
方案详情
文
迄今为止,经临床批准的大多数单克隆抗体类生物治疗药物,主要是IgG1形式,其次是IgG2和IgG4形式。糖基化是导致单克隆抗体翻译后修饰异质性的主要原因,会影响药物的治疗作用机制(MOA)。同时,糖基化也是影响药物溶解度、动力学、稳定性和疗效的关键因素之一。因此,监测聚糖的关键质量属性(CQA)在药物开发阶段显得至关重要。
IgG与其细胞Fc受体(FcR)的结合亲和力取决于其IgG亚类和Fc区的糖基化修饰。并且,N-聚糖的结构也与抗体ADCC活性密切相关,因此需要对单克隆抗体制剂及其生物类似药进行糖基化修饰的监测。
东曹生命科学(Tosoh Bioscience)开发出了一种基于FcγIIIa的亲和色谱法来评价单克隆抗体糖型差异的全新方法。在文章中,首先简要概述了Fc受体的功能。然后,阐述了基于FcR的亲和色谱的原理、新型FcR色谱柱根据抗体N-聚糖分离多种单克隆抗体具有高选择性。最后,我们举例介绍了FcR色谱柱在细胞株筛选、糖工程监测、工艺开发和生产过程监控中的应用。
方案详情

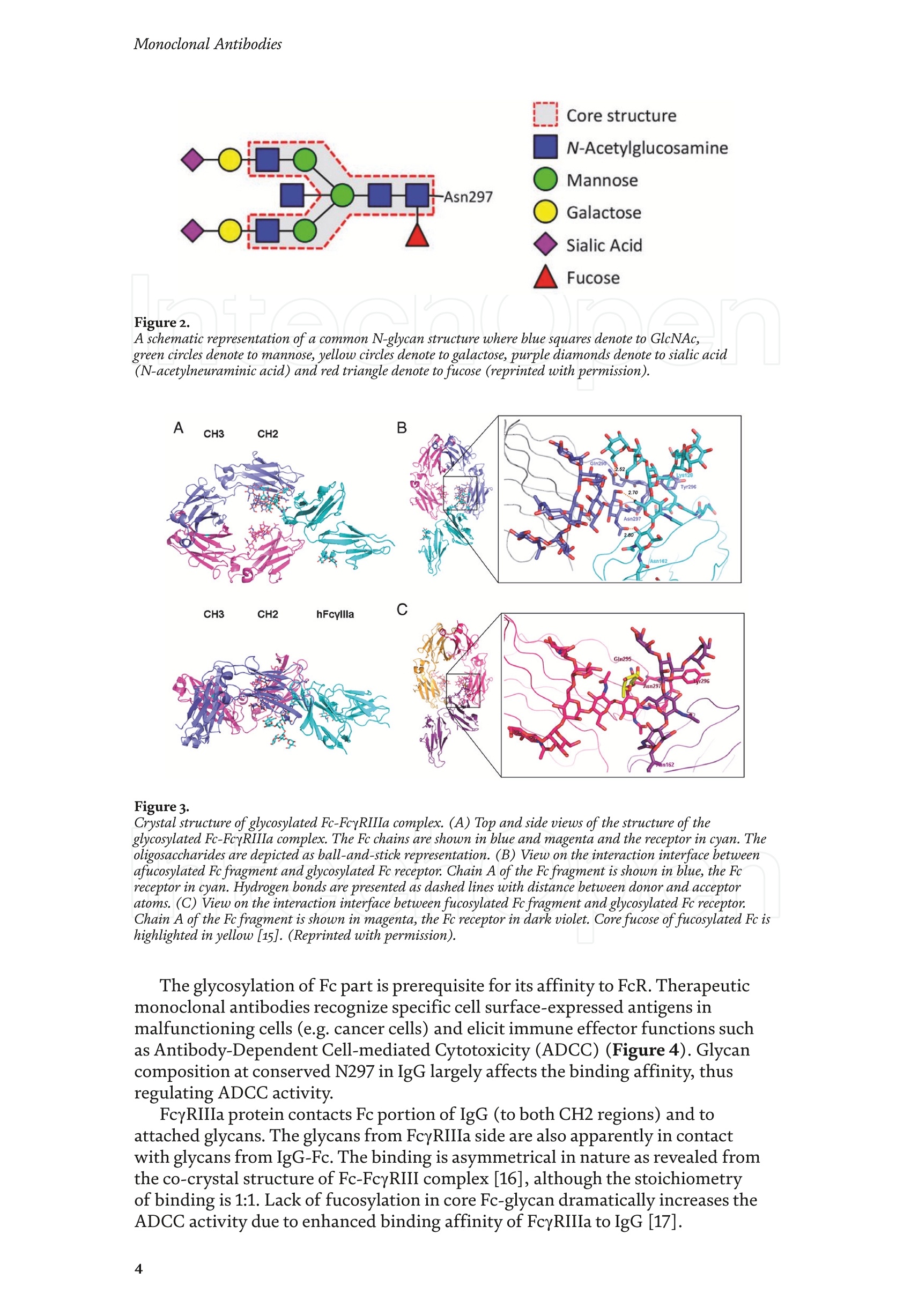
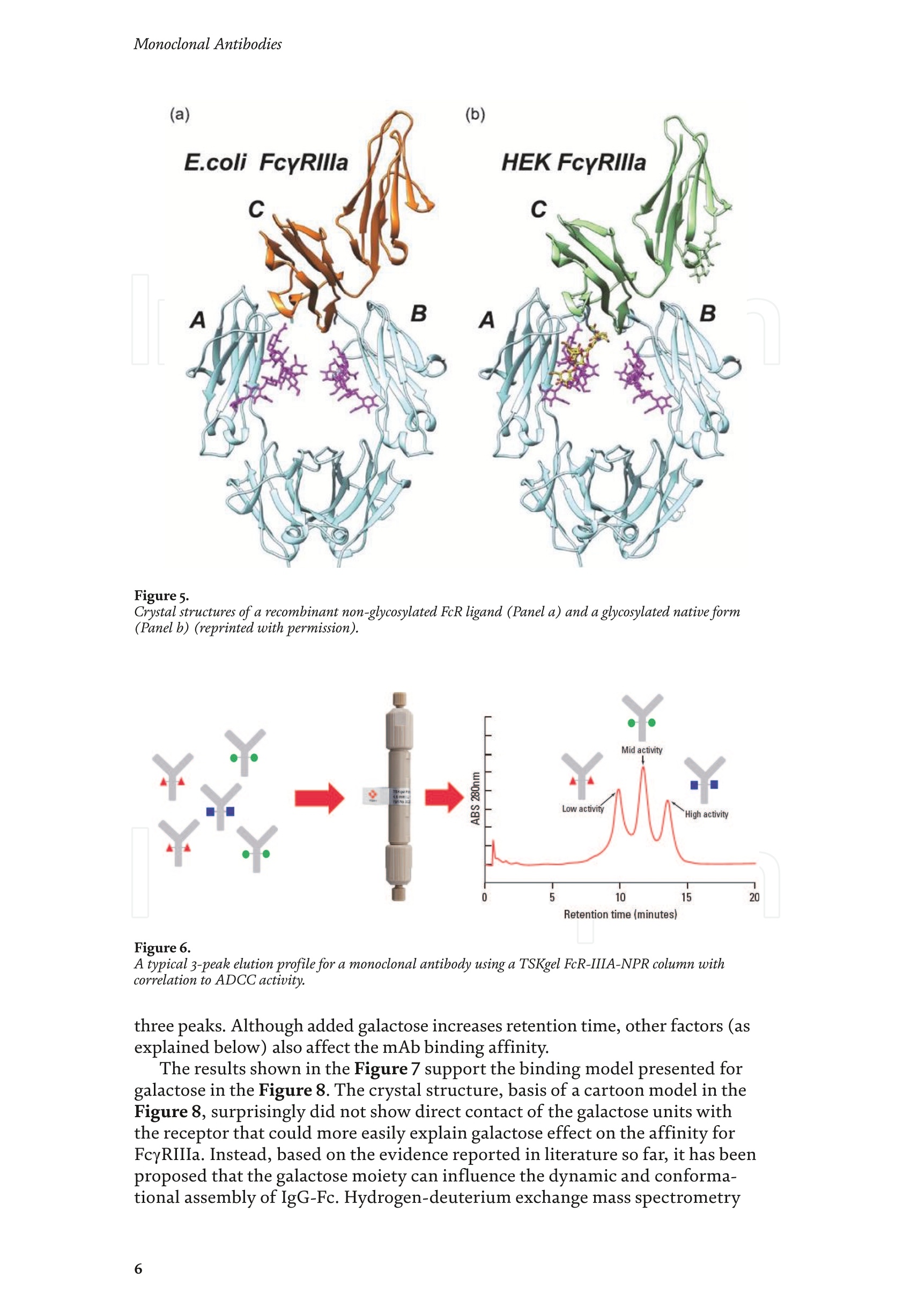
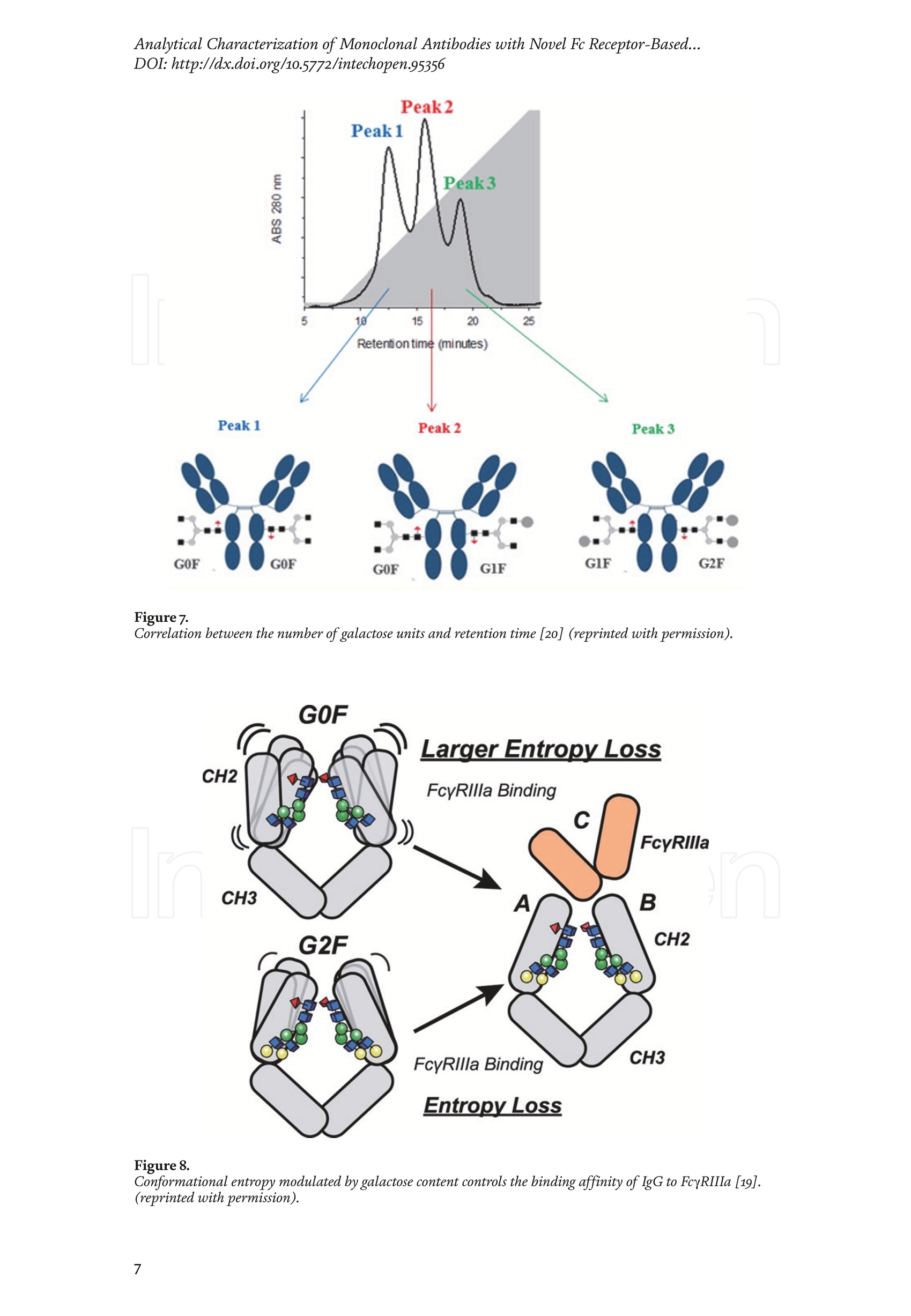
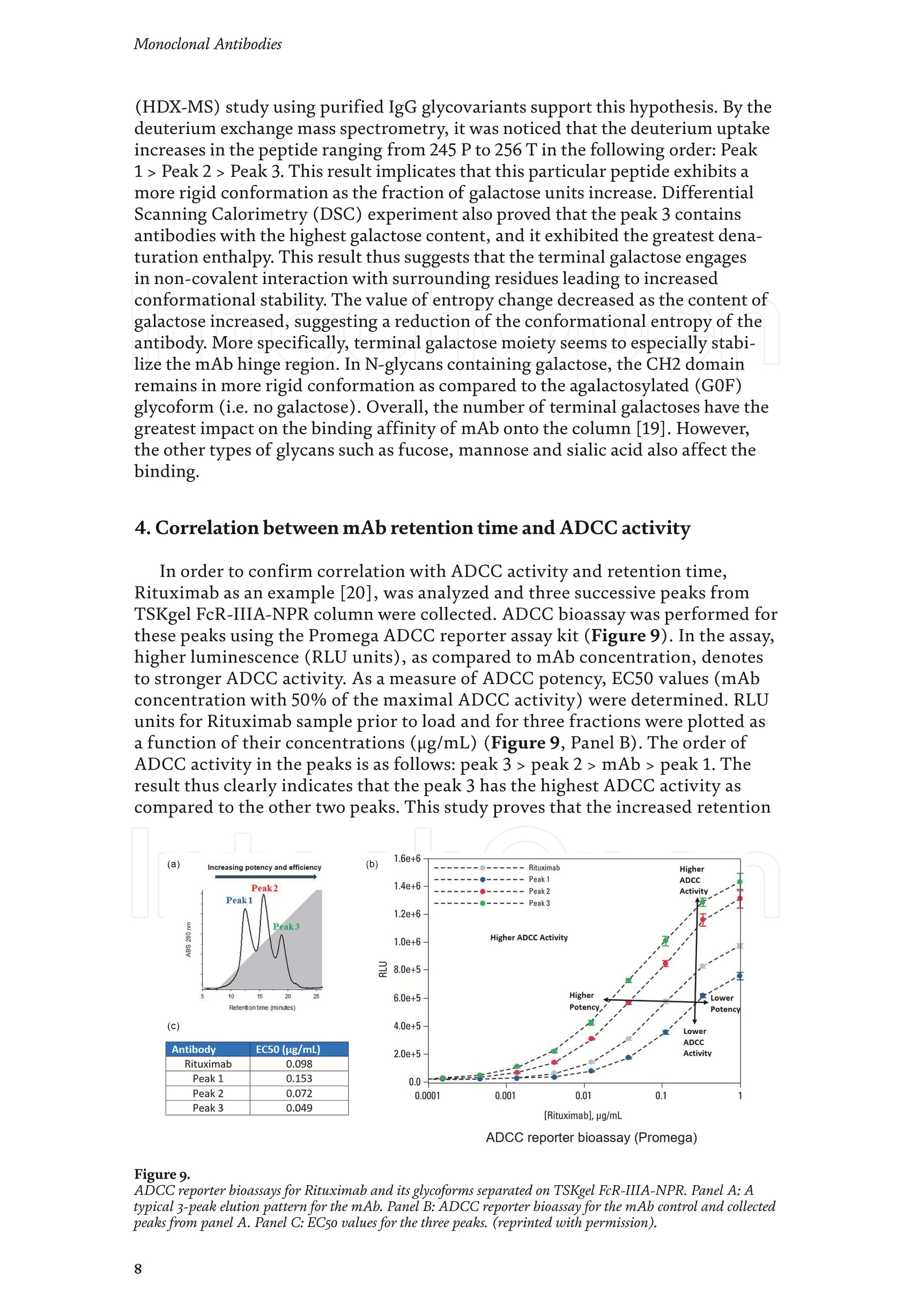
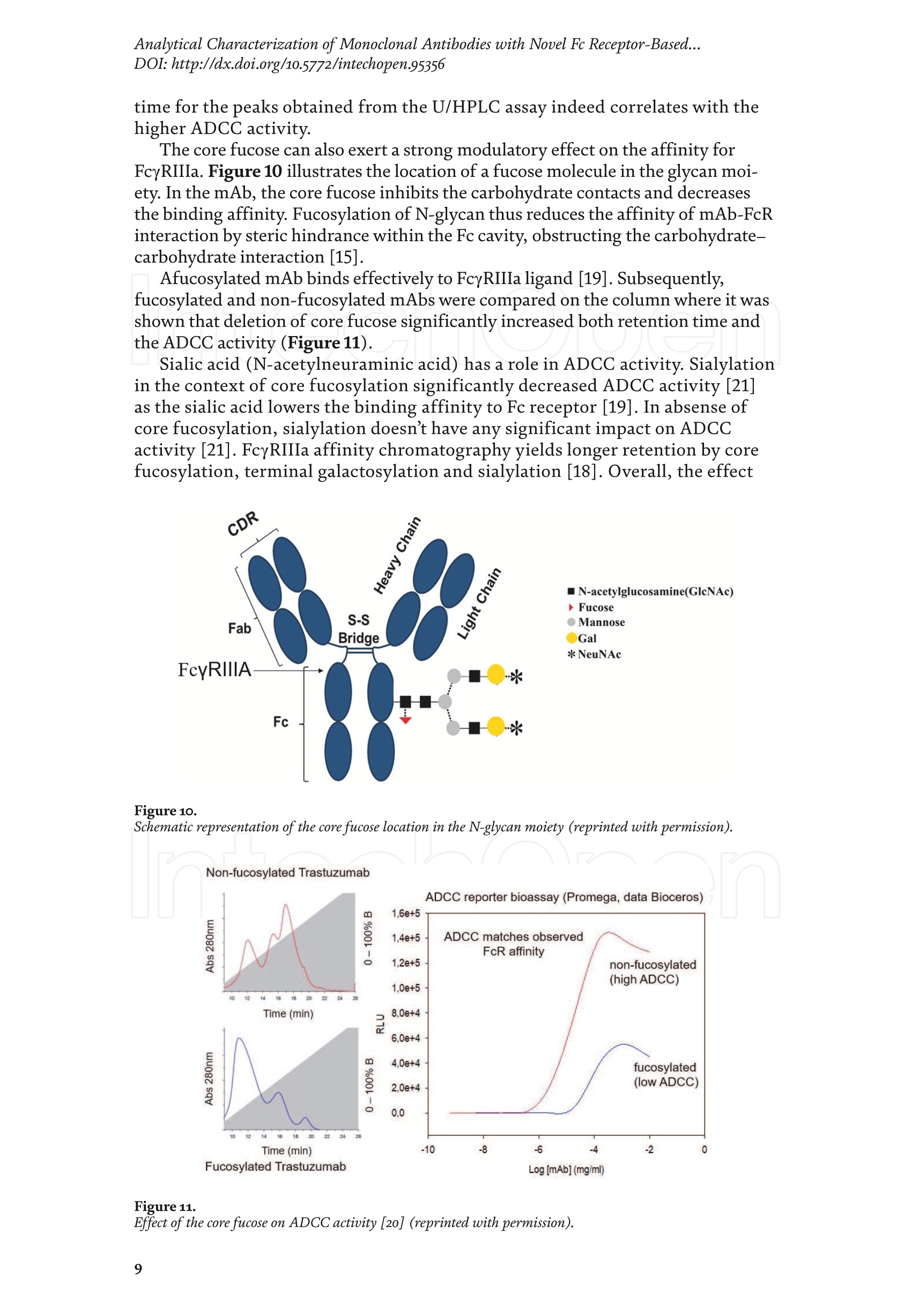
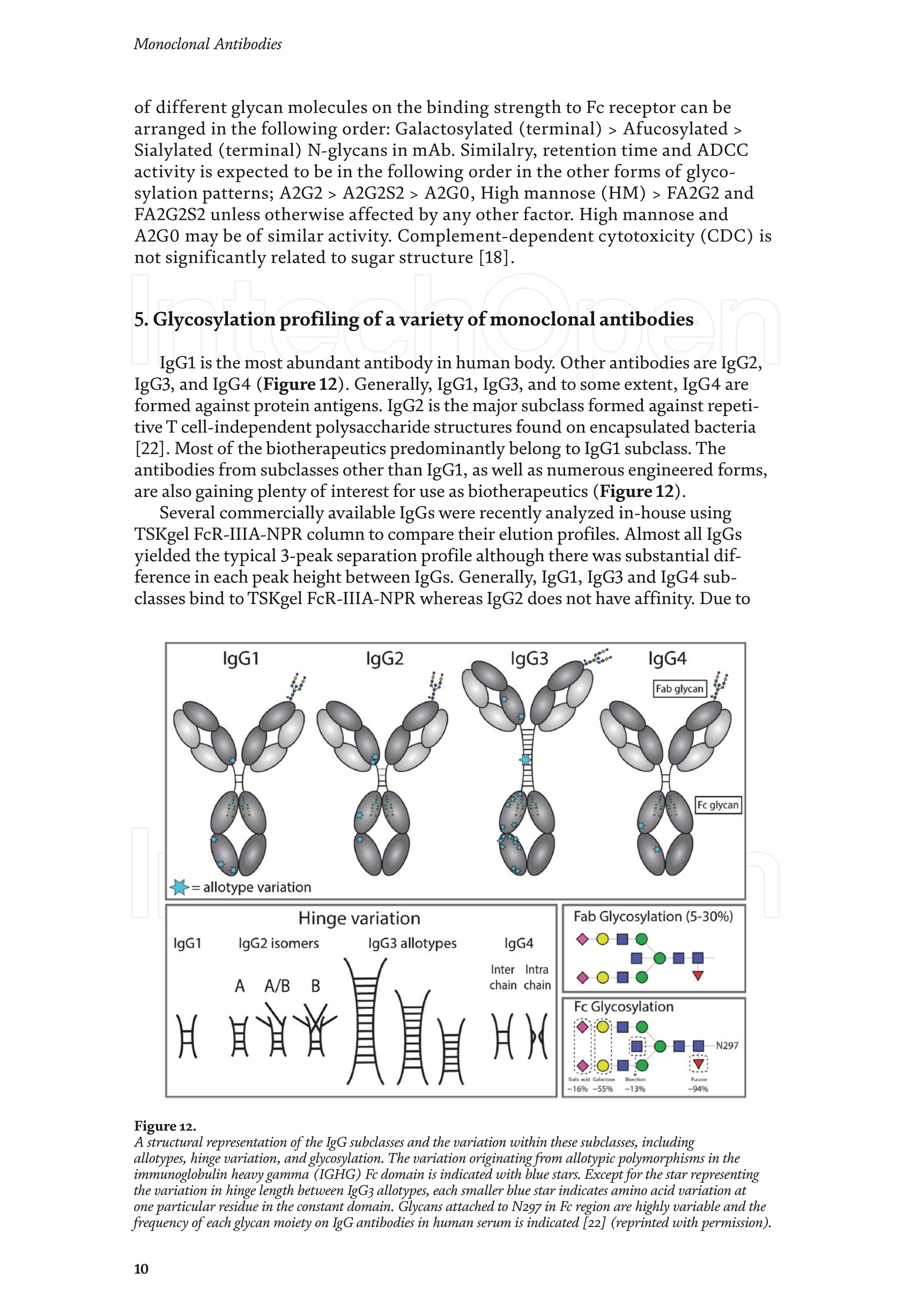
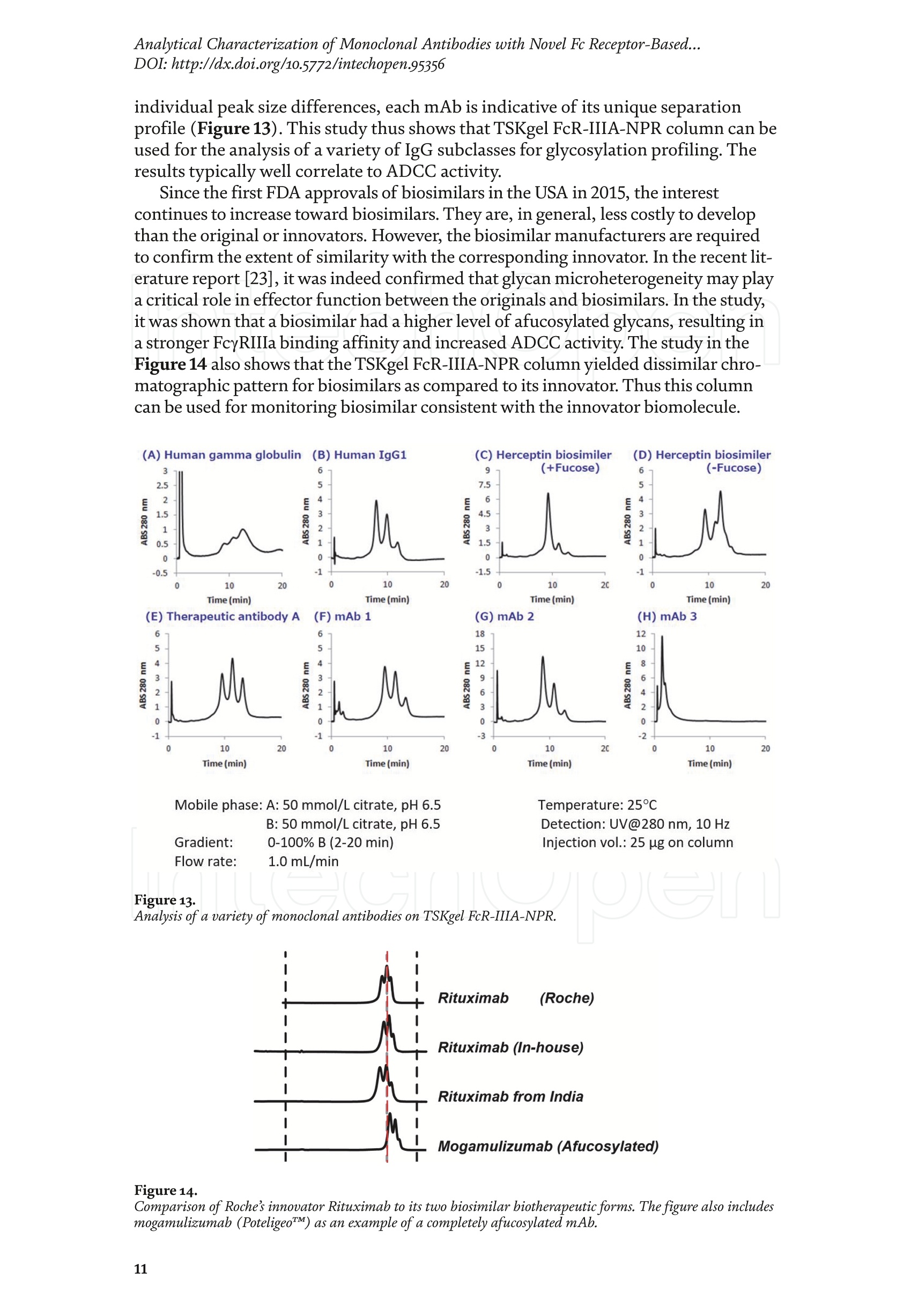
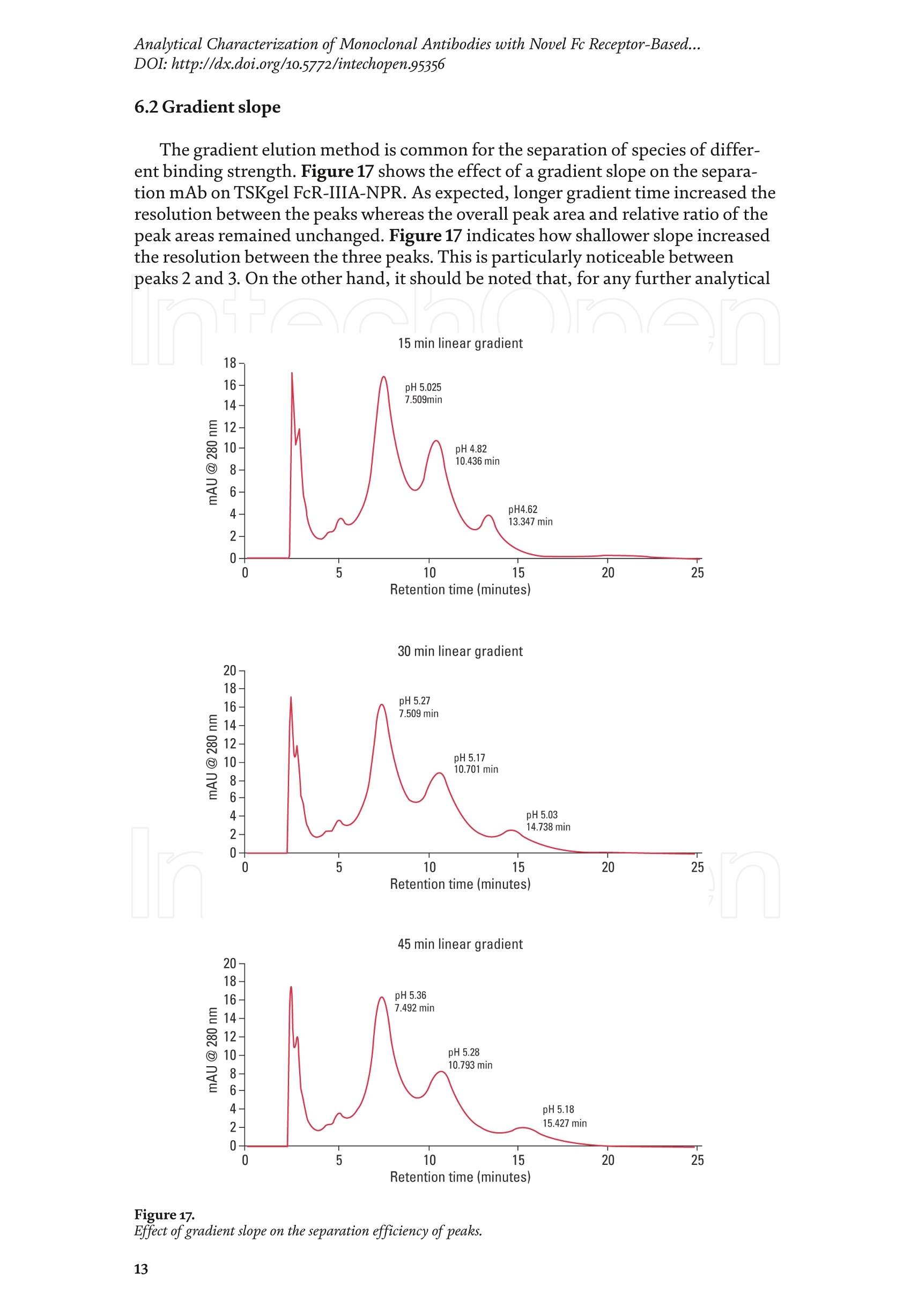
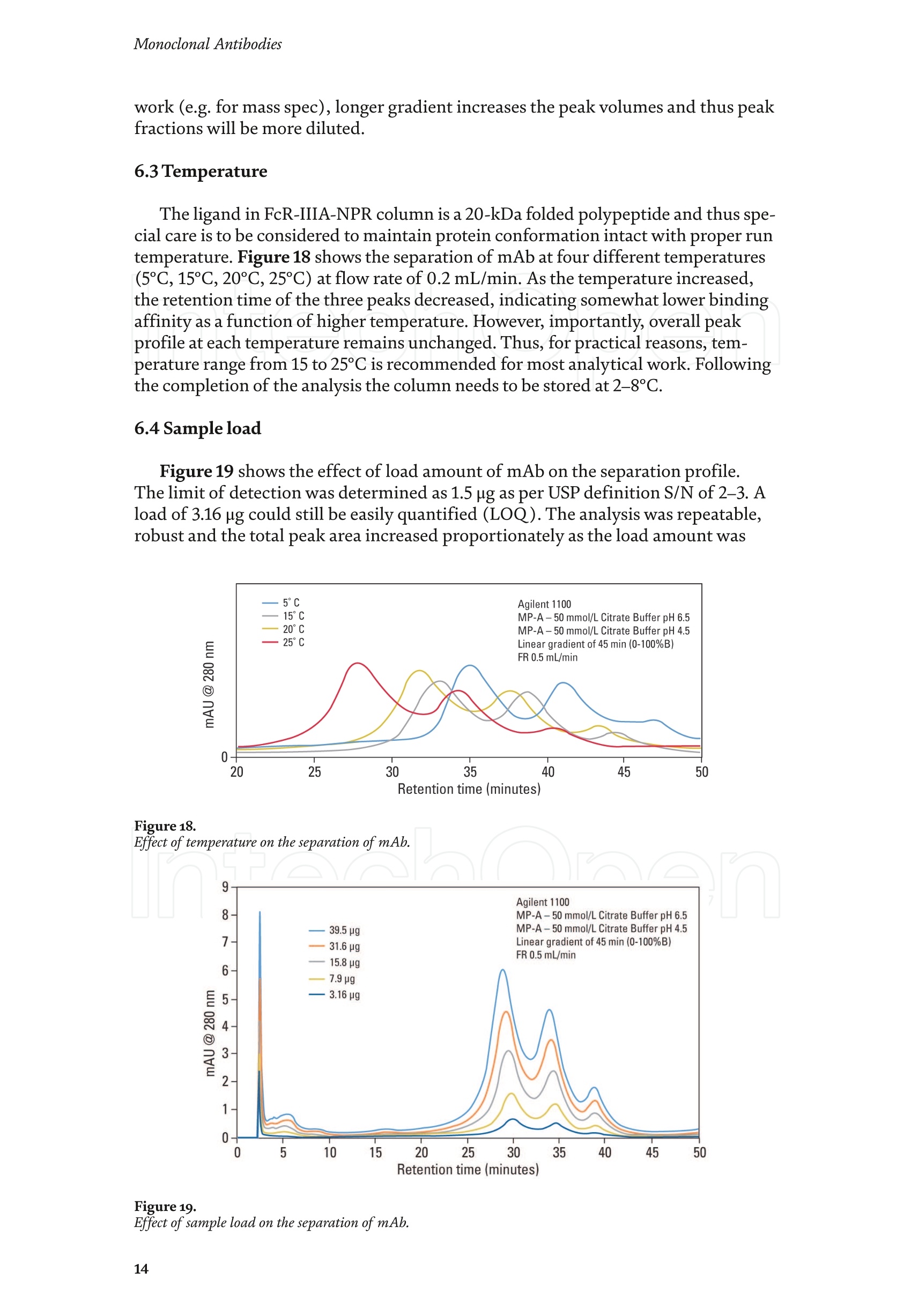
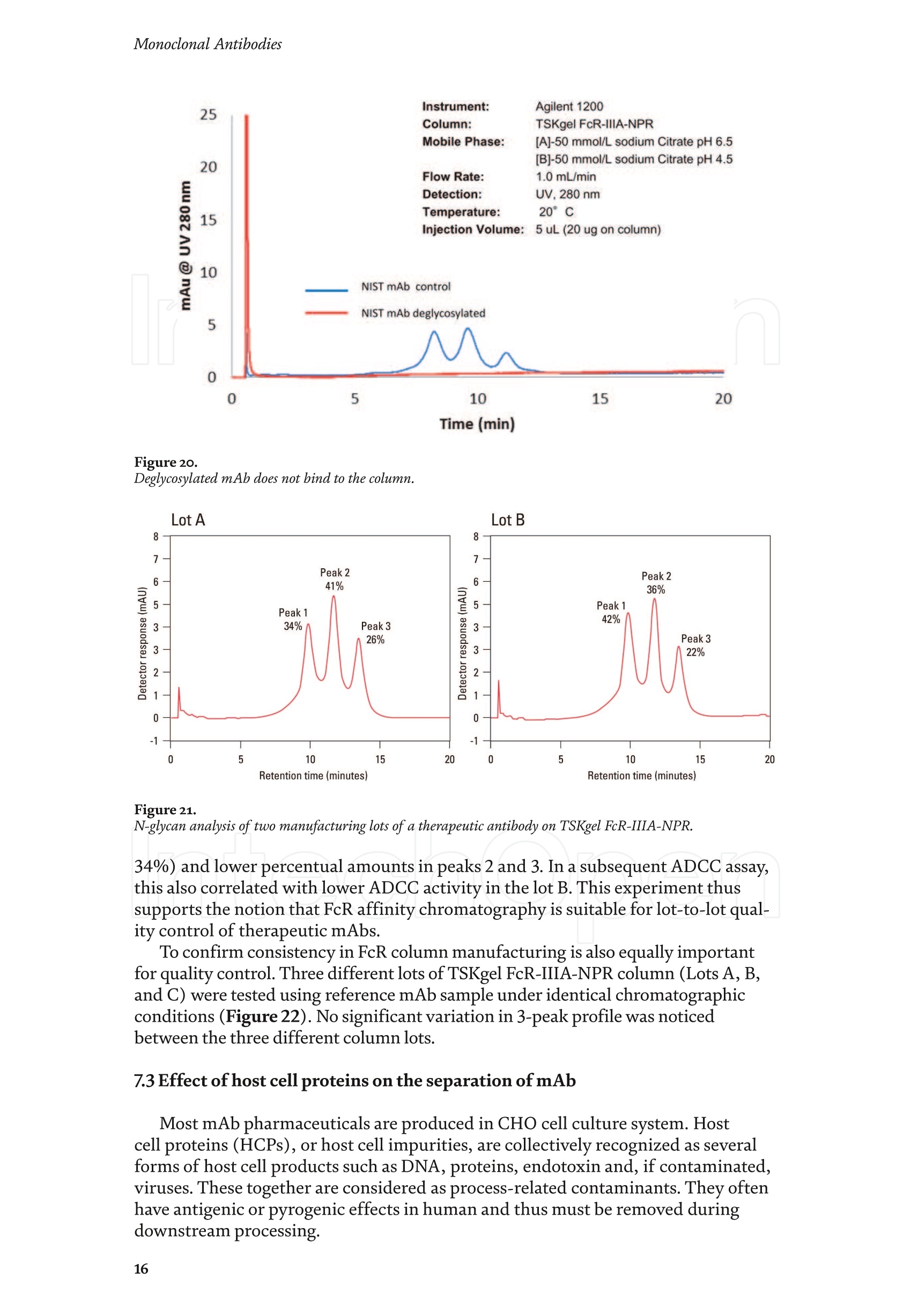
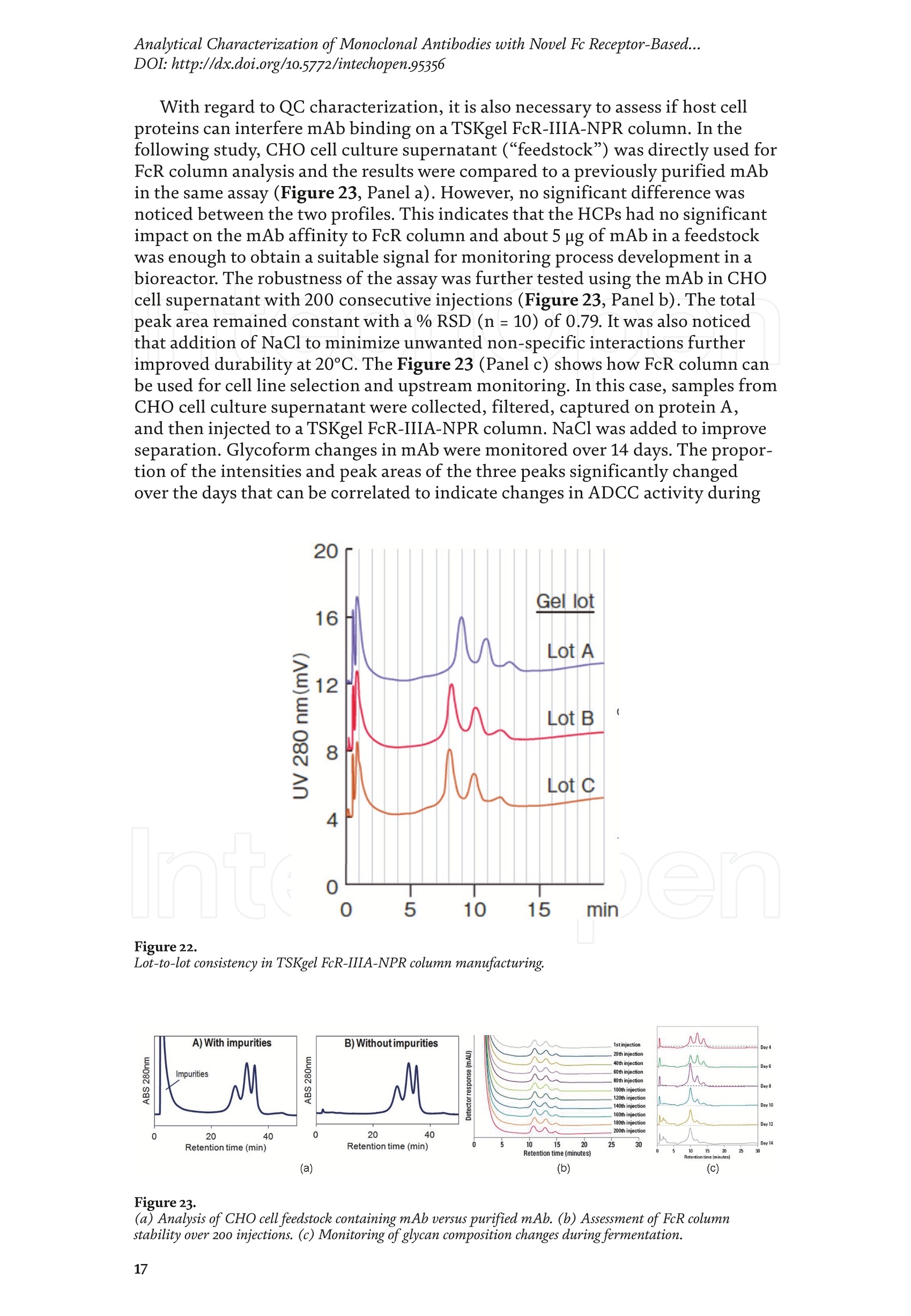
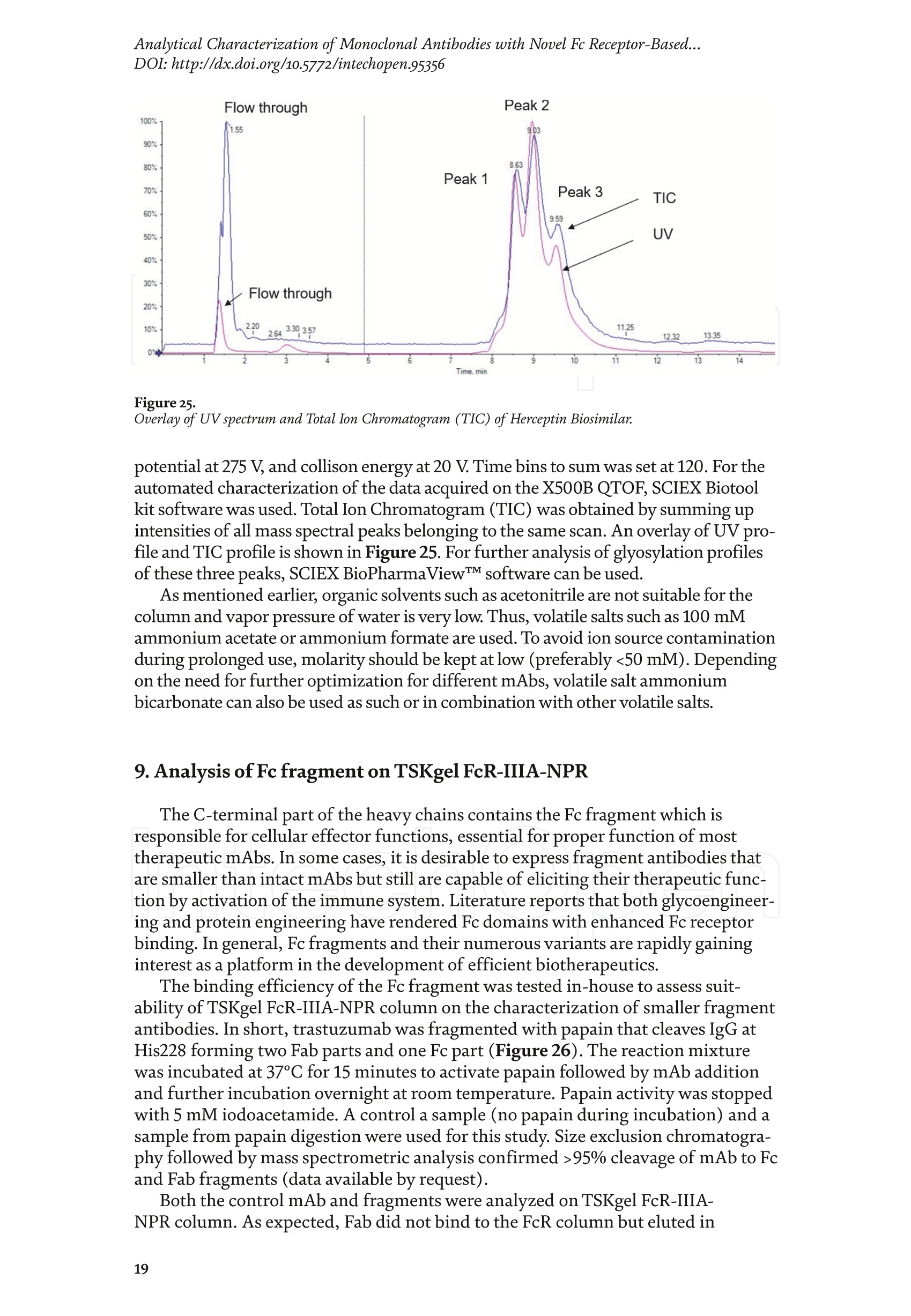
Chapter Monoclonal Antibodies Analytical Characterizationof Monoclonal Antibodieswith Novel Fc Receptor-BasedChromatography Technique Atis Chakrabarti, Jukka Kervinen, Egbert Miller,Toru Tanaka and Kazuaki Muranaka Abstract Most clinically approved large biotherapeutics are monoclonal antibodies (mAbs),primarily belonging to immunoglobulin G subclass-1 (IgG1) and, to a lesser extent,IgG2 and IgG4. Glycosylation is the main source of post-translational heterogeneity ofmAbs, impacting their drug therapeutic mechanism of action (MOA). Glycosylationis also one of the critical factors in drug product solubility, kinetics, stability andefficacy. Thus, monitoring glycan critical quality attributes (CQAs) is an essential partof any biopharmaceutical development. The binding affinity of an IgG to its cellular Fcreceptor (FcR) depends on both its IgG subclass and Fc domain glycosylation pattern.Since composition of the N-glycans also correlates to the Antibody-Dependent CellularCytotoxicity (ADCC), the glycosylation pattern needs to be monitored for consis-tency in potency and efficacy. This applies for the original mAb biologics as well asbiosimilars. In this chapter, we present a truly novel way to assess the variances in mAbglycoforms using FcyRIIIa-based affinity chromatography. First, a brief overview of theFc receptor function is presented. Then, the principle of FcR-based affinity chromatog-raphy is explained including how this column's potential to analyze a variety of mAbsaccording to their N-glycan content is highly selective and robust. Finally, we provideexamples of the FcR column’s potential to improve analytical characterization of mAbswith practical applications such as effective cell line screening, monitoring of glycoengi-neering, process development and process control in manufacturing. Keywords: FcR, glycoform,N-glycan, monoclonal antibody, mAb, biosimilar,affinity chromatography, antibody-dependent cellular cytotoxicity, ADCC 1. Introduction Affinity chromatography is a popular method for the purification of biomol-ecules. The purification involves interaction of the biomolecule with a ligandcovalently immobilized to a solid stationary phase. Elution occurs as a function ofbinding strength of the biomolecule to the stationary phase, tighter the bindinglater the elution time in a linear gradient. Due to high selectivity and fast separa-tion, affinity chromatography is regarded as the most widely used purificationmethod for capture step in biopharmaceutical industry. Different types of affinity chromatography ligands are available as resins and pre-packed columns for purifi-cation at various scales. Similarly, different types of U/HPLC affinity chromatogra-phy columns are available for analytical characterization and quality control. Thischapter focuses on a recently introduced novel FcR-based affinity column, TSKgel-FcR-IIIA-NPR, that enables chromatographic characterization of mAbs based ontheir N-glycan content attached to a highly conserved Asn-297 in Fc region. 2. Brief overview of Fc receptor A brief overview of the Fc Receptor (FcR) structure and function is provided tobest understand the chromatography principle of the column discussed in this chap-ter. FcR proteins belong to immunoglobulin (Ig) superfamily [1]. Interest in FcRs forbiotherapeutic research has gained momentum since 1980s. The purification of FcRfrom the glycoprotein fraction of the placental membranes by chromatography wasreported in 1982 [2]. A functional 40 kDa FcR, with low affinity for native IgG, waspurified from the human peripheral nerve extract using F(ab)2 fragments of mAbagainst placental FcR as affinity agent in 1989 [3]. Important role of FcR in IgG dis-tribution to the brain [4], inhibition of cell activation [5] and in enhancement andsuppression of the effector function [6] have also been reported. Overall, it becameevident that FcRs are important for numerous biological functions. 2.1 FcyRs The FcRs binding to immunoglobulin G (IgG) are known as Fc-gamma recep-tors (FcyR). FcyRs play essential role in immunity, inflammatory and infectiousdiseases [7]. Immune enhancement and suppression are influenced by binding tothese FcyRs [6]. Additional interaction between hyaluronic acid (HA) and sialicacids on immune cells helps to optimize the FcR-mediated effector function [8]. Fcyreceptors do not bind to IgA or IgM [9]. 2.2 Fcy receptor binding to IgG Typical IgG is Y-shaped protein of ~150 kDa in size, containing two heavy chainsand two light chains (Figure 1). The heavy chain (HC) contains three constantdomains (CH1-CH3) and a variable domain (VH) with three complementarity-determining regions (CDRs). The light chain (LC) has only one constant domain(CL) and a variable domain (VL) with CDRs. The Fd consists of VH and CH1. LCand Fd together form the antigen binding fragment (Fab). The CH2 domain of eachheavy chain of IgG has a highly conservative asparagine (N) residue at position 297(Asn297 or N297) that is almost invariably glycosylated. Fcy receptor binding siteis located near the hinge region of IgG, close to N297 in the CH2 domain. The mostflexible portion of the hinge region is between CH1 and CH2 domains of a heavychain. The four chains are covalently connected via disulfide bridges [10]. Fractioncrystallizable (Fc) is composed of CH2 and CH3 domains of the two heavy chains.The highly conserved glycan moiety at position N297 infers structural changes tothe Fc-region required for binding to FcyR. Subtle differences in the glycan compo-sition at this site, thus, can affect the conformational rigidity of the Fc-structure,and may also alter the interaction with FcyR by direct contact [11]. 2.3 FcyR subclasses FcyRs are divided into three subclasses, abbreviated as FcyRI, FcyRII and FcyRIII.Extracellular regions of all the FcyRs are extremely homologous, whereas the Analytical Characterization of Monoclonal Antibodies with Novel Fc Receptor-Based...DOI: http://dx.doi.org/10.5772/intechopen.95356 Figure 1. Schematic overview of human IgG1 with a detailed sequence view into the hinge region [10] (reprinted withpermission). cytoplasmic domains differ considerably from each other [12]. FcyRI exhibits the high-est affinity for IgG, K 10-10°M-whereas FcyRII and FcyRIII show a weaker affinity[13] for monomeric IgG,Ka≤10M-. Receptor clustering is essential for FcyR signal-ing. FcyRIII (also known as CD16) is a cluster of differentiation molecule found on thesurface of natural killer (NK) cells, neutrophils, monocytes and macrophages. 2.4 FcyRIII isoforms FcyRIII exists in two different isoforms, (a) FcyRIIIa or CD16a and (b) FcyRIIIbor CD16b. Both forms take part in intracellular signal transduction. Two nearlyidentical genes in human encode these two isoforms. FcyRIIIa is a 50-65 kDa type-1transmembrane protein whereas FcyRIIIb is a 48 kDa glycosylphosphatidylino-sitol (GPI)-anchored protein. This chapter focuses on the modified recombinantFcyRIIIa protein ligand, immobilized on a polymethacrylate stationary phase andpacked into an analytical chromatography column that can be used for characteriza-tion of antibodies based on their N-glycan content on Asn297. 2.5 FcyRIIIa and glycosylation mode ofIgG Post-translational modifications, particularly glycosylation, of both IgGantibodies and Fcy receptors modulate the affinity of their interaction. N-glycan(Figure 2) is a well-defined complex biantennary structure composed of a corehepta-saccharide, made up of N-acetylglucosamine (GlcNAc) and mannose,followed by variable additions of galactose, sialic acid (N-acetylneuraminic acid),fucose, and bisecting GlcNAc residues [11]. The attached glycans play various crucial roles on the function of immunoglobu-lins. Fc sialylation prolongs serum half-life of therapeutic antibodies [14]. Figure3 shows details of the structure of the glycosylated Fc fragment complexed to aFcyRIIIa receptor [15]. In non-fucosylated mAb, the carbohydrate-carbohydrateinteractions increase binding affinity between N-Glycan of IgG-Fc and N-Glycan ofFcR (Kp=7.2×10-M) while in fucosylated mAb the carbohydrate-carbohydrateinteraction is weekened or non-existent depending on the extent of steric hindranceof the fucose moiety (Kp=3.0×10-’M) [15]. Figure 2. A schematic representation of a common N-glycan structure where blue squares denote to GlcNAc,green circles denote to mannose, yellow circles denote to galactose, purple diamonds denote to sialic acid(N-acetylneuraminic acid) and red triangle denote to fucose (veprinted with permission). Figure3. Crystal structure of glycosylated Fc-FcyRIIIa complex. (A) Top and side views of the structure of theglycosylated Fc-FcyRIIIa complex. The Fc chains are shown in blue and magenta and the receptor in cyan. Theoligosaccharides are depicted as ball-and-stick representation.(B) View on the intevaction interface betweenafucosylated Fc fragment and glycosylated Fc receptor. Chain A ofthe Fc fragment is shown in blue, the Fcreceptor in cyan. Hydrogen bonds are presented as dashed lines with distance between donor and acceptoratoms. (C) View on the interaction interface between fucosylated Fc fragment and glycosylated Fc receptor.Chain A of the Fc fragment is shown in magenta, the Fc receptor in dark violet. Core fucose of fucosylated Fc ishighlighted in yellow[15]. (Reprinted with permission). The glycosylation of Fc part is prerequisite for its affinity to FcR. Therapeuticmonoclonal antibodies recognize specific cell surface-expressed antigens inmalfunctioning cells (e.g. cancer cells) and elicit immune effector functions suchas Antibody-Dependent Cell-mediated Cytotoxicity (ADCC) (Figure 4). Glycancomposition at conserved N297 in IgG largely affects the binding affinity, thusregulating ADCC activity. FcyRIIIa protein contacts Fc portion of IgG (to both CH2 regions) and toattached glycans. The glycans from FcyRIIIa side are also apparently in contactwith glycans from IgG-Fc. The binding is asymmetrical in nature as revealed fromthe co-crystal structure of Fc-FcyRIII complex [16], although the stoichiometryof binding is 1:1. Lack of fucosylation in core Fc-glycan dramatically increases theADCC activity due to enhanced binding affinity of FcyRIIIa to IgG [17]. Analytical Characterization of Monoclonal Antibodies with Novel Fc Receptor-Based...DOI: http://dx.doi.org/10.5772/intechopen.95356 Figure4.Schematic representation of the antibody-Fc receptor interaction resulting in ADCC activity. 3. TSKgel FcR-IIIA-NPR affinity column TSKgel FcR-IIIA-NPR affinity column contains non-porous polymethacrylatebase beads as stationary phase. The ligand is a modified recombinant non-glyco-sylated FcyRIIIa of 20 kDa, produced in E. coli expression system. RecombinantFcyRIIIa ligand has eight amino acid substitutions as compared to its wild-type form.These changes were necessary for stabilization of the ligand structure [18]. The 2.7 Acrystal structure of recombinant FcyRIIIa protein verifies the molecular basis of theIgG-FcR complex formation. No significant difference was found between the crystalstructures of glycosylated wild-type FcyRIIIa expressed in human embryonic kidney(HEK) cells vs. non-glycosylated recombinant FcyRIIIa from E. coli (Figure 5). Thisconfirms suitability of E. coli-produced non-glycosylated FcyRIIIa to be used as anaffinity ligand in a chromatography resin. Notably, no direct contact of the terminalAsn297 N-glycan galactose of IgG and modified non-glycosylated FcyRIIIa wasobserved [19]. Both proteins were crystallized as complex with Fc [19]. The dimension of the TSKgel FcR-IIIA-NPR column is 4.6 mm ID ×7.5 cm(l)with a total bed volume of 1.25 mL. Polymethacrylate-based matrix is composed ofnon-porous material with ~5 um particle size. Maximum pressure limit of the columnis 90 bar (9MPa). The column is suitable for both HPLC and UHPLC instrumentsettings. The recommended run temperature is 15-25℃. The operational pH rangeis from pH 4 to 8. In general, most monoclonal antibodies bind effectively on thecolumn at pH 6.5. Typically, 50 mM ammonium citrate (or ammonium acetate)buffer is used. A linear pH gradient from pH 6.5 to 4.5 over 16 column volumes (CV)at the flow rate of 1.0 mL/min is recommended. Figure 6 shows a typical three-peak chromatographic profile for a monoclonal antibody in these settings. Sodiumchloride can be added to buffer to enhance the separation if needed. Longer retentiontime indicates stronger mAb affinity to the ligand. However, as to the general compo-sition of glycans, it should be noted that all the three peaks still contain a mixture ofglycoforms with variable amounts of galactose and other sugar molecules (Figure7). From a related experiment (Figure 7), the three peaks were collected for glycananalysis and ADCC activity assay. Determination of the glycan structures revealedthat the retention time increase correlates with increased number of the terminalgalactose. Terminal galactose tends to stabilize conformation of the Fc region,providing tighter binding onto FcyRIIIa affinity ligand [20]. However, FcR columnis not designed for quantitation of only galactose but to obtain a more generalunderstanding of the variation in distribution of the glycan content among the Figure5. Crystal structures of a vecombinant non-glycosylated FcR ligand (Panel a) and a glycosylated native form(Panel b) (reprinted with permission). Figure 6. A typical 3-peak elution profile for a monoclonal antibody using a TSKgel FcR-IIIA-NPR column withcorrelation to ADCC activity. three peaks. Although added galactose increases retention time, other factors (asexplained below) also affect the mAb binding affinity. The results shown in the Figure 7 support the binding model presented forgalactose in the Figure 8. The crystal structure, basis of a cartoon model in thure8, surprisin+11eFig,gly did not show direct contact of the galactose units withthe receptor that could more easily explain galactose effect on the affinity forFcyRIIIa. Instead, based on the evidence reported in literature so far, it has beenproposed that the galactose moiety can influence the dynamic and conforma-tional assembly of IgG-Fc. Hydrogen-deuterium exchange mass spectrometry Analytical Characterization of Monoclonal Antibodies with Novel Fc Receptor-Based...DOI: http://dx.doi.org/10.5772/intechopen.95356 Figure7. Correlation between the number of galactose units and retention time [20] (reprinted with permission). Figure 8. Conformational entropy modulated by galactose content controls the binding affinity of IgG to FcyRIIIa [19].(reprinted with permission). (HDX-MS) study using purified IgG glycovariants support this hypothesis. By thedeuterium exchange mass spectrometry, it was noticed that the deuterium uptakeincreases in the peptide ranging from 245 P to 256 T in the following order: Peak1> Peak 2> Peak 3. This result implicates that this particular peptide exhibits amore rigid conformation as the fraction of galactose units increase. DifferentialScanning Calorimetry (DSC) experiment also proved that the peak 3 containsantibodies with the highest galactose content, and it exhibited the greatest dena-turation enthalpy. This result thus suggests that the terminal galactose engagesin non-covalent interaction with surrounding residues leading to increasedconformational stability. The value of entropy change decreased as the content ofgalactose increased, suggesting a reduction of the conformational entropy of theantibody. More specifically, terminal galactose moiety seems to especially stabi-lize the mAb hinge region. In N-glycans containing galactose, the CH2 domainremains in more rigid conformation as compared to the agalactosylated (G0F)glycoform (i.e. no galactose). Overall, the number of terminal galactoses have thegreatest impact on the binding affinity of mAb onto the column [19]. However,the other types of glycans such as fucose, mannose and sialic acid also affect thebinding. 4. Correlation between mAb retention time and ADCC activity In order to confirm correlation with ADCC activity and retention time,Rituximab as an example [20], was analyzedand three successive peaks fromTSKgel FcR-IIIA-NPR column were collected. ADCC bioassay was performed forthese peaks using the Promega ADCC reporter assay kit (Figure 9). In the assay,higher luminescence (RLU units), as compared to mAb concentration, denotesto stronger ES50yADCC activity. As a measure of ADCC potency, EC50 values (mAbconcentration with 50% of the maximal ADCC activity) were determined. RLUunits for Rituximab sample prior to load and for three fractions were plotted asa function of their concentrations (rg/mL) (Figure 9, Panel B). The order ofADCC activity in the peaks is as follows: peak 3> peak 2>mAb> peak 1. Theresult thus clearly indicates that the peak 3 has the highest ADCC activity ascompared to the other two peaks. This study proves that the increased retention ADCC reporter bioassay (Promega) Figure 9. ADCC reporter bioassays for Rituximab and its glycoforms separated on TSKgel FcR-IIIA-NPR. Panel A: Atypical 3-peak elution pattern for the mAb. Panel B: ADCC reporter bioassay for the mAb control and collectedpeaks from panel A. Panel C: EC50 values for the three peaks. (reprinted with permission). DOI: http://dx.doi.org/10.5772/intechopen.95356 time for the peaks obtained from the U/HPLC assay indeed correlates with thehigher ADCC activity. The core fucose can also exert a strong modulatory effect on the affinity forFcyRIIIa. Figure 10 illustrates the location of a fucose molecule in the glycan moi-ety. In the mAb, the core fucose inhibits the carbohydrate contacts and decreasesthe binding affinity. Fucosylation of N-glycan thus reduces the affinity of mAb-FcRinteraction by steric hindrance within the Fc cavity,obstructing the carbohydrate-carbohydrate interaction [15]. Afucosylated mAb binds effectively to FcyRIIIa ligand [19]. Subsequently,fucosylated and non-fucosylated mAbs were compared on the column where it wasshown that deletion of core fucose significantly increased both retention time andthe ADCC activity (Figure 11). Sialic acid (N-acetylneuraminic acid) has a role in ADCC activity. Sialylationin the context of core fucosylation significantly decreased ADCC activity [21]as the sialic acid lowers the binding affinity to Fc receptor [19]. In absense ofcore fucosylation, sialylation doesn't have any significant impact on ADCCactivity [21].FcyRIIIa affinity chromatography yields longer retention by corefucosylation, terminal galactosylation and sialylation [18].Overall, the effect Figure 10. Schematic representation of the core fucose location in the N-glycan moiety (reprinted with permission). Non-fucosylated Trastuzumab Ec< 14 24 Time (min) c Time (min) Fucosylated Trastuzumab Figure11. Effect of the core fucose on ADCC activity [20] (reprinted with permission). of different glycan molecules on the binding strength to Fc receptor can bearranged in the following order: Galactosylated (terminal)>Afucosylated>Sialylated (terminal) N-glycans in mAb. Similalry, retention time and ADCCactivity is expected to be in the following order in the other forms of glyco-sylation patterns; A2G2 >A2G2S2> A2G0, High mannose (HM)>FA2G2 andFA2G2S2 unless otherwise affected by any other factor. High mannose andA2G0 may be of similar activity. Complement-dependent cytotoxicity (CDC) isnot significantly related to sugar structure [18]. 5. Glycosylation profiling of a variety of monoclonal antibodies IgG1 is the most abundant antibody in human body.Other antibodies are IgG2,IgG3, and IgG4 (Figure 12). Generally,IgG1, IgG3, and to some extent, IgG4 areformed against protein antigens. IgG2 is the major subclass formed against repeti-tive T cell-independent polysaccharide structures found on encapsulated bacteria[22]. Most of the biotherapeutics predominantly belong to IgG1 subclass. Theantibodies from subclasses other than IgG1, as well as numerous engineered forms,are also gaining plenty of interest for use as biotherapeutics (Figure 12). Several commercially available IgGs were recently analyzed in-house usingTSKgel FcR-IIIA-NPR column to compare their elution profiles. Almost all IgGsyielded the typical 3-peak separation profile although there was substantial dif-ference in each peak height between IgGs. Generally, IgG1, IgG3 and IgG4 sub-classes bind to TSKgel FcR-IIIA-NPR whereas IgG2 does not have affinity. Due to Figure12. A structural representation of the IgG subclasses and the variation within these subclasses, includingallotypes, hinge variation, and glycosylation. The variation originating from allotypic polymorphisms in theimmunoglobulin heavy gae6127mma (IGHG) Fc domain is indicated with blue stars. Except for the star vepresentingthe variation in hinge length between IgG3 allotypes, each smaller blue star indicates amino acid variation atone particular residue in the constant domain. Glycans attached to N297 in Fc region are highly variable and thefrequency of each glycan moiety on IgG antibodies in human serum is indicated [22] (reprinted with permission). Analytical Characterization of Monoclonal Antibodies with Novel Fc Receptor-Based...DOI: http://dx.doi.org/10.5772/intechopen.95356 individual peak size differences,each mAb is indicative of its unique separationprofile (Figure 13). This study thus shows that TSKgel FcR-IIIA-NPR column can beused for the analysis of a variety of IgG subclasses for glycosylation profiling. Theresults typically well correlate to ADCC activity. Since the first FDA approvals of biosimilars in the USA in 2015, the interestcontinues to increase toward biosimilars. They are, in general, less costly to developthan the original or innovators. However, the biosimilar manufacturers are requiredto confirm the extent of similarity with the corresponding innovator. In the recent lit-erature report [23], it was indeed confirmed that glycan microheterogeneity may playa critical role in effector function between the originals and biosimilars. In the study,it was shown that a biosimilar had a higher level of afucosylated glycans, resulting ina stronger FcyRIIIa binding affinity and increased ADCC activity. The study in theFigure 14 also shows that the TSKgel FcR-IIIA-NPR column yielded dissimilar chro-matographic pattern for biosimilars as compared to its innovator. Thus this columncan be used for monitoring biosimilar consistent with the innovator biomolecule. (A) Human gamma globulin (B) Human IgG1 (C) Herceptin biosimiler (D) Herceptin biosimiler (+Fucose) (-Fucose) 2.5 69655cEE4 巧 E 41 的cN2 的c6332巧 0 0 0 -0.5 -1 -1.5 -1 0 10 20 0 10 20 10 20 0 10 20 Time (min) Time (min) Time (min) Time (min) (E) Therapeutic antibody A(F) mAb 1 (H)mAb 3 6 (G) mAb 2招8巧 12 453 2 E 1 的 552 10 cEE 6 c64 0 0 0 -1 1 -3 -2 0 10 20 0 10 20 0 10 2( 10 20 Time (min) Time (min) Time (min) Time (min) Mobile phase: A: 50 mmol/L citrate, pH 6.5B: 50 mmol/L citrate, pH 6.5Gradient 0-100% B (2-20 min)Flow rate: 1.0mL/min Temperature: 25°℃ Detection: UV@280 nm, 10 Hz Injection vol.: 25 ug on column Figure 13.Analysis of a variety ofmonoclonal antibodies on TSKgel FcR-IIIA-NPR. Figure 14. Comparison of Roches innovator Rituximab to its two biosimilar biotherapeutic forms. The figure also includesmogamulizumab (PoteligeoTM) as an example of a completely afucosylated mAb. 6.Factors affecting the chromatographic separation 6.1 Salt concentration Salts affect the separation of mAb on TSKgel FcR-IIIA-NPR. To best controlthe pH in the linear gradient, mobile phase should consist of a buffer with suitablebuffering capacity whereas neutral salts are used to increase the ionic strength. Bothcomponents affect the binding affinity. Buffer provides pH control and salt ionsprovide charge shielding or stoichiometric ion bonding on the stationary phase andmAbs. Salts impart specific or non-specific effects by modulating protein-proteinand protein-surface interactions. Binding affinity to the column depends on thebinding constant. Increasing salt concentration have shown to lead to the elutionat earlier retention time (Figure 15, Panel A), although the intensity of the effectprobably is also related to the individual mAb studied. The binding strength is alsodependent on the buffer used such as sodium citrate or sodium acetate. Citrateyielded stronger binding and hence higher retention time. Acetate buffer insteadyielded better resolution of the peaks as compared to citrate (Figure 15, Panel B). In the affinity chromatography, the optimum flow rate of elution may be depen-dent on the molecule-specific interaction with the ligand. Irrespective of the flowrates (Figure 16), all the three glycoform peaks eluted within 67% of mobile phaseB when the analysis was carried out using a linear gradient of 50 mM sodium citratebuffer from pH 6.5 to 4.5 over 50 minutes at 20℃. Although flow rate did not haveeffect on elution pH, lower flow rate may be used to increase the sensitivity due tolonger residence time in the flow cell. Figure 15.Effect of salt (Panel A) and buffer (Panel B) on the separation of mAb glycoforms. Figure 16. Increased sensitivity at lower flow rate. DOI: http://dx.doi.org/10.5772/intechopen.95356 6.2 Gradient slope The gradient elution method is common for the separation of species of differ-ent binding strength. Figure 17 shows the effect of a gradient slope on the separa-tion mAb on TSKgel FcR-IIIA-NPR. As expected, longer gradient time increased theresolution between the peaks whereas the overall peak area and relative ratio of thepeak areas remained unchanged. Figure 17 indicates how shallower slope increasedthe resolution between the three peaks. This is particularly noticeable betweenpeaks 2 and 3. On the other hand, it should be noted that, for any further analytical Figure 17. Effect of gradient slope on the separation efficiency ofpeaks. work (e.g. for mass spec), longer gradient increases the peak volumes and thus peakfractions will be more diluted. 6.3 Temperature The ligand in FcR-IIIA-NPR column is a 20-kDa folded polypeptide and thus spe-cial care is to be considered to maintain protein conformation intact with proper runtemperature. Figure 18 shows the separation of mAb at four different temperatures(5°C,15℃,20°C, 25°C) at flow rate of 0.2 mL/min. As the temperature increased,the retention time of the three peaks decreased, indicating somewhat lower bindingaffinity as a function of higher temperature. However, importantly, overall peakprofile at each temperature remains unchanged. Thus, for practical reasons, tem-perature range from 15 to 25℃ is recommended for most analytical work. Followingthe completion of the analysis the column needs to be stored at 2-8°C. 6.4Sample load Figure 19 shows the effect of load amount of mAb on the separation profile.The limit of detection was determined as 1.5 rg as per USP definition S/N of 2-3. Aload of 3.16 rg could still be easily quantified (LOQ). The analysis was repeatable,robust and the total peak area increased proportionately as the load amount was Figure 18. Effect of temperature on the separation ofmAb. Figure 19.Effect of sample load on the separation ofmAb. Analytical Characterization of Monoclonal Antibodies with Novel Fc Receptor-Based...DOI: http://dx.doi.org/10.5772/intechopen.95356 increased in a linear manner in consecutive injections. Relative ratio of the indi-vidual peak areas in the three peaks remained constant. The column can generallybe used up to 100 rg protein load. However, 5-50 pg load of mAb is recommendedfor the best resolution and for maintaining the lifetime of the column. Presence of aggregates in IgG samples impact the binding to the Fcy receptors. Arecently published article reports that deamidated IgG samples caused aggregation orformation of higher molecular weight (HMW) species and thereby impacted the bind-ing affinity. Asparagine deamidation led to reduced binding of IgG to the low affinityFcyRIIIa receptor [24]. IgGs may also be more prone to aggregation when glycans areabsent, which in turn has an effect on Fc effector functions. Lack of glycan and its effecton binding is explained below in Section 7.1. IgG dimers and aggregates may also bindstronger to different types of Fc receptors and thus have significant impact on affinitydetermination. Accumulated strength of multiple non-covalent affinities between theligand and the receptor is known as avidity effect. This effect can alter the binding tothe receptor and should be considered during the analysis mAb with dimer and higherorder aggregates. The interaction, if any, needs to be evaluated in case-by-case basis.Up to 5% of aggregates in IgG samples changed the binding and kinetics to each of theFc receptors [24].Methionine is easily oxidized to methionine sulfoxide which can alsolead to light chain aggregation. Oxidation has impact on the binding to the Fcy receptorsand depends on the extent of oxidation. As reported [24],methionine oxidation below7% did not impact on binding to the receptors. Taken together, all above factors shouldbe considered when using this column, especially during analysis method developmentformAbs containing any amount of aggregates or oxidized forms. 7.Robustness of TSKgel FcR-IIIA-NPR affinity column 7.1 Selectivity The usefulness of any affinity chromatography column depends on severalrobustness factors. Here, selectivity is dependent on the nature of N-glycan. This isclearly demonstrated by analyzing enzymatically deglycosylated mAb. PNGase-Fdeglycosidase reacts between asparagine residue and the innermost N-acetyl glucos-amine (GlcNAc) of the complex oligosaccharide or high mannose content. Figure 20shows that enzymatically deglycosylated NIST mAb does not bind to the column andthus elutes in void volume. 7.2Lot-to-lot variability Scope of quality control of the therapeutic antibodies is expanding rapidly due tothe emergence of biosimilars,“biobetter”forms and numerous other kinds of biolog-ics in the biotherapeutic market. Lot-to-lot difference in the activity of innovatormAb may vary up to 20% in the manufacturing process [25]. Although substantialimprovement has been attained in CHO cell engineering during recent years, anddifferent strategies are there e.g., to produce afucosylated antibody drugs, still notenough technology is available to fully control in vivo glycosylation during production[26]. The lot-to-lot difference in N-glycan content may give rise to a wide variety ofrisk and thus N-glycan heterogeneity is a key factor to be monitored in quality control. To demonstrate importance of the mAb lot-to-lot quality control, two manufac-turing lots of mAb were analyzed using the TSKgel FcR-IIIA-NPR column (Figure21). Both lots yielded a similar 3-peak elution profile. However, when percentualpeak areas of the individual peaks were compared to check the consistency betweenthe two mAb lots. Lot B showed a higher glycan percentage in peak 1 (42% versus Figure 2o. Deglycosylated mAb does not bind to the column. N-glycan analysis oftwo manufacturing lots ofa therapeutic antibody on TSKgel FcR-IIIA-NPR. 34%) and lower percentual amounts in peaks 2 and 3. In a subsequent ADCC assay,this also correlated with lower ADCC activity in the lot B. This experiment thussupports the notion that FcR affinity chromatography is suitable for lot-to-lot qual-ity control of therapeutic mAbs. To confirm consistency in FcR column manufacturing is also equally importantfor quality control. Three different lots of TSKgel FcR-IIIA-NPR column (Lots A, B,and C) were tested using reference mAb sample under identical chromatographicconditions (Figure 22). No significant variation in 3-peak profile was noticedbetween the three different column lots. 7.3 Effect of host cell proteins on the separation of mAb Most mAb pharmaceuticals are produced in CHO cell culture system. Hostcell proteins (HCPs), or host cell impurities, are collectively recognized as severalforms of host cell products such as DNA, proteins, endotoxin and, if contaminated,viruses. These together are considered as process-related contaminants. They oftenhave antigenic or pyrogenic effects in human and thus must be removed duringdownstream processing. With regard to QC characterization, it is also necessary to assess if host cellproteins can interfere mAb binding on a TSKgel FcR-IIIA-NPR column. In thefollowing study, CHO cell culture supernatant (“feedstock”) was directly used forFcR column analysis and the results were compared to a previously purified mAbin the same assay (Figure23, Panel a). However, no significant difference wasnoticed between the two profiles. This indicates that the HCPs had no significantimpact on the mAb affinity to FcR column and about 5 rg of mAb in a feedstockwas enough to obtain a suitable signal for monitoring process development in abioreactor. The robustness of the assay was further tested using the mAb in CHOcell supernatant with 200 consecutive injections (Figure 23, Panel b). The totalpeak area remained constant with a%RSD (n=10) of 0.79. It was also noticedthat addition of NaCl to minimize unwanted non-specific interactions furtherimproved durability at 20°C. The Figure 23 (Panel c) shows how FcR column canbe used for cell line selection and upstream monitoring. In this case, samples fromCHO cell culture supernatant were collected, filtered, captured on protein A,and then injected to a TSKgel FcR-IIIA-NPR column. NaCl was added to improveseparation. Glycoform changes in mAb were monitored over 14 days. The propor-tion of the intensities and peak areas of the three peaks significantly changedover the days that can be correlated to indicate changes in ADCC activity during nt er Figure 22.Lot-to-lot consistency in TSKgel FcR-IIIA-NPR column manufacturing. Figure 23. (a) Analysis of CHO cell feedstock containing mAb versus purified mAb. (b)Assessment of FcR columnstability over 200 injections. (c) Monitoring of glycan composition changes during fermentation. Figure24. Acid stability ofa recombinant FcyRIII ligand as compared to a wild type ligand. fermentation. Thus, by monitoring samples from the bioreactor using TSKgelFcR-IIIA-NPR, process engineers can approximate the optimal day for desiredyield and ADCC activity. 7.4 Column pH stability and cleaning Recommended working pH for the FcR column as mentioned in operationalconditions and specifications (OCS) is from pH 8 to 4. As mentioned earlier, theprotein ligand contains eight amino acid substitutions for improved stability. Tofurther test acid stability, the column was held at pH3 for 200 hours. The modifiedligand did not lose its binding affinity and the selectivity while the wild-type lostthe binding affinity and selectivity within one hour (Figure 24). Based on this andother studies, a pH range of 3-8 is can be used for short term and pH 4.5-7 for longterm usage. Due to a protein nature of the ligand, acetonitrile and other organicsolvents are not suitable for the column. For cleaning, 3-5 injections of 0.5-2 mLof a buffer containing 500 mM NaCl or 20% ethanol can safely be used in reversedirection at half the normal flow rate. Once the cleaning procedure is complete,it is necessary to equilibrate the column in mobile phase for at least 45 minutes.Cleaning with alkalic solutions above pH 8 are not recommended since this willdenature the protein ligand. Sodium azide (0.05%) can be used in the mobile phaseas antibacterial agent. 8. Mass spectrometric characterization of glycoform peaks separated byTSKgel FcR-IIIA-NPR column Mass spectrometric characterization is becoming an integral part of the liquidchromatography analysis. As an example how TSKgel FcR-IIIA-NPR column can beutilized in mass spec work, we describe here an in-line LC-MS intact mAbanalysisof trastuzumab (Herceptin Biosimilar). The analysis was carried out using 100 mMvolatile ammonium acetate buffer and a linear pH gradient from pH 6.5 to pH 4.5 atthe flow rate of 0.4 mL/min. The wavelenght of detection was 280 nm. The columntemperature during the analysis was maintained at 20℃. Three glycoform peaks couldbe detected by UV detector. Mass spectrometric detection was carried out using SCIEXX500B Q-TOF in ESI positive mode, within mass/charge (m/z) range of 5000-7000.Ion source gases 1 and 2 were maintained at 50 psi, curtain gas at 30 psi, CAD gas at7 psi and temperature at 450℃. Spray voltage was maintained at 5200 V, declustering Figure 25. Overlay of UV spectrum and Total Ion Chromatogram (TIC) of Herceptin Biosimilar potential at 275 V, and collison energy at 20 V.Time bins to sum was set at 120. For theautomated characterization of the data acquired on the X500B QTOF, SCIEX Biotoolkit software was used. Total Ion Chromatogram (TIC) was obtained by summing upintensities of all mass spectral peaks belonging to the same scan. An overlay of UV pro-file and TIC profile is shown in Figure 25. For further analysis of glyosylation profilesof these three peaks, SCIEX BioPharmaViewTM software can be used.1. As mentioned earlier, organic solvents such as acetonitrile are not c_..suitable for thecolumn and vapor pressure of water is very l1ow. Thus,, volatile salts such as 100 mMammonium acetate or ammonium formate are used111. To avoid ion source contaminationduring prolonged use, molarity should be kept at low (preferably <50 mM). Dependingon the need for further optimization for different mAbs, volatile salt ammoniumbicarbonate can also be used as such or in combination with other volatile salts. 9. Analysis of Fc fragment on TSKgel FcR-IIIA-NPR The C-terminal part of the heavy chains contains the Fc fragment which isresponsible for cellular effector functions, essential for proper function of mosttherapeutic mAbs. In some cases, it is desirable to express fragment antibodies thatare smaller than intact mAbs but still are capable of eliciting their therapeutic func-tion by activation of the immune system. Literature reports that both glycoengineer-ing and protein engineering have rendered Fc domains with enhanced Fc receptorbinding. In general, Fc fragments and their numerous variants are rapidly gaininginterest as a platform in the development of efficient biotherapeutics. The binding efficiency of the Fc fragment was tested in-house to assess suit-ability of TSKgel FcR-IIIA-NPR column on the characterization of smaller fragmentantibodies. In short, trastuzumab was fragmented with papain that cleaves IgG atHis228 forming two Fab parts and one Fc part (Figure 26). The reaction mixturewas incubated at 37°C for 15 minutes to activate papain followed by mAb additionand further incubation overnight at room temperature. Papain activity was stoppedwith 5 mM iodoacetamide. A control a sample (no papain during incubation) and asample from papain digestion were used for this study. Size exclusion chromatogra-phy followed by mass spectrometric analysis confirmed >95% cleavage of mAbto Fcand Fab fragments (data available by request). Both the control mAb and fragments were analyzed on TSKgel FcR-IIIA-NPR column. As expected, Fab did not bind to the FcR column but eluted in Figure 26. Schematic representation of monoclonal antibody fragmentation with papain. Figure 27. Analysis of intact mAb and Fc fragment on TSKgel FcR-IIIA-NPR column. flow-through. Fc fragment efficiently bound to the column and yielded threeglycoform peaks similar to intact mAb (Figure27). Same sample volumes fromthe control sample and digestion reaction mixture were loaded onto the column.Lower peak heights for the Fc fragment were due to loss of Fab (2x48 kDa) fromthe protein mass during analysis. Interestingly, slightly longer retention times weredetected for Fc fragment peaks, thus suggesting more rigid conformational stabil-ity for the Fc fragment leading to stronger binding as compared to the intact mAb.In summary, this experiment confirms that fragment antibodies, as long as theycontain intact unobstructed Fc region,can be tested using the FcR column. 10. Novelty of TSKgel FcR-IIIA-NPR column The mechanism of binding for IgG and other Fc engaging molecules is shownin the Figure 28. Complement component (C1q), Fc gamma receptors (FcyR), theNeonatal Fc receptor (FcRn), Tripartite motif 21 (Trim21), and Fc receptor-like(FcRL) molecules bind to various locations of mAb for the exertion of biologicalactivity. For each ligand, the stoichiometric ratio of binding is also reported (Panela). Recently, a biotinylated recombinant human FcRn immobilized to a StreptavidinSepharose matrix and packed in a low pressure FPLC column has been introducedby Roche. A prepacked analytical protein-A affinity column (TSKgel ProteinA5-PW) marketed by Tosoh also interacts with Fc region. Site of interaction of FcyRis separate from the site of interaction for FcRn or Protein A as seen in the Figure 28Panel b. Figure 28. Intevaction of IgG with Fc effector molecules and protein A. TSKgel FcR-IIA-NPR Figure 29. Selectivity of the modified recombinant FcyRIIIa ligand us. Protein A ligand. TSKgel FcR-IIIA-NPR and TSKgel Protein A-5PW columns were tested for bindingaffinity of mAb with and without N-glycan using surface plasmon resonance tech-nique (Figure 29). Protein A affinity chromatography column showed similar bindingto mAb regardless of N-glycan whereas TSKgel FcR-IIIA-NPR column did not bind tomAb withoutN-glycan, similarly to the result in the Figure 20. Thus, the FcR columnis unique due to its capability to analyze mAbs solely on the basis of their glycosylation. 11. Preparative scale purification of antibodies using FcR-basedchromatography technique Here, we provide a quick preview of the preparative scale TSKgel FcR-IIIA-5PWcolumn that will be commercially available soon. The preparative column is manu-factured using the same recombinant FcyRIIIA protein ligand, however, the ligandin this column is bound to porous (~100 nm nominal pore size) polymethacrylatepolymer base beads. The column is suited for mAb purification in a significantlylarger scale (loading 0.5-5 mg) as compared to the analytical FcR column (loading≤100 rg). Chromatographic profile in the Figure 30, panel A was obtained usingU/HPLC instru..ment and the analytical FcR column whereas the panel B shows peakseparation with the preparative FcR column connected to a FPLC instrument. Thepeak separation profiles are closely similar with both columns. However, the prepar-ative scale column allows collection of much more material for further experimenta-tion such as glycan release, labeling and HILIC analysis, among other assays. Figure 3o. Separation ofmAb glycoforms using (A) analytical FcR column and (B) preparative FcR column. 12. Conclusions TSKgel FcR-IIIA-NPR affinity chromatography column is a unique tool separat-ing monoclonal antibodies into three peaks based on their glycosylation profile.Selectivity of the genetically engineered FcyRIIIa ligand .is very specif..1.ic to the mAbbased on its glycan composition on highly conserved Asn-297 residue. IgG1, IgG3and IgG4 subclasses bind to the FcR column whereas IgG2 subclass does not haveaffinity.IgA and IgM also don’t bind to this column. Non-glycosylated mAb alsodoes not bind to the column. Importantly, this column can be used for fast evalu-ation of antibody's ADCC effector function since the peak profile correlates wellwith the ADCC activity. Longer the retention time, higher is the ADCC activity. The generally accepted workflow for mAb characterization, based on its glycancontent typically follows the three different pathways (Figure 31, panel A). These arereporter bioassay for monitoring ADCC activity, Surface Plasmon Resonance (SPR)for measuring FcR affinity and U/HPLC-MS analysis for characterization of the glycanstructure. Characterization of mAb on TSKgel FcR-IIIA-NPR column can combinethese three pathways to one workflow (Figure 31, Panel B) in most circumstances. The column is expected to be useful in several application areas (Figure 32)including (a) early screening of ADCC activity, (b) upstream (cell culture) opti-mization, (c) quality control of the mAb lot-to-lot consistency and (d) comparisonbetween innovator and biosimilar products. Overall, this novel FcR affinity columnis anticipated to be useful in research and development, characterization,manufac-turing and quality control. Figure 31.Workflow for the characterization of mAb. Analytical Characterization of Monoclonal Antibodies with Novel Fc Receptor-Based...DOI: http://dx.doi.org/10.5772/intechopen.95356 Application areas of analytical and preparative FcR columns. Acknowledgements Chromatogram showing separation of mAb glycoforms using preparativeTSKgel FcR-IIIA-5PW column (Figure 30, Panel B) was kindly provided by ScottL. Melideo, Ph.D., Tosoh Bioscience LLC. We sincerely thank Oscar Yamasaki andHiroshi Tomizawa (Tosoh Bioscience, Tokyo, Japan) for their valuable commentsand suggestions to the manuscript. Conflict of interest All the authors are current employees of Tosoh Bioscience, part of the TosohCorporation, that markets the TSKgel FcR-IIIA-NPR affinity column. Beyond this,the authors are not aware of any affiliations, memberships, funding, or financialholding that might be perceived as affecting the objectivity of this work. Author details Atis Chakrabarti *, Jukka Kervinen , Egbert Muller , Toru Tanaka’and Kazuaki Muranaka’ 1Tosoh Bioscience LLC, King of Prussia, PA, USA 2 Tosoh Bioscience GmbH, Darmstadt, Germany 3 Bioscience Division, Tosoh Corporation, Japan *Address all correspondence to: atis.chakrabarti@tosoh.com IntechOpen ◎ 2020 The Author(s). Licensee IntechOpen.This chapter is distributed under the termsof the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium,provided the original work is properly cited.LcoBY References [1] Fanger MW, Graziano RF,Guyre PM. Production and useof anti-FcR bispecific antibodies.Immunomethods. 1994;4:72-81.DOI:10.1006/immu.1994.1009 Investigation of the role of FcgammaRandFcRn in mAb distribution to thebrain. Mol Pharm. 2013;10:1505-1513.DOI:10.1021/mp300214k [5] Anderson CC, Sinclair NR. FcR-mediated inhibition of cell activationand other forms of coinhibition.Crit Rev Immunol.1998;18:525-544.DOI:10.1615/critrevimmunol.v18.i6.30 [6] Bell CG. Characterization of theFc receptors (FcR) functioning asbridge between antibody and cell-effector function; antibody-FcR-linkedactivation of immune responsiveness.Biochem Soc Trans. 1998;26:ArticleS200. DOI:10.1042/bst026s200 ( [7] Anania JC, Chenoweth AM,Wines BD, Hogarth PM. The HumanFcyRI (CD32) Family of Leukocyte FcR in Health and Disease. Frontiers in Immunology. 2019; Article 464. DOI:10.3389/fimmu.2019.00464 ) [8] Wang H, Coligan JE, MorseHC 3. Frontiers in Immunology.Emerging Functions of Natural IgM and Its Fc Receptor FCMR in ImmuneHomeostasis. 2016;7:Article 99.DOI:10.3389/fimmu.2016.00099 [9] Kimberly RP, Salmon JE. and Edberg JC. Receptors for[2] Mikulska J, BoratynskiJ,Immunoglobulin G. Arthritis andNiezgodka M, Lisowski, J. HumanRheumatism. 1995;38:306-314.placental membrane receptorDOI:10.1002/art.1780380303for IgG. Purification of thereceptor and its subunit structure.[10] LippoldS, Nicolardi S, WuhrerImmunology Letters. 1982;5:137-143.M and Falck D. Proteoform-ResolvedDOI:10.1016/0165-2478(82)90098-0FcrRIIIa Binding Assay for FabGlycosylated Monoclonal Antibodies[3] Vedeler CA, Matre R andAchieved by Affinity ChromatographyKristoffersen EK. SolubilizationMass Spectrometry of Fc Moieties.of human peripheral nerve Fc7Frontiers in Chemistry. 2019; 7:Articlereceptors and purification ofna1401m698.DOI:10.3389/fchem.2019.00698a functional 40 kDa receptor.Immunology Letters. 1989;22:281-286.[11] Dekkers G, PlompR,DOI:10.1016/0165-2478(89)90166-1Koeleman C.A.M, Visser R, vonHorsten H.H, Sandig V,Rispens T,[4] Abuqayyas L, Balthasar JP.Wuhrer M and Vidarsson G. Multi-level ( glyco-engineering techniques to generate IgG with defined Fc-glycans. Nature. Scientific Reports. 2016;6:Article36964. DOI:10.1038/srep36964 ) [12] Indik ZK, Park J, Hunter S andSchreiber A.D. Structure/functionrelationships of Fey receptors inphagocytosis. Seminars in Immunology.1995;7:45-54. DOI:10.1016/1044-5323(95)90007-1 ( [ 13] Radaev S, Motyka S, Fridman WH, Sautes-Fridman C, Sun PD. The structure of a human type III Fcgamma receptor in complex with Fc. The J ournal of Biological Chemistry. 2001;276:16469-16477.DOI:10.1074/jbc. M100350200 ) ( [14] Bas M, Terrier A, Jacque E,Dehenne A, Pochet-Beghin V, Beghin C,Dezetter AS, Dupont G,Engrand A,Beaufils B, Mondon P, F ournier N,de Romeuf C, Jorieux S, Fontayne A,Mars LT, Monnet C. Fc SialylationProlongs Serum Half-Life of Therapeutic Antibodies. The Journal ) of Immunology. 2019;202:1582-1594. DOI:10.4049/jimmunol.1800896 [15] Ferrara C, Grau S, Jager C,Sondermann P, Brinker P,Waldhauer I, Hennig M, Ruf A,Rufer AC, Stihle M, Umana P andBenz J.Unique carbohydrate-carbohydrate interactions are requiredfor high affinity binding betweenFcyRIII and antibodies lacking corefucose.PNAS. 2011;108:12669-12674.DOI:10.1073/pnas.1108455108 [16] Liu Z, Gunasekaran K,Wang W,Razinkov V, Sekirov L, Leng E, HeatherSweet H, Foltz I, Howard M, Rousseau A,Kozlosky C, Fanslow W, and Yan W.Asymmetrical Fc Engineering GreatlyEnhances Antibodydependent CellularCytotoxicity (ADCC) Effector Functionand Stability of the Modified Antibodies.The journal of Biological Chemistry.2014;289:3571-3590.DOI:10.1074/jbc.M113.513366 [17] Mizushima T, Yagi H,Takemoto E, Shibata-Koyama M,Isoda Y, Iida S, Masuda K, Satoh M andKato K. Structural basis for improvedefficacy of therapeutic antibodies ondefucosylation of their Fc glycans.Genes to Cells. 2011;16:1071-1080.DOI:10.1111/j.1365-2443.2011.01552.x ( [18] Wada R, Matsui M, Kawasaki N.Influence of N-glycosylation on effector functions and thermal stability of glycoengineered IgG1 monoclonal antibody with homogeneous glycoforms. MABS. 2019;11:350-372. DOI:10.1080/19420862.2018.1551044 ) [19] Kiyoshi M, Caaveiro J. M.M, ( Tada M, Tamura H, Tanaka T,TeraoY, Morante K,HarazonoA,HashiiN, Shibata H, Kuroda D,Nagatoishi S, Oe S, Ide T, Tsumoto K, I shii-Watabe A.Assessing the Heterogeneity of the Fc-Glycan of aTherapeutic Antibody Using an engineered FcgammaReceptor IIIa-Immobilized Column. Scientific reports. 2018;8:Article 3955. DOI:10.1038/ s41598-018-22199-8 ) [20] Master Thesis (2019) of LeilaGhaleh, Technischen UniversitatDarmstadt, Rituximab kindlyprovided by Rentschler Biopharma in.collaboration with Tosoh BioscienceGmbH ( [ 21] Li T, DiLillo DJ, Bournazos S,Giddens JP, Ravetch JV, Wang LX.Modulating IgG effector function by Fc glycan engineering. Proc Natl Acad Sci USA. 2017;114:3485-3490. DOI:10.1073/pnas.1702173114 ) ( [22] de Taeye SW, Rispens T, Vidarsson G. The Ligands for HumanIgG and Their Effector Functions Antibodies (Basel). 2019;8:2-18.DOI:10.3390/antib8020030 ) ( [23] KangJ, Kim SY, Vallejo D, Hageman TS, White DR, Benet A,CoghlanJ, S en KI, Ford M , Saveliev S, Tolbert TJ, Weis DD, Schwendeman SP,Ruotol BT, Schwendeman A. Multifacetedassessment of rituximab biosimilarity:The impact of glycan microheterogeneityon F c function. European Journal ofPharmaceutics and B iopharmaceutics. 2020;146:111-124. DOI:10.1016/j. ejpb.2019.12.003 ) ( [24] Geuijen KPM, Oppers-Tiemissen C, Egging DF, Simons PJ, Louis Boon L, Schasfoort RBM and Eppink MHM. Rapid screening of IgG qualityattributes- effects on Fc receptorbinding. FEBS Open Bio. 2017;7:1557- 1574. DOI:10.1002/2211-5463.12283 ) ( [25] Schiestl M, Stangler T, Torella C,Cepeljnik T, Toll H, Grau R. Acceptable changes in quality attributes ofglycosylated biopharmaceuticals. Nature Biotech.2011;29:310-312. DOI:10.1038/ nbt.1839 ) ( [26] Pereira NA, Chan KF, Lin PC, and Song Z. The“less-is-more”in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellularcytotoxicity.MAbs. 2018;10:693-711 DOI:10.1080/19420862.2018.1466767 ) IntechOpen 迄今为止,经临床批准的大多数单克隆抗体类生物治疗药物,主要是IgG1形式,其次是IgG2和IgG4形式。糖基化是导致单克隆抗体翻译后修饰异质性的主要原因,会影响药物的治疗作用机制(MOA)。同时,糖基化也是影响药物溶解度、动力学、稳定性和疗效的关键因素之一。因此,监测聚糖的关键质量属性(CQA)在药物开发阶段显得至关重要。IgG与其细胞Fc受体(FcR)的结合亲和力取决于其IgG亚类和Fc区的糖基化结构。并且,N-聚糖的结构也与抗体ADCC活性密切相关,因此需要对单克隆抗体制剂及其生物类似药进行糖基化修饰的监测。东曹生命科学(Tosoh Bioscience)开发出了一种基于FcγIIIa的亲和色谱法来评价单克隆抗体糖型差异的全新方法。在文章中,首先简要概述了Fc受体的功能。然后,阐述了基于FcR的亲和色谱的原理、新型FcR色谱柱根据抗体N-聚糖分离多种单克隆抗体具有高选择性。最后,我们举例介绍了FcR色谱柱在细胞株筛选、糖工程监测、工艺开发和生产过程监控中的应用。
确定












还剩23页未读,是否继续阅读?
东曹(上海)生物科技有限公司为您提供《单克隆抗体中分析表征检测方案 》,该方案主要用于其他中理化性质检测,参考标准--,《单克隆抗体中分析表征检测方案 》用到的仪器有TSKgel FcR-ⅢA-NPR高性能亲和色谱柱
相关方案
更多
该厂商其他方案
更多









