方案详情
文
乳酸依沙吖啶含量测定 应用资料
根据中国药典2020年版,乳酸依沙吖啶【含量测定】取本品约0.27g,精密称定,加无水甲酸5.0ml使溶解,加冰醋酸60ml,照电位滴定法(通则0701),用高氯酸滴定液(0.1mol/L)滴定,并将滴定的结果用空白试验校正。每1ml高氯酸滴定液(0.1mol/L)相当于34.34mg的C15H15N3O·C3H6O3。
方案详情

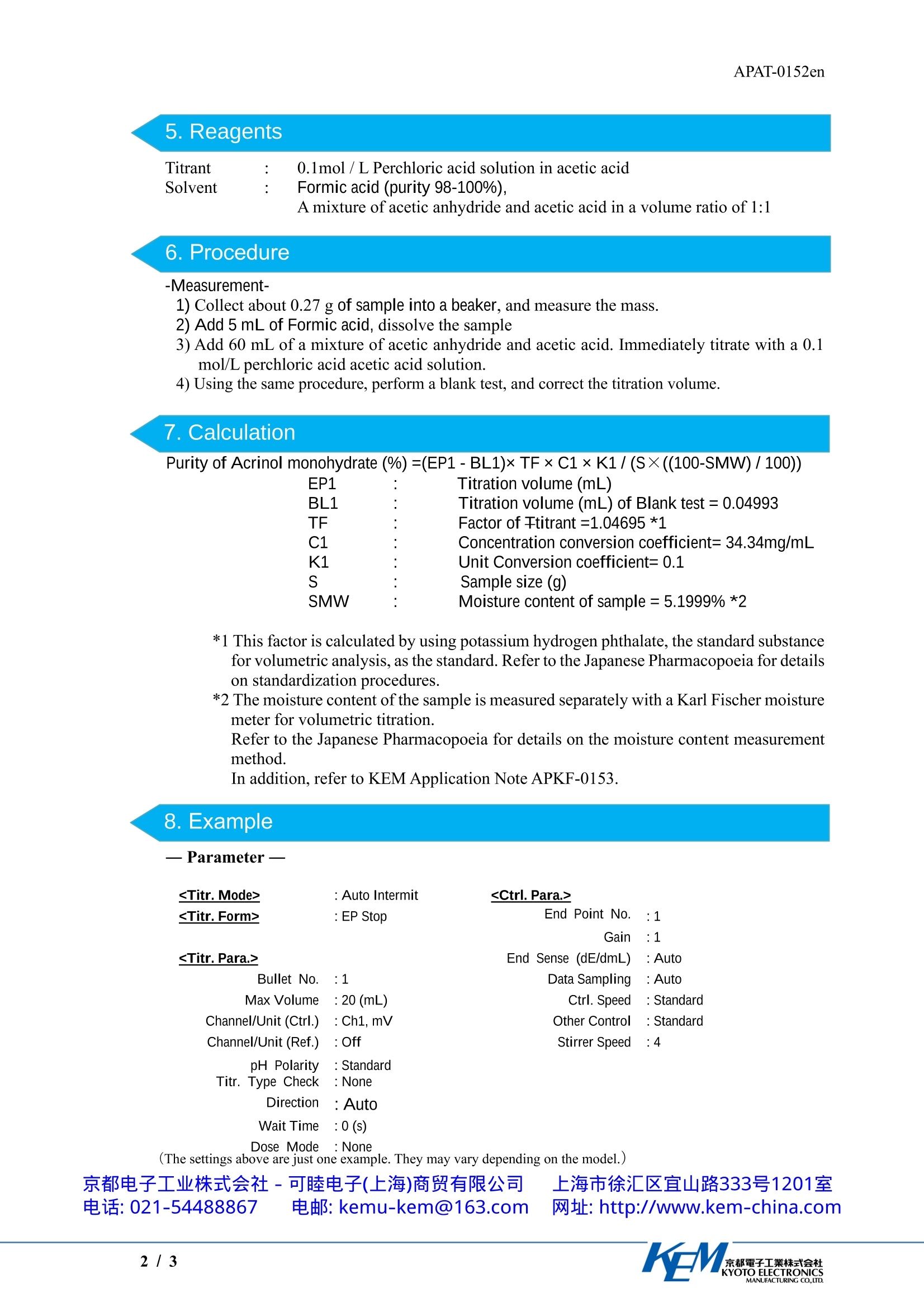
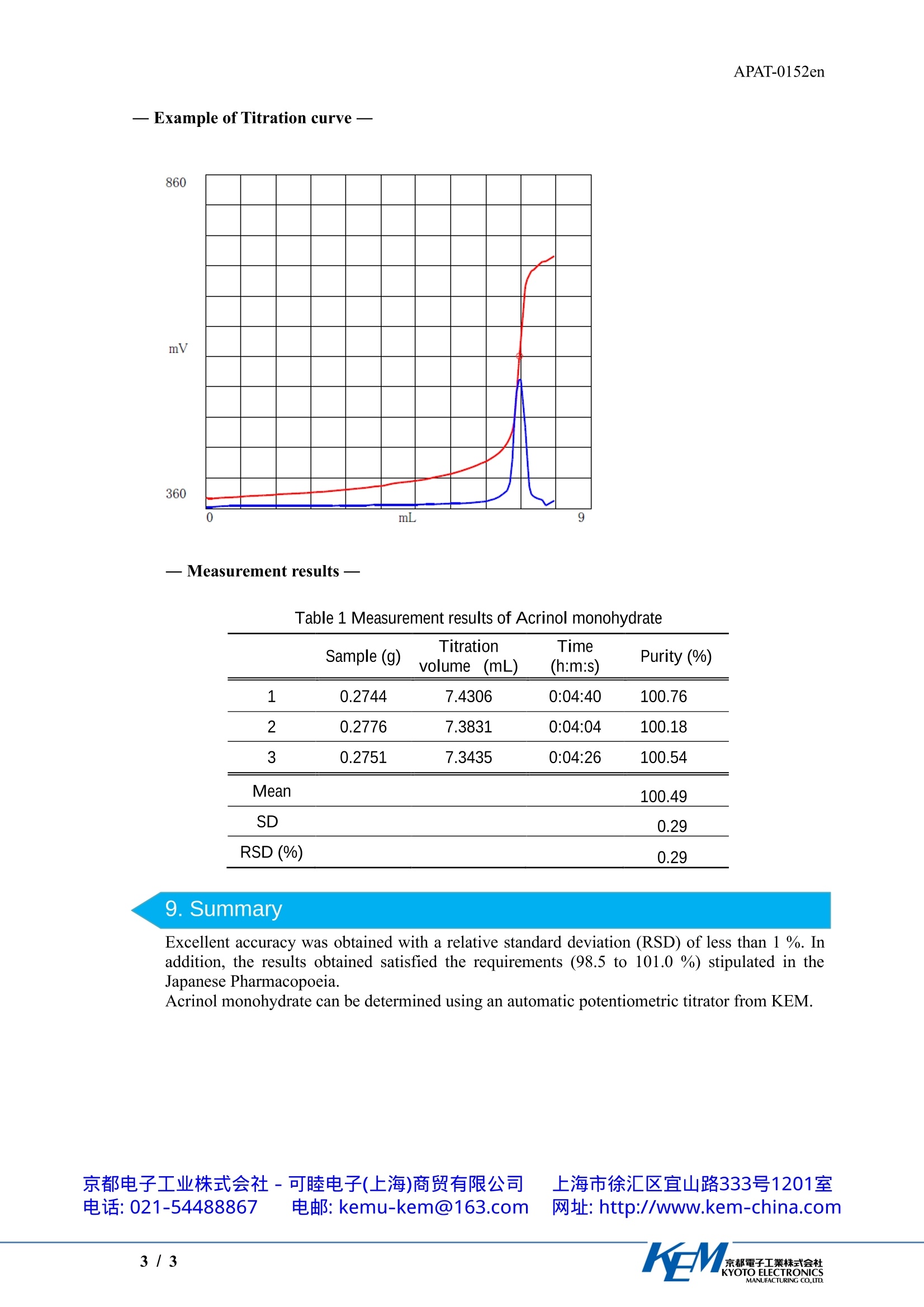
APAT-0152en京都電子工業株式会社KYOTO ELECTRONICSMANUFACTURING CO.,LTD. APAT-0152en Application Note Determination of Acrinol Monohydrate by Titrationwith Acetic Acid / Acetic Anhydride as Solvent Industry Instrument Measurement method Standards PharmaceuticalAutomatic potentiometric titratorPotentiometric titration /Neutralization titrationJapanese Pharmacopoeia 1.Scope Acrinol monohydrate is one of the pharmaceuticals listed in the Japanese Pharmacopoeia. Thisapplication introduces an example of the determination of acrinol monohydrate based on JapanesePharmacopoeia. The Japanese Pharmacopoeia stipulates,“Acrinol Hydrate contains not less than 98.5% and notmore than 101.0 % of acrinol, calculated on the anhydrous basis.”Accordingly, the moisturecontent must be measured separately by the Karl Fischer method. 2. Precautions The sample is not easily dissolved in the solvent, so stir it thoroughly, and do not start themeasurements until the sample is completely dissolved. In this measurement, water contamination causes an error. In order to avoid water contamination,use one of the following electrolyte for the reference electrode. (1)1mol/L Lithium chloride solution in acetic acid (2) Saturated sodium perchlorate solution in acetic acid Electrolyte (1) is available from KEM, so contact us if you would like to order it. Electrolyte (2)must be prepared by the operator. When preparing this solution, saturate acetic acid withanhydrous sodium perchlorate, and use the supernatant liquid. 3. Post-measurement procedure Seal the refill port for internal liquid of reference electrode by rubber septum so that electrolyteis prevented from leaking out or concentrating, and store the electrode. The performance of the glass electrode quickly degrades if it is stored while dry. The followingstorage methods are recommended. · Short-term storage (less than one month): Store it immersed in pure water. ·Long-term storage (one month or longer): Store it immersed in a 1:1 volume ratio mixture of astandard solution of pH4 and a 3.3 mol/LKCl solution. 4. Apparatus Main unit Automatic potentiometric titrator (preamplifier :STD)Electrode Glass electrodeDouble junction reference electrode type (Electrolyte: 1mol/L Lithiumchloride in acetic acid) 京都电子工业式会社-可睦电子(上海)商贸有限公司 上海市徐汇区宜山路333号1201室电话:021-54488867 电邮: kemu-kem@163.com 网址: http://www.kem-china.com 5.Reagents Titrant 0.1mol/L Perchloric acid solution in acetic acid Solvent Formic acid (purity 98-100%). A mixture of acetic anhydride and acetic acid in a volume ratio of 1:1 6. Procedure -Measurement- 1) Collect about 0.27 g of sample into a beaker, and measure the mass. 2) Add 5 mL of Formic acid, dissolve the sample 3) Add 60 mL of a mixture of acetic anhydride and acetic acid. Immediately titrate with a 0.1mol/L perchloric acid acetic acid solution. 4) Using the same procedure, perform a blank test, and correct the titration volume. 7.Calculation Purity of Acrinol monohydrate(%)=(EP1-BL1)xTF×C1×K1/(S×((100-SMW)/100)) EP1 Titration volume (mL) BL1 : Titration volume (mL) of Blank test=0.04993 TF : Factor of Ttitrant =1.04695 *1 C1 : Concentration conversion coefficient=34.34mg/mL K1 : Unit Conversion coefficient=0.1 : Sample size (g) SMW Moisture content of sample=5.1999%*2 *1 This factor is calculated by using potassium hydrogen phthalate, the standard substancefor volumetric analysis, as the standard. Refer to the Japanese Pharmacopoeia for detailson standardization procedures. *2 The moisture content of the sample is measured separately with a Karl Fischer moisturemeter for volumetric titration. Refer to the Japanese Pharmacopoeia for details on the moisture content measurementmethod. In addition, refer to KEM Application Note APKF-0153. 8. Example —Measurement results Table 1 Measurement results of Acrinol monohydrate Sample (g) Titration Time Purity (%) volume (mL) (h:m:s) 1 0.2744 7.4306 0:04:40 100.76 2 0.2776 7.3831 0:04:04 100.18 3 0.2751 7.3435 0:04:26 100.54 Mean 100.49 SD 0.29 RSD (%) 0.29 9. Summary Excellent accuracy was obtained with a relative standard deviation (RSD) of less than 1 %. Inaddition, the results obtained satisfied the requirements (98.5 to 101.0 %) stipulated in theJapanese Pharmacopoeia. 京都電子工業株式会社KYOTO ELECTRONICSMANUFACTURING CO.,LTD. 乳酸依沙吖啶含量测定 应用资料(英文版)根据中国药典2020年版,乳酸依沙吖啶【含量测定】取本品约0.27g,精密称定,加无水甲酸5.0ml使溶解,加冰醋酸60ml,照电位滴定法(通则0701),用高氯酸滴定液(0.1mol/L)滴定,并将滴定的结果用空白试验校正。每1ml高氯酸滴定液(0.1mol/L)相当于34.34mg的C15H15N3O·C3H6O3。
确定

还剩1页未读,是否继续阅读?
可睦电子(上海)商贸有限公司-日本京都电子(KEM)为您提供《乳酸依沙吖啶中有效成分含量检测方案(自动电位滴定)》,该方案主要用于化药制剂中含量测定检测,参考标准--,《乳酸依沙吖啶中有效成分含量检测方案(自动电位滴定)》用到的仪器有AT-710M四通道旗舰型自动电位滴定仪、AT-710S豪华型自动电位滴定仪
推荐专场
相关方案
更多
该厂商其他方案
更多