方案详情
文
The degradation of monochlorobenzene (MCB) was assessed in a constructed wetland treating MCB contaminated groundwater using a detailed geochemical characterisation, stable isotope composition analysis and in situ microcosm experiments. A correlation between ferrous iron mobilisation, decreasing MCB concentration and enrichment in carbon isotope composition was visible at increasing distance from the inflow point, indicating biodegradation of MCB in the wetland. Additionally, in situ microcosm systems loaded with 13C-labelled MCB were deployed for the first time in sediments to investigate the biotransformation of MCB. Incorporation of 13C-labelled carbon derived from the MCB into bacterial fatty acids substantiated in situ degradation of MCB. The detection of 13C-labelled benzene indicated reductive dehalogenation of MCB. This integrated approach indicated the natural attenuation of the MCB in a wetland system. Further investigations are required to document and optimise the in situ biodegradation of MCB in constructed and natural wetland systems treating contaminated groundwater.
方案详情

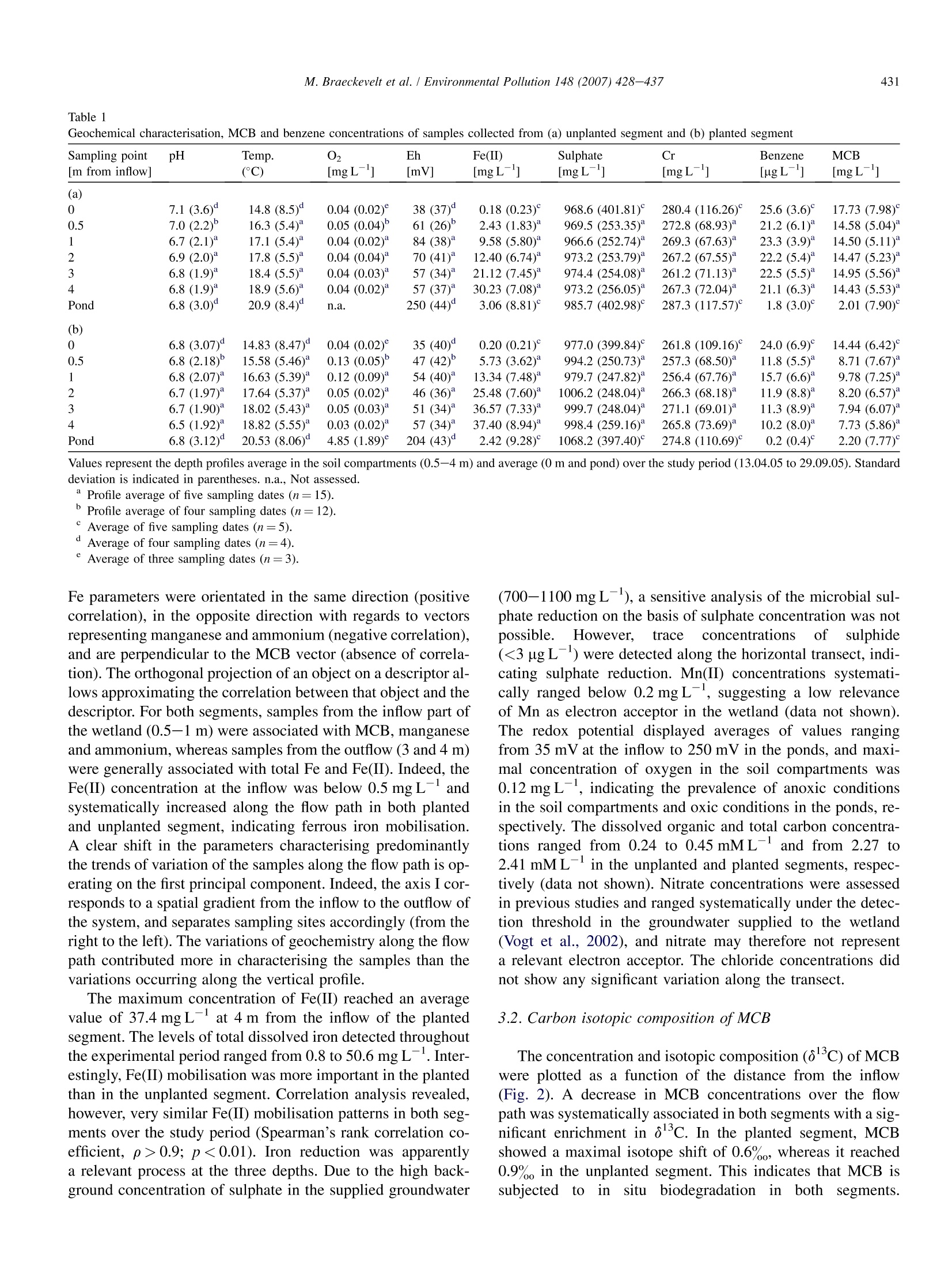
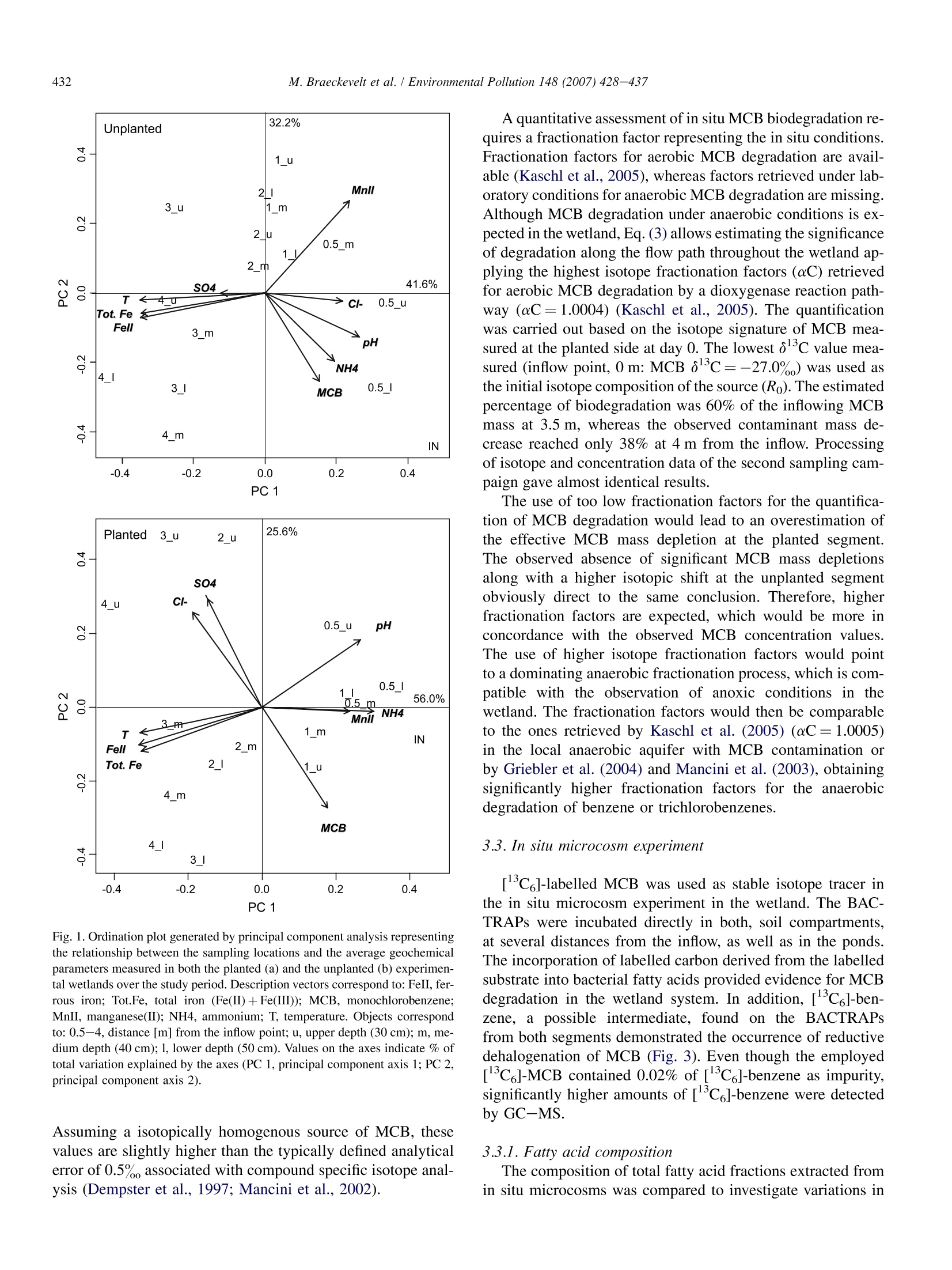
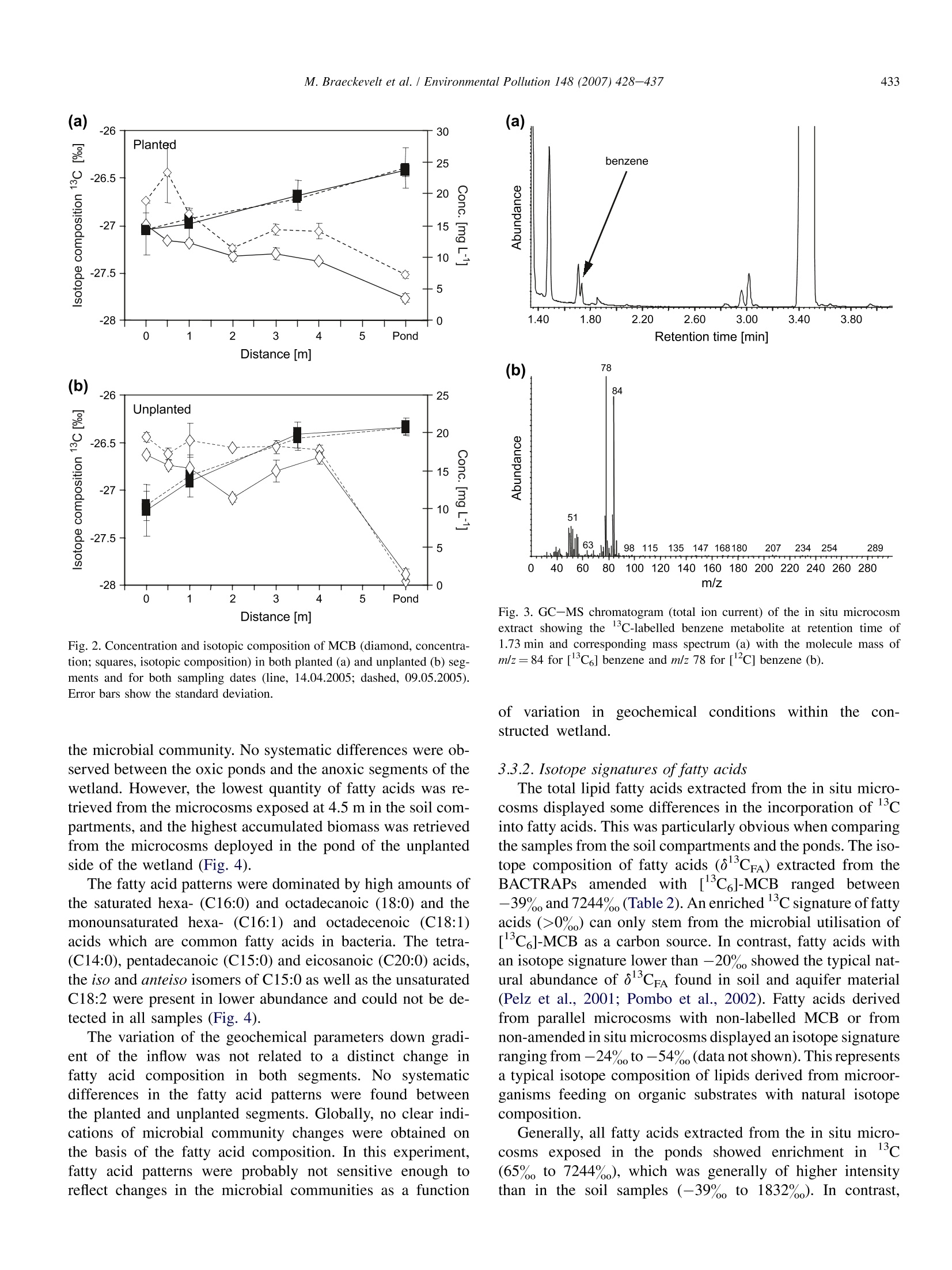
Available online at www.sciencedirect.comEnvironmental Pollution 148 (2007) 428-437 429M. Braeckevelt et al. / Environmental Pollution 148 (2007) 428-437 ENVIRONMENTALPOLLUTION www.elsevier.com/locate/envpol Assessment of in situ biodegradation of monochlorobenzene incontaminated groundwater treated in a constructed wetland Mareike Braeckevelt, Hemal Rokadia , Gwenael Imfeld b.*,Nicole Stelzer b,Heidrun Paschke, Peter Kuschk, Matthias Kastner , Hans-H. Richnow , Stefanie Weber ADepartments of Bioremediation, Helmholtz Centre for Environmental Research -UFZ, Permoserstrasse 15, Leipzig D-04318, Saxonia, Germany ° Department of Isotope Biogeochemistry, Helmholtz Centre for Environmental Research-UFZ, Permoserstrasse 15, Leipzig D-04318, Saxonia, Germany Department of Analytical Chemistry, Helmholtz Centre for Environmental Research-UFZ, Permoserstrasse 15, Leipzig D-04318, Saxonia, Germany Received 2 June 2006; received in revised form 27 November 2006; accepted 11 December 2006 An integrated approach including isotope composition analysis and in situ microcosm experimentsprovided evidences for in situ biodegradation of MCB in a wetland system. Abstract The degradation of monochlorobenzene (MCB) was assessed in a constructed wetland treating MCB contaminated groundwater using a de-tailed geochemical characterisation, stable isotope composition analysis and in situ microcosm experiments. A correlation between ferrous ironmobilisation, decreasing MCB concentration and enrichment in carbon isotope composition was visible at increasing distance from the inflowpoint, indicating biodegradation of MCB in the wetland. Additionally, in situ microcosm systems loaded with C-labelled MCB were deployedfor the first time in sediments to investigate the biotransformation of MCB. Incorporation of C-labelled carbon derived from the MCB intobacterial fatty acids substantiated in situ degradation of MCB. The detection of C-labelled benzene indicated reductive dehalogenation ofMCB. This integrated approach indicated the natural attenuation of the MCB in a wetland system. Further investigations are required to doc-ument and optimise the in situ biodegradation of MCB in constructed and natural wetland systems treating contaminated groundwater.@2007 Elsevier Ltd. All rights reserved. Keywords: Constructed wetland; Monochlorobenzene; Biodegradation; In situ microcosms; Isotopic fractionation 1.Introduction Monochlorobenzene (MCB) is encountered worldwide asa groundwater pollutant, and persists in the essentially anaer-obic aquifer at the large-scale contaminated site in Bitterfeld,Germany (Heidrich et al., 2004; Wycisk et al., 2004). In recentyears, interest has grown in using phytoremediation processesfor the elimination of recalcitrant organic substances from waste- and groundwater (Macek et al.,1998; Schnoor et al.,1995; Shimp et al., 1993; Trapp, 2000) including chloroaro-matics (Gilbert and Crowley, 1997). Wetland systems repre-sent an effective and inexpensive option to treat groundwaterpolluted with organic compounds by taking advantage of thegeochemical and biological processes (e.g. Baker, 1998; Dun-babin and Bowmer, 1992; Gumbricht, 1993). Indeed, rapiddegradation of chlorinated organics has been observed in therhizosphere (Anderson anddWalton, 1995; Jordahl et al.,1997; Lorah and Olsen, 1999; Pardue et al., 1996). ( * Corresponding author. Tel.: + 49 0341 235 2638; fax: + 4 9 0341 2 3 5 2492.E-mail a ddress: gwenael.im f eld@ufz . de (G. Imfeld). ) ( 0269-7491/$- se e fr o nt matter @ 200 7 Else v ier Ltd. All r ights reserved.doi:10.1016/j.envpol.2006.12.008 ) While aerobic degradation of MCB has been well studied(e.g. Van Agteren et al., 1998), only some evidence for MCB transformation under anoxic conditions has been presented yetand the degradation pathway is unknown (Kaschl et al.,2005;Liang and Gribic-Galic, 1990; Nowak et al.,1996). Moreover,only very few studies focus on the anaerobic microbial transfor-mation of MCB under field conditions. Recently, indications ofanaerobic MCB degradation taking place in the Bitterfeld con-taminated aquifer were provided on the basis ofisotope fraction-ation patterns (Kaschl et al., 2005). Kinetic isotope fractionationprocesses have been employed to demonstrate the biologicaltransformation of various contaminants (Richnow et al.,2003a,b;Sherwood Lollar et al., 2001; Song et al., 2002). A sub-stantial enrichment of c in the non-degraded fraction in thecourse of a contaminant plume indicates microbial degradation,as dilution and sorption do not affect the isotope composition ofcontaminants significantly (Harrington et al., 1996; Schuthet al., 2003; Slater et al., 2000). Combining stable isotope com-position analysis with information obtained from simple in situmicrocosm experiments (BACTRAPs) using isotope labelledsubstrate may provide a suitable approach to qualitatively sup-port in situ biotransformation and to monitor spatial and tempo-ral natural attenuation processes.Previously, BACTRAPs wereexclusively installed in groundwater monitoring wells (Geyeret al., 2005; Kastner et al., 2006; Stelzer et al., 2006) and weredeployed in sediment for the first time in the framework ofthis study. For the assessment of in situ biodegradation in constructedwetlands and wetlands treating contaminated groundwater, itmay be necessary to use several methods providing more thanone line ofevidence. A combined approach may be of additionalbenefit in particular when systems are complex, possess severalcompartments and convincing evidence is required. Moreover,a better understanding of the controlling geochemical processesin wetland systems is necessary to reliably predict the retentionand transformation of contaminant. In this study, we evaluatedthe natural attenuation of MCB in a constructed wetland treatingMCB contaminated groundwater using a detailed geochemicalcharacterisation, stable isotope composition analysis and insitu microcosm experiments. The spatial variations of geochem-ical parameter were studied with the help of multivariate statis-tics to investigate the main processes controlling the wetlandsystem. The concentration and carbon stable isotope composi-tion of MCB was analysed to monitor the in situ contaminantdegradation and in situ microcosms were used to provide qual-itative evidences of in situ biotransformation of MCB. 2. Materials and methods 2.1. Design and characteristics of the wetland The pilot scale constructed wetland at the experimental site in Bitterfeldwas set up in December 2002. The horizontal subsurface flow wetland con-sisted of a stainless steel tank divided into two segments. Each segment was6m×1 m and was filled to an average depth of 0.5 m with autochthonousquaternary aquifer material consisting predominantly of Bitterfeld mica sand(25%) and gravel (67%), which was embedded in lignite (10%) with an effec-tive porosity of 28% (Vogt et al., 2002). The hydrogeochemical characteristicsof the study site and the filling material originating from the local aquifer aredescribed in previous studies (e.g. Vogt et al., 2002; Weiss et al., 2001). One ofthe segments was planted with common reed (Phragmites australis, Cav.), whereas the other side was left unplanted. In both segments, a 1 m longopen water pond at the outflow side allows direct contact between the atmo-sphere and the water surface. The water level was maintained at approximately10 cm below the surface of the wetland. The groundwater was collected fromthe MCB contaminated aquifer and conveyed from 16 to 22 m depth directlyto the wetland. Both segements were operated in a flow-through mode at a flowrate of 4.7Lh, corresponding to a retention time of 6 days. 2.2. Sampling In the period from April to September 2005, pore water samples were col-lected five times (d0, d53, d66, d143, d172) in order to investigate the geo-chemical processes and the contaminant behaviour in the wetland system.The pore water was collected in both segments along a transect from the inflowup to the outflow of the wetland, at respectively 0 (inflow valves), 0.5, 1,2, 3and 4 m using a stainless steel lance. At each sampling point, three depths, 30,40 and 50 cm were systematically investigated. Water samples were also col-lected at the ponds (6 m). In addition, to assess the in situ biodegradation using isotope compositionanalysis, pore water samples from both segments were collected at day 0 andday 53, at 0, 1 and 3.5 m along the wetland at 0.5 m depth, as well as in theponds. 2.3. Physico-chemical and geochemical parameters of the porewater samples The redox potential was measured on-line in the field using a SenTix ORPelectrode (PT 1000, PreSens, Regensburg, Germany). The temperature wasdetermined by a temperature sensor (PT 1000, PreSens, Regensburg, Germany).Samples for the pH analysis and the quantitative ions were filtered through a5-um syringe filter (Ministart NML, Sartorius) for particle removal. The pHvalue was measured with a SenTix41 electrode with pH 537 Microprocessor(WTW, Weilheim, Germany). Oxygen measurement was carried out usingan optical oxygen trace sensor system (oxygen meter Fibox-3-trace andflow-through cell type sensor FTC-TOS7) with automatic temperature com-pensation (temperature sensor PT 1000) (PreSens, Regensburg, Germany).For the analysis of Mn(II), total iron and Fe(II), hydrochloric acid was addedand samples were diluted with deionised water (1:10, v:v). Total iron andMn(II) concentrations were analysed by atomic emission spectrometry withICP excitation and CCD detection (Spectro Ciros Vision CCD, Spectro Ana-lytical Instruments, Kleve, Germany). Photometric analysis of ferrous ironwas carried out at 562 nm after derivatisation with ferrocin using a Cadas100 photometer (Hach Lange, Diisseldorf, Germany). Chloride and sulphateconcentrations were determined by ion chromatography (DX 500) with con-ductivity detection (CD 20) and an IonPacAG11 (4×250 mm) column (Dio-nex Corporation, Sunnyvale, USA). For the analysis of sulphide concentrationssamples were spiked with sulphide anti-oxidant buffer (200 ml L-10 MNaOH, 35 gL- ascorbic acid, 67 gL-EDTA) (1:1, v:v) and measuredwith an ion selective Ag/S 500 electrode and reference electrode R 503(WTW, Weilheim, Germany). 2.4. Analysis of benzene, MCB and metabolites Pore water samples for the analysis of benzene and MCB concentrationswere collected in 20 mL glass flasks (Supelco, Bellefonte, USA), and sealedwith Teflon-lined septa. Sodium azide solution was added to the samples toinhibit microbial activity. Benzene and MCB concentrations were quantifiedby automatic headspace gas chromatography using an HP 6890 gas chromato-graph with flame ionisation detector (Agilent Technologies, Palo Alto, USA).For headspace analysis a volume of 1000 ul was injected at an injection tem-perature of 250 °C with split 1:5 (measurements in duplicates). The chromato-graphic separation was achieved on an HP-1 capillary column (AgilentTechnologies, Palo Alto, USA) (30 m×0.32 mm ×5 pm) with the followingoven temperature program: 45℃ (1 min), 20°C min-to 200C (2.5 min),65°C min- to 250℃ (1 min) and a detector temperature of 280℃. For the determination of the carbon isotope composition of MCB, 1 L glassbottles (Schott, Mainz, Germany) containing NaOH pellets to prevent microbial growth were filled completely with groundwater, stored at 4°C andextracted within 24 h using 2 mL n-pentane as described previously (Richnowet al.,2003b). The analysis of volatile metabolites obtained in the in situ mi-crocosm experiments was carried out using an HP 6890 gas chromatographwith HP 5973 mass spectrometer (Agilent Technologies, Palo Alto, USA).Aliquots of 1 pl liquid samples were injected at a temperature of 280°C withsplit 1:40 and separated on a Zebron BPX-5 column (30 m x 0.32 mm ×0.25 um)(Phenomenex, Torrance, USA). The following oven temperature programwas applied: 40℃(2.5 min), 10°C min-to 70℃ (0 min), 60C min- to280°C (4 min). 2.5. In situ microcosms (BACTRAPs) 2.5.1. Preparation of microcosms and derivatisation of fatty acids The in situ microcosms were prepared as described previously (Stelzeret al., 2006). Different sets of in situ microcosm experiments were prepared.One set was loaded with [C ]-labelled MCB (Cambridge Isotope Laborato-ries,Andover, USA) and another one with natural abundance MCB. A third setwas kept unloaded to observe the background effects. The loading was donevia gas phase under reduced pressure with approximately 40 mg MCB per gBio-Sep. The microcosms were deployed at 1.5, 2.5 and 4.5 m from the in-flow in both planted and unplanted segments at 50 cm depth. The microcosmswere collected after 6 weeks and fatty acid extraction was carried out accord-ing to Bligh and Dyer (1959). The derivatisation to obtain fatty acid methylesters (FAME) was done according to Thiel et al. (2001). After evaporationto complete dryness and addition of heneicosanoic acid methyl ester(C21:0) as an internal standard the FAME fraction was dissolved in n-hexanefor further identification, structural characterisation and carbon isotope com-position analysis. 2.5.2. GC-MS analysis For identification and structural characterisation of FAME,an HP 6890 gaschromatograph coupled with an HP 5973 quadrupole mass spectrometer (Agi-lent Technologies, Palo Alto, USA) was used. The FAME were separated ona Zebron BPX-5 column (30 m ×0.32mm×0.25 um) (Phenomenex, Tor-rance, USA) with the following temperature program: 70℃ (1 min),20°C min-to 130°C,2°C minto 150C (5 min), 2°C minto 165°C(5 min), 2℃ min-to 230°℃, 20℃ min-to 300℃ (5 min). FAME wereidentified by comparing with the retention time and mass spectra of an authen-tic standard mix (bacterial acid methylesters mix, Sigma-Aldrich, Germany)and quantified relatively to the internal standard. 2.6. Isotopic composition analysis The carbon isotope composition of MCB and the FAME was measuredwith a gas chromatography-combustion-isotope ratio mass spectrometry sys-tem (GC-C-IRMS) consisting of a GC unit (HP 6890, Agilent Technologies,Palo Alto, USA), a combustion device (Finnigan MAT GC III, ThermoFinni-gan Bremen Germany) with water-removal assembly (Nafion membrane,50 cm long,T=0°C) and a mass spectrometer (Finnigan MAT 252; Thermo-Finnigan, Bremen, Germany), as previously described (Richnow et al., 2003a).Helium was used as carrier gas at a flow rate of 1.5 ml min. Stable isotope samples were measured in triplicates and the analyses werecarried out immediately after each sampling. Aliquots of 1 ul liquid sampleswere injected at 250°C with split 1:10 to the GC-C-IRMS and separatedon a capillary column (Zebron ZB-1, 60 mx0.32m×1 um; Phenomenex,Torrance, USA). The following chromatographic conditions were applied:injector temperature 250°C, oven temperature program: 40°C (1 min),4°C min-to 150°C, 20°C min to 250C (2 min). The carbon isotopecomposition is reported in the delta notation as o3c values [%] relative toVienna Pee Dee Belemnite Standard (V-PDB, IAEA-Vienna) (Eq. 1) (Hoefs,1997). Eq.(2) is applied to calculate the remaining substrate fraction (f ) using theisotope fractionation factor (aC). The R, and Ro give the isotope composition of MCB at time t and zero.Eq. (3) is applied to calculate the percentage of biodegradation of the residualsubstrate fraction (B,). For the separation of the FAME fractions, a Restek RTX 5 column(60 m×0.32 mm ×0.1 um; Restek, Bellefonte, USA) was used with thesame temperature program applied for the GC-MS-analysis of FAME. Ali-quots of 1 pl were injected with split 1:5. The methylation of fatty acids forgas chromatographic analysis introduces an additional carbon atom into thestructure of the fatty acid molecules which affects its isotopic composition.Therefore the isotope signature of fatty acids (8CFA) was corrected for theisotope effect upon derivatisation to FAME with methanol as described previ-ously (Abraham et al., 1998; Abrajano et al., 1994; Goodmann and Brenna,1992). The methanol used for the derivatisation had an isotope compositionof -38.2% 2.7. Statistical analysis Statistical analyses were carried out using the R Software (R, Version2.1.1, 2005). Statistical significance of the difference in geochemical parame-ters as well as concentrations and isotopic compositions of MCB between theplanted and unplanted segment was determined with the unpaired Wilcoxon orthe Kruskal-Wallis rank sum tests. Correlation analyses were carried out us-ing the Spearman rank sum coefficient. Principal component analysis (PCA)was used to analyse the relationship between the different samples with refer-ence to their respective pore water parameters. The sampling location corre-sponds to the object and the chemical parameters to the descriptors(represented by the vectors) of the multivariate analysis. The PCA were scaledas correlation biplots. 3.Results 3.1. Characterisation of pore water chemistry 3.1.1. Distribution of MCB and benzene The MCB concentration was measured as a function of thedistance from the inflow point in both the planted andunplanted segment (Table 1). The average amount of MCBranged from 14.4-17.7 mg L- at the inflow down to 2.0-2.2 mg L-in the ponds for the planted and the unplantedsegment, respectively. No significant difference in MCB con-centration among the three depths over the study period wasgenerally observed (p<0.05). Benzene was found in low con-centration in both segments (<26 pg L), with generallyhigher concentration values in the unplanted segment (Table 1). 3.1.2. Pore water geochemistry The evolution of oxygen, redox potential, manganese, sul-phide, sulphate, ferrous iron and total iron were monitoredalong the flow path at three depths in order to characterisethe geochemical conditions prevailing in the wetland system.The average values of the three depths investigated at eachsampling point (0, 0.5, 1,2, 3,4 m from the inflow) were com-puted (Table 1). To investigate the differences between thesampling locations and to explore existing gradients, the datasets were analysed by principle component analysis, separatelyfor the unplanted and the planted segments (Fig. 1). In bothcases, the vectors representing the temperature,Fe(II), and total Table i Geochemical characterisation, MCB and benzene concentrations of samples collected from (a) unplanted segment and (b) planted segment Sampling point pH Temp. 02 Eh Fe(II) Sulphate Cr Benzene MCB [m from inflow] (C) [mgL-] [mV] [mgL-] [mgL] [mg L-l] [ugL ] [mgL ] (a) 0 7.1(3.6)° 14.8(8.5) 0.04 (0.02) 38 (37)° 0.18(0.23) 968.6(401.81) 280.4 (116.26) 25.6(3.6) 17.73(7.98) 0.5 7.0(2.2) 16.3(5.4)* 0.05 (0.04)° 61(26) 2.43 (1.83) 969.5 (253.35) 272.8(68.93) 21.2(6.1) 14.58(5.04) 1 6.7(2.1)“ 17.1(5.4)* 0.04(0.02) 84(38)* 9.58(5.80) 966.6(252.74) 269.3(67.63) 23.3(3.9)* 14.50(5.11) 2 6.9 (2.0) 17.8(5.5) 0.04(0.04) 70(41) 12.40(6.74) 973.2(253.79) 267.2(67.55)° 22.2(5.4)* 14.47(5.23)* 3 6.8(1.9)* 18.4(5.5)* 0.04(0.03) 57(34)* 21.12(7.45) 974.4(254.08)° 261.2(71.13)* 22.5(5.5)* 14.95 (5.56) 4 6.8(1.9)* 18.9(5.6)* 0.04 (0.02) 57 (37)* 30.23(7.08)* 973.2(256.05)° 267.3(72.04) 21.1(6.3)* 14.43(5.53) Pond 6.8(3.0) 20.9(8.4) n.a. 250(44)° 3.06(8.81) 985.7(402.98) 287.3(117.57) 1.8(3.0)° 2.01(7.90) (b) 0 6.8(3.07)° 14.83(8.47) 0.04(0.02) 35 (40)" 0.20(0.21) 977.0(399.84) 261.8 (109.16) 24.0(6.9) 14.44(6.42)° 0.5 6.8(2.18) 15.58(5.46) 0.13 (0.05) 47 (42) 5.73(3.62)* 994.2(250.73) 257.3(68.50) 11.8(5.5)* 8.71(7.67) 1 6.8(2.07) 16.63 (5.39)* 0.12(0.09) 54(40)* 13.34(7.48)* 979.7 (247.82) 256.4(67.76)° 15.7(6.6) 9.78(7.25)° 2 6.7(1.97) 17.64(5.37)* 0.05(0.02)* 46 (36) 25.48 (7.60)* 1006.2(248.04)" 266.3(68.18) 11.9(8.8)* 8.20(6.57)* 3 6.7(1.90) 18.02(5.43)* 0.05(0.03) 51(34) 36.57(7.33)* 999.7 (248.04) 271.1(69.01) 11.3(8.9) 7.94(6.07) 4 6.5(1.92) 18.82(5.55)* 0.03 (0.02) 57(34)* 37.40(8.94) 998.4(259.16) 265.8(73.69) 10.2(8.0) 7.73(5.86) Pond 6.8(3.12) 20.53 (8.06) 4.85(1.89) 204(43) 2.42(9.28) 1068.2(397.40)° 274.8(110.69) 0.2(0.4) 2.20(7.77) Fe parameters were orientated in the same direction (positivecorrelation), in the opposite direction with regards to vectorsrepresenting manganese and ammonium (negativecorrelation),and are perpendicular to the MCB vector (absence of correla-tion). The orthogonal projection of an object on a descriptor al-lows approximating the correlation between that object and thedescriptor. For both segments, samples from the inflow part ofthe wetland (0.5-1 m) were associated with MCB, manganeseand ammonium, whereas samples from the outflow (3 and 4 m)were generally associated with total Fe and Fe(II). Indeed, theFe(II) concentration at the inflow was below 0.5 mg Landsystematically increased along the flow path in both plantedand unplanted segment, indicating ferrous iron mobilisation.A clear shift in the parameters characterising predominantlythe trends of variation of the samples along the flow path is op-erating on the first principal component. Indeed, the axis I cor-responds to a spatial gradient from the inflow to the outflow ofthe system, and separates sampling sites accordingly (from theright to the left). The variations of geochemistry along the flowpath contributed more in characterising the samples than thevariations occurring along the vertical profile. The maximum concentration of Fe(II) reached an averagevalue of 37.4 mg L- at 4 m from the inflow of the plantedsegment. The levels of total dissolved iron detected throughoutthe experimental period ranged from 0.8 to 50.6 mg L-. Inter-estingly, Fe(II) mobilisation was more important in the plantedthan in the unplanted segment. Correlation analysis revealed,however, very similar Fe(II) mobilisation patterns in both seg-ments over the study period (Spearman’s rank correlation co-efficient, p>0.9; p<0.01). Iron reduction was apparentlya relevant process at the three depths. Due to the high back-ground concentration of sulphate in the supplied groundwater (700-1100 mg L-), a sensitive analysis of the microbial sul-phate reduction on the basis of sulphate concentration was notpossible.. However, tracey concentrations ofsulphide(<3ug L-) were detected along the horizontal transect, indi-cating sulphate reduction. Mn(II) concentrations systemati-cally ranged below 0.2 mg L-, suggesting a low relevanceof Mn as electron acceptor in the wetland (data not shown)The redox potential displayed averages of values rangingfrom 35 mV at the inflow to 250 mV in the ponds, and maxi-mal concentration of oxygen in the soil compartments was0.12 mg L, indicating the prevalence of anoxic conditionsin the soil compartments and oxic conditions in the ponds, re-spectively. The dissolved organic and total carbon concentra-tions ranged from 0.24 to 0.45 mML- and from 2.27 to2.41 mMLin the unplanted and planted segments, respec-tively (data not shown). Nitrate concentrations were assessedin previous studies and ranged systematically under the detec-tion threshold in the groundwater supplied to the wetland(Vogt et al., 2002), and nitrate may therefore not representa relevant electron acceptor. The chloride concentrations didnot show any significant variation along the transect. 3.2. Carbon isotopic composition of MCB The concentration and isotopic composition (83c) of MCBwere plotted as a function of the distance from the inflow(Fig. 2). A decrease in MCB concentrations over the flowpath was systematically associated in both segments with a sig-nificant enrichment in ol3C. In the planted segment, MCBshowed a maximal isotope shift of 0.6%, whereas it reached0.9%。 in the unplanted segment. This indicates that MCB issubjected to in situbiodegradation inbothsegments. 32.2% Unplanted 3 u 2u 2_m SO4 T <4u 32.2% 1_u 2_1 Mnll1_m才 0.5_m1_ 41.6% Tot. FeS Fell3 m 4_I 3_1 4_m >Cl- 0.5_u pH NH4 MCB0.5_ IN -0.4 -0.2 0.0 0.2 0.4 PC 1 Planted 3_u 2_u SO4 4_uCI- 25.6% 0.5_u pH 0.5I110.5m56.0% 3_m-Fell 2_mTot. Fe 2_1 4_m 4_ 31 NH4MnlI 1_m IN 1_u MCB Fig.1. Ordination plot generated by principal component analysis representingthe relationship between the sampling locations and the average geochemicalparameters measured in both the planted (a) and the unplanted (b) experimen-tal wetlands over the study period. Description vectors correspond to: FeII, fer-rous iron; Tot.Fe, total iron (Fe(II)+Fe(III)); MCB, monochlorobenzene;MnII, manganese(II); NH4, ammonium; T, temperature. Objects correspondto: 0.5-4, distance [m] from the inflow point; u, upper depth (30 cm); m, me-dium depth (40 cm); l, lower depth (50 cm). Values on the axes indicate % oftotal variation explained by the axes (PC 1, principal component axis 1; PC 2,principal component axis 2). Assuming a isotopically homogenous source of MCB, thesevalues are slightly higher than the typically defined analyticalerror of 0.5% associated with compound specific isotope anal-ysis (Dempster et al., 1997; Mancini et al., 2002). A quantitative assessment of in situ MCB biodegradation re-quires a fractionation factor representing the in situ conditions.Fractionation factors for aerobic MCB degradation are avail-able (Kaschl et al., 2005), whereas factors retrieved under lab-oratory conditions for anaerobic MCB degradation are missing.Although MCB degradation under anaerobic conditions is ex-pected in the wetland, Eq. (3) allows estimating the significanceof degradation along the flow path throughout the wetland ap-plying the highest isotope fractionation factors (C) retrievedfor aerobic MCB degradation by a dioxygenase reaction path-way (aC=1.0004) (Kaschl et al., 2005). The quantificationwas carried out based on the isotope signature of MCB mea-sured at the planted side at day 0. The lowest olc value mea-sured (inflow point, 0 m: MCB 813c=-27.0%) was used asthe initial isotope composition of the source (Ro). The estimatedpercentage of biodegradation was 60% of the inflowing MCBmass at 3.5 m, whereas the observed contaminant mass de-crease reached only 38% at 4 m from the inflow. Processingof isotope and concentration data of the second sampling cam-paign gave almost identical results. The use of too low fractionation factors for the quantifica-tion of MCB degradation would lead to an overestimation ofthe effective MCB mass depletion at the planted segment.The observed absence of significant MCB mass depletionsalong with a higher isotopic shift at the unplanted segmentobviously direct to the same conclusion. Therefore, higherfractionation factors are expected, which would be more inconcordance with the observed MCB concentration values.The use of higher isotope fractionation factors would pointto a dominating anaerobic fractionation process, which is com-patible with the observation of anoxic conditions in thewetland.The fractionation factors would then be comparableto the ones retrieved by Kaschl et al. (2005)(αC=1.0005)in the local anaerobic aquifer with MCB contamination orby Griebler et al. (2004) and Mancini et al. (2003), obtainingsignificantly higher fractionation factors for the anaerobicdegradation of benzene or trichlorobenzenes. 3.3.In situ microcosm experiment [Co]-labelled MCB was used as stable isotope tracer inthe in situ microcosm experiment in the wetland. The BAC-TRAPs were incubated directly in both, soil compartments,at several distances from the inflow, as well as in the ponds.The incorporation of labelled carbon derived from the labelledsubstrate into bacterial fatty acids provided evidence for MCBdegradation in the wetland system. In addition, [Co]-ben-zene, a possible intermediate, found on the BACTRAPsfrom both segments demonstrated the occurrence of reductive.d1e3h,alogenation of MCB (Fig. 3). Even though the employed[c]-MCB contained 0.02% of [c]-benzene as impurity,significantly higher amounts of [C6]-benzene were detectedby GC-MS. 3.3.1. Fatty acid composition The composition of total fatty acid fractions extracted fromin situ microcosms was compared to investigate variations in 3厂 Retention time [min] Distance [m] 3厂二 Fig. 3. GC-MS chromatogram (total ion current) of the in situ microcosmextract showing the 1C-labelled benzene metabolite at retention time of1.73 min and corresponding mass spectrum (a) with the molecule mass ofm/z=84 for [3C] benzene and m/z 78 for [’c] benzene (b). Fig. 2. Concentration and isotopic composition of MCB (diamond, concentra-tion; squares, isotopic composition) in both planted (a) and unplanted (b) seg-ments and for both sampling dates (line, 14.04.2005; dashed, 09.05.2005).Error bars show the standard deviation. of variation in1geochemical conditionswithin the con-structed wetland. the microbial community. No systematic differences were ob-served between the oxic ponds and the anoxic segments of thewetland. However, the lowest quantity of fatty acids was re-trieved from the microcosms exposed at 4.5 m in the soil com-partments, and the highest accumulated biomass was retrievedfrom the microcosms deployed in the pond of the unplantedside of the wetland (Fig. 4). 3.3.2. Isotope signatures of fatty acids The fatty acid patterns were dominated by high amounts ofthe saturated hexa- (C16:0) and octadecanoic (18:0) and themonounsaturated hexa- (C16:1) and octadecenoic (C18:1)acids which are common fatty acids in bacteria. The tetra-(C14:0),pentadecanoic (C15:0) and eicosanoic (C20:0) acids,the iso and anteiso isomers of C15:0 as well as the unsaturatedC18:2 were present in lower abundance and could not be de-tected in all samples (Fig. 4). The variation of the geochemical parameters down gradi-ent of the inflow was not related to a distinct change infatty acid composition in both segments. No systematicdifferences in the fatty acid patterns were found betweenthe planted and unplanted segments. Globally, no clear indi-cations of microbial community changes were obtained onthe basis of the fatty acid composition. In this experiment,fatty acid patterns were probably not sensitive enough toreflect changes in the microbial communities as a function The total lipid fatty acids extracted from the in situ micro-cosms displayed some differences in the incorporation of 13cinto fatty acids. This was particularly obvious when comparingthe samples from the soil compartments and the ponds. The iso-tope composition of fatty acids (òCFA) extracted from theBACTRAPss amended with[c]-MCB ranged between-39%and 7244%。 (Table 2). An enriched C signature of fattyacids (>0%) can only stem from the microbial utilisation of[C]-MCB as a carbon source. In contrast, fatty acids withan isotope signature lower than -20%showed the typical nat-ural abundance of CFA found in soil and aquifer material(Pelz et al.,2001; Pombo et al., 2002). Fatty acids derivedfrom parallel microcosms with non-labelled MCB or fromnon-amended in situ microcosms displayed an isotope signatureranging from -24%。to-54%(data not shown). This representsa typical isotope composition of lipids derived from microor-ganisms feeding on organic substrates with natural isotopecomposition. Generally, all fatty acids extracted from the in situ micro-cosms exposed in the ponds showed enrichment in13C(65%。to 7244%), which was generally of higher intensitythan in the soil samples (-39% to 1832%). In contrast, Fig. 4. Absolute abundance of extracted fatty acids [ug FA per gram Bio-Sep"beads] from in situ microcosms exposed in the soil compartment at differentdistance from the inflow and ponds at the unplanted (a) and planted (b) seg-ments of the constructed wetland. only fatty acids with up to 16 carbon atoms showed C incor-poration in the soil samples with highest enrichment in C16species. Fatty acids with longer carbon chains such as C18:0or C18:1 displayed no enrichment in c (-22%to -39%),indicating that the microbial community was not exclusively growing on the [C]-MCB. Comparing the samples fromthe planted and unplanted segments no significant differencesin oCFA were observed. Therefore, the putative impact ofplants on the microbial community involved in MCB degrada-tion could not be assessed using our test system. Some fatty acids can be used as biomarker to identify spe-cific groups of microorganisms (Kaur et al., 2005; Zelles,1999). In some of the samples labelled iso and anteisobranched fatty acids with 15 carbon atoms could be identified,indicating that Gram-positive bacteria were involved in thebiodegradation of MCB. Labelled C18:2 was only found insamples of the ponds. Linoleic acids (C18:2) can serve asa biomarker for fungi or other eukaryotic organisms (Losel,1988) and their presence may lead to the hypothesis that graz-ing organisms, not involved in the biodegradation of MCB,may feed on the microbial biofilm. However, the appliedGC-MS procedure did not allow conclusive identification ofthe position of the double bonds. 4. Discussion The geochemical parameters indicated the overall preva-lence of anoxic conditions associated with iron mobilisationin the soil parts of the wetland, whereas an aerobic milieucharacterised the ponds. In the in situ microcosm experiments,the level of incorporation of labelled carbon into bacterial bio-mass was used as direct indicator of in situ MCB degradation.Interestingly, the analysis of the BACTRAPs incubated in theponds revealed fatty acids patterns and’C incorporationlevels differing from the ones retrieved from the soil compart-ments. The higher c incorporation level observed in theponds of both segments is indicative of a more effective micro-bial transformation of the [C]-MCB under the prevailingaerobic conditions. Along with a significant accumulation ofbiomass on the microcosms retrieved from the ponds, these re-sults suggest that some change in the microbial communitydynamics may operate between the anoxic soil compartmentsand the more aerobic ponds. Moreover, the fact that all theextracted fatty acids showed incorporation in13C suggests Carbon isotope composition of fatty acids extracted from in situ microcosms incubated for 6 weeks with C6 labelled monochlorobenzene at 1.5, 2.5 and 4.5 mfrom the inflow point as well as at the pond [m] From Planted 8CFA [%] Unplanted 8CFA [%] inflow fatty acid 1.5 2.5 4.5 Pond 1.5 2.5 4.5 Pond C13:0 n.d. n.d. n.d. n.d. n.d. n.d. n.d. 118 C14:0 -6 40 n.d. n.d. 138 204 116 238 iso C15:0 121 89 n.d. n.d. n.d. 742 n.d. 1597 anteiso C15:0 23 68 n.d. n.d. n.d. 3 n.d. n.d. C15:0 n.d. n.d. n.d. n.d. n.d. n.d. -37 252 C16:1 411 453 n.d. 7244 n.d. 1832 n.d. 806 C16:0 2 9 93 1711 260 95 192 639 C17:0 n.d. n.d. n.d. n.d. n.d. n.d. n.d. 242 C18:2 -31 -30 n.d. 65 n.d. -27 -30 216 C18:1 -30 -29 -28 276 -23 -23 -24 175 C18:0 -27 -28 -24 449 -22 -27 -26 266 C20:0 -37 -39 n.d. n.d. n.d. n.d. n.d. n.d. that the microbial community established on the microcosmswas mostly involved in contaminant degradation. However,it should be considered that cross-feeding by metabolites orrecycling of dead biomass within the biofilm may also channellabelled carbon into individual members of the microbial com-munity, explaining the variation in the labelling of specificfatty acids. In general, fatty acids displaying a higher incor-poration of 13c were very likely synthesised by organismsfeeding on [}c]-MCB, whereas organisms synthesisingnon-labelled fatty acids were likely not involved in the degrada-tion of the labelled MCB and used different carbon sources. Theanalysis of the composition of total fatty acid fractions showedthat this method might not be sensitive enough for investigatingdetail changes in microbial communities between soil and wa-ter compartments as well as down gradient the flow path. Additionally, the MCB degradation processes in the wet-land were investigated by carbon stable isotope compositionanalysis. A correlation between decreasing MCB concentra-tion and a shift in the carbon isotope signature towards theheavier isotope was visible along the flow path, suggestingthe degradation of MCB. Toxic effects of MCB on theMCB-degrading population can be reasonably excluded atthe observed range of concentration values (Fritz et al.,1992; Vogt et al., 2002,2004). In the transition from the soil compartments to the ponds,a substantial contaminant mass depletion without concomitantisotope enrichment was observed. In this open system, it islikely that part of the contaminant may partition into theatmosphere, affecting the MCB concentration values withoutgenerating a significant isotope shift. Moreover, under oxic-prevailing conditions, several bacteria have the ability to useMCB as sole carbon and energy source, and may putativelyadopt the well-known and described aerobic degradation path-ways (Van Agteren et al., 1998). These bacteria may degradeMCB at faster rate than the degrading bacteria associated withanoxic conditions, contributing to the observed contaminantmass decrease. These oxygen-driven degradation reactionswould lead to MCB mass decrease without a significant asso-ciated isotope effect (Kaschl et al., 2005). In parallel, biogeo-chemical processes such as oxidation of ferrous iron ormineral surfaces may compete for oxygen (Ehrlich, 1998;Spgaard et al., 2001; Warren and Haack, 2001), leading totransient conditions and oxygen gradients, which may affectthe composition of the existing microbial community andrate of degradation reactions. However, the anaerobic degrada-tion pathway of MCB is not elucidated yet. In the anoxic soil compartments, two major hypotheticaldegradation pathways would come into consideration: (1)reductive dechlorination of MCB; and (2) degradation of MCBas an electron donor molecule. First, an initial dechlorinationof MCB, followed by the degradation of benzene, is likely tooccur in the soil compartments. Indeed, the presence of 13C-labelled benzene detected in all the microcosms along withthe detection of low benzene concentrations suggested thatMCB is degraded reductively to benzene under anoxic condi-tions prior mineralisation. This pathway would suggest a simi-lar pattern as observed previously by Nowak et al. (1996). The pH values were, however, constant, and the relatively highbackground level of chloride in the groundwater hindered thedirect verification of MCB degradation by an increasing chlo-ride concentration along the flow path. Bacterial breakdown ofbenzene could lead to the formation of benzoate or phenol asintermediates (Chakraborty and Coates, 2005; Edwards andGrbic-Galic, 1992; Lovley, 2000; Phelps et al., 2001; Ulrichet al.,2005), which were not detected in this study. Soluble or-ganic carbon species, which could serve as electron donors fordechlorination processes, were found in low concentration inthe unplanted segment, which might effectively limit the extentof degradation. Conversely, dechlorination activity in theplanted segment may be partly related to the abundance of hy-drogen and reduced organic acids such as acetate and propio-nate (Holliger et al., 1992; Middeldorp et al., 1997). Reductive dechlorination reaction of MCB to benzene isexpected to be associated with a pronounced primary isotopeeffect (Griebler et al., 2004). In contrast, the isotope composi-tion presented in this study displayed a slight but significantenrichment ranging from 0.4 to 0.7 8 units, in the soil com-partments of the planted and the unplanted segments, respec-tively. However, the isotope effect at zones of preferential insitu degradation reaction can be substantially higher. If othernon-fractionating processes such as sorption and volatilisationalso contribute to a decrease in MCB concentrations, the iso-tope effect upon in situ degradation is expected to be relativelyhigh. The observed isotope effect points to dominating anaer-obic processes, as inferred by the estimation of biodegradationlevels over the flow path. In the planted segment, the oxygensupplied by the plant at the rizosphere level may favour theestablishment of aerobic zones. Although this process is notrelevant for the electron budget, oxygen may contribute tothe MCB degradation reactions, leading to MCB decreasewithout concomitant isotope effect. A fractionating anaerobicprocess and a less fractionating aerobic process may both con-tribute to in situ degradation, resulting in a mixed overall frac-tionation at the planted segment. Conversely, the isotopiccomposition shift observed at the unplanted segment suggeststhe occurrence of a more fractionating process. Alternatively, MCB may be degraded as an electron donormolecule under ferric iron- or sulphate-reducing conditions.Anaerobic oxidation of benzene under these conditions hasbeen previously observed (Anderson et al., 1998; Andersonand Lovley, 2000). For instance, geochemical footprints ofiron reduction processes were found, and the ferrous iron mo-bilisation was increasing as a function of the flow path. Dis-solved Fe in pore waters can be a result of differentprocesses such as Fe(III) reduction (Lovley, 1991,1997), py-rite oxidation (Lord and Church, 1983), or Fe complexation(Luther et al., 1996). Fe(II) may be precipitated with sulphideoriginating from sulphate reduction activity or form com-plexes. Therefore the concentrations may not reflect the trueextent of iron reduction in the presence of sulphate reduction.A low extent of sulphate reduction process and the availabilityof reactive iron may prevent accumulation of H2S in the near-neutral conditions of the wetland. A MCB mineralisation bysulphate reduction contributing to the contaminant mass decrease is feasible and may be possible. However, due to thehigh concentrations of sulphate in the supplied water, a reliableestimation of the extent of sulphate reduction could not be car-ried out. However, a reduction of ferric iron directly linkedstoichiometrically to MCB oxidation in the wetland may the-oretically account for a MCB mass decrease of about 18% be-tween the inflow and the outflow of the system at both theplanted and unplanted segment. Additionally, other unknown degradation pathways cannotbe excluded. These hypotheses will require further detailed in-vestigations, along with further isolation and identification ofmicroorganisms involved in the anaerobic MCB degradationin wetlands treating MCB contaminated water. 5. Conclusion The integrated approach provided evidence for in situ MCBbiodegradation in both, soil compartments and ponds of theplanted and unplanted segments of a horizontal subsurfaceflow constructed wetland. This was supported by isotopic frac-tionation analysis, combined with in situ microcosm experi-ments, which can be utilized to document further the in situdegradation of MCB and other contaminants in wetland sys-tems. Further investigations to elucidate the microbial degra-dation of MCB, facilitated by an integrated approach andcombined with a high resolution sampling, are required toevaluate zones of enhancedl inn situbiodegradation ofMCB and to optimise wetland systems treating contaminatedgroundwater. Acknowledgements The Department of Groundwater Remediation, the SAFIRAProject, in particular Dr. A. Kaschl and Dr. H. Weiss, the ANA-NAS Project and the Department of Analytical Chemistry of theUFZ are acknowledged for assistance in the field and laboratorywork. We are grateful to S. Taglich, J. Ahlheim,O. Thiel, G.Mirschel, I. Mausezahl and T. Nullmeyer. We are thankful toDr. A. Miltner, Dr. M. Gehre, U. Giinther, K. Ethner, K. Pu-schendorf, and A. Fischer, for their technical support in our lab-oratory and iisotope measurements. Dr. Kerry Sublette,University of Tulsa, USA provided the Bio-Sepbeads forthis work. We thank the EU Marie Curie Host Fellowships forEarly Stage Training Project “AXIOM”(MEST-CT-2004-8332), the Virtual Institute for Isotope Biogeochemistry(VIBE) and the Deutsche Bundesstiftung Umwelt (20004/751) for financial support. References ( Abraham, W.R., Hesse, C., Pelz,O., 199 8 . Rat i os of carbon isot o pes in m i cro- b ial lipids as an indicator of substrat e usage. Applied and Environmental Microbiology 64, 4202-4209. ) ( Abrajano , T.A. , Murphy, D.E., F ang, J., Comet, P ., Brook, J.M., 1994. 13C/12C r atios i n individual fatty acids of marine mytilids with and w ithoutbacterial symbionts. Organic Geochemistry 21, 611-617. ) Anderson, R.T., Lovley, D.R., 2000. Anaerobic biodegradation of benzeneunder sulfate-reducing conditions in a petroleum-contaminated aquifer.Environmental Science and Technology 34, 2261-2266. Anderson, R.T.,Rooney-Varga, J.N., Gaw, C.V., Lovley, D.R., 1998. Anaero-bic benzene oxidation in the Fe(III) reduction zone of petroleum-contam-inated aquifers. Environmental Science and Technology 32, 1222-1229. Anderson, T.A., Walton, B.T., 1995. Comparative fate of [C]trichloroethy- ( lene i n the root zone of plants from a former s olvent d isposal s ite. Environ- m ental Toxicology and Chemistry 14, 2041-2047. ) ( Baker, L ., 1 998. D esign considerations a n d applications for wetland tr e atment of high-nitrate waters. Wate r Scienc e and Technology 31, 389-395. ) Bligh, E.G., Dyer, W.J., 1959. A rapid method of total lipid extraction and puri-fication. Canadian Journal of Biochemistry and Physiology 37 (8),911-917. ( Chakraborty, R ., Coates, J .D., 2005. Hydroxylation and carboxylation - twocrucial steps of anaerobic benzene degradation by Dechloromonas strainRCB. Applied E nvironmental Microbiology 71,5427-5432. ) ( Dempster, H .S., S herwood Lo l lar, B . , F e enstra, S . , 1 9 97. T r acing organic contaminant in groundwater : a new methodology using compound-specific isotopic analysis. Environmental S cience and T e chnology 31, 3193-3197. ) ( Dunbabin, J., B owmer, K., 1992. Po t ential u s e of constructed wetlands fo r treatment of industrial wastewaters c ontaining metals. Science of the Total Environment 1 11, 151-168. ) ( Edwards, E.A., Grbic-Galic,D., 1 9 92. C o mplete mineralization of benzene by aquifer microorganisms u nder strictly a naerobic c onditions. Applied Environmental Microbiology 58, 2663-2666. ) Ehrlich, H.L., 1998. Geomicrobiology: its significance for geology. Earth-Science Review 45, 45-60. ( Fritz, H., Reineke, W., Schmidt, E.,1992.Toxicity of chlorobenzene on Pseu-domonas s p . strain RHO1, a chlorobenzene-degrading strain. Biodegrada- tion 2. 165-170. ) ( Geyer, R., Peacock, A .D., M iltner, A ., R ichnow, H . H., White, D . C., Sublette, K.L., Kastner, M., 2005. I n s itu assessment of biodegradation potential using biotrap s amended with C-labeled benzene o r t o luene. Environmental S cience a nd T echnology 39, 4983-4989. ) ( Gilbert, E.S., Crowley, D .E., 1 9 97. P l ant compounds that induce poly c hlori-nated biphenyl biodegradation by Arthrobacter sp. strain B1B. AppliedEnvironmental Microbiology 63, 1933-1938. ) ( Griebler, C., Adrian, L ., M eckenstock, R., Richnow, H., 200 4 . Stable carbon isotope f ractionation during a erobic and a n aerobic transformation oftrichlorobenzene. FEMS M icrobiology Ecology 48, 3 13-321. ) ( Goodmann, K. J ., Br e nna, J.T., 1992. Hi g h sensitivity tracer detection using high-precision gas chromatography-combustion i s otope r atio mass spec-trometry and h ighly e nriched [U-C]-labeled pr e cursors. Analytical Chemistry 64 (10), 1088-1096. ) ( Gumbricht, T., 1 9 9 3. N u trient r e moval processes in fr e sh-water su b mersed m acrophyte systems. Ecological Engineering 2, 1-30. ) ( Harrington, R.R., Poulson, S.R., Drever, J.I., Colberg, P.J.S., Kel l y, E.F., 199 6 . Carbon isotope s ystematics of monoaromatic hydrocarbons: v aporizationand a dsorption experiments. Organic Geochemistry 30, 765-775. ) Heidrich, S., Weiss, H., Kaschl, A., 2004. Attenuation reactions in a multiple-contaminated aquifer in Bitterfeld (Germany). Environmental Pollution129,277-288. ( Hoefs, J., 1997. Stable Isotope Geochemistry. p. 201. ) ( Holliger, C., Schraa, G., Stams, A ., Zehnder, A., 1 9 92. E n richment and pro p -erties of an a naerobic m i xed cu l ture reductively dechlorinating 1,2 , 3- trichlorobenzene to 1,3-dichlorobenzene . Applie d and EnvironmentalMicrobiology 58, 1636-1644. ) ( J ordahl, J.L., F oster, L., Schnoor, J. L ., Alvarez, P.J . J., 19 9 7. Eff e ct of hybridpopular t rees on m icrobial populations i mportant t o h azardous w a stebioremediation. Environmental T oxicology Chemistry 16, 1 3 18-1321. ) ( Kaschl, A ., Vogt, C., U h lig, S ., N i jenhuis, I. , Weiss, H. , Ka s tner, M.,Richnow, H.H., 2 005. I s otopic f ractionation indicate s anaerobi c mono-chlorobenzene biodegradation. E nvironmental T o xicology Chemistry 24,1315 - 1324. ) Kastner, M., Fischer, A., Nijenhuis, I., Geyer, R., Stelzer, N., Bombach, P.,Tebbe, C.C., Richnow, H.H., 2006. Assessment of microbial in situ activityin contaminated aquifers. Engineering in Life Science 6 (3), 234-251. ( Kaur, A ., Chaudhary, A ., K aur, A ., C houdhary, R . , K a ushik, R . , 2 0 05.Phospholipid f atty acid -a bioindicator of environment monitoring andassessment in soil ecosystem. Current Science 89 (7) , 1103-11 1 2. ) ( Liang, L .N., Gribic-Galic, D., 1 990. Anaerobic transformation o f chloroben- zene and mixtures with aromatic acids by aquifer microorganisms and sta - ble suspended consortia. Abstracts of the Annual Meeting of the American Society for Microbiology 90, 198-202. ) ( L orah, M .M., O l sen, L . D., 1 9 99. Natural attenuation o f c h lorinated v o latileorganic compounds in a freshwater tidal wetland: field evidence of anaerobic biodegradation. Water Resources 3 5, 3811-3827. ) ( Lord, C ., Church, T.A.,1983.Quantitative model for p yritization in s alt-marsh sediments. Estuaries 6 , 295-296. ) ( Losel, D .M., 1 9 88. Fungal lip i ds. In : Ra t ledge, C., Wil k inson, S.G. (Eds.),Microbial L ipids, Vol. 1. Academic Press, London, pp. 699-806. ) ( Lovley,D.R., 1991. Dissimilatory Fe(III) and Mn(IV) r e duction. Microbiolog- ical Reviews 55, 259-287. ) Lovley, D.R., 1997. Microbial Fe(III) reduction in subsurface environments.FEMS Microbiology Reviews 20, 305-313. Lovley, D.R., 2000. Anaerobic benzene degradation. Biodegradation 11,107-116. ( Luther, G.W., S hellenbarger, P.A., B rendel, P .J. , 1996. Dissolved F e(III) and F e(II) complexes in salt marsh porewaters.Geochimica and CosmochimicaActa 60, 951-960. ) ( Macek, T . , M ackova, M . , B u rkhard, J., Demnerova, K., 1998. Introduction o fg reen plants for t he control of metals a nd organics in environmental reme-diation. In: Holm, F.W. ( E d.), Effluents from Alternative De m ilitarisation T echnologies. Kluwer Academic Publishers, D o rdrecht, pp. 71-84. ) ( Mancini, S .A., L acrampe-Couloume, G . , J o n ker, H . , van B r eukelen, B.M.,Groen,J., Volkering, F . , Sherwood Lollar, B., 2 0 02. Hydrogen isotopic enrich- ment: an indicator of biodegradation at a petroleumhydrocarbon contaminated field site. Environmental Science and Technology 36 (11), 2464-2470. ) ( Mancini , S.A., U lrich , A.C., Lacrampe-Couloume, G., , 、 S leep, B..Edwards, E.A., S herwood Lollar, B.S., 2 003. C arbon a n d h y drogen is o to-pic fractionation during anaerobic biodegradation of benzene. Applied En-vironmental Microbiology 69, 191-198. ) ( Middeldorp, P ., deWolf, J . , Zehnder, A., Schraa, G., 1997. Enrichment and properties of a 1,2,4-trichlorobenzene-dechlorinating methanogenic mi c ro- bial consortium. Applied Environmental Microbiology 63, 1225-1229. ) Nowak, J., Kirsch, N.H., Hegemann, W., Stan, H.-J., 1996. Total reductivedechlorination of chlorobenzenes to benzene by a methanogenic mixedculture enriched from the Saale river sediment. Applied Microbiologyand Biotechnology 45, 700-709. ( P ardue, J .H., Kongara, W .A., Jones,W . J., 1 9 96. E f fect o f cadmium on red u c-tive dechlorination of trichloroaniline. Environmental T oxicology Chemis-try 15, 1083-1088. ) ( Pelz, O., Chatzinotas, A., Zarda-Hess, A., Abraham, W . R., Zeyer, J. , 2 0 0 1.Tracing t oluene-assimilating sulfate-reducing bacteria using C-13 incorpo-ration in fatty acid s and whole-cell hybridization . FEMS M icrobiology Ecology 32, 123-131. ) ( Phelps, C.D., Zhang, X., Young, L.Y., 2001. Use of s table isotope to identify benzoate as a m etabolite of benzene d e gradation in a s u lphidogenic co n - sortium. Environmental Microbiology 3, 600-603. ) ( Pombo, S.A., Pe l z, O., Schroth, M.H., Zey e r, J., 2002. Field-scale C-13-lab e l-ing of phospholipid fatty acids (PFLA) and dissolved inorganiccarbon: tracing acetate assimilation and mineralization in a p etroleumhydrocarbon-contaminated a quifer . FEMS M i crobiology Ec o logy 41,259-267. ) ( Richnow, H.H., Annweiler, E., M ichaelis, W., Meckenstock, R.U.,20 0 3a. Mi- crobial in sit u degradation of aromatic hydrocarbons in a contaminated aquifer monitored b y c a rbon isotope fractionation. Journal of Contaminant Hydrology 1 771, 239-257. ) ( Richnow, H. H ., Meckenstock, R.U., Ask, L ., Baun, A ., L edin, A.,Christensen, T.H., 2003b. In s itu biodegradation determined by carbon iso-tope f raction o f a romatic h y drocarbons in a n ana e robic land f ill lea c hateplume (Vejen, D enmark). J o urnal of Contaminant Hydrology 64, 59-72. ) ( Schnoor, J.L., L icht, L .A., M cCutcheon, S. C ., Wo l fe, N.L . ,Carreira, L.H. , 1995. Phytoremediation of organic and n u trient contaminants. Environ-mental S cience a nd Technology 29 (7), 318-323. ) ( Schiith, C.,Taubald, H., Bolano, N.,Maciejczykn, S.C . , 2003. Carbon and hy-drogen isotope effects during sorption of organic contaminants on carbona-ceous m aterials. Journa l of Contaminant Hydrology 64, 269-281. ) Sherwood Lollar, B.S., Slater, G.F., Witt, M.B.,Klecka, G.M.,Harkness, M.R.,Spivack,J., 2001. Stable carbon isotope evidence for intrinsic bioremedi-ation of tetrachloroethene and trichloroethene at Area 6, Dover Air ForceBase. Environmental Science and Technology 35, 261-269. ( Shimp, J.F., Tracy, J.C., Davis, L.C., Lee, E., Huang, W., E rickson, L .E.,Schnoor, J.L., 1 993. B e neficial e f fects o f plants i n t h e r e mediation ofsoil and groundwater contaminated with organic materials. C ritical Review i n Environmental Science and Technology 23, 41-77. ) ( Slater, G.F., Ahad, J.M.E., Sherwood-Lollar, B., All e n-King, R., Slee p , B., 2000. Carbon is o tope effects re s ulting from equilibrium sorption of dis-solved VOCs. A nalytical Chemistry 72 , 5669-5672. ) ( Spgaard, E.G., Remgigijus, A.,Abraham-Peskir, J., Koch, B. C ., 20 0 1. Co n di-tions for biological precipitation of iron by Gallionella ferruginea ina slightly polluted groundwater. Applied Geochemistry 16, 1129-1137. ) ( Song, D .L., C onrad, M.E., Sorenson, K . S., Alvarez-Cohen, L . , 2002. S t ablecarbon isotope fractionation d uring enhanced in situ bioremediation of tri- chloroethene. Environmental Science and Technology 36,2262-2268. ) ( Stelzer, N ., B uining, C., P f eifer, F., T ebbe, C.C., Ni j enhuis, I, K astner, M., Richnow, H . H., 2 0 06. In situ m i crocosms to evaluate natural attenuationpotentials in contaminated aquifers . Organi c Geochemistry 37 ( 10),1394-1410. ) Thiel, V., Peckmann, J., Richnow, H.H., Luth, U., Reithner, J.,Michaelis, W.,2001. Molecular signals for anaerobic methane oxidation in Black Seaseep carbonates and microbial mat. Marine Chemistry 73, 97-112. Trapp, S., 2000. Aspekte der Phytoremediation organischer Schadstoffe.USWF- Z. Umweltchemie und Okotoxikologie 12 (5), 246-255. Urich, A.C., Beller, H.R., Edwards, E.A.,2005. Metabolites detected duringbiodegradation of Co-benzene in nitrate-reducing and methanogenicenrichment cultures. Environmental Science Technology 39, 6681-6691. Van Agteren, M.H., Keuning, S., Janssen, D.B., 1998. Handbook on Biodeg-radation and Biological Treatment of Hazardous Organic Compounds.Kluwer Academic, Dordrecht, The Netherlands. Vogt, C., Alfreider, A., Lorbeer, H., Ahlheim, J., Feist, B., Boehme, O.,Weiss, H., Babel, W., Wuensche, L., 2002. Two pilot plant reactors de-signed for the in situ bioremediation of chlorobenzene-contaminatedground water: hydrogeological and chemical characteristics and bacterialconsortia. Water, Air and Soil Pollution 2, 161-170. Vogt, C., Simon, D., Alfreiden, A., Babel, W., 2004. Chlorobenzene degrada-tion under oxygen-limited conditions leads to accumulation of 3-chloroca-techol. Environmental Toxicology and Chemistry 23, 265-270. Warren, L.A., Haack, E.A., 2001. Biological control on metal behaviour infreshwater environments. Earth-Science Review 44, 261-320. Weiss, H., Schirmer, M., Teutsch, G., Merkel, P., 2001. Sanierungsforschungin regional kontaminierten Aquiferen (SAFIRA)-2. Projektuiberblick undPilotanlage. Grundwasser 3,135-139. Wycisk, P., Weiss, H., Kaschl, A., 2004. Groundwater pollution and remedia-tion options for multisource-contaminated aquifer (Bitterfeld/Wolfen,Germany). Toxicology Letters 140, 343-351. ( Zelles, L., 1999. Fatty acid patterns of phospholipids and lipopolysaccharides i n t he c haracterisation of m icrobial c o mmunities in soil: a review. Bio l ogy and F ertility of Soils 29, 1 1 1- 1 29. )
确定







还剩8页未读,是否继续阅读?
科艺仪器有限公司为您提供《地下水中生物降解一氯代苯检测方案(氧分析仪)》,该方案主要用于环境水(除海水)中有机污染物检测,参考标准--,《地下水中生物降解一氯代苯检测方案(氧分析仪)》用到的仪器有光学O2、pH 和 CO2测量仪
推荐专场
相关方案
更多