
方案详情
文
甲酰胺(Formamide)是甲酸衍生出的酰胺,分子式为HCONH2。它是无色液体,与水混溶,有与氨类似的气味。主要用于生产磺胺类药物,合成维生素及用作纸张和纤维的软化剂。纯的甲酰胺可以溶解许多不溶于水的离子化合物,因此也被用作溶剂。
方案详情

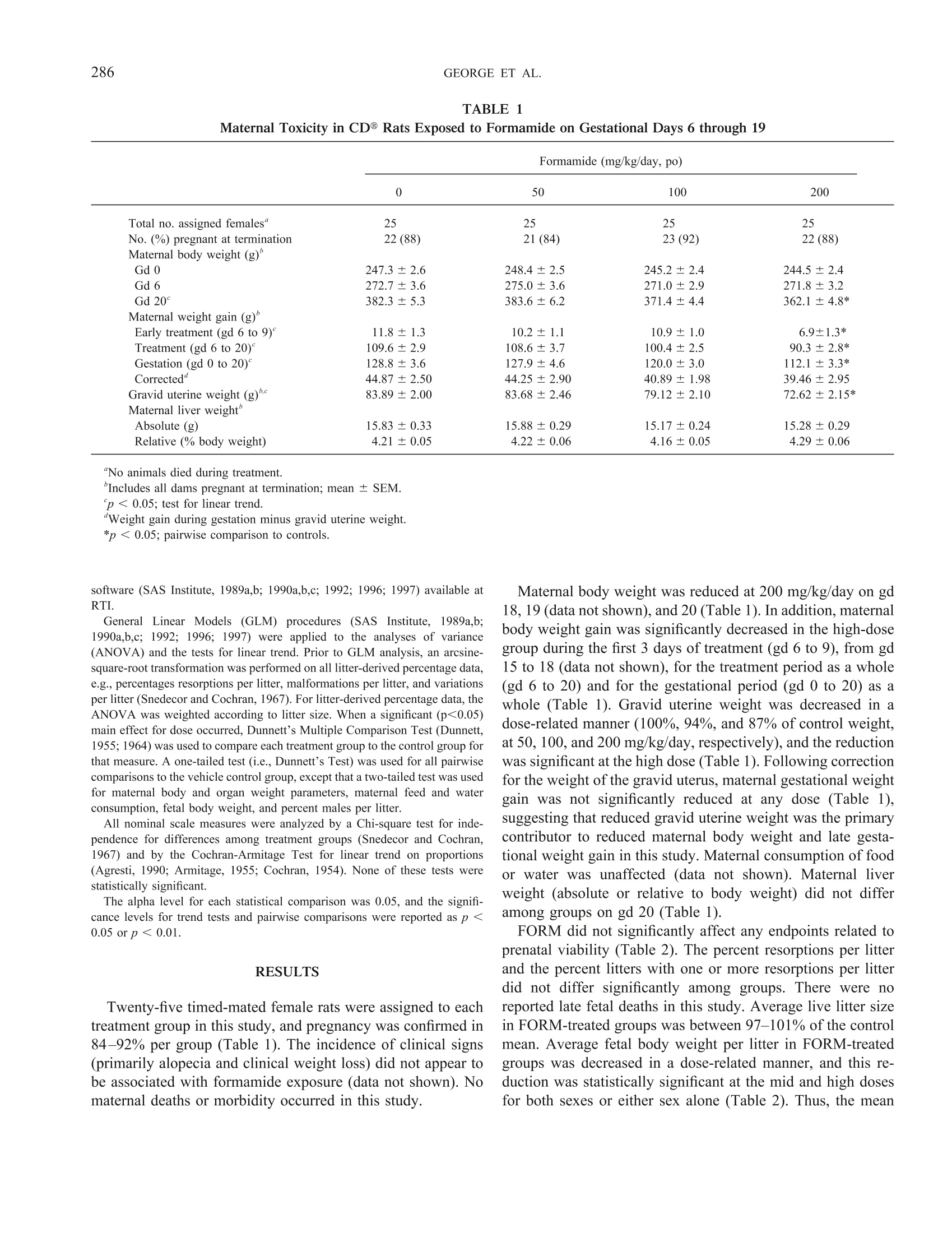
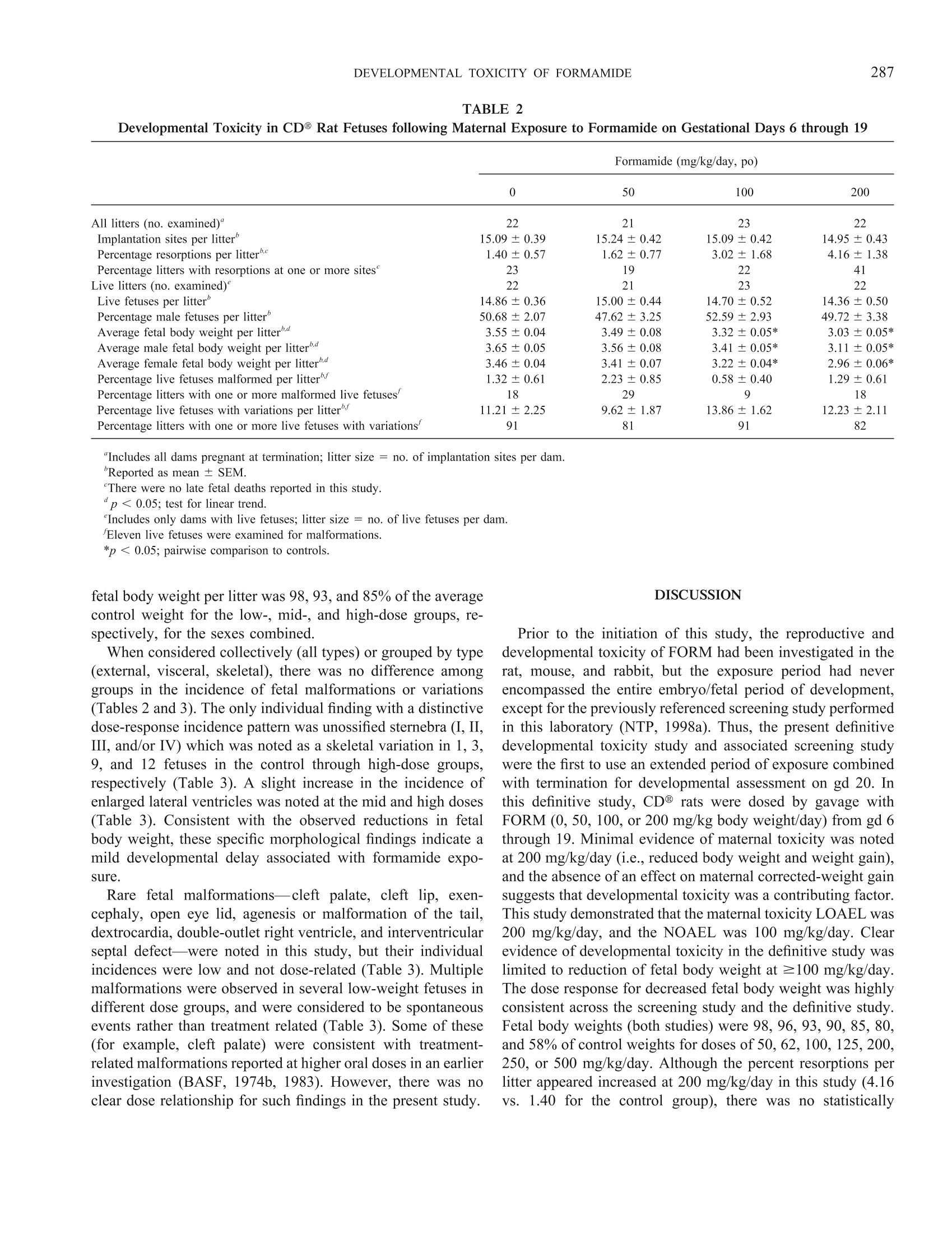
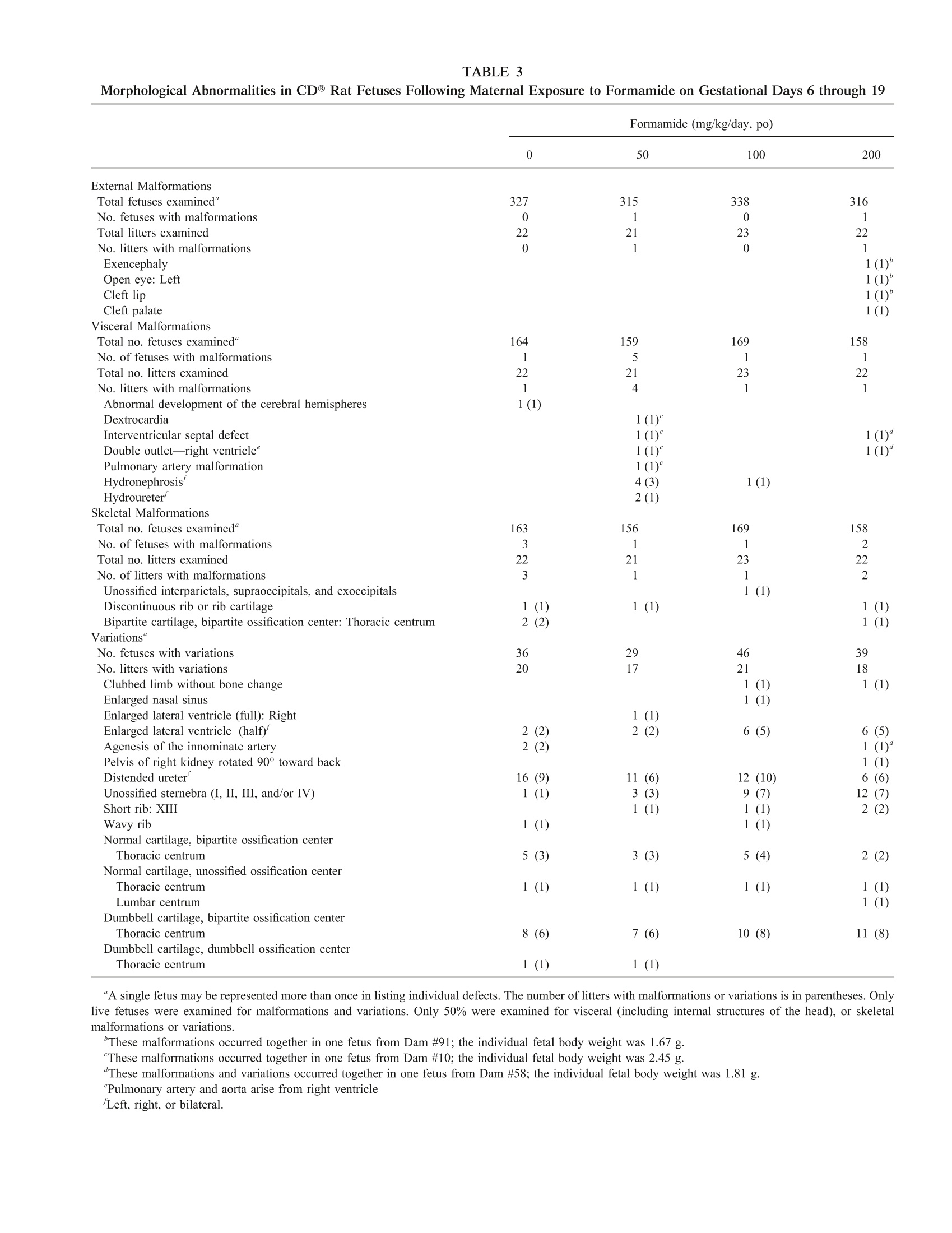
TOXICOLOGICAL SCIENCES 57, 284-291 (2000)Copyright C 2000 by the Society of Toxicology 285DEVELOPMENTAL TOXICITY OF FORMAMIDE 284 Evaluation of the Developmental Toxicity of Formamidein Sprague-Dawley (CD) Rats Julia D. George, Catherine J. Price, Melissa C. Marr, Christina B. Myers, and Gloria D. Jahnke* Chemistry and Life Sciences, Research Triangle Institute, Post Office Box 12194, Research Triangle Park, North Carolina 27709-2194; and*Developmental and Reproductive Toxicology Group, National Toxicology Program, National Institute of Environmental Health Sciences,Post Office Box 12233, Research Triangle Park, North Carolina 27709 Received April 10, 2000; accepted June 28, 2000 Timed-pregnant CD@ outbred albino Sprague-Dawley rats re-ceived formamide (50, 100, or 200 mg/kg/day) or vehicle (5 ml/kgdeionized/distilled water, po) on gestational days (gd) 6 through19. Maternal food and water consumption (absolute and relative),body weight, and clinical signs were monitored at regular intervalsthroughout gestation. At termination (gd 20), confirmed-pregnantfemales (21-23 per group) were evaluated for clinical status andgestational outcome; live fetuses were examined for external,vis-ceral, and skeletal malformations and variations. There were nomaternal deaths and no dose-related clinical signs. At 200 mg/kg/day, maternal body weight on gd 20, weight gain, and graviduterine weight were significantly decreased. Maternal weight gain,corrected for gravid uterine weight, liver weight (absolute or rel-ative), and food and water consumption (absolute or relative),were not affected. Formamide did not affect prenatal viability orincidences of fetal malformations or variations. Average fetal bodyweight/litter was decreased at 100 and 200 mg/kg/day. Fetal bodyweight was affected at lower daily doses than in previously pub-lished studies, possibly due to the longer total exposure periodand/or lack of a recovery period between cessation of exposure andtermination. In summary, the maternal toxicity no-observed-ad-verse-effect level (NOAEL) was 100 mg/kg/day and the low ob-served adverse effect level (LOAEL) was 200 mg/kg/day under theconditions of this study. Similarly, the developmental toxicityNOAEL was 50 mg/kg/day and the LOAEL was 100 mg/kg/day. Key Words: formamide; developmental toxicity; teratogenicity;rats; morphological development. Formamide (FORM) is used as an industrial solvent inorganic synthesis reactions, and as an intermediate in themanufacture of dyes and pigments (ITII, 1988; Sax and Lewis,1987; Windholz et al., 1983). It is also used as a softener forpaper, gums, and animal glues; as an ionizing and pharmaceu-tical solvent; in ink solutions; and as a versatile syntheticreagent. ACGIH (1999) has set the TLV for formamide at 10 ( T his study was p resented a t the 39th Annual M eeting of the Te r atology S ociety, Keystone, C olorado [Teratology 5 9(6), 41 2 (Abstract p. 49) 1 999]. ) ( To whom co r respondence should be addressed a t Hermann Laboratory B uilding, P .O. B ox 1 2 194, R e search Tr i angle Institute, R esearch T r iangle P ark, NC 27709-2194. Fax: (9 1 9) 54 1 -6499. E-m a il: jdg 0 1@rti.org. ) ppm, and the NIOSH TWA is also 10 ppm (15 mg/m’for skin)(NIOSH, 2000). Documented incidences of exposure or quan-titative occupational monitoring for formamide exposures werenot found in the recently published literature, except by indi-rect exposure to alkylformamides (see below). Formamide is generated in vivo as a metabolite of structur-ally related alkylformamides, which have medicinal and/orindustrial uses. In mice, formamide was one of several metab-olites found in the plasma and urine after exposure to N-methylformamide (NMF; Ross et al., 1981; Gescher et al.,1982), a potential antitumor medication studied in recent clinictrials (Cody et al., 1992; Del Bufalo et al., 1994). N-methyl-formamide is also a potential photoproduct of the aqueousherbicide, fluridone, but environmental concentrations arelikely to be quite low (ppb range; Liu et al., 1989; West andTurner, 1988). More importantly, formamide is found as one ofthe major metabolites following exposure to N,N-dimethylfor-mamide (DMF; Mraz et al., 1989; Saillenfait et al., 1997),another widely used industrial solvent (Cheng et al., 1999;Lareo and Perbellini, 1995; Major et al., 1998; Ogata et al.,1997; Sakai et al., 1995). Human volunteers were exposed toDMF by a common route of occupational exposure (inhala-tion), and formamide accounted for ~8-24% of the total doseexcreted in the urine, and in laboratory animals (mouse, rat,hamster), formamide accounted for ~8-38% of the total dose(Mraz et al., 1989). The literature pertaining to the biologicaland toxicological effects of alkylformamides and their metab-olites is extensive, and a thorough review exceeds the scope ofthe present manuscript. Developmental toxicity of NMF andDMF has been reported in laboratory animals (see a review byKennedy 1986, and more recent studies including Hellwig etal., 1991;Kelich et al, 1995;Liu et al., 1989; Rickard et al.,1995; Saillenfait et al., 1997). The reproductive and developmental toxicity of FORM hasalso been investigated in mammalian species, including rats,mice, and rabbits (BASF, 1974a,b, 1983; Fail et al., 1998;Gliech 1974; Kennedy, 1986; Klauss et al., 1967, Merkle andZeller, 1980; Oettel and Frohberg, 1964; Oettel and Wilhelm,1957; Stula et al., 1992; Thiersch, 1962, 1971). However, treatment periods in previous developmental toxicity studiesdid not extend throughout the embryo/fetal period, as sug-gested by current guidelines for prenatal developmental toxic-ity testing (U.S. EPA, 1997, 1998; U.S. FDA, 1994b). Forexample, oral exposure to formamide (318 mg/kg/day) duringmajor organogenesis (gd 6-15) was associated with decreasedfetal body weight and increased fetal malformations in rats,including malformations of the vertebral column and ribs(BASF, 1974b, 1983). The widespread uses of FORM, as well as prior evidence ofdevelopmental toxicity for FORM and structurally related al-kylformamides, warranted determination of developmentaltoxicity LOAEL and NOAEL values based on a study designedin accordance with current regulatory guidelines. The results ofthe proposed investigation provide additional information rel-evant to the safety assessment of FORM exposure duringpregnancy, with particular focus on in utero growth, viability,and morphological development. The present study was de-signed to establish NOAELs and LOAELs for maternal anddevelopmental toxicity following daily oral exposure through-out the embryo/fetal period. Because formamide had beenpreviously studied using the shorter exposure period requiredby predecessor testing guidelines (i.e., major organogenesis),the influence of the longer exposure period on dose-rangeselection and outcome for various toxicity endpoints was alsoof interest. MATERIALS AND METHODS Animals and animal husbandry..This study was conducted in accordancewith the Food and Drug Administration's“Good Laboratory Practice Regula-tions for Nonclinical Laboratory Studies”(U.S. FDA, 1988, 1994a). Copies ofthe final study report (NTP, 1998b) are available for a fee from the NationalTechnical Information Service, Springfield, VA 22161. The experimental animals were Sprague-Dawley-derived outbred albino rats[Crl:CD@ (SD)BR VAF/Plus@] (Charles River Laboratories, Inc., Raleigh,NC). Animals were individually identified by ear tag (National Band and TagCo., Newport, KY) during a 10-day quarantine. After quarantine, individualfemales were placed overnight in the home cage of a singly housed male of thesame stock for mating, and then examined by vaginal lavage the next morningfor the presence of sperm (Hafez, 1970). Female rats weighed from 222 to273 g on gd 0 (i.e., day of vaginal sperm detection). During the study, femaleswere housed singly in solid-bottom, polycarbonate cages with stainless steellids (Laboratory Products, Rochelle Park, NJ)@ and Certified Sani-Chip@hardwood cage litter (P.J. Murphy, Montville, NJ). Food (Purina CertifiedRodent Chow (#5002), PMI, St. Louis, MO) and tap water were provided adlibitum throughout the study. The light cycle (12-h light:dark), temperature,and relative humidity (RH) in the animal rooms were monitored, recorded, andcontrolled (Siebe/Barber-Colman Network 8000 System with SIGNAL@Software [Version 4.1]) throughout the study. The means (ranges) for temper-ature and humidity were 72°F (70.7-73.4°F) and 53.5%(48.2-58.5% RH),respectively. Time-mated females were assigned to dose groups by stratified randomiza-tion for body weight on gd 0, so that mean body weights across dose groupswere not significantly different on gd 0. Test chemical and treatment, The doses chosen for this study were 0, 50,100, and 200 mg FORM/kg/day. FORM (Obtained by Midwest ResearchInstitute through the sponsor, from Aldrich Chemical Company, Inc., Lot No. 15231AN) (CAS No. 75-12-7) of ≥99% purity was dissolved in deionized/distilled water. Identity and purity of the test material were confirmed byMidwest Research Institute (NIEHS Contract NO1-ES-55385).Identity wasconfirmed by infrared (IR) spectroscopy and H nuclear magnetic resonancespectroscopy. Purity was determined by gas chromatography (GC) and GC/mass spectrometry (NTP, 1998b). Bulk chemical re-analysis was performedwithin one week of study initiation. Identity was reconfirmed by IR spectros-copy and relative purity was 103.6% when compared by HPLC to a frozenreference sample (NTP, 1998b). Stability of the dosing solutions was verifiedfor the period of use, and they were verified to be within 97.9-100.3% of theirtheoretical concentrations by high performance liquid chromatography, prior toand after the period of administration (NTP, 1998b). Each dosing solution was coded so that treatment and examiTniantion ofanimals were performed without knowledge of the dose levels. The volumeadministered (5 ml/kg) was based on body weight, taken daily prior to dosing,during the dosing period. Dose selection was based on a screening study inwhich CD rats were exposed to FORM (0, 62,125,250,500,or 1000mg/kg/day) by gavage from gd 6 through 19 (NTP, 1998a). At 1000 mg/kg/day, excessive maternal toxicity resulted in termination of all dams by gd 14.At ≥250 mg/kg/day, maternal food and water intake were transiently reducedduring early treatment, with full recovery after gd 15. Maternal body weightand weight gains, including corrected maternal weight gain, were reduced at≥250 mg/kg/day. Incidences of prenatal mortality and morphological anom-alies were not affected. However, two fetuses in the 500-mg/kg/day groupexhibited unusual malformations (either cleft palate or agenesis of the tail).Gravid uterine weight was reduced at 500 mg/kg/day. Average fetal bodyweight per litter was 96,90, 80, and 58% of the control weight in the 62through 500-mg/kg/day groups, respectively. Thus, fetal body weight wassignificantly lower than controls at ≥125 mg/kg/day (NTP, 1998a). Based onthese results, the target doses for the definitive study were 0, 50, 100,and 200mg/kg/day. Observations.Animals were weighed on gd 0, then daily during treatmentfrom gd 6 through 19, and on gd 20. Food and water weights were taken at1-6-day intervals throughout the study, beginning on gd 0. Clinical signs wererecorded once daily prior to initiation of treatment, and twice daily during thetreatment period. Following termination by CO, asphyxiation on gd 20,maternal liver weight and gravid uterine weight were measured and ovariancorpora lutea were counted. Uterine contents were evaluated for the number ofimplantation sites, resorptions, late fetal deaths (i.e., fetuses with discernibledigits and weighing greater than 0.9 g, but displaying no vital signs at the timeof uterine dissection), and live fetuses. The uterus was stained to revealpossible early resorptions (Salewski, 1964) when visible evidence of preg-nancy was not apparent. Live fetuses were dissected from the uterus andanesthetized by inducing hypothermia (Blair, 1971; Danneman and Mandrell,1997; Lumb and Jones, 1973; Wixson and Smiler,1997). Each live fetus wascounted, weighed, and examined for external morphological abnormalities,including cleft palate. Approximately one-half of the fetuses were terminatedby decapitation and the remaining by evisceration under terminal cold anes-thesia. Approximately one-half of the fetal carcasses were sexed and examined forvisceral morphological abnormalities using a fresh tissue dissection method(Staples, 1974; Stuckhardt and Poppe, 1984). The same fetal carcasses weredecapitated prior to dissection. Fetal heads were fixed and decalcified inBouin’s solution and subsequently examined using a free-hand sectioningtechnique (Wilson, 1965). All fetal carcasses were eviscerated (and sex deter-mined for those not scheduled for a full visceral morphological examination),and the skeletons macerated and stained with alcian blue/alizarin red-S stain(Marr et al., 1988). Intact fetal skeletons (i.e., those fetuses that were notdecapitated) were examined for skeletal morphological abnormalities. Statistical analyses.The unit for statistical measurement was the pregnantfemale or the litter. Quantitative continuous data (e.g., maternal body weights,fetal body weights, feed consumption, etc.) were compared among treatmentgroups by parametric statistical tests whenever Bartlett’s test for homogeneityof variance was not significant. Statistical analyses were based on SASO TABLE BMaternal Toxicity in CD@ Rats Exposed to Formamide on Gestational Days 6 through 19 Formamide (mg/kg/day, po) 0 50 100 200 Total no. assigned females" 25 25 25 25 No. (%) pregnant at termination 22(88) 21(84) 23(92) 22(88) Maternal body weight (g)" Gd0 247.3±2.6 248.4±2.5 245.2±2.4 244.5±2.4 Gd 6 272.7±3.6 275.0±3.6 271.0±2.9 271.8±3.2 Gd 20° 382.3±5.3 383.6±6.2 371.4±4.4 362.1±4.8* Maternal weight gain (g)' Early treatment (gd 6 to 9)" 11.8±1.3 10.2±1.1 10.9±1.0 6.9±1.3* Treatment (gd 6 to 20)° 109.6±2.9 108.6±3.7 100.4±2.5 90.3±2.8* Gestation (gd 0 to 20)° 128.8±3.6 127.9±4.6 120.0±3.0 112.1±3.3* Corrected" 44.87±2.50 44.25±2.90 40.89±1.98 39.46±2.95 Gravid uterine weight (g) 83.89±2.00 83.68±2.46 79.12±2.10 72.62±2.15* Maternal liver weight Absolute (g) 15.83±0.33 15.88±0.29 15.17±0.24 15.28±0.29 Relative (% body weight) 4.21±0.05 4.22±0.06 4.16±0.05 4.29±0.06 “No animals died during treatment."Includes all dams pregnant at termination; mean ± SEM.p< 0.05; test for linear trend.“Weight gain during gestation minus gravid uterine weight. software (SAS Institute, 1989a,b; 1990a,b,c; 1992; 1996;1997) available atRTI. GeneralLLinear Models (GLM) procedures (SAS IInstitute, 1989a,b;1990a,b,c; 1992;1996;1997) were applied to the analyses of variance(ANOVA) and the tests for linear trend. Prior to GLM analysis, an arcsine-square-root transformation was performed on all litter-derived percentage data,e.g.,percentages resorptions per litter, malformations per litter, and variationsper litter (Snedecor and Cochran, 1967). For litter-derived percentage data, theANOVA was weighted according to litter size. When a significant (p<0.05)main effect for dose occurred, Dunnett’s Multiple Comparison Test (Dunnett,1955;1964) was used to compare each treatment group to the control group forthat measure. A one-tailed test (i.e., Dunnett’s Test) was used for all pairwisecomparisons to the vehicle control group, except that a two-tailed test was usedfor maternal body and organ weight parameters, maternal feed and waterconsumption, fetal body weight, and percent males per litter. All nominal scale measures were analyzed by a Chi-square test for inde-pendence for differences among treatment groups (Snedecor and Cochran,1967) and by the Cochran-Armitage Test for linear trend on proportions(Agresti, 1990; Armitage, 1955; Cochran, 1954). None of these tests werestatistically significant. The alpha level for each statistical comparison was 0.05, and the signifi-cance levels for trend tests and pairwise comparisons were reported as p <0.05 or p< 0.01. RESULTS Twenty-five timed-mated female rats were assigned to eachtreatment group in this study, and pregnancy was confirmed in84-92% per group (Table 1). The incidence of clinical signs(primarily alopecia and clinical weight loss) did not appear tobe associated with formamide exposure (data not shown). Nomaternal deaths or morbidity occurred in this study. Maternal body weight was reduced at 200 mg/kg/day on gd18, 19 (data not shown), and 20 (Table 1). In addition, maternalbody weight gain was significantly decreased in the high-dosegroup during the first 3 days of treatment (gd 6 to 9), from gd15 to 18 (data not shown), for the treatment period as a whole(gd6 to 20) and for the gestational period (gd 0to 20) as awhole (Table 1). Gravid uterine weight was decreased in adose-related manner (100%, 94%, and 87% of control weight,at 50, 100, and 200 mg/kg/day, respectively), and the reductionwas significant at the high dose (Table 1). Following correctionfor the weight of the gravid uterus, maternal gestational weightgain was not significantly reduced at any dose (Table 1),suggesting that reduced gravid uterine weight was the primarycontributor to reduced maternal body weight and late gesta-tional weight gain in this study. Maternal consumption of foodor water was unaffected (data not shown). Maternal liverweight (absolute or relative to body weight) did not differamong groups on gd 20 (Table 1). FORM did not significantly affect any endpoints related toprenatal viability (Table 2). The percent resorptions per litterand the percent litters with one or more resorptions per litterdid not differ significantly among groups. There were noreported late fetal deaths in this study. Average live litter sizein FORM-treated groups was between 97-101% of the controlmean. Average fetal body weight per litter in FORM-treatedgroups was decreased in a dose-related manner, and this re-duction was statistically significant at the mid and high dosesfor both sexes or either sex alone (Table 2). Thus, the mean Developmental Toxicity in CD Rat Fetuses following Maternal Exposure to Formamide on Gestational Days 6 through 19 Formamide (mg/kg/day, po) 0 50 100 200 All litters (no. examined)" 22 21 23 22 Implantation sites per litter 15.09±0.39 15.24±0.42 15.09±0.42 14.95±0.43 Percentage resorptions per litter 1.40±0.57 1.62±0.77 3.02±1.68 4.16±1.38 Percentage litters with resorptions at one or more sites 23 19 22 41 Live litters (no. examined) 22 21 23 22 Live fetuses per litter’ 14.86±0.36 15.00±0.44 14.70±0.52 14.36±0.50 Percentage male fetuses per litter’ 50.68±2.07 47.62±3.25 52.59±2.93 49.72±3.38 Average fetal body weight per litter 3.55±0.04 3.49±0.08 3.32±0.05* 3.03±0.05* Average male fetal body weight per litterd 3.65±0.05 3.56±0.08 3.41±0.05* 3.11±0.05* Average female fetal body weight per litter 3.46±0.04 3.41±0.07 3.22±0.04* 2.96±0.06* Percentage live fetuses malformed per litter ..b.f 1.32±0.61 2.23±0.85 0.58±0.40 1.29±0.61 Percentage litters with one or more malformed live fetuses 18 29 9 18 Percentage live fetuses with variations per litter 11.21±2.25 9.62±1.87 13.86±1.62 12.23±2.11 Percentage litters with one or more live fetuses with variations 91 81 91 82 "Includes all dams pregnant at termination; litter size = no. of implantation sites per dam. fetal body weight per litter was 98, 93, and 85% of the averagecontrol weight for the low-, mid-, and high-dose groups, re-spectively, for the sexes combined. When considered collectively (all types) or grouped by type(external, visceral, skeletal), there was no difference amonggroups in the incidence of fetal malformations or variations(Tables 2 and3). The only individual finding with a distinctivedose-response incidence pattern was unossified sternebra (I, II,III, and/or IV) which was noted as a skeletal variation in 1, 3,9, and 12 fetuses in the control through high-dose groups,respectively (Table 3). A slight increase in the incidence ofenlarged lateral ventricles was noted at the mid and high doses(Table 3). Consistent with the observed reductions in fetalbody weight, these specific morphological findings indicate amild developmental delay associated with formamide expo-Sure. Rare fetal malformations-cleft palate, cleft lip, exen-cephaly, open eye lid, agenesis or malformation of the tail,dextrocardia, double-outlet right ventricle, and interventricularseptal defect-were noted in this study, but their individualincidences were low and not dose-related (Table 3). Multiplemalformations were observed in several low-weight fetuses indifferent dose groups, and were considered to be spontaneousevents rather than treatment related (Table 3). Some of these(for example, cleft palate) were consistent with treatment-related malformations reported at higher oral doses in an earlierinvestigation (BASF, 1974b,1983). However, there was noclear dose relationship for such findings in the present study. DISCUSSION Prior to the initiation of this study, the reproductive anddevelopmental toxicity of FORM had been investigated in therat, mouse, and rabbit, but the exposure period had neverencompassed the entire embryo/fetal period of development,except for the previously referenced screening study performedin this laboratory (NTP, 1998a). Thus, the present definitivedevelopmental toxicity study and associated screening studywere the first to use an extended period of exposure combinedwith termination for developmental assessment on gd 20. Inthis definitive study, CD@ rats were dosed by gavage withFORM (0, 50, 100, or 200 mg/kg body weight/day) from gd 6through 19. Minimal evidence of maternal toxicity was notedat 200 mg/kg/day (i.e., reduced body weight and weight gain),and the absence of an effect on maternal corrected-weight gainsuggests that developmental toxicity was a contributing factor.This study demonstrated that the maternal toxicity LOAEL was200 mg/kg/day, and the NOAEL was 100 mg/kg/day. Clearevidence of developmental toxicity in the definitive study waslimited to reduction of fetal body weight at≥100 mg/kg/day.The dose response for decreased fetal body weight was highlyconsistent across the screening study and the definitive study.Fetal body weights (both studies) were 98, 96, 93, 90, 85, 80,and 58% of control weights for doses of 50, 62, 100,125,200,250, or 500 mg/kg/day. Although the percent resorptions perlitter appeared increased at 200 mg/kg/day in this study (4.16vs. 1.40 for the control group), there was no statistically "A single fetus may be represented more than once in listing individual defects. The number of litters with malformations or variations is in parentheses. Onlylive fetuses were examined for malformations and variations. Only 50% were examined for visceral (including internal structures of the head), or skeletalmalformations or variations. "These malformations occurred together in one fetus from Dam #91; the individual fetal body weight was 1.67 g. “These malformations occurred together in one fetus from Dam #10; the individual fetal body weight was 2.45 g. "These malformations and variations occurred together in one fetus from Dam #58; the individual fetal body weight was 1.81 g. Pulmonary artery and aorta arise from right ventricle Left, right, or bilateral. TABLE 4Maternal and Developmental Toxicity in Evaluations of Formamide Administered to CD@ Rats BASF studies" NTP studies' 177-1588 mg/kg/day (gd 6-15) 0-200 mg/kg/day (gd 6-19) Maternal NOAEL 100 mg/kg/day Maternal LOAEL 177 mg/kg/day 200 mg/kg/day Developmental NOAEL 177 mg/kg/day 50 mg/kg/day Developmental LOAEL 318 mg/kg/day ≥100 mg/kg/day Prenatal mortality 个≥794 mg/kg/day Fetal body weight J≥a318 mg/kg/day ↓≥100 mg/kg/day Malformations 个≥318 mg/kg/day significant difference from control, and the value was wellwithin the range of historical control values for this parameter(1.18-6.82 percent resorptions per litter). Thus, under theconditions of this study, the developmental toxicity for theformamide LOAEL was 100 mg/kg/day and the NOAEL was50 mg/kg/day. Except for the length of exposure, the study design ofpreviously reported studies (BASF,1974b,1983) was quitesimilar to the present definitive study and the associatedscreening study (NTP, 1998a,b). Results of these studies aresummarized in Table 4. In the BASF study, pregnant Sprague-Dawley rats were administered technical grade FORM (0, 156,280,463, 700, or 1400 ul/kg/day) by gavage during majororganogenesis (gd 6-15 day; BASF, 1974b,1983). Doses wereequivalent to 0, 177,318,525,794, or 1588 mg/kg/day. Ma-ternal weight gain was depressed at all doses (i.e., ≥177mg/kg/day), and other indicators of maternal toxicity werereported at ≥794 mg/kg/day. Corrected maternal weight gainwas not reported; the contribution of decreased fetal weight todecreased maternal gravid weight is not known. Nearly all(99%) of the fetuses died at 1588 mg/kg/day, and some pre-natal mortality was reported at 794 mg/kg/ day. No prenatalmortality was reported at 525 mg/kg/day, a dose more thantwice that used in the present NTP-sponsored definitive study.Reduced fetal body weight, as well as an increased incidenceof fetal malformations, were reported at ≥318 mg/kg/day.Skeletal malformations, including vertebral cleaving, aplasiaand hypoplasia, and fused ribs were noted, although data werenot provided. Thus, in the BASF study,maternal toxicity wasnoted at all doses ≥177 mg/kg/day (maternal LOAEL), thedevelopmental toxicity NOAEL was equivalent to 177 mg/kg/day, and the developmental toxicity LOAEL was equivalent to318 mg/kg/day. In contrast to the BASF study, the results of the studydescribed in this manuscript indicate that the conceptus is moresensitive than the adult to the adverse effects of FORM whenlower doses are used and the exposure period is extended overthe full period of embryo/fetal development. Fetal body weight was the most sensitive indicator of developmental toxicity,exhibiting significant reductions at ≥ 100 mg/kg/day form-amide. A significant increase in malformations was not seen atthis dose level. Thus it appears that these two developmentalendpoints (fetal weight/growth and malformations) responddifferently to the presence of formamide. Whereas the dose-response curve for fetal weight/growth exhibited a downshift atlower doses, the dose-response curve for malformations didnot. The observation of fetal body weight as the most sensitiveendpoint for the developmental effects of formamide is notsurprising, since fetal body weight is often the most sensitivedevelopmental endpoint in rodents (Schwetz and Harris, 1993).The extended treatment period in this study (gd6-19) was alsoassociated with significant developmental toxicity at lowerNOAEL and LOAEL values relative to those reported in theBASF studies (BASF 1974b, 1983) after exposure duringmajor organogenesis (gd 6-15). Thus, in the BASF studies, thedevelopmental toxicity NOAEL was 177 mg/kg/day (gd 6-15)vs. 50 mg/kg/day (gd 6-19) in the present study. Likewise,BASF identified a developmental toxicity LOAEL at 318 mg/kg/day (gd 6-15), whereas the present study sets the develop-mental LOAEL at 100 mg/kg/day (gd 6-19). Although thepossible influence of other experimental factors cannot be ruledout entirely, it seems likely that the observation of the devel-opmental toxicity NOAEL and LOAEL at lower-dose levels inthis study reflect the combined impact of a longer exposureperiod on embryo/fetal growth, as well as no recovery period(versus 5 days for the BASF study) between the end of expo-sure and termination of the experiment. The comparison ofresults between studies illustrates the potential influence ofexperimental design on dose selection, relative sensitivity ofthe maternal and developing organism, and characterization ofthe toxic response. In summary, the maternal toxicity LOAEL was 200 mg/kg/day, and the NOAEL was 100 mg/ kg/day in this study. Thedevelopmental toxicity LOAEL and NOAEL were 100 and 50mg/kg/ day, respectively. These data provide a more completeevaluation of the developmental toxicity of formamide in rats. ACKNOWLEDGMENTS The present study was conducted at Research Triangle Institute (RTI),Research Triangle Park, North Carolina, under contract to the National Tox-icology Program and the National Institute of Environmental Health Sciences(NIEHS/NTP Contract NO1-ES-65405). The authors express their apprecia-tion for chemistry support to Cynthia S. Smith of NIEHS, as well as EvelynMurrill, Robert E. Smith, Robert Moor, James G. Greaves, Linda Siemann,Michael Kozak, and J. Michael Cannon of Midwest Research Institute. Inaddition, our appreciation goes to the following RTI professional and technicalpersonnel who contributed to the completion of this study: Patricia A. Fail,Donald B. Feldman, Frank N. Ali, Frieda S. Gerling, Vickie I. Wilson, BettyT. McTaggart, Lawson B. Pelletier,Mal-Selika H. Perry, Marian V. Chees-borough, Dee A. Wenzel, M. Michael Veselica, Randy A. Price, T. DouglasBurnette, and Donald L. Hubbard. ( REFERENCES ) ( ACGIH (1999). 1999 TLVs@ and BEIs@Threshold Limit Values for C hem- ical Substances a nd P h ysical A g ents, and Bi o logical Ex p osure Ind i ces.American C o nference of Governmental Indu s trial Hygienists, Cincinnati, Ohio. ) ( Agresti, A. (1990). Categorical Data Analysis. John Wiley and Sons, NewYork. ) ( A rmitage, P. (1 9 55). T e st f o r linear t rends i n p roportions a nd frequencies.Biometrics 11,375 - 386. ) ( BASF (1 9 74a).“Re p ort of t h e study of formamide for teratogenic effect in th e rat after repeated work-day , percutaneous application. " Report Number NCI 008271T6, report date: 9/24/1974. L ette r fro m BASF Corporation submit-ting information (5/27/1992 , in German) , N TIS Number: OTS0539634. ) ( BASF (1974b).“Report on the examination of formamide and acetamide forteratogenic e ffect i n rats after oral application”. Report N umber XI X /197, report date: 9/24/1974. Letter from BASF Corporation su b mitting informa- tion (5/27/1992, in German), NTIS Number: O TS0539636. ) ( B ASF (1983). C o ver letter and E nglish t ranslation of“Report on the Exami-nation o f Formamide for Teratogenic Effects in Ra t s af t er Oral Application."BASF Wyandotte Corp. (1/1 1/ 83), NTIS Number: O T S0512663. ) ( Blair, E. (1971). Hypothermia. I n Textbook of Veterinary Anesthesia, (L. S oma, E d .), pp. 5 55- 57 9. Williams and Wilkins, Co., B a ltimore. ) ( Cheng, T . J., Hwang, S . J., Kuo, H. W., L u o, J. C., and Chang, M. J. (1999). E x posure to e pichlorohydrin a nd dimethylformamide, glutathione S-trans-ferases, and siste r chromatid exchange f requencies in peripheral lympho- cytes. Arch. Toxicol . 73,282 - 287. ) ( Cochran, W . (1954). Some methods fo r strengthening the common x’t e sts.Biometrics 10, 417-451. ) ( Cody, R . L . , S e id, J . E . , and N a tale, R . B. (1 9 92). Ph a se II t rial of N-methylformamide in l u ng cancer. I n vest New Drugs 10, 21 5 -216. ) ( Danneman, P. J. , a n d Ma n drell, T. D . ( 19 9 7). Eva l uation of five agen t s/methods f o r anesthesia of n e onatal r o dents. L a b. A n im. Sci. 4 7 , 386-395. ) ( Del B ufalo,D., B u cci, B., D’Agnano, I., and Zup i , G. ( 19 9 4). N-m e thylfor-mamide a s a p o tential t h erapeutic a pproach i n c o lon cancer. D i s. C o lonRectum 37, S133 - S137. ) ( Dunnett, C. W. ( 1 955). A m ultiple comparison procedure for comparingseveral treatments with a control . J . Am. Stat . Asso c . 50, 1096-1121. ) ( Dunnett, C . W . ( 1 964). N e w tab l es for multiple comparisons with a co n trol.Biometrics 20, 482- 4 91. ) ( Fail, P. A., George,J. D., Grizzle, T . B., and Heindel, J.J. (1998 ) . Formamide and d imethylformamide: Re p roductive assessment by con t inuous breeding in mice. R eprod. Toxicol. 1 2, 31 7 -332. ) Atassi, G. (1982). N-methylformamide: Antitumour activity and metabolismin mice. Br. J. Cancer 45, 843-850. Gliech, J. (1974). Proceedings: The influence of simple acid amides on fetaldevelopment of mice. Naunyn. Schmiedebergs. Arch. Pharmacol 282, R25. Hafez, E. S., Ed. (1970). Reproduction and Breeding Techniques for Labora-tory Animals. Lea and Febiger, Philadelphia. Hellwig, J., Merkle, J., Klimisch, H. J., and Jackh, R. (1991). Studies on theprenatal toxicity of N,N-dimethylformamide in mice, rats, and rabbits. FoodChem. Toxicol. 29, 193-201. ( I TII ( 1 988). T oxic and Hazardous Industrial Chemicals Saf e ty Manual forHandling and D i sposal With To x icity and Hazard Data. The Int e rnational T echnical I nformation I nstitute, Tokyo. ) ( Jonckheere, A . R. ( 1 954). A d i stribution-free k -sample test a gainst orderedalternatives . Biometrika 41,133- 1 45. ) ( K elich, S. L., Mercieca, M. D., and Pohland, R . C . ( 1995). D evelopmentaltoxicity of N-methylformamide administered by gavage to CD rats and NewZealand w hite rabbits. Fundam. Appl. Toxicol. 2 7, 239- 4 6. ) ( K ennedy, G. L. , Jr. (1 9 86). Biological effects of a cetamide, formamide, an d their monomethyl and dimethyl derivatives. Cr i t. Rev. Toxicol. 17, 129-18 2 . ) ( K rauss, W . C . , Stula, E . F. , a nd Clayton, J. W ., Jr. ( 1967). T he effects o fdimethylformamide (DMF), monomethylformamide (MMF), an d fo r mamide (F) on e mbryonic development in t he rat. Haskell La b oratory for Tox i cology and I ndustrial Medicine. E. I . DuPont de Nemours, Wilmington, D E. ) ( Lareo, A . C . , a n d Perbellini, L . ( 1 995). Bi o logical mo n itoring of wor k ers exposed t o N -N-dimethylformamide: II. Dimethylformamide and its metab- olites in urine o f exposed workers. Int . Arch . Occup. Environ. Health 67, 47-52. ) ( L iu, S. L., Mercieca, M. D . , Markham, J. K., Pohland, R . C., and Kenel, M . F . (1 9 89). Developmental t oxicity studie s w it h N-methylformamide (NMF)administered o r ally t o rats a nd r a bbits. T e ratology 39, 466. ) ( L umb, W. V . , a n d J o nes, E . W . ( 1 973). A n esthesia i n l aboratory and zoo animals. In Veterinary Anesthesia,pp. 452-4 5 3. Lea and Fe b iger, Ph i ladel- phia. ) ( M ajor, J., H udak, A . , Kiss, G . , J a kab, M . G. , Sz a niszlo, J., Naray, M., Nagy, I ., and T o mpa, A . (1 9 98). F ollow-up b i ological a nd g enotoxicological monitoring of acrylonitrile - and dimethylformamide-exposed viscose rayon plant workers. Environ . Mol. Mutagen 31 , 301-310. ) ( M arr , M . C. , M yers, C . B ., George,J. D ., and Price, C . J . (1988). Comparisonof s ingle and double staining f or evaluation of skeletal development: the effects of ethylene glycol (EG) i n C D r a ts. T eratology 37, 476. ) ( M erkle, J., and Z eller, H. (1980). Studies o n a cetamides a n d formamides for embryotoxic a n d te r atogenic activities in the r a bbit. A rzeimittelforschung 30, 1557 - 1562. ) ( M raz, J. , Cross, H ., G escher, A . , T h readgill, M. D . , a n d Fl e k, J. (1989). Differences between r odents a nd humans in the metabolic toxification o f N,N-dimethylformamide. Toxicol. Appl. Pharmaco l . 9 8, 507-516. ) ( NIOSH (2000). National In s titute of O ccupational S a fety and Health. Inter-national Chemical S afety C ards, Formamide. A c cessible o n -line at w ww.cdc.gov/niosh/ipcsneng/neng0891.html. ) ( NTP ( 1 9 98a). D evelopmental toxicity screen for formamide (CAS N o. 75- 12-7) administered by gavage to Sprague-Dawley (CD@) ra t s on g es t ationaldays 6 t hrough 19. Final S tudy R eport (April 1 3, 1 998). ) ( N TP (1998b). D e velopmental t o xicity evaluation of f ormamide ( CAS No. 75-12-7) administered by gavage to Sprague-Dawley (CD@) ra t s on gesta- tional days 6 through 19. Fina l Study Report , 12/30/98 . NTIS Number: PB98- 1 09213. ) ( N TP (2000). N a tional Toxicology Program. Chemical Heal t h and Safety Data.Formamide. Accessed on-line at h t tp://ntp-server.niehs.nih.gov/, on May 23, 2000. ) ( tostatisc h Wirksmen Produkten an 7 Tiertumorem. Naunyn S chmiedeberg Arch. Pharmacol. 2 30, 559. ) ( Oettel, H., and Frohberg, H. (1 9 64). Teratogenic ac t ion of the elementary acid amides i n experiments with animals. Naunyn Schmiedebergs Arch. Phar-macol. 2 47, 363- 3 64. ) ( Ogata, M ., N umano, T . , Hosokawa, M., and Michitsuji,H. (1997). L a rge-scale b iological monitoring in Japan. Sci. Total Environ . 199, 197-204. ) ( Rickard, L . B ., D riscoll, C. D ., K ennedy, G. L. , Jr . , S t aples, R . E . , a n d Valentine, R . (1995). D evelopmental to x icity of inhaled N- m ethylform- amide in t he rat . Fundam. Appl. Toxicol. 28,167-176. ) ( R oss, D ., G escher, A . , and H i ckman, J. A . (1981). Metabolic stud i es on the antitumor a gent N-methylformamide. Br. J . C a ncer 44, 278. ) ( S aillenfait, A. M., P ayan, J . P., B eydon, D., Fabry,J. P., Langonne, I., S a bate, J . P., and Gallissot , F.(1997). Assessment of the developmental to x icity, metabolism, a nd p lacental transfer of N,N-dimethylformamide administeredto pregnant r ats. Fundam. Appl. T o xicol. 39, 33-43. ) ( S akai, T . , Kageyama, H., Araki, T ., Y osida, T., Kuribayashi, T ., a nd Ma-suyama, Y . (1995). Biological m onitoring of w orkers e x posed to N, N - dimethylformamide by determination of the urinary metabolites, N-methyl- formamide and N-acetyl-S-(N-methylcarbamoyl) cy s teine. I n t. Arch. Occup. Environ. Health 67, 125-129. ) ( Salewski, E . (1964). Farbemethode zum makroskopischen Nachweis von Im-plantationsstellen am Uterus der Ratte. Naunyn Schmiedebergs Arch. P har- macol.247,367. ) ( S AS I n stitute. ( 1989a) . SAS Language and Procedures: Usage, V ersion 6, F irst Edition. SAS Institute, Cary, NC. ) ( S AS I n stitute. (1989b). SAS/STAT U sers’ Guide, V e rsion 6 , Fourth E d ition,Volumes 1 a nd 2. S AS I n stitute, Cary, NC. ) ( SAS Institute. ( 1 990a). SAS Language: Reference, Ve r sion 6, Fi r st Edition.SAS Institute,Cary, NC. ) ( SAS I n stitute. (1990b). SAS L anguage: Procedures Gu i de, Version 6, T hi r d Edition.SAS Institute, Cary, NC. ) ( SAS I n stitute. (1990c). SAS Companion for the VMSTM Environment, Version 6, F irst Edition. SAS I n stitute, Cary, NC. ) ( SAS I n stitute. (1992). SAS T echnical Report P - 229, S A S/STAT Sof t ware: Changes and Enhancements, R e lease 6 . 0 7 . SAS Institute, Cary, N C. ) ( S AS I n stitute. (1996). SAS Companion fo r the Microsoft Wi n dows Env i ron-ment. SAS I n stitute, Cary, NC. ) ( SAS I n stitute . (1997). SAS/STA T Software : Changes and Enhancements Through Release 6 . 12. S AS Institute, C ary, NC. ) ( Sax, N. I ., and Lewis, R. J., Sr . (1987). Hawley’ s Condensed Chemical Dictionary. 11th Ed. Van Nostrand Reinhold, New York. ) ( Schwetz, B. A., and H a rris, M. W. (1993). D e velopmental toxicology: Status o f the f ield and contribution o f the N ational Toxicology Program. Environ.Health Perspect. 1 00, 269- 2 82. ) Snedecor, G. W., and Cochran, W.G. (1967). Statistical Methods,6th ed. IowaState University Press, Ames, IA. Staples, R. E. (1974). Detection of visceral alterations in mammalian fetuses.Teratology 9, A37-38. ( Stuckhardt, J. L., and Poppe, S . M. ( 1984). F resh visceral examination of r at and r abbit f etuses used in teratogenicity testing. Teratog. Carcinog. Muta- gen. 4, 181 - 188. ) ( S tula, E . F ., K rauss, W . C., Zapp, J. A. , Jr . , Clayton, J. W. , Jr., Maritz, R. S., and Aftosmis, J. G . ( 1 992). Embryotoxicity in rat s and rabbits from appl i - cation of formamide and o t her ch emicals t o skin d u ring organogenesis. H askell Laboratory for Toxicology and Ind u strial Medicine, E. I. DuPont deNemours, W ilmington, DE. ) ( T hiersch, J.B . (1962). Effect of substituted mercaptopurines on the r a t l i tter inutero. J. Reprod. F e rtil. 4 , 2 91. ) ( T hiersch, J. B . ( 1971). I n vestigations into the differential e f fect o f compounds on r at litter and mother. Congenital Malformation s of Mammals, Massonand C i e , P aris. ) ( U.S. E PA ( 1 997). U . S. E n vironmental Pr o tection Agency (Part II I ), 4 0 C F R Part 799. TSCA Prenatal Developmental Toxicity (S799.9370), Toxic Sub- stances Control Act T e st G u idelines; F i nal R u le. F e deral Register 62,43832-43834. ) ( U.S. E PA ( 1 998). Prenatal D e velopmental Toxicity Study: Hea l th Effects Tes t Guidelines, OPPTS 870.3700, EPA 712-C, pp. 98-2 0 7. U.S. Environmental Protection Agency, Office of Pr e vention, Pesticides, an d Toxi c Sub s tances. ) ( U.S. F D A (1988). Good Lab o ratory Practice Regulations f or N onclinical Laboratory S tudies, Code o f Federal Regulations (CFR), p p. 229-243.U.S.Food and Drug Administration. ) ( U.S. FD A ( 1 994a).“ Go od Lab o ratory Pra c tice Regulations for No n clinicalL a boratory Studies." U.S. Food a nd D rug Administration. C ode of Federal Regulations (CFR) (March 2 1) 56FR1 2 300. ) ( U.S. F DA ( 1 994b). I n ternational Conference on Harmonization; Guideline on Detection of Toxicity to Reproduction for Medicinal Products. U.S. Food and Drug Administration.Federal Register 59 (183) , 48746-48752,9/22/94. ) ( West, S . D ., a nd T u rner, L. G . ( 1 988). R e sidue le v el d e termination of th e aquatic herbicide fluridone and a potential photoproduct (N-methylform- amide) in w ater.J. Assoc. O ff. Anal. C h em. 7 1, 1 049-1053. ) ( Wilson, J. G . (1965). E m bryological con s iderations in t e ratology. In Ter a tol-ogy: P rinciples a nd T e chniques, (J. G. W i lson and J. Wa r kany, Eds.), pp.251-277. University of C hicago P r ess, Chicago, I L . ) ( Windholz, M . , B u davari, S., Blumetti, R. F., and O tterbein, E. S., E ds. (1 983). The M erck Index: A n E n cyclopedia of Chemicals and D r ugs. T e nth E d .Rahway, NJ. ) ( Wixson, S. K., and Smiler, K. L. (1997). A nesthesia and analgesia i n rodents. In Anesthesia and Analgesia in Laboratory Animals (American College of L a boratory Animal M edicine S eries) (D. F. Kohn, S. K. Wixson, W. J.White, and G. J. Benson, Eds.) , pp. 16 5 -203 . Academic Press, New York. )
确定





还剩6页未读,是否继续阅读?
海德创业(北京)生物科技有限公司为您提供《甲酰胺对SD大鼠发育毒性的毒理研究》,该方案主要用于其他中--检测,参考标准--,《甲酰胺对SD大鼠发育毒性的毒理研究》用到的仪器有甲酰胺(超高纯)
相关方案
更多
该厂商其他方案
更多









