方案详情
文
用户多,使用频繁,涵盖相关应用面广。
XH-100型祥鹄电脑微波催化合成/萃取仪,是应用先进的微波技术作为物理催化手段的新型化学反应装置。主要由微波催化仪主机、微电脑智能控制系统、高精度温度传感器、回流冷凝系统等组成。仪器使用先进的温度传感器,对反应温度进行实时精确监测;采用独创的电脑自学习技术,自动调节微波功率,智能控温保温,控温精度达±1℃。大容量不锈钢腔体,耐腐蚀,耐高温。反应容积微波泄漏符合国家标准。仪器操作简单,界面友好,您可轻松制订各种实验方案,并对实验过程进行全程监控。
方案详情

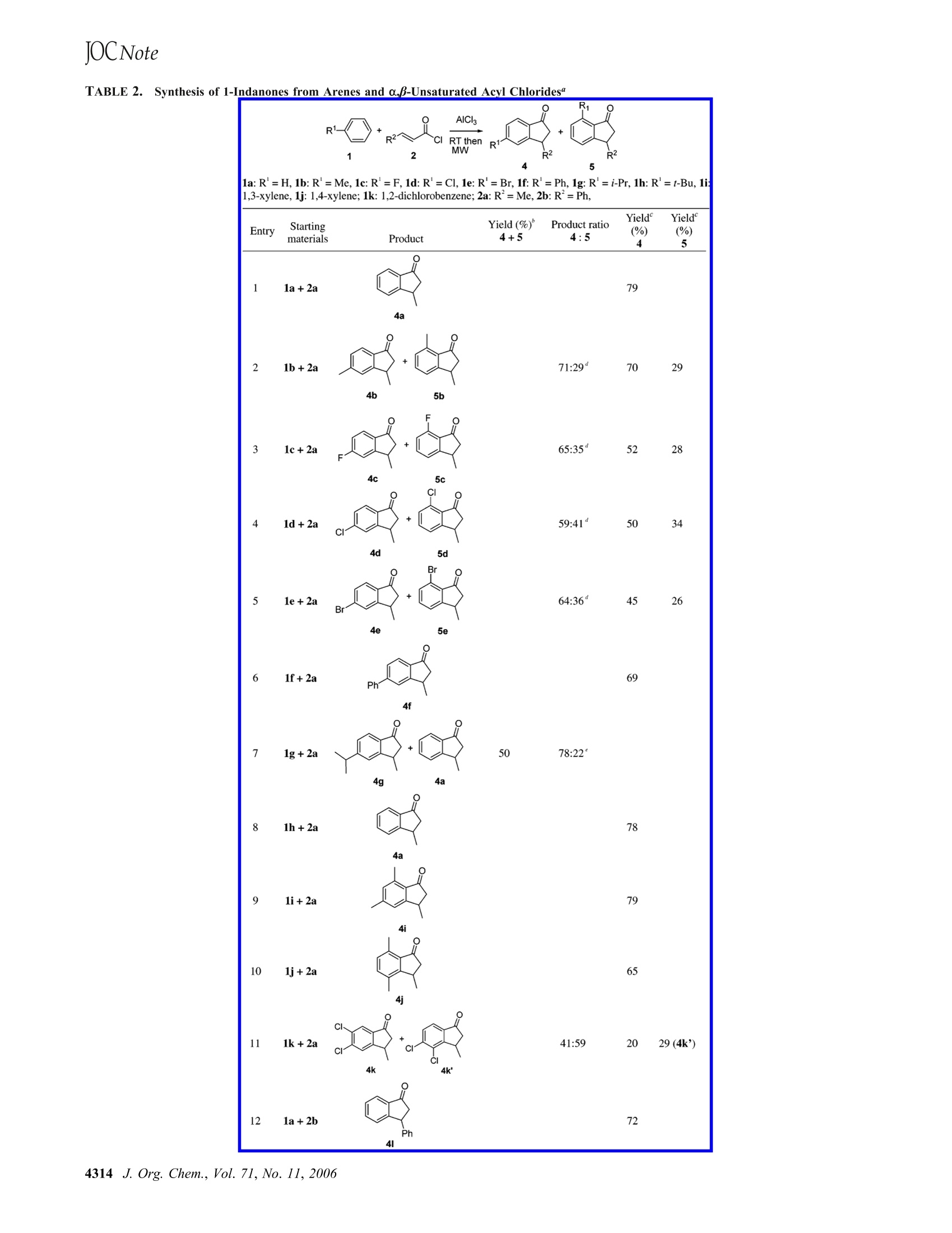
JOCNote Table 2 (Continued) Microwave-Assisted One-Pot Synthesis of1-Indanones from Arenes and a,B-UnsaturatedAcyl Chlorides Wei Yin,t Yuan Ma,*,t Jiaxi Xu,* and Yufen Zhaot Key Laboratory of Bioorganic Phosphorus Chemistry andChemical Biology, Ministry of Education, Department ofChemistry, Tsinghua University, Beijing 100084, People'sRepublic ofChina, and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, People’s Republic of China mayuan@mail.tsinghua.edu.cn Received January 5, 2006 A series of 1-indanones were synthesized in good yields viatandem Friedel-Crafts acylation and Nazarov cyclization ofarenes and a,B-unsaturated acyl chlorides in the presenceof aluminum chloride under microwave irradiation. 1-Indanones are important synthetic intermediates for phar-maceuticals and biologically active compounds and ligands ofolefin polymerization catalysts. There are numerous methodsavailable for the preparation of 1-indanones. The intramolecularFriedel-Crafts acylation of 3-aryl carboxylic acids or thecorresponding acyl chlorides catalyzed by Lewis acids and/orprotic acids4 has been one ofthe oldest and most widely usedapproaches because of its efficiency and convenience. Theeducts were prepared generally from arenes and o,B-unsaturatedcarboxylic acids. Actually, the intermolecular Friedel-Craftsreaction of arenes and a,B-unsaturated carboxylic acids has alsobeen attempted to prepare 1-indanones.1d,5,6 However, except ( * To whom correspondence should be addressed . Phone : +86-10-62792673.Fax: +86-10-62792673. ) ( TT singhua University. ) ( P eking University. ) ( ( 1 ) For examples, s e e: ( a ) Bogeso, K. P. ; Christensen, A. V.; Hyttel, J. ; Liljefors,T . 1 985,2 8 ,18 1 7-18 2 8. (b) Bogeso, K. P. ; A r n t, J.; Frederiksen, K.; Hansen, H. O.; Hyttel, J.; Pedersen,H.1995,38,4380-4392.(c) Sugimoto, H. 1 999, 71,2031- ) ( 2037.( d ) Guillon, J.; D a llemagne, P . ; Leger, J.-M.; S opkova, J . ; B ovy, P. R.; Jarry, C.; Rault,S. 2002, 1 0, 1 043-1050. ) ( (2) For examples, s ee: ( a) Schumann, H.; S t enzel, O.; G irgsdies, F . 2001,20,1 7 43-1751.(b) Herzog, M. N.; Chien, J. C. ) W.; Rausch, M. D. 2002,654,29-35. ( (3) For a recent summary, see: Wessig, P.; Gl o mbitza, C.; Muller, G.;Teubner, J. 2004,69,7582- 7 591. ) ( ( 4) For recent examples, see: (a) Premasagar, V.; Palaniswamy, V. A.;Eisenbraun, E. J. 1 981,46,2974-2976 and references therein. ) ( (b) Cui, D. M.; Zhang, C.; Kawamura, M . ; S h imada,S. 2004, 45, 1 741-1745.(c) Rendy,R.; Zhang, Y . ;M c Elrea, A . ; G omez, A.; Klumpp, D . A. 2004,69,2340-2347. ) for superacidic trifluoromethanesulfonic acid-induced cyclia-cyalkylation of arenes, competitive Friedel-Crafts alkylationand/or acylation led to mixtures of 1-indanones, a.,B-unsaturatedaromatic ketones, and 3-aryl carboxylic acids, with the latteras major products in most cases. Furthermore, Friedel-Craftsalkylation of 3-aryl a,B-unsaturated carboxylic acids affords 3,3-diaryl carboxylic acids, which will produce different structuralisomers during the subsequent cyclization when the two arylgroups are different. Because the acyl chloride predominantlyundergoes a Friedel-Crafts acylation rather than a Friedel-Crafts alkylation, to avoid competitive intermolecular Friedel-Crafts alkylation at the first step, active acyl chlorides shouldbe more suitable reactants instead of the corresponding acids.However, although some 1-indanones could be prepared in goodyields by the reaction of substituted benzenes and a,B-unsaturated acyl chloride under the catalysis of AlCls ormethanesulfonic acid,the substituted benzenes are limited tobenzene and electron-rich substituted benzenes.8 Microwave irradiation has been widely applied in organicsynthesis recently and achieved great success for many reac-tions, including Friedel-Crafts acylationsl0 and Nazarovcyclizations,l with high efficiency and yields. Herein, wepresent our results on the microwave-assisted one-pot synthesisof 1-indanones from substituted benzenes and a,B-unsaturatedacyl chlorides in good yields. First, we used identical conditions as reported by Koelsch etal. with crotonic acid and benzene as starting materials.However, we failed to achieve the literature’s yield (81.5%; avery low yield was obtained) in repeating the reaction severaltimes. Instead, crotonic acid was recovered in all cases. Toimprove reactivity, we used more active crotonoyl chlorideinstead of crotonic acid and obtained a mixture of 3-methyl-1- ( (5) Surya Prakash, G. K .; Yan, P.; Toeroek, B .; Olah, G. A . C a t a l L 2003, 87, 109-112. ) ( (6) Klumpp, D . A.; Rendy, R.; Zhang, Y.;Gomez, A .; McElrea, A. Org. Le t t . 2004 , 6 , 1789-1792. ) (7)(a) Johnston, K. M.; Shotter, R. G. Tatrom 1974,30,4059-4064. (b) Shotter, R. G.; Johnston, K. M.; Jones, J. F.Tatuaad 1978. 34, 741-746.(8)(a) Ready, T. E.; Chien, J. C. W.;Rausch,M.D.1999,583,11-27.(b) Ranu, B. C.; Jana, U. 1999, 64,6380-6386.(c) Camps, F.; Coll,J.; Colomina, O.; Messeg七uer, A.Oro Chom 1984.39B.1801-1805.(d) Boberg. F.; Deters, K.; Schulz, J.;Torges, K. 1994, 91, 69-80. (e)Jefferson, A.; Wangchareontrakul, S.Il 1985, 38, 605-614.(f) Bhattacharyya, S.;Basu, B.; Mukherjee, D.Totowll. 1983,39,4221-4224. (g) Dupin, J. F. E.; Chenault, J. oo一al.1983,20,2401-2404.(h) Miyamoto, T. K.; Tsutsui, M.; Chen, L.Clhom Lott..1981,729-730.(i) Conover, L. H. 1953,75,4017-1020.(j) Colonge,J.; Daunis, H. 1961,2238-2241.(k) Granger, I.;Corbier, M.; Vinas, J.; Nau, P. 1957,810-814.(1)Knorr, I; Lattke,E.; Raepple, E. 1980, 1207-1215.(9) For recent reviews. see: (a)Kappe,,C. O.; Stadler, A. In Mionefrom the series, Methods andprinciples in Medicinal Chemistry; Mannhold, R.,Kubinyi, H., Folker, G.,Eds.; Wiley-VCH: Weinheim, 2005; Vol.25. (b) Haves.B. AL.LLl.Acta 2004, 37,66-77. (c) Loupy, A., Ed.Wiley-VCH: Weinheim, 2002. For recent examples, see: (d) Liang, Y.;Jiao, L.; Zhang, S. W.; Xu, J. X.CL2005,70,334-33.27. (e)Wetter, C.; Studer, A. 2004,174-175.(10) Paul, S.; Nanda, P.; Gupta, R.; Loupy, A. SSuntlaosic 2003,2877-2881 and references therein.(11) Lawrence,N. J.; Armitage, E. S. M.; Greedy, B.; Cook, D.; Ducki, S.;McGown, A. T. 2006,47,1637-1640. (12) Koelsch, C. F.; Hochmann, H.; Le Claire, C. D.1943,65,59-60. AlCl reaction yield of yield of entry solvent conditions reaction time 3(%) 4a(%) 1 PhH reflux 5h 55 trace 2 CS2 reflux 5h 42 3 PhNO2 reflux 5h 4 PhNO2 80°C 5h 3 11 5 PhNO2 80°C 16h 12 6 PhNO2 80°C 18 h 10 7 PhNO2 rt+80°Ca 5h+18h 22 8 PhNO2 rt+80°C 24h+18h 22 9 0-C6H4Cl2 rt+80°C 5h+18h 41 10 o-C6H4Cl2 MW6 3x 1.5 min 36 11 o-C6H4Cl2 MW 5× 5 min 58 12 o-C6H4Cl2 rt+MWb 5h+5x 5min 77 3 o-C6H4Cl2 rt+MW 5h+10 ×2 min 79 a Stirred at rt for 5 h and then at 80 C for 18 h.Irradiated undermicrowave at185°C at intervals. It takes about 30 s to achieve 185 °Cunder microwave conditions. indanone, 1-phenyl-but-2-en-1-one, and 1,3-diphenyl-1-butanone(23% yield, not shown in Table 1) (entry 1, Table 1). Tosuppress the Friedel-Crafts alkylation of 1-phenyl-but-2-en-1-one and benzene, we decreased the amount of benzene toequimolar amount of acyl chloride. The one-pot reaction ofcrotonoyl chloride and benzene was selected as a model reactionto optimize the reaction conditions. The results are summarizedin Table 1. 1-Phenyl-2-buten-1-one was obtained as a majorproduct under refluxing in carbon disulfide as solvent. Toimprove the reaction temperature, nitrobenzene was used assolvent. Under refluxing conditions, the reaction mixturedarkened, but no desired product was found. Lowering thereaction temperature to 80 °C provided the desired 3-methyl-1-indanone (4a), albeit in low yields. The yield of 4a wasimproved when the solution of crotonoyl chloride and benzenein nitrobenzene was stirred at room temperature for 5 h andthen heated at 80 °C for 18 h. A longer reaction time at roomtemperature of 24 h did not improve yields (entries 7 and 8,Table 1). Under the same conditions, but using o-dichloroben-zene as solvent, the yield of 4a was remarkably increased (entry9, Table 1). The yield was further improved when the reactionmixture was irradiated in a microwave field for 5 × 5 min (entry11, Table 1). Finally, an optimized yield of 79% was achievedunder microwave irradiation (10 x2 min) at intervals. Pulsedmicrowave irradiation gave higher yields than irradiating thereaction mixture continuously for the same period of time. Thisphenomenon has also been discovered by other investigators,although the reason is not stated.13 In our case, the continuousirradiation overheated the reaction mixture and caused blacktarlike products. In recent years, an alternative method forperforming microwave-assisted organic reactions, termed“en-hanced microwave synthesis”(EMS), has been examined.9b Inthis method, microwave irradiation is administered whilesimultaneously externally cooling the reaction vessel withcompressed air. It enables a greater amount of microwave energyto be introduced into a reaction while keeping the reactiontemperature low.14 This may offer an alternative operation tothe pulsed approach. After successful optimization, we expanded the scope of thereaction to various substituted benzenes (Table 2). Halobenzenes 1c-e expectedly provided both structural isomers 4 and 5. Mostof them could be chromatographically separated and character-ized via 'H NMR. The 3.5-disubstituted isomers 4 were isolatedas the major products, clearly indicating that the initial Friedel-Crafts acylation occurred predominantly para to the substituent.Biphenyl 1f provided the 3,5-isomer 4f exclusively. Toluene1b provided both isomers 4b and 5b. Interestingly, with thehigher alkyl benzene homologues ethyl benzene (not shown),isopropyl benzene (1g), and tert-butyl benzene (1h), an increas-ing rate of concomitant dealkylation was observed. Conse-quently, ethyl benzene provided a mixture of the 5- and7-isomers accompanied by 4a. 1g reacted to form a mixture of4g and 4a, whereas 1h suffered from complete dealkylationproviding 4a in 78% yield (entries 7 and 8, Table 2). Second, 1a and halobenzenes 1c-e were reacted withcinnamoyl chloride 2b under optimized conditions providingmixtures of the 5- and 7-isomers of the desired 1-indanones inyields of 72-97%. When reacting the less-reactive halobenzenes with 2a or 2b,small amounts of dichloro-1-indanones were detected via massspectroscopy, indicating that the solvent participated in thereaction. We evaluated the reactivity of the solvent by reactingo-dichlorobenzene with 2a and provided actually only two ofthree possible isomers. 5,6-Dichloro-3-methyl-1-indanone (4k)and 4,5-dichloro-3-methyl-1-indanone (4k') were obtained inyields of 20% and 29%, respectively, and were identified bytheir H NMR spectra and NOE experiments (see SupportingInformation). Both of them were formed from para acylationof o-dichlorobenzene and subsequent cyclization. Althougho-dichlorobenzene can react with crotonoyl chloride and cin-namoyl chloride, it does not affect the desired reaction in mostcases because of its lower reactivity. The reaction process proceeds as follows: first, Friedel-Crafts acylation between a substituted benzene and an a,B-unsaturated acyl chloride occurs (stirring the reaction mixtureat room temperature favors this process), and then an aromaticNazarov cyclization gives rise to a 1-indanone. Although thecyclization of aryl vinyl ketones generally is considered aNazarov cyclization15 and it is generally considered that anaromatic ketone hardly undergoes a Friedel-Crafts alkylation,the Friedel-Crafts alkylation of 1-phenyl-but-2-en-1-one andbenzene was observed (Table 1, entry 1). In the current case,the formed ketone 3 generally exists favorably in the s-cisconformation. Its AlCls-coordinated complex 6 should predomi-nately convert to the s-trans conformation 7 because of theincreasing size of the oxygen group. The carbon atom of theAlCl3-coordinated carbonyl group could act as a carbocationas suggested in a recent report,6 which could induce a C=Cbond rearrangement to form an allylic or an allylic and benzyliccarbocation 8. The enolic carbocation 8 could easily undergoan intramolecular Friedel-Crafts alkylation to yield an indene-type intermediate 9, which gives rise to the desired 1-indanone ( (13) Baxendale, I. R.; Lee, A.-L.; L e y,S. V.12002,1850-1857. ) (14) (a) Chen, J. J.; Deshpande, S. V. 2003, 44,8873- ( 8 876. (b) Humphrey, C. E.; Ea s son, M. A . M.; Tier n ey, J.P.; Turn e r, N. J . Oro Lett x .2003,5,849-852.(c) Kat r itzky,A. R.; Zhang, Y.; Singh, S. K.; Steel, P. J. Arkivoc.200 3 ,47-64. ) ( (15) Fo r recent reviews, see: ( a) Habermas,K. L. ; Denmark, S. E.; Jones, T . K. O ro R o a c t 1 994, 4 5 , 1 - 158. (b ) Pe l lissier, H . T 2005. 2005,61, ) ( 61, 6479-6517. (c) F r ontier, A . J .; C o llison, C. Tatmoo drom 7577-7606. ) ( (16) Xu, J . X .; Xia, J . K.; L a n, Y. 2005,35,2347- 2353. ) TABLE 2.Synthesis of 1-Indanones from Arenes and a.B-Unsaturated Acyl Chlorides" Entry Yield (%)' Product ratio Yield Yield Starting materials Product 4 + 5 4 : 5 (%) 4 (%) 5 F 13 1c+2b 79 41:59 30 4m Ph 5m Ph 1 14 1d+2b CI 88 56:44 45 4n Ph 5n Ph Br 15 1e+2b Br' Ph Ph 55:45 53 44 4o 5o a Stirred at rt for 5 h and then irradiated under microwave at 185 °C for 10 × 2 min at intervals. It takes about 30 s to achieve 185°C under microwaveconditions.Total yield of the mixture of 4 and 5. Isolated yields after column chromatography. dMeasured by lH NMR of the crude products. Measuredby HPLC analysis of the crude products on a ZORBAX Sil column (4.6×150 mm) with a mixture of hexane and 2-propanol (98:2, v/v) as eluent monitoredat 254 nm. SCHEME 1. Proposed Reaction Mechanism (ElectrophilicAttack at Para Position Shown) after hydrolysis and tautomerization (Scheme 1). It is impossibleto distinguish whether the cyclization is an aromatic Nazarovcyclization or an intramolecular Friedel-Crafts alkylation. Weconsidered it as a dual process. In conclusion, a series of 1-indanones were synthesized ingood yields via the tandem Friedel-Crafts acylation andNazarov cyclization of substituted benzenes and a,B-unsaturatedacyl chlorides in the presence of aluminum chloride undermicrowave irradiation. The current route is a practical andconvenient method for the synthesis of 1-indanone derivativesfrom simple starting materials in a one-pot reaction, albeit itshows low regioselectivity for toluene and halobenzene. Experimental Section General Procedure for the Preparation of 1-Indanones. Adried round-bottom flask (50 mL) equipped with a condenser cappedwith an anhydrous CaCl2 drying tube was charged with a solutionof arene 1 (3 mmol), a,B-unsaturated acyl chloride (3.3 mmol),and anhydrous aluminum chloride (9 mmol) in 6 mL of driedo-dichlorobenzene. The solution was stirred at room temperaturefor 5 h and then irradiated under microwave at 185°C for 10 x 2min at intervals. It takes about 30 s to achieve 185°C under microwave conditions. Between the two irradiations, the reactiontemperature was allowed to cool to about 60 °C. After hydrolysisby addition of concentrated hydrochloric acid (15 mL) and ice, theresulting mixture was extracted with diethyl ether (3× 25mL).The combined organic phase was washed by 2 mol/L HCl (30 mL)twice, water, saturated sodium bicarbonate, and water and driedover anhydrous sodium sulfate. After removal of solvent, the residuewas separated on a silica gel column with a mixture of petroleumether (60-90°C) and ethyl acetate (40:1 to 25:1, v/v)as eluent toafford the desired 1-indanone as product. To obtain pure product,most products were purified twice on the silica gel column. 5-Bromo-3-phenyl-1-indanone (40). Colorless crystals. Mp 98-100°℃. Ry= 0.25 [ethyl acetate/ petroleum ether (60-90℃) 1:15,v/v, silica gel plate]. IR (KBr) v (cm-): 1713.4(C=O). 1HNMR(300 MHz, CDCl3): 07.84-6.93 (m,8H), 4.58 (dd, J= 8.2, 3.8Hz, 1H), 3.23 (dd,J= 19.1, 8.2 Hz, 1H), 2.62 (dd,J=19.1,3.8Hz, 1H).13CNMR (75.5 MHz, CDCl3): 0 43.6,46.4,123.7,126.7,127.0,128.3,129.6,130.9,135.3,136.7,143.9, 156.5, 204.8. MS(EI) m/z (rel intensity, %):286 (M+, 84), 207 (M+ - Br,97),77(100). HRMS cacld for CisHnBrO (M+), 285.9993; found,285.9985. 7-Bromo-3-phenyl-1-indanone (50). Colorless crystals. Mp 68-70 °C. R = 0.38 [ethyl acetate/ petroleum ether (60-90℃) 1:15,v/v, silica gel plate]. IR (KBr) v(cm-): 1713.4(C=0). IH NMR(300 MHz, CDCl3):0 7.83-7.11 (m,8H),4.58 (dd,J=7.9,3.7Hz, 1H), 3.23 (dd,J=19.3, 7.9 Hz, 1H), 2.69 (dd,J=19.3, 3.7Hz, 1H). 13C NMR (50 MHz, CDCl3): 0 44.4,46.8,123.4, 126.8,126.9,127.6,127.8,128.9,135.0,136.7,143.6,157.9,205.9. MS(EI) m/z (rel intensity,%):286 (M+,7), 208 (M+- PhH,100),207(M+-Br, 27),193 (12),179(38),165(27),77(18). HRMScacld for C1sHBro (M+), 285.9993; found, 285.9985. Acknowledgment..This work was supported in part byTsinghua University. Supporting Information Available: The experimental details,the analytical data of products 4 and 5, lH NMR and 13C NMRspectra for the unknown 1-indanones 4g, 4k, 4k', 4m, 4o, 5c-e,and 50, and the H NMR NOE spectrum for the unknown1-indanone 4k'. This material is available free of charge via theInternet at http://pubs.acs.org. ( JO060022P ) jo CCC: $O American Chemical SocietyPublished on Web . Org.Chem. J. Org. Chem, Vol. No.
确定



还剩2页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《南瓜果肉中胡芦巴碱检测方案(微波萃取仪)》,该方案主要用于蔬菜中营养成分检测,参考标准--,《南瓜果肉中胡芦巴碱检测方案(微波萃取仪)》用到的仪器有电脑微波催化合成萃取仪 祥鹄XH-100A
推荐专场
相关方案
更多
该厂商其他方案
更多