方案详情
文
Cimps是Zahner公司潜心研发的光电化学测试设备,是世界上第一套系统的将各种光电化学测试技术集合到一起的仪器。由于目前该仪器对于国内的用户还比较陌生,所以我们特别将Zahner公司与德国某大学合作的科研结果介绍给大家。希望可以起到抛砖引玉的作用,让目前世界上最热门的动态光电化学测试技术尽快在国内得到普及。这是一份现场报告的摘要,里面简要介绍了在光照条件下采用EIS技术测试不同的光电极的反应性质的过程及结论,从中可以看到,Cimps实际上是采用光信号作为激发信号的阻抗谱技术,所以它可以用于研究光电极的动态工作状况(类似于采用EIS技术研究电极的工作状况)。最后,通过运用拟合技术还可以研究光电极的能量结构,这是Cimps对IMVS/IMPS等技术的扩展与深化。
方案详情

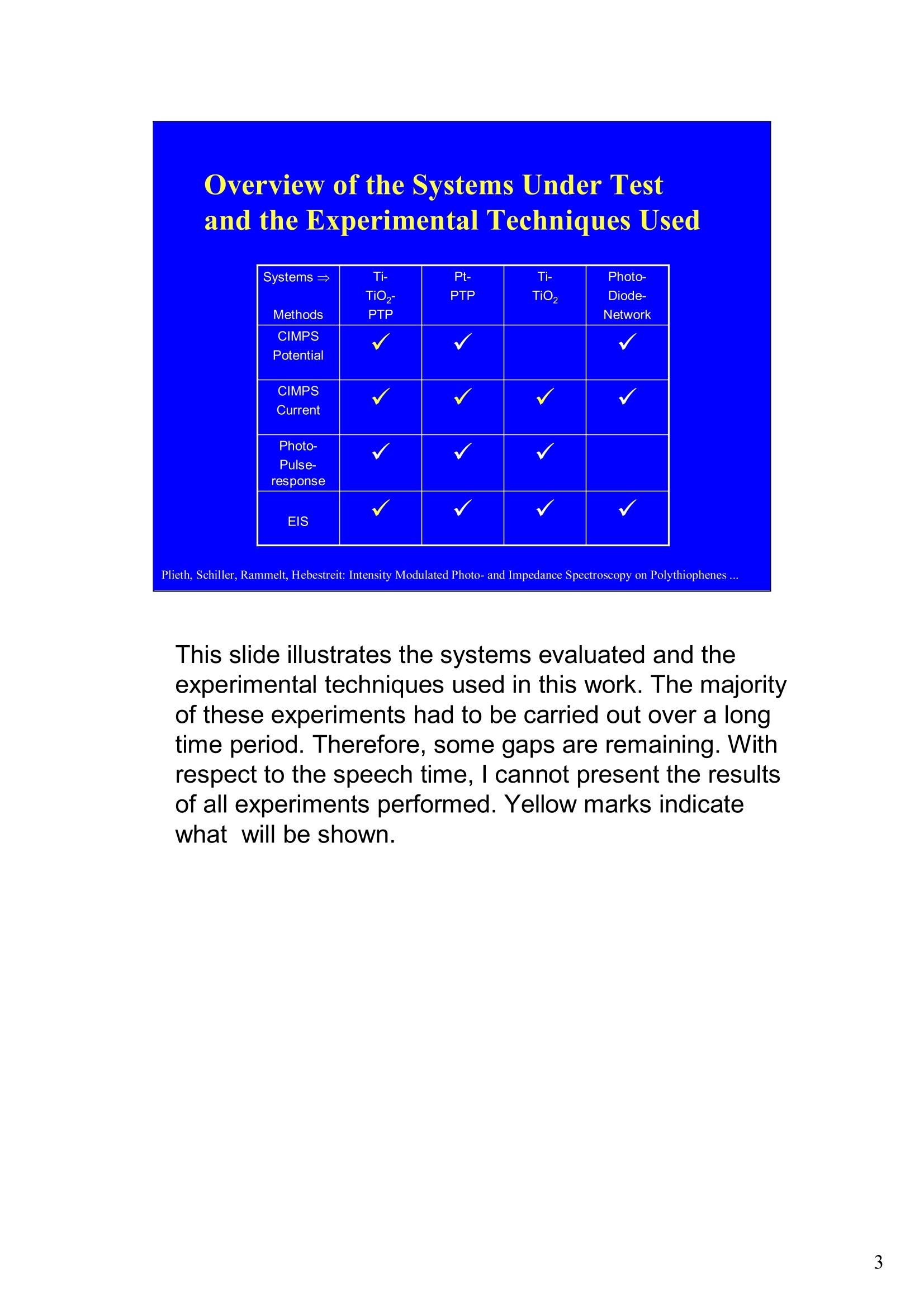
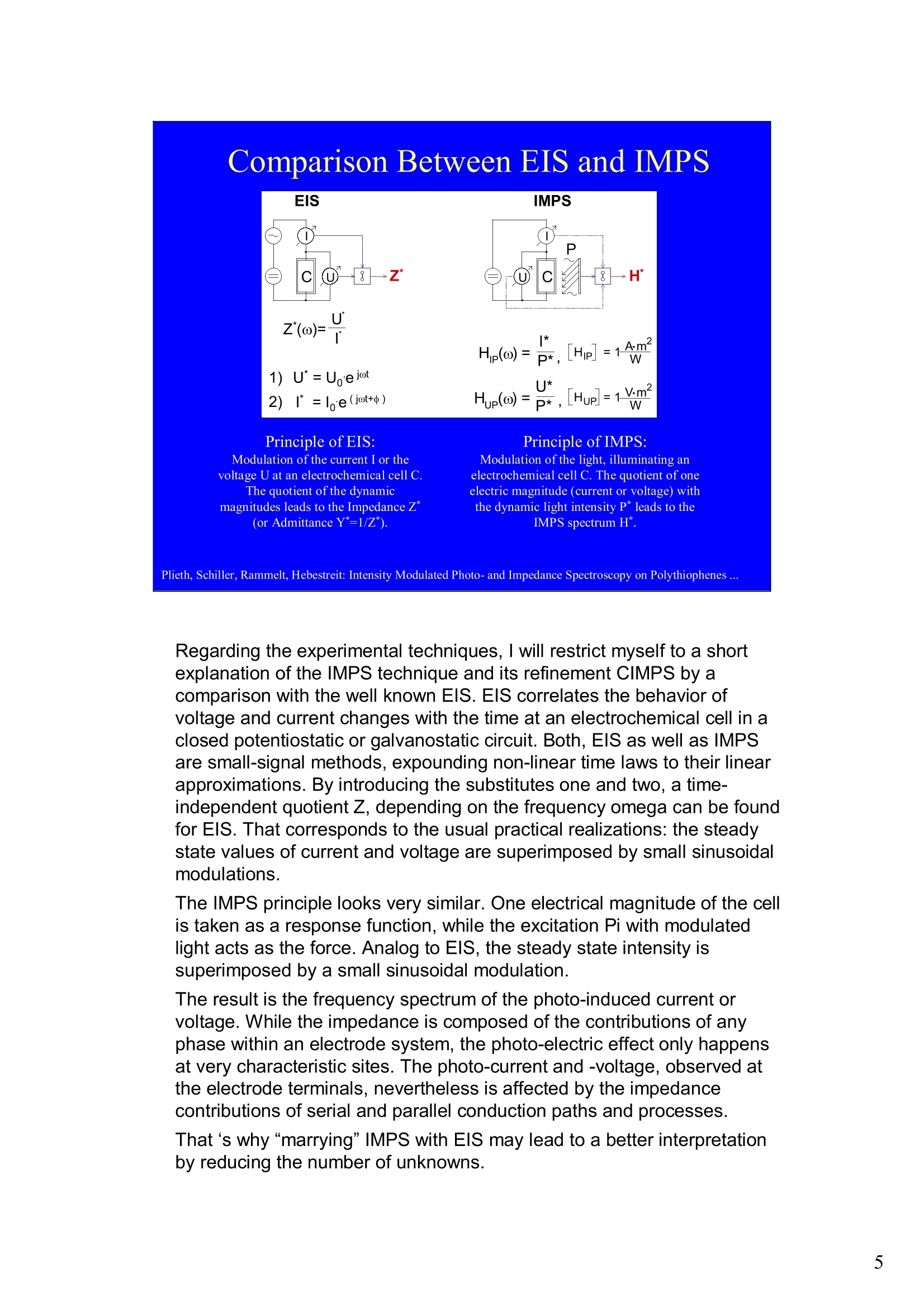
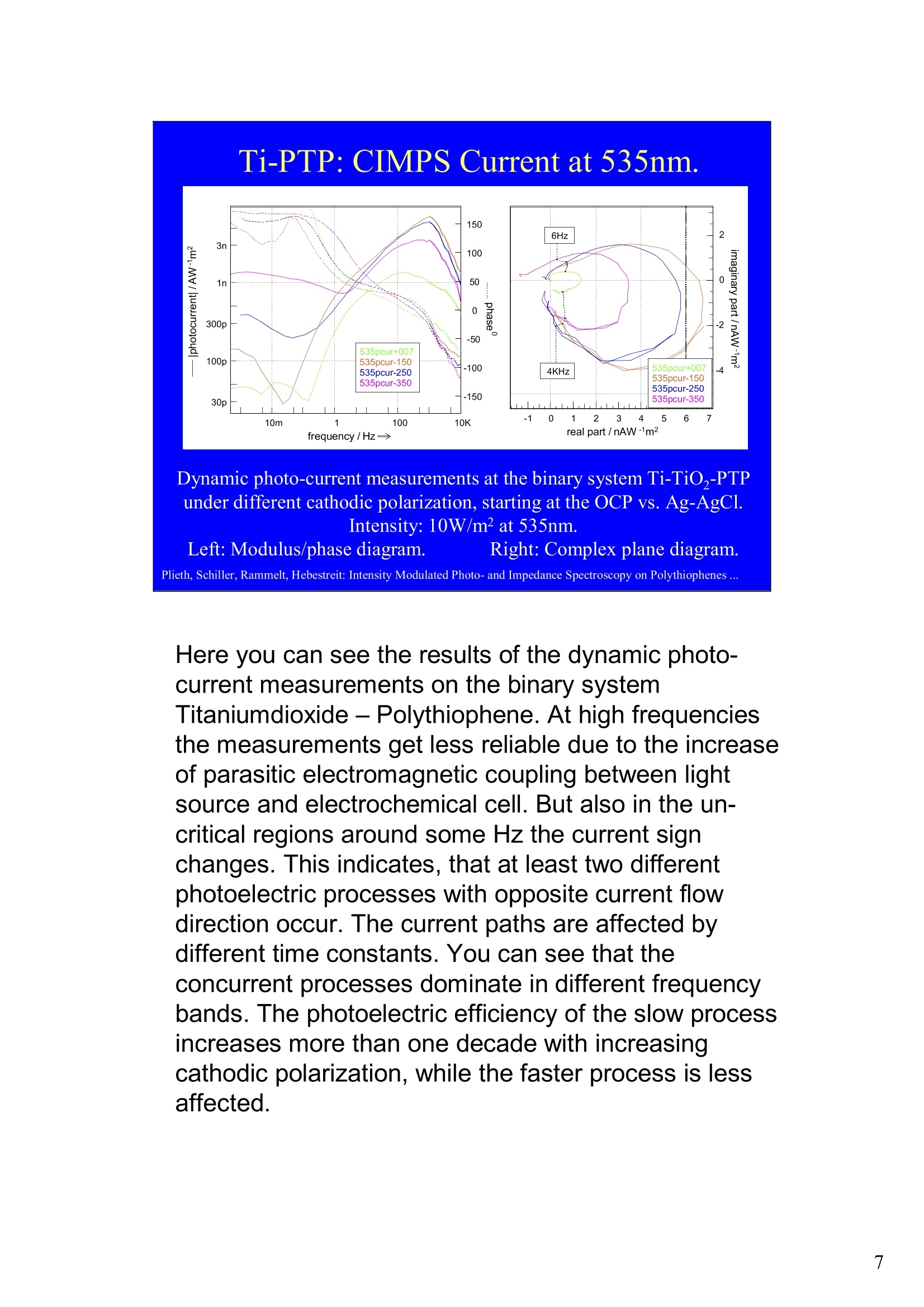
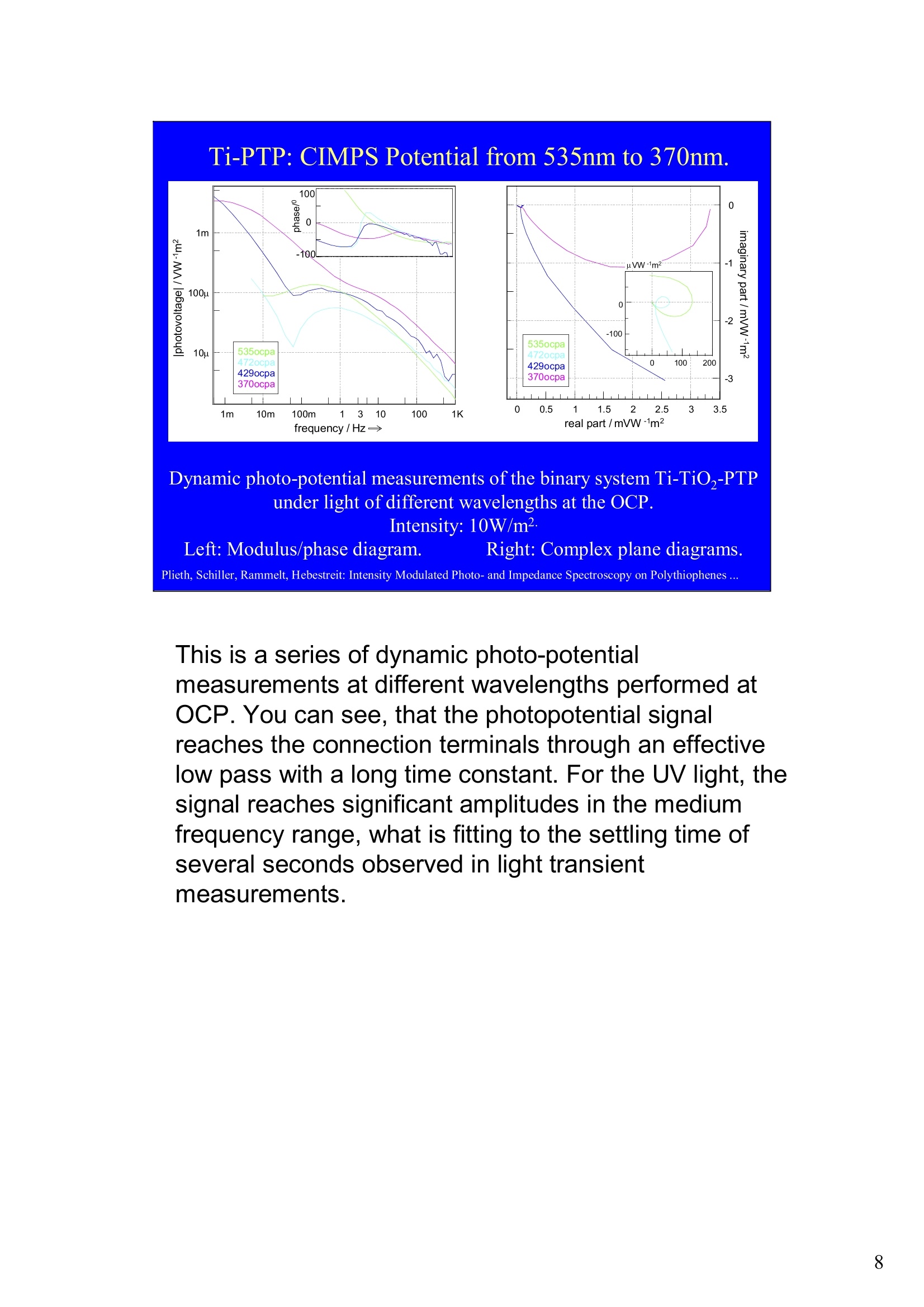
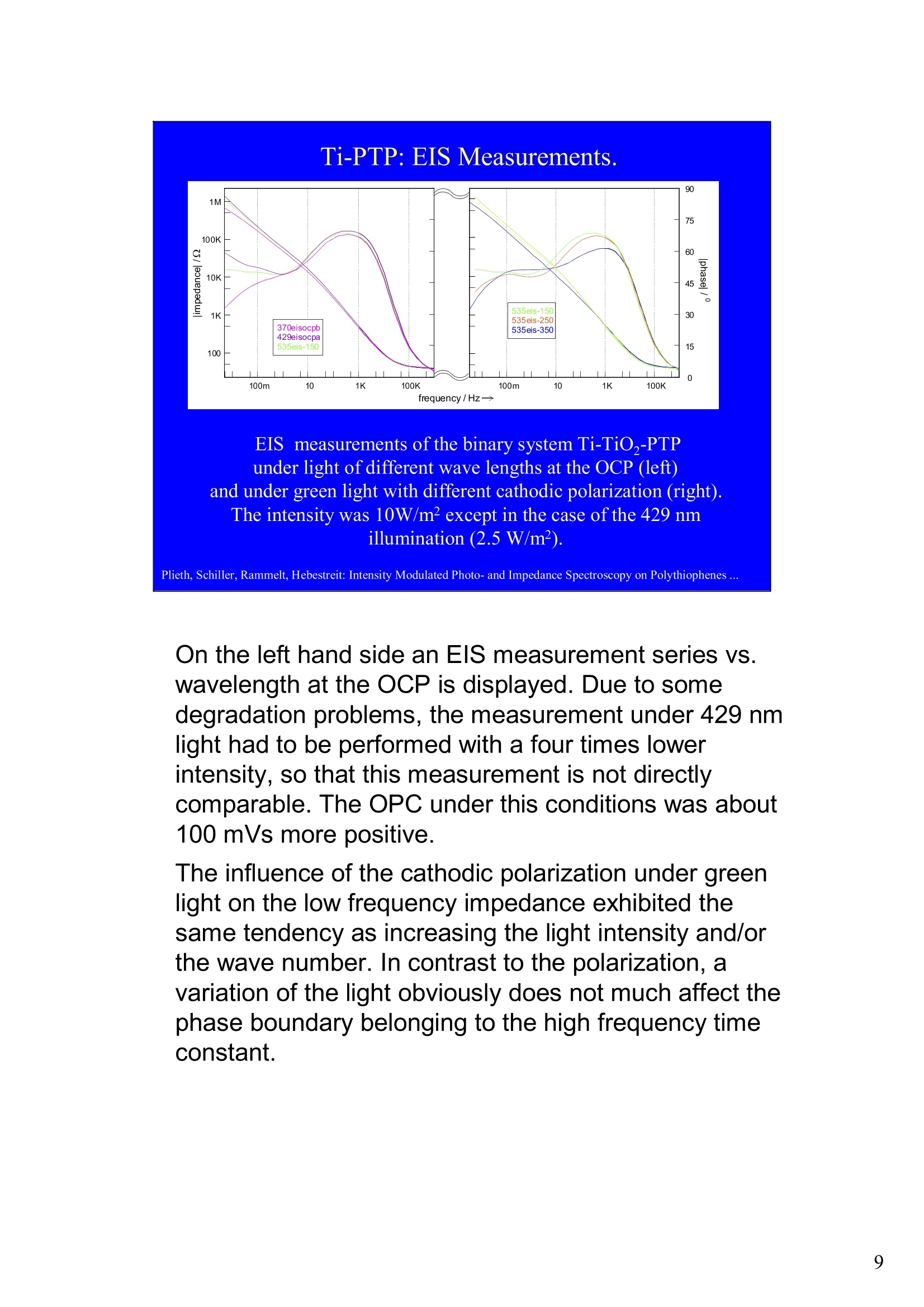
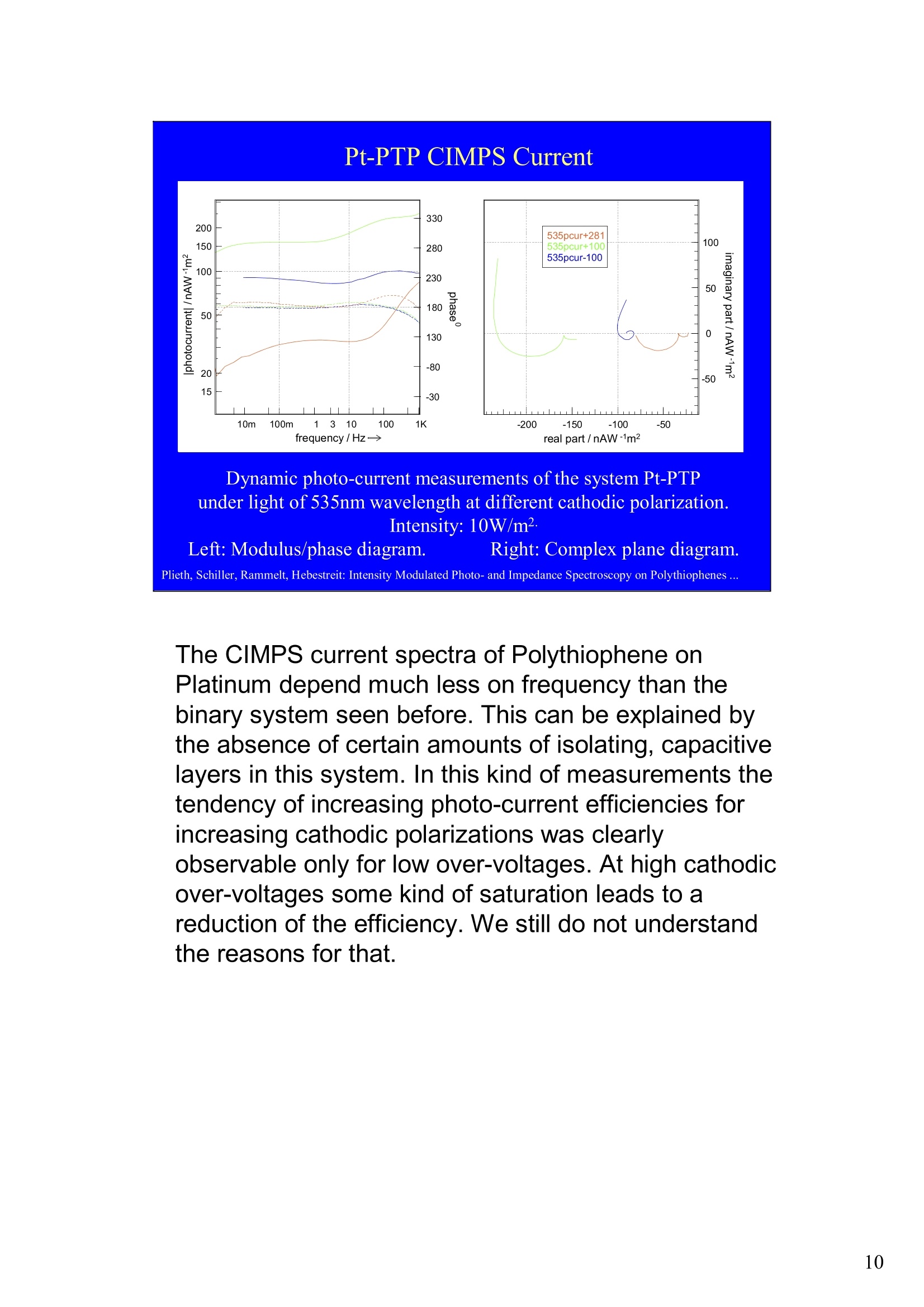
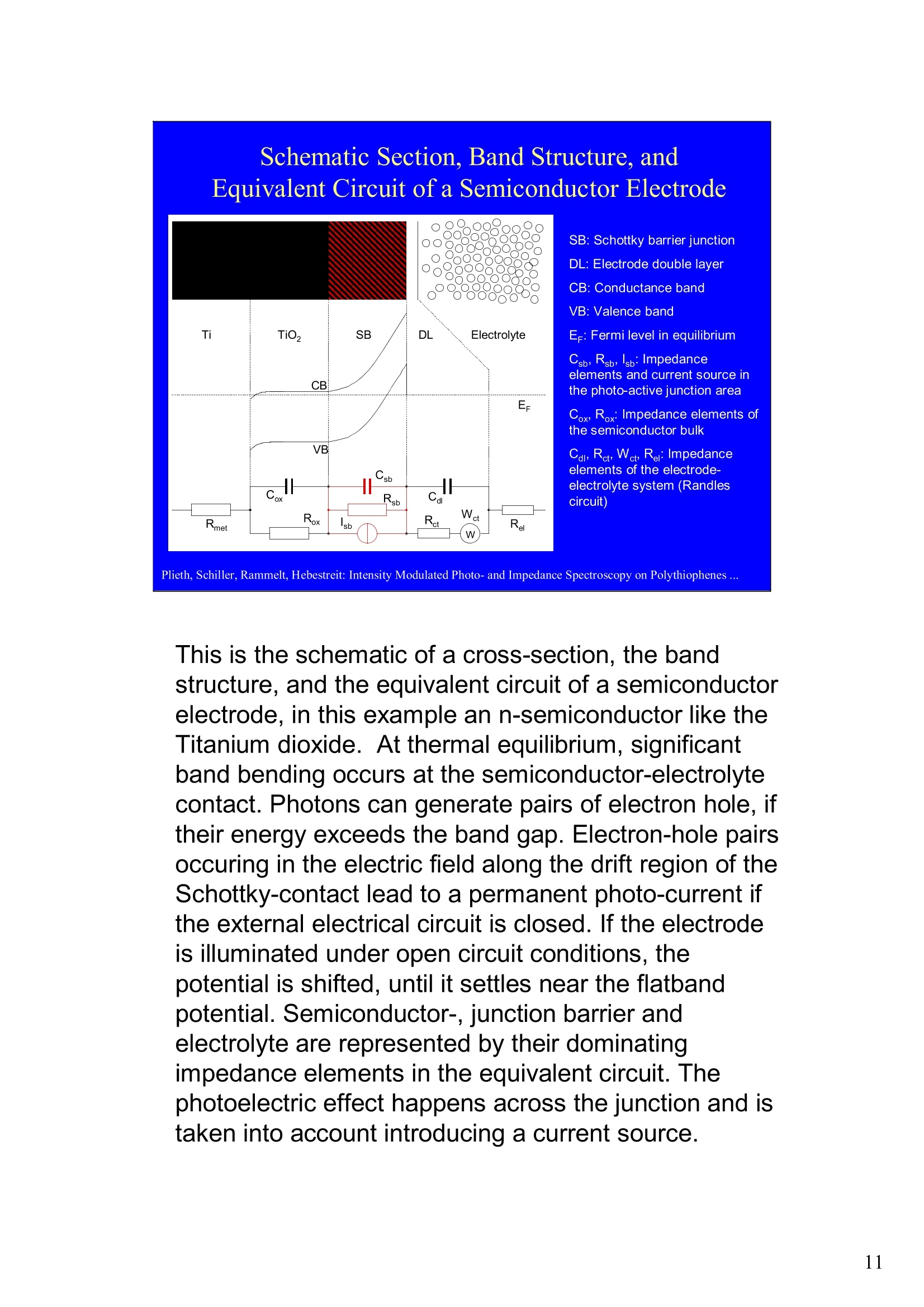
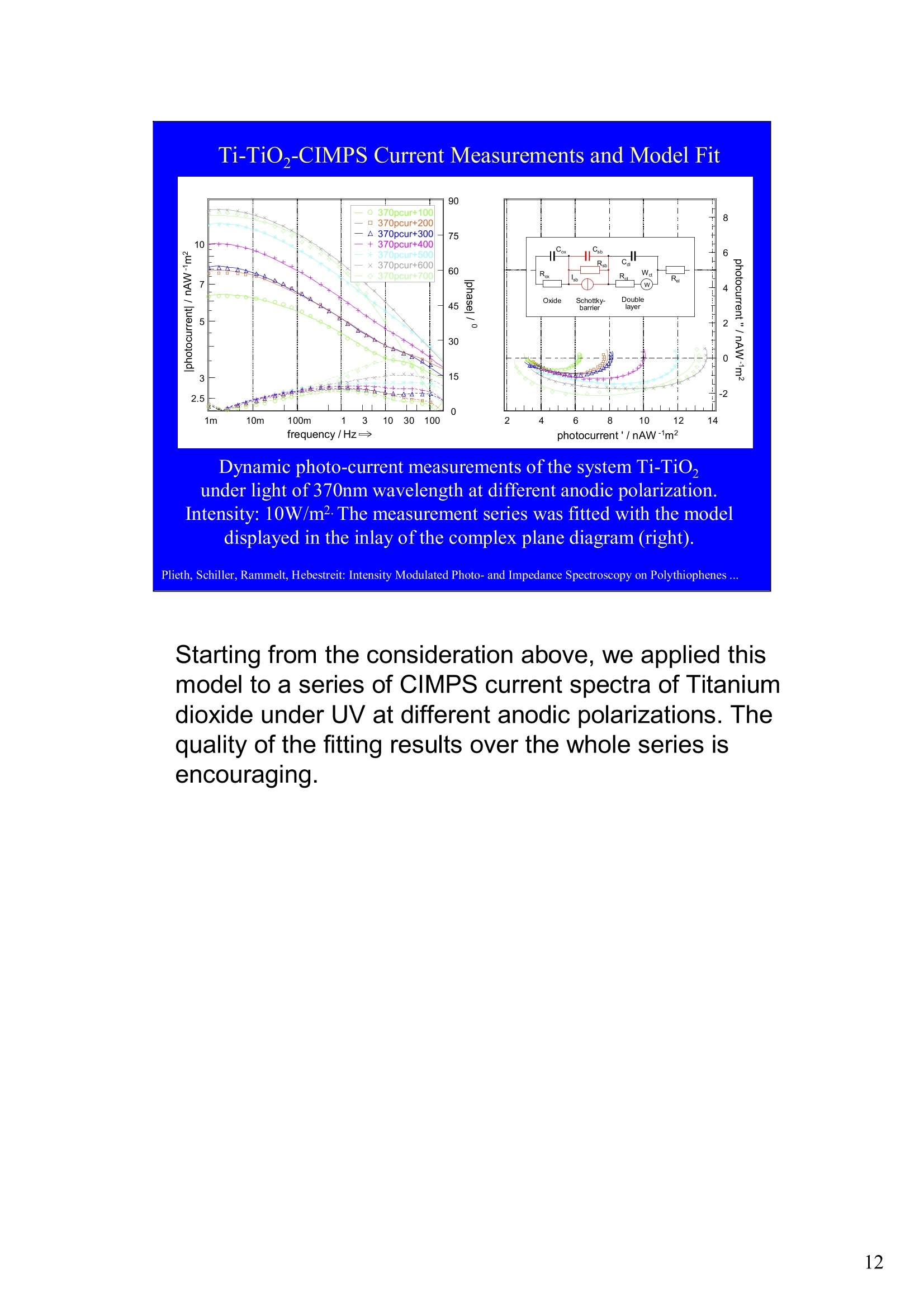
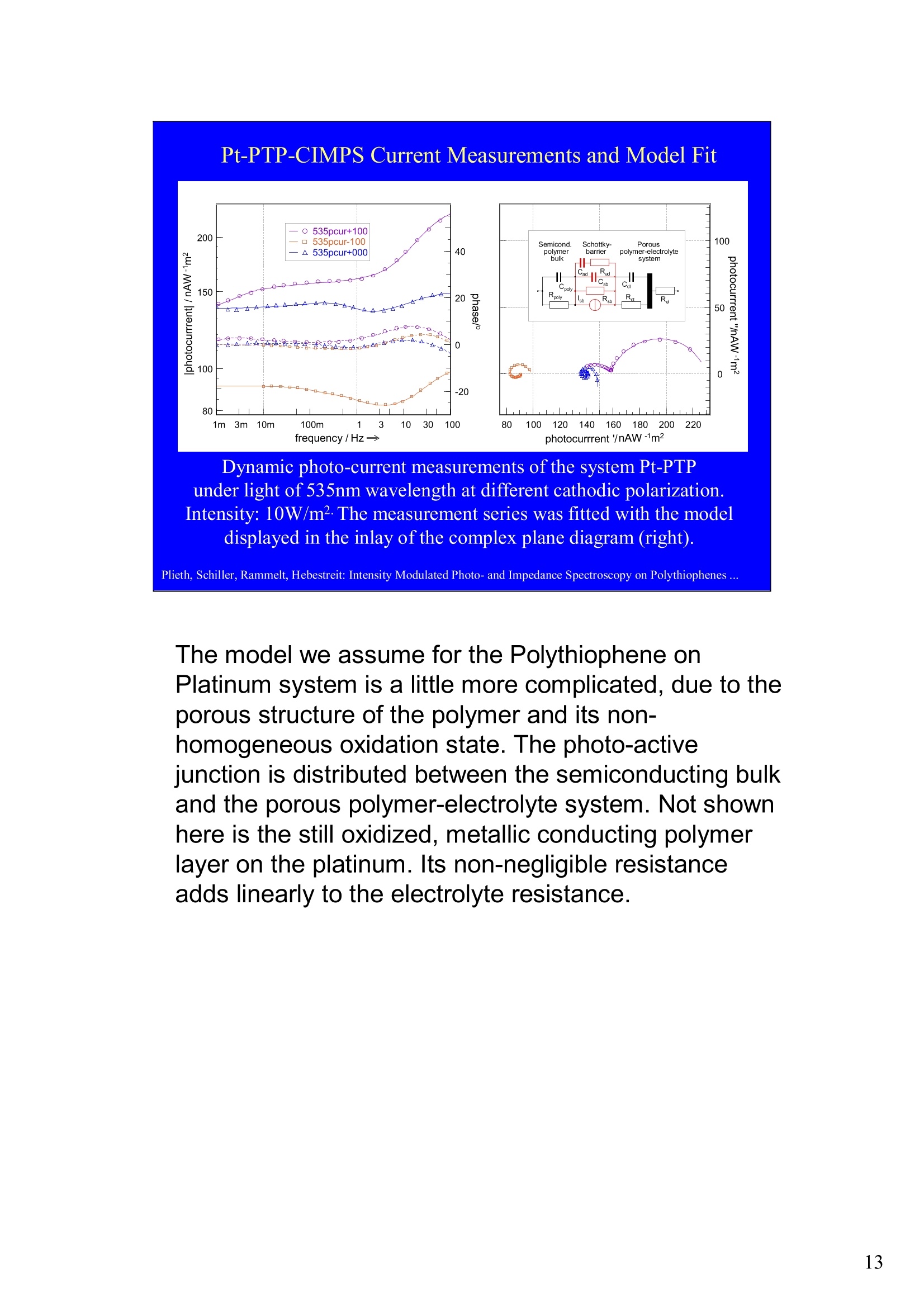
Intensity Modulated Photo- andImpedance Spectroscopy atPolythiophenes on TiO, and Pt in theReduced State W. Plieth, C.A. Schiller,U. Rammelt, N. Hebestreit 'Dresden University of Technology, Institute ofPhysical Chemistry andElectrochemistry, Mommsenstr. 13, D-010602 Dresden, Germany 2ZAHNER-Messtechnik, Thiiringer Str. 12, D-96317 Kronach, Germany cas@zahner.de Ladies and gentlemen, The work I will present now was performed at the Institute ofPhysical Chemistry and Electrochemistry in Dresden, Germany,and at the labs of Zahner-Messtechnik in Kronach, Germany. Conducting polymers are still in the focus of research interest.To name only two potential applications: while the highlyconductive oxidized states are candidates for protective layers,the semi-conducting properties of the reduced substancesnourish ideas of omnipresent printed low-cost electronics.Compared to silicon semiconductors, the polymer versions are highly complex systems. Mainly their non-homogeneousstructure and the potential coupling of the charge conductionwith redox reactions are the source of this complexity. Inside these systems, certain phase boundaries behave assemi-conductor junction barriers. The photo-electrical effecttaking place within this barriers can be used as a very directindicator for their evaluation. The basic aim of this work is tocontribute to a better understanding of organic polymers byevaluating their photo-electric and electrochemical properties.Intensity modulated photo-spectroscopy was chosen as apromising tool. Let’s cut down this long-term objective into single steps.Compared to traditional electrochemical techniques like EIS,the dynamic photo-spectroscopy techniques using modulatedlight are less wide spread as well as less developed. One steptowards a better understanding should be the refinement of theIMPS technique. Photo-potential-, photo-current- and EIS measurements allshow up little different dynamic aspects of the system undertest. A plausible model should be able to explain the results ofall three measurements. As a second step towards a betterunderstanding we tried to reduce the description of the differentresults to one common model. This slide illustrates the systems evaluated and theexperimental techniques used in this work. The majorityof these experiments had to be carried out over a longtime period.Therefore, some gaps are remaining.Withrespect to the speech time, I cannot present the resultsof all experiments performed. Yellow marks indicatewhat will be shown. Preparation of Electrodes Tio, Electrolysis of 0.5 M K,SO_ /10 V, ca. 10 min. (thickness: 25 nm) PTP/Pt Electropolymerisation of 0.1 M 2,2 bithiophene /TBAPF,(‘CV-scan :0 - 1.3 V vs SCE, scanrate =20mV/s, 15 min.thickness 200 nm) PTP/TiO, - Pretreatment of TiO,-electrode with adhesion promotor TUTS (11-(3-thienyl)undecyltrichlorsilane), ca. 30 min. Oxidation of Thiophene with FeCl, in CHCl, ca. 90 min., (thickness: 50-100 nm) Plieth, Schiller, Rammelt, Hebestreit: Intensity Modulated Photo- and Impedance Spectroscopy on Polythiophenes ... For the same reason, I will go through the preparationtechniques of the electrodes very roughly. Typical electrode systems for the experiments presented herewere prepared according to the following recipes: -The titanium oxide electrode was prepared by electrochemicalanodic oxidation, electrolyzing a point five molar solution ofPotassiumsulfate at ten Volts. -The Poly-Thiophene electrode on platinum was prepared byelectrochemical cycling of a solution of bithiophene withTetrabutylammonium-hexafluorophosphate as supportingelectrolyte between zero and plus one point three Volts at ascanrate of twenty milivolts per second. -The Poly-Thiophene electrode on Titanium oxide was preparedusing a Titanium oxide electrode sample prepared as explainedabove. An adhesion promotor TUTS forced the precipitation onthe electrode of the polymer, produced by chemical oxidation ofthe thiophene solution in chloroforme. Regarding the experimental techniques, I will restrict myself to a shortexplanation of the IMPS technique and its refinement CIMPS by acomparison with the well known EIS. EIS correlates the behavior ofvoltage and current changes with the time at an electrochemical cell in aclosed potentiostatic or galvanostatic circuit. Both, EIS as well as IMPSare small-signal methods, expounding non-linear time laws to their linearapproximations. By introducing the substitutes one and two, a time-independent quotient Z, depending on the frequencyomega can be foundfor EIS. That corresponds to the usual practical realizations: the steadystate values of current and voltage are superimposed by small sinusoidalmodulations. The IMPS principle looks very similar.One electrical magnitude of the cellis taken as a response function, while the excitation Pi with modulatedlight acts as the force. Analog to EIS, the steady state intensity issuperimposed by a small sinusoidal modulation. The result is the frequency spectrum of the photo-induced current orvoltage. While the impedance is composed of the contributions of anyphase within an electrode system, the photo-electric effect only happensat very characteristic sites. The photo-current and -voltage, observed atthe electrode terminals, nevertheless is affected by the impedancecontributions of serial and parallel conduction paths and processes. That's why“marrying”IMPS with EIS may lead to a better interpretationby reducing the number of unknowns. What is the difference between IMPS and CIMPS?IMPS uses LEDs as light sources. The intensity ofLEDs can be modulated fast and easily by their supplycurrent. With IMPS the function generator output of anFRA supplies the LED with modulated current. Mostly, thecell current is correlated with the LED current by the FRA tocalculate the photo-electrochemical transfer function H*.CIMPS differs in two aspects. The first is, that the LEDcurrent is regulated by an OPAmp construction in a way, thatthe intensity Pact measured by a photodetector at the site ofthe cell fits exactly a given intensity value Pset· Thisguarantees linearity of the modulation and the high stabilityof the average intensity, necessary for measurements over along period of time. The second is, that using the actualmeasured intensity instead of the control current forcalculating the transfer function yields much more accurateresults. Here you can see the results of the dynamic photo-current measurements on the binary systemTitaniumdioxide - Polythiophene. At high frequenciesthe measurements get less reliable due to the increaseof parasitic electromagnetic coupling between lightsource and electrochemical cell. But also in the un-critical regions around some Hz the current signchanges. This indicates, that at least two differentphotoelectric processes with opposite current flowdirection occur. The current paths are affected bydifferent time constants. You can see that theconcurrent processes dominate in different frequencybands. The photoelectric efficiency of the slow processincreases more than one decade with increasingcathodic polarization, while the faster process is lessaffected. This is a series of dynamic photo-potentialmeasurements at different wavelengths performed atOCP. You can see, that the photopotential signalreaches the connection terminals through an effectivelow pass with a long time constant. For the UVlight, thesignal reaches significant amplitudes in the mediumfrequency range, what is fitting to the settling time ofseveral seconds observed in light transientmeasurements. On the left hand side an EIS measurement series vs.wavelength at the OCP is displayed. Due to somedegradation problems, the measurement under 429 nmlight had to be performed with a four times lowerintensity, so that this measurement is not directlycomparable. The OPC under this conditions was about100 mVs more positive. The influence of the cathodic polarization under greenlight on the low frequency impedance exhibited thesame tendency as increasing the light intensity and/orthe wave number. In contrast to the polarization, avariation of the light obviously does not much affect thephase boundary belonging to the high frequency timeconstant. The CIMPS current spectra of Polythiophene onPlatinum depend much less on frequency than thebinary system seen before. This can be explained bythe absence of certain amounts of isolating, capacitivelayers in this system. In this kind of measurements thetendency of increasing photo-current efficiencies forincreasing cathodic polarizations was clearlyobservable only for low over-voltages. At high cathodicover-voltages some kind of saturation leads to areduction of the efficiency. We still do not understandthe reasons for that. This is the schematic of a cross-section, the bandstructure, and the equivalent circuit of a semiconductorelectrode, in this example an n-semiconductor like theTitanium dioxide. At thermal equilibrium, significantband bending occurs at the semiconductor-electrolytecontact. Photons can generate pairs of electron hole, iftheir energy exceeds the band gap.Electron-hole pairsoccuring in the electric field along the drift region of theSchottky-contact lead to a permanent photo-current ifthe external electrical circuit is closed. If the electrodeis illuminated under open circuit conditions, thepotential is shifted, until it settles near the flatbandpotential. Semiconductor-,junction barrier andelectrolyte are represented by their dominatingimpedance elements in the equivalent circuit. Thephotoelectric effect happens across the junction and istaken into account introducing a current source. Starting from the consideration above, we applied thismodel to a series of CIMPS current spectra of Titaniumdioxide under UV at different anodic polarizations. Thequality of the fitting results over the whole series isencouraging. The model we assume for the Polythiophene onPlatinum system is a little more complicated, due to theporous structure of the polymer and its non-homogeneous oxidation state. The photo-activejunction is distributed between the semiconducting bulkand the porous polymer-electrolyte system. Not shownhere is the still oxidized, metallic conducting polymerlayer on the platinum. Its non-negligible resistanceadds linearly to the electrolyte resistance. Conclusions Semiconductor-electrodeslikeconductingl polymerscan be modeled with the same strategy as their two-pole impedance:for that. current sources.representingthe photoactive |process, have to beintroduced. Photocurrent and photovoltage reflect different parts ofthe total electrode network. An equivalent circuit model, common to potentiostaticand galvanostatic produced CIMPS and ElS spectraunder identical current load conditions promise toprove a proper modeling. Measurements at the OCP under illumination as well asmeasurements with resistance load complicate the Plieth, Schiller, Rammelt, Hebestreit: Intensity Modulated Photo- and Impedance Spectroscopy on Polythiophenes ..
确定





还剩12页未读,是否继续阅读?
香港环球分析测试仪器有限公司为您提供《光电材料中光电极的动态检测方案(电化学工作站)》,该方案主要用于其他中光电极的动态检测,参考标准--,《光电材料中光电极的动态检测方案(电化学工作站)》用到的仪器有CIMPS 可控强度调制光电化学谱仪/光电化学测试系统
推荐专场
相关方案
更多
















