方案详情
文
多环芳烃是一组因为潜在致癌性而一直引起别人关注的化学物质。该物质通过不同的渠道进入环境,但是绝大多数是因为不完全的高温燃烧所产生。因此,在空气,水和土壤中都能发现多环芳烃。由于不同的样品都各具复杂性,所以必须开发一套稳定可靠的方法用于分析多环芳烃。此外,由于样品量的不足,对多环芳烃的检测灵敏度也是一个问题。所以有效的样品前处理也显得极为重要。
为了开发出高回收率,重复性的方法,可以消除水中基质效应对分析的影响。因此我们研究了使用高分子据水固相萃取吸附剂来优化多环芳烃的在线萃取过程。
方案详情

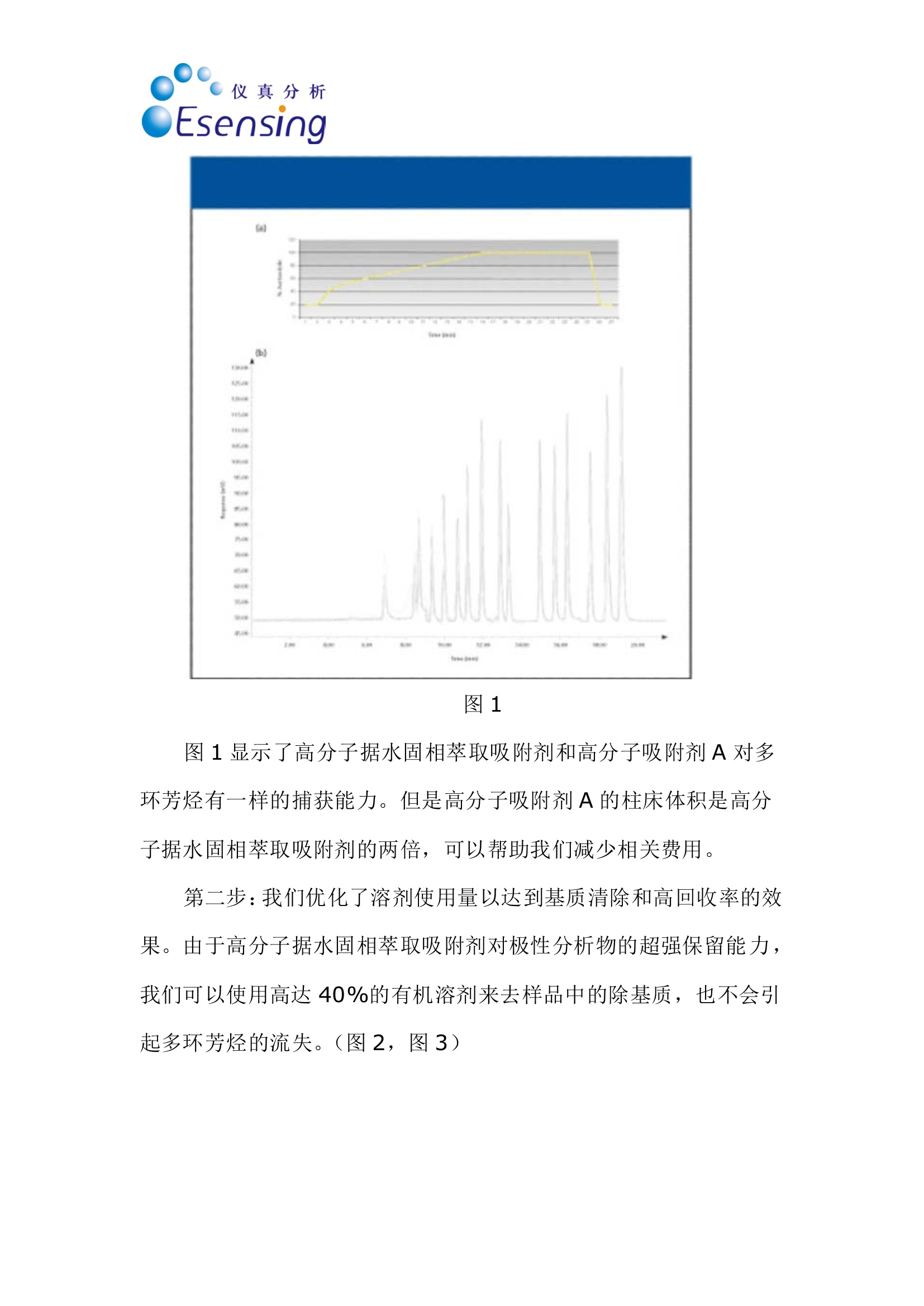
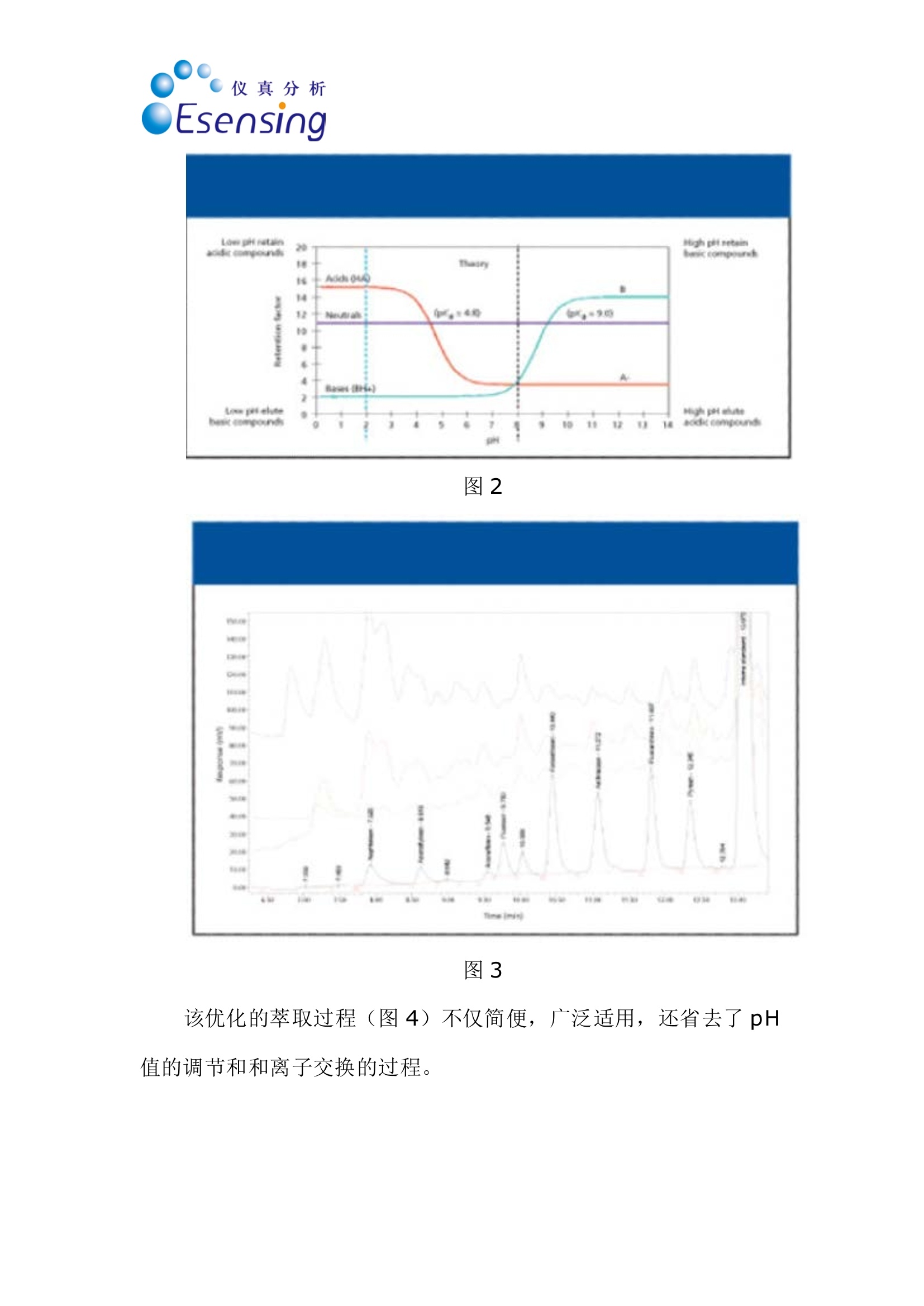
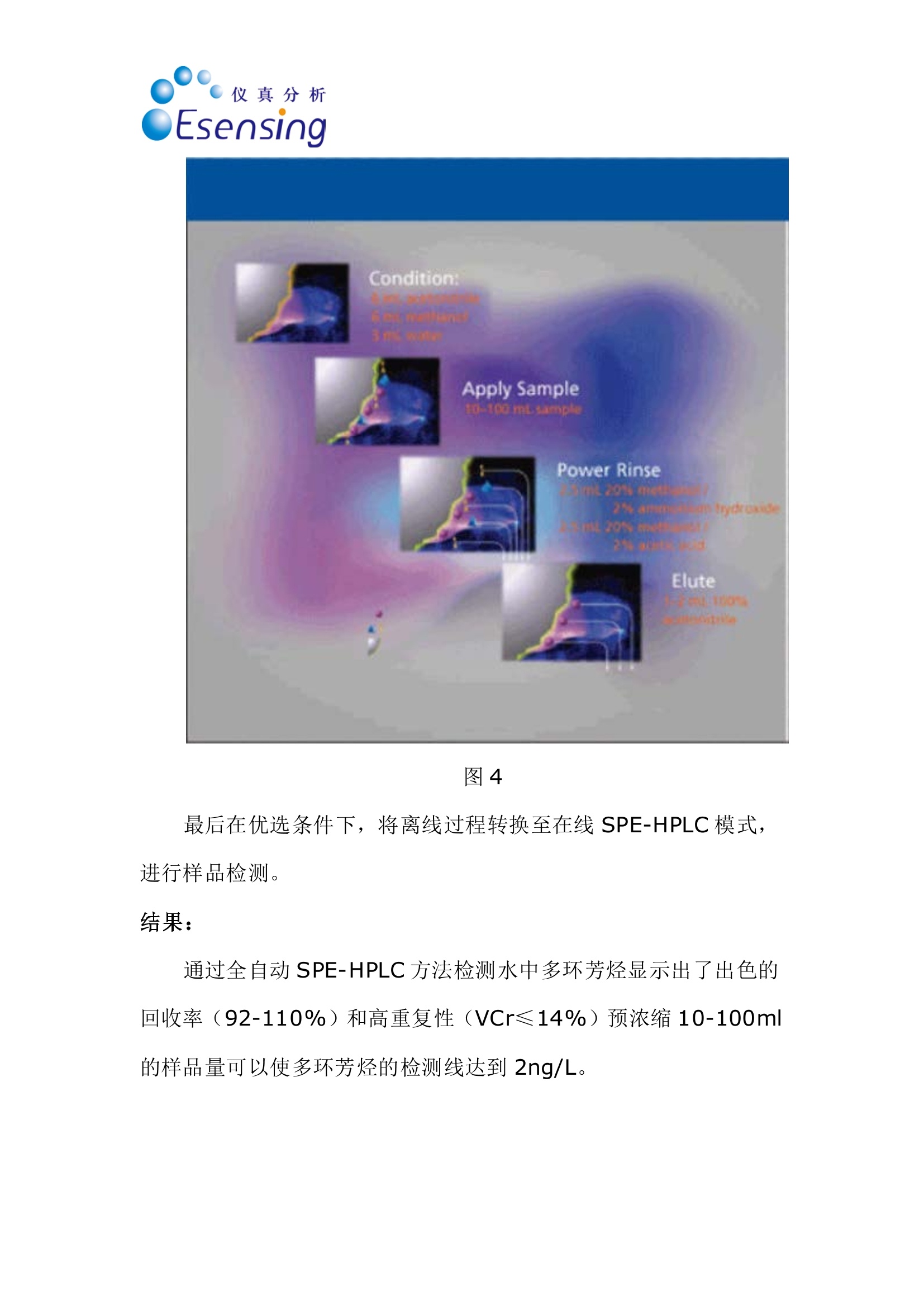
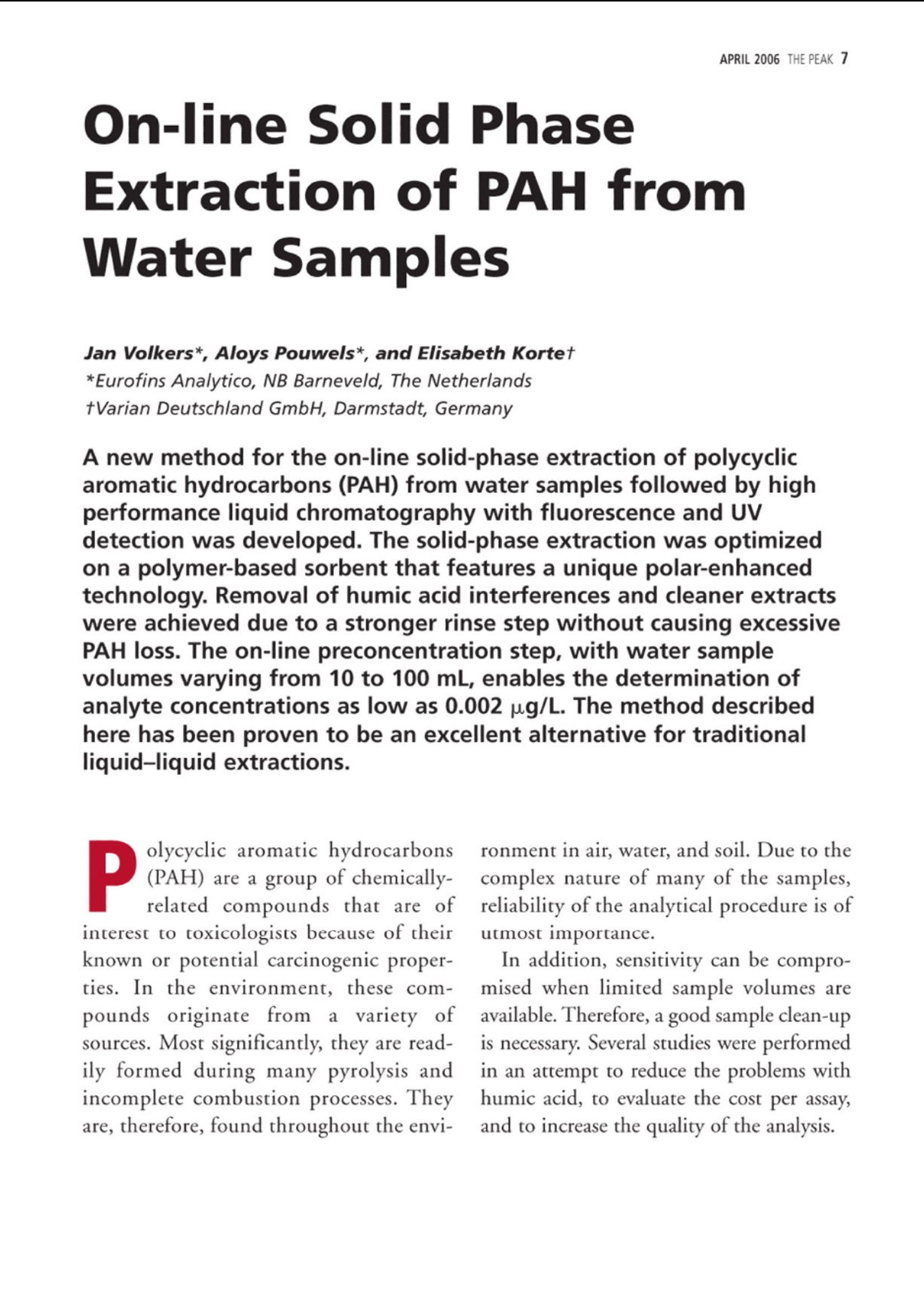
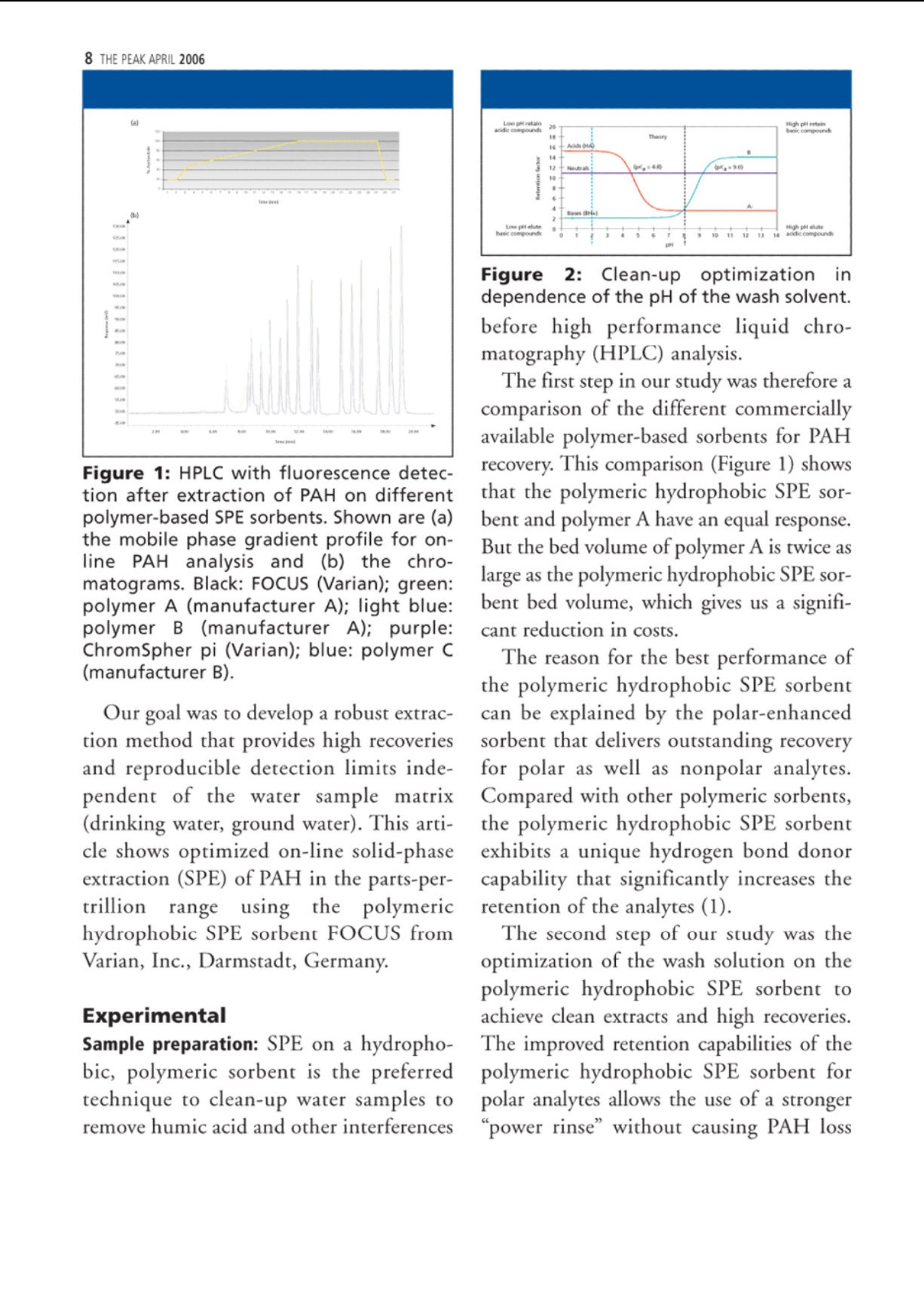
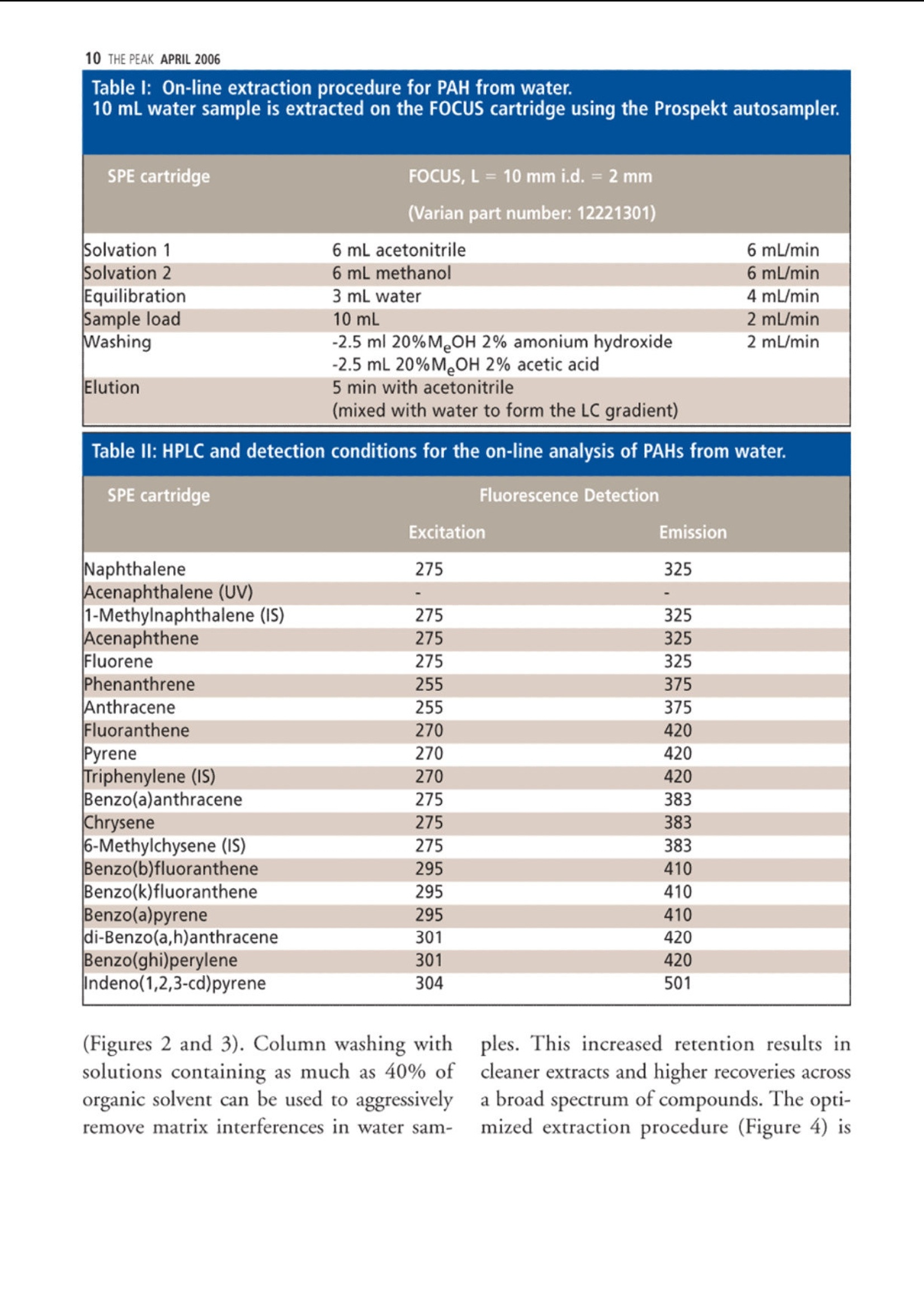
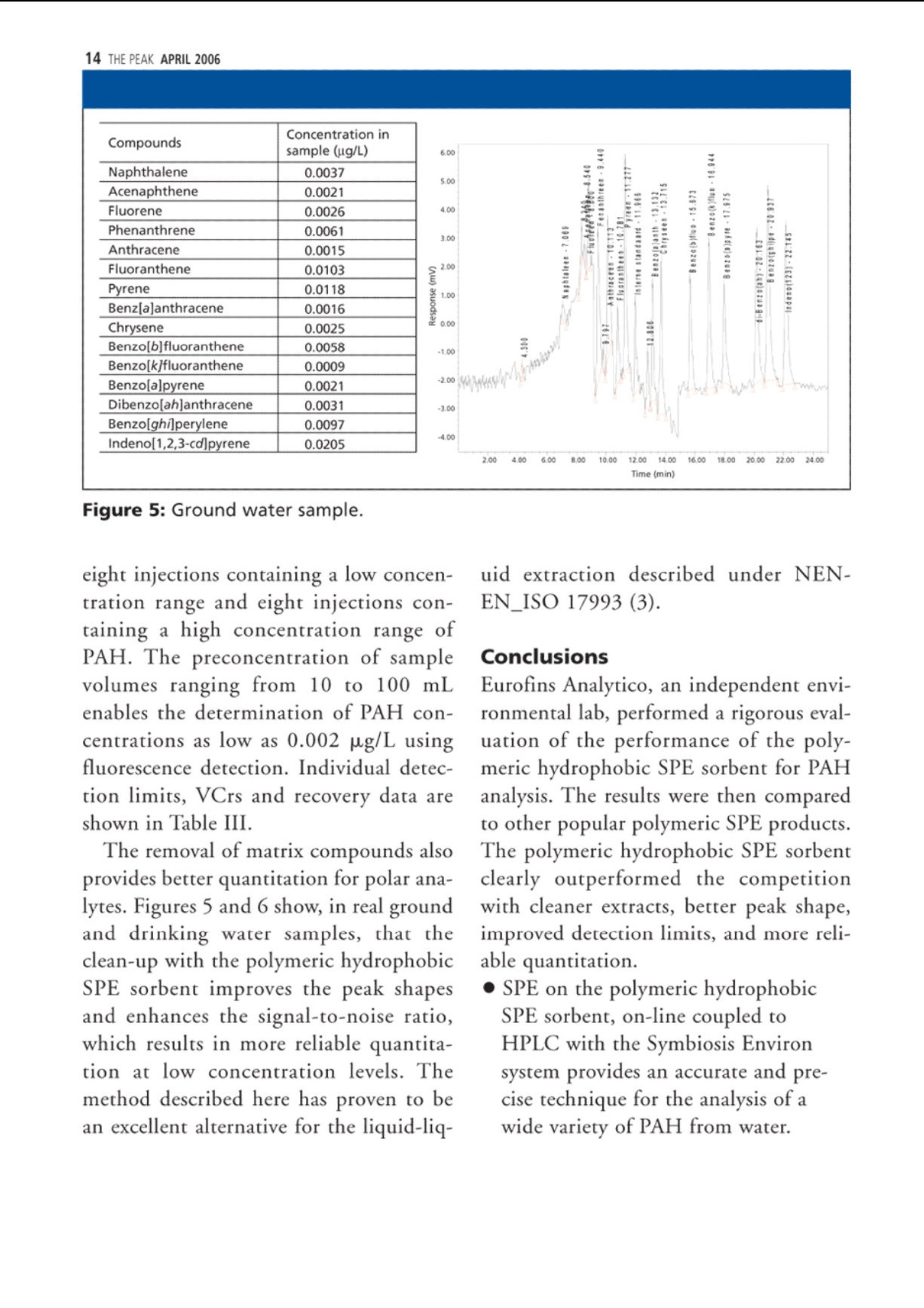
仪真分析)EEsensing 仪真分析)EEsensing 在线 SPE-LC-FLD或 UV检测水中的多环芳烃 背景: 多环芳烃是一类由于具有潜在致癌性而引起公众关注的化学物质。该物质通过不同的渠道进入环境,但是绝大多数是因为不完全的高温燃烧所产生。因此,在空气,水和土壤中都能发现多环芳烃。由于不同的样品都各具复杂性,所以必须开发一套稳定可靠的方法用于分析多环芳烃。此外,由于样品量的不足,对多环芳烃的检测灵敏度的提升也存在较大困难,故有效的样品前处理即显得极为重要。 为开发出高回收率,高重复性的方法,需要消除水中基质效应对分析的影响。因此我们亚开发了使用高分子拒水固相萃取吸附剂来优化化多环芳烃的在线萃取过程。 实验过程: 样品制备:样品进入HPLC之前,使用拒水高分子吸附剂和固相萃取来除去水样中的腐植酸和其他干扰物是目目流行的方法。 第一步:我们研究了不同品牌的高分子吸附剂对多环芳烃的捕获能力 图1 图1显示了高分子据水固相萃取吸附剂和高分子吸附剂A对多环芳烃有一样的捕获能力。但是高分子吸附剂A的柱床体积是高分子据水固相萃取吸附剂的两倍,,可以帮助我们减少相关费用。 第二步:我们优化了溶剂使用量以达到基质清除和高回收率的效果。由于高分子据水固相萃取吸附剂对极性分析物的超强保留能力,我们可以使用高达40%的有机溶剂来去样品中的除基质,也不会引起多环芳烃的流失。(图2,图3) 图2 图3 该优化的萃取过程(图4)不仅简便,广泛适用,还省去了 pH值的调节和和离子交换的过程。 图4 最后在优选条件下,将离线过程转换至在线 SPE-HPLC模式,进行样品检测。 结果: 通过全自动 SPE-HPLC 方法检测水中多环芳烃显示出了出色的回收率(92-110%)和高重复性 (VCr≤14%)预浓缩10-100ml的样品量可以使多环芳烃的检测线达到2ng/L。 On-line Solid PhaseExtraction of PAH fromWater Samples Jan Volkers*, Aloys Pouwels*, and Elisabeth Kortet *Eurofins Analytico, NB Barneveld, The Netherlands tVarian Deutschland GmbH, Darmstadt, Germany A new method for the on-line solid-phase extraction of polycyclicaromatic hydrocarbons (PAH) from water samples followed by highperformance liquid chromatography with fluorescence and UVdetection was developed.The solid-phase extraction was optimizedon a polymer-based sorbent that features a unique polar-enhancedtechnology. Removal of humic acid interferences and cleaner extractswere achieved due to a stronger rinse step without causing excessivePAH loss. The on-line preconcentration step, with water samplevolumes varying from 10 to 100 mL, enables the determination ofanalyte concentrations as low as 0.002 ug/L. The method describedhere has been proven to be an excellent alternative for traditionalliquid-liquid extractions. olycyclic aromatic hydrocarbonsP(PAH) are a group of chemically-related compounds that are ofinterest to toxicologists because of theirknown or potential carcinogenic proper-ties. In the environment, these com-poundsoriginate from aa variety ofsources. Most significantly, they are read-ily formed during many pyrolysis andincomplete combustion processes. Theyare, therefore, found throughout the envi- ronment in air, water, and soil. Due to thecomplex nature of many of the samples,reliability of the analytical procedure is ofutmost importance. In addition, sensitivity can be compro-mised when limited sample volumes areavailable. Therefore, a good sample clean-upis necessary. Several studies were performedin an attempt to reduce the problems withhumic acid, to evaluate the cost per assay,and to increase the quality of the analysis. Figure 1: HPLC with fluorescence detec-tion after extraction of PAH on differentpolymer-based SPE sorbents. Shown are (a)the mobile phase gradient profile for on-line PAH analysisand (b) the chro-matograms. Black: FOCUS (Varian); green:polymer A (manufacturer A); light blue:polymer B(manufacturer A); purple:ChromSpher pi (Varian);blue: polymer C(manufacturer B). Our goal was to develop a robust extrac-tion method that provides high recoveriesand reproducible detection limits inde-pendent of the water sample matrix(drinking water, ground water). This arti-cle shows optimized on-line solid-phaseextraction (SPE) of PAH in the parts-per-trillionrange using the polymerichydrophobic SPE sorbent FOCUS fromVarian, Inc.,Darmstadt, Germany. Experimental Sample preparation: SPE on a hydropho-bic, polymeric sorbent is the preferredtechnique to clean-up water samples toremove humic acid and other interferences Figure 2: Clean-up optimization itdependence of the pH ofthe wash solvent.before high performance liquid chro-matography (HPLC) analysis. The first step in our study was therefore acomparison of the different commerciallyavailable polymer-based sorbents for PAHrecovery. This comparison (Figure 1) showsthat the polymeric hydrophobic SPE sor-bent and polymer A have an equal response.But the bed volume of polymer A is twice aslarge as the polymeric hydrophobic SPE sor-bent bed volume, which gives us a signifi-cant reduction in costs. The reason for the best performance ofthe polymeric hydrophobic SPE sorbentcan be explained by the polar-enhancedsorbent that delivers outstanding recoveryfor polar as well as nonpolar analytes.Compared with other polymeric sorbents,the polymeric hydrophobic SPE sorbentexhibits a unique hydrogen bond donorcapability that significantly increases theretention of the analytes (1). The second step of our study was theoptimization of the wash solution on thepolymeric hydrophobic SPE sorbent toachieve clean extracts and high recoveries.The improved retention capabilities of thepolymeric hydrophobic SPE sorbent forpolar analytes allows the use of a stronger“power rinse”without causing PAH loss SPE cartridge FOCUS, L= 10 mm i.d.=2 mm (Varian part number: 12221301) Solvation 1 6 mL acetonitrile 6 mL/min Solvation 2 6 mL methanol 6 mL/min Equilibration 3 mL water 4 mL/min Sample load 10 mL 2 mL/min 2 mL/min Washing -2.5 ml 20%MOH 2% amonium hydroxide Elution 2.5 mL 20%MOH 2% acetic acid 5 min with acetonitrile (mixed with water to form the LC gradient) SPE cartridge Fluorescence Detection Excitation Emission Naphthalene 275 325 Acenaphthalene (UV) 1-Methylnaphthalene (IS) 275 325 Acenaphthene 275 325 Fluorene 275 325 Phenanthrene 255 375 Anthracene 255 375 Fluoranthene 270 420 Pyrene 270 420 Triphenylene (IS) 270 420 Benzo(a)anthracene 275 383 Chrysene 275 383 6-Methylchysene (IS) 275 383 Benzo(b)fluoranthene 295 410 Benzo(k)fluoranthene 295 410 Benzo(a)pyrene 295 410 di-Benzo(a,h)anthracene 301 420 Benzo(ghi)perylene 301 420 Indeno(1,2,3-cd)pyrene 304 501 ples. This increased retention results incleaner extracts and higher recoveries acrossa broad spectrum of compounds. The opti-mized extraction procedure (Figure 4) is (Figures 2 and 3). Column washing withsolutions containing as much as 40% oforganic solvent can be used to aggressivelyremove matrix interferences in water sam- high concentrated ground water samples. Component Detection limit VCr% VCr% low concentration range high concentartion range Flu UV Concentration Flu UV Concentration Flu UV (ug/L) (ug/L)(p.g/L) (ug/L) Naphthalene 0.004 0.009 0.3 4.2 7.8 3.000 2.3 4.2 Acenaphthalene - 0.0070.4 6.7 4.004 - 3.3 Acenaphthene 0.003 0.004 0.1 4.8 10.6 1.002 2.5 4.9 Fluorene 0.002 0.0060.1 4.2 4.3 1.004 1.2 2.9 Phenanthrene 0.00300.005 0.125 5.2 7.8 1.252 1.1 4.0 Anthracene 0.002 0.0100.05 4.4 13.8 0.501 1.6 7.8 Fluoranthene 0.003 0.006 0.43 2.7 3.4 4.253 0.7 2.9 pyrene 0.004 0.0070.3 2.5 2.8 3.004 2.0 4.1 Benzo(a)anthracene 0.002 0.005 c0.075 2.3 6.6 0.751 2.0 6.3 Chrysene 0.002 0.0050.1 4.3 3.1 1.000 0.7 3.0 Benzo(b)fluoranthene 0.002 0.0070.3 1.2 2.3 2.998 1.0 3.0 Benzo(k)fluoranthene 0.005 0.0070.03 2.0 13.1 0.300 1.0 13.6 Benzo(a)pyrene 0.005 0.005 0.06 6.2 11.8 0.600 12.1 11.4 di-Benzo(a,h)anthracene 0.001 0.0090.15 4.3 13.1 1.504 6.1 3.3 Benzo(ghi)perylene 0.003 0.0070.3 10.7 10.0 3.000 4.7 3.0 Indeno(1,2,3-cd)pyrene 0.00550.0061.2 3.8 3.4 6.000 2.8 2.9 simple, universal, and eliminates the com-plex pH adjustments necessary with ionexchange and mixed mode methods. The third step was the transfer of the off-line method to an on-line SPE-HPLC sys-tem. For this application we used the poly-meric hydrophobic SPE sorbent material in10 mm (length) ×2 mm (i.d.) cartridges.The SPE conditions for the on-line extrac-tion are shown in Table I. On-line SPE-HPLC The PAH-method includes on-line SPEon a Prospect (Spark Holland Emmen,The Netherlands) autosampler followedby HPLC analysis with a programmablefluorescence and UV detection under rel- ative fast gradient conditions. The analy-sis conditions are listed in Table II. Theswitching of the autosampler valves, topreconcentrate and analyze PAH in watersamples, allows the use of hydrophobicSPE cartridges and can be transferred toother applicationsas well, especiallywhere the HPLC mobile phase is tooweak for on-line desorption (2). Results PAH analysis in ground water matrixusing the automated on-line SPE-HPLCsystem shows excellent recoveries(92-110%) andd1hignh reproducibility(variation coefficient VCr ≤ 14%). Thevariation coefficient was calculated from Figure 3: Humic acid clean-up in sample.The chromatogram shows that some com-ponent become visual by using a wash with60% A (2% ammonia, 2% acetic acid) and40% B (methanol) (red). Acenaphthylene,acenaphthene and fluorene become visual.With 25% of ammonia (green) some com-ponents are washed away. Black: standard;blue: wash step 10% methanol; red: washstep with 60% A (2 %ammonia, 2% aceticacid) and 40% (b) (methanol); green: washstep 60% A (25% ammonia, 2% acetic acid)and 40% B (methanol). Figure 4: Off-line extraction procedure forPAHs from water on the FOCUS polymerichydrophobic SPE sorbent.To preventadsorption of PAH on the sample flask, thewater sample was diluted with isopropanolto obtain a sample with 25% isopropanol. eight injections containing a low concen-tration range and eight injections con-taining a high concentration range ofPAH. The preconcentration of samplevolumes ranging from 10 to 100 mLenables the determination of PAH con-centrations as low as 0.002 pg/L usingfluorescence detection. Individual detec-tion limits, VCrs and recovery data areshown in Table III. The removal of matrix compounds alsoprovides better quantitation for polar ana-lytes. Figures 5 and 6 show, in real groundand drinking water samples, that theclean-up with the polymeric hydrophobicSPE sorbent improves the peak shapesand enhances the signal-to-noise ratio,which results in more reliable quantita-tion at low concentration levels.Themethod described here has proven to bean excellent alternative for the liquid-liq- uid extraction described under NEN-EN_ISO 17993 (3). Conclusions Eurofins Analytico, an independent envi-ronmental lab, performed a rigorous eval-uation of the performance of the poly-meric hydrophobic SPE sorbent for PAHanalysis. The results were then comparedto other popular polymeric SPE products.The polymeric hydrophobic SPE sorbentclearly outperformed the competitionwith cleaner extracts, better peak shape,improved detection limits, and more reli-able quantitation. ●SPE on the polymeric hydrophobicSPE sorbent, on-line coupled toHPLC with the Symbiosis Environsystem provides an accurate and pre-cise technique for the analysis of awide variety of PAH from water. The polymeric hydrophobic SPE sorbentcan be applied in the majority of watersamples that are analyzed at EurofinsAnalytico.With a proper washing step alarge amount of possible contaminantscan be removed. PAH analysis can be fully automatedwith good recoveries (92-110 %) andacceptable reproducibility (VCr=0.7-13.8 %) in the ground watermatrix. ● The preconcentration of 10-100 mL ofsample volume enables the determinationof PAH concentrations as low as 2 ng/L. ●The polymeric hydrophobic SPE sorbentgenerally shows an improved analyticalperformance as well as a significantreduction in costs. · The method described here has provento be an excellent alternative for the liq-uid-liquid extraction described underNEN-EN_ISO 17993 (3). References (1))Varian, brochure “Focus enhanced polarrecovery. (2)Spark Holland, application note, “Fully auto-mated sample preparation and HPLC analysisof Polycyclic Aromatic Hydrocarbons in waterSamples." (3)NEN-EN-ISO 17993: Water quality -Determination of 15 PAH in water by HPLCwith fluorescence detection after liquid-liquidextraction.■ 多环芳烃是一组因为潜在致癌性而一直引起别人关注的化学物质。该物质通过不同的渠道进入环境,但是绝大多数是因为不完全的高温燃烧所产生。因此,在空气,水和土壤中都能发现多环芳烃。由于不同的样品都各具复杂性,所以必须开发一套稳定可靠的方法用于分析多环芳烃。此外,由于样品量的不足,对多环芳烃的检测灵敏度也是一个问题。所以有效的样品前处理也显得极为重要。为了开发出高回收率,重复性的方法,可以消除水中基质效应对分析的影响。因此我们研究了使用高分子据水固相萃取吸附剂来优化多环芳烃的在线萃取过程。
确定




还剩9页未读,是否继续阅读?
仪真分析仪器有限公司为您提供《环境水中多环芳烃检测方案(固相萃取仪)》,该方案主要用于环境水(除海水)中有机污染物检测,参考标准--,《环境水中多环芳烃检测方案(固相萃取仪)》用到的仪器有超高压液相在线SPE色谱联用系统CHRONECT Symbiosis
推荐专场
相关方案
更多
该厂商其他方案
更多
















