and 92.5%, respectively, and the chemicalstructure identification was carried out by UV, MS and NMR. This separation method was more effective than some conventional techniques.O 2006 Elsevier B.V. All rights reserved. Keywords: Curculigo orchioides; Preparative chromatography; Counter-current chromatography; Plant material; Separation; Glycosides 1. Introduction Generally, preparative isolation and separation of pure prod-ucts from medicinal plants by some classical methods aretedious, time consuming, requiring multiple chromatographicsteps on silica gel, polyamide, macroporous resin column, etc.Thus, an efficient method for the preparative isolation is war-ranted. High-speed counter-current chromatography (HSCCC),a support free liquid-liquid partition chromatographic tech-nique, eliminates irreversible adsorption of the sample onto solidsupport, has an excellent sample recovery. The method permitsdirectly introduction of crude samples into the column withoutmore preparation, so it has been successfully applied to isolateand purify a number of natural products [1-5]. ( *C orresponding au t hor. Tel.: +86 21 2 5 07 0388; fax: + 8 6 212 50 7 0388.E-mail address: Guor f a n @ y ahoo. c om . c n ( G . F an). ) ( 1 3 83-5866/$-see front matter @ 20 0 6 Else v ier B. V . All rights reserved.doi: 1 0. 1 016/j.sep p ur.20 0 6 .0 3. 0 10 ) Curculigo orchioides (XianMao in Chinese), the rhizome ofC. orchioides Gaertn, family of Amaryllidaceae, is a famoustraditional Chinese medicine and listed in the Chinese Phar-macopoeia [6]. This plant has been exhibited a widely varietyof active effects including anti-osteoporosis, anti-aging effects,anti-inflammatory, enhancement immune function and someactions to the central nervous system [7-9]. The major com-ponents of C. orchioides are considered to be curculigoside andcurculigoside B (shown in Fig. 1). The aim of the present paper, therefore, was to develop anefficient method to isolate and purify curculigoside and cur-culigoside B from C. orchioides by HSCCC. The best isolationconditions were optimized after investigation the effects of someseparation parameters including two-phase solvent system, col-umn temperature, flow rate of the mobile phase and revolutionspeed of the apparatus. To our best knowledge, this is the firstreport of using HSCCC to isolate and separate curculigoside andcurculigoside B from natural resources. Fig. 1. The chemical structures of curculigoside and curculigoside B. 2. Experimental 2.1. Apparatus Preparative HSCCC was carried out with a model TBE-300A HSCCC (Shenzhen, Tauto Biotech, China). The apparatusequipped with a polytetrafluoroethylene three preparative coils(diameter of tube, 1.6mm; total volume, 300 ml) and a 20 mlsample loop. The revolution radius or the distance between theholder axis and central axis of the centrifuge (R) was 5cm,and the B value varied from 0.5 at the internal terminal to0.8 at the external terminal (B=r/R where r is the distancefrom the coil to the holder shaft). The HSCCC system wasequipped with a model S constant-flow pump, a model UV-IIdetector operating at 280nm, and a model N2010 workstation(Zhejiang University, Hangzhou, China). The experimentaltemperature was adjusted by HX 1050 constant temperature cir-culating implement (Beijing Boyikang Lab Implement, Beijing,China). The analytical HPLC system used throughout this study con-sisted of 515 pump (Waters, USA), 2487 detector (Waters,USA), and a model N2000 workstation (Zhejiang University,Hangzhou, China) and a 20 pal sample loop. D101 macroporousresin was purchased from the Chemical Plant of Nankai Univer-sity (Tianjin, China). 2.2. Reagents Ethyl acetate, n-hexane, chloroform, n-butanol, ethanol andacetic acid were analytical grade and purchased from WuLianChemical Factory (Shanghai, China), and the standards cur-culigoside B and curculigoside were purchased from theNational Institute for the Control of Pharmaceutical and Biolog-ical Products, Ministry of Health, Beijing, China. Acetonitrileand methanol was HPLC grade (Merck, Germany). Reverseosmosis Milli-Q water (18MQ) (Millipore, USA) was used forall solutions and dilutions. The C. orchioides was purchasedfrom a local drug store (Shanghai,China). 2.3. Preparation of the crude extract The C. orchioides was ground into powder (20-40 mesh),1000 g of the powder was extracted by 8000 ml 60% aqueousethanol for two times and 2.0h for each. United the filter andevaporated to no ethanol under reduced pressure at 60°C and400 ml residue was obtained, then the residue was redissolvedin water (total volume 800 ml), which was added into a glasscolumn (6.0cmx80.0 cm, contained 1500g D101 macrop-orous resin). An amount of 6000 ml water was first used to elutethe resin until the elution was nearly no color, then 5000 ml 30%aqueous ethanol was used to elute the target compounds, and 10elution fractions (500 ml for each) were collected and 4 (from 4to 7 fraction) were united and evaporated to dryness accordingto HPLC analysis, which was used for further HSCCC isolationand separation. 2.4. Preparation oftwo-phase solvent system and samplesolution Two-phase solvent system composed of ethyl acetate-ethanol-water (5:1:5, v/v/v) was used in the present study andprepared. The solvent mixture was thoroughly equilibrated in aseparated funnel at room temperature and the two phases wereseparated shortly before use. The sample solution was preparedby dissolving the sample in 15 ml lower phase of the solventsystem for isolation and purification. 2.5. HSCCC separation procedure The coil column was first entirely filled with the upper phaseofthe solvent system. Then the apparatus was rotated at 800 rpm,while the lower phase was pumped into the column at a flowrate of 2.0 ml/min. After the mobile phase front emerged andhydrodynamic equilibrium was established in the column, about15 ml sample solution containing 300 mg of the crude extractwas injected through the injection value. The effluent of thecolumn was continuously monitored with a UV-vis detector at280nm. Peak fractions were collected according to the elutionprofile. The temperature of the apparatus was set at 30°C. 2.6. Analysis and identification ofHSCCC peak fractions The crude extract after cleaning-up by macroporous resin andthe fractions obtained by HSCCC were all analyzed by HPLC. The analysis was performed with a reversed-phase Lichro-spher C18 (150 mmx6.0 mmI.D., 5 pum) (Hanbang Science,Jiang Su Province, China). The mobile phase composed ofCHCN-H2O-HAC (20:80:2, v/v/v) was eluted isocratically ata flow rate of 1.0 ml/min. The column temperature and detectionwavelength were set at 25°C and 280 nm. 3. Results and discussion 3.1. HPLC analysis of the crude extract In resin column chromatography, water was first used toremove some co-extracted matrix components, which have noor little retention on AB-8 macroporous resin, 30% aqueousethanol was then used to yield target sample. The crude extract after resin column chromatography wasfirst analyzed by HPLC. After a lot of experiments, an excellentseparation of curculigoside and curculigoside B was achievedunder the following conditions: the mobile phase composed ofacetonitrile-water-acetic acid (20:80:2, v/v/v) was eluted iso-cratically at a flow rate of 1.0 ml/min, the column temperatureand detection wavelength were set at 25C and 280 nm. No com-plex gradient of mobile phase and no buffer were necessary. TheHPLC chromatogram of the crude extract is shown in Fig. 2.Peaks 1 and 2 correspond to curculigoside B and curculigoside,which presented at the contents of 5.09% and 26.08%, respec-tively. 3.2. Selection suitable two-phase solvent system forHSCCC isolation The most important step in HSCCC is to optimize suitableconditions for an efficient separation. Various parameters includ-ing two-phase solvent system,column temperature, flow rate ofthe mobile phase and revolution speed are generally consideredto be the most important factors. The optimization of suitableHSCCC conditions can be carried out step-by-step or by using Fig. 2. HPLC chromatogram of crude extract from C. orchioides after cleaning-up by D101 macroporous resin. Column: reversed-phase Lichrospher C18(150 mmx6.0 mm I.D., 5 um); mobile phase: CH3CN-H2O-HAC (20:80:2,v/v/v); flow rate: 1.0 ml/min; UV wavelength: 280 nm; column temperature:25°C. Peaks 1 and 2 correspond to curculigoside B and curculigoside, respec-tively. Table 1The partition coefficients (K) of curculigoside and curculigoside B in differentsolvent systems Solvent systems Curculigoside Separation Curculigoside B (Kj) factor (a) (K2) n-Hexane-ethyl acetate- 0.034 2.94 0.10 methanol-water1:2:2:5 (v/v)a Chloroform-methanol- 3.92 1.59 2.46 water 4:3:2 (v/v/v) Ethyl acetate-n-butanol- 6.04 1.71 10.33 water 2:1:3 (v/v/v) Ethyl acetate-n-butanol- 7.70 1.69 12.99 water 2:3:5 (v/v/v)" Ethyl acetate-n-butanol- 3.58 1.55 5.54 water 4:1:5 (v/v/v)a Ethyl acetate-ethanol- 1.34 1.81 2.42 water 5:1:5 (v/v/v)a n-Butanol-water 1:1 (v/v) 4.43 1.71 7.57 n-Butanol-acetic acid- 3.09 2.04 6.30 water 4:1:5(v/v/v) Ethyl acetate-methanol- 5.68 2.20 12.48 water4:1:4(v/v/v) Experimental protocol: 4 ml of each phase of the equilibrated two-phase solventsystem was added to approximately 2 mg of crude sample placed in a 10 ml testtube. The test tube was caped and shaken vigorously for 2 min to equilibratethe sample thoroughly. An equal volume of each phase was then analyzed byHPLC to obtain the partition coefficient (K). The partition coefficient (K) valuewas expressed as the peak area of the compound in the upper phase divided bythe peak area of the compound in the lower phase. Separation factor,α=K/K2(K>K2). an experimental design. In the present study, all selected factorswere optimized step-by-step. In HSCCC, the selection of two-phase solvent system isthe most important for successful separation, and is also themost difficult step. In the present paper, nine kinds of two-phase solvent systems were tested and their partition coeffi-cients (K) and separation factors (a) were all examined andlisted in Table 1. The results indicated that the solvent systemcomposed of n-hexane-ethyl acetate-methanol-water (1:2:2:5,v/v/v) had small K value and the solvent systems composedofchloroform-methanol-water (4:3:2, v/v/v), ethyl acetate-n-butanol-water (2:1:3,2:3:5,4:1:5, v/v/v), n-butanol-water(1:1,v/v), n-butanol-acetic acid-water (4:1:5, v/v/v) and ethylacetate-methanol-water (4:1:4,v/v/v)had large K values, whichwere not suitable for the isolation and separation. At last, thesolvent system composed of ethyl acetate-ethanol-water at avolume ratio of 5:1:5 (v/v/v) was selected for our purification inthe present research. 3.3. Selection suitable column temperature for HSCCCisolation Apart from a suitable two-phase solvent system, other param-eters may affect the isolation results in HSCCC, such as columntemperature, flow rate of the mobile phase and revolution speedAmong them, column temperature might influence the retentionof the stationary phase significantly. High temperature wouldproduce high retention of the stationary phase and low temper 45 Fig. 3. The influence of the separation temperature to the retention of the sta-tionary phase and the purities of curculigoside and curculigoside B in HSCCCseparation. Solvent system: ethyl acetate-ethanol-water (5:1:5, v/v/v); station-ary phase: upper phase; mobile phase: lower phase; flow rate: 2.0 ml/min;revolution speed: 800 rpm; sample size: 300 mg; detection wavelength:280 nm.(A) The relationship between the retention of the stationary phase with thecolumn temperature; (B) the relationship between the purities of the target com-pounds with the column temperature. ature would cause the stationary phase loss, which would affectthe purities of the target compounds. In the present paper, fivekinds of column temperature (10, 20, 25, 30 and 40°C) weretested. The highest retention (50%) of the stationary phase ofthe selected solvent system was obtained at 40°C and lowestretention (20%) was obtained at 10C (Fig. 3A). The puri-ties of curculigoside and curculigoside B decreased when thecolumn temperature was lower than 30C (Fig. 3B), and morebadly, only one peak was obtained and the purities of the twocompounds decreased to 37.4% and 7.2%, respectively at 10C(Fig.6B), which was similar to the crude extract after cleaning-upby D101 macroporous resin without any HSCCC purification.Although the result was satisfactory at 40 °C, a large quantity ofair bubble was produced. Furthermore, high temperature wouldlessen the life of the apparatus. So, the column temperaturewas set at 30°C in our isolation mainly considering the aboveaspects. 3.4. Selection suitable flow rate of the mobile phase forHSCCC isolation The flow rate might influence the HSCCC separation, too.Different flow rates (1.0, 1.5,2.0, 2.5 and 3.0 ml/min) of themobile phase of the selected system were examined in thepresent paper. Different retentions of the stationary phase(Fig. 4A) and different purities of the compounds were obtained(Fig. 4B). High flow rate was unfavorable to the retention ofthe stationary phase and the purities of the compounds, and (B) Flow rate (ml/min) Fig. 4. The influence of the flow rate of the mobile phase to the retention ofthe stationary phase and the purities of curculigoside and curculigoside B inHSCCC separation. Solvent system: ethyl acetate-ethanol-water (5:1:5, v/v/v);stationary phase: upper phase; mobile phase: lower phase; column temperature:30°C; revolution speed: 800 rpm; sample size: 300 mg;detection wavelength:280 nm. (A) The relationship between the retention of the stationary phase withthe flow rate of the mobile phase; (B) the relationship between the purities ofthe target compounds with the flow rate of the mobile phase. curculigoside and curculigoside B were not separated andcombined in one peak (Fig.6C). On the contrary, low flow rate(1.0 ml/min) was satisfactory to our aim, but the elution timewas long and more mobile phase was required. Thus, the flowrate was set at 2.0 ml/min in the present separation accordingto the overall analysis. 3.5. Selection suitable revolution speed of the apparatus forHSCCC isolation High revolution speed has the ability to increase the retentionof the stationary phase, and it could not be reserved in HSCCCcolumn at low revolution speed. After trying a lot of experiments(700,800, 900 and 1000 rpm),the curves were draw according tothe retention of the stationary phase and the compounds’ puritiesto the revolution speed of the apparatus. Low revolution speedwas unfavorable to the retention of the stationary phase (Fig.5A)and the purities of the compounds (Fig.5B), and curculigosideand curculigoside B were not separated and combined in onepeak (similar to the Fig. 6C and not shown). At last, 800 rpmwas selected to separate the target compounds in our paper. The most suitable isolation conditions, therefore, wereselected as follows: the two-phase solvent system was com-posed of ethyl acetate-ethanol-water (5:1:5, v/v/v), the lowerphase was used as the mobile phase at 2.0 ml/min, the columntemperature and revolution speed were set at 30C and 800 rpm.Under the optimized conditions, three peaks were isolated andtwo (I and II) were collected in less than 210 min. The HSCCC Fig. 5. The influence of the revolution speed to the retention of the stationaryphase and the purities of curculigoside and curculigoside B in HSCCC sep-aration. Solvent system: ethyl acetate-ethanol-water (5:1:5, v/v/v); stationaryphase: upper phase; mobile phase: lower phase; column temperature: 30°C; flowrate: 2.0 ml/min; sample size: 300 mg; detection wavelength:280 nm. (A) Therelationship between the retention of the stationary phase with the revolutionspeed of the apparatus; (B) the relationship between the purities of the targetcompounds with the revolution speed of the apparatus. Fig. 7. HPLC chromatograms and the UV spectra of the fractions obtainedby HSCCC from C. orchioides. Column: reversed-phase Lichrospher C18(150 mmx6.0 mm I.D., 5 pm); mobile phase: CH3CN-H2O-HAC (20:80:2,v/v/v); flow rate: 1.0 ml/min; UV wavelength: 280 nm; column temperature:25C. (A) Fraction I obtained by HSCCC; (B) fraction II obtained by HSCCC;peaks 1 and 2 correspond to curculigoside B and curculigoside. Ecc 200 (B) Fig. 6. HSCCC chromatograms of the crude extract from C. orchioides after cleaning-up by D101 macroporous resin. Solvent system: ethyl acetate-ethanol-water(5:1:5, v/v/v); stationary phase: upper phase; mobile phase: lower phase; revolution speed: 800 rpm; sample size: 300 mg; detection wavelength: 280 nm. (A) Columntemperature: 30 °C; flow rate of the mobile phase: 2.0 ml/min. (B) Column temperature: 30°C; flow rate of the mobile phase: 3.0 ml/min. (C) Column temperature:10°C; flow rate of the mobile phase: 2.0 ml/min. Fig. 8. HSCCC chromatograms of the ethanol extract from C. orchioideswithout D101 macroporous resin cleaning-up step. Solvent system: ethylacetate-ethanol-water (5:1:5, v/v/v); stationary phase: upper phase; mobilephase: lower phase; revolution speed: 800 rpm; column temperature: 30°C; flowrate of the mobile phase: 2.0 ml/min; detection wavelength: 280 nm. (A) Samplesize: 300 mg; (B) sample size: 80 mg. chromatogram is shown in Fig.6A, which was glossy and morebeautiful than others. After the two compounds were eluted out,in order to save solvents and time, the remaining compounds inthe column were removed by forcing out the stationary phasewith pressurized nitrogen gas instead of eluting them with themobile phase because the stationary phase was not to be reused.The isolation produced a total of 14.5 mg curculigoside B and72.8 mg curculigoside with purities of 96.5% and 99.4%,respec-tively as determined by HPLC with external standard method(Fig. 7) from 300 mg crude extract. The recoveries of the twocompounds were 91.6% and 92.5%, respectively. Generally, a cleaning-up step was required after extractionin HSCCC, and suitable samples were obtained and someco-extracted matrix components were eliminated to minimizeadverse effects affecting our isolation. In the present paper, thestep of D101 resin column chromatography was important forthe successful purification. Firstly, we tried to introduce theethanol extract to HSCCC column without the cleaning-up step,however, the outcome was bad and no pure fraction was obtainedunder the optimized conditions (Fig.8A), and even the samplesize was decreased to 80 mg (Fig.8B). Apart from the optimizedconditions, a lot of experiments were carried out to isolate thetwo compounds without resin step, but the results were not sat-isfactory. Thus, we can say without resin step without excellentpurification. 3.6. Chemical structure identification The chemical structure identification was carried out by UV,MS, and NMR as follows. Fraction I: UV MeOH: 283, 245max, (B) m/z Fig.9. The MS spectra of the fractions obtained by HSCCC from C. orchioides. 225 nm (Fig. 7A). ESI-MS: 475[M+Na]* (Fig. 9A), it showedthe molecular was 452, which was in agreement with the molecu-lar formula C21H24O11.1HNMR (500 MHz, DMSO-d6)8: 6.97(1H,d,J=9.0Hz, H-3),6.63 (1H,dd,J=9.0,3.0Hz,H-4),9.05(1H, s, 5-OH), 6.86 (1H, d,J=3.0Hz, H-6), 5.30 (2H, s,H-7),10.02 (1H, s,2-OH), 6.53 (1H, d,J=8.3Hz, H-3), 7.18(1H,t, J=8.3Hz, H-4'), 6.50 (1H,d,J=8.0Hz, H-5), 3.75 (3H, s,6'-OCH3),4.63 (1H,d,J=7.0Hz, H-1");CNMR(125MHz,DMSO-d6)8:127.7(C-1),147.8(C-2),117.7(C-3),115.2(C-4),152.7 (C-5),114.8(C-6),61.3(C-7), 102.6(C-1),156.2(C-2'),111.4 (C-3'), 131.7(C-4), 108.8(C-5),157.8 (C-6'), 166.7(C-7'),56.3(-OCH3-),103.2(C-1"),73.7(C-2"),77.4(C-3"), 70.2(C-4"), 76.7 (C-5"), 61.7 (C-6"). Comparing with the reporteddata, the UV, MS and NMR data are in agreement with that ofcurculigoside B in literature [10]. Fraction I: UVAMeOH: 282,230 nm (Fig. 7B). ESI-MS: 489 [M+Na]+(Fig.9B), it showedthe molecular was 466, which was in agreement with the molecu-lar formula C22H26O11.1HNMR (500MHz,DMSO-d6)8:6.98(1H,d,J=9.0Hz,H-3), 6.65 (1H,dd,J=9.0,3.0Hz,H-4), 9.05(1H, s,5-OH), 6.80(1H,d,J=3.0Hz,H-6), 5.31(2H, s, H-7),3.77 (3H,s,2'-OCH3),6.73 (1H, d,J=8.5Hz,H-3), 7.39 (1H,t,J=8.5Hz, H-4'), 6.75 (1H, d,J=8.5Hz, H-5'), 3.78 (3H, s,6'-OCH3), 4.60(1H, d,J=7.5Hz,H-1");13C NMR (125MHz,DMSO-d6)8: 127.7(C-1), 147.8 (C-2), 117.6 (C-3), 115.2 (C- 4), 152.7 (C-5), 114.8 (C-6),61.7 (C-7), 104.8 (C-1'), 157.0(C-2'), 113.1 (C-3'), 131.6 (C-4'), 113.1 (C-5'), 157.1 (C-6'),166.0(C-7), 56.2 (2'-OCH3), 56.3 (6'-OCH3), 103.0 (C-1"),73.7 (C-2"),77.5(C-3"), 70.2 (C-4"), 76.8 (C-5"), 61.4 (C-6").Comparing with the reported data, the UV, MS and NMR dataare in agreement with that of curculigoside in literature [10]. 4. Conclusion In the present paper, some parameters including solvent sys-tem, column temperature, flow rate and revolution speed wereoptimized step-by step. Under the selected isolation conditions,two glycosides were successfully separated by HSCCC from thetraditional Chinese medicinal plant C. orchioides. The resultsalso indicated that HSCCC is a powerful technique to isolateand purify pure materials from natural plants. Acknowledgement Financial support from Ministry of Science and Technologyof China (863 project) is gratefully acknowledged. References ( [ 1 ] J.Y. Peng, G.R. Fan, Z.Y. H o ng, Y. F . Ch a i, Y.T. Wu, Preparative sepa- ration of i sovitexin and i soorientin f rom Patrinia v i llosa Juss by high- ) speed counter-current chromatography, J. Chromatogr. A 1074 (2005)111-115. ( [2] J.Y . Peng, G.R . Fan, L.P. Qu, Z . X in, Y .T. W u, A p plication o f preparative h igh-speed c ounter-current c hromatography fo r is o lation a n dseparation o f s chizandrin and gomisin A f ro m Sch i sa n dra chinensis, J.Chromatogr. A 1 082 ( 2005) 203- 2 07. ) ( [ 3 ] X. W ang, Y . Q. W a ng, Y. L . Geng, F.W . Li, C.C . Zhe n g, Isolation and p urification of honokiol and m agnolol from co r tex Magnoliae off i cinalisby h igh-speed c ounter-current chromatography, J . Chromatogr. A 1 036 ( 2 004) 171-175. ) ( [4]Y . J i a ng , H.T. L u. F. Chen, P reparative p urification n O fo f g ly cyrrhizin extracte d from t he root o f l i quorice using hi gh-speed counter-current chromatography, J . Chromatogr. A 1 033 ( 2 0 04) 1 8 3-186. ) ( [5] J .Y. Peng, G .R. Fan, Y . T. W u , Su p ercritical fl u id extraction o f a u renti- a mide a c etate f r om Patrinia vil l osa Jus and su b sequent iso l ation by sil i cagel and h igh-speed c ounter-current chromatography, J. Chromatogr. A 1083 (2005)52- 5 7. ) ( [6] Committee of National Pharnacopoeia, Pharmacopoeia o f China, Chem- i cal I ndustry P ress, B eijing, 1 995, p . 8 1. ) ( [7] X.Y. Gao, X.J. Du, C . Y. Z h ao, Ef f ect of ki d ney-tonifying traditional Chinese m e dicine on proliferation of osteoblast-like ce l ls U M R106, J. Chengde M ed. College 1 8 (2001) 283-285. ) ( [8] Q.S. C hen, P harmacological r e search of C u rcul i go o r chioides, C h inaChin. Mater. Med. 1 4 (1989) 4 2-4 3 . ) [9] Y.L. Huang, Advanced research of Curculigo orchioides, ZhongYaoCai26 (2003)225-228. ( [10] D.X. F u, G .Q. Lei, X .W. Cheng, J.K. C h en, T. S . Z h ou, CurculigosideC, a n ew phenolic g lucoside f rom Rhizomes of C u rculigo orchioides, A cta B ot. Sin. 46 (2004) 621-624. )
确定



还剩5页未读,是否继续阅读?
上海同田生物技术有限公司-高速逆流色谱仪HSCCC为您提供《仙茅中仙茅甙和仙茅甙乙的分离检测方案(高速逆流色谱)》,该方案主要用于中药材和饮片中含量测定检测,参考标准--,《仙茅中仙茅甙和仙茅甙乙的分离检测方案(高速逆流色谱)》用到的仪器有TBE-300A高速逆流色谱仪/制备色谱仪、TBE-200V 高速逆流色谱仪、TBE-20A分析型高速逆流色谱仪/萃取仪/制备色谱仪、TBE300B+AKTA高速逆流色谱仪/离心分配色谱/萃取仪/制备色谱仪、TBE-1000A制备色谱仪/萃取仪/快速分离制备色谱仪
推荐专场
相关方案
更多
该厂商其他方案
更多




















