方案详情
文
本研究旨在开发一种可促进吞咽过程的可口多奈哌齐(DP)或分散膜(ODF),并从动力学过程和体内药物吸收的角度探讨环糊精对味觉掩藏的影响。
方案详情

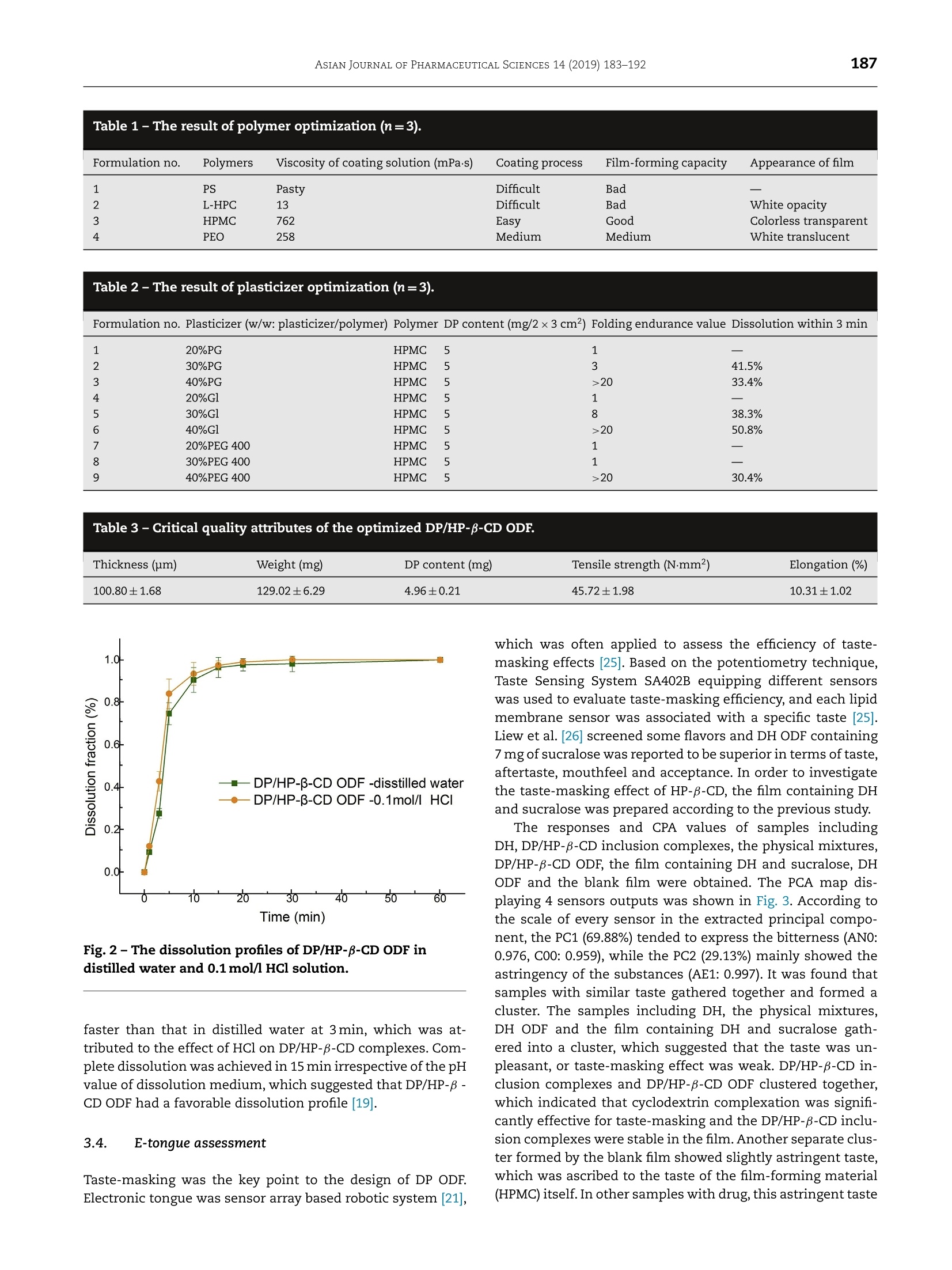
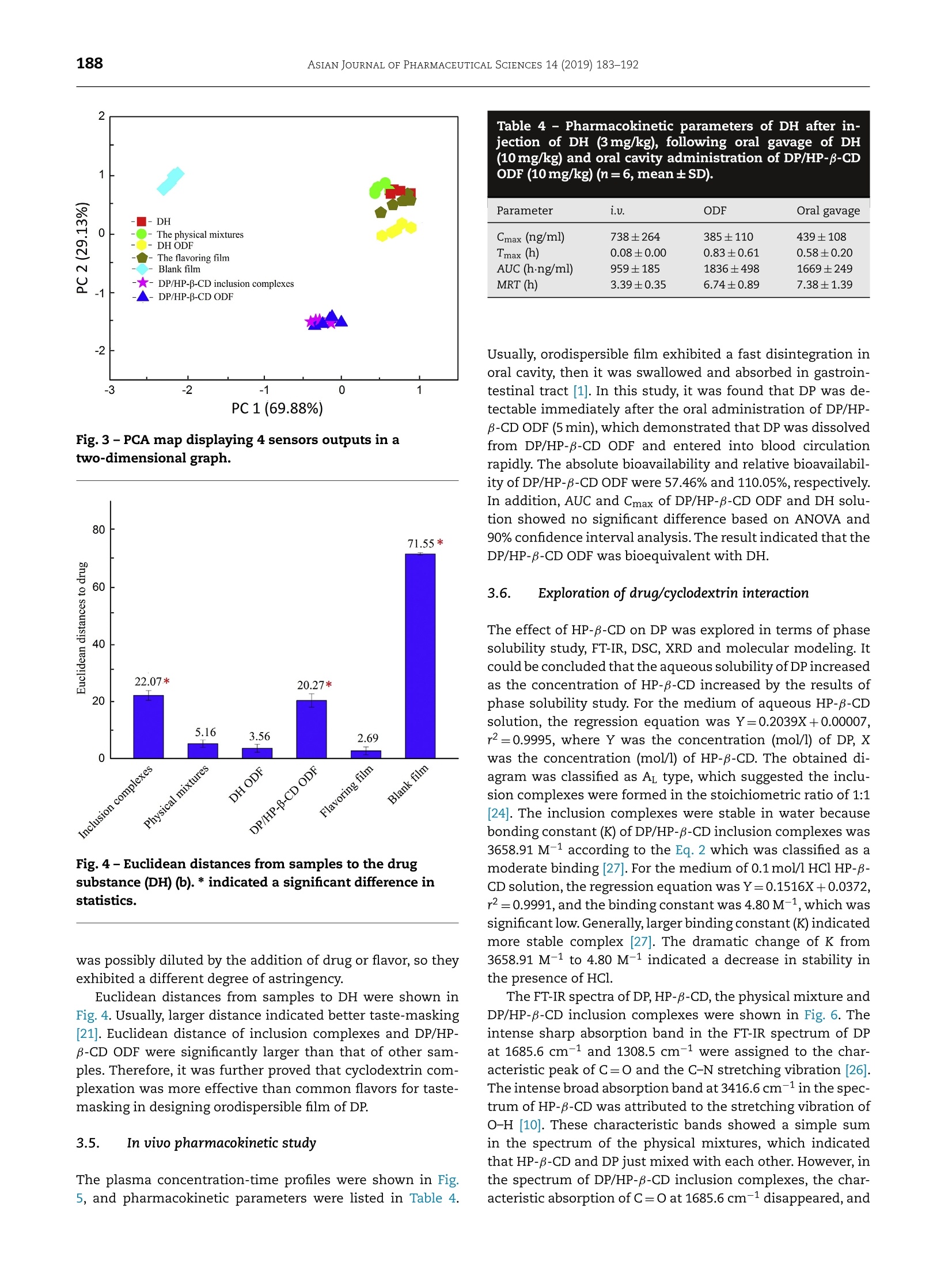
ASIAN JOURNAL OF PHARMACEUTICAL SCIENCES 14 (2019) 183-192 https://doi.org/10.1016/j.ajps.2018.05.001 Available online at www.sciencedirect.com ScienceDirectjournal homepage: www.elsevier.com/locate/AJPS Original Research Paper A donepezil/cyclodextrin complexationorodispersible film: Effect of cyclodextrin ontaste-masking based on dynamic process and inviuo drug absorption Tingting Liu", Xiaocao Wan,Zheng Luob, Chao Liu, Peng Quan',Dongmei Cun"*, Liang Fangb.,b,** aWuya college of Innouation, Shenyang Pharmaceutical University, 103 Wenhua Road, Shenyang 110016, China'School of Pharmacy, Shenyang Pharmaceutical University, 103 Wenhua Road, Shenyang 110016, China ARTICLE INFO Article history:Received 26 December 2017Revised 15 March 2018 Accepted 5 May 2018 Available online 24 May 2018 Keywords: Donepezil Orodispersible film Taste-masking Dynamic process E-tongue Absorption ABSTRACT The aim of this study was to develop a palatable donepezil (DP) orodispersible film (ODF)to facilitate the swallowing process and investigate the effect of cyclodextrin on taste-masking based on dynamic process and in vivo drug absorption. Complexation of DP withhydroxypropyl-B-cyclodextrin (HP-B-CD) was applied to mask the bitter taste then the pre-pared complexes were incorporated into ODF using solvent casting method. The taste-masking effciency was evaluated by e-tongue; meanwhile the pharmacokinetic behavior ofDP/HP-B-CD ODF was investigated by in viuo study. Results showed the optimized film wasmore palatable than donepezil hydrochloride (DH) film and was bioequivalent with DH. Themolecular mechanism was revealed by phase solubility study, Fourier-transform infraredspectrometer (FT-IR), Differential scanning calorimeter (DSC), X-ray diffraction (XRD) andmolecular modeling. Taste-masking was attributed to the formation of DP/HP-B-CD whichwas due to moderate interaction between DP and HP-B-CD. The stability of DP/HP-B-CD wasdecreased because of the acid environment in stomach,which facilitated the absorption ofDP. These results extended our understanding about the application of cyclodextrin com-plexation and provided guidance for the design of ODF especially for drugs with disgusting C 2018 Shenyang Pharmaceutical University. Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license. Recently, orodispersible film (ODF) gets growing attention be-cause of its unique superiorities for the target groups includ-ing children and the geriatric population who have difficultyin swallowing [1-2]. ODF allows drug to disperse rapidly inoral cavity [3], thus provides many advantages such as rapiddisintegration, improved dosing accuracy and stability com-pared to ordinary oral dosage forms [4-5]. Donepezil (DP) isa commonly used drug for the treatment of Alzheimer's dis-ease. Commercial oral products of DP (tablets or capsules)may result in swallowing difficulties, which causes distressto the major group of Alzheimer’s patients, the elders.There-fore, in order to improve patient compliance, it is meaning-ful to develop donepezil oral orodispersible film as an alterna-tive to commercial DP products. Moreover, the recommendeddosages of donepezil are 5 mg and 10 mg per day [6], which isappropriate to develop DP ODF [7]. DP has unacceptable taste such as bitterness, leading topoor compliance during the development process of DP ODFdue to its rapid dissolution in oral cavity. Adding flavors intothe formulation is the simplest and most commonly usedtaste-masking method [8]. However, the addition of flavor isnot effective enough to mask the taste of drugs like DP withextremely disgusting taste [9]. Cyclodextrin and its derivativesare widely used for preparing inclusion complexes due to itsnon-toxicity, high biodegradability and various other advan-tages [10]. It was reported that when drugs formed stable in-clusion complexes with cyclodextrin, the disgusting taste ofdrugs could be reduced, even fully masked [11]. Therefore,cyclodextrin complexation was a useful method for taste-masking in the development of ODF. Additionally, HP-B-CDshowed high solubility (60% or more, w/w) in water [12], whichwas suitable to be applied to developODF. When drug/cyclodextrin inclusion complexes are used tomask taste in a formulation, the effect of cyclodextrin be-comes complicated due to multiple factors influencing drugabsorption. Except for the drug itself, one of the factors isthe physicochemical properties of cyclodextrins. If cyclodex-trin has a low solubility, it possibly results in a slow dis-solution of drug in uivo. Another factor, which is consid-ered as the most important one, is the in vivo dynamic pro-cess of drug/cyclodextrin inclusion complexes. It is signifi-cantly influenced by drug/cyclodextrin interaction and theen-vironment in vivo. The existence of moderate interaction willachieve a successful taste-masking, but if the interaction forcebetween drug and cyclodextrin is too strong, it will lead tothe decrease of free drug concentration in the intestine,caus-ing low absorption [13-14]. Additionally, drug molecules travelthrough various physiological environment from mouth togastrointestinal tract. Changing of the location may affect theform of drug or the stability of complexes, which affects drugabsorption. In a previous research focusing on the effect of cyclodex-trin concentration on the oral bioavailability, variations onpharmacokinetic parameters and profiles were observed af-ter drug/cyclodextrin solution with different cyclodextrin con-centration was dosed in rats [15]. Another research found thesignificant influence of HP-B-CD and sucrose on the absorp- Fig. 1- The structures of DP (a) and HP-B-CD (b). tion of midazolam in rabbits [16]. These studies showed theimportant role of cyclodextrin in the enhancement of solu-bility or taste-masking and suggested the complicated effectof cyclodextrin on drug absorption. Nevertheless, the effectof drug/cyclodextrin dynamic process in vivo was rarely men-tioned or studied. Therefore, the knowledge of how cyclodex-trin affected drug absorption while taste-masking and howthe dynamic process carried out was still limited. In this study, DP/HP-B-CD inclusion complexes were pre-pared to mask the disgusting taste of DP, and subsequentlythe complexes were incorporated into ODF by solvent cast-ing method. The structures of DP and HP-B-CD were shownin Fig. 1. The taste-masking efficiency was evaluated by e-tongue, and the pharmacokinetic behavior of the film was in-vestigated by in uivo study. More importantly, the effect of cy-clodextrin was explored via series of technologies based ondynamic process and in uiuo drug absorption. 2. Materials and methods 2.1. Chemicals and animals Donepezil hydrochloride (DH) was obtained from Jinan Dex-injia Biological Products Co., Ltd. (Jinan, China). HP-B-CD waspurchased from Xian Deli Biological Chemical Co., Ltd. (Xi'an,China). Hypromellose (HPMC, Methocel E5 premium LV) was agift from Colorcon (Shanghai, China). Polyethylene glycol 40Cand glycerol were provided by Bodi Chemical Co., Ltd, (Tian-jin, China). All other chemicals or solvent were of the highestreagent grade available. Wistar rats (male, 200±20 g) were provided by the Experi-mental Animal Center of Shenyang Pharmaceutical University(Shenyang, China). The experiments were performed in accor-dance with the Institutional Animal Care and Use Committeewith the approval of No. SYPU-IACUC-C2016-1121-201. 2.2. Quantitative analysis of DP DP in various samples was determined using a HPLC system(Hitachi High-Technologies Corporation, Tokyo, Japan) con-sisting of a L-2130 pump, a L-2420 ultraviolet absorbance de-tector and a L-2200 automatic injector. An ODS-C18 column(200 mmx4.6mm, 5 um, Dikma Technologies Inc., USA) was used with the temperature set at 40C. For the in uitro samples,the mobile phase was 0.1% glacial acetic acid and 0.2% triethy-lamine in water-methanol (40:60,v/v). For the in uivo study,the mobile phase was 60% methanol and 40% glacial aceticacid solution (0.5%) adjusted to pH 6.5 with triethylamine. Thequantitative analysis was performed under the condition ofthe flow rate at 1.0 ml/min and the wavelength at 271nm. Theinjection volume was 20 ul. All of the analytical methods havebeen validated. 2.3. Development of DP/HP-B-CD ODF 2.3.1..Preparation of DP/HP-B-CD inclusion complexesDP free base was prepared using liquid-liquid extractionmethod [17]. After that, 6.90g of HP-B-CD and 1g of DP werecompletely dissolved in alcohol and the solution was stirredfor 12 h at room temperature. After the evaporation of alcohol,the product was collected for further use. 2.3.2.. Formulation design of DP/HP-B-CD ODF One-factor optimization method was used to obtain the fi-nal optimized formulation. Film forming materials includinghydroxypropyl methyl cellulose (HPMC), pregelatinized starch(PS), polyethylene oxide (PEO) and low-substituted hydrox-ypropyl cellulose (L-HPC) were optimized according to thestandards of film-forming capacity and appearance of films.Plasticizers such as polyethylene glycol (PEG), glycerin andpropylene glycol, were optimized with the standards of disso-lution within 3 min and folding endurance value, which wasdetermined by repeatedly folding the film at the same pointuntil broken [18]. Moreover, the drug content was controlledby adjusting the drug loading. 2.3.3. Preparation of DP/HP-B-CD ODF Solvent casting method was used to prepare DP/HP-B-CD ODF.Certain amount of polymer, plasticizer and DP/HP-B-CD inclu-sion complexes were dissolved in distilled water and stirredfor 1 h at room temperature to obtain the casting solution. Af-ter degassing, the casting solution was casted onto a plasticplate using a laboratory coating unit (SLT200; Kaikai Company,Ltd., Shanghai, China) with casting height of 1mm, and thenthe formed film was dried at 54°C for 1h to obtain the DP/HP-B-CD ODF. Then the film was cut into 2x3 cm. As control samples, DH ODF, the film containing DH andsucralose, blank film (pure polymer) were prepared using thesame method. 2.4. Critical quality attributes of DP/HP-B-CD ODF Some critical quality attributes were controlled in the devel-opment of ODF, including thickness, weight, drug content andmechanical properties using micrometer (Mitutoyo, Japan)(n=6), electronic analytical balance CP225D (Sartorius Instru-ments system, Ltd., Beijing, China) (n=20), HPLC (n=20) andauto stripping tester (Labthink instruments Co., Ltd., China)(n=6) [19], respectively. 2.5. In vitro dissolution test In uitro dissolution test was performed to investigate the dis-solution behavior of film in different conditions. According to the United States Pharmacopeia (USP), the paddle method wasapplied, and distilled water as well as 0.1 mol/l HCl were usedas dissolution medium.DP/HP-B-CD ODF (2×3cm2,n=6)wasplaced at the bottom of the dissolution cup (250 ml), then dis-tilled water of 100 ml at 37±1°℃ was poured into the cup andstirred at a rotation speed of 50rpm.At preselected time in-tervals of 1, 3, 5, 10, 15,20,30 and 60min, dissolution mediumof 5 ml was withdrawn and replaced by equal volume of freshdistilled water immediately. After centrifuging at 16000 rpm,the withdrawn samples were analyzed by the HPLC. For thedissolution medium of 0.1 mol/l HCl, all the operations werethe same as that of distilled water. 2.6. E-tongue assessment E-tongue assessment was conducted to evaluate the taste ofsamples using a Taste Sensing System SA402B (IntelligentSensor Technology, Ltd., Japan). Four kinds of medicinal sen-sors, BTO (for specific bitterness of hydrochloride drugs), AN0(for alkaline bitterness), C00 (for acidic bitterness) and AE1 (forastringency) were used separately. Before the assessment, thesamples were dissolved, and the concentration of DP was at0.05 mg/ml. Potassium chloride solution of 10 mM was usedas the reference and rinse solution. According to relative sensor outputs, the difference be-tween the test sample and reference solution in potential(Vs-Vr) and CPA values which was defined as the dif-ference (Vr’-Vr) between the potentials of the referencesolution before and after sample assessment (change ofmembrane potential caused by adsorption of samples) wereobtained [20-21]. Principal component analysis (PCA) wasused to analyze these data [22-23]. Meanwhile, Euclideandistance from samples to DH was calculated according to thefollowing Eq.1: Where p and q were the Vs-Vr value of samples and DH,respectively, i represented different sensors. 2.7. In uiuo pharmacokinetic study 2.7.1. Administration and sampling Eighteen Wistar rats were divided into three groups randomly,No.1, No.2 and No.3 (6 rats for each group), which were fas-tened and only allowed free access to water for 12 h prior tothe experiment. DHaqueous solution was administered to the rats of groupNo.1 intravenously (3mg/kg). The rats of the group No.2 wasgiven DH aqueous solution via intragastrical administrationroute (10 mg/kg), and for the group No.3,DP/HP-B-CD ODFsstrips were set on the tongue of the rat (10mg/kg). After ad-ministration, blood samples of 0.2 ml were taken from orbitat 5, 10, 15, 30, 60,90, 120,180,240,300,360,420,480,600 and720 min for group No.1 and at 0.25,0.5,1.0,1.5,2.0,3.0, 4.0,6.0,8.0, 12.0 and24.0 h for group No.2 and No.3. 2.7.2. Plasma sample and pharmacokinetic analysis Plasma was :separated immediately by centrifuging2at16,000rpm and stored at-60 C before analysis. The Win- Nonlin (Version 5.2.1; Pharsight Co., Moutain View, USA) wasused to calculate pharmacokinetic parameters based on thenon-compartmental model. 2.8. Statistical analysis Results were expressed as the mean ±standard deviation. Sta-tistical analysis of data was carried out based on two-wayanalysis of variance (ANOVA) and 90% confidence intervalanalysis.The level of significance was taken as p <0.05. Forparameters of animal study, AUC and Cmax were logarithmic,and then simply used to evaluate the bioequivalence of thesetwo preparations. 2.9. Exploration of drug/cyclodextrin interaction 2.9.1. Phase solubility study In order to investigate the effect of HP-B-CDon the solubilityof DP and explore the stability of inclusion complexes in dif-ferent conditions, phase solubility study was conducted ac-cording to the method established by Higuchi and Connors[24]. Aqueous solution and 0.1 mol/l HCl solution of HP-B-CDwere used as medium, respectively. Excess amounts of DPwere added into 10 ml of aqueous HP-B-CD solution with dif-ferent concentrations (from 0 to 100 mM). The obtained sus-pensions were shaken at 37°C for 48h. The samples were col-lected and filtered through a Millipore filtration of 0.22 umtoremove the undissolved DP. The amount of DP in filtrate wasthen determined by HPLC and all experiments were carriedout in triplicate. The solubility diagram was plotted with themolar concentration of DP as a function of the molar concen-tration of HP-B-CD. The value of binding constant (K) was cal-culated through the Eq. 2. Where So was the solubility of DP in water. For the medium of0.1 mol/l HCl solution of HP-B-CD, all the operations were thesame as aqueous HP-B-CD solution. 2.9.2. Fourier-transform infrared spectroscopy (FT-IR) FT-IR study was conducted to explore the interaction betweenDP and HP-B-CD. The FT-IR spectra of DP, HP-B-CD, physicalmixtures and DP/HP-B-CD inclusion complexes were obtainedin the region of 400-4000 cm-1 by Bruker Vertex 70 spec-tromerter (Billerica, USA). All the powders were measured bythe KBr disk technique. 2.9.3. Thermal analysis (DSC) Thermal analysis was conducted using a DSC-1 system (Met-tler Toledo International Inc.,Switzerland). The samples of DP,HP-B-CD, physical mixtures and DP/HP-B-CD inclusion com-plexes were accurately weighed (about 5mg) and placed in analuminum pan with the cover sealed. The heat procedure wasat a scanning rate of 10 °C/min from 25 to 150°C under a ni-trogen purge. 2.9.4. X-ray diffractometry (XRD) XRD study was conducted to investigate the crystal form ofsubstance. XRD patterns of DP, HP-B-CD, physical mixturesand DP/HP-B-CD inclusion complexes were obtained at roomtemperature using a DX2700 X-ray diffractometer (Aolong Ra-diative Instrument Group Co. Ltd, Dandong, China) under avoltage of 40 kV and a current of 40 mA. All the samples wereanalyzed in an angle range of 5-50° (20) with a scanning stepwidth of 2°per minute. 2.9.5. Molecular modeling Molecular modeling was conducted to investigate interactionbetween HP-B-CD and DP. AutoDock 4.2 software was used tccalculate the molecular model. The initial structures of DP andHP-B-CD were obtained using ChemBioDraw Ultral 14.0, andthen these structures were optimized using Discovery Studic4.0 software.Additionally, the Lamarckian Genetic Algorithm(LGA) in AutoDock 4.0 was used to perform the docking studyin a vacuum environment. Furthermore, in order to probe theDP/HP-B-CD dynamic process in viuo, the DP/HCl interactionwas explored using same operations as DP/HP-B-CD. 3. Results and discussion 3.1. Formulation design of DP/HP-B-CD ODF Results of the optimization process were listed in Tables 1and 2. HPMC had proper film forming capacity and the filmprepared by HPMC had a good appearance; meanwhile, thefilm containing glycerin of 40% (accounting for the propor-tion of polymer, 16.7% of the formulation) was excellent inboth mechanical properties and dissolution behavior.There-fore, HPMC was selected as the optimized polymer, and glyc-erin of 40% was chosen as the optimized plasticizer. Addi-tionally, DP content was controlled as 5 mg/2x3cm²by ad-justing DP/HP-B-CD inclusion complexes loading as 41.4% ofthe formulation. The optimized formulation of DP/HP-B-CDODF contained DP/HP-B-CD inclusion complexes (41.4%, DPaccounted for 4.7% of the formulation), HPMC (41.9%), andglycerol (16.7%). 3.2. Critical quality attributes of DP/HP-B-CD ODF The critical quality attributes of the optimized film werelisted in Table 3. The DP content and the weight of filmwere 4.96±0.21 mg, 129.02±6.29 mg (RSD<5%) (n=20), re-spectively, which would be used for further studies. 3.3. In vitro dissolution test An orodispersible film should dissolve quickly to ensure therapid dissolution of drug. In this study, two different dissolu-tion media were selected to investigate the effect of HCl ondissolution behavior. Meanwhile, 0.1 mol/l HCl solution wasused to simulate the gastric environment. The dissolution be-haviors of DP/HP-B-CD ODF were shown in Fig. 2. DP dissolvedrapidly from the film in both distilled water and 0.1 mol/l HClsolution. The dissolution rate in HCl solution was significantly Table 1- The result of polymer optimization (n=3). Formulation no. Polymers Viscosity of coating solution (mPas) Coating process Film-forming capacity Appearance of film 1 PS Pasty Difficult Bad 一 2 L-HPC 13 Difficult Bad White opacity 3 HPMC 762 Easy Good Colorless transparent 4 PEO 258 Medium Medium White translucent Table 2-The result of plasticizer optimization (n=3). Formulation no. Plasticizer (w/w: plasticizer/polymer) Polymer DP content (mg/2×3 cm²) Folding endurance value Dissolution within 3 min 1 20%PG HPMC 5 1 — 2 30%PG HPMC 5 3 41.5% 3 40%PG HPMC 5 >20 33.4% 4 20%G1 HPMC 5 1 5 30%Gl HPMC 5 8 38.3% 6 40%Gl HPMC 5 >20 50.8% 7 20%PEG 400 HPMC 5 1 8 30%PEG 400 HPMC 5 1 9 40%PEG 400 HPMC 5 >20 30.4% Table 3-Critical quality attributes of the optimized DP/HP-B-CD ODF. Thickness (um) Weight(mg) DP content (mg) Tensile strength (N-mm2) Elongation (%) 100.80±1.68 129.02±6.29 4.96±0.21 45.72±1.98 10.31±1.02 Fig. 2-The dissolution profiles of DP/HP-B-CD ODF indistilled water and 0.1 mol/l HCl solution. faster than that in distilled water at 3min, which was at-tributed to the effect of HCl on DP/HP-B-CD complexes. Com-plete dissolution was achieved in 15 min irrespective of the pHvalue of dissolution medium, which suggested that DP/HP-B-CD ODF had a favorable dissolution profile [19]. 3.4. E-tonque assessment Taste-masking was the key point to the design of DP ODF.Electronic tongue was sensor array based robotic system [21], which was often applied to assess the efficiency of taste-masking effects [25]. Based on the potentiometry technique,Taste Sensing System SA402B equipping different sensorswas used to evaluate taste-masking efficiency,and each lipidmembrane sensor was associated with a specific taste [25].Liew et al. [26] screened some flavors and DHODF containing7 mg of sucralose was reported to be superior in terms oftaste,aftertaste, mouthfeel and acceptance. In order to investigatethe taste-masking effect of HP-B-CD, the film containing DHand sucralose was prepared according to the previous study. The responses and CPA values of samples includingDH,DP/HP-B-CD inclusion complexes, the physical mixtures,DP/HP-B-CD ODF, the film containing DH and sucralose, DHODF and the blank film were obtained. The PCA map dis-playing 4 sensors outputs was shown in Fig. 3. According tothe scale of every sensor in the extracted principal compo-nent, the PC1 (69.88%) tended to express the bitterness (ANO:0.976, C0O: 0.959), while the PC2 (29.13%) mainly showed theastringency of the substances (AE1: 0.997). It was found thatsamples with similar taste gathered together and formed acluster. The samples including DH, the physical mixtures,DH ODF and the film containing DH and sucralose gath-ered into a cluster, which suggested that the taste was un-pleasant, or taste-masking effect was weak.DP/HP-β-CD in-clusion complexes and DP/HP-B-CD ODF clustered together,which indicated that cyclodextrin complexation was signif-cantly effective for taste-masking and the DP/HP-B-CD inclu-sion complexes were stable in the film. Another separate clus-ter formed by the blank film showed slightly astringent taste,which was ascribed to the taste of the film-forming material(HPMC) itself. In other samples with drug, this astringent taste Fig.3-PCA map displaying 4 sensors outputs in atwo-dimensional graph. Fig. 4-Euclidean distances from samples to the drugsubstance (DH) (b). * indicated a significant difference instatistics. was possibly diluted by the addition of drug or flavor, so theyexhibited a different degree of astringency. Euclidean distances from samples to DH were shown inFig. 4. Usually,larger distance indicated better taste-masking[21]. Euclidean distance of inclusion complexes and DP/HP-B-CD ODF were significantly larger than that of other sam-ples. Therefore, it was further proved that cyclodextrin com-plexation was more effective than common flavors for taste-masking in designing orodispersible film of DP. 3.5. In uiuo pharmacokinetic study The plasma concentration-time profiles were shown in Fig.5, and pharmacokinetic parameters were listed in Table 4. Table 4 - Pharmacokinetic parameters of DH after in-jection of DH (3mg/kg), following oral gavage of DH(10mg/kg) and oral cavity administration of DP/HP-B-CDODF (10mg/kg)(n=6, mean±SD). Parameter i.u. ODF Oral gavage Cmax (ng/ml) 738±264 385±110 439±108 Tmax (h) 0.08±0.00 0.83±0.61 0.58±0.20 AUC (hng/ml) 959±185 1836±498 1669±249 MRT (h) 3.39±0.35 6.74±0.89 7.38±1.39 Usually, orodispersible film exhibited a fast disintegration inoral cavity, then it was swallowed and absorbed in gastroin-testinal tract [1]. In this study, it was found that DP was de-tectable immediately after the oral administration of DP/HP-β-CD ODF (5 min), which demonstrated that DP was dissolvedfrom DP/HP-B-CD ODF and entered into blood circulationrapidly. The absolute bioavailability and relative bioavailabil-ity of DP/HP-B-CD ODF were 57.46% and 110.05%, respectivelyIn addition, AUC and Cmax of DP/HP-B-CD ODF and DH solu-tion showed no significant difference based on ANOVA and90% confidence interval analysis. The result indicated that theDP/HP-B-CD ODF was bioequivalent with DH. 3.6. Exploration of drug/cyclodextrin interaction The effect of HP-B-CD on DP was explored in terms of phasesolubility study, FT-IR, DSC,XRD and molecular modeling. Itcould beconcluded that the aqueous solubility of DP increasedas the concentration of HP-B-CD increased by the results ofphase solubility study. For the medium of aqueous HP-B-CDsolution, the regression equation was Y=0.2039X+0.00007,r2=0.9995, where Y was the concentration (mol/l) of DP, Xwas the concentration (mol/l) of HP-B-CD. The obtained di-agram was classified as AL type, which suggested the inclu-sion complexes were formed in the stoichiometric ratio of 1:1[24]. The inclusion complexes were stable in water becausebonding constant (K) of DP/HP-B-CD inclusion complexes was3658.91 M-1 according to the Eq. 2 which was classified as amoderate binding [27]. For the medium of 0.1 mol/l HCl HP-B-CD solution, the regression equation wasY=0.1516X+0.0372,r2=0.9991, and the binding constant was 4.80 M-1, which wassignificant low.Generally,larger binding constant (K) indicatedmore stable complex [27]. The dramatic change of K from3658.91 M-1 to 4.80 M-1 indicated a decrease in stability inthe presence of HCl. The FT-IR spectra of DP,HP-B-CD, the physical mixture andDP/HP-B-CD inclusion complexes were shown in Fig. 6. Theintense sharp absorption band in the FT-IR spectrum of DPat 1685.6 cm-1 and 1308.5 cm-1 were assigned to the char-acteristic peak ofC=0 and the C-N stretching vibration [26].The intense broad absorption band at 3416.6 cm-1in the spec-trum of HP-B-CD was attributed to the stretching vibration ofO-H [10]. These characteristic bands showed a simple sumin the spectrum of the physical mixtures, which indicatedthat HP-B-CD and DP just mixed with each other. However, inthe spectrum of DP/HP-B-CD inclusion complexes, the char-acteristic absorption of C=0 at 1685.6 cm-1 disappeared, and Fig. 5-Pharmacokinetic profiles of DH solution after intragastrical administration and DP/HP-B-CD ODF after oral cavityadministration (A); Pharmacokinetic profile of DP after injection (B). Fig.6-The FT-IR spectra of samples: DP (a), HP-B-CD (b), thephysical mixtures (C) and DP/HP-B-CD inclusion complexes(d). the absorption of C-N at 1308.5 cm-1 diminished greatly. Itdemonstrated that some groups of DP were embedded into thecavity of HP-B-CD successfully. Additionally, it showed about2.0 cm-1 wavenumber variations of O-H absorption of HP-B-CD, which indicated the existence of interaction between DPand HP-B-CD. The thermograms of DP, HP-B-CD, physical mixtures andDP/HP-B-CD inclusion complexes were shown in Fig. 7. Theendothermic peak starting from 94.33℃ in the profile of DPwas assigned to the melting point of DP [17]. The peak in theprofile of HP-B-CD indicated that HP-B-CD was amorphous.The thermal peak from 60 ℃ to 110 C was attributed tothe evaporation of water molecules. The physical mixtures ofDP and HP-B-CD showed a simple superposition of the en-dothermic peaks of DP and HP-B-CD, which suggested thatthere was no interaction between DP and HP-B-CD. In thecurve of DP/HP-B-CD inclusion complexes, the endothermicpeak of DP disappeared. It was reported that melting point Fig. 7-The DSC curves of samples: DP (a), HP-B-CD (b), thephysical mixtures (c)and DP/HP-B-CD inclusion complexes(d). of guest molecules shifted to different temperature or disap-peared when they inserted into cavities or crystal lattice of CD[28].Therefore, the results mentioned above indicated the for-mation of DP/HP-B-CD complexes. The XRD profiles of DP, HP-B-CD, the physical mixturesand DP/HP-B-CD inclusion complexes were shown in Fig. 8.Again, DP alone presented the typical XRD pattern of crys-talline state, i.e., sharp characteristic diffraction peaks,whileHP-B-CD existed as amorphous form. The disappearance ofdiffraction peaks of DP in the pattern of DP/HP-B-CD inclu-sion complexes, which were still detectable in the pattern ofphysical mixtures, indicated the complete amorphization ofDP after it inserted into the cavity of HP-B-CD. The DP/HP-B-CD interaction was further explored bymolecular modeling. As shown in Fig. 9, the most prob- Fig. 8-The XRD patterns of samples: DP (a), HP-B-CD (b),the physical mixtures (c) and DP/HP-B-CD inclusioncomplexes (d). able modes of DP/HP-B-CD inclusion complexes were de-termined by the program of Autodock. Hydrogen bondingwas found between the carbonyl, tertiary nitrogen groupsof the guest molecule and the hydroxyl group of hostmolecule, respectively, which was in accordance with theresults of FT-IR. Additionally, the minimum energy DP/HP-B-CD was-6.51kcal/mol. However, the minimum energy ofDP/HCl was -23.46 kcal/mol,which was far less than DP/HP-B-CD inclusion complexes. There is no definite regulation aboutthe minimum energy value in molecular modeling. Generally,lower energy value indicated stronger stability [29]. These re-sults suggested that DP/HCl interaction was much strongerthan DP/HP-B-CD interaction, which verified the results ofphase solubility study. Therefore, the reason for successfultaste-masking was that DP molecule with unpleasant tasteentered into the cavity of HP-B-CD in whole or in part, andthe complexes was relatively stable in mouth due to moder-ate DP/HP-B-CD interaction. After the DP/HP-B-CD ODF was applied to the tongue of rat,a series of changes occurred in DP/HP-B-CD inclusion com-plexes and the dynamic process was closely related to drugabsorption. For the optimized DP/HP-B-CD ODF, the pharma-cokinetic profile was similar to the DH solution according toin vivo study.The reason was explained from the followingtwo aspects.On one hand, the environment had changed dra-matically from mouth to stomach, which led to a variationof DP/HP-B-CD inclusion complexes. As shown in phase sol-ubility study, the binding constant (K) changed from 3658.91M-1 to 4.80 M-1 in the presence of HCl.From the results ofmolecule modeling, DP was easier to interact with HCl thanHP-B-CD. Meanwhile, the film exhibited better dissolution be-havior in 0.1 mol/l HCl solution according to the results of invitro dissolution test (Fig. 2), indicating that most of hydro-gen bonding between DP and HP-B-CD may be broken dueto the acidic environment of stomach, which facilitated the c Fig. 9-Models of the DP/HP-B-CD inclusion complexes andDP/HCl from docking calculation: DP/HP-B-CD inclusioncomplexes (A) and DP/HCl (B). DP absorption. On the other hand, the absorption of cyclodex-trin occurred in the colon where cyclodextrin was metabolizedby colonic bacteria [27].DP/HP-B-CD inclusion complexes dis-solved quickly from film due to the amorphous form of DP af-ter it inserted into the cavity of HP-B-CD. After that,the DP/HP-B-CD inclusion complexes quickly passed through the staticwater layer with the help of HP-B-CD and reached to the sur-face of biofilm. Since cyclodextrins could not pass through thelipid biofilm, there was a dynamic balance between the inclu-sion and the free DP. A small binding constant (K=4.80 M-was beneficial for DP across the biofilm [27]. Therefore, rapiddissolution, and the decreased stability of DP/HP-B-CD inclu- sion complexes in stomach, explained why the DP/HP-B-CDODF was bioequivalent with DH solution while taste-masking. In summary, taste-masking was achieved by moderate in-teraction between DP and HP-B-CD, which made DP/HP-B-CDstable in oral cavity. Moreover, DP/HP-B-CD ODF showed fa-vorable pharmacokinetic behavior in rat, which was mainlyattributed to the decrease of stability of complexes in stom-ach based on DP/HP-B-CD dynamic process. 4. Conclusions A rapid dissolving DP/HP-B-CD ODF was developed, and thetaste was masked successfully. Apart from fulfilling the orig-inal aim of improving the bitter taste and solving the swal-lowing difficulties, the effect of cyclodextrin on taste-maskingwas illustrated at molecular level based on dynamic pro-cess and drug absorption in uivo. It was found that taste-masking was achieved successfully by the moderate DP/HP-β-CD interaction. Additionally, the varied stability of DP/HP-B-CD inclusion complexes from oral cavity to stomach fa-cilitated drug absorption. These conclusions extended ourunderstanding about the application of cyclodextrin com-plexation in the development of ODF and provided guid-ance for the design of ODF, especially for the drugs neededtaste-masking. Conflict of Interest The authors declare that there is no conflicts of interest. Acknowledgments This research did not receive any specific grant from fundingagencies in the public,commercial, or not-for-profit sectors. Supplementary materials Supplementary material associated with this article can befound,in the online version, at doi:10.1016/j.ajps.2018.05.001. REFERENCES ( [3] Di n ge A, N ag arsenker M. Formula t ion a n d e v aluation of f a st d i s sol v ing films f o r delivery o f triclosan to the o r al c avity. AAPS PharmSciTech 2008;9(2 ):34 9- 5 6. ) ( [4B] a la R, Pawar P, Khanna S, Arora S. Orally dis solving strips: a n ew approach to oral drug delivery system. Int J P h arm I nvest 2 013;3( 2):6 7- 7 6. ) ( [5] | K arki S , Kim H , Na SJ, et a l . Review Th in fi l ms as an emergi ng p l a tform for drug del i very . Asian J Pharm S ci 2016;11(5) : 559-74. ) ( [6] D ooley M, L a mb HM. Do ne pezil: a rev i ew of its use in Alzheimer's disease. D ru gs Ag ing 2 000;16(3): 1 99- 2 26. ) ( [7] Hof f mann E M , Breitenbach A, Breitkr e utz J. Advances in orodispersible fil m s for drug d elivery. Expert Op i n Drug D e liv2011;8(3):299-31 6 . ) ( [8] N ilesh M R, Shiva ji DM, R ameshrao KD. Taste m asking methods and agents in p h armaceutical formulations. IRJP 2012;3 (8): 67-70. ) ( [9] | Choi DH, Kim NA, Nam TS, Le e S, Jeong SH. Evaluation of t aste-maskin g effects of pharmaceut ic al sweeteners w ith a nelectronic to ngu e s y stem. Dru g D ev I nd P harm 2013;40:308-17. ) ( [10 ] ] Yang Y, Gao J, Ma X, Huang G. Incl u sion compl e x of t amibarotene with hydroxypropyl-p - cyclodex t rin: preparat i on,characterization, in-vitro a n d in-vivoevaluation. Asian JPh a rm Sci 2 0 17;2:18 7 -92. ) ( [1 1 ] Szejt l i J, Sz e nte L . E l imina t ion of bitter , disgusting tastes of dr u gs and f oods by cyclodextrins. Eur J Phar m Biopharm 2005;61:1 1 5-25. ) ( [12]B r ewster ME, Loftsson T . Cyc l odextr i ns as pharmaceuticalsolubilizes. Adv Drug Del Rev 2007;59:645-66. ) ( [13 | ] Szejtl i J . Medic i nal applications of cyclodextrin. Med Res R e v 1994;14:353- 8 6. ) ( [14 | ] Bekers O, Uijtendaal EV, Beijnen JH, Bult A, Underberg WJM. Cyc lodextrins in the p harmaceutical fel d. D rug D ev Ind P harm 1991;17:1503-49. ) ( [15]] Holm R, O lesen NE, Hartvig RA, et al. Effect o f cyclodextr i n c oncentra t io n on t he oral bio a vailabilit y o f danazol and cinnar i zine in rats. Eur J Pharm Biopharm 2016;101: 9 -14. ) ( [16 ] ] Kaartama R , Turunen E, To l jamo K, et al . The effect o f hy drox ypr op yl- beta-cyc l odextrin and sucrose on the sublingual absorption of midazolam in rabbits. Eur JP h arm Bi o phar m 2012;81:178- 8 3. ) ( [17] G u o W, Quan P, Fang L , Cun D, Yang M . Sustained re l ease DP l oaded P L GA microspheres for injec t ion: Preparation, i n vitro a nd in vivo stu dy . Asian J Pha r m Sci 2015;10:405-14. ) ( [18 ] ] Kumar GP, Ph a ni A R , Prasad RG, et al. P ol y vinylpyrrolidone o ral f i l ms of enrofloxacin: Fil m characteri z ation and drug r elease. Int J Ph a rm 2014;471:146-52. ) ( [19|] Zhao Y , Quan P , Fang L. P reparation of an o r al t hin f i lm containi n g meclizine hydrochloride: I n ui t ro and in viuo evaluation. Int J Pharm 2 015;496:314-22. ) ( [20]] Harada T,Uchid a T , Yoshid a M, et a l . A new method fo r evaluati ng the bitte r ness of medicines in development using a taste sensor and a disint eg ratio n tes t i n g app aratus . Chem Pharm B ull 2010;58(8):1009-14. ) ( [21] | Woertz K, Tissen C,Kl e i n ebudde P, Breitkreutz J. Taste sensing systems (electronic tongues) for pharmaceu t ical app li c ations. I nt J Pharm 2011;41 7 :256-71. ) ( [22 ] ] L enik J , Wesoly M, Ciosek P , Wroblewski W. E valuation o f t aste masking effect o f di cl ofenac usin g sweeteners and c yclodextrin by a potentiometric e lectronic tongue. J Electroanal Chem 2016;780:153- 9 . ) ( [23] Rachid O, Simons F E, Rawasqalaji M , Simons KJ. An 上 上 electronic t on gu e: evaluation o f the masking efficacy of sweetenin g a nd/or flavoring ag en ts on the bitter taste o f epinephrine. AAPS P harmSciTech 2010;11(2):550-7. ) ( [24 H ] iguchi T, Conn o rs KA. Advances in analytical chemistry and instru m e n tation.chapter 4. P has e Solubility Studies;1965. p . 1 17-212. ) ( [25] H ara gu chi T , Yoshida M, Koj im a H,U c hida T. U s efulne s s and l imi t ations o f taste sensors in the evaluation of palatability and t aste-masking i n oral dosag e f orms. Asian J Pharm Sci 2016;4:479-85. ) ( [26 ] L iew K B , Tan YT, Peh KK. Characteriza t ion of oral disintegrating film containing donepezil for Alzheimer disease. AAPS P harmSciTech 2 0 12;13(1 ):13 4-42. ) ( [27] Carrier RL , Miller LA, Ahmed I. The utili t y o f cyc l odextrins fo r enhancing ora l bi o availability . J Control Releas 2007;123:78-99. ) ( [28 ! 1 Sun Y , Du L , Liu Y, et a l. T r ansdermal delivery of th e in- s itu h ydrogels o f c u rcumin and its incl u sion complexes of h y droxy p rop yl- p-cyclo d extrin for m elanoma tr e atment. In t J P harm 2014;469:31 - 9. ) ( [29] S ong W, Quan P, Li S, Z h ao Y, Fa n g L. Probing the rol e of chemical enhancers in f a c ilitating drug release from p atches:mechanist i c insights based o n FT-IR s p ectroscopy, m olecular modeling and therma l an a lysis.J Control R e leas2016;227:13-22. ) 本研究旨在开发一种可促进吞咽过程的可口多奈哌齐(DP)或分散膜(ODF),并从动力学过程和体内药物吸收的角度探讨环糊精对味觉掩藏的影响。将DP与羟丙基-β-环糊精(HP-β-CD)络合,掩盖其苦味,然后采用溶剂铸造法将其加入ODF中。用电子舌法评价其味觉掩蔽效果;同时对DP/HP-β-CD ODF的体内药代动力学行为进行了研究。结果表明,优化后的膜优于盐酸多奈哌齐(DH)膜,且与DH膜具有生物等效性。通过相溶解度研究、傅里叶变换红外光谱仪(FT-IR)、差示扫描量热仪(DSC)、x射线衍射(XRD)和分子模型分析,揭示了分子机理。DP/HP-β-CD的形成是由于DP和HP-β-CD相互作用适度所致。胃内酸性环境降低了DP/HP-β-CD的稳定性,促进了DP的吸收。这些结果拓展了我们对环糊精络合物应用的认识,并对ODF的设计特别是对E心味药物的ODF设计提供了指导。
确定





还剩8页未读,是否继续阅读?
产品配置单
北京盈盛恒泰科技有限责任公司为您提供《药物环糊精中味觉掩蔽检测方案(感官智能分析)》,该方案主要用于化药制剂中其他检测,参考标准--,《药物环糊精中味觉掩蔽检测方案(感官智能分析)》用到的仪器有电子舌、日本INSENT味觉分析系统(电子舌)
推荐专场
感官智能分析系统(电子鼻/电子舌)
相关方案
更多
该厂商其他方案
更多