
方案详情
文
瑞士万通非常荣幸的发布温度滴定在食品行业的两个最新应用:一个是人造黄油生产中的钠离子监测,第二个是豆奶中钠离子的检测。两个应用均可在www.metrohm.com.cn下载。
应用报告:
AN-H-124 人造黄油生产中钠离子监测
AN-H-125 豆奶中钠离子检测
温度滴定较传统电位滴定速度快(可3min完成),无需电极维护,一支电极可用于所有滴定(酸碱、氧化还原、EDTA、沉淀、非水滴定)。更重要的是电极无需校正,即使在苛刻条件下也能保持极高灵敏度。
方案详情

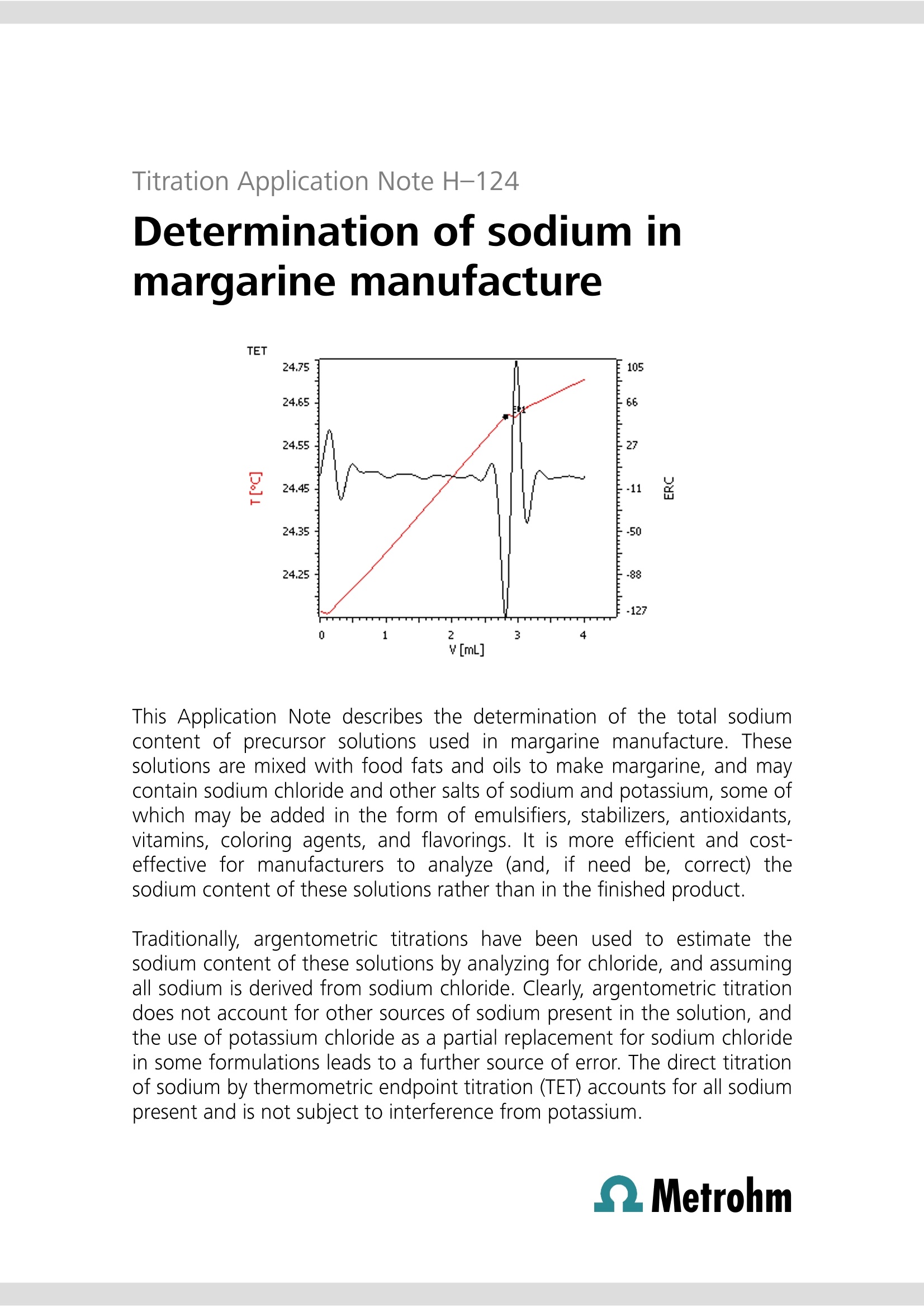
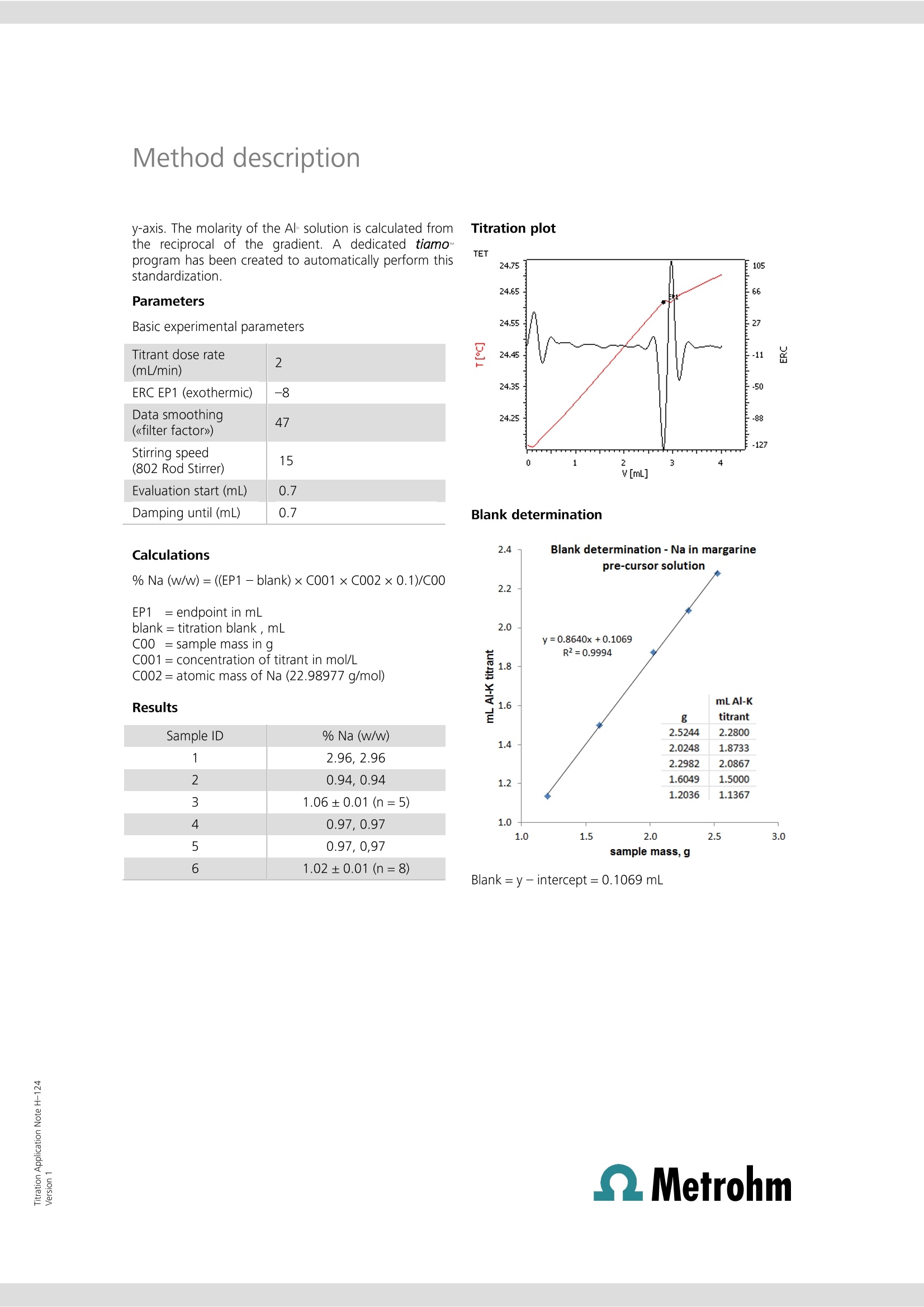
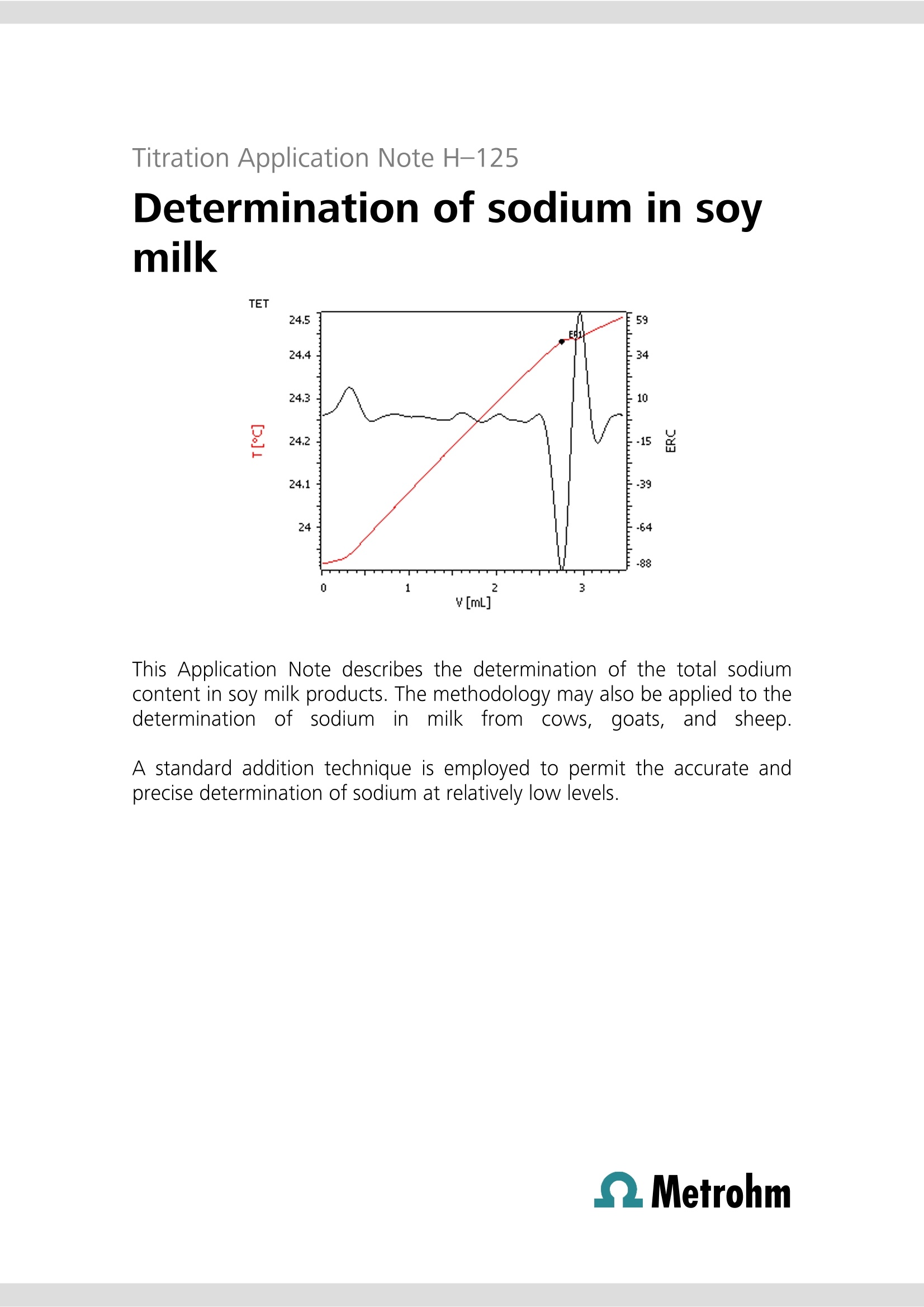
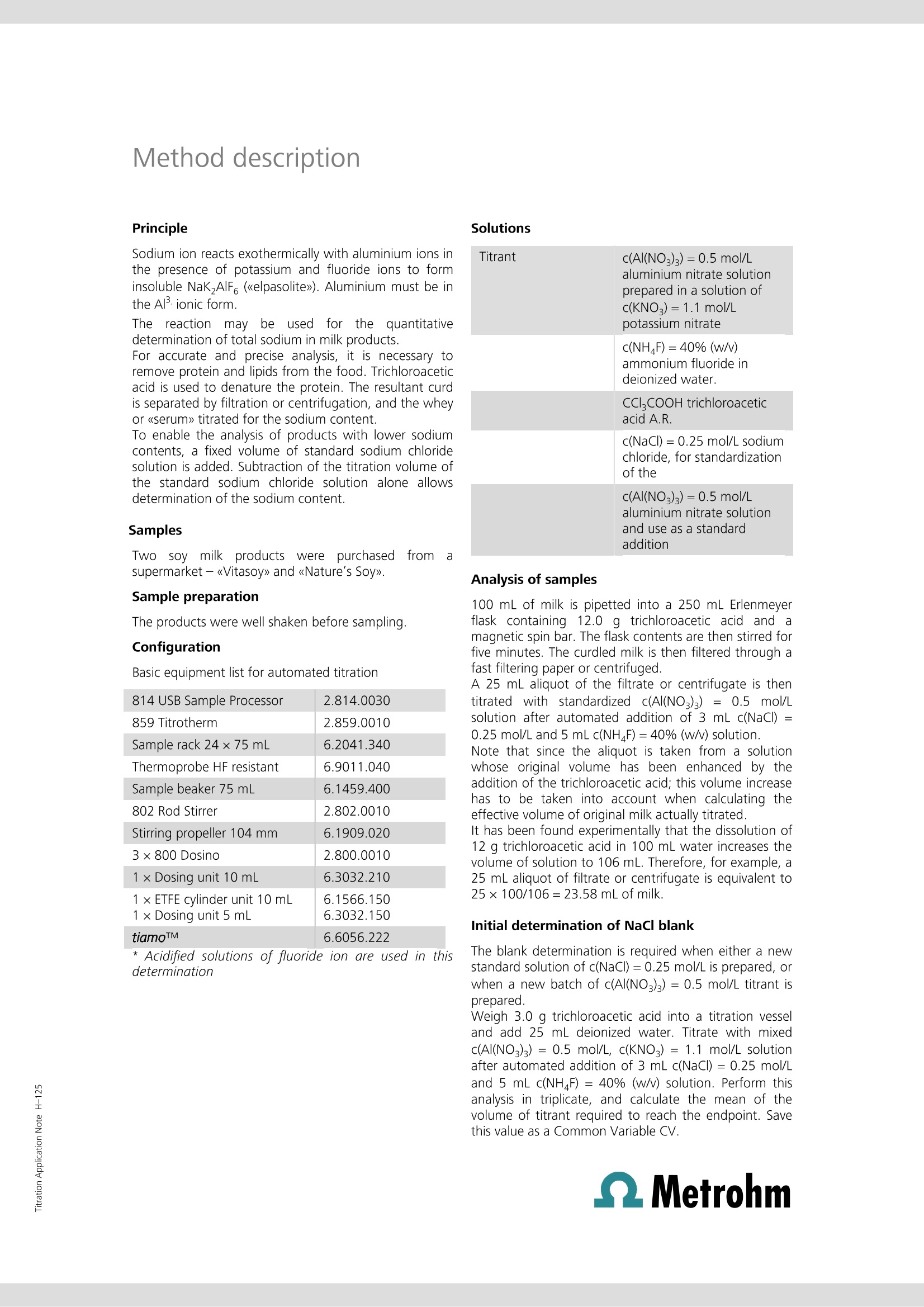
Titration Application Note H-124 Determination of sodium inmargarine manufacture This Application Note describes the determination of the total sodiumcontent of precursor solutions used in margarine manufacture. Thesesolutions are mixed with food fats and oils to make margarine, and maycontain sodium chloride and other salts of sodium and potassium, some ofwhich may be added in the form of emulsifiers, stabilizers, antioxidants,vitamins, coloring agents, and flavorings. It is more efficient and cost-effective for manufacturers to analyze (and, if need be, correct) thesodium content of these solutions rather than in the finished product. Traditionally, argentometric titrations have been used to estimate thesodium content of these solutions by analyzing for chloride, and assumingall sodium is derived from sodium chloride. Clearly, argentometric titrationdoes not account for other sources of sodium present in the solution, andthe use of potassium chloride as a partial replacement for sodium chloridein some formulations leads to a further source of error. The direct titrationof sodium by thermometric endpoint titration (TET) accounts for all sodiumpresent and is not subject to interference from potassium. Principle Solutions Sodium ion reacts exothermically with aluminium ions inthe presence of potassium and fluoride ions to forminsoluble NaK,AlF6 (《elpasolite》). Aluminium must be inthe Al+ ionic form. The reaction maybe usedfor the quantitativedetermination of total sodium in various foodstuffs. Samples Randomly selected samples of aqueous margarine pre-cursor solutions were provided by a food manufacturer. Sample preparation The samples required no special preparation. Configuration 814 USB Sample Processor 2.814.0030 859 Titrotherm 2.859.0010 Sample rack 24x75 mL 6.2041.340 Thermoprobe HF resistant 6.9011.040 Sample beaker 75 mL 6.1459.400 802 Rod Stirrer 2.802.0010 Stirring propeller 104 mm 6.1909.020 2×800 Dosino 2.800.0010 1× Dosing unit 10 mL 6.3032.210 1×ETFE cylinder unit 10 mL1 × Dosing unit 5 mL 6.3032.150 tiamoTM 6.6056.222 * Acidified solutions of fluoride ion are used in thisdetermination c(Al(NO3)3)=0.5 mol/L aluminiumnitrate solution prepared in a solution ofc(KNO)=1.1mol/L potassium nitrate c(NH F)=40% (w/v) ammonium fluoridein deionized water. Analysis of samples Between 0.5 and 2.5 g of sample is weighed into aclean, dry titration vessel to obtain a titration volume atthe endpoint of between approximately 1 to 2.5 mL oftitrant. The amount required may vary according to theformulation and may require some initialexperimentation to obtain optimal sample masses fordifferent solution formulations. The solution is dilutedwith 25 mL of c(HCI)=~1 mol/L. Alternatively, 2 mLconcentrated HCl and 25 mL deionized water may beused to dilute and condition the sample. The suspension is then titrated with standardizedc(Al(NO3)3)= 0.5 mol/L solution after automatedaddition of 5 mL c(NHF)=40%(w/v) solution. Initial determination of reagent blank The blank determination is only required for the initialsetup for analysis of this type of food product. Five separate titrations are performed on a sampleregarded as typical by the analyst. For example, for asolution where the sodium content is approximately 1%(w/w), samples masses ranging from ~1 to ~2.5 g inroughly equal increments may be used. A regression analysis is performed on the results, withthe x-axis denoting sample mass in g, and y-axis titrationendpoint volumes in mL. Standardization of titrant Aliquots of 5, 10, 15, 20, and 25 mL of 0.1 mol/Lc(NaCl) are pipetted into titration vessels and dilutedwith deionized water to bring the volume to~25 mL. To each vessel, 1 mL of concentrated HCl is added. Thesolutions are titrated under the same conditions as forthe samples. A regression analysis is performed, with the amount ofNaCl titrated (as mmol) plotted on the x-axis, and thevolume of c(Al(NO3)3)=0.5 mol/L in mL plotted on the y-axis. The molarity of the Al solution is calculated fromthe reciprocal of the gradient. A dedicated tiamoprogram has been created to automatically perform thisstandardization. Titration plot Parameters Basic experimental parameters Titrant dose rate2(mL/min)ERC EP1 (exothermic) -8Data smoothing47(《filter factor》)Stirring speed15(802 Rod Stirrer)Evaluation start (mL) 0.7 Damping until (mL) 0.7 Blank determination 2.44 Blank determination-Na in margarine Calculations % Na (w/w)= ((EP1-blank)×C001×C002×0.1)/C00 EP1 = endpoint in mL blank= titration blank , mL C00 = sample mass in g C001=concentration of titrant in mol/L C002= atomic mass of Na (22.98977 g/mol) Results Sample ID % Na (w/w) 1 2.96,2.96 2 0.94,0.94 3 1.06±0.01(n=5) 4 0.97,0.97 5 0.97,0,97 6 1.02±0.01(n=8) Blank=y-intercept=0.1069 mL 3.0 Determination of sodium in sovmilk This Application Note describes the determination of the total sodiumcontent in soy milk products. The methodology may also be applied to thedetermination of sodium in milkfrom cows.goats,and sheep. A standard addition technique is employed to permit the accurate andprecise determination of sodium at relatively low levels. Principle Sodium ion reacts exothermically with aluminium ions inthe presence of potassium and fluoride ions to forminsoluble NaKAlF6 (《elpasolite》). Aluminium must be inthe Alionic form. Thereaction may beusedffoorr the quantitativedetermination of total sodium in milk products. For accurate and precise analysis, it is necessary toremove protein and lipids from the food. Trichloroaceticacid is used to denature the protein. The resultant curdis separated by filtration or centrifugation, and the wheyor《serum》titrated for the sodium content. To enable the analysis of products with lower sodiumcontents, a fixed volume of standard sodium chloridesolution is added. Subtraction of the titration volume ofthe standard sodium chloride solution alone allowsdetermination of the sodium content. Samples Twosoy milkproductswere purchasedfromasupermarket -《Vitasoy》and 《Nature’s Soy》. Sample preparation The products were well shaken before sampling. Configuration Basic equipment list for automated titration 814 USB Sample Processor 2.814.0030 859 Titrotherm 2.859.0010 Sample rack 24×75 mL 6.2041.340 Thermoprobe HF resistant 6.9011.040 Sample beaker 75 mL 6.1459.400 802 Rod Stirrer 2.802.0010 Stirring propeller 104 mm 6.1909.020 3×800 Dosino 2.800.0010 1× Dosing unit 10 mL 6.3032.210 1 × ETFE cylinder unit 10 mL 1 × Dosing unit 5 mL 6.1566.150 6.3032.150 tiamoTM 6.6056.222 * Acidified solutions of fluoride ion are used in thisdetermination CCl,COOH trichloroaceticacid A.R. c(NaCl)=0.25 mol/L sodiumchloride, for standardizationof the c(AI(NO3)3)=0.5 mol/Laluminium nitrate solutionand use as a standard addition Analysis of samples 100 mL of milk is pipetted into a 250 mL Erlenmeyerflask containing 12.0 g trichloroacetic acid and amagnetic spin bar. The flask contents are then stirred forfive minutes. The curdled milk is then filtered through afast filtering paper or centrifuged. A 25 mL aliquot of the filtrate or centrifugate is thentitrated with standardized c(Al(NOg)3)= 0.5 mol/Lsolution after automated addition of 3 mL c(NaCl)= 0.25 mol/L and 5 mL c(NHF)=40% (w/v) solution.Note that since the aliquot is taken from a solutionwhose original volume has been enhanced by theaddition of the trichloroacetic acid; this volume increasehas to be taken into account when calculating theeffective volume of original milk actually titrated.It has been found experimentally that the dissolution of 12 g trichloroacetic acid in 100 mL water increases thevolume of solution to 106 mL. Therefore, for example, a25 mL aliquot of filtrate or centrifugate is equivalent to25×100/106=23.58 mL of milk. Initial determination of NaCl blank The blank determination is required when either a newstandard solution of c(NaCl)= 0.25 mol/L is prepared, orwhen a new batch of c(Al(NO)3)=0.5 mol/L titrant isprepared. Weigh 3.0 g trichloroacetic acid into a titration vesseland add 25 mL deionized water. Titrate with mixedc(Al(NO:)3)=0.5 mol/L, c(KNO:)= 1.1 mol/L solutionafter automated addition of 3 mL c(NaCl)=0.25 mol/Land 5 mL c(NHF)=40% (w/v) solution. Perform thisanalysis in triplicate, and calculate the mean of thevolume of titrant required to reach the endpoint. Savethis value as a Common Variable CV. Method description Standardization of titrant Titration plot Prepare five titration vessels, each containing 25 mLdeionized water and 3 g trichloroacetic acid. Aliquots of2, 4, 6, 8, and 9 mL of c(NaCl)= 0.25 mol/L aredispensed by a Dosino as part of an automated tiamoTMstandardization program, which also0 adds5 mL c(NHF)=40% (w/v)):solutionbeforeethecommencement of titration with mixed c(Al(NO3)3)=0.5mol/L, c(KNO,)=1.1 mol/L solution. w A regression analysis is performed automatically bytiamoTM to compute the strength of the Al solution. Parameters Basic experimental parameters Titrant dose rate (mL/min) 2 ERC EP1 (exothermic) -40 Data smoothing (《filter factor》) 47 Stirring speed (802 Stirrer) 15 Evaluation start (mL) 1 Damping until (mL) 1 Results Blank determination (titration of 3.0 mL c(NaCl)=0.2502 mol/L): 1.5000,1.5033,1.5000, 1.4967 mL Mean= 1.5000 mL Analysis of soy milks Na mg/100 mL Soy milk Nutrition information average value TET analysis Nature’s Soy 25 24.9±0.2(n=7) Vitasoy 44 65.1±0.1(n=8) QMetrohm
确定

还剩4页未读,是否继续阅读?
瑞士万通中国有限公司为您提供《豆奶中钠离子检测方案(自动电位滴定)》,该方案主要用于乳粉中营养成分检测,参考标准--,《豆奶中钠离子检测方案(自动电位滴定)》用到的仪器有瑞士万通859 温度滴定仪
推荐专场
相关方案
更多
该厂商其他方案
更多















