方案详情
文
Infrared-visible sum-frequency generation (SFG) vibrational spectra were obtained from the conducting form of polyaniline (PANI), emeraldine salt (ES). Observation of the SFG signal indicated the presence of oriented non-planar structures in ES. It was suggested that the additional resonant enhancement due to the absorption of the visible beam by the polymer film operated in the investigated system. Resonances characteristic to different structural units, benzenoid rings and polarons, were detected in the SFG spectra. The C-C stretching and C-H deformation vibrations of benzenoid rings were detected at 1623 and 1192 cm–1, while the polaronic ν(C~N+) vibrational mode was observed at 1336 cm–1. The stretching vibration of N-H bond was detected at 3273 cm–1 in the ssp-polarized SFG spectrum, indicating that the neighboring polymer chains in ES are connected by the N-H•••N interchain bridges
方案详情

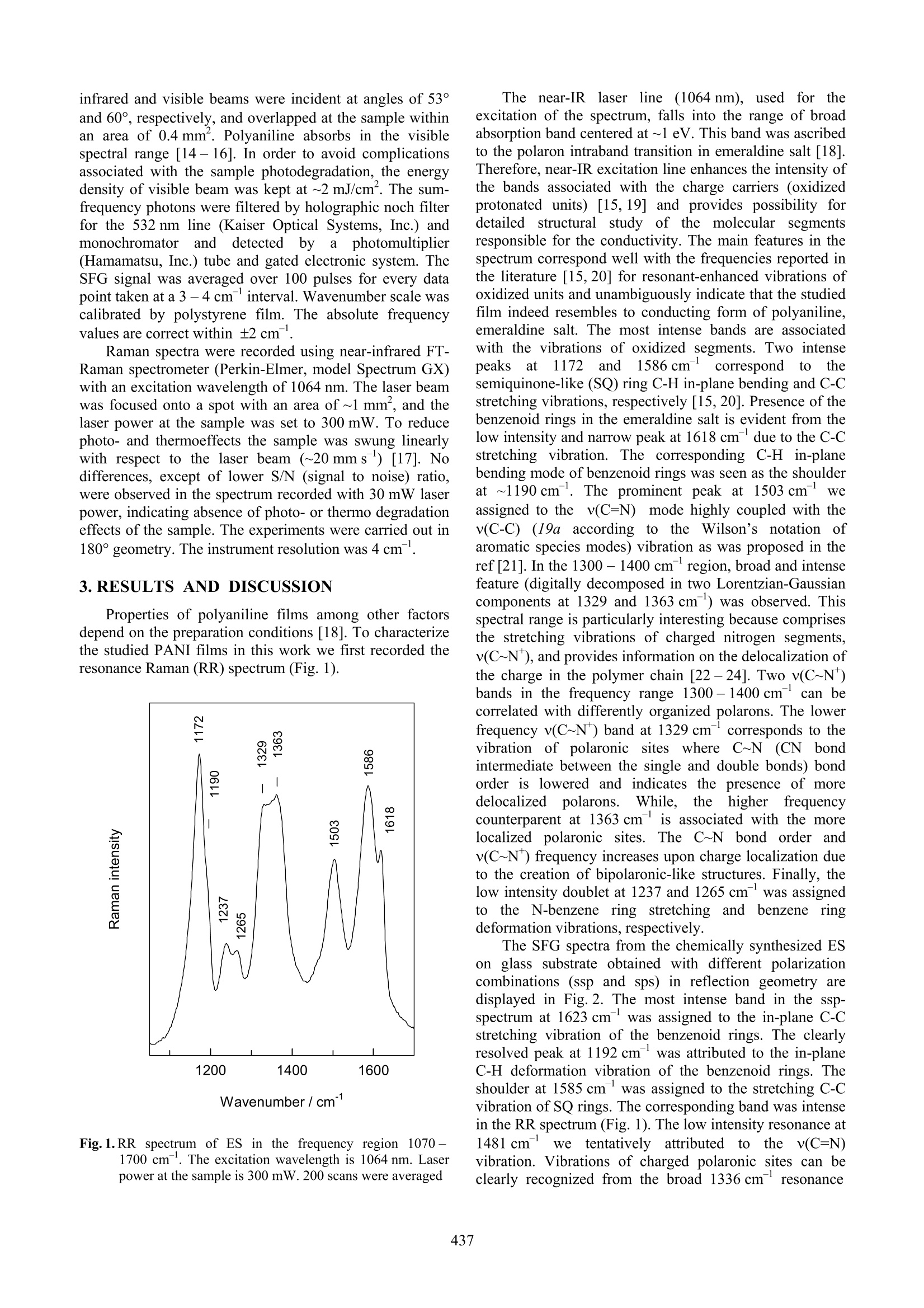
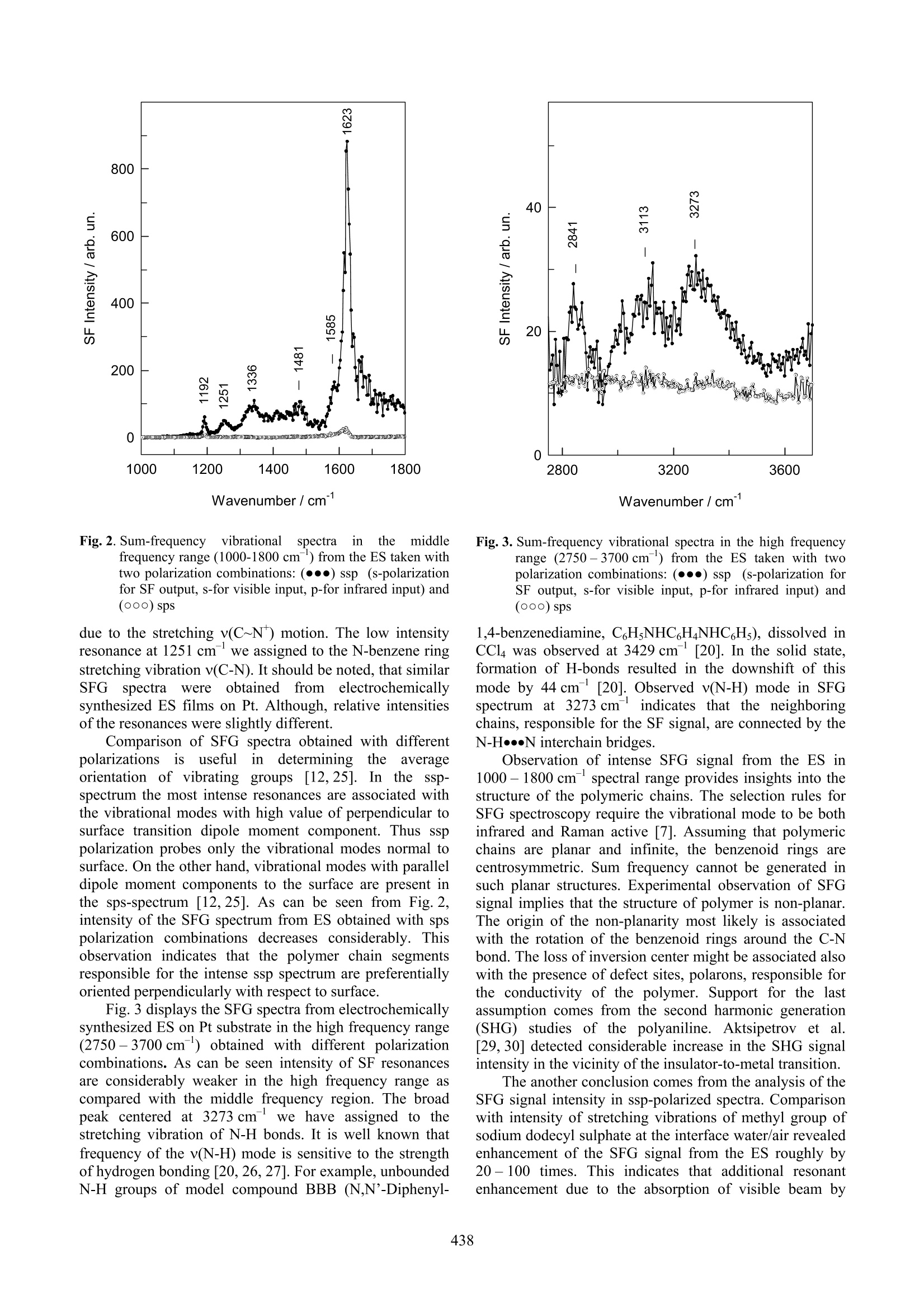
ISSN 1392-1320 MATERIALS SCIENCE (MEDZIAGOTYRA). Vol.9,No. 4. 2003 Application of Sum-Frequency Generation Spectroscopyfor the Structural Studies of Polyaniline G.Niaural*, Z. Kuprionis’, V.Kod,R. Mazeikiene, A. Malinauskas Institute of Chemistry, Gostauto 9, LT-2600 Vilnius, Lithuania EKSPLA Ltd., Savanoriu Av. 231, LT-2053 Vilnius, Lithuania Received 30 September 2003;accepted 21 October 2003 Infrared-visible sum-frequency generation (SFG) vibrational spectra were obtained from the conducting form ofpolyaniline (PANI), emeraldine salt (ES). Observation of the SFG signal indicated the presence of oriented non-planarstructures in ES. It was suggested that the additional resonant enhancement due to the absorption of the visible beam bythe polymer film operated in the investigated system. Resonances characteristic to different structural units, benzenoidrings and polarons, were detected in the SFG spectra. The C-C stretching and C-H deformation vibrations of benzenoidrings were detected at 1623 and 1192 cm , while the polaronic v(C~N*) vibrational mode was observed at 1336 cm .The stretching vibration of N-H bond was detected at 3273 cm in the ssp-polarized SFG spectrum, indicating that theneighboring polymer chains in ES are connected by the N-H·N interchain bridges. Keywords: sum frequency generation, SFG, polyaniline, emeraldine salt. 1.INTRODUCTION Conducting polymer polyaniline (PANI) has beenamong the most studied polymers, because of its excellentenvironmental stability [1] and technological importance inthe fields of catalysis [2], biosensors [3], batteries [4], andelectronic technology[5]. An understanding of thestructure of the polymer in the conducting state requiresexperimental technique sensitive to the organization ofpolymer at molecular-level. Detailed information on thestate and structure of polyaniline backbone might beobtained from vibrational spectroscopic studies. Among the experimental techniques suitable for suchstudies.,VvibrationalalSsum-frequency generation(SFG)spectroscopy seems particularly attractive. SFG emerges in1987 [6] as the nonlinear interface specific vibrationalprobe with submonolayer sensitivity. Surface sensitivityand molecular specificity are the major advantages of SFGtechnique [7]. Surface sensitivity comes from the fact thatthe nonlinear generation of the sum-frequency signal fromthe overlapped visible and infrared beams is forbidden inthe media of randomly oriented molecules or in thecentrosymmetric media, but is allowed at the interfaceswhere inversion symmetry is necessary broken [7].Molecular specificity of the technique arises from thepossibility to record vibrational spectrum. Intensity of SFGsignal enhances resonantly whenever the frequency ofinfrared or visible beam coincides with vibrational orelectronic transition in molecule. Because frequency of theinfrared beam is tunable in the wide frequency range theobserved resonances correspondto the vibrationalspectrum of molecule. Only vibrations those allowed bothin the infrared and Raman spectra are monitored by SFGtechnique [7]. The successful applications of SFG spectroscopy forthe analysis of the surfaces of polymers have been recently ( C orresponding author. Tel.:+370-5-2729642; fa x : +370-5-2617018. E-mail address: gniaura@ktl.mii.lt (G.Niaura) ) reported [8-12]. In such studies the surface compositionand surface structure of biopolymer blends as a function oftemperature and bulk blend concentration [8 -11], and theorientation as well as conformation of alkyl side chains ofpolyimide [12] were analyzed. In this manuscript we report the SFG spectra ofemeraldine salt (ES). To the best of our knowledge, therewas no report regarding the SFG study of polyaniline. 2.EXPERIMENTAL Aniline has been distilled before use. PI-50-1 modelpotenciostat, arranged with a PR-8 model programmer wasused in the electrochemical experiments. In this work wehave used two type of polyaniline samples: (a) electro-chemically synthesized, and (b) chemically synthesized.Electrochemical polymerization has been performed bydeposition of the polyaniline on the Pt plate (10×50 mm)from the 0.5 M H2SO4 +0.05M aniline solution, at aconstant anodic current density of~ 1.5 mA/cm, appliedfor 5 min. Chemical polymerization has been performed bytwo steps [13]. It was demonstrated that the processproceeds through the autocatalytic mechanism [13]. First,polymer was deposited on the glass plates (25×76 mm)from the 22 mL solution containing 0.5 M HSO4 +0.05 Maniline +0.0025 MK2Cr2O, for 1 hour. In the second step.an additional layer was deposited from the newly preparedthe same solution for 15 min. After the polymerization thesamples were placed in the 1 M HCl solution for 5 min andthen dried. The SFG spectrometer is based on a model-lockedPL2143A Nd:YAG laser (EKSPLA Co.) generating 20 pspulses at 1064 nm with 10 Hz repetition rate. The secondharmonic radiation (532 nm) from this laser was used asvisible beam. The tunable infrared pulses in the 2.3-10um range with the energies of 200 -50 uJ respectivelywere produced in parametric generator PG401VIR/DFG(EKSPLA Co) by using third harmonic (355 nm) andfundamental radiations of the laser. The bandwidth ofinfrared beam was 6 cm. To produce SFG spectra the infrared and visible beams were incident at angles of 53°and 60°, respectively, and overlapped at the sample withinan area of 0.4 mm’. Polyaniline absorbs in the visiblespectral range [14-16]. In order to avoid complicationsassociated with the sample photodegradation, the energydensity of visible beam was kept at ~2 mJ/cm’. The sum-frequency photons were filtered by holographic noch filterfor the 532 nm line (Kaiser Optical Systems, Inc.) andmonochromator and detected by aphotomultiplier(Hamamatsu, Inc.) tube and gated electronic system. TheSFG signal was averaged over 100 pulses for every datapoint taken at a 3-4 cm-interval. Wavenumber scale wascalibrated by polystyrene film. The absolute frequencyvalues are correct within ±2 cm. Raman spectra were recorded using near-infrared FT-Raman spectrometer (Perkin-Elmer, model Spectrum GX)with an excitation wavelength of 1064 nm. The laser beamwas focused onto a spot with an area of~1 mm, and thelaser power at the sample was set to 300 mW. To reducephoto- and thermoeffects the sample was swung linearlywith respect to the laser beam (~20 mms ) [17]. Nodifferences, except of lower S/N (signal to noise) ratio,were observed in the spectrum recorded with 30 mW laserpower, indicating absence of photo- or thermo degradationeffects of the sample. The experiments were carried out in180° geometry. The instrument resolution was 4 cm . 3.RESULTS AND DISCUSSION Properties of polyaniline films among other factorsdepend on the preparation conditions [18]. To characterizethe studied PANI films in this work we first recorded theresonance Raman (RR) spectrum (Fig. 1). Fig.1.RR spectrum of ES in the frequency region 1070 -1700 cm . The excitation wavelength is 1064 nm. Laserpower at the sample is 300 mW. 200 scans were averaged The near-IRlaser line (1064 nm),, usedf. for theexcitation of the spectrum, falls into the range of broadabsorption band centered at ~1 eV. This band was ascribedto the polaron intraband transition in emeraldine salt [18].Therefore, near-IR excitation line enhances the intensity ofthe bands associated with the charge carriers (oxidizedprotonated units) [15, 19] and provides possibility fordetailed structural study of the molecular segmentsresponsible for the conductivity. The main features in thespectrum correspond well with the frequencies reported inthe literature [15,20] for resonant-enhanced vibrations ofoxidized units and unambiguously indicate that the studiedfilm indeed resembles to conducting form of polyaniline,emeraldine salt. The most intense bands are associatedwith the vibrations of oxidized segments. Two intensepeaksat11722and1586cm correspondtootlthesemiquinone-like (SQ) ring C-H in-plane bending and C-Cstretching vibrations, respectively [15,20]. Presence of thebenzenoid rings in the emeraldine salt is evident from thelow intensity and narrow peak at 1618 cm due to the C-Cstretching vibration. The corresponding, C-H in-planebending mode of benzenoid rings was seen as the shoulderat ~1190 cm. The prominent peak at 1503 cm weassigned to the v(C=N) mode highly coupled with thev(C-C) (19a according to the Wilson’s notation ofaromatic species modes) vibration as was proposed in theref [21]. In the 1300 -1400 cm region, broad and intensefeature (digitally decomposed in two Lorentzian-Gaussiancomponents at 1329 and 1363 cm ) was observed. Thisspectral range is particularly interesting because comprisesthe stretching vibrations of charged nitrogen segmentsv(C~N), and provides information on the delocalization ofthe charge in the polymer chain [22-24]. Two v(C~N)bands in the frequency range 1300-1400 cmcan becorrelated with differently organized polarons. The lowerfrequency v(C~N*) band at 1329 cmcorresponds to thevibration of polaronic sites where C~N (CN bondintermediate between the single and double bonds) bondorder is lowered and indicates the presence of moredelocalizedpolaronSs. While.tthehigherrfrequencycounterparent at 1363 cm is associated with the morelocalized polaronic sites. TThhee CC~N bond order andv(C~N) frequency increases upon charge localization dueto the creation of bipolaronic-like structures. Finally, thelow intensity doublet at 1237 and 1265 cm-was assigned. to the N-benzene ring stretching and benzeneringdeformation vibrations, respectively. The SFG spectra from the chemically synthesized ESon glass substrate obtained with different polarizationcombinations (ssp and sps) in reflection geometry aredisplayed in Fig. 2. The most intense band in the ssp-spectrum at 1623 cm was assigned to the in-plane C-Cstretching vibration of the benzenoid rings. The clearlyresolved peak at 1192 cmwas attributed to the in-planeC-H deformation vibration of the benzenoid rings. Theshoulder at 1585 cm-’ was assigned to the stretching C-Cvibration of SQ rings. The corresponding band was intensein the RR spectrum (Fig. 1). The low intensity resonance at1481 cmwetentatively attributedl to tthhee v(C=N)vibration. Vibrations of charged polaronic sites can beclearly recognized from the broad 1336 cmresonance Wavenumber/cm-1 Fig.2. Sum-frequencyy vibrationalspectrain themiddlefrequency range (1000-1800 cm) from the ES taken withtwo polarization combinations: (·●) ssp (s-polarizationfor SF output, s-for visible input, p-for infrared input) and(ooo) sps due to the stretching v(C~N) motion. The low intensityresonance at 1251 cmwe assigned to the N-benzene ringstretching vibration v(C-N). It should be noted, that similarSFGspectra were(obtained from electrochemicallysynthesized ES films on Pt. Although, relative intensitiesof the resonances were slightly different. Comparison of SFG spectra obtained with differentpolarizations1is usefulirindetermining theaverageorientation of vibrating groups[12,25]. In the ssp-spectrum the most intense resonances are associated withthe vibrational modes with high value of perpendicular tosurface transition dipole moment component. Thus ssppolarization probes only the vibrational modes normal tosurface. On the other hand, vibrational modes with paralleldipole moment components to the surface are present inthe sps-spectrum [12,25]. As can be seen from Fig. 2,intensity of the SFG spectrum from ES obtained with spspolarization combinations decreases considerably. Thisobservation indicates that the polymer chain segmentsresponsible for the intense ssp spectrum are preferentiallyoriented perpendicularly with respect to surface. Fig.3 displays the SFG spectra from electrochemicallysynthesized ES on Pt substrate in the high frequency range(2750-3700cm) obtained with different polarizationcombinations. As can be seen intensity of SF resonancesare considerably weaker in the high frequency range ascompared with the middle frequency region. The broadpeak centered at 3273 cm we have assigned to thestretching vibration of N-H bonds. It is well known thatfrequency of the v(N-H) mode is sensitive to the strengthof hydrogen bonding [20, 26,27]. For example, unboundedN-H groups of model compound BBB (N,N'-Diphenyl- Fig.3. Sum-frequency vibrational spectra in the high frequencyrange (2750-3700cm) from the ES taken with twopolarization combinations: (·●) ssp (s-polarization forSF output, s-for visible input, p-for infrared input) and(ooo)sps 1,4-benzenediamine, CHNHC,HNHC,Hs), dissolved inCCl was observed at 3429 cm [20]. In the solid state,formation of H-bonds resulted in the downshift of thismode by 44 cm[20]. Observed v(N-H) mode in SFGspectrum at3273 cm indicates that the neighboringchains, responsible for the SF signal, are connected by theN-H···N interchain bridges. Observation of intense SFG signal from the ES in1000-1800cm'spectral range provides insights into thestructure of the polymeric chains. The selection rules forSFG spectroscopy require the vibrational mode to be bothinfrared and Raman active [7]. Assuming that polymericchains are planar and infinite, the benzenoid rings arecentrosymmetric. Sum frequency cannot be generated insuch planar structures. Experimental observation of SFGsignal implies that the structure of polymer is non-planar.The origin of the non-planarity most likely is associatedwith the rotation of the benzenoid rings around the C-Nbond. The loss of inversion center might be associated alscwith the presence of defect sites, polarons, responsible forthe conductivity of the polymer. Support for the lastassumption comes from the second harmonic generation(SHG) studies of the polyaniline. Aktsipetrov et al.[29,30] detected considerable increase in the SHG signalintensity in the vicinity of the insulator-to-metal transition. The another conclusion comes from the analysis of theSFG signal intensity in ssp-polarized spectra. Comparisonwith intensity of stretching vibrations of methyl group ofsodium dodecyl sulphate at the interface water/air revealedenhancement of the SFG signal from the ES roughly by20 -100 times. This indicates that additional resonantenhancement due to the absorption of visible beam by the polymer operates in the case of ES. Possibility ofsuch double-resonant infrared-visible sum-frequencygeneration spectroscopyy was recently demonstrated byRasche et al. [31]. 4.CONCLUSIONS We have studied the structure of the conducting formof polyaniline, emeraldine salt, by the non-linear infrared-visible sum-frequency generation technique. Intense ssp-polarized SFG spectrum in the 1000-1800 cm-frequencyregion from the ES was observed for the first timee. It was suggesteddthat the additionalsignalenhancement comes from the resonance of visible beamwith the electronic transition in the polymer. Because SFG is forbidden in centrosymmetric orrandomly oriented media, the observation of SFG spectraindicates the deformation of the polymeric chains from thecentrosymmetric geometry. Itwassuggested that therotation of benzenoid rings around the C-N bond and thepresence of charged defects, polarons, are responsible forthe structural transformations in the ES. Characteristic stretchingand in-plane deformationvibrations of benzenoid rings were observed in the SFGspectra at 1623 and 1192 cm ,respectively. The stretchingvibration of C~N (CN bond intermediate between thesingle and double bonds) bond was detected at 1336 cm. Considerably lower intensity SFG resonances wereobserved in the high frequency region (2750-3700 cm).Based on the stretching N-H bond vibrational frequency,detectedatit3273 cm, itwasscconcluded1thattheneighboring chains in the polymer are connected by theN-HN interchain bridges. Presented data show that SFG spectroscopy opens thenew and sensitive approach for studies of structural defectsand non-planarity of polyaniline backbone. Acknowledgments This work was supported by Lithuanian State Scienceand Studies Foundation. We gratefully acknowledge theDepartment of Bioelectrochemistry and Biospectroscopy atthe Institute of Biochemistry (Vilnius) for the possibility touse the FT-Raman spectrometer. REFERENCES ( 1. C hen, S. A., Fang, W. G. E lectrically y C onductive P olyaniline Poly(vinyl A lcohol) Composite Films - P hysical P roperties and Morphological S tructures Macromolecules24 1 991:pp.1242-1248. ) ( 2. M alinauskas, A. E l ectrocatalysis at Conducting Polymers Synth. Met. 107 1 1999: pp.75-83. ) ( 3. M cQuade, D. T., Pullen, A. E., S wager, T. M . Conjugated Polymer-Based Chemical Senso s r s Chem. Rev. 1 1002000: pp.2537- 2 574. ) ( 4. Novak,P., Muller, K., Santhanam,K. S. V ., H aas, O . E lectrochemically Active Polymers for Rechar geable Batteries Chem.Rev. 97 1997: p p.207-281. ) ( 5. MacDiarmid, A. G. Polyaniline and Polypyrrole: W here areWe Headed? S ynth. Met. 84 1997:pp.27-34. ) 6. Zhu, X.D., Suhr, H., Shen, Y. R. Surface VibrationalSpectroscopy by Infrared-Visible Sum FrequencyGeneration Phys. Rev. B 355 1987:pp.3047-3050. ( 7. Shen, Y. R. Wave Mixing Spectroscopy for Surface StudiesSolid State Commun.102 1997: pp.221-22 9 . ) ( 8. Zhang,D., G r acias,D. H .,Ward,R., G a uckler, M.,Tian, Y ., Shen, Y. R., S omorjai, G. A. Surface S t udies o f Polymer Blends by S un Frequency V ibrational Spectroscopy, Atomic M icroscopy, and ( Contact AngleGoniometry J. Phys. Chem. B 102 1998: pp.6225-6230. ) ( 9. Gracias, D. H., Chen, Z., S hen,Y. R., Somorjai, G. A. M olecular Characterization of Polymer and Polymer BlendSurfaces. Combined S um F r equency Generation S u rfaceVibrational Spectroscopy and Scanning Force Microscopy Studies Acc. Chem.Res. 32 1999: pp.930-940. ) ( 10. C hen, Z., Ward, R. , Tian, Y., Eppler, A. S. , Shen, Y. R.,Somorjai, G. A . S u rface Composition n of B iopolymer Blends Biospan-SP/Phenoxy yand Biospan-F/Phenoxy Observed with SFG, XPS, and Contact Angle GoniometryJ. Phys. Chem . B 103 1 999: pp.2935-2942. ) ( 1. Chen, Z., G racias,D. H., S omorjai, G. A. Sum Frequency Generation (SFG)-Surface Vibrational Spectroscopy Stu d ies of B uried I n terfaces: C atalytic R e action In t ermediates on T ransition Metal Crystal Surfaces at High R eactant P res- sures; Polymer Surface Structures at t h e S o lid-Ga s a n dSolid-Liquid I nterfaces Appl. P hys. B 68 1999:pp. 549 - 557. ) ( 12. ( . Oh-e,M., L vovsky,A. I., Wei, X., Shen, Y. R. R . s Sum- F requency G eneration (SFG) V i brational Spectroscopy of Side : Alkyl C hain Structuresof P olyimide Surfaces J. Chem. Phys. 19 2000: pp.8827-8832. ) ( 13. 5 . M azeikiene, R., Malinauskas, A. Deposition of Polyanilineon G lass and P latinum b y Autocatalytic Oxidation of Aniline with Dichromate Synth. Met . 1 08 2 000: pp. 9- 14. ) ( 14 + . . J Malinauskas, A. Chemical Deposition o f C o nducting Polymers Polymer 4 2 2 001: p p.3957-3972. ) ( 15. Q uillard,S.,Berrada,K., L ouarn, G., .,1 L efrant, S.,Lapkowski, M., P ron, A. In Situ Raman Spectroscopic Studies of the Electrochemical Behavior of Polyaniline New J. Chem . 19 1995: pp.365-374. ) ( 16. N Jekrasov, A . A., Ivanov, V. F., Vannikov, A. V. I solation of Individual Components i n t h e E l ectronic A b sorptionSpectra of Polyaniline from t he Spectroelectrochemical DataRuss. J. Electrochem. 36 2000: p p.883-888. ) ( 17. N iaura, G.,Gaigalas, A. K., Vilker, V. L. Moving Spectroelectrochemica l Cell for Surface Raman S p ectroscopyJ. Raman Spectrosc . 28 1997: pp. 1009- 1 011. ) ( 18. C hinn,D . , D u Bow,J., Li,J., J an ata,J., Josowicz, M.Comparison of Chemically an d Electrochemically Prepared P olyaniline F ilms. 1 . E lectrical Properties Chem. M atter. 7 1 1995: pp.1504-1509. ) ( 19. E] ngert, C., Umapathy,S., Ki efer, W., Hamaguchi, H. Dynamic Structure o f Charge Ca r rier in Po l yaniline by Near-Infrared Excited R esonance R aman S p ectroscopyChem. Phys. Lett.218 1994: pp. 87-92. ) 20. Gruger, A., Novak, A., Regis, A., Colomban, Ph. Infraredand Raman Study of Polyaniline. Part II: Influence of OrthoSubstituents on Hydrogen Bonding and UV/VIS-Near-IRElectron Charge Transfer J. Mol. Struct. 328 1994:pp.153-167. ( 21. F olch, S., Gruger, A., Regis, A. , Colomban, Ph. Optical and V ibrational S pectra o f S o ls/Solutions of Polyaniline: Water as Secondary Dopant Synth. Met. 81 1996: pp.221 - 225. ) ( 22. B ernard,M. C., Hugot-Le Goff, A. Electrochromic P erformance of Polyaniline Films du r ing their Cycling in apH 3 Electrolyte J. Electrochem. Soc. 141 1994: pp.2682 -2 689. ) ( 2 3 . Bernard,M. C., Bich,V.T., Hugot-Le Goff, A. Resonant R aman Identification o f the Polaronic Organization in PANI Synth. Met. 1 101 1 1999: pp.811- 8 12. ) ( 24. B oyer,M.L., Quillard,S., Louarn, G., , Froyer, G., L efrant, S. Vibrationa l Study of t h e FeCl-Doped Dimmerof Polyaniline; A Good Model Compound o f E meraldine Salt J. Phys. Chem. B 104 2 000: pp.8952-8961. ) ( 25. W alker, R. A., Smiley, B. L., Richmond, G. L. V ibrationalSum F r equency Spectroscopy at Li q uid: Liquid In t erfaces Spectroscopy 1 41 999: pp.18-28. ) ( 26 . F urukawa, Y ., Ueda,F., Hyodo,Y., Harada, I., Nakajima,T., Kawagoe, T. l. V ibrational S pectra and Structure o f P olyaniline Macromlecules 21 1988:pp. 1297- 1 305. ) ( 27. Angelopoulos,M., Dipietro, R., Zheng, W. G.,MacDiarmid, A. G., Epstein, A. J. Effect of SelectedProcessing on Solution Propertie s an d Morphology of Polyaniline and I mpact on Conductivity Synth. Met. 841997: pp. 35-39. ) ( 28. Wei, X., H ong, S. C., Lvovsky, A. I., Held, H., Shen, Y . R . Evaluation of Surface vs Bulk Contributions in Sum-Frequency V i brational S p ectroscopy Us i ng Reflection andTransmission GeometriesJ. P hys. Chem. B 104 2000: pp. 3349 - 3354. ) ( 29. . A ktsipetrov,O. A., V e reta, V.V., Daikhin,L. I., Levi,M. D., Ermushev, A. V., Petukhov, A. V. Generation of R eflected 2nd Harmonic and I n sulator-Metal Transition i n Conducting Polymer Films JETP Lett. 54 1991: pp. 171 - 174. ) 30. Aktsipetrov,O., Ermushev, A., Petukhov, A., Daikhin,L., Levi, M., Vereta, V.2"dOptical Harmonic Generation inthe Vicinity of Insulator-to-Metal Transition in PolyanilineFilm Synth. Met. 54i41993: pp.327-330. ( 31. R ] aschke, M. B., Hayashi,M., L Lin,S. H. , S hen, Y. R. Doubly-Resonant Sum- Frequency Generation Spectroscopy for Surface Studies C hem. Phys. Lett. 359 2002: pp.367-3 7 2. )
确定



还剩3页未读,是否继续阅读?
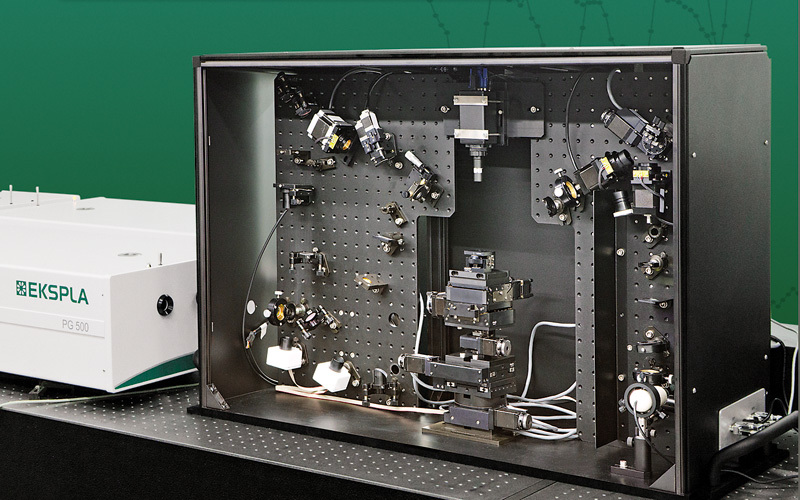
北京欧兰科技发展有限公司为您提供《聚苯胺中聚苯胺结构检测方案(其它光谱仪)》,该方案主要用于其他中聚苯胺结构检测,参考标准--,《聚苯胺中聚苯胺结构检测方案(其它光谱仪)》用到的仪器有Ekspla SFG 表面和频光谱分析系统、Ekspla PL2230型高能量皮秒激光器、Ekspla CARS 相干反斯托克斯拉曼显微光谱仪
推荐专场
相关方案
更多
该厂商其他方案
更多