方案详情
文
由荷兰Spark Holland公司生产的系列仪器“全自动在线固相萃取-液相色谱联用系统”为食品检测行业的用户带来了一种全新的样品前处理技术。该仪器与传统意义的离线手动固相萃取以及目前流行的全自动固相萃取仪器相比,具有颠覆性的创新技术含量,目前已在全球拥有超过1500家的实验室用户。自2009年进入中国市场以来,产品销量成大幅上升趋势,目前已有包括各级国家食品检验检疫机构,临床药代实验室,省级公安毒理实验室,烟草集团理化实验室,环境监测站以及中科院研究院所等超过三十家用户使用该设备进行各种检测项目和方法开发并获得了广泛的好评。
方案详情

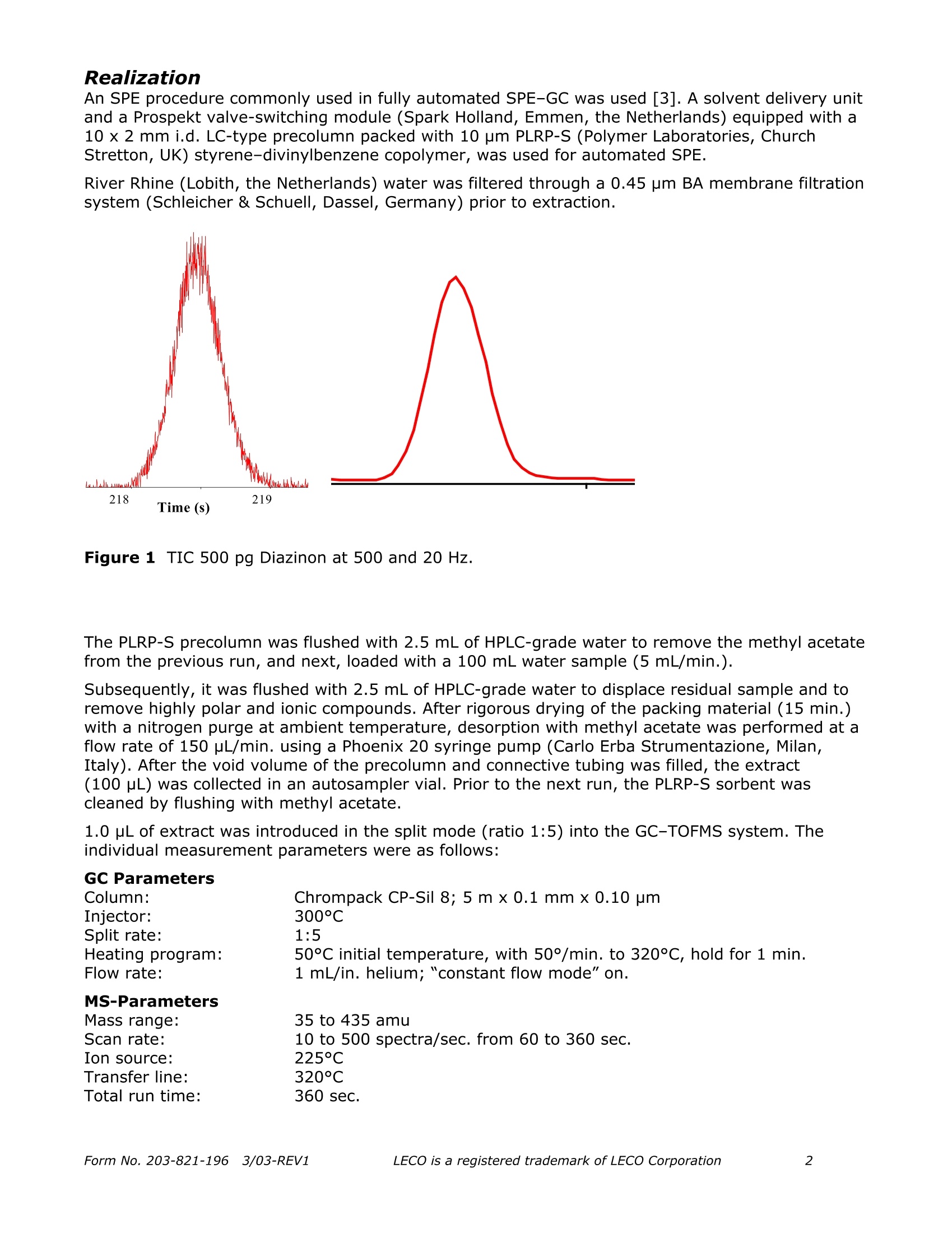
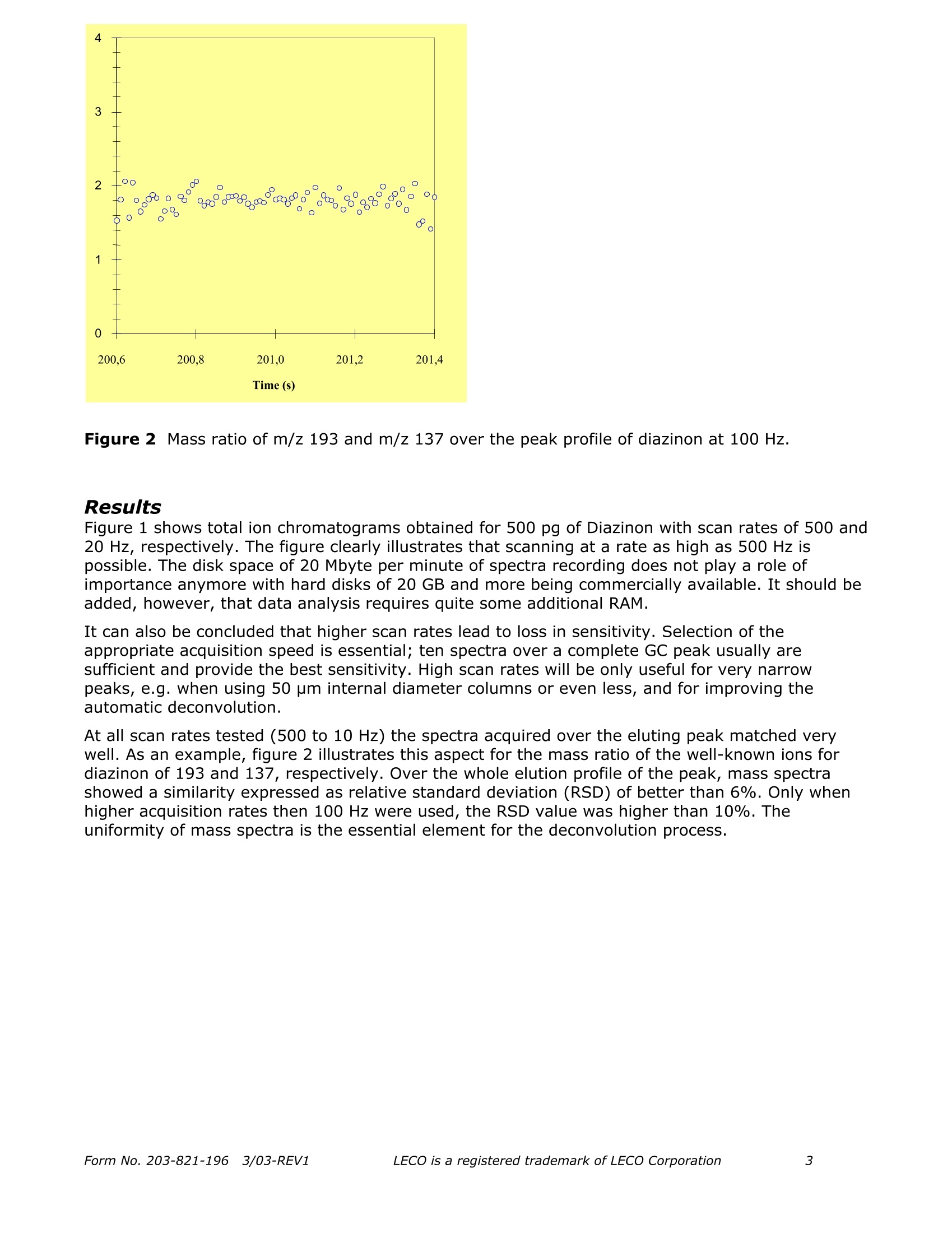
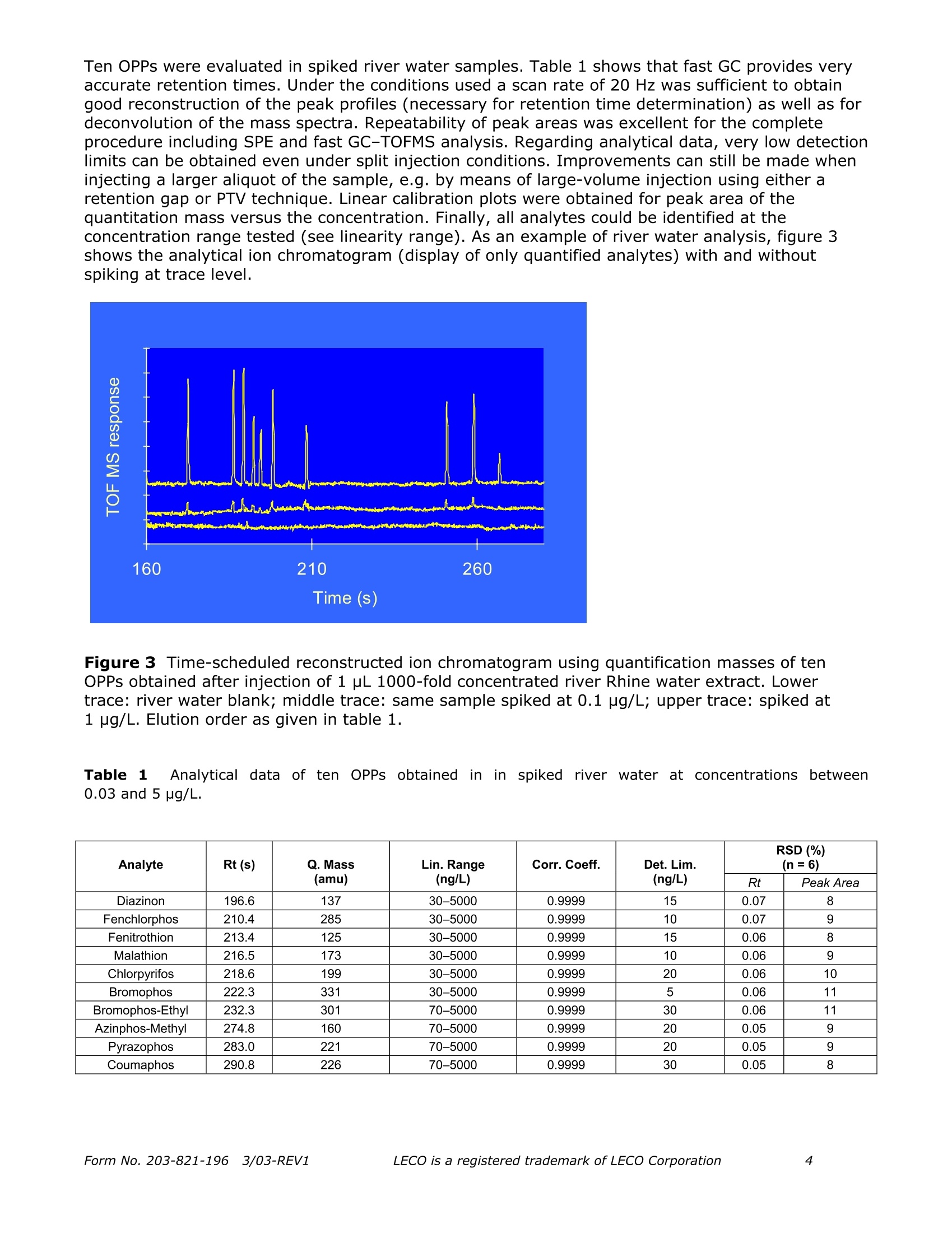
Separation Science Application Note Pegasus Fast quantitative determinationof organophosphorus pesticidesin water samples ReneJ.J. Vreuls, Jens Dalluige, and Udo A.Th. Brinkman Vrije Universiteit, Department of Analytical Chemistry and Applied Spectroscopy,de boelelaan 1083, 1081 HV Amsterdam, the Netherlands;e-mail: vreuls@chem.vu.nl Introduction The determination of organophosphorus pesticides (OPPs) in water samples still presents significantproblems. To reach the required EU level of 0.1 part-per-billion(ppb) for drinking water, and the1-3 ppb level in surface water, a significant concentration step and very sensitive detection arerequired. Properties of the OPPs such as good solubility in water, a wide polarity range,thermolability and chemolability, suggest column liquid chromatography (LC) as the method ofchoice for the analysis-cum-detection of these compounds. However, the use of LC is not reallysatisfactory because many OPPs do not have a chromophoric group-let alone that, generallyspeaking, UV absorption does not provide sufficient selectivity and/or sensitivity in environmentaltrace analysis. Next, identification with modern mass spectrometric detection suffices due to thelow ionisation in the electrospray and APCI interfaces. In actual practice, therefore, capillary gaschromatography (GC) is the preferred separation technique, especially because it can be easilycombined with the selective and sensitive thermionic, flame-photometric and MS detectors. In onerather laborious example, described by the Environmental Protection Agency (EPA), thedetermination is carried out by liquid-liquid extraction of 1 L of water sample with dichloromethane,evaporation of this solvent, exchange to methyl tert-butyl ether and injection of a few microliterson a capillary GC column equipped with an NPD [1]. Solid-phase extraction can solve some of thedisadvantages of liquid-liquid extraction, such as laboriousness, the use of large quantities ofextraction solvents and sample. Carried out in a miniaturised fashion, the required concentrationstep does not need any solvent evaporation step [2]. SPE is carried out on small cartridges filledwith a polymeric sorbent using 50 mL of sample. After drying by applying a nitrogen pressure, theanalytes are eluted with 50 to 100 pL of ethyl acetate. The small sample volume prevents thatanalytes, among them the relatively polar OPPs show breakthrough during trace enrichment. Foridentification purposes, MS is required. The slow scanning instruments usually prevent the use offast GC analysis. In this note, the GC analysis of extracts by means of fast GC with MS detection is the mainobjective. Since fast GC often leads to loss of resolution a solution has to be found. Time-of-flightMS (TOF MS) appears to be the MS of choice, because it provides a high rate of acquisition, whichenables chemometrica1YSIl approaches for data analysis. In this paper, real-life water sampleextracts-spiked at (sub) ppb-level-have been subjected to fast GC-TOFMS. Special attention hasbeen paid to the acquisition rate and to the similarity of the acquired spectra (absence of skewing).Next analytical data were evaluated with respect to linearity and detection limits. Realization An SPE procedure commonly used in fully automated SPE-GC was used [3]. A solvent delivery unitand a Prospekt valve-switching module (Spark Holland, Emmen, the Netherlands) equipped with a10x 2 mm i.d. LC-type precolumn packed with 10 pm PLRP-S (Polymer Laboratories, ChurchStretton, UK) styrene-divinylbenzene copolymer, was used for automated SPE. River Rhine (Lobith, the Netherlands) water was filtered through a 0.45 pm BA membrane filtrationsystem (Schleicher & Schuell, Dassel, Germany) prior to extraction. 218 Time (S) Figure 1 TIC 500 pg Diazinon at 500 and 20 Hz. The PLRP-S precolumn was flushed with 2.5 mL of HPLC-grade water to remove the methyl acetatefrom the previous run, and next, loaded with a 100 mL water sample (5 mL/min.). Subsequently, it was flushed with 2.5 mL of HPLC-grade water to displace residual sample and toremove highly polar and ionic compounds. After rigorous drying of the packing material (15 min.)with a nitrogen purge at ambient temperature, desorption with methyl acetate was performed at aflow rate of 150 pL/min. using a Phoenix 20 syringe pump (Carlo Erba Strumentazione, Milan,Italy). After the void volume of the precolumn and connective tubing was filled, the extract(100 pL) was collected in an autosampler vial. Prior to the next run, the PLRP-S sorbent wascleaned by flushing with methyl acetate. 1.0 uL of extract was introduced in the split mode (ratio 1:5) into the GC-TOFMS system. Theindividual measurement parameters were as follows: GC Parameters Figure 2Mass ratio of m/z 193 and m/z 137 over the peak profile of diazinon at 100 Hz. Results Figure 1 shows total ion chromatograms obtained for 500 pg of Diazinon with scan rates of 500 and20 Hz, respectively. The figure clearly illustrates that scanning at a rate as high as 500 Hz ispossible. The disk space of 20 Mbyte per minute of spectra recording does not play a role ofimportance anymore with hard disks of 20 GB and more being commercially available. It should beadded, however, that data analysis requires quite some additional RAM. It can also be concluded that higher scan rates lead to loss in sensitivity. Selection of theappropriate acquisition speed is essential; ten spectra over a complete GC peak usually aresufficient and provide the best sensitivity. High scan rates will be only useful for very narrowpeaks, e.g. when using 50 pm internal diameter columns or even less, and for improving theautomatic deconvolution. At all scan rates tested (500 to 10 Hz) the spectra acquired over the eluting peak matched verywell. As an example, figure 2 illustrates this aspect for the mass ratio of the well-known ions fordiazinon of 193 and 137, respectively. Over the whole elution profile of the peak, mass spectrashowed a similarity expressed as relative standard deviation (RSD) of better than 6%. Only whenhigher acquisition rates then 100 Hz were used, the RSD value was higher than 10%. Theuniformity of mass spectra is the essential element for the deconvolution process. Ten OPPs were evaluated in spiked river water samples. Table 1 shows that fast GC provides veryaccurate retention times. Under the conditions used a scan rate of 20 Hz was sufficient to obtaingood reconstruction of the peak profiles (necessary for retention time determination) as well as fordeconvolution of the mass spectra. Repeatability of peak areas was excellent for the completeprocedure including SPE and fast GC-TOFMS analysis. Regarding analytical data, very low detectionlimits can be obtained even under split injection conditions. Improvements can still be made wheninjecting a larger aliquot of the sample, e.g.by means of large-volume injection using either aretention gap or PTV technique. Linear calibration plots were obtained for peak area of thequantitation mass versus the concentration. Finally, all analytes could be identified at theconcentration range tested (see linearity range). As an example of river water analysis, figure 3shows the analytical ion chromatogram (display of only quantified analytes) with and withoutspiking at trace level. Figure 3 Time-scheduled reconstructed ion chromatogram using quantification masses of tenOPPs obtained after injection of 1 pL 1000-fold concentrated river Rhine water extract. Lowertrace: river water blank; middle trace: same sample spiked at 0.1 pg/L; upper trace: spiked at1 pg/L. Elution order as given in table 1. Table 1 Analytical data of ten OPPs obtained in in spiked river water at concentrationsbetween0.03 and 5 ug/L. Analyte Rt (s) Q. Mass (amu) Lin. Range(ng/L) Corr. Coeff. Det. Lim. (ng/L) RSD(%) (n=6) Rt Peak Area Diazinon 196.6 137 30-5000 0.9999 15 0.07 8 Fenchlorphos 210.4 285 30-5000 0.9999 10 0.07 9 Fenitrothion 213.4 125 30-5000 0.9999 15 0.06 8 Malathion 216.5 173 30-5000 0.9999 10 0.06 9 Chlorpyrifos 218.6 199 30-5000 0.9999 20 0.06 10 Bromophos 222.3 331 30-5000 0.9999 5 0.06 11 Bromophos-Ethyl 232.3 301 70-5000 0.9999 30 0.06 11 Azinphos-Methyl 274.8 160 70-5000 0.9999 20 0.05 9 Pyrazophos 283.0 221 70-5000 0.9999 20 0.05 9 Coumaphos 290.8 226 70-5000 0.9999 30 0.05 8 Conclusions As demonstrated in this application, the Pegasus is ideal for performing fast, sensitivedetermination of water analysis. The data processing software detects and identifies the targetcompounds by comparison of complete spectra (even when the components are buried in thebaseline) as well as performA..ing a search for unknown substances after separating overlappingspectra. A proper library identification can also be achieved using derived (background subtracted)spectra. Further acceleration and increase in sensitivity could easily be accomplished by means ofhigher scan rates, larger injection volume, etc. Gas chromatographic analyses can be performed within 5 minutes. Regarding quantitative aspects,detection at very low limits (low pg absolute amounts) can be conducted and linear response overa wide concentration, range assures an excellent method for analyzing water extracts. Literature K.W. Edgell, E. Jenkins, V. Lopez-Avila and J.E. Longbottom, J. Assoc. Off. Anal. Chem. 74 (1991)295. L.L.P. v. Stee, P.E. Leonards, R.J.J. Vreuls and U.A.Th. Brinkman, The Analyst 124 (1999) 1547. Th. Hankemeier, S.P.J. van Leeuwen, R.J.J. Vreuls and U.A.Th. Brinkman, Journal ofChromatography. A 811(1998)117. QLeciLECO Corporation· 3000 Lakeview Ave.· St. Joseph, MI 49085-2396 LECO is a registered trademark of LECO CorporationForm No. REV Form No. REVLECO is a registered trademark of LECO Corporation 由荷兰Spark Holland公司生产的系列仪器“全自动在线固相萃取-液相色谱联用系统”为食品检测行业的用户带来了一种全新的样品前处理技术。该仪器与传统意义的离线手动固相萃取以及目前流行的全自动固相萃取仪器相比,具有颠覆性的创新技术含量,目前已在全球拥有超过1500家的实验室用户。自2009年进入中国市场以来,产品销量成大幅上升趋势,目前已有包括各级国家食品检验检疫机构,临床药代实验室,省级公安毒理实验室,烟草集团理化实验室,环境监测站以及中科院研究院所等超过三十家用户使用该设备进行各种检测项目和方法开发并获得了广泛的好评。
确定


还剩3页未读,是否继续阅读?
仪真分析仪器有限公司为您提供《环境水中Diazinon检测方案(固相萃取仪)》,该方案主要用于环境水(除海水)中Diazinon检测,参考标准--,《环境水中Diazinon检测方案(固相萃取仪)》用到的仪器有超高压液相在线SPE色谱联用系统CHRONECT Symbiosis
推荐专场
相关方案
更多
该厂商其他方案
更多
















