方案详情
文
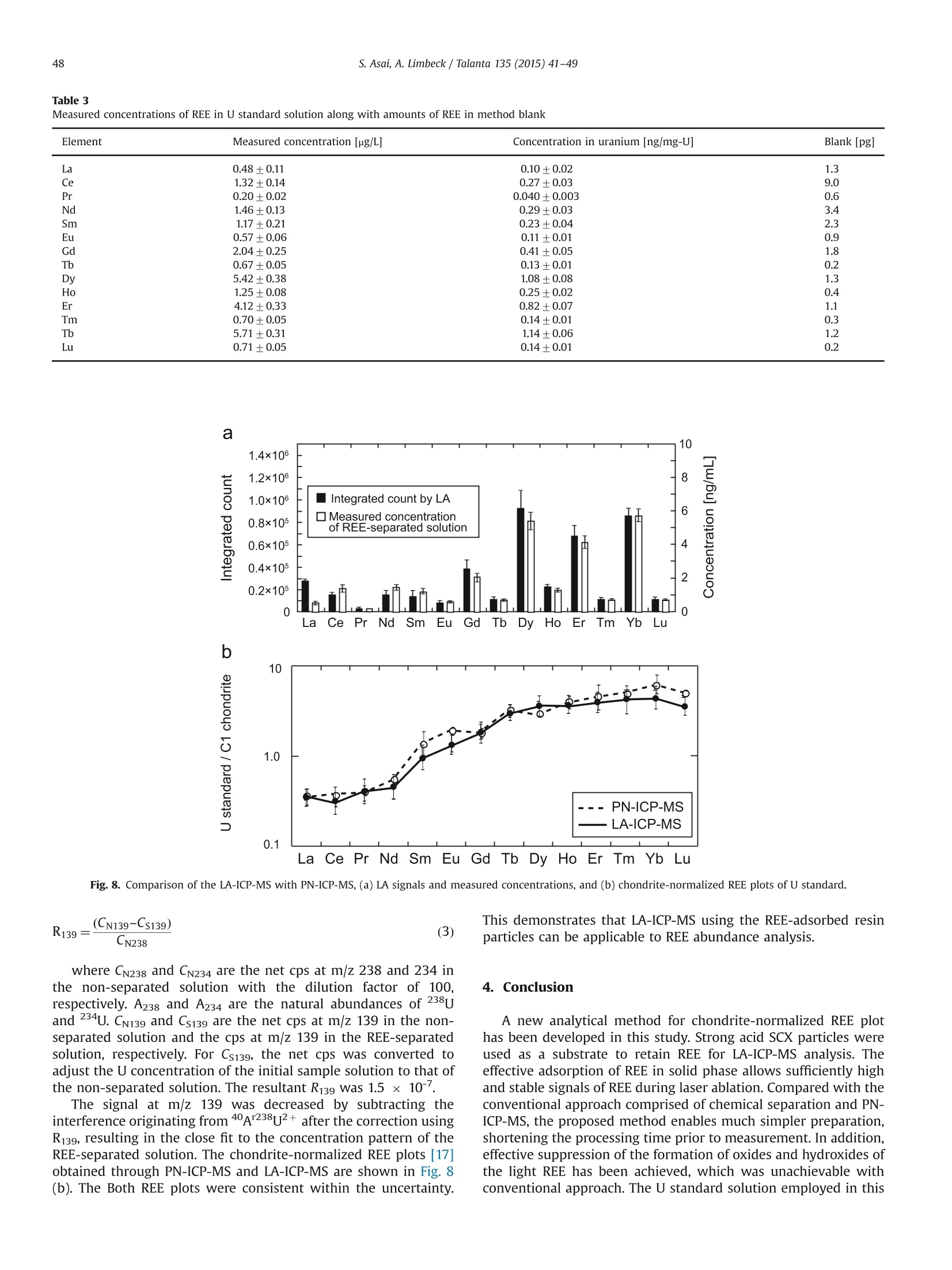
根据气动雾化ICP-MS (PN-ICP-MS)的灵敏度变化趋势,稀土元素的灵敏度随原子序数的增加而增大。稀土元素的信号强度与用于颗粒制备的浸渍溶液中稀土元素的浓度几乎成正比。由稀土元素净信号计算出的*可测浓度约为1ng /g,对应于配制颗粒溶液中的0.1 ng。与传统的PN-ICP-MS相比,LA分析在重稀土测量中,由于无溶剂干扰,从而使光谱干扰的轻质稀土和Ba的氧化物及氢氧根的形成得到了有效的抑制。为评价该方法的适用性,采用LA-ICP-MS测定了将树脂吸附颗粒浸泡在含U溶液(市售U标准溶液)中制备的树脂吸附颗粒。除LA分析外,采用传统的PN-ICP-MS法对同一U标准溶液中的稀土元素进行阳离子交换色谱分离,测定其浓度。稀土元素的浓度从0.04(Pr)到1.08(Dy)μg / gu。U标准样品LA-ICP-MS分析得到的球粒状归一化图与不确定条件下REE分离溶液PN-ICP-MS分析得到的球粒状归一化图吻合较好。
方案详情

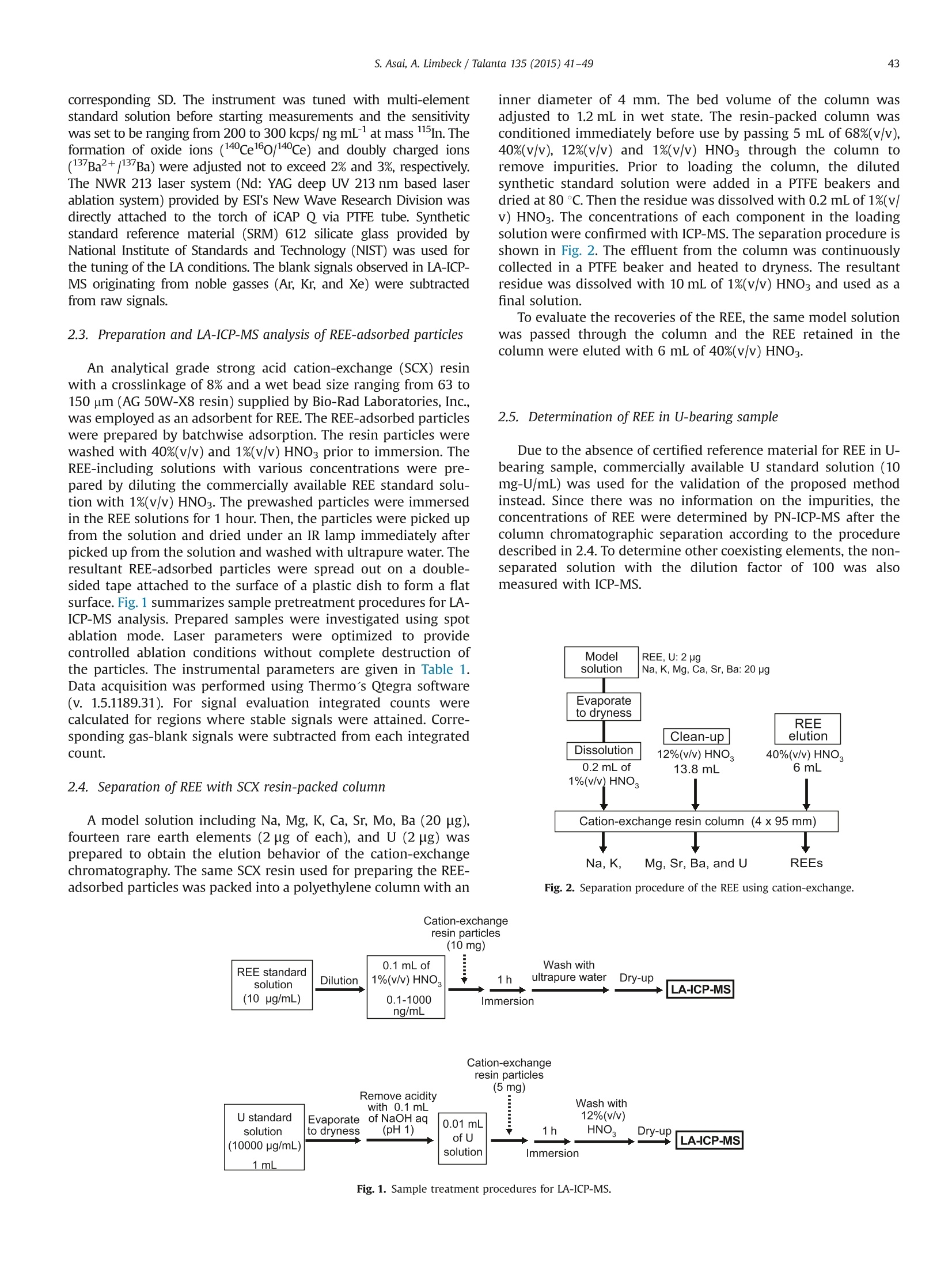
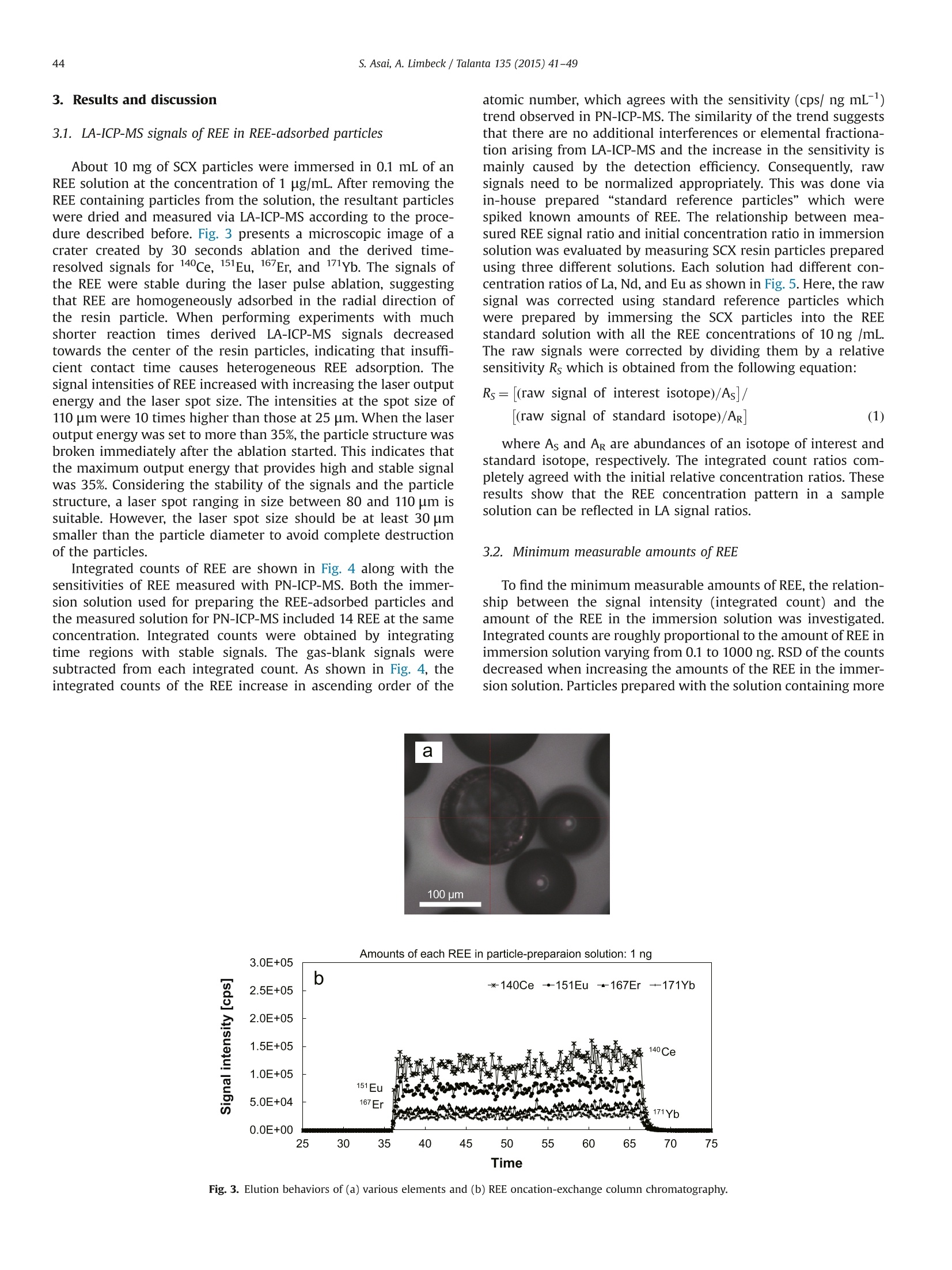
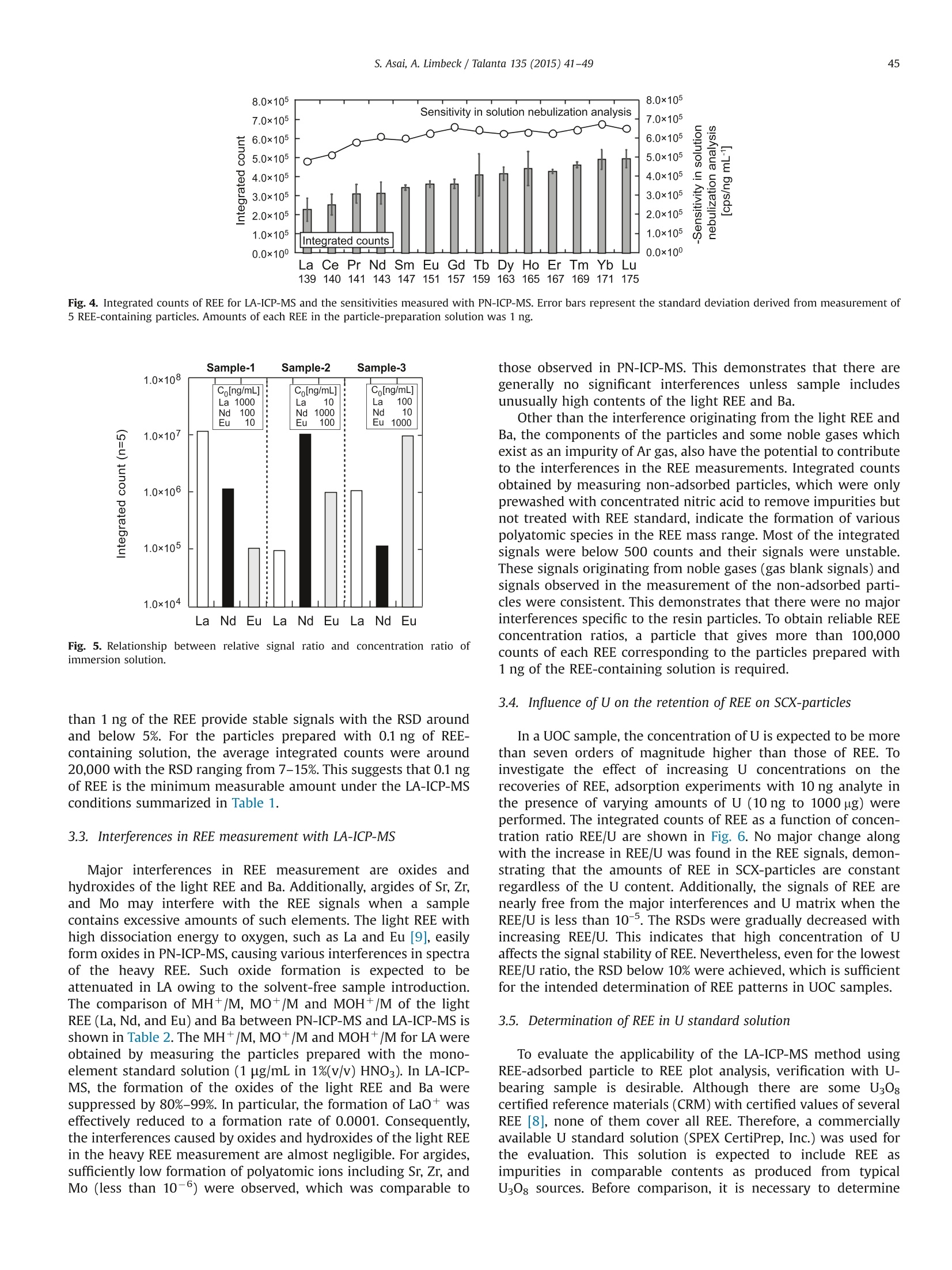
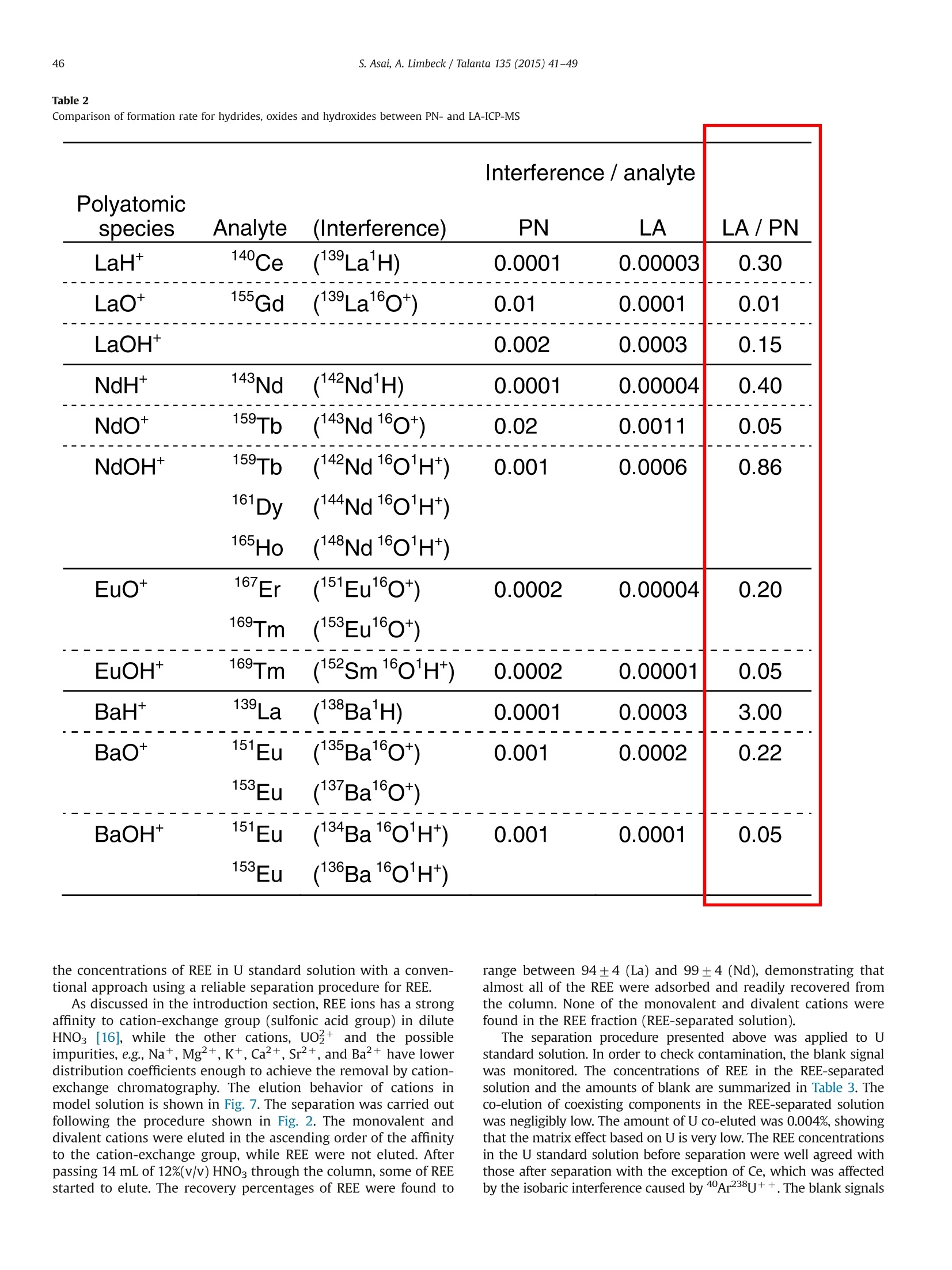
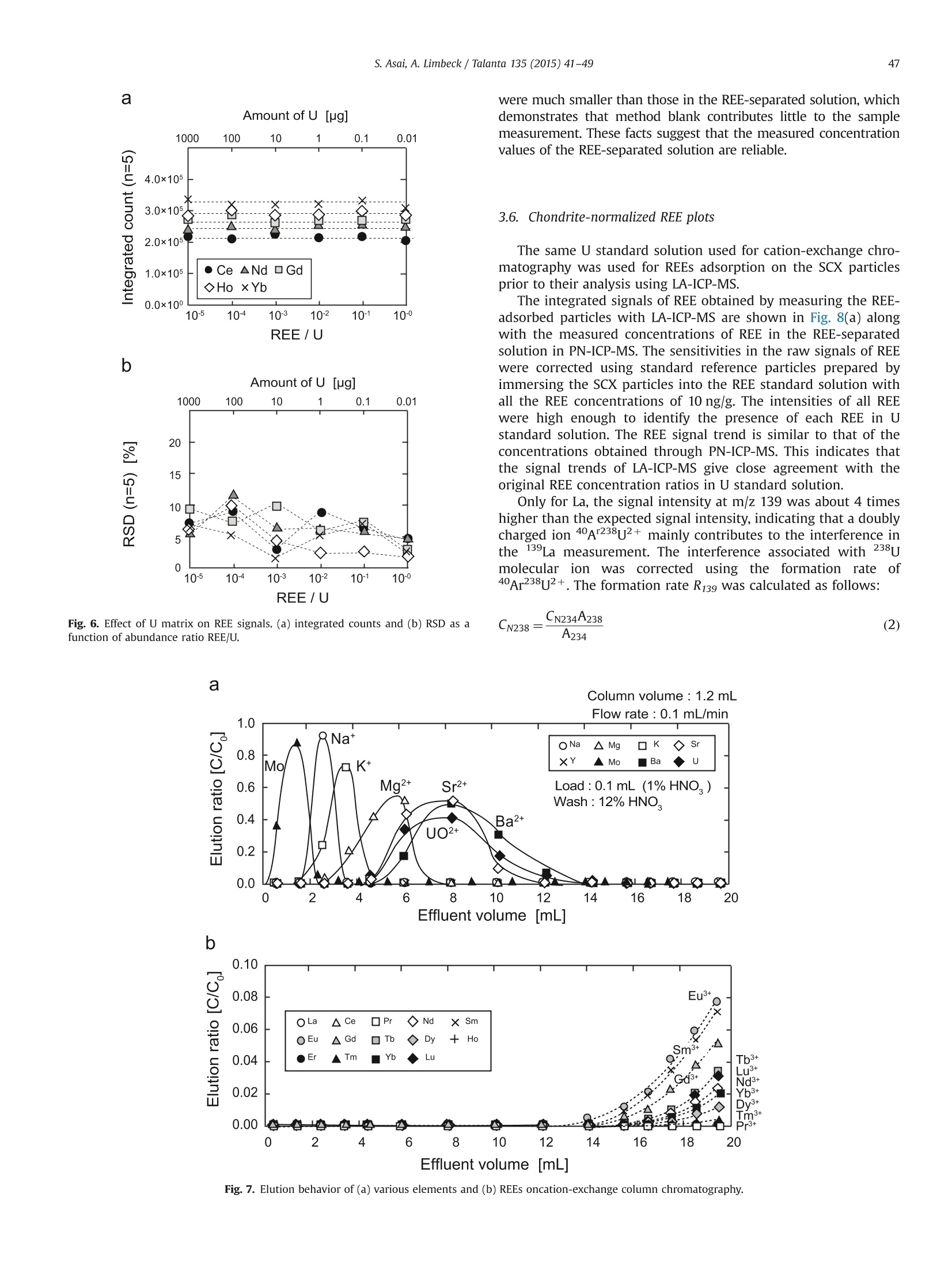
Talanta 135 (2015) 41-49Contents lists available at ScienceDirect 42S. Asai, A. Limbeck / Talanta 135 (2015)41-49 http://dx.doi.org/10.1016/j.talanta.2014.12.009 Talanta ELSEVIER journal homepage: www.elsevier.com/locate/talanta LA-ICP-MS of rare earth elements concentrated in cation-exchangeresin particles for origin attribution of uranium ore concentrate CrossMark Shiho Asai*, Andreas Limbeck” Japan Atomic Energy Agency, Tokai-mura, Naka-gun, Ibaraki 319-1195, Japan" Vienna University of Technology, Getreidemarkt 9/164, A-1060 Vienna, Austria ARTICLEINFO ABSTRACT Article history:Received 29 October 2014Received in revised form8 December 2014 Accepted 9 December 2014Available online 18 December 2014Keywords: Rare earth elementsChondrite-normalized REE plotUranium ore concentrate Cation-exchange resin LA-ICP-MS Nuclear forensics Rare earth elements (REE) concentrated on cation-exchange resin particles were measured with laserablation inductively coupled plasma mass spectrometry (LA-ICP-MS) to obtain chondrite-normalizedREE plots. The sensitivity of REE increased in ascending order of the atomic number, according to thesensitivity trend in pneumatic nebulization ICP-MS (PN-ICP-MS). The signal intensities of REE werenearly proportional to the concentrations of REE in the immersion solution used for particle-preparation.Minimum measurable concentration calculated from the net signals of REE was approximately 1 ng/gcorresponding to 0.1 ng in the particle-preparation solution. In LA analysis, formation of oxide andhydroxide of the light REE and Ba which causes spectral interferences in the heavy REE measurementwas effectively attenuated due to the solvent-free measurement capability, compared to conventionalPN-ICP-MS. To evaluate the applicability of the proposed method,the REE-adsorbed particles preparedby immersing them in a U-bearing solution (commercially available U standard solution) were measuredwith LA-ICP-MS. Aside from the LA analysis, each concentration of REE in the same U standard solutionwas determined with conventional PN-ICP-MS after separating REE by cation-exchange chromatography.The concentrations of REE were ranging from 0.04 (Pr) to 1.08 (Dy) ug/g-U. The chondrite-normalizedplot obtained through LA-ICP-MS analysis of the U standard sample exhibited close agreement with thatobtained through the PN-ICP-MS of the REE-separated solution within the uncertainties. 1. Introduction Nuclear forensic analysis plays an important role in nuclearcounter-terrorism and counter-proliferation. There has been anincreasing concern about smuggling of interdicted nuclear materi-als that could be altered into nuclear weapons. Uranium oreconcentrate (UOC), which is better known as yellow cake, drawsspecial attention owing to its high concentration of U, ease ofdistribution, and fungibility [1]. For specifying the traffickingroute, the identification of the geographic origin of UOC is aneffective approach. Accumulation of forensic signatures on thematerial origin derived from laboratory-based analysis, for exam-ple, U age determination, isotope ratio analysis, morphologicalobservation, and impurity analysis is the key to achieve a credibleattribution of UOC, resulting in the enhancement of nuclearsecurity measures. Impurities in UOCs, such as, Fe [2], Sr [3], Pb[3], and rare earthelements (REE) [4-6], which exhibit inherent characteristics ofUOC have been determined for origin attribution of UOCs. ( *Corresponding author. Tel. : +81 29282 6367. ) Chondrite-normalized REE plots obtained through the impurityREE abundances can be one of the helpful predictive indicators forthe source origin of the UOC. Owing to their similar chemicalproperties based on the stable oxidation state of +3 with theexceptions of Ce and Eu, REE behave as a coherent group oversome geological or industrial processes. Inductively coupled plasma mass spectrometry (ICP-MS) hasbeen one of the leading techniques for acquiring chondrite-normalized REE plots which require simultaneous detection of14 elements or more at trace or ultratrace levels. However, thespectral interference caused by isobars and isobaric molecules is amajor problem in ICP-MS analysis [4,7]. The interfering species inREE measurement are roughly classified into three types: (1)plasma-based polyatomic species associated with Sr, Zr, and Mo,(2) oxides and hydroxides of the light REE, and (3) hydride, oxideand hydroxide of Ba. The plasma-based polyatomic species, such as 95Mo40Ar16o+,88Sr40Ar160+ 90zr40Ar1601H+,88Sr40Ar1601H+,and 100Mo40Ar+,may interfere with 151Eu, 144Nd, 147Sm, 145Nd, and 140Ce. However,REE are less subjected to such plasma-based polyatomic speciesdue to generally low abundances of Sr, Zr, and Mo [8] and theinstability of the formed species. In the case of the sample whichcontains unusually high amounts of Sr, Zr, and Mo (six or more orders of magnitude higher than REE), the removal of them or thesuppression of the formation of the plasma-based polyatomicspecies is necessary. With regard to the second group, the light REE may interfere onthe heavy REE, forming some oxides, for example, 139La10 and143Nd160, which have the nominal mass of 155Gd and 159Tb,respectively. Light REE oxides such as those of Ce, La, and Pr [9]have high dissociation energy and may cause interferences in themeasurement of the heavier REE. The interferences associatedwith the light REE are more problematic than those of the plasma-based polyatomic species. Chemical separation of the light REEfrom the heavy REE with sufficiently high recoveries for all REE isdifficult because of the similarity of their chemical properties. The third group of polyatomic ions, hydrides, oxides andhydroxides of Ba are known to cause interferences in the mea-surements of Eu and Gd. As Ba is likely accompanied by the otheralkaline earth metals, the determination of REE in samples withhigh contents of alkaline earth metals can be hampered from theinterferences caused by Ba. To eliminate the above described interferences, coexistingelements are chemically separated by solid phase extraction incolumn packed with commercial resins such as TRU[4-6],UTEVA [2], Ln@ [10] or TODGA [11]. These resins lead to theeffective removal of U along with the coexisting elements in UOCsand sufficiently low limits of detection (LoD)in the pg per g range.The removal of the interfering elements is the key to realize areliable determination. However, the separation procedure is time-consuming. Laser ablation (LA) ICP-MS is a promising technique with abenefit of simple sample preparation. The LA system enables solidsample introduction to ICP-MS by ablating and evaporating frag-ments of the solid sample surface, generating fine particles to bedirectly transported to the ICP torch. The LA technique providesspatially resolved detection of trace elements with LoDs in the ngper g range or below. Various substrates including organic materi-als [12,13] have been applied to LA-ICP-MS. In LA, quantitative analysis by LA-ICP-MS is still problematicdue to the lack of standards. The preferential vaporization ofvolatile elements during ablation causes time-dependent sensitiv-ity change as the depth of the crater increases [14,15]. The degree Table 1Operating conditions of ICP-MS (iCAP Q) for PN-and LA- Common plasma settings RF power 1550 W Plasma gas flow rate 14.0 L/min Auxiliary gas flow rate 0.80 L/min Solution nebulization conditions Nebulizer gas flow rate 0.80 L/min Dwell time 0.3 s No. replicate 5 Laser ablation conditions Laser ablation device NWR 213 Wave length 213 nm Carrier He gas flow rate 0.75 mL/min Nebulizer gas flow rate 0.80 L/min Laser energy fluence 1.2 J/cm² Output energy 35% Pulse repetition rate 10 Hz Ablation pattern Spot scan Spot diameter 100 pm Dwell time 0.01 s Measured isotopes 23Na, 24Mg, 88sr, 91Zr, 95Mo, 137Ba, 139La, 140Ce, 41Pr, 143Nd, 147Sm, 151Eu,157Gd,159Tb, 163Dy, 165Ho,167Er, 169Tm, 171Yb, 175Lu, 234u of such elemental fractionation is, in general, affected by samplematrix. Consequently, complete matrix-matching between a sam-ple and a standard is essential for the determination of theconcentrations, which makes the accurate and precise determina-tion more difficult than that with solution nebulization ICP-MS(PN-ICP-MS). As for the analysis of chondrite-normalized REE plotsof UOC samples, since only the relative relationships in theirabundances are required, raw signal corrections are expected tobe much simpler than that for quantitative analysis. Another practical advantage in LA-ICP-MS is that mass spectraof heavy REE are lower susceptible to the interferences caused bythe oxides and hydroxides of the light REE. Formation of suchoxide ions during measurement is estimated to be much less thanthat in PN-ICP-MS owing to the capability of solvent-free sampleintroduction. LA-ICP-MS is advantageous over conventional PN-ICP-MS in alleviating the interferences and minimizing the samplepreparation procedure. However, the concentrations of REE in UOCmay be lower than the LoD for quadrupole ICP-MS. This indicatesthat direct measurement of REE in UOC is difficult without anvpreconcentration procedure. The objective of this study is the development of an LA-ICP-MSmethod for accurate analysis of REE in U-bearing solution. Theproposed approach is based on the preliminary pre-concentrationof REE on commercially available strong acid cation-exchange(SCX) resin particles. REE show strong affinity to the cation-exchange group (sulfonic acid group) in dilute acid media. Thedistribution coefficient of the SCX resin for REE in 0.1 M HNO3 ismore than 10000, while those of U and Ba, which are majorinterfering components in REE detection, are about 5000 and 660,respectively [16]. Therefore, roughly selective adsorption of REE onSCX resin particles is expected, enabling the separation frominterfering sample constituents. Finally, the REE containing parti-cles were analyzed with LA-ICPMS using optimized ablationconditions. U standard solution including unknown amounts ofREE was used to check the accuracy, comparing the resultantchondrite-normalized REE plots with that obtained through con-ventional PN-ICP-MS of REE-separated solution. 2. Experimental 2.1. Reagents Multi-element standard solutions including fifteen rare earthelements (Y,La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb andLu, 10 mg/L in 5%(v/v) HNO3, Agilent technologies), alkaline metal-mix standard solution (250 mg-Li/L, 1,000 mg-Na/L, and 10,000mg-K/L in dilute HNO3, Merck & Co., Inc.,), alkaline earth metal-mix standard solution (Mg, Ca, Sr, and Ba, 1000 mg/L in diluteHNO3, Merck & Co., Inc.,), U standard solution (10,000 mg/L in 5%(v/v) HNO3, SPEX CertiPrep, Inc.), were used to prepare the modelsample solution and standard solutions for ICP-MS. Nitric acidused for dilution was of analytical grade and used without anyfurther purification. 2.2. Instrumentation All the measurements were conducted with quadrupole ICP-MS(iCAP Qc, Thermo Fisher Scientific Inc.). Sample introduction for PN-ICP-MS measurements was performed employing a peltier-cooledspray chamber equipped with a concentric quartz glass nebulizer.The final solutions for PN-ICP-MS were prepared by diluting with 1%(v/v) HNO3. The operation conditions of ICP-MS are summarized inTable 1. The uncertainty of each measurement was set to be twice thestandard deviation (SD) of five repeated measurements. The relativestandard deviation (RSD) was calculated using averaged signals and corresponding SD. The instrument was tuned with multi-elementstandard solution before starting measurements and the sensitivitywas set to be ranging from 200 to 300 kcps/ ng mL-at mass 115In. Theformation of oxide ions (140Ceo/140Ce) and doubly charged ions(13Ba2+(13Ba) were adjusted not to exceed 2% and 3%, respectively.The NWR 213 laser system (Nd: YAG deep UV 213 nm based laserablation system) provided by ESI’s New Wave Research Division wasdirectly attached to the torch of iCAP Q via PTFE tube. Syntheticstandard reference material (SRM) 612 silicate glass provided byNational Institute of Standards and Technology (NIST) was used forthe tuning of the LA conditions. The blank signals observed in LA-ICP-MS originating from noble gasses (Ar, Kr, and Xe) were subtractedfrom raw signals. 2.3. Preparation and LA-ICP-MS analysis of REE-adsorbed particles An analytical grade strong acid cation-exchange (SCX) resinwith a crosslinkage of 8% and a wet bead size ranging from 63 to150 pm (AG 50W-X8 resin) supplied by Bio-Rad Laboratories, Inc.,was employed as an adsorbent for REE. The REE-adsorbed particleswere prepared by batchwise adsorption. The resin particles werewashed with 40%(v/v) and 1%(v/v) HNO3 prior to immersion. TheREE-including solutions with various concentrations were pre-pared by diluting the commercially available REE standard solu-tion with 1%(v/v) HNO3. The prewashed particles were immersedin the REE solutions for 1 hour. Then, the particles were picked upfrom the solution and dried under an IR lamp immediately afterpicked up from the solution and washed with ultrapure water. Theresultant REE-adsorbed particles were spread out on a double-sided tape attached to the surface of a plastic dish to form a flatsurface. Fig. 1 summarizes sample pretreatment procedures for LA-ICP-MS analysis. Prepared samples were investigated using spotablation mode. Laser parameters were optimized to providecontrolled ablation conditions without complete destruction ofthe particles. The instrumental parameters are given in Table 1.Data acquisition was performed using Thermo's Qtegra software(v. 1.5.1189.31). For signal evaluation integrated counts werecalculated for regions where stable signals were attained. Corre-sponding gas-blank signals were subtracted from each integratedcount. 2.4. Separation ofREE with SCX resin-packed column A model solution including Na, Mg, K, Ca, Sr, Mo, Ba (20 pg),fourteen rare earth elements (2 ug of each), and U (2 ug) wasprepared to obtain the elution behavior of the cation-exchangechromatography. The same SCX resin used for preparing the REE-adsorbed particles was packed into a polyethylene column with an inner diameter of 4 mm. The bed volume of the column wasadjusted to 1.2 mL in wet state. The resin-packed column wasconditioned immediately before use by passing 5 mL of 68%(v/v),40%(v/v), 12%(v/v) and 1%(v/v) HNO3 through the column toremove impurities. Prior to loading the column, the dilutedsynthetic standard solution were added in a PTFE beakers anddried at 80C. Then the residue was dissolved with 0.2 mL of 1%(v/v) HNO3. The concentrations of each component in the loadingsolution were confirmed with ICP-MS. The separation procedure isshown in Fig. 2. The effluent from the column was continuouslycollected in a PTFE beaker and heated to dryness. The resultantresidue was dissolved with 10 mL of 1%(v/v) HNO3 and used as afinal solution. To evaluate the recoveries of the REE, the same model solutionwas passed through the column and the REE retained in thecolumn were eluted with 6 mL of 40%(v/v) HNO3. 2.5. Determination of REE in U-bearing sample Due to the absence of certified reference material for REE in U-bearing sample, commercially available U standard solution (10mg-U/mL) was used for the validation of the proposed methodinstead. Since there was no information on the impurities, theconcentrations of REE were determined by PN-ICP-MS after thecolumn chromatographic separation according to the proceduredescribed in 2.4. To determine other coexisting elements, the non-separated solution with the dilution factor of 100 was alsomeasured with ICP-MS. Fig.2. Separation procedure of the REE using cation-exchange. Fig. 1. Sample treatment procedures for LA-ICP-MS. 3. Results and discussion 3.1. LA-ICP-MS signals of REE in REE-adsorbed particles Integrated counts of REE are shown in Fig. 4 along with thesensitivities of REE measured with PN-ICP-MS. Both the immer-sion solution used for preparing the REE-adsorbed particles andthe measured solution for PN-ICP-MS included 14 REE at the sameconcentration. Integrated counts were obtained by integratingtime regions with stable signals. The gas-blank signals weresubtracted from each integrated count. As shown in Fig. 4, theintegrated counts of the REE increase in ascending order of the atomic number, which agrees with the sensitivity (cps/ ng mL-)trend observed in PN-ICP-MS. The similarity of the trend suggeststhat there are no additional interferences or elemental fractiona-tion arising from LA-ICP-MS and the increase in the sensitivity ismainly caused by the detection efficiency. Consequently, rawsignals need to be normalized appropriately. This was done viain-house prepared “standard reference particles" which werespiked known amounts of REE. The relationship between mea-sured REE signal ratio and initial concentration ratio in immersionsolution was evaluated by measuring SCX resin particles preparedusing three different solutions. Each solution had different con-centration ratios of La, Nd, and Eu as shown in Fig. 5. Here, the rawsignal was corrected using standard reference particles whichwere prepared by immersing the SCX particles into the REEstandard solution with all the REE concentrations of 10 ng /mL.The raw signals were corrected by dividing them by a relativesensitivity Rs which is obtained from the following equation: where As and Ar are abundances of an isotope of interest andstandard isotope, respectively. The integrated count ratios com-pletely agreed with the initial relative concentration ratios. Theseresults show that the REE concentration pattern in a samplesolution can be reflected in LA signal ratios. 3.2. Minimum measurable amounts of REE To find the minimum measurable amounts of REE, the relation-ship between the signal intensity (integrated count) and theamount of the REE in the immersion solution was investigated.Integrated counts are roughly proportional to the amount of REE inimmersion solution varying from 0.1 to 1000 ng. RSD of the countsdecreased when increasing the amounts of the REE in the immer-sion solution. Particles prepared with the solution containing more Amounts of each REE in particle-preparaion solution: 1 ng Fig. 3. Elution behaviors of (a) various elements and (b) REE oncation-exchange column chromatography. Fig. 4. Integrated counts of REE for LA-ICP-MS and the sensitivities measured with PN-ICP-MS. Error bars represent the standard deviation derived from measurement of5 REE-containing particles. Amounts of each REE in the particle-preparation solution was 1 ng. Fig. 5. Relationship between relative signal ratio and concentration ratio ofimmersion solution. than 1 ng of the REE provide stable signals with the RSD aroundand below 5%. For the particles prepared with 0.1 ng of REE-containing solution, the average integrated counts were around20,000 with the RSD ranging from 7-15%. This suggests that 0.1 ngof REE is the minimum measurable amount under the LA-ICP-MSconditions summarized in Table 1. 3.3. Interferences in REE measurement with LA-ICP-MS Major interferences in REE measurement are oxides andhydroxides of the light REE and Ba. Additionally, argides of Sr, Zr,and Mo may interfere with the REE signals when a samplecontains excessive amounts of such elements. The light REE withhigh dissociation energy to oxygen, such as La and Eu [9],easilyform oxides in PN-ICP-MS, causing various interferences in spectraof the heavy REE. Such oxide formation is expected to beattenuated in LA owing to the solvent-free sample introduction.The comparison of MH+/M, MO+/M and MOH+/M of the lightREE (La, Nd, and Eu) and Ba between PN-ICP-MS and LA-ICP-MS isshown in Table 2. The MH+/M,MO+/M and MOH+/M for LA wereobtained by measuring the particles prepared with the mono-element standard solution (1 pg/mL in 1%(v/v) HNO3). In LA-ICP-MS, the formation of the oxides of the light REE and Ba weresuppressed by 80%-99%. In particular, the formation of LaO+ waseffectively reduced to a formation rate of 0.0001.Consequently,the interferences caused by oxides and hydroxides of the light REEin the heavy REE measurement are almost negligible. For argides,sufficiently low formation of polyatomic ions including Sr, Zr, andMo (less than 10-6) were observed, which was comparable to those observed in PN-ICP-MS. This demonstrates that there aregenerally no significant interferences unless sample includesunusually high contents of the light REE and Ba. Other than the interference originating from the light REE andBa, the components of the particles and some noble gases whichexist as an impurity of Ar gas, also have the potential to contributeto the interferences in the REE measurements. Integrated countsobtained by measuring non-adsorbed particles, which were onlyprewashed with concentrated nitric acid to remove impurities butnot treated with REE standard, indicate the formation of variouspolyatomic species in the REE mass range. Most of the integratedsignals were below 500 counts and their signals were unstableThese signals originating from noble gases (gas blank signals) andsignals observed in the measurement of the non-adsorbed parti-cles were consistent. This demonstrates that there were no majorinterferences specific to the resin particles.To obtain reliable REEconcentration ratios,a particle that gives more than 100,000counts of each REE corresponding to the particles prepared with1 ng of the REE-containing solution is required. 3.4. Influence of U on the retention of REE on SCX-particles In a UOC sample,the concentration of U is expected to be morethan seven orders of magnitude higher than those of REE. Toinvestigate the effect of increasing U concentrations on therecoveries of REE, adsorption experiments with 10 ng analyte inthe presence of varying amounts of U (10ng to 1000 ug) wereperformed. The integrated counts of REE as a function of concen-tration ratio REE/U are shown in Fig. 6. No major change alongwith the increase in REE/U was found in the REE signals, demon-strating that the amounts of REE in SCX-particles are constantregardless of the U content. Additionally, the signals of REE arenearly free from the major interferences and U matrix when theREE/U is less than 10-5. The RSDs were gradually decreased withincreasing REE/U. This indicates that high concentration of Uaffects the signal stability of REE. Nevertheless, even for the lowestREE/U ratio, the RSD below 10% were achieved, which is sufficientfor the intended determination ofREE patterns in UOC samples. 3.5. Determination of REE in U standard solution To evaluate the applicability of the LA-ICP-MS method usingREE-adsorbed particle to REE plot analysis, verification with U-bearing sample is desirable. Although there are some U30gcertified reference materials (CRM) with certified values of severalREE [8], none of them cover all REE. Therefore, a commerciallyavailable U standard solution (SPEX CertiPrep, Inc.) was used forthe evaluation. This solution is expected to include REE asimpurities in comparable contents as produced from typicalU30: sources. Before comparison, it is necessary to determine Comparison of formation rate for hydrides, oxides and hydroxides between PN- and LA-ICP-MS Interference / analyte Polyatomic species Analyte (Interference) PN LA LA/PN LaH+ 140/Ce (139La'H) 0.0001 0.00003 0.30 LaO+ 155/Gd (139La160*) 0.01 0.0001 0.01 LaOH* 0.002 0.0003 0.15 NdH+ 143Nd (142Nd'H) 0.0001 0.00004 0.40 NdO* 159-Tb (143Nd160*) 0.02 0.0011 0.05 NdOH+ 159-Tb (142Nd 11601H*) 0.001 0.0006 0.86 61Dy (144Nd11601H*) 165Ho 148Nd11601H*) EuO+ 167Er (151Eu16O*) 0.0002 0.00004 0.20 169-lm 153Eu160*) EuOH+ 169-Tm 152Sm1601H*) 0.0002 0.00001 0.05 BaH+ 139iLa.a /1388rBa'H) 0.0001 0.0003 3.00 BaO+ 151rEu 135Ba160*) 0.001 0.0002 0.22 153rEu (137Ba160*) BaOH* 151rEu 134Ba1601H*) 0.001 0.0001 0.05 153rEu 136Ba11601H*) the concentrations of REE in U standard solution with a conven-tional approach using a reliable separation procedure for REE. As discussed in the introduction section, REE ions has a strongaffinity to cation-exchange group (sulfonic acid group) in diluteHNO3 [16], while the other cations, UO+ and the possibleimpurities, e.g.,Na+,Mg2+, K+, Ca2+,Sr+,and Ba2+ have lowerdistribution coefficients enough to achieve the removal by cation-exchange chromatography. The elution behavior of cations inmodel solution is shown in Fig. 7. The separation was carried outfollowing the procedure shown in Fig. 2. The monovalent anddivalent cations were eluted in the ascending order of the affinityto the cation-exchange group, while REE were not eluted. Afterpassing 14 mL of 12%(v/v) HNO3 through the column, some of REEstarted to elute. The recovery percentages of REE were found to range between 94±4 (La) and 99±4 (Nd), demonstrating thatalmost all of the REE were adsorbed and readily recovered fromthe column. None of the monovalent and divalent cations werefound in the REE fraction (REE-separated solution). The separation procedure presented above was applied to Ustandard solution. In order to check contamination, the blank signalwas monitored. The concentrations of REE in the REE-separatedsolution and the amounts of blank are summarized in Table 3. Theco-elution of coexisting components in the REE-separated solutionwas negligibly low. The amount of U co-eluted was 0.004%, showingthat the matrix effect based on U is very low. The REE concentrationsin the U standard solution before separation were well agreed withthose after separation with the exception of Ce, which was affectedby the isobaric interference caused by 40Ar238U++. The blank signals were much smaller than those in the REE-separated solution, whichdemonstrates that method blank contributes little to the samplemeasurement. These facts suggest that the measured concentrationvalues of the REE-separated solution are reliable. 3.6. Chondrite-normalized REE plots The same U standard solution used for cation-exchange chro-matography was used for REEs adsorption on the SCX particlesprior to their analysis using LA-ICP-MS. The integrated signals of REE obtained by measuring the REE-adsorbed particles with LA-ICP-MS are shown in Fig. 8(a) alongwith the measured concentrations of REE in the REE-separatedsolution in PN-ICP-MS. The sensitivities in the raw signals of REEwere corrected using standard reference particles prepared byimmersing the SCX particles into the REE standard solution withall the REE concentrations of 10 ng/g. The intensities of all REEwere high enough to identify the presence of each REE in Ustandard solution. The REE signal trend is similar to that of theconcentrations obtained through PN-ICP-MS.This indicates thatthe signal trends of LA-ICP-MS give close agreement with theoriginal REE concentration ratios in U standard solution. Only for La, the signal intensity at m/z 139 was about 4 timeshigher than the expected signal intensity, indicating that a doublycharged ion 40Ar238u2+ mainly contributes to the interference inthe 139La measurement. The interference associated with 238umolecular ionnwascorrected using the formation rate of40Ar238u2+. The formation rate R139 was calculated as follows: Effluent volume [mL] Fig. 7. Elution behavior of (a) various elements and (b) REEs oncation-exchange column chromatography. Element Measured concentration [ug/L] Concentration in uranium [ng/mg-U] Blank [pg] La 0.48+0.11 0.10+0.02 1.3 Ce 1.32+0.14 0.27+0.03 9.0 Pr 0.20+0.02 0.040+0.003 0.6 Nd 1.46±0.13 0.29±0.03 3.4 Sm 1.17±0.21 0.23±0.04 2.3 Eu 0.57±0.06 0.11±0.01 0.9 Gd 2.04±0.25 0.41±0.05 1.8 Tb 0.67±0.05 0.13±0.01 0.2 Dy 5.42+0.38 1.08+0.08 1.3 Ho 1.25±0.08 0.25+0.02 0.4 Er 4.12±0.33 0.82±0.07 1.1 Tm 0.70+0.05 0.14+0.01 0.3 Tb 5.71±0.31 1.14±0.06 1.2 Lu 0.71±0.05 0.14±0.01 0.2 Fig.8. Comparison of the LA-ICP-MS with PN-ICP-MS,(a) LA signals and measured concentrations, and(b) chondrite-normalized REE plots of U standard. where CN238 and CN234 are the net cps at m/z 238 and 234 inthe non-separated solution with the dilution factor of 100,respectively. A238 and A234 are the natural abundances of 238uand 234U. CN139 and Cs139 are the net cps at m/z 139 in the non-separated solution and the cps at m/z 139 in the REE-separatedsolution, respectively. For Cs139, the net cps was converted toadjust the U concentration of the initial sample solution to that ofthe non-separated solution. The resultant R139 was 1.5 x 10'. This demonstrates that LA-ICP-MS using the REE-adsorbed resinparticles can be applicable to REE abundance analysis. 4. Conclusion A new analytical method for chondrite-normalized REE plothas been developed in this study. Strong acid SCX particles wereused as a substrate to retain REE for LA-ICP-MS analysis. Theeffective adsorption of REE in solid phase allows sufficiently highand stable signals of REE during laser ablation. Compared with theconventional approach comprised of chemical separation and PN-ICP-MS, the proposed method enables much simpler preparation,shortening the processing time prior to measurement. In addition,effective suppression of the formation of oxides and hydroxides ofthe light REE has been achieved, which was unachievable withconventional approach. The U standard solution employed in this study for checking the accuracy contains Sr, Zr, Mo, and Ba at lowconcentrations, resulting in the interference-free measurement ofREE with LA-ICP-MS. Even if a sample contains a significant levelof such elements, they may be removed by an additional washingstep of the resin particles with appropriate solution prior to LA-ICP-MS analysis. The most important finding in this study is thatthe signal trend of REE in LA analysis is in consistency to theamount ratios of REE adsorbed in the resin particles and the initialconcentration ratios in the immersion solution. This demonstratesthat the REE signals are practically free from elemental fractiona-tions and major interferences caused by resin matrix. Acknowledgments The authors are grateful to Dr. Sergei F. Boulyga of SafeguardsAnalytical Services, International Atomic Energy Agency for help-P-ful comments on this work. Furthermore thanks should beaddressed to Vienna University of Technology and Japan AtomicEnergy Agency for providing a collaborative work environment.The authors greatly appreciate the contribution of Mr. WinfriedNischkauer for reviewing and proof reading. References [4] Z. Varga, R. Katona,Z. Stefanka, M. Wallenius, K. Mayer, A. Nicholl, Talanta 80(2010)1744-1749. Z. Varga, M. Wallenius, K. Mayer, Radiochim. Acta 98 (2010)771-778. ] J Krajko, Z. Varga, E. Yalcintas, M. Wallenius, K. Mayer, Talanta 129 (2014)499-504. [7] J. Baker, T. Waight, D. Ulfbeck, Geochim. Cosmochim. Acta 66 (2002)3635-3646. 8D. Roudil, C. Rigaux, C. Rivier, J.C. Hubinois, L. Aufore, Proc. Chem 7 (2012)709-715. [9] S.Aries, M. Vallandon, M. Polve, Correction of interferences caused by oxideand hydroxide analyte species in ICP-MS: development and limits of a newmethod applied to transition metals, In: Proceedings of the GoldschmidtConference, Toulouse, France, 30 August-3 September 1998, pp. 1711-1712. [10]C.H. Gonzalezm, A.J.Q. Cavezas, M.F. Diaz, Talanta 68 (2005) 47-53 (2009) 1188-1197.). 111A. Pourmand,N. Dauphas, T.J. Ireland, Chem. Geol 291 (2012) 28-54.[12].] Y. Gao, N. Lehto, Talanta 92 (2012)78-83. [13]NV.orapalawut,M.M. Labrador, P. Pohl, M.Caetano, J. Chirinos,C. Arnaudguilhem, B. Bouyssiere, J. Shiowatana, R. Lobinski, Talanta 97(2012)574-578. [14]]M. Gaboardi, M. Humayun,J. Anal. At. Spectrom. 24 (2009) 1188-1197. [15] S. Johnstone,J. Hourigan,C. Gallagher, Geochim. Cosmochim. Acta 109 (2014)143-161. [16]F.W.E. Strelow, R. Rethemeyer, C.J.C. Bothma, Anal. Chem. 37 (1965) 106-111. [17]]1 H. Palme, Chemical abundances in meteorites, in: G. Klare, (Ed.), Reviews inModern Astronomy, Springer, Berlin, 1988, pp. 28-51. 根据气动雾化ICP-MS (PN-ICP-MS)的灵敏度变化趋势,稀土元素的灵敏度随原子序数的增加而增大。稀土元素的信号强度与用于颗粒制备的浸渍溶液中稀土元素的浓度几乎成正比。由稀土元素净信号计算出的最小可测浓度约为1ng /g,对应于配制颗粒溶液中的0.1 ng。与传统的PN-ICP-MS相比,LA分析在重稀土测量中,由于无溶剂干扰,从而使光谱干扰的轻质稀土和Ba的氧化物及氢氧根的形成得到了有效的抑制。为评价该方法的适用性,采用LA-ICP-MS测定了将树脂吸附颗粒浸泡在含U溶液(市售U标准溶液)中制备的树脂吸附颗粒。除LA分析外,采用传统的PN-ICP-MS法对同一U标准溶液中的稀土元素进行阳离子交换色谱分离,测定其浓度。稀土元素的浓度从0.04(Pr)到1.08(Dy)μg / gu。U标准样品LA-ICP-MS分析得到的球粒状归一化图与不确定条件下REE分离溶液PN-ICP-MS分析得到的球粒状归一化图吻合较好。
确定



还剩7页未读,是否继续阅读?
上海凯来仪器有限公司为您提供《稀土元素在阳离子交换树脂颗粒中铀检测方案(激光剥蚀进样)》,该方案主要用于司法鉴定中铀检测,参考标准--,《稀土元素在阳离子交换树脂颗粒中铀检测方案(激光剥蚀进样)》用到的仪器有ESL213 灵活的激光剥蚀系统
推荐专场
相关方案
更多















