方案详情
文
一种简单、快速的一步电沉积方法在激光诱导石墨烯(LIG)上修饰Au/NiO/Rh三金属复合材料,并用于亚硝酸盐(NO2-)的检测。
方案详情

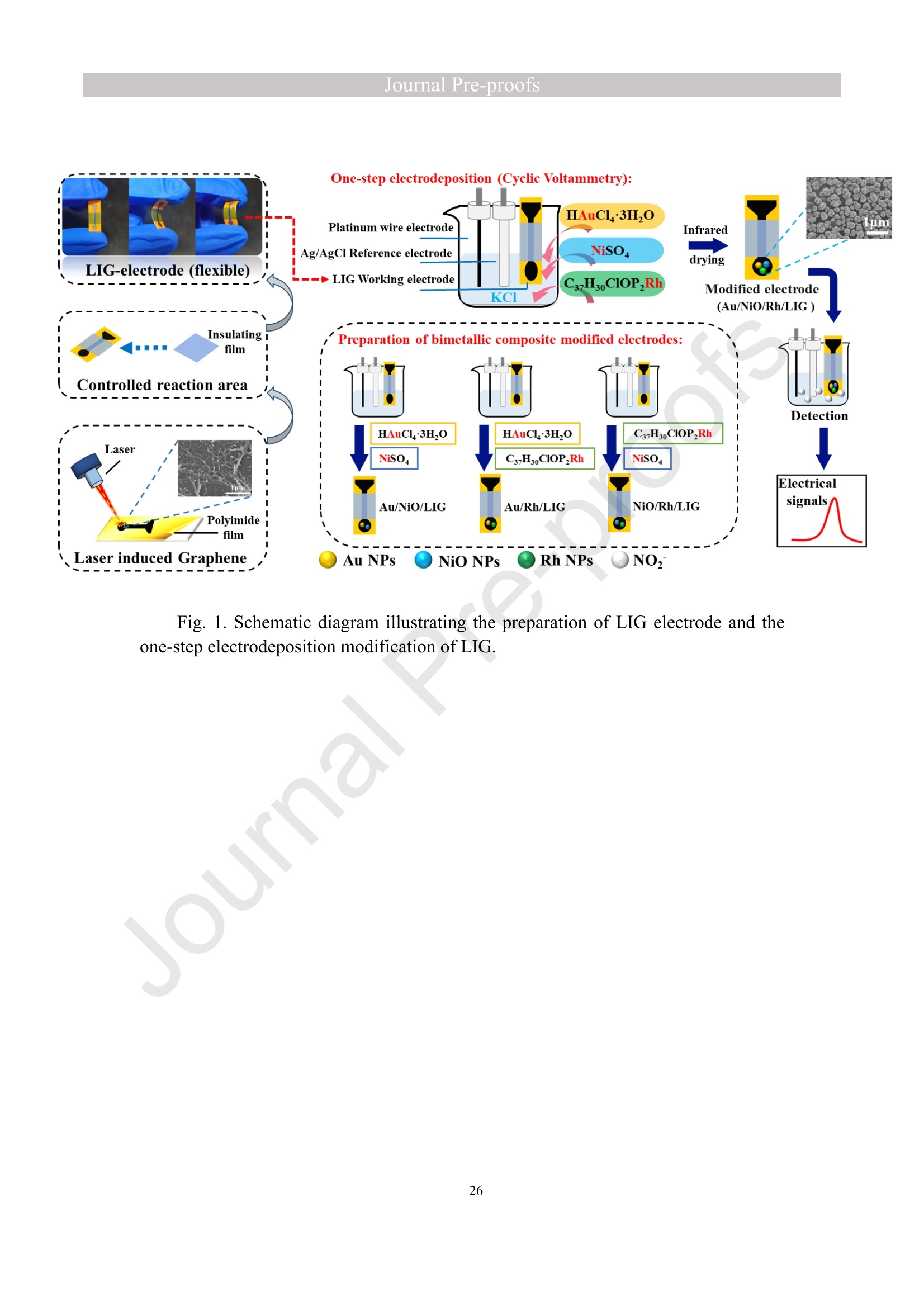
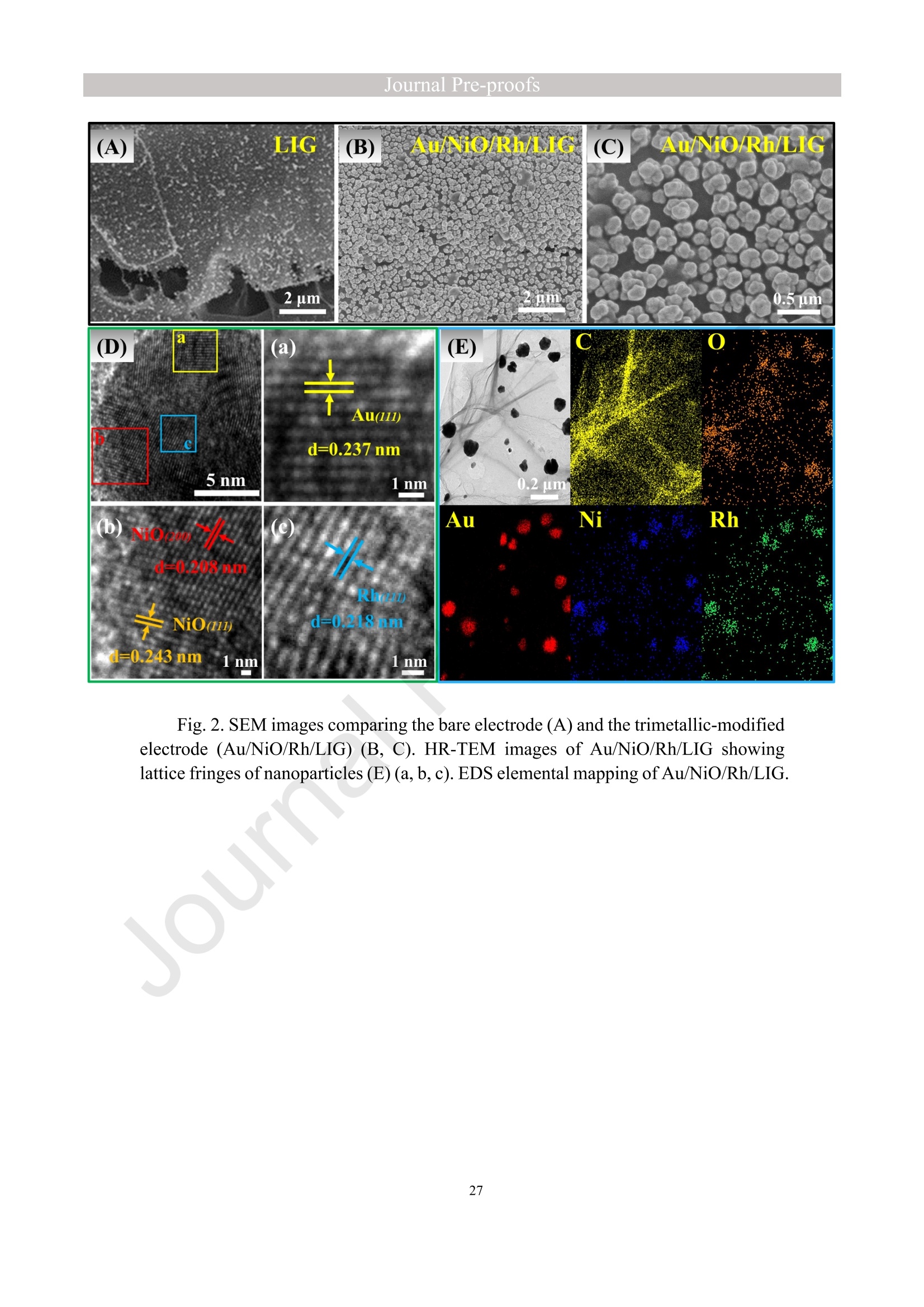
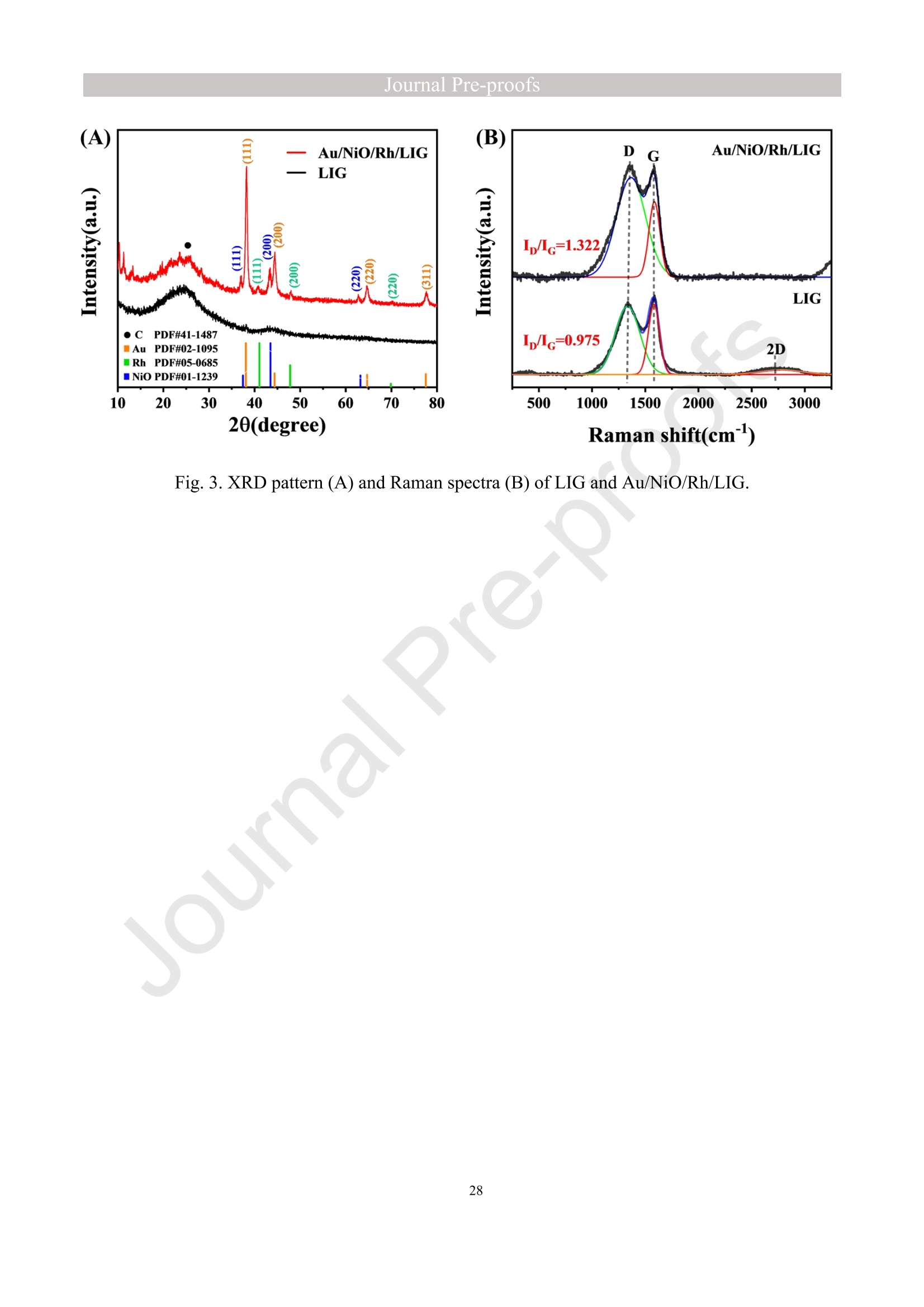
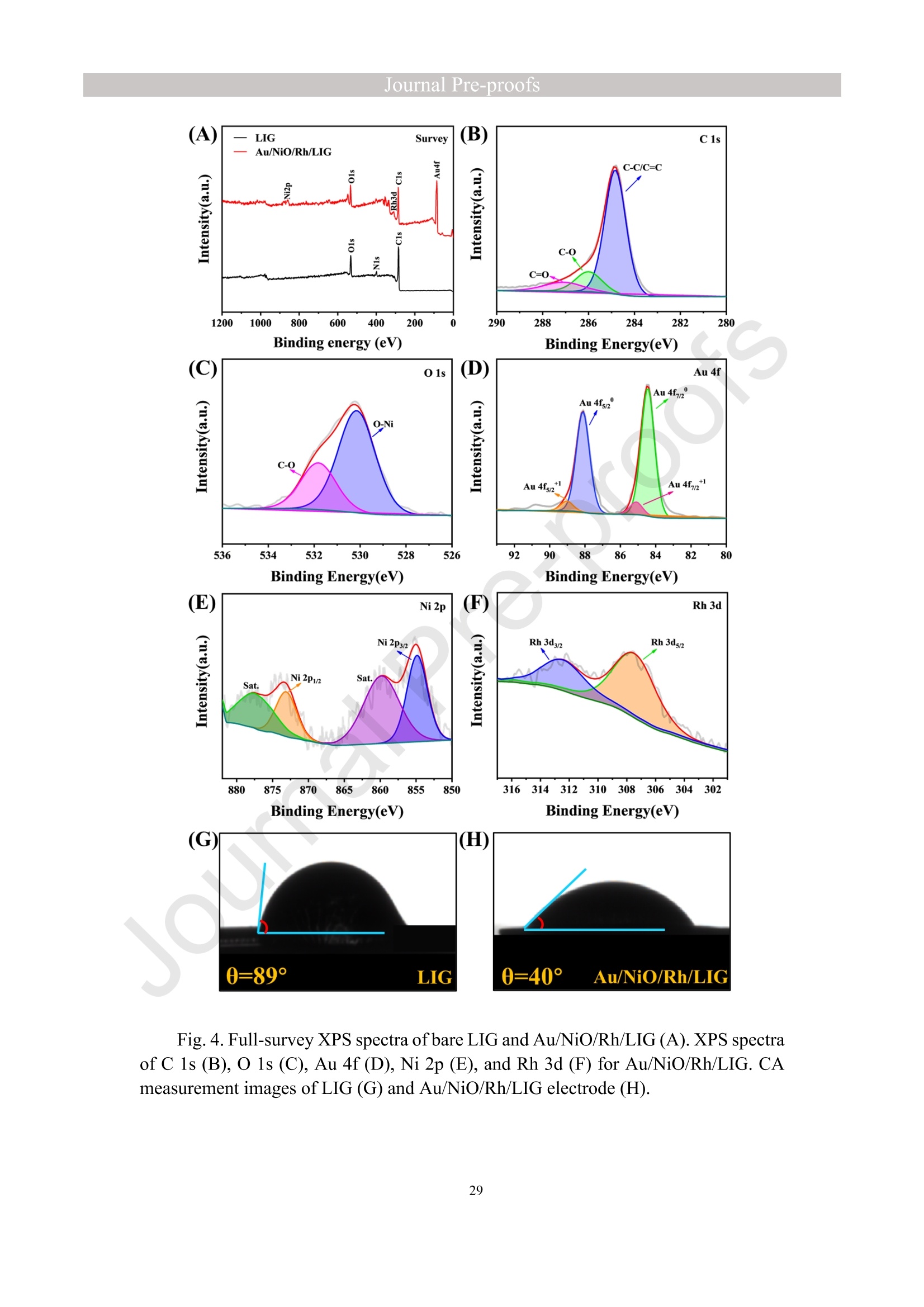
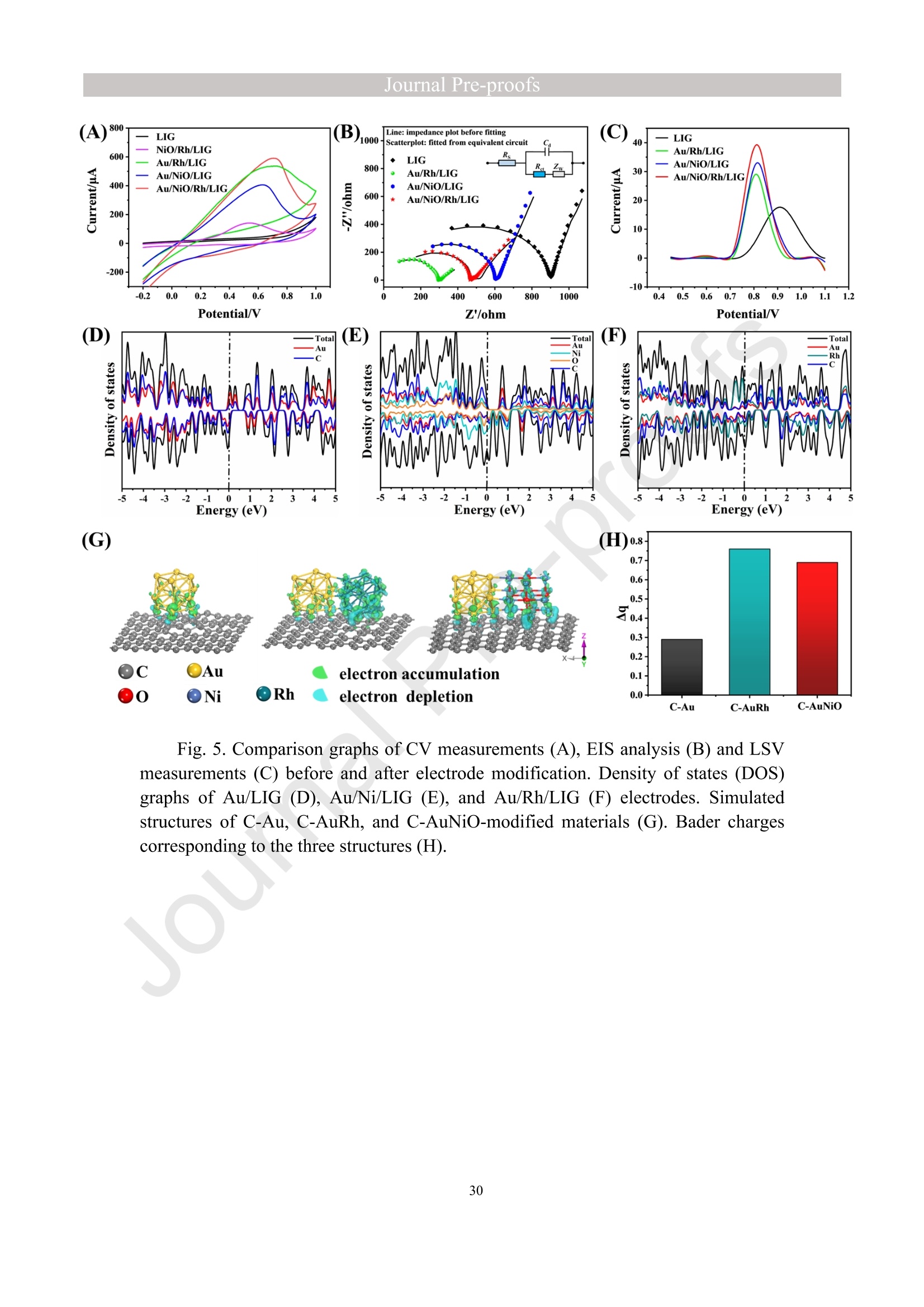
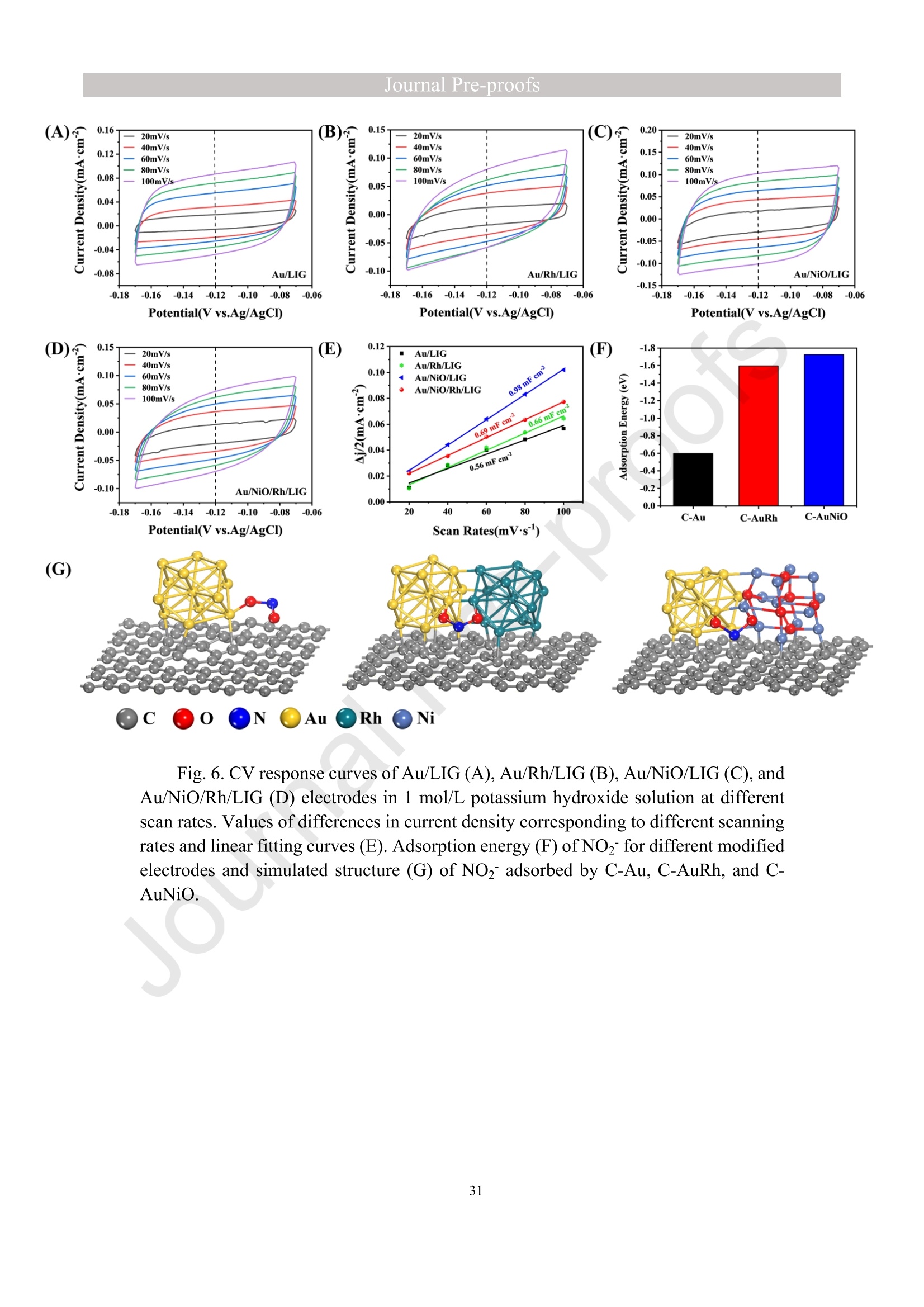
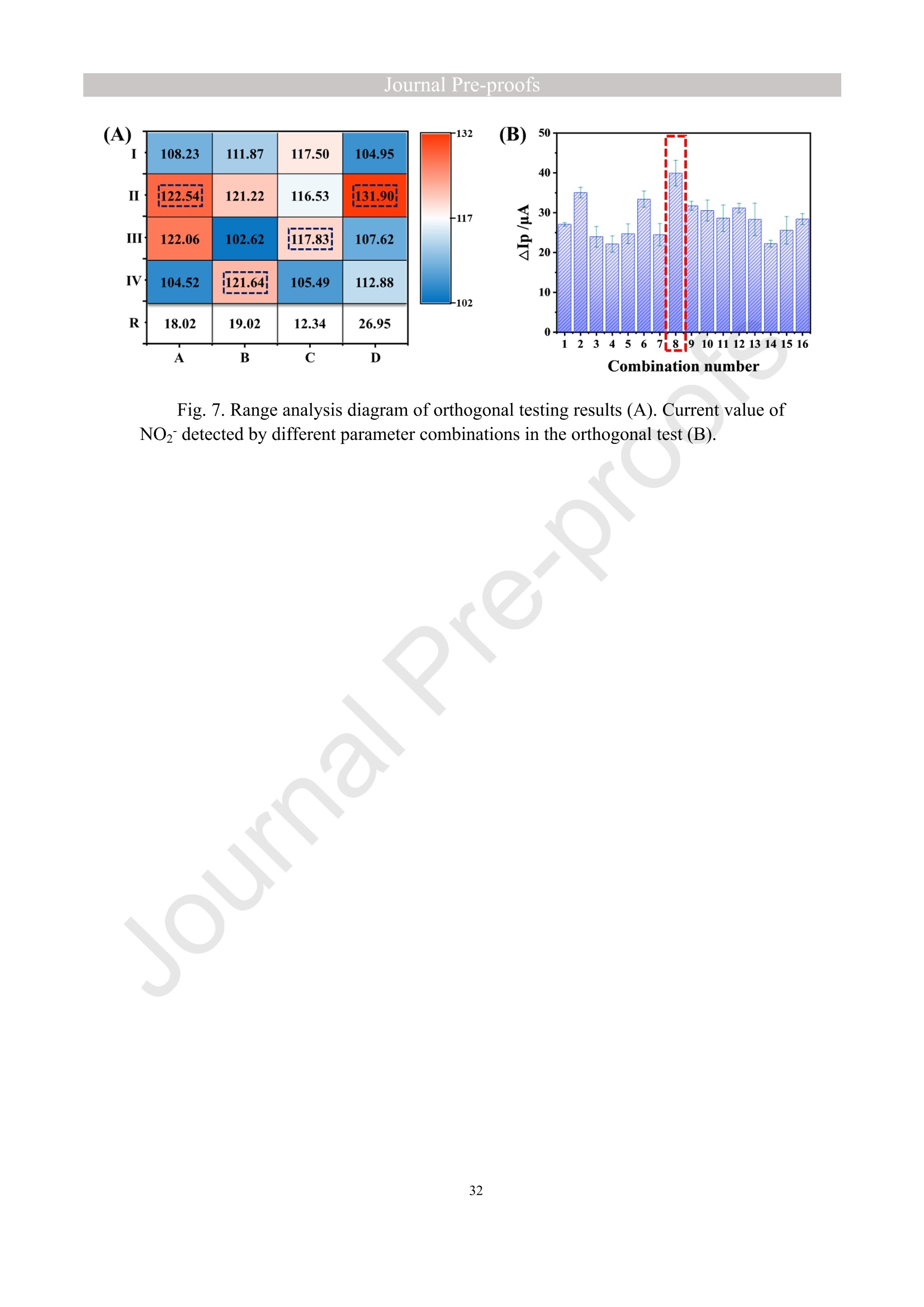
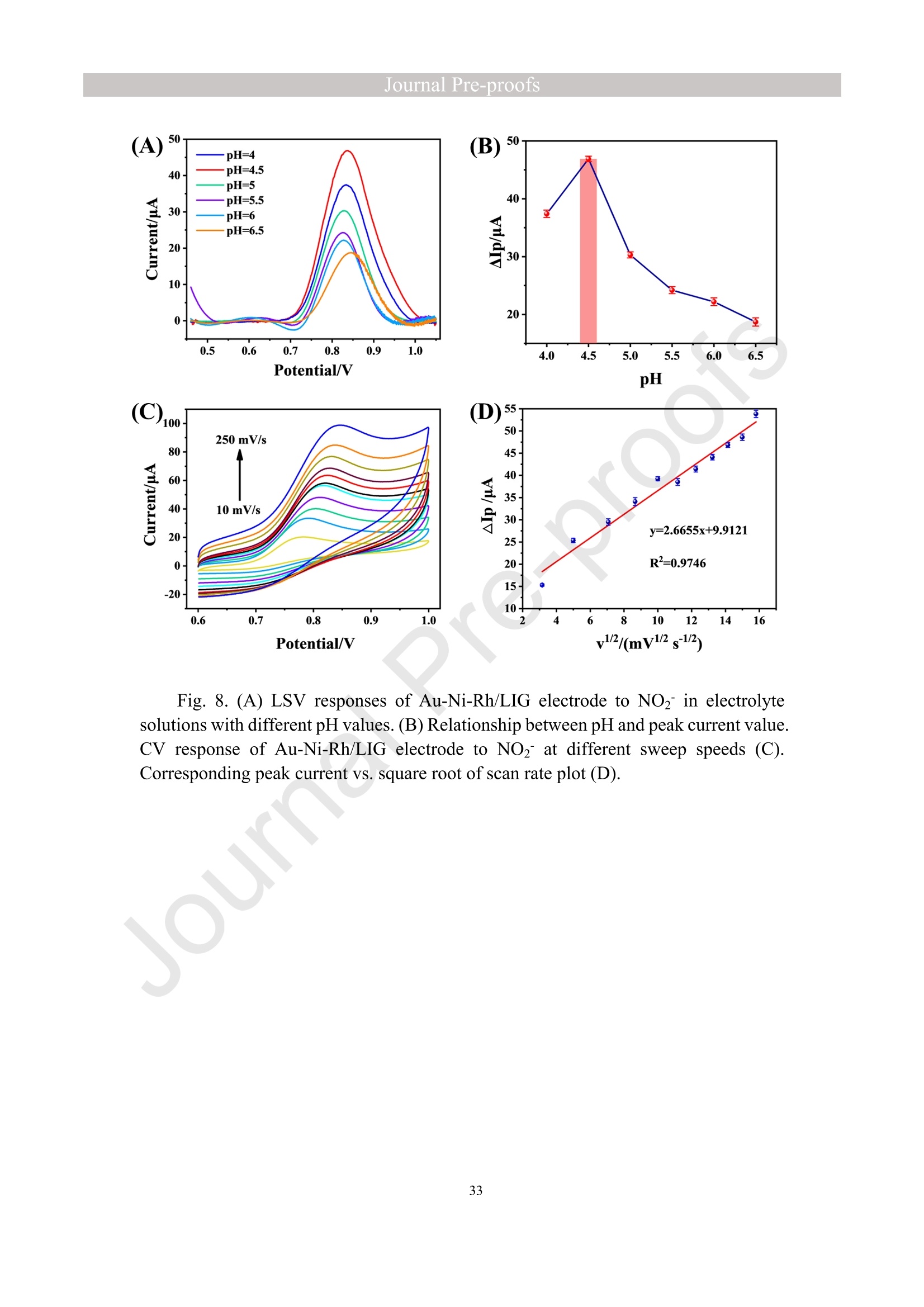
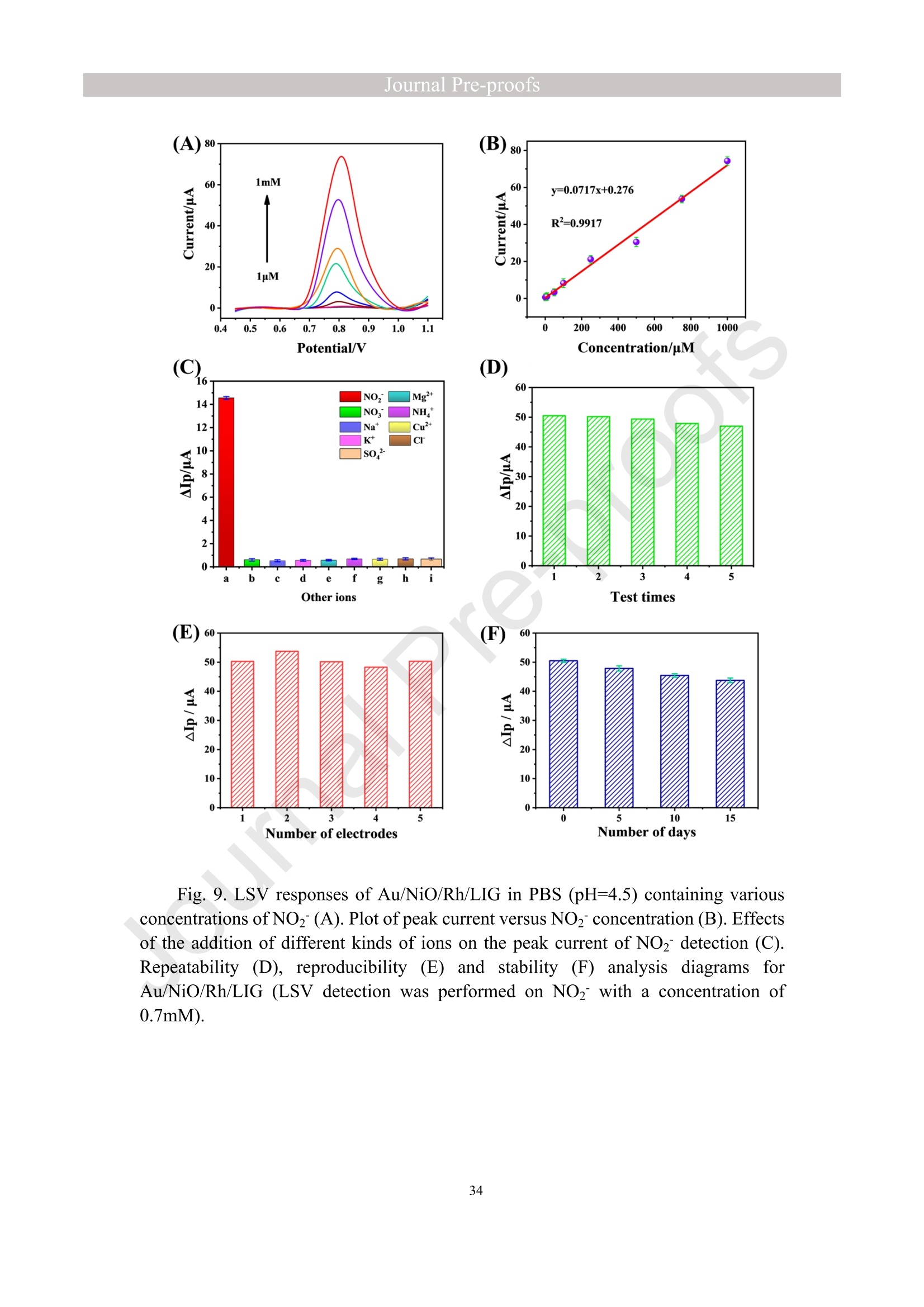
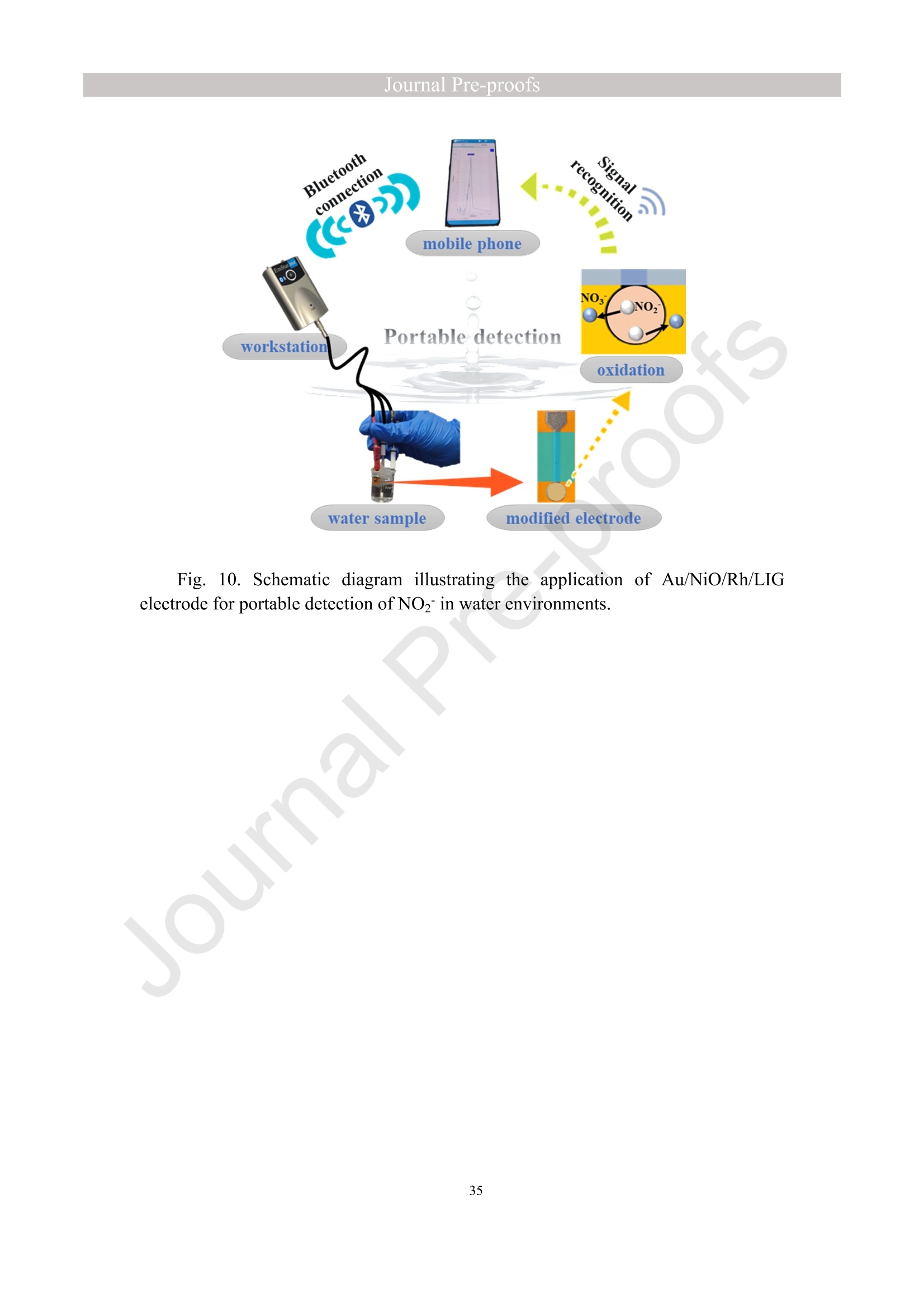
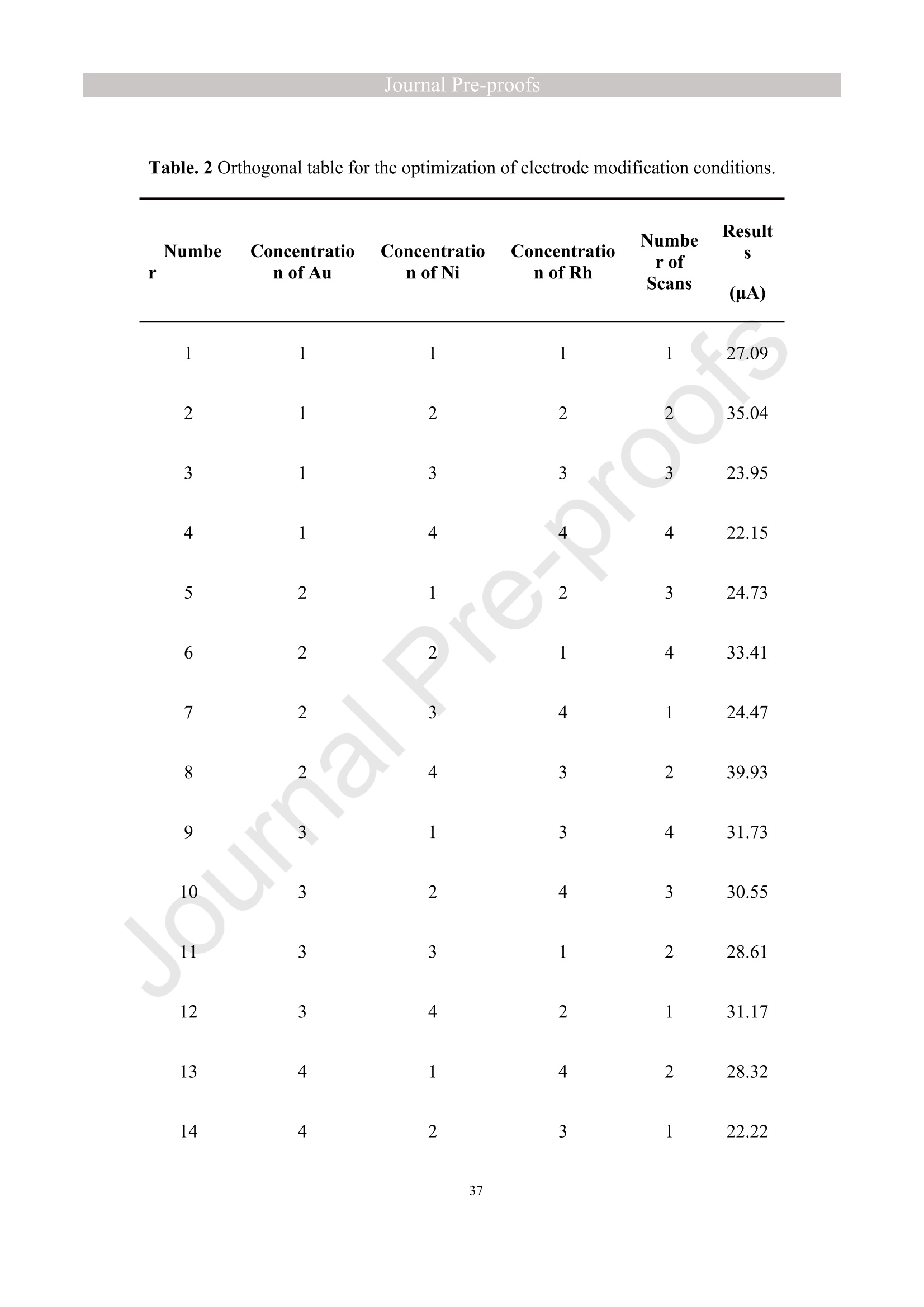
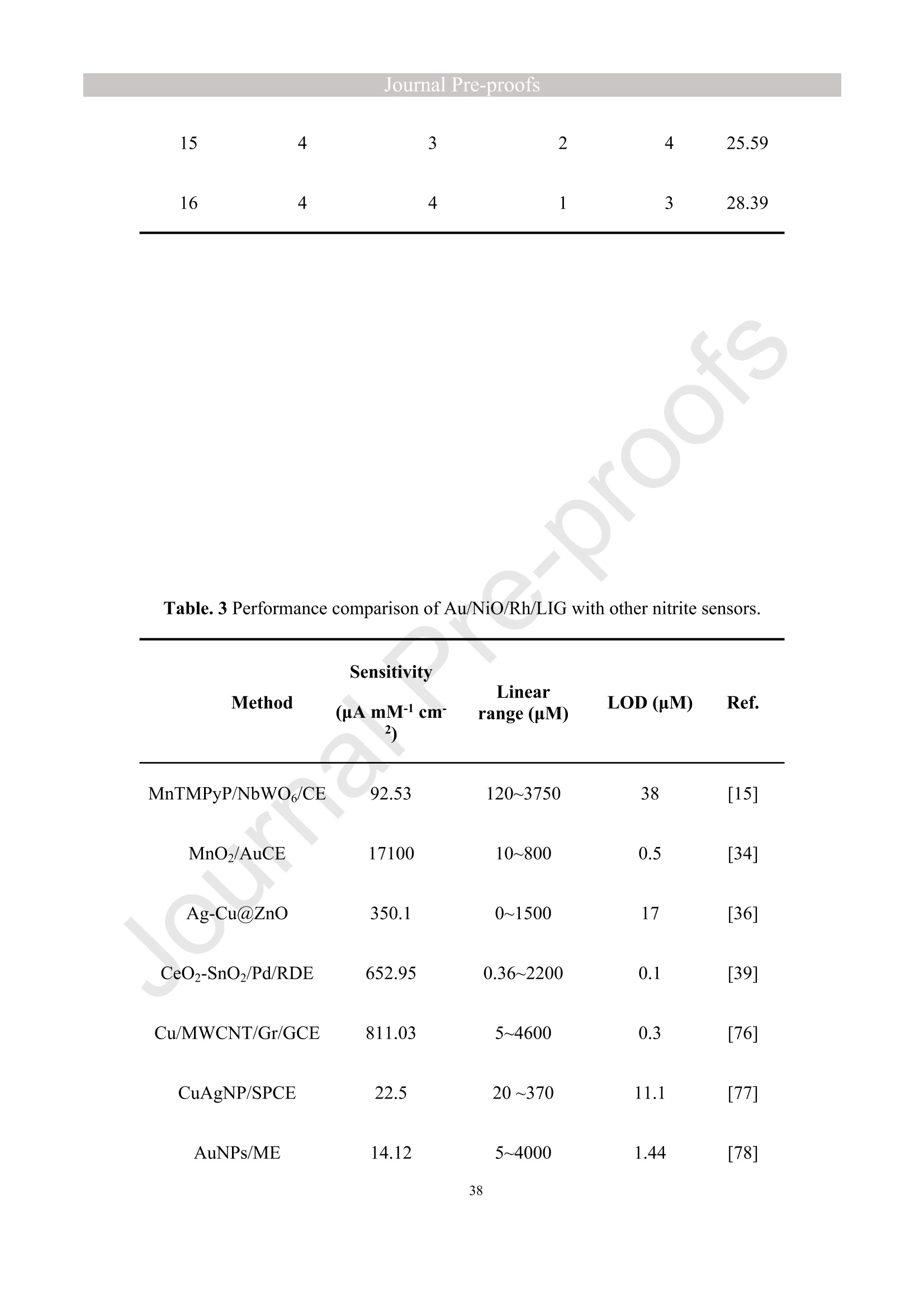
亮点:1、 通过一步电沉积法在LIG上沉积了Au、NiO和Rh纳米颗粒2、 DFT计算证实了吸附能和电子转移的增强。3、 三种金属在催化和吸附方面促进了亚硝酸盐的检测。4、 所开发的传感器对亚硝酸盐的检测限非常低,为0.3μM。5、 该传感器可用于实际水样中NO2-的便携式测定在这项研究中,中国地质大学薛强课题组采用了一种简单、快速的一步电沉积方法在激光诱导石墨烯(LIG)上修饰Au/NiO/Rh三金属复合材料,并用于亚硝酸盐(NO2-)的检测。利用扫描电子显微镜等技术对电极的形态组成和电化学特性进行了大量的表征和测试。通过循环伏安等实验比较了电极改性前后的电化学活性面积。此外,通过密度泛函理论计算(DFT)证实了改性电极在电子传递性能和吸附能方面的变化。实验结果表明,用铑纳米颗粒(RhNPs)和金纳米颗粒(AuNPs)对 LIG 进行协同改性,可以改善电极界面的电子传输能力,提高电极的电催化性能。氧化镍(NiO)的加入为NO2-的检测提供了更大的反应面积和更多的活性位点。这三种改性组分的共同作用大大提高了电极对 NO2-的检测能力,其性能超过了双金属修饰电极。在最佳实验条件下,该传感器的线性范围为 1 μmol/L ~ 1 mmol/L,检测限为 0.3 μmol/L。同时,该传感器还具有出色的抗干扰性和可重复性。我们成功地将该传感器用于测定自来水中的NO2-,结果令人满意。这项研究为利用金属基电化学传感器便携、灵敏、原位检测水环境中的NO2-提供了一种具有前景的策略。原文链接:https://doi.org/10.1016/j.cej.2023.145486 本文中进行电化学测试的仪器为荷兰PalmSens,型号:EmStat3Blue便携式电化学分析仪,由雷迪美特中国有限公司提供。需要了解更多电化学方面的知识,请关注我们或进入PalmSens中文网站:“https://www.palmsens.cn/”。Journal Pre-proofs Efficient detection of nitrite in water based on an Au/NiO/Rh trimetallic com‐posite modified laser-induced graphene electrode prepared by one-step elec‐trodeposition Zeyu Liu, Xiaohan Shan, Qiang Xue, Yao Liu, Lin He, Haijiao Xie Please cite this article as: Z. Liu, X. Shan, Q. Xue, Y. Liu, L. He, H. Xie, Efficient detection of nitrite in water based on an Au/NiO/Rh trimetallic composite modified laser-induced graphene electrode prepared by one-step electrodeposition, Chemical Engineering Journal (2023), doi: https://doi.org/10.1016/j.cej.2023.145486 This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. Efficient detection of nitrite in water based on an Au/NiO/Rh trimetallic composite modified laser-induced graphene electrode prepared by one-step electrodeposition Zeyu Liu a,b #, Xiaohan Shan a,b #, Qiang Xue a,b,*, Yao Liu a,b, , Lin He a,b, Haijiao Xie c a MOE Key Laboratory of Groundwater Circulation and Environmental Evolution, SchoolofWaterResourcesandEnvironment,ChinaUniversityofGeosciences (Beijing), Beijing, 100083, PR China b Beijing Key Laboratory of Water Resources and Environmental Engineering, School of Water Resources and Environment, China University of Geosciences (Beijing), Beijing 100083, PR China cHangzhouYanquInformationTechnologyCo.,Ltd.,HangzhouCity,Zhejiang Province 310003, PR China *Correspondence Author: Qiang Xue E-mail: xueqiang@cugb.edu.cn # Indicates equal contribution. Abstract In this study, we employed a straightforward and rapid one-step electrodeposition method to enhance modify an Au/NiO/Rh composite on laser-induced graphene (LIG) for nitrite (NO2-) detection. We extensively characterized and tested the morphological composition and electrochemical properties of the electrode using scanning electron microscopy and other characterization techniques. To compare the electrochemical active surface areas of the electrode before and after modification, we conducted cyclic voltammetry experiments. Additionally, we confirmed the changes in electron transfer performance and adsorption energy of the modified electrode through by density functional theory calculations. The experimental results demonstrate that the combined modification of LIG with rhodium nanoparticles (RhNPs) and gold nanoparticles (AuNPs) improves electron transport ability at the electrode interface and enhances the electrode's electrocatalytic performance. The incorporation of nickel oxide (NiO) also provides a larger reaction area and more active sites for NO2- detection. The synergistic effects of these three modified components significantly enhance NO2- detection at the electrode, surpassing the performance of the bimetallic modified electrode. Under optimized experimental conditions, the sensor exhibits a linear range of 1 μmol/L to 1mmol/L and a detection limit of 0.3 μmol/L. Furthermore, the sensor demonstrates excellent anti-interference and repeatability properties. We successfully applied the sensorfor determination ofNO2- intapwater,yieldingsatisfactory results.This research presents a promising strategy for the portable, sensitive, and in-situ detection of NO2- in water environments using metal-based electrochemical sensors. Keywords:Electrochemicalsensor;Nitrite;Trimetallic;Laser-inducedgraphene; Water environment 1. Introduction Nitrite (NO2-) is a common nitride found widely in the natural environment. While it plays a crucial role in the nitrogen cycle [1], it is also extensively used in various industries,includingchemicals,food,andpharmaceuticals[2-4].Ingroundwater, although nitrite levels are lower compared to nitrate and ammonia nitrogen levels, its environmental toxicity is relatively high. Excessive ingestion of NO2- through drinking water and its accumulation in the human body can lead to severe health risks [5, 6], suchasacutepoisoning,braindamage,andcarcinogenesis.TheWorldHealth Organization (WHO) has classified nitrite as a carcinogen [7]. Due to its significant detrimental effects on both the environment and human health, NO2- has gained increasing attention worldwide [8]. Therefore, the efficient and sensitive detection of environmental concentrations of NO2- holds practical importance. ExistingmethodsfordetectingNO2-includechemiluminescence[9,10], spectrophotometry[11],high-performanceliquidchromatography[12,13]and electrochemical methods [14-16]. Although these methods offer reliable detection, they possess limitations, such as the need for expensive equipment, complex detection processes, and incompatibility with on-site detection. Electrochemical methods have been widely employed for the qualitative and quantitative detection of NO2- due to their affordability,simplicity,sensitivity,reliability,andabilitytofacilitate in-situ monitoring. Graphene (GR) has been utilized as an electrode modification material due to its exceptional electrocatalytic activity, large surface area, and straightforward preparation process [17-19]. Laser-induced graphene (LIG) is a graphene-like material produced through the instantaneous photothermal catalysis of a precursor polymer via laser etching[20,21],providingexcellentelectricalconductivityandelectrocatalytic properties [22]. Additionally, this novel flexible and paper-based electrode significantly reduces the cost of electrode fabrication, enhances portability, and offers a new avenue in-situ detection of contaminants using electrochemical sensors [23]. Modifying of the electrode interface with appropriate materials is crucial for enhancing the detection signal and performance of sensors [24-26]. Previous studies have demonstrated the significance of carbon nanomaterials (such as carbon nanotubes(CNTs) and graphene (GR) in the field of electrochemical nitrite sensors, owing to their exceptionalproperties[2,27,28].Thesematerialspossesswideelectrochemical windows, excellent stability, and large specific surface areas [7]. In recent years, researchers have increasingly focused on the utilization of metal (or metal oxide) nanoparticles to facilitate electron transfer dynamics. Additionally, the exploration of nano-metal oxides and multi-metal nanocomposites has gained attention [29, 30]. The high catalytic activity of certain metals [17, 31], combined with the unique morphology and structure of metal oxide nanoparticles [32-34], is beneficial for promoting electron transfer and improving electrode sensitivity. For instance, Hameed, R. M. Abdel et al. successfully enhanced nitrite detection performance by utilizing bimetallic Cu@Pt nanoparticles with a core-shell structure, combined with graphene. Their approach resulted in a low detection limit [35]. Similarly, G. Manjari et al. employed plant extracts(acaciacaesiaflowerextract)toreducethreemetalstonanoparticles, fabricatingmetalormetalnanocomposites(Ag-Cu@ZnO)andforGCelectrode modification.Ag-Cu@ZnO/GCexhibitedremarkablecatalyticactivityfor NO2-oxidation,achievingalowdetectionlimit(17μM),andoutperformingother nanocomposites [36]. In summary, the incorporation of multi-metal materials into the electrode modification process demonstrates superior versatility and synergistic effects comparedtosinglemetalcompetitors.Italsooffersadditionaladvantagesfor electrochemical detection [37-39]. However, the application of trimetallic composite modified electrodes in the field of NO2- detection remains limited, and the preparation methods for materials and electrode modification are often laborious and complex. Therefore, in order to ensure high sensitivity and accuracy of electrochemical sensors, exploring simple and effective methods for multi-metal modification essential for promoting the rapid development of NO2- nanosensors. Au nanoparticles (AuNPs) are widely employed as modification materials due to their high catalytic activity, demonstrating excellent catalytic oxidation effects for the electrochemicaldetectionofNO2-[40,41].Nano-nickeloxide(NiO)isanideal material for sensor fabrication, possessing a high specific surface area and relatively uniform dispersion, which facilitates the loading of the measured substance [42, 43]. Rhodium(Rh),aplatinumgroupmetal,exhibitsstablechemicalpropertiesand remarkable electrocatalytic activity in the field of electrochemistry [44]. Therefore, in this study, we utilized a one-step electrodeposition method to co-modify three metals (Au, NiO, and Rh) on the surface of a laser-induced graphene (LIG) electrode, resulting in the formation of nanoscale particles. The roles played by NiO and Rh in the performance of these Au-modified electrodes were analyzed through comprehensivecharacterizations.Experimentaloptimizationwasconductedto determine the electrode parameters for NO2- detection. The synergistic effects of the three metal components, including the excellent electrocatalytic performance of Au and Rh, combined with the large specific surface area provided by NiO significantly enhance the electrochemical properties of the LIG surface. Consequently, the oxidation of NO2- to NO3- is catalyzed more effectively, leading to enhanced the detection signals and reduced detection limits. This electrochemical sensor allows for portable detection of NO2- in real water samples. Additionally, the deposition of the three metals on the electrode interface through one-step electrodeposition method ensures cost-effective fabrication and simplicity of operation. This study also presents a novel strategy for the preparation of multi-metal composite modified electrodes. 2. Experimental materials and methods 2.1 Reagents NaNO2, HAuCl4·3H2O, NiSO4, and bis(triphenylphosphine)rhodium(I) carbonyl chloride (C37H30ClOP2Rh) were obtained from Aladdin Biochemical Technology Co., Ltd. KCl and K3[Fe(CN)6] were acquired from Sinopharm Beijing Reagent Co., Ltd. All chemicals used in this study were of analytical grade. The electrolyte utilized in the experiments was phosphate buffer solution (PBS). PBS solutions with varying pH values were prepared by combing 0.2 M Na2HPO4 and 0.2 M NaH2PO4 stock solutions in different proportions. 2.2 Preparation of polymetallic modified electrodes 2.2.1 Preparation of laser-induced graphene (LIG) The LIG used in this study was prepared using a computer-controlled laser scribing micromachining system (Nano Pro-III, Tianjin Jiayin Nano Technology Co., Ltd). Firstly, a polyimide (PI) film was tightly adhered to a treated high-temperature-resistant paper. The surface of the PI film was then wiped with alcohol to ensure it was flat and clean. The treated PI film was securely positioned within the laser action zone of the instrument, and the laser emitter was adjusted to the correct position. A computer was employed to import the desired pattern for printing and to set optimal parameters for laser-etching power (90) and depth (25). Laser induction was then performed to produce the LIG electrode. Subsequently, the prepared LIG electrode was cut to the appropriate size, and an insulated blue film was applied to control the LIG reaction area, limiting the reaction interface to a circular area with a diameter of 2 mm. Finally, the processed LIG electrodes were placed in a vacuum box for preservation until further use. 2.2.2 Fabrication of Au/NiO/Rh/LIG Topreparetheelectrodemodificationsolution,10mmol/LHAuCl4·3H2O,20mmol/L NiSO4 and 15 mg/L C37H30ClOP2Rh were added to a 0.2 mol/L KCl solution. The solution was thoroughly mixed and then subjected to ultrasonic shaking for 30 min. This solution served as the electrode modification solution. The prepared LIG was connected to the three-electrode working system as the working electrode. The three-electrodesystemwasimmersedintheabove-mentionedsolution.Sixcyclesof deposition were performed using cyclic voltammetry (CV) within a potential range of-0.9 V to 1.2 V. After deposition, the working electrode was removed and subjected to infrareddrying.Inthisstudy,aone-stepelectrochemicaldepositionmethodwas employed to produce the trimetallic modified LIG (Au/NiO/Rh/LIG). Similarly, 10 mL of KCl solution was placed in two small beakers. One beaker contained HAuCl4·3H2O and NiSO4, while the other contained HAuCl4·3H2O and C37H30ClOP2Rh. The three-electrode system was placed in each beaker, respectively, and cyclic voltammetric scanning was performed in the potential range of -0.9 V to 1.2 V. Finally, the prepared bimetallic composite modified electrodes (Au/NiO/LIG, Au/Rh/LIG) were dried for further use. The electrode preparation procedure illustrated in Fig. 1. 2.3 Electrochemical detection of NO2- All electrochemical methods in this study utilized a three-electrode working system. The working electrode chosen was LIG, the counter electrode was a platinum wire electrode, and the reference electrode was an Ag/AgCl electrode. For the detection of NO2-, CV, linear sweep voltammetry (LSV) and amperometric(i-t) techniques were employed. The LSV parameter settings were as follows: potential scanning range of +0.4 V to 1.1 V and a scan rate of 100 mV/s. The i-t method is mainly applied to the analysis of sensor response times. NO2- solution (0.5 M) was added to the electrolyte every 50 s interval after the current stabilization, and the measurement time was 300 s, the applied voltage was 0.9 V. The electrolyte solution used was 0.2 M PBS. Different concentrations of NO2- solution were prepared using 0.2 M PBS. The electrochemical detection of NO2- by the sensor and the optimization of experimental parameters were conducted using a 1 mM NO2- solution. 2.4 Pretreatment of actual water samples Tap water samples were collected from the laboratory of China University of Geosciences (Beijing) for this experiment. To ensure the removal of physical impurities, the tap water samples to be tested underwent filtration using a filter membrane. Subsequently, the filtered tap water was prepared as a solvent by mixing it with 0.2 M PBS buffer. NaNO2 powder was precisely weighed and dissolved in the filtered tap water to create a stock solution of NO2- with a concentration of 1 mM. The pH of the solution was carefully measured and adjusted to the optimal value of pH 4.5 using a pH meter. This adjustment ensured the best ionic strength and a strong current response signal. Throughout the measurement process, the same water sample was used to dilute the stock solution to the desired concentration. 2.5 Instruments and analysis Electrochemical measurements, including CV, LSV and electrochemical impedance spectroscopy (EIS), were conducted using a CHI660 workstation (Chenhua Instruments,China)equippedwithaconventionalthree-electrodesystem.The electrodesurfaceswerecharacterizedusingscanningelectronmicroscopy(SEM)(SU8020, Hitachi, Japan), transmission electron microscopy (TEM) (Tecnai G2 F30, FEIcompany,USA),andX-raydiffraction(XRD)(D8ADVANCEX-Ray Diffractometer, Bruker company, Germany) to evaluate their surface morphology and elemental composition. X-ray photoelectron spectroscopy (XPS) measurements were obtained using a Thermo Escalab 250Xi spectrometer (Thermo Fisher Scientific, USA). The contactangles(CA)oftheelectrodes weremeasured usingacontactangle measuring instrument (JY-82A, Dingsheng, China). The BET surface area measurement (Mac ASAP 2460, USA) was employed to analyze the surface area and pore size of the electrodes. For the electrochemical detection of nitrite in actual water samples, a handheld potentiometer (Palmsens, Netherlands) was used. LIG electrodes with different metal modifications were immersed in H2O2 solution for CV scanning. The potential range of the scan was set from -0.2 V to +1 V, with a scan rate of 100 mV/s. CV scans were also performed in a solution containing 5 mmol/L K3[Fe(CN)6]. The scan interval was set from -0.5 V to +0.8 V, and the scan rate was 100 mV/s. EIS measurements were conducted in a 0.1 M KCl solution containing 5mM K3[Fe(CN)6]. The parameters were set as follows: open-circuit voltage of 0.3 V, amplitude voltage of 5.0 mV, and a frequency range of 1 Hz to 1000 kHz. 2.6. DFT calculation method WeemployedthePBEformulation[45]withinthegeneralizedgradient approximation for density functional theory (DFT) calculations of various electrode structures. Simulations were performed using the Vienna Ab Initio Simulation Package[46, 47] in conjunction with the projected augmented wave algorithm [48, 49]. Gaussian smearing was applied to broaden the energy levels near the Fermi level. Grimme's DFT-D3 correction method [50] was utilized to calculate the dispersion correction energy based on the geometric structure. The electron density was computed by integrating the Brillouin zone using K-point sampling. The adsorption energies (Eads) before and after metal modification of the electrodes were determined using the equation: Eads =Ead/sub - Ead - Esub [51], where Ead/sub, Ead and Esub denote the energy of the optimized adsorbate adsorbed in the substrate system, the energy of the adsorbate and the energy of the substrate, respectively. 3. Results and Discussion 3.1 Characterization of electrode morphology, structure and composition The results of SEM morphological characterization of the interface before and after LIG electrode modification are presented in Fig. 2. Fig. 2(A) displays the partial morphology of flaky graphitic carbon on the pristine LIG surface, exhibiting a laminar structurewitharelativelysmoothsurface.Fig.2(B,C)illustratesthesurface morphology of LIG after the deposition of the three metals (Au/NiO/Rh). It is evident that the metal nanoparticles are distributed on the LIG surface without being affected by its layered structure. The metal nanoparticles show uniform distribution on the LIG surface, and the presence of Ni and Rh effectively prevents the agglomeration of AuNPs (Figure S1) and enhances the available reaction space for AuNPs to interact with NO2-. Moreover, the deposited NiO forms irregular bulges on the LIG surface, thereby increasing the electrode surface area and providing additional reaction sites for NO2-. Additionally, a small amount of Rh is observed to be attached to AuNPs and NiONPs in the form of RhNPs. The metal nanoparticles on the modified electrode surface were further characterized using high-resolution transmission electron microscopy (HR-TEM), as shown in Fig. 2(D)(E). The lattice spacings of Au and Rh were measured to be 0.237 nm and 0.218 nm, respectively, corresponding to the (111) crystal planes of Au(PDF#02-1095)andRh(PDF#05-0685),respectively.Mostofthenickel electrodeposited on the LIG is observed in the form of NiO, and the measured lattice spacings of 0.243 nm and 0.208 nm are consistent with the (111) and (200) crystal planesofNiO(PDF#01-1239)onthePDFstandardcard.EnergyDispersive Spectroscopy (EDS) scanning was performed on the modified electrode Au/NiO/Rh/LIG. The results of the element distribution are shown in Fig. 2(E) and Figure. S2 (B) (C), clearly revealing the presence of Au, Ni, and Rh elements loaded on the graphite carbon surface. And the elemental mapping of Au, Ni, O and Rh show similarmorphologicaldistributions,whichalsoindicatethatthethreemetal nanoparticles are attached to each other. Fig. 3(A) depicts the XRD patterns of the bare LIG and Au/NiO/Rh/LIG electrode. The diffraction peak at 2θ=25.3° is attributed to the (002) plane of the graphite crystal, resulting from the hexagonal conjugated carbon structure (PDF#41-1487). The high resolution and diffraction peaks observed in Au/NiO/Rh/LIG indicate a certain degree of crystallinity in the obtained material. Strong diffraction peaks corresponding to the crystalline planes of Au were observed at 38.1°, 44.4°, 64.7°, and 77.5°, representing the(111),(200),(220),and(311)planesofAu,respectively(PDF#02-1095). Furthermore, characteristic peaks were observed at 2θ=37.4°, 43.5°, and 63.2° in the XRD pattern, corresponding to the (111), (200), and (220) crystal planes of NiO(PDF#01-1239). The presence of a small amount of Rh in Au/NiO/Rh/LIG resulted in lower intensity peaks corresponding to the (111) and (200) crystal planes observed at2θ=41.1° and 47.8° (PDF#05-0685). In conclusion, the XRD analysis confirmed the successful modification of AuNPs and other metallic nanomaterials onto the LIG substrate, further validating the presence of metallic substance morphology consistent with the TEM scanning results. The structures of bare LIG and nanocomposite-modified LIG were verified using Raman spectroscopy, as shown in Fig 3. (B) and Fig. S2. Two prominent peaks can be observedintheRamanspectraatapproximately1350cm−1and1580cm−1, corresponding to the D-band and G-band, respectively. The measured Raman spectra were fitted and calculated, revealing that the ID/IG values (ID/IG =1.322) of the metal nano-modified LIG were higher than those of the bare LIG (ID/IG =0.975). This increase is attributed to the presence of structural defects when metal nanoparticles are inserted into the porous carbon matrix [52]. Additionally, a 2D peak occasionally appears at around 2700 cm-1, and analysis of the I2D/IG ratio led to the conclusion that the prepared LIG electrodes have randomly stacked graphene layers [53]. 3.2 Chemical properties of the electrodes XPS analysis was employed to investigate the elemental composition and valence states of the modified electrode. Fig. 4(A) presents the XPS measurement spectra of the bare LIG and Au-Ni-Rh/LIG electrode. The detailed XPS spectra for each element on the modified electrode are depicted in Fig. 4(B)-(F). In Fig. 4(B), the C 1s spectrum reveals three distinct peaks at 284.8 eV, 286 eV, and 287.1 eV, corresponding to C-C, C-O, and C=O, respectively. Fig. 4(C) displays the XPS spectrum of O 1s, where the characteristic peak at a binding energy of 530.02 eV corresponds to lattice oxygen bonded with the metal. Additionally, the presence of oxygen-containing functional groups (such as C-O bonds) gives rise to a characteristic peak at 531.9 eV [54, 55]. The Au 4f spectrum (Fig. 4(D)) primarily exhibits two peaks at approximately 84.4 eV and 88.1 eV, representing Au 4f7/2 and Au 4f5/2, respectively, indicating metallic Au [56-58]. Moreover, two characteristic peaks at 85.1 eV and 89 eV suggest the presence of a small amount of Au+ [59]. The high-resolution XPS spectrum of Ni 2p (Fig. 4(E)) can bedeconvolutedintotwopeaks,alongwithtwosatellitepeaks.Thepeaks corresponding to Ni2+ are observed at binding energies of 855 eV and 873.2 eV, corresponding to Ni 2p3/2 and Ni 2p1/2, respectively [60]. Satellite peaks are also evident at binding energies of 859.7 eV and 877.8 eV [61]. Regarding the Rh 3d region shown in Fig. 4(F), the binding energies of Rh 3d5/2 and Rh 3d3/2 for metallic Rh were determined to be 307.6 eV and 312.7 eV, respectively [62, 63]. CA characterization experiments were conducted to assess the hydrophilicity and hydrophobicity of the LIG interface before and after modification. A highly hydrophilic electrode interface can facilitate the adsorption of the target at the electrode interface by reducing the contact time between pollutants and the electrode interface [64]. The contact angle is inversely related to hydrophilicity. As depicted in Fig. 4(G, H), the bare LIG electrode exhibits a large contact angle (θ=89°), whereas the electrode modified withtrimetallic(Au/NiO/Rh/LIG)showsasignificantreductionincontactangle(θ=40°). These characterization results indicate that the modified electrodes exhibit enhanced hydrophilicity, primarily due to the incorporation of a large number of metal nanoparticles through electrodeposition. 3.3 Electrochemical characteristics of modified LIG electrodes The different metal-modified LIG electrodes were immersed in an H2O2 solution for CV scanning. The oxidation peak current at a potential of +0.6 V was analyzed and to evaluate the catalytic oxidation effect of each metal component [65], as depicted in Fig. 5(A). The unmodified LIG electrode exhibited a very low response current to H2O2, while the Ni-Rh/LIG electrode showed a higher peak current compared to bare LIG. These experimental results clearly demonstrate a significant increase in the response currentoftheAu-basedmodifiedelectrodes(Au/Rh/LIG,Au/NiO/LIG,and Au/NiO/Rh/LIG).Toinvestigatetheelectrocatalyticactivitiesofthedifferent electrodesfurther,theywereimmersedinK3[Fe(CN)6]probesolutionforCV measurements, as shown in Figure. S5. The results obtained were consistent with those in Fig. 5(A), indicating that among the various metal nanoparticles obtained through electrodeposition, AuNPs play a major catalytic role. Subsequent experiments focused only on the performance of Au-modified electrodes. In summary, it can be inferred that thecatalyticperformanceoftheAu/NiO/Rh/LIGelectroderesultsfromamajor contribution by AuNPs and an auxiliary contribution by RhNPs, while the catalytic effect of metal Ni is weak in this study. To analyze the impedance values of the different electrodes, EIS was performed in a 0.1 M KCl solution containing 5 mM K3[Fe (CN)6]. The diameter of the semicircle in the high-frequency region indicates the level of electrochemical impedance of the electrode. The high-frequency region of the obtained EIS image shows an incomplete semicircle (Fig. 5B (black line graph)), which is mainly influenced by the properties of the laser-induced graphene electrode itself and the capacitance of the solution system. The horizontal distance between the right end point and the highest point of the semicircular arc in the high-frequency area is approximately 1/2 Rct. The experimental data were fitted and analyzed using ZView software and the equivalent circuit diagram. The obtained image is presented in Fig. 5(B) scatterplot, and the data are shown in Table S1. The fitting results revealed that bare LIG had the highest impedance value with Rct=705.28 Ω, while Au/Rh/LIG exhibited the lowest impedance value (Rct=260 Ω). This reduction in impedance can be attributed to the effects of Rh, indicating that the presence of RhNPs effectively reduces the electrode impedance and facilitates the rate of electron transfer in the reaction. Although the impedance value of Au/NiO/Rh/LIG was not the lowest (Rct=410 Ω), it was significantly lower than that of bare LIG, demonstrating that the trimetallic composite-modified LIG effectively enhances the electron transport ability of the electrode in this experiment. Toinvestigatetheelectrochemicalactivityofthetrimetallic-modifiedLIG electrode (Au/NiO/Rh/LIG), we compared and analyzed the electrochemical performances of the modified and unmodified electrodes for detecting NO2-. Fig. 5(C) displays the scanning images obtained from LSV detection of NO2- solution using different modified electrodes.Compared to bare LIG, all the metal-nanomodified electrodes exhibited varying degrees of enhancement in the oxidation peak current for NO2- detection. Among them, the Au/NiO/Rh/LIG electrode achieved the highest peak currentvaluewhendetectingNO2-,withanoxidationpeakcurrentof39μA, approximatelytwiceashighasthatofbareLIG.Theseexperimentalresults demonstrate that co-modification with three metals is more effective in promoting nitrite detection compared to bimetallic modification. Insummary,thetrimetalliccomposite-modifiedelectrode(Au/NiO/Rh/LIG) displayedthehighestdetectioncurrentamongthegold-basedLIGelectrodes (Au/Rh/LIG, Au/NiO/LIG) for electrochemical NO2- detection. This suggests that both RhNPs and NiONPs contribute to the detection process. Therefore, further exploration oftheindividualadvantagesofthesetwometallicsubstancesinthetrimetallic composites is warranted. 3.4 Electron transfer of the electrodes To further demonstrate the electrocatalytic capacity of Ni and Rh in the Au-based modified LIG electrode, we utilized Density Functional Theory (DFT) to evaluate electrontransferattheinterfacesofdifferentmodifiedelectrodes.Fig.5(D)-(F) correspond to the density of states (DOS) of Au/LIG, Au/NiO/LIG, and Au/Rh/LIG materials, respectively. These results reveal that the Au/Rh/LIG structure exhibits a greater electron occupancy. In other words, the Au/Rh/LIG electrode demonstrates the highest electron density near the Fermi level (Fig. 5(F)). The differential charge densities of the C-Au, C-AuRh and C-AuNiO structures are shown in Fig. 5(G), it is evident that the C-AuRh structure exhibits more pronounced aggregation and electron transfer compared to the C-Au and C-AuNiO structures. This provides evidence that metal Rh plays a pivotal role in enhancing the electron transfer rate at the LIG interface. 3.5 Studies of electrode adsorption properties To investigate the active sites of different modified electrodes, CV experiments were conducted in a 1 M KOH solution at various sweep rates, with a potential sweep range of -0.18 V to -0.06 V selected as the non-Faraday potential interval. Fig. 6(A-D) illustrates the CV curves of Au/LIG, Au/Rh/LIG, Au/NiO/LIG, and Au/NiO/Rh/LIG electrodes at different scan speeds. Fitting calculations were performed to determine the correlation between scan speed and the difference in current density at a potential of -0.12V. The results of the fitting are presented in Fig. 6(E). The capacitance value of amodifiedmaterialcanbedeterminedfromtheslopeofthelinearequation, representingthedouble-layercapacitance(Cdl)valuesofAu/LIG,Au/Rh/LIG, Au/NiO/LIG, and Au/NiO/Rh/LIG as 0.56, 0.66, 0.98, and 0.69 mF cm-2, respectively. According to the formula for electrochemical active surface area (ECSA) = Cdl/Cs, ECSA is proportional to Cdl [66, 67]. Therefore, the electrochemically active area of Au/NiO/LIG is the largest, followed by that of the Au/NiO/Rh/LIG electrode. To further verify the specific surface area of each metal-modified LIG, the BET methodwasemployed,involvingtheadsorption/desorptionofnitrogen (N2). Parameters such as specific surface area, pore volume, and average pore size were obtained, as shown in Table S2. Figures S6 (A-D) display type IV isotherms and type-H3hysteresisforeachelectrodematerialataconstantbathtemperatureof approximately 77 K and a relative pressure range of P/P0 from 0.025 to 1.00. Figures S6 (E-H) reveal the BET surface areas of Au/LIG, Au/Rh/LIG, Au/NiO/LIG, and Au/NiO/Rh/LIG as 16.8876, 37.7611, 105.6937, and 58.7952 m2.g-1, respectively. The BET surface area of Au/NiO/LIG is the largest, being nearly 2-5 times that of the other samples, which aligns with the results of the ECSA experiment. These findings indicate that the presence of NiONPs further increases the specific surface area of the trimetallic composites (58.7952 m2.g-1). Additionally, the pore volume of Au/NiO/Rh/LIG is0.0973 cm3.g-1, with an average particle size of 6.6208 nm. To further validate the NO2- adsorption capacity of the electrodeposited metals Ni and Rh in the modified Au-based LIG electrode, we simulated the structures of C-Au, C-AuRh and C-AuNiO for NO2- adsorption, as shown in Fig. 6(G). Adsorption energy refers to the energy released during the adsorption process, with higher adsorption energyindicatinggreaterstabilityofthecorrespondingstructure.Therefore,we calculated the adsorption energies of the three structures for NO2- using DFT, and the results are presented in Fig. 6(F). Among the structures, C-AuNiO exhibited the highest adsorption energy (-1.7263 eV), indicating its strongest adsorption capacity for NO2-. This aligns with the previous experimental findings regarding BET and ECSA. The predominantpresence of nano-nickeloxide(NiONPs),resultingfromthe electrodeposition of metal Ni on LIG, may explain this observation. These rounded NiO NPs are uniformly distributed on the electrode surface, effectively increasing the surface area [68]. As a result, there is more space available for the deposition of AuNPs and RhNPs, along with additional reaction sites for the oxidation of NO2-. Consequently, the electrode's adsorption capacity for NO2- is enhanced. 3.6 Optimization of experimental parameters 3.6.1 Optimization of modification conditions The trimetallic modified electrode (Au/NiO/Rh/LIG) used in the electrochemical detection experiments for NO2- was prepared through one-step electrodeposition. The modificationconditionsthatinfluencethedetectioneffectmainlyincludethe concentration of each component in the deposition solution and the deposition time (i.e., the number of CV scans). Since the three components are present in the same solution and may have mutual influences, and to simplify the optimization process, an orthogonalexperimentalmethodwasemployedtoanalyzeandoptimizethe Journal Pre-proofs modification conditions. In this optimization scheme, there are four factors to be considered: the deposition concentrations of Au, Ni and Rh, and the number of CV scans. Each factor was set at four levels, resulting in a 4-factor and 4-level orthogonal experiment. An L16(45) orthogonal table was selected for the experiment design. For ease of reference, the four factors are denoted by letters (A, B, C, and D), and the four levels of each factor are represented by numbers (1, 2, 3, and 4), as shown in Table 1. The designed orthogonal test table is presented in Table 2. The LIG electrode was modified based on the experimental conditions specified in the orthogonal table. Subsequently, NO2- was detected by LSV scanning, and the values of the recorded oxidation peak currents were analyzed in the experimental results column of Table 2 (rightmost column). Range analysis was conducted on the experimental data presented in Table 2. The data obtained at the same levels for each factor were summed. For instance, the data corresponding to level 1 (5 mM) of Factor A (Au concentration) were summed, and the result was recorded as IA (i.e., IA = 27.09 + 35.04 + 23.95 + 22.15 = 108.23). The value of IA reflects the effect of different levels of Factors B, C, and D on level A1. Similarly, IIA, IIIA, and IVA can be calculated. By comparing IA, IIA, IIIA and IVA vertically, differences among them can be attributed to the four different levels of Factor A. The I, II, III and IV of Factors B, C, and D can be calculated following the same process, as illustrated in Fig. 7(A). Subsequently, the range R was calculated, representing the difference between the maximum and minimum values of each factor in I, II, III, and IV. This range reflects the influence of each specific factor on the experimental results at different levels. The ranges (RA, RB, RC, and RD) for the four factors were computed and depicted in the figure. The results indicate that RD has the largest value, while RC has the smallest. Therefore, the order of influence for each factor can be ranked as D >B ≈ A > C (alternatively expressed as DABC, considering the small difference between A and B). Consequently, the number of scans is the primary influencing factor, followed by the concentrations of Ni and Au, and finally, the concentration of Rh. To determine the optimal combination of deposition parameters, we selected the maximum values of I, II, III and IV for each factor, with the corresponding level of the maximum value considered as the best experimental condition. As depicted in Fig. 7(A)(black dashed box), the optimal combination of experimental conditions is A2B4C3D2. In summary, deposition concentrations of 10 mM Au, 20 mM Ni, and 15 mg/L Rh were selected as the optimal values for the metal ions, while 6 cycles were chosen as the optimal number of deposition scans. To further validate this conclusion, LSV was employed to detect NO2- under different parameter combinations, with four repeated experiments conducted for each group, as shown in Fig. 7(B). The experimental results indicate that Combination 8 (Table 2) represents the best experimental condition, which aligns with the conclusions drawn from the orthogonal testing and analysis. 3.6.2 Optimization of detection parameters The pH value of the solution also affects electrochemical detection. To determine the optimal pH value for detection, Au/NiO/Rh/LIG electrodes were immersed in NO2--containing solutions (500 μmol/L) with different pH values for detection (Fig. 8A). In acidic solutions, the peak response current of NO2- gradually increased with increasing pH, reaching its maximum at pH=4.5 (Fig. 8B). This is because at low pH (<4.5), the hydrogen evolution reaction occurs at the electrode interface, generating hydrogen ions, that occupy active sites at the reaction interface [69] and affect the adsorption of NO2-. As the pH value continues to increase, the peak current gradually decreases. This is primarily due to hydrolysis of the metal ions and the influence on certain functional groups at the electrode reaction interface at higher pH values [70, 71]. Consequently, a pH value of 4.5 was chosen as the optimal condition for the PBS solution. And at this pH value, the metal nanoparticles remain stable on the surface of the LIG electrode (Figure S8). Fig. 8(C) illustrates the CV curves of Au/NiO/Rh/LIG for NO2- detection at different scan rates (10 mV/s to 250 mV/s). As the scan rate increases, the oxidation peak current of NO2- gradually rises, indicating that very low scan rates can affect the detectionsensitivity.However,excessivelyhighscanratesintroduceadditional charging current in the system, which also affects the detection results. Therefore, considering overall factors, a scan rate of 100 mV/s was generally selected as the optimal value. Fig. 8(D) shows a plot of the peak oxidation current of NO2- versus scanning rates. After fitting calculations, it was observed that the peak current (Ipa) and the square root of the scan rate follow the equation: ΔIpa(μA) = 2.6655v(mV1/2·s-1/2) +9.9121 (R2 = 0.9746). A significant linear relationship exists between these two factors. Hence, the oxidation process of NO2- on the electrode surface can be considered diffusion-controlled. The peak potential of the NO2- oxidation peak shifts with the scan rate, indicating that the reaction of NO2- on Au/NiO/Rh/LIG is irreversible. Furthermore, the linear relationship between the anodic peak potential (Epa) and the logarithm of the scan rate (v) was investigated, as depicted in Figure. S10(B). The results were analyzed using the Laviron equation, as shown in equations (1) and (2)[72, 73]: Where, Ep represents the peak potential, E0’ is the formal potential, R is the universalgasconstant,Tistheabsolutetemperature,αistheelectrontransfer coefficient, n is the number of electrons transferred, ν is the scan rate, F is Faraday's constant, and ks is the standard rate constant of the reaction. A higher electron transfer rate constant (ks) is obtained from formula (1) indicating a significant enhancement in the catalytic performance of the electrode and the electron transfer process on the electrode surface when NO2- is detected using the Au/NiO/Rh/LIG electrode [74]. Additionally,accordingtoequation(2),theslopeofthefittedcurveallowsthe calculation of n when α is approximately 0.5, revealing that the electron transfer number is 2. Therefore, the oxidation reaction principle of NO2- can be described by the following equation [73, 75]: 3.7 Analytical results 3.7.1 NO2- detection by Au/NiO/Rh/LIG C Under the optimal modification and detection conditions, a stock solution of NO2-wasdilutedwithPBS(pH=4.5)todifferentconcentrationsfortesting.LSV electrochemicaldetectionwasperformedusinganAu/NiO/Rh/LIGelectrodein solutions with varying NO2- concentrations. As shown in Fig. 9(A) and (B), a good linear relationship between the concentration and peak current value was observed when the NO2- concentration was gradually increased from 1 μmol/L to 1 mmol/L. Afterfitting,thefollowinglinearregressionequationwasderived:Ip(µA)=0.0717CNO2-(µM) + 0.276 (R2 = 0.9917). The limit of detection (LOD) was calculated tobe0.3μmol/Latasignal-to-noiseratio(S/N)of3.OurAu/NiO/Rh/LIG electrochemical sensor demonstrates a wider linear detection range and a lower LOD compared to previous similar studies (Table 3). The amperometric test was carried out to analyze the response time of the modified electrode to NO2-, and the typical steady-state current-time response images were obtained as shown in Figure S12. The results show that the bare LIG can recognize the signal quickly after the injection of NO2- into the solution, while the current value is improved. The response time of LIG to NO2- was about 1.8 s calculated after repeating the operation three times. However, under the same experimental conditions, the LIG electrode modified with modified materials showed high and stable current values, which proved the reaction and electron transfer of NO2- on the surface of the working electrode. TheresponseoftheAu/NiO/Rh/LIGelectrodewasveryfast,withanaverage response time of 0.9 s. This result proves that the electrode has a fast response time in detecting NO2-. 3.7.2 Analysis of electrode anti-interference capacity, repeatability, reproducibility and stability To assess the anti-interference performance of the Au/NiO/Rh/LIG electrode, multiplesetsofLSVexperimentswereconductedbyaddinginterferingionsin concentrations more than 50 times higher than that of NO2- to PBS containing 0.2 mM NO2-. The response current values of NO2- and the changes in current values after the addition of other ions are depicted in Fig. 9(C). The experimental data indicated that the response peak current was little affected by the introduction of interfering ions into the target solution. These results demonstrate that common anions and cations present in water environments have negligible effects on the NO2- detection signal of the Au/NiO/Rh/LIG electrode. Thus, the trimetallic-modified LIG electrode (Au/NiO/Rh/LIG)preparedinthisexperimentexhibitsexcellentanti-interference ability. Under optimal detection conditions, the same electrode (Au/NiO/Rh/LIG) was used to detect NO2- five times, as shown in Fig. 9(D). The results show that the current response signal of NO2- is basically stable after multiple detections. This proves that the modified electrode prepared in this study can be continuously tested for many times and has a certain repeatability. Five Au/NiO/Rh/LIG electrodes were prepared using the one-step electrodeposition method and employed to detect a constant concentration of NO2-. As illustrated in Fig. 9(E), the detected current values of the five electrodes exhibited no significant differences, with a relative standard deviation (RSD) of 3.9%. This indicates that the prepared electrodes possess good reproducibility. Electrode stability was assessed by testing the same electrode at five-day intervals, as shown in Fig. 9(F). The peak oxidation current of the Au/NiO/Rh/LIG electrode in response to 0.7mM NO2- on the 15th day was 86.7% of the initial measured value (the modified LIG electrodes are all stored in a vacuum drying oven), indicating that the modified electrode maintains good stability over a certain period of time. 3.7.3 Portable detection of nitrite in real water environments Due to its advantages of low fabrication cost and a simple, fast fabrication process, the Au/NiO/Rh/LIG electrode is highly suitable for on-site detection of real water samples. To assess its practicality and portability, the electrode was utilized as a working electrode in conjunction with a handheld potentiometer for electrochemical detectionofrealwatersamples.Asmartphonewaswirelesslyconnectedtothe potentiometer via Bluetooth, allowing the phone to set relevant detection parameters and receive detection signals. The portable system was employed to detect tap water samples, and the detection procedure is illustrated schematically in Fig. 10. Following the standard addition method, concentrations of 5 µM, 10 µM and 20 µM of NO2- were addedtowatersamples,andtheNO2-concentrationinthewatersampleswas determined under optimal conditions. The experimental results are presented in Table4. The results revealed a recovery rate of 97-101% for NO2- in tap water samples, with a relative standard deviation (RSD) of less than 4.52%. These findings demonstrate the feasibility of utilizing the Au/NiO/Rh/LIG sensor for NO2- detection in real water environments. 4. Conclusions In this study, a one-step electrodeposition method was used to deposit three metals(Au, Ni, and Rh) onto a flexible LIG electrode. By incorporating AuNPs as the primary catalytic materials, a novel electrochemical sensor (Au/NiO/Rh/LIG electrode) was successfully developed for the detection of NO2- in water environments. Characterization techniques such as SEM, TEM, XRD, and XPS were employed to analyze the surface morphology and elemental composition of the modified electrode. The electrochemical performance of the electrode was evaluated using EIS and CV measurements. Additionally, DFT calculations, ECSA, and BET experiments were conducted to investigate the underlying mechanisms responsible for the improved electrocatalysis and adsorption capabilities. The results of our study demonstrate that AuNPs serve as the main catalytic materials for the NO2- oxidation reaction. The introduction of RhNPs enhances the electrode's electrocatalytic activity and reduces impedance, facilitating electron transfer. NiO NPs effectively increase the electrode's surface area and the number of reactive sites. The synergistic effects of these three metal substances contribute to the improved sensitivity of the electrode for NO2- detection. The parameters for electrode preparation were optimized using the orthogonal test method, and experimental conditions such as pH value and sweep speed were also optimized.Undertheoptimalexperimentalconditions,thesensorexhibitedhigh sensitivity (2292.99 μA mM-1 cm-2), surpassing other NO2- sensors by 1.5 to 100 times. The linear range for nitrite detection with the Au/NiO/Rh/LIG electrode was 1 μmol/L to 1 mmol/L, with a limit of detection (LOD) of 0.3 μmol/L. Moreover, the relative standard deviations of the peak current values for NO2- detection were below 10% in the presence of other ions in the solution. The sensor maintained good detection results even after 15 days, showcasing excellent anti-interference capacity, reproducibility, and stability.ThecombinationoftheAu/NiO/Rh/LIGelectrodewithahandheld potentiometer enabled the successful portable detection of nitrite in real water samples. In conclusion, the trimetallic-modified electrode developed in this research presents a novel solution for efficient, portable, and in -situ detection of NO2- in real water environments. Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Acknowledgment This work was financially supported by the National Natural Science Foundation of China (No. 42177061), and the National Key Research and Development Program ofChina(2020YFC1808300).TheauthorswouldliketothankSanZhangfrom Shiyanjia Lab (wwwshiyanjia.com) for the DFT calculation. [1] R. Madhuvilakku, S. Alagar, R. Mariappan, S. Piraman, Glassy carbon electrodes modified with reducedgrapheneoxide-MoS2-poly(3,4-ethylenedioxythiophene)nanocompositesforthenon-enzymatic detection of nitrite in water and milk, Analytica chimica acta 1093 (2020) 93-105. [2] W. Zhao, H.Y. Yang, S. Xu, X.J. Li, W. Wei, X.Y. Liu, "Olive-Structured" Nanocomposite Based on Multiwalled Carbon Nanotubes Decorated with an Electroactive Copolymer for Environmental Nitrite Detection, Acs Sustainable Chemistry & Engineering 7 (2019) 17424-17431. [3] Q.-H. Wang, L.-J. Yu, Y. Liu, L. Lin, R.-g. Lu, J.-p. Zhu, L. He, Z.-L. Lu, Methods for the detection and determination of nitrite and nitrate: A review, Talanta 165 (2017) 709-720. [4] S. Radhakrishnan, K. Krishnamoorthy, C. Sekar, J. Wilson, S.J. Kim, A highly sensitive electrochemical sensor for nitrite detection based on Fe2O3 nanoparticles decorated reduced graphene oxide nanosheets, Applied Catalysis B-Environmental 148 (2014) 22-28. [5] M. Annalakshmi, S. Kumaravel, S.-M. Chen, P. Balasubramanian, T.S.T. Balamurugan, A straightforward ultrasonic-assisted synthesis of zinc sulfide for supersensitive detection of carcinogenic nitrite ions in water samples, Sensors and Actuators B-Chemical 305 (2020) 127387. [6] Y. Ma, X. Song, X. Ge, H. Zhang, G. Wang, Y. Zhang, H. Zhao, In situ growth of alpha-Fe2O3nanorod arrays on 3D carbon foam as an efficient binder-free electrode for highly sensitive and specific determination of nitrite, Journal of Materials Chemistry A 5 (2017) 4726-4736. [7]X.J.Li,J.F.Ping,Y.B.Ying,Recentdevelopmentsincarbonnanomaterial-enabled electrochemical sensors for nitrite detection, Trac-Trends in Analytical Chemistry 113 (2019) 1-12. [8] M.R. Awual, A.M. Asiri, M.M. Rahman, N.H. Alharthi, Assessment of enhanced nitrite removal and monitoring using ligand modified stable conjugate materials, Chemical Engineering Journal 363(2019) 64-72. [9] J. Dai, D. Deng, Y. Yuan, J. Zhang, F. Deng, S. He, Amperometric nitrite sensor based on a glassycarbonelectrodemodifiedwithmulti-walledcarbonnanotubesandpoly(toluidineblue), Microchimica Acta 183 (2016) 1553-1561. [10] A. Gill, J. Zajda, M.E. Meyerhoff, Comparison of electrochemical nitric oxide detection methodswithchemiluminescenceformeasuringnitriteconcentrationinfoodsamples,Analytica Chimica Acta 1077 (2019) 167-173. [11] R. Ahmad, T. Mahmoud, M.-S. Ahn, J.-Y. Yoo, Y.-B. Hahn, Fabrication of sensitive non-enzymatic nitrite sensor using silver-reduced graphene oxide nanocomposite, Journal of Colloid and Interface Science 516 (2018) 67-75. [12] S. Wang, M. Liu, S. He, S. Zhang, X. Lv, H. Song, J. Han, D. Chen, Protonated carbon nitride induced hierarchically ordered Fe2O3/H-C3N4/rGO architecture with enhanced electrochemical sensing of nitrite, Sensors and Actuators B-Chemical 260 (2018) 490-498. [13] J. Chen, S. Pang, L. He, S.R. Nugen, Highly sensitive and selective detection of nitrite ions using Fe3O4@SiO2/Au magnetic nanoparticles by surface-enhanced Raman spectroscopy, Biosensors &Bioelectronics 85 (2016) 726-733. [14] K. Zhang, W. Shi, C. Yang, X. Zhou, Diphenylmethane-based cross-linked polyisocyanide:synthesis and application as nitrite electrochemical probe and N-doped carbon precursor, Journal of Materials Science 55 (2020) 5021-5037. [15] Z.C. Fan, L.X. Sun, S.N. Wu, C. Liu, M.J. Wang, J.S. Xu, X.B. Zhang, Z.W. Tong, Preparation of manganese porphyrin/niobium tungstate nanocomposites for enhanced electrochemical detection of nitrite, Journal of Materials Science 54 (2019) 10204-10216. [16] M. Wang, C. Liu, X. Zhang, Z. Fan, J. Xu, Z. Tong, In situ synthesis of CsTi2NbO7@g-C3N4core-shell heterojunction with excellent electrocatalytic performance for the detection of nitrite, Journal of Materials Research 33 (2018) 3936-3945. [17] Y. Liu, Q. Xue, C. Chang, R. Wang, Q. Wang, X. Shan, Highly efficient detection of Cd(II) ions by a stannum and cerium bimetal-modified laser-induced graphene electrode in water, Chemical Engineering Journal 433 (2022) 133791. [[1188]] C C .. C C h h a a n n g g ,, Q Q .. W W a a n n g g ,, Q Q .. X X u u e e ,, F F.. L L i i u u ,, L L .. H H o o u u ,, S S .. P P u u ,, H H i i g g h h l l y y e e f f f f i i c c i i e e n n t t d d e e t t e e c c t t i i o o n n o o f f chloramphenicol in water using Ag and TiO2 nanoparticles modified laser-induced graphene electrode, Microchemical Journal 173 (2022) 107037. [19] H. Dai, N. Wang, D. Wang, H. Ma, M. Lin, An electrochemical sensor based on phytic acid functionalized polypyrrole/graphene oxide nanocomposites for simultaneous determination of Cd(II) and Pb(II), Chemical Engineering Journal 299 (2016) 150-155. [20] Christos Kokkinosa, Anastasios Economoua, Dimosthenis Giokas, Paper-based device with a sputtered tin-film electrode for the voltammetric determination of Cd(II) and Zn(II), Sensors and Actuators B: Chemical 260 (2018) 223–226. [21] Z. Liu, Q. Wang, Q. Xue, C. Chang, R. Wang, Y. Liu, H. Xie, Highly efficient detection of ofloxacin in water by samarium oxide and β-cyclodextrin-modified laser-induced graphene electrode., Microchemical Journal 186 (2023) 108353. [22] D.P. Rocha, V.N. Ataide, A. de Siervo, J.M. Gonsalves, R.A.A. Munoz, T.R.L.C. Paixa, L. Angnes,Reagentlessandsub-minutelaser-scribingtreatmenttoproduceenhanceddisposable electrochemical sensors via additive manufacture, Chemical Engineering Journal 425 (2021) 108353. [23] F. Cui, H. Sun, X. Yang, H. Zhou, Y. Wu, J. Li, H. Li, J. Liu, C. Zeng, B. Qu, J. Zhang, Q. Zhou, Laser-induced graphene (LIG)-based Au@CuO/V2CTx MXene non-enzymatic electrochemical sensors for the urine glucose test Chemical Engineering Journal 457 (2023) 141303. [24] Z. Liu, R. Wang, Q. Xue, C. Chang, Y. Liu, L. He, Highly efficient detection of Cd(II) ions in water by graphitic carbon nitride and tin dioxide nanoparticles modified glassy carbon electrode, Inorganic Chemistry Communications 148 (2023) 110321. [25] Q. Wang, Q. Xue, T. Chen, J. Li, Y. Liu, X. Shan, F. Liu, J. Jia, Recent advances in electrochemical sensors for antibiotics and their applications, Chinese Chemical Letters 32 (2021) 609-619. [26] Z. Lu, Y. Li, T. Liu, G. Wang, M. Sun, Y. Jiang, H. He, Y. Wang, P. Zou, X. Wang, Q. Zhao, H. Rao, A dual-template imprinted polymer electrochemical sensor based on AuNPs and nitrogen-doped grapheneoxidequantumdotscoatedonNiS2/biomasscarbonforsimultaneousdeterminationof dopamine and chlorpromazine, Chemical Engineering Journal 389 (2020) 124417. [27] Mounesh, K.R.V. Reddy, Sensitive and reliable electrochemical detection of nitrite and H2O2embellish-CoPc coupled with appliance of composite MWCNTs, Analytica Chimica Acta 1108 (2020)98-107. [28] M.Y. Xu, X.J. Zhu, H. Hong, K.Z. Hu, R. Yang, X.Y. Wang, X.J. Li, X.Y. Liu, An electro-active amphiphilic copolymer to functionalize carbon nanotubes for highly sensitive determination of nitrite in water, Colloids and Surfaces a-Physicochemical and Engineering Aspects 576 (2019) 123-129. [29] X.R. Lin, Y.F. Zheng, X.C. Song, Electrocatalysis and Detection of Nitrite on a Pd/Fe2O3Nanocomposite Modified Glassy Carbon Electrode, Journal of Nanoscience and Nanotechnology 18(2018) 4858-4864. [30] N. Comisso, S. Cattarin, P. Guerriero, L. Mattarozzi, M. Musiani, L. Vazquez-Gomez, E. Verlato,StudyofCu,Cu-NiandRh-modifiedCuporouslayersaselectrodematerialsforthe electroanalysis of nitrate and nitrite ions, Journal of Solid State Electrochemistry 20 (2016) 1139-1148. [31] Z.T. Zhao, J. Zhang, W.D. Wang, Y.J. Sun, P.W. Li, J. Hu, L. Chen, W.P. Gong, Synthesis and electrochemical properties of Co3O4-rGO/CNTs composites towards highly sensitive nitrite detection, Applied Surface Science 485 (2019) 274-282. [32] Y. Gao, R. Li, S. Chen, L. Luo, T. Cao, W. Huang, Morphology-dependent interplay of reduction behaviors, oxygen vacancies and hydroxyl reactivity of CeO2 nanocrystals, Physical Chemistry Chemical Physics 17 (2015) 31862-31871. [33] E. Rezvani, A. Hatamie, M. Berahman, M. Simchi, S. Angizi, R. Rahmati, J. Kennedy, A. Simchi, Synthesis, First-Principle Simulation, and Application of Three-Dimensional Ceria Nanoparticles/Graphene Nanocomposite for Non-Enzymatic Hydrogen Peroxide Detection, Journal of the Electrochemical Society 166 (2019) H3167-H3174. [34] Y.F. Dai, J.Z. Huang, H.C. Zhang, C.C. Liu, Highly sensitive electrochemical analysis of tunnel structured MnO2 nanoparticle-based sensors on the oxidation of nitrite, Sensors and Actuators B-Chemical 281 (2019) 746-750. [35] R.M.A. Hameed, S.S. Medany, Sensitive nitrite detection at core-shell structured Cu@Pt nanoparticles supported on graphene, Applied Surface Science 458 (2018) 252-263. [36] G. Manjari, S. Saran, S. Radhakrishanan, P. Rameshkumar, A. Pandikumar, S.P. Devipriya, Facile green synthesis of Ag-Cu decorated ZnO nanocomposite for effective removal of toxic organic compounds and an efficient detection of nitrite ions, Journal of Environmental Management 262 (2020)110282. [37] S.S. Zhao, J.H. Tong, Y. Li, J.Z. Sun, C. Bian, S.H. Xia, Palladium-Gold Modified Ultramicro Interdigital Array Electrode Chip for Nitrate Detection in Neutral Water, Micromachines 10 (2019) 223. [38] T. Chen, Y.X. Li, L.Y. Li, Y.J. Zhao, S.H. Shi, R.Y. Jiang, H.Y. Ma, Cu Modified Pt Nanoflowers with Preferential (100) Surfaces for Selective Electroreduction of Nitrate, Catalysts 9 (2019)536. [39] A. Mahmoodi, A.A. Ensafi, B. Rezaei, Fabrication of Electrochemical Sensor Based on CeO2-SnO2 Nanocomposite Loaded on Pd Support for Determination of Nitrite at Trace Levels, Electroanalysis 32 (2020) 1025-1033. [40] C.X. Yin, W.J. Weng, R. Gao, J. Liu, Y.Y. Niu, G.J. Li, W. Sun, Investigation of the direct electrochemistry and electrocatalysis of myoglobin on gold nanorods modified electrode, Journal of the Chinese Chemical Society 66 (2019) 1341-1346. [41] Y. Zhou, M. Ma, H. He, Z. Cai, N. Gao, C. He, G. Chang, X. Wang, Y. He, Highly sensitive nitrite sensor based on AuNPs/RGO nanocomposites modified graphene electrochemical transistors, Biosensors & bioelectronics 146 (2019) 111751. [42]H.Y.Yin,Y.Wan,L.Wang,Au/NiO/Multi-WalledCarbonNanotubesNanocomposite Electrode for Nitrite Electrochemical Determination, Journal of Nanoscience and Nanotechnology 19(2019) 5279-5286. [43] S. Dong, Z. Li, Y. Fu, G. Zhang, D. Zhang, M. Tong, T. Huang, Bimetal-organic framework Cu-Ni-BTCanditsderivativeCuO@NiO:Constructionofthreeenvironmentalsmall-molecule electrochemical sensors, Journal of Electroanalytical Chemistry 858 (2020) 113785. [44] H. Bao, S. Xia, F. Wu, F. Li, L. Zhang, Y. Yuan, G. Xu, W. Niu, Surface engineering of Rh-modified Pd nanocrystals by colloidal underpotential deposition for electrocatalytic methanol oxidation, Nanoscale 13 (2021) 5284-5291. [45] G. Kresse, D. Joubert, From ultrasoft pseudopotentials to the projector augmented-wave method, Physical Review B 59 (1999) 1758-1775. [46] G. Kresse, J. Furthmuller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Physical Review B 54 (1996) 11169-11186. [47] J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple, Physical Review Letters 77 (1996) 3865-3868. [48] P.E. Blochl, PROJECTOR AUGMENTED-WAVE METHOD, Physical Review B 50 (1994)17953-17979. [49] S. Grimme, J. Antony, S. Ehrlich, H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu, Journal of Chemical Physics 132 (2010) 154104. [50] G. Henkelman, B.P. Uberuaga, H. Jonsson, A climbing image nudged elastic band method for finding saddle points and minimum energy paths, Journal of Chemical Physics 113 (2000) 9901-9904. [51] C. Chang, Q. Xue, R. Wang, Z. Liu, Y. Liu, L. He, F. Liu, H. Xie, Development of a novel sensor based on Bi2O3 and carbonized UIO-66-NH2 nanocomposite for efficient detection of Pb(II) ion in water environment, Applied Surface Science 616 (2023) 156510. [52] G. Meng, F. Long, Z. Zeng, L. Kong, B. Zhao, J. Yan, L. Yang, Y. Yang, X.-Y. Liu, Z. Yan, N. Lin, Silk fibroin based wearable electrochemical sensors with biomimetic enzyme-like activity constructed for durable and on-site health monitoring, Biosensors & Bioelectronics 228 (2023) 115198. [53]J.-H.Han,S.H.Park,S.Kim,J.J.Pak,Aperformanceimprovementofenzyme-based electrochemical lactate sensor fabricated by electroplating novel PdCu mediator on a laser induced graphene electrode, Bioelectrochemistry 148 (2022) 108259. [54] Z. Xing, Z. Ju, Y. Zhao, J. Wan, Y. Zhu, Y. Qiang, Y. Qian, One-pot hydrothermal synthesis of Nitrogen-doped graphene as high-performance anode materials for lithium ion batteries, Scientific Reports 6 (2016) 26146. [55] T.A. Amollo, G.T. Mola, V.O. Nyamori, Germanium quantum dot/nitrogen-doped graphene nanocomposite for high-performance bulk heterojunction solar cells, Rsc Advances 8 (2018) 21841-21849. [56] L. Wang, W. Lu, W. Zhu, H. Wu, F. Wang, X. Xu, A photoelectrochemical sensor for highly sensitive detection of glucose based on Au-NiO1-x hybrid nanowires, Sensors and Actuators B-Chemical 304 (2020) 127330. [57] A.J. Young, M. Sauer, G.M.D.M. Rubio, A. Sato, A. Foelske, C.J. Serpell, J.M. Chin, M.R. Reithofer,One-stepsynthesisandXPSinvestigationsofchiralNHC-Au(0)/Au(i)nanoparticles, Nanoscale 11 (2019) 8327-8333. [58] M. Govindasamy, S. Manavalan, S.-M. Chen, U. Rajaji, T.-W. Chen, F.M.A. Al-Hemaid, M.A. Ali, M.S. Elshikh, Determination of Neurotransmitter in Biological and Drug Samples Using Gold Nanorods Decorated f-MWCNTs Modified Electrode, Journal of the Electrochemical Society 165 (2018) B370-B377. [59] J. Feng, J. Liu, X. Cheng, J. Liu, M. Xu, J. Zhang, Hydrothermal Cation Exchange Enabled Gradual Evolution of Au@ZnS-AgAuS Yolk-Shell Nanocrystals and Their Visible Light Photocatalytic Applications, Advanced Science 5 (2018) 1700376. [60] T. Zhan, H. Yin, J. Zhu, J. Chen, J. Gong, L. Wang, Q. Nie, Ni-3(PO4)(2) nanoparticles decorated carbon sphere composites for enhanced non-enzymatic glucose sensing, Journal of Alloys and Journal Pre-proofs Compounds 786 (2019) 18-26. [61] C. Cao, L. Wei, G. Wang, J. Shen, In-situ growing NiCo2O4 nanoplatelets on carbon cloth as binder-free catalyst air-cathode for high-performance microbial fuel cells, Electrochimica Acta 231(2017) 609-616. [62] M. Akbayrak, A.M. Onal, High Durability and Electrocatalytic Activity Toward Hydrogen Evolution Reaction with Ultralow Rhodium Loading on Titania, Journal of the Electrochemical Society 167 (2020) 156501. [63] S. Xu, Y. Zhang, M. Du, S. Wang, Y. Wei, T. Cheng, Hollow Ag@Au-Rh core-frame nanocubes for electrochemical sensing and catalytic degradation of environmental pollutants, Journal of the Taiwan Institute of Chemical Engineers 129 (2021) 118-127. [64] X. Mao, L. Yang, Z. Zou, L. Luo, X. Zhang, D. Tian, H. Deng, H. Li, Dye responsive optical-electrochemical-wettability on a naphthalene-appended calix 4 arene clicking surface, Sensors and Actuators B-Chemical 212 (2015) 371-376. [65] F. Zhao, S. Zhou, Y. Zhang, Ultrasensitive Detection of Hydrogen Peroxide Using Bi2Te3Electrochemical Sensors, Acs Applied Materials & Interfaces 13 (2021) 4761-4767. [66] T. Gao, Z. Jin, M. Liao, J. Xiao, H. Yuan, D. Xiao, A trimetallic V-Co-Fe oxide nanoparticle as an efficient and stable electrocatalyst for oxygen evolution reaction, Journal of Materials Chemistry A 3 (2015) 17763-17770. [67] T.Y. Ma, S. Dai, M. Jaroniec, S.Z. Qiao, Metal-Organic Framework Derived Hybrid Co3O4-Carbon Porous Nanowire Arrays as Reversible Oxygen Evolution Electrodes, Journal of the American Chemical Society 136 (2014) 13925-13931. [68] F.J. Garcia-Garcia, P. Salazar, F. Yubero, A.R. Gonzalez-Elipe, Non-enzymatic Glucose electrochemical sensor made of porous NiO thin films prepared by reactive magnetron sputtering at oblique angles, Electrochimica Acta 201 (2016) 38-44. [69] Z. Lu, W. Dai, X. Lin, B. Liu, J. Zhang, J. Ye, J. Ye, Facile one-step fabrication of a novel 3D honeycomb-like bismuth nanoparticles decorated N-doped carbon nanosheet frameworks: Ultrasensitive electrochemical sensing of heavy metal ions, Electrochimica Acta 266 (2018) 94-102. [70] G. Ran, Y. Li, Y. Xia, Graphene oxide and electropolymerized p-aminobenzenesulfonic acid mixed film used as dopamine and serotonin electrochemical sensor, Monatshefte Fur Chemie 151 (2020)293-299. [71] P.W. Sayyad, T.R. Ansari, N.N. Ingle, T. Al-Gahouari, G.A. Bodkhe, M.M. Mahadik, S.M. Shirsat, M.D. Shirsat, L-Cysteine peptide-functionalized PEDOT-PSS/rGO nanocomposite for selective electrochemical detection of lead Pb(II) ions, Applied Physics a-Materials Science & Processing 127(2021) 381. [72] S. Sahoo, P.K. Sahoo, A. Sharma, A.K. Satpati, Interfacial polymerized RGO/MnFe2O4/polyaniline fibrous nanocomposite supported glassy carbon electrode for selective and ultrasensitive detection of nitrite, Sensors and Actuators B-Chemical 309 (2020) 127763. [73] C.C. Li, D.L. Chen, Y.Y. Wang, X.Y. Lai, J. Peng, X.H. Wang, K.X. Zhang, Y. Cao, Simultaneous Electrochemical Detection of Nitrite and Hydrogen Peroxide Based on 3D Au-rGO/FTO Obtained Through a One-Step Synthesis, Sensors 19 (2019) 1304. [74] P. Li, Y. Ding, A. Wang, L. Zhou, S. Wei, Y. Zhou, Y. Tang, Y. Chen, C. Cai, T. Lu, Self-Assembly of Tetrakis (3-Trifluoromethylphenoxy) Phthalocyaninato Cobalt(II) on Multiwalled Carbon Nanotubes and Their Amperometric Sensing Application for Nitrite, Acs Applied Materials & Interfaces 5 (2013) 2255-2260. [75] G. Xu, B. Li, X.T. Cui, L. Ling, X. Luo, Electrodeposited conducting polymer PEDOT doped with pure carbon nanotubes for the detection of dopamine in the presence of ascorbic acid, Sensors and Actuators B-Chemical 188 (2013) 405-410. [76]A.Abo-Hamad,M.A.AlSaadi,M.A.Hashim,Eutecticmixture-functionalizedcarbon nanomaterials for selective amperometric detection of nitrite using modified glassy carbon electrode, Journal of Electroanalytical Chemistry 812 (2018) 107-114. [77] N.-C. Lo, I.W. Sun, P.-Y. Chen, CuAg nanoparticles formed in situ on electrochemically pre-anodized screen-printed carbon electrodes for the detection of nitrate and nitrite anions, Journal of the Chinese Chemical Society 65 (2018) 982-988. [78] V.S. Manikandan, Z. Liu, A. Chen, Simultaneous detection of hydrazine, sulfite, and nitrite based on a nanoporous gold microelectrode, Journal of Electroanalytical Chemistry 819 (2018) 524-532. [79] D. Manoj, R. Saravanan, J. Santhanalakshmi, S. Agarwal, V.K. Gupta, R. Boukherroub, TowardsgreensynthesisofmonodisperseCunanoparticles:Anefficientandhighsensitive electrochemical nitrite sensor, Sensors and Actuators B-Chemical 266 (2018) 873-882. [80] M.A.T. Masoud Ghanei-Motlagh, A novel electrochemical sensor based on silver/halloysite nanotube/molybdenum disulfide nanocomposite for efficient nitrite sensing, Biosensors and Bioelectronic 109 (2018) 279-285. 0 Figure legends Fig. 1. Schematic diagram illustrating the preparation of LIG electrode and the one-step electrodeposition modification of LIG. Fig. 2. SEM images comparing the bare electrode (A) and the trimetallic-modified electrode (Au/NiO/Rh/LIG) (B, C). HR-TEM images of Au/NiO/Rh/LIG showing lattice fringes of nanoparticles (E) (a, b, c). EDS elemental mapping of Au/NiO/Rh/LIG. Fig. 3. XRD pattern (A) and Raman spectra (B) of LIG and Au/NiO/Rh/LIG. Fig. 4. Full-survey XPS spectra of bare LIG and Au/NiO/Rh/LIG (A). XPS spectra of C 1s (B), O 1s (C), Au 4f (D), Ni 2p (E), and Rh 3d (F) for Au/NiO/Rh/LIG. CA measurement images of LIG (G) and Au/NiO/Rh/LIG electrode (H). Fig. 5. Comparison graphs of CV measurements (A), EIS analysis (B) and LSV measurements (C) before and after electrode modification. Density of states (DOS) graphs of Au/LIG (D), Au/Ni/LIG (E), and Au/Rh/LIG (F) electrodes. Simulated structures of C-Au, C-AuRh, and C-AuNiO-modified materials (G). Bader charges corresponding to the three structures (H). Fig. 6. CV response curves of Au/LIG (A), Au/Rh/LIG (B), Au/NiO/LIG (C), and Au/NiO/Rh/LIG (D) electrodes in 1 mol/L potassium hydroxide solution at different scan rates. Values of differences in current density corresponding to different scanning rates and linear fitting curves (E). Adsorption energy (F) of NO2- for different modified electrodes and simulated structure (G) of NO2- adsorbed by C-Au, C-AuRh, and C-AuNiO. Fig. 7. Range analysis diagram of orthogonal testing results (A). Current value of NO2-detected by different parameter combinations in the orthogonal test (B). Fig. 8. (A) LSV responses of Au-Ni-Rh/LIG electrode to NO2- in electrolyte solutions with different pH values. (B) Relationship between pH and peak current value. CV responseofAu-Ni-Rh/LIGelectrodetoNO2-atdifferentsweepspeeds(C). Corresponding peak current vs. square root of scan rate plot (D). Fig.9.LSVresponsesofAu/NiO/Rh/LIGinPBS(pH=4.5)containingvarious concentrations of NO2- (A). Plot of peak current versus NO2- concentration (B). Effects of the addition of different kinds of ions on the peak current of NO2- detection (C). Repeatability(D),reproducibility(E)andstability(F)analysisdiagramsfor Au/NiO/Rh/LIG (LSV detection was performed on NO2- with a concentration of0.7mM). Fig. 10. Schematic diagram illustrating the application of Au/NiO/Rh/LIG electrode for portable detection of NO2- in water environments. ot s ro re-p l P na ourJ 1m Fig. 1. Schematic diagram illustrating the preparation of LIG electrode and the one-step electrodeposition modification of LIG. Fig. 2. SEM images comparing the bare electrode (A) and the trimetallic-modified electrode (Au/NiO/Rh/LIG) (B, C). HR-TEM images of Au/NiO/Rh/LIG showing lattice fringes of nanoparticles (E) (a, b, c). EDS elemental mapping of Au/NiO/Rh/LIG. Jou rn Raman shift(cm) Fig. 3. XRD pattern (A) and Raman spectra (B) of LIG and Au/NiO/Rh/LIG.p -e r P l a nr u o J Fig. 4. Full-survey XPS spectra of bare LIG and Au/NiO/Rh/LIG (A). XPS spectra of C 1s (B), O 1s (C), Au 4f (D), Ni 2p (E), and Rh 3d (F) for Au/NiO/Rh/LIG. CA measurement images of LIG (G) and Au/NiO/Rh/LIG electrode (H). Fig. 5. Comparison graphs of CV measurements (A), EIS analysis (B) and LSV measurements (C) before and after electrode modification. Density of states (DOS) graphs of Au/LIG (D), Au/Ni/LIG (E), and Au/Rh/LIG (F) electrodes. Simulated structures of C-Au, C-AuRh, and C-AuNiO-modified materials (G). Bader charges corresponding to the three structures (H). O U J Fig. 6. CV response curves of Au/LIG (A),Au/Rh/LIG (B),Au/NiO/LIG (C), and Au/NiO/Rh/LIG (D) electrodes in 1 mol/L potassium hydroxide solution at different scan rates. Values of differences i n current density corresponding to di f ferent scanning rates and l inear f i tting curves (E). Adsorption energy (F) of NOz for different modified electrodes and simulated structure (G) of NO2 adsorbed by C-Au, C-AuRh, and C-AuNiO. Fig. 7. Range analysis diagram of orthogonal testing results (A). Current value of NO2- detected by different parameter combinations in the orthogonal test (B). Fig. 8. (A) LSV responses of Au-Ni-Rh/LIG electrode to NO2- in electrolyte solutions with different pH values. (B) Relationship between pH and peak current value. CVresponseofAu-Ni-Rh/LIGelectrodetoNO2-atdifferentsweepspeeds(C). Corresponding peak current vs. square root of scan rate plot (D). Fig. 9. LSV responses of Au/NiO/Rh/LIG in PBS (pH=4.5) containing various concentrations of NO2- (A). Plot of peak current versus NO2- concentration (B). Effects of the addition of different kinds of ions on the peak current of NO2- detection (C). Repeatability(D),reproducibility(E)andstability(F)analysisdiagramsfor Au/NiO/Rh/LIG (LSV detection was performed on NO2- with a concentration of0.7mM). Fig.10.SchematicdiagramillustratingtheapplicationofAu/NiO/Rh/LIG electrode for portable detection of NO2- in water environments. l P rnaou J fSo l P re -our na J Table. 2 Orthogonal table for the optimization of electrode modification conditions. Numbe Result Numbe r Concentratio n of Au Concentratio n of Ni Concentratio n of Rh r of Scans s (μA) 1 1 1 1 1 27.09 2 1 2 2 2 35.04 3 1 3 3 3 23.95 4 1 4 4 2 仑21 4 22.15 5 2 1 3 24.73 6 2 2 4 33.41 7 2 公 3 4 1 24.47 8 2 4 3 2 39.93 9 3 1 3 4 31.73 10 3 2 4 3 30.55 11 3 3 1 2 28.61 12 3 4 2 1 31.17 13 4 1 4 2 28.32 14 4 2 3 1 22.22 15 4 3 2 4 25.59 16 4 4 1 3 28.39 16 4 4 1 3 28.39 Table. 3 Performance comparison of Au/NiO/Rh/LIG with other nitrite sensors. Method Sensitivity (μA mM-1 cm- 2) Linear range (μM) LOD (μM) Ref. MnTMPyP/NbWO6/CE 92.53 120~3750 38 [15] MnO2/AuCE 17100 10~800 0.5 [34] Ag-Cu@ZnO 350.1 0~1500 17 [36] CeO2-SnO2/Pd/RDE 652.95 0.36~2200 0.1 [39] Cu/MWCNT/Gr/GCE 811.03 5~4600 0.3 [76] CuAgNP/SPCE 22.5 20 ~370 11.1 [77] AuNPs/ME 14.12 5~4000 1.44 [78] 38 Journal Pre-proofs Cu/MWCNTs/GC 455.84 5~1260 1.8 [79] Ag/HNT/MoS2/CPEs 89.9 2~425 0.7 [80] Au/Rh/LIG 1850.32 3~800 1.06 This work Au/NiO/LIG 2104.64 2~850 0.98 This work Au/NiO/Rh/LIG 2292.99 1~1000 0.3 This work re-0l Table. 4 NO2- recovery determination in tap water samples. Sample RSD (%) Recovery (%) Sample Added/μM Found/μM RSD (%) Recovery (%) 5 4.88 4.52 97 Tap water 10 9.96 3.94 97 20 20.32 3.53 101 Declaration of interests ☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Journal Pre-proofs ☐The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: fs r oQ e-pr l P na ou r J Graphical Abstract NO2- detection based on Au-Ni-Rh/LIG electrochemical sensor. l a rnu oJ Journal Pre-proofs Highlights Au, NiO, and Rh nanoparticles were deposited onto LIG by one-step electrodeposition. DFT calculation confirmed the enhanced adsorption energy and electron transfer. Three metals promoted the detection of nitrite in terms of catalysis and adsorption. The developed sensor obtained a very low detection limit of 0.3 μM for nitrite. This sensor can be used for portable determination of NO2- in real water samples.%o rp -e r P l a n r u o J
确定






























还剩41页未读,是否继续阅读?
雷迪美特中国有限公司为您提供《【EmStat3Blue电化学应用】一步电沉积法制备Au/NiO/Rh三金属复合材料修饰激光诱导石墨烯电极,高效检测水中亚硝酸盐》,该方案主要用于饮用水中无机阴离子检测,参考标准--,《【EmStat3Blue电化学应用】一步电沉积法制备Au/NiO/Rh三金属复合材料修饰激光诱导石墨烯电极,高效检测水中亚硝酸盐》用到的仪器有EmStat4X便携式电化学分析仪
推荐专场
相关方案
更多
该厂商其他方案
更多
















