盐酸氯丙嗪含量测定 应用资料
根据中国药典2020年版,盐酸氯丙嗪【含量测定】取本品约0.2g,精密称定,加冰醋酸10ml与醋酐30ml溶解后,照电位滴定法(通则0701),用高氯酸滴定液(0.1mol/L)滴定,并将滴定的结果用空白试验校正。每1ml高氯酸滴定液(0.1mol/L)相当于35.53mg的C17H19ClN2S·HCl。
方案详情

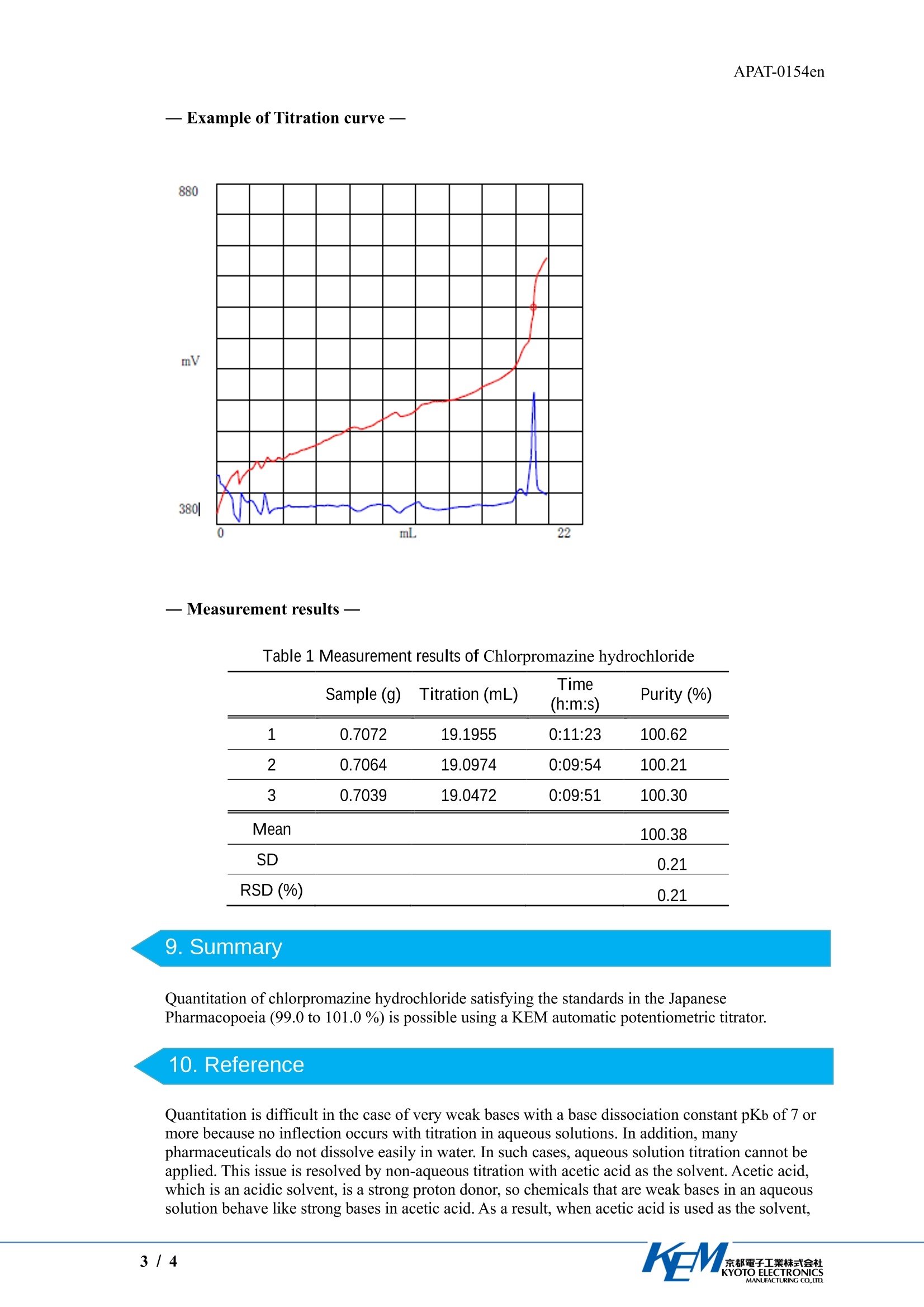
APAT-0154er京都電子工業株式会社KYOTO ELECTRONICSMANUFACTURING CO.,LTD. APAT-0154en5.Reagents Application Note Quantitation of chlorpromazine hydrochloride bytitration with acetic acid/acetic anhydride as thesolvent Industry Pharmaceutical Instrument Automatic potentiometric titrator Measurement method . Potentiometric titration / Neutralization titration Standards Japanese Pharmacopoeia 1.Scope Chlorpromazine hydrochloride is one of the pharmaceuticals listed in the Japanese PharmacopoeiaThis Application Note describes an example of the quantitation of this compound based on theJapanese Pharmacopoeia. 2. Precautions ·Desiccate the sample prior to use. The desiccation conditions are as follows. (Desiccation conditions: Introduce 1 g of the sample. Heat it for 2 hours at 105 C. Then cool it tcroom temperature in a desiccator.) · The sample is not easily dissolved in the solvent, so stir it thoroughly, and start the measurementsafter the sample is completely dissolved. · The sample gradually deteriorates when exposed to light,so refrigerate and shade it from lightduring storage. In this titration, if water is mixed in, it will lead to errors. To avoid water contamination, use one ofthe following for the electrolyte in the reference electrode. 1)1 mol/L Lithium chloride acetic acid solution 2) Saturated sodium perchlorate acetic acid solution Electrolyte (1) is available from KEM, so contact us if you would like to order it. Electrolyte(2)must be prepared by the operator. When preparing this solution, saturate acetic acid with anhydroussodium perchlorate, and use the supernatant liquidd. 3.Post-measurement procedure To prevent leakage of the electrolyte, when the combined glass electrode is not in use, seal theelectrolyte filling port with a rubber stopper.The performance of the electrodes quickly deteriorates if they are stored while dry. The followingstorage methods are recommended. · Short-term storage (less than one month): Store in pure water. .·Long-term storage (one month or longer): Store in a mixture of pH4 standard solution and 3.3 mol/Ipotassium chloride solution in a 1:1 volume ratio. 4. Apparatus Main unit Automatic potentiometric titrator (preamplifier: STD) Electrode Combined glass electrode double junction type Titration liquid: 0.1 mol/L perchloric acid acetic acid solution* Solvent: Solution combining acetic anhydride and acetic acid in a 7:3 volume ratio * Refer to the Japanese Pharmacopoeia for details on the preparation procedures. 6.Procedure -Pretreatment- 1) Desiccate the sample. (Desiccation conditions:1 g, 105C, 2 hours) -Measurement- 1) Introduce approximately 0.7 g of the sample into a beaker, and weigh it. 2) Add 50 mL of the solvent, and stir until the sample is completely dissolved. 3) Titrate using a 0.1 mol/L perchloric acid acetic acid solution, with the inflection point in thetitration curve as the end point. 4) In addition, perform a blank test separately to correct the titration volume whenmeasuring the sample. 7.Calculation Purity of chlorpromazine hydrochloride (%)=(EP1-BL1)×TF×C1×K1/S EP11Titration volume (mL)1 BL1 Titration volume during a blank test =0.0662 mL Titration liquid factor= 1.0469* a1 Concentration conversion coefficient =35.53 mg/mLs Unit conversion coefficient = 0.1 Amount of sample introduced (g) * This factor is calculated by using potassium hydrogen phthalate, the standard substance forvolumetric analysis, as the standard. Refer to the Japanese Pharmacopoeia for details on standardization procedures. 一Measurement results Table 1 Measurement results of Chlorpromazine hydrochloride Sample (g) Titration (mL) Time Purity (%) (h:m:s) 1 0.7072 19.1955 0:11:23 100.62 2 0.7064 19.0974 0:09:54 100.21 3 0.7039 19.0472 0:09:51 100.30 Mean 100.38 SD 0.21 RSD(%) 0.21 9. Summary Quantitation of chlorpromazine hydrochloride satisfying the standards in the JapanesePharmacopoeia (99.0 to 101.0%) is possible using a KEM automatic potentiometric titrator. 10. Reference Quantitation is difficult in the case of very weak bases with a base dissociation constant pKb of 7 ormore because no inflection occurs with titration in aqueous solutions. In addition, manypharmaceuticals do not dissolve easily in water. In such cases, aqueous solution titration cannot beapplied. This issue is resolved by non-aqueous titration with acetic acid as the solvent. Acetic acid,which is an acidic solvent, is a strong proton donor, so chemicals that are weak bases in an aqueoussolution behave like strong bases in acetic acid. As a result, when acetic acid is used as the solvent, inflections near the end point become apparent, thus enabling quantitation. For this reason, non-aqueous titration with acetic acid as the solvent is specified as a quantitation method for manypharmaceuticals listed in the Japanese Pharmacopoeia. / 都電子工業株式会社KYOTO ELECTRONICSMANUFACTURING CO.,LTD. 盐酸氯丙嗪含量测定 应用资料(英文版)根据中国药典2020年版,盐酸氯丙嗪【含量测定】取本品约0.2g,精密称定,加冰醋酸10ml与醋酐30ml溶解后,照电位滴定法(通则0701),用高氯酸滴定液(0.1mol/L)滴定,并将滴定的结果用空白试验校正。每1ml高氯酸滴定液(0.1mol/L)相当于35.53mg的C17H19ClN2S·HCl。
确定



还剩2页未读,是否继续阅读?
可睦电子(上海)商贸有限公司-日本京都电子(KEM)为您提供《盐酸氯丙嗪中有效成分含量检测方案(自动电位滴定)》,该方案主要用于化药制剂中含量测定检测,参考标准--,《盐酸氯丙嗪中有效成分含量检测方案(自动电位滴定)》用到的仪器有AT-710M四通道旗舰型自动电位滴定仪、AT-710S豪华型自动电位滴定仪
推荐专场
相关方案
更多
该厂商其他方案
更多