许多湿地植物都面临着严峻的土壤问题。化工生产使土壤产生缺氧症和植物毒性化合物。为了维持一个有氧呼吸的湿地植物根系产生通气组织,证植物体通过地下器官有效组织氧气输送。此外湿地植物能够向土壤中释放氧气,这反过来又影响湿地植物曝气,可以大大影响土壤化学,通过需氧根围保护根部。通过植物通气组织的地下器官释放氧气的实验在实验室已经得到很好的验证并记录,但没有在野外条件下的相关实验记录。
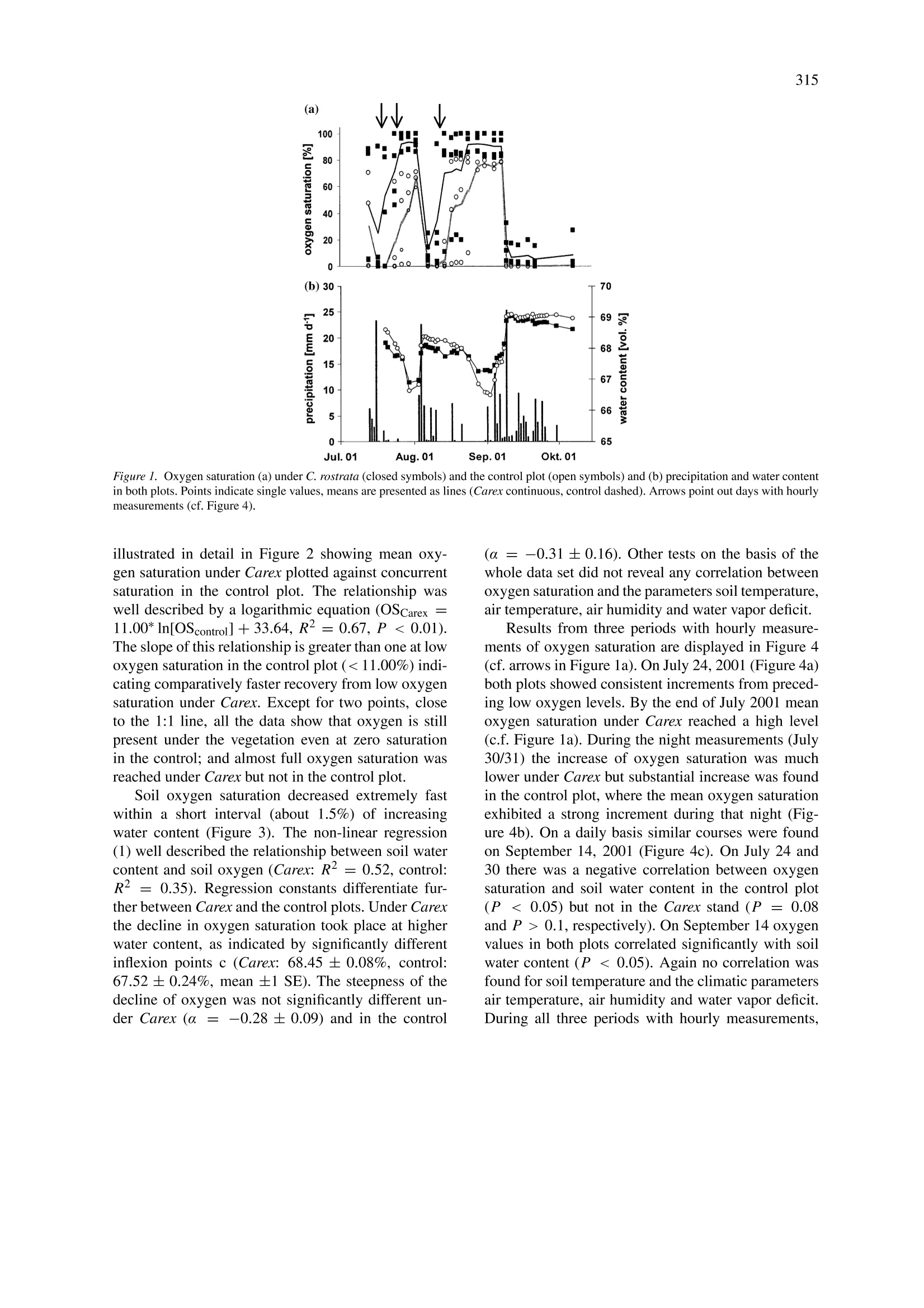
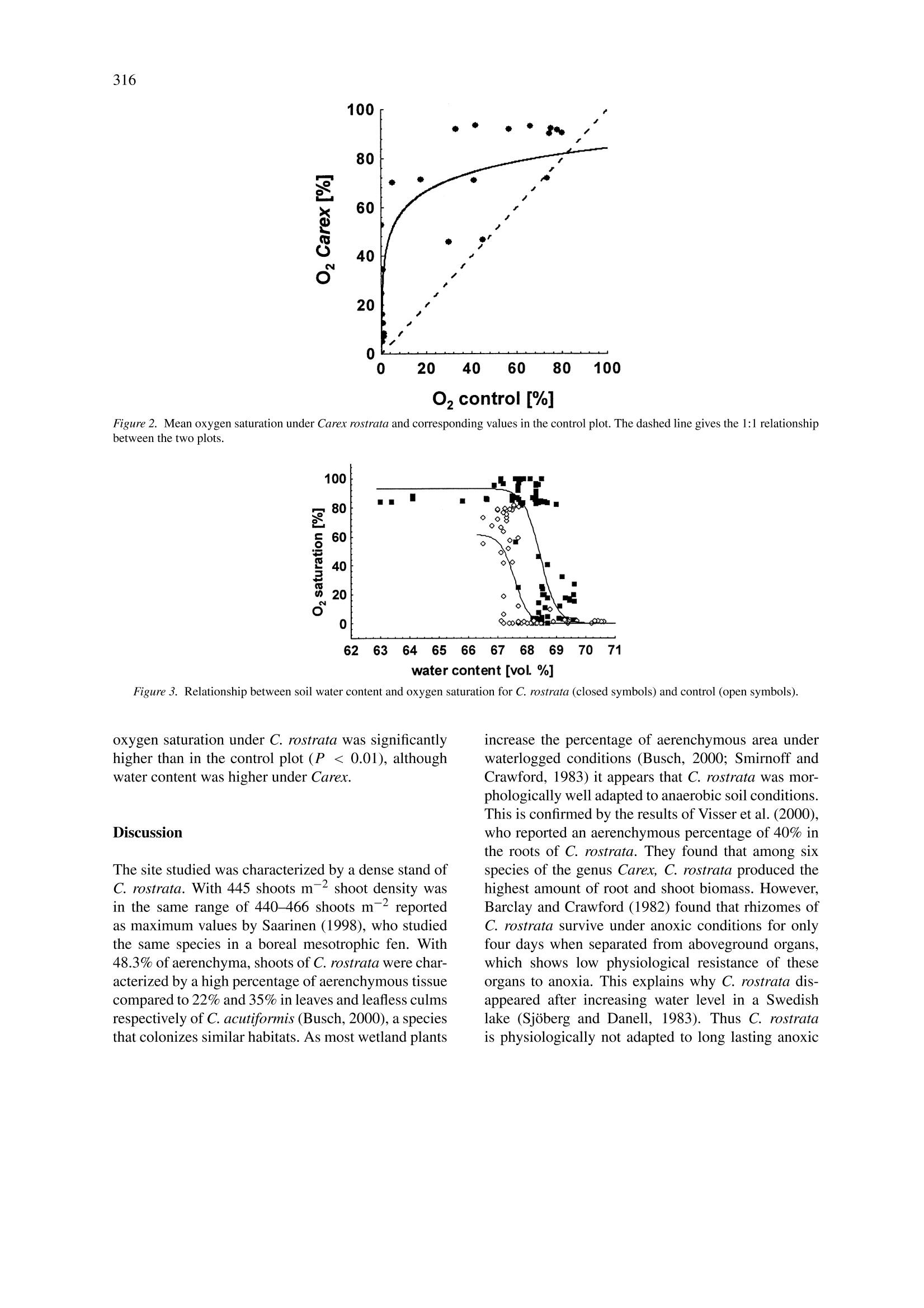
在这项研究中,我们进行氧饱和度动态测量,同时测量了土壤含水量和小气候参数。测量时间为2001年7月至10月,实验对象为盖有 Carex rostrata Stokes的一些低地泥炭。氧饱和度量化使用最新才(德国Presens 公司microoptrodes)光学传感器。 C. rostrata的存在显著提高了土壤中的氧气含量,在有Carex rostrata覆盖时的氧饱和度 (56.0%) 明显高于无植被(26.6%)。含水量波动(变化)时,两块地的氧饱和度变化都很明显。增加土壤水分含量在两地块引起氧饱和度剧烈下降,并导致缺氧的对照区。在有C. rostrata存在时,含水量较高时土壤中的氧气显著下降(68.5%,较对照区的67.5%),这是因为在测量时水的平均含量在67至69%之间浮动。
方案详情

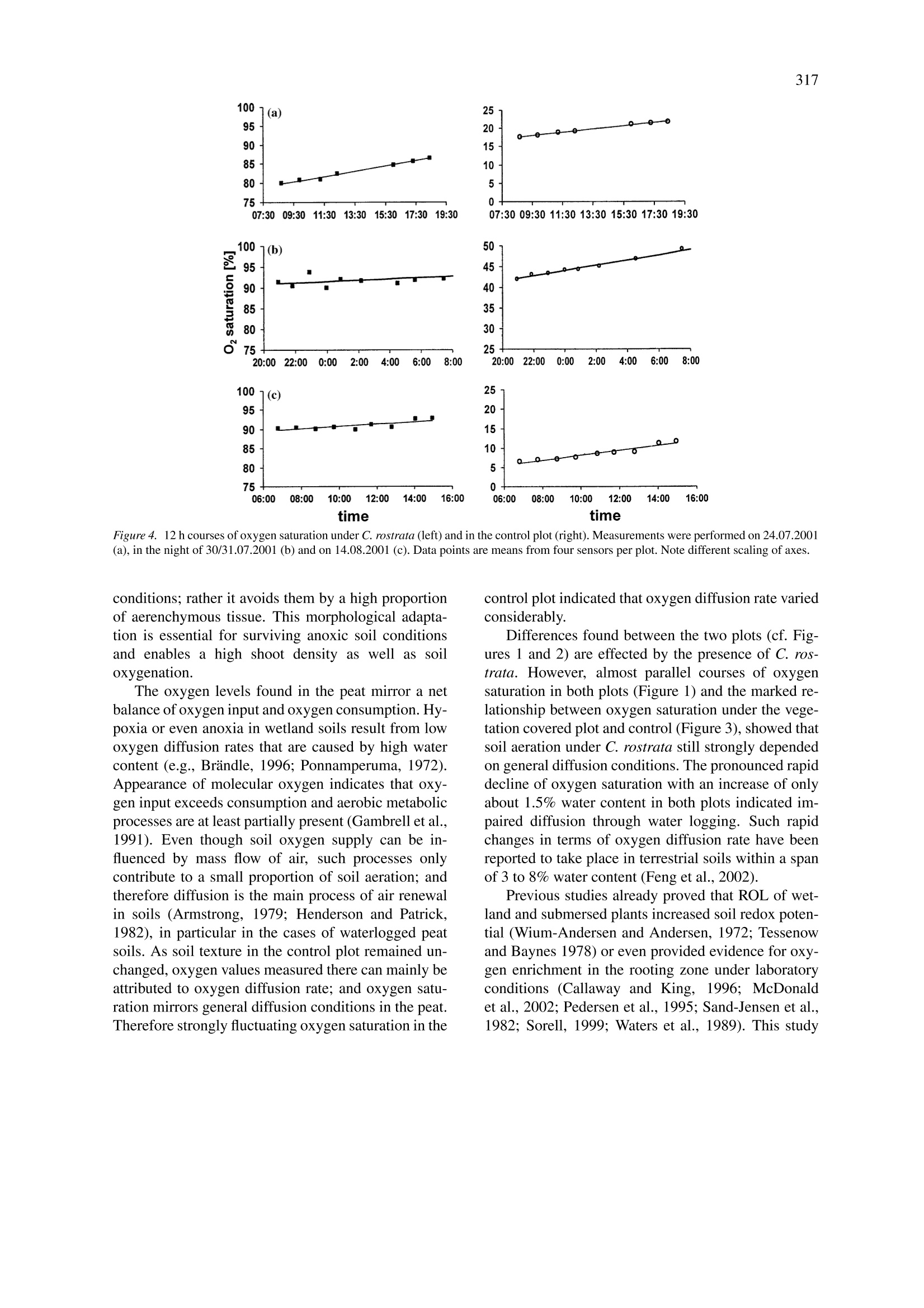
Plant and Soil (2004) 270:311-320DOI 10.1007/s11104-004-1724-zO Springer 2004 312 Effects of Carex rostrata on soil oxygen in relation to soil moisture Raphael Mainiero1,2& Marian Kazdal 'Department of Systematic Botany and Ecology, University of Ulm, Albert-Einstein-Allee 11, D-89069 Ulm,Germany. Corresponding author* Received 29 March 2004. Accepted in revised form 2 August 2004 Key words: aerenchyma, microclimate, microoptrodes, oxygenation, soil water, wetlands Abstract Many wetland plants are faced with severe edaphic problems. Long term flooding effects a sequence of chemicalprocesses that result in soil anoxia and production of several phytotoxic compounds. In order to maintain anaerobic root respiration wetland plants produce aerenchyms that enable oxygen conduction through the plant bodyto underground organs. Moreover wetland plants are able to release oxygen into the soil. This aeration effect ofwetland plants in turn can influence soil chemistry considerably and protects roots by an aerobic rhizosphere.Oxygen release by underground organs of aerenchymous plants has been well documented in laboratory investi-gations but not under field conditions. In this study, dynamics of oxygen saturation were measured together withsoil water content and microclimatic parameters. Measurements were carried out on some lowland peat coveredby Carex rostrata Stokes from July to October 2001. Oxygen saturation was quantified using novel optical sensors(microoptrodes). The presence of C. rostrata significantly increased oxygen content in the soil. Mean oxygensaturation under Carex rostrata (56.0%) was significantly higher than in a control plot without vegetation (26.6%).Due to fluctuating water content, oxygen saturation in both plots was characterized by pronounced time variation.Increasing soil water content caused an extreme decline of oxygen saturation in both plots and led to anoxia inthe control plot. In the presence of C. rostrata, the decline in soil oxygen took place at significantly higher watercontent (68.5% compared to 67.5% in the control plot) which is substantial as the mean water contents variedbetween 67 and 69% during the measurement period. Abbreviations: ROL- radial oxygen loss; SEM-scanning electron microscope; TDR -time domain reflectrome-try. Introduction Plants growing on at least temporarily flooded soilshave to overcome severe edaphic problems. With in-creasing water content gases are displaced from thesoil pores. As gas diffusion in water is about 10times lower than in air, oxygen supply to the under-ground plant organs is greatly impeded (Feng et al.,2002). Residual oxygen is then rapidly consumed byrespiration and chemical processes so that the soil be-comes hypoxic or anoxic within a few days or evensome hours after submergence (Ernst, 1990; Ponnam-peruma, 1972). Long lasting water logging result in ( *FAX No:+49-731/50-22720. ) ( E -mail: raphael.mainiero@biologie.uni-ulm.de ) decrease of soil redox potential so that the elementsbecome successively reduced. Some of the reducedcompounds like ammonium, hydrogen sulfide, loweralcohols and lower fatty acids are phytotoxins (Arm-strong, 1979). Additionally, heavy metal ions likeiron or manganese become soluble and plant availableand accumulate to phytotoxic concentrations (Brandle,1996). Different adaptations to these effects have been de-scribed. In addition to physiological adaptations likedifferent kinds of fermentation (Brandle,1996) themost apparent adaptation of wetland plants is the cre-ation of aerenchyma. Aerenchyma enable transportof oxygen from the atmosphere and/or oxygen origi-nating from photosynthesis through the plant body to the buried organs (e.g., Armstrong, 1979; Tessenowand Baynes, 11978). Already Conway (1937) hadshown that oxygen in roots of wetland species occursat nearly atmospheric saturation. Moreover, wetlandplants, as well as submersed plants, are known to re-lease oxygen from their roots, a phenomenon calledradial oxygen loss’(ROL) (e.g., Armstrong, 1979).ROL can take place in different amounts and at dif-ferent locations of roots (Colmer, 2003; Sand-Jensenet al., 1982) but often it occurs at the laterals and closeto the root tip. As a result the soil redox potentialincreases and possibly harmful molecules become re-oxidized and thus detoxified. Thus key locations fornutrient absorption and root elongation are protectedby aerobic conditions. Apparent evidence for ROLare iron-plaques in the close vicinity around roots ofwetland species (e.g., Brandle, 1996; Crowder andMacFie,1986). Since molecular oxygen in soils has a net balanceof supply (supply from the soil surface and ROL)and consumption (respiration by underground plantorgans, heterotrophs and chemical bonding) molec-ular oxygen may appear despite low diffusion ratesin the soil, provided that input via ROL is highenough to exceed consumption. Since both oxygensupply and consumption are not spatio-temporallyconstant, the appearance of molecular oxygen de-pends on environmental factors (e.g., Callaway andKing, 1996, Christensen et al., 1994). Several stud-ies have reported pressurized ventilation systems inwetland plants that provide buried rhizomes with oxy-gen effectively (Armstrong et al., 1992; Dacey, 1981).However, studies dealing with such influence on soilproperties are rare. Submersed plants were provedto significantly increase redox potential in the root-ing zone (Flessa, 1994; Tessenow and Baynes, 1978;Wium-Andersen and Andersen, 1972). Studies onROL of emergent wetland plants focused on the roleof redox potential around the roots (Kludze and De-Laune, 1996; WieBner et al., 2002) or on locationsof oxygen release (Chabbi et al., 2000; Conlin andCrowder, 1989). They clearly showed the ability torelease oxygen; however, they were mainly carried outunder static and artificial conditions. Hacker and Bertness (1995) found in a field study that the less adaptedIva frutescens L. benefited from ROL of Juncus ger-ardi Loisel.Callaway and King (1996) came to similarconclusions in a greenhouse experiment with Typhalatifolia L., albeit they could not provide evidence foroxygen enrichment under natural conditions. Investigations of soil oxygen content in the presentstudy were done in a lowland peat fen covered byCarex rostrata Stokes (bottle sedge). This sedge isknown to tolerate flooding (Fagerstedt, 1992; Steedet al., 2002) and it survives in soils with low redoxpotential (Visser et al., 2000). Rhizomes apparentlybenefit from internal oxygen transport from motherplants (Armstrong and Boatman, 1967). The specieswas already shown to release oxygen under labora-tory conditions (Conlin and Crowder, 1989) and ironplaques were found around its roots (Crowder and Mc-Fie, 1986) indicating a considerable amount of ROL.According to Saarinen (1996), who studied C. rostratain a boreal mesotrophic fen, the investment in fineroot biomass is high (78% of total biomass) with rootsfound at more than 2 m depth, indicating good adapt-ability for supplying buried organs with oxygen. Thusthe influence of anoxic soil conditions on C. rostrataand its adaptations are relatively well documented.However, it is not known to what extent this speciesinfluences soil oxygen content. The objective of this work was to study the poten-tial influence of ROL by C. rostrata on soil oxygencontent in a lowland peatfen under naturally fluctu-ating field conditions. The hypothesis was that thiswetland species is able to release so much oxygenthat in spite of consumption (respiration by under-ground plant organs, heterotrophs and chemical bond-ing) molecular oxygen appears in the soil. A furtheraim was to clarify to what extent soil water content,soil temperature, air humidity and air temperature caninfluence the oxygen saturation in wetland peat. Materials and methods Study site The study plot is located in South Germany in the largewetland area Schwaibisches Donaumoos (48°29'N,10°12' E, 450 m a.s.l.). It is colonized by differentrepresentatives of marsh (Phragmites australis Trin.ex Steud, Typha latifolia L.) and fen vegetation (Carexpaniculata L., C. nigra (L.) Reichard, C. pseudocype-rus L., C. rostrata). The nutrient-rich peat of this areais characterized by high nitrogen content of up to 3%and pH(H2O) values between 6.0 and 6.9. The inves-tigated Carex rostrata Stokes colonizes wet or evenflooded moderately chalky banks and shores (Griese,1998). In the habitat under study, it grew on peatin monospecific stands or associated with T. latifo-lia. Measurements were done in a dense monospecific stand of C. rostrata of about 100 m² in area. Plantheight ranged between 40 and 60 cm and intensiverooting was found between 0 and 15 cm. A controlplot was created within the same stand. Here, all plantswere removed over an area of about 3 m by cuttingthe stems at ground level with a knife. Connectionsvia rhizomes and roots to the surrounding plants weresevered by spade cuts. Seedlings and tillers were re-moved when they appeared. All these activities wereperformed from the margins of the control plot so thatno soil compaction or other changes of physical prop-erties occurred. Glass fibers of the probes were longenough to permit oxygen measurements as much as2 m distance from the plot. Thus the physical soilproperties remained unchanged and oxygen detectedin the control plot could have reached the sensors onlyby diffusion from the soil surface. However, remov-ing the plants increased the mean soil temperature by1.5 K. Oxygen measurements Optical sensors (microptodes), working on the basisof fluorescence were used to determine soil oxygen.The optical sensors used (type B2, Presens, Regensburg, Germany) consist of a glass fiber with a luminophor applied to its tip. Oxygen interacts with theluminophor by reversible binding, thereby changingthe fluorescence characteristics (Holst et al., 1997;Klimant et al., 1995). A light wave is emitted froma gauge (Microx TX, Presens, Regensburg, Germany)through the glass fiber to the luminophor. The remittedlight wave is conducted back to the gauge. The shift inphase angle between the stimulating light wave and thefluorescence response is compared and directly relatedto oxygen saturation (see Gansert et al., 2001 for moreinformation). Apart from cross sensitivity for gaseoussulfur dioxide and gaseous chlorine, microoptodesare exclusively sensitive to oxygen. Oxygen is notconsumed by probes themselves (Holst et al., 1997;Klimant et al., 1995). There is no interaction withcarbon dioxide, hydrogen sulfide, ammonia or anyionic species; and sensors are applicable from pH 1to pH 14. Due to their extremely small dimensionsmicrooptodes enable a high spatial resolution. Two calibration values are required to convertphase angles to oxygen saturation, one in total ab-sence of oxygen and one in water-vapor saturated air.Since these values depend on temperature and everyprobe has its own characteristic values, all microop-todes were calibrated separately. Values in the absence of oxygen were obtained in a 1% Na2S2O4 solution.Bleaching of the luminophor due to numerous mea-surements causes shifts of the calibration values andcollecting 100,000 data points results in an error of1.6% air saturation. In this study each probe was usedfor about 500 single measurements and therefore per-manent drift was not expected and sensors were notre-calibrated. Therefore the oxygen probes were leftinstalled permanently at the measuring points. Oxygen probes were placed at 10-cm depth, inaccordance with the results of Saarinen (1996), whofound the greatest proportion of underground biomassof C. rostrata in a boreal fen in the uppermost 30 cm.Furthermore, Allen et al. (2002) recently reported thatROL of C. rostrata is highest at 5-10 cm depth. Inorder to assess spatial variation within the plots, fourprobes were installed in each plot (vegetation and con-trol). Data were collected twice a week between 9.00and 11.00 a.m. from July 13 to October 7, 2001. Eventhough this study focused on the medium-term dynam-ics, three 12 h courses were measured on July 24 andAugust 14 and one course during the night of July30, 2001. The readings started in the early morning orlate evening, respectively; and were continued roughlyat hourly intervals for each sensor. These short-termdata were treated separately from the medium termdata. The starting value from each course was usedfor further calculation of the general relationship be-tween medium-term soil oxygen saturation and othervariables. Soil temperature in the control and in the vegeta-tion plot were collected separately. These data wereentered manually in the measurement program (pro-gram Microx TX, Presens, Regensburg, Germany).The latter enabled individual calibration values of eachsensor (phase angles and calibration temperature) aswell as automatic temperature compensation and cal-culation of oxygen saturation. Each measurement persensor was an average of 3 to 10 readings (coefficientof variation about 3%, increasing to 10% for oxygensaturation close to zero). These average values werelater processed by statistical analysis. Soil water content and microclimatic parameters Volumetric water content in the soil was measuredwith time domain reflectrometry probes (TDR probes,type ML2x, Delta T Devices, Burwell, UK). EachTDR probe was installed close to each oxygen probeat a distance of about 10 cm. Soil temperature wasmeasured in both the control and the vegetation plot with one temperature probe (Delta T, Burwell,UK). Air temperature and air humidity instruments(Delta T, Burwell, UK) were installed at a heightof 1.70 m. These climatic parameters were collectedevery 30 min by a DL2 data logger (Delta T, Burwell,UK). Water vapor pressure deficit was calculated ac-cording to procedures recommended by Jones (1992).Precipitation data were obtained from a station of theZweckverband Landeswasserversorgung at a distanceof about 7 km from the study plot. Plant sampling Eleven plants were randomly selected and harvestedafter the completion of measurements. Cross sectionsof plants at the soil surface were made and immedi-ately fixed in 50% ethanol solution. Residual waterwas removed by increasing the ethanol content to70% and 100%. Test samples were transferred intoformaldehyd dimethyl acetal solution (CH2(OCH3)2)for 24 h afterwards, dried by critical point drying(Polaron, Watford, England) and covered with gold(Balzers FL-9496, Balzers Union,Fiirstentum Liechtenstein). SEM images were made with a Zeiss DSM942 (Zeiss, Oberkochen, Germany). Scaled SEM-micrographs were converted to black/white imagesturning intracellular spaces black. Whole stem crosssections were colored black on duplicated images. Dif-ferences in the black areas between the two typesof images revealed the absolute area of aerenchyma.Quantification of black areas was done using the pro-gram‘Delta T Scan’(Delta T, Burwell, UK). Thepercentage of aerenchyma was obtained by dividingthe absolute aerenchymous area by the total cross sec-tion area for each shoot. The total number of shootswas assessed in three 0.5 m × 0.5 m squares withinthe vegetation plot at the end of the period studied. Evaluation procedure Statistical tests were performed using the Statisticaprogram, Rel. 6.1 (StatSoft Inc., Tulsa, Okla, USA).Differences in oxygen content between the two plotswere determined using ANCOVA with water contentas covariate. The regression package was used to testthe relationship between the parameters studied. Therelationship between soil oxygen and soil water con-tent (Figure 2) was fitted using equation (1) within thenon-linear models package. OS: oxygen saturation; W: soil water content; A, c, a:estimated constants. The use of this equation is in line with the results ofFeng et al.(2002), who reported a very rapid decline ofoxygen diffusion rate with increasing soil water con-tent. These regressions were calculated separately forthe Carex and the control plot. Parameter c providesthe inflexion point of the curve. This point was usedto determine the different characteristics of oxygendynamics between the Carex and the control plot. Pa-rameter a of the equation represents the steepness ofdeclining oxygen saturation with increasing soil watersaturation. Results C. rostrata produced a dense stand with 445 shoots mand had a high percentage of aerenchymous tis-sue (48.3%). These two figures result in 0.013 maerenchymous area m-2 soil; i.e., more than one per-cent of the soil surface consists of aerenchyma foraeration of the rooting zone. Oxygen saturation (means from four sensors perplot) ranged from 5.3% to 93.3% under C. rostratain comparison to 0.0% to 80.2% in the control plot(Figure 1a). In the latter, mean oxygen saturation of26.6±4.2% was significantly lower (P <0.01) thanunder C. rostrata (56.0±3.9%, mean ±SE) but nosignificant differences were found between the plotsregarding the mean water content (C. rostrata 68.06%,control 68.05%). Mean oxygen saturation in both plotsshowed strong temporal variation during the studiedperiod. Both curves of mean oxygen concentrationsshow an almost parallel course; however, due to dif-ferent dynamics, single values of the probes withinthe each plot differed considerably. There were twopronounced maxima, the first around 30 July the sec-ond by the end of August/beginning of September.These maxima coincided with the ‘drier’conditions(Figure 1b) even though water content in the fen un-der study was generally high. The influence of groundwater on soil moisture was further increased by pre-cipitation (Figure 1b). Both heavy rainfall (greaterthan 20 mm d-) and smaller but frequent amountsof rainfall (e.g., in September 2001) resulted in an in-crease in soil water content. Therefore periods of highoxygen saturation were terminated simultaneously inboth plots by high amounts of rainfall. Following theserain periods, mean oxygen saturation under Carexrecovered earlier than in the control plot. This is Figure 1. Oxygen saturation (a) under C. rostrata (closed symbols) and the control plot (open symbols) and (b) precipitation and water contentin both plots. Points indicate single values, means are presented as lines (Carex continuous, control dashed). Arrows point out days with hourlymeasurements (cf. Figure 4). illustrated in detail in Figure 2 showing mean oxy-gen saturation under Carex plotted against concurrentsaturation in the control plot. The relationship waswell described by a logarithmic equation (OSCarex =11.00*ln[OScontroi]+33.64,R2= 0.67, P <0.01).The slope of this relationship is greater than one at lowoxygen saturation in the control plot (<11.00%) indi-cating comparatively faster recovery from low oxygensaturation under Carex. Except for two points, closeto the 1:1 line, all the data show that oxygen is stillpresent under the vegetation even at zero saturationin the control; and almost full oxygen saturation wasreached under Carex but not in the control plot. Soil oxygen saturation decreased extremely fastwithin a short interval (about 1.5%) of increasingwater content (Figure 3). The non-linear regression(1) well described the relationship between soil watercontent and soil oxygen (Carex: R2=0.52, control:R²= 0.35). Regression constants differentiate fur-ther between Carex and the control plots. Under Carexthe decline in oxygen saturation took place at higherwater content, as indicated by significantly differentinflexion points c (Carex: 68.45 ±0.08%, control:67.52 ±0.24%, mean ±1 SE). The steepness of thedecline of oxygen was not significantly different un-der Carex (a =-0.28±0.09) and in the control (a = -0.31±0.16). Other tests on the basis of thewhole data set did not reveal any correlation betweenoxygen saturation and the parameters soil temperature,air temperature, air humidity and water vapor deficit. Results from three periods with hourly measure-ments of oxygen saturation are displayed in Figure 4(cf. arrows in Figure 1a). On July 24, 2001 (Figure 4a)both plots showed consistent increments from preced-ing low oxygen levels. By the end of July 2001 meanoxygen saturation under Carex reached a high level(c.f. Figure 1a). During the night measurements (July30/31) the increase of oxygen saturation was muchlower under Carex but substantial increase was foundin the control plot, where the mean oxygen saturationexhibited a strong increment during that night (Fig-ure 4b). On a daily basis similar courses were foundon September 14, 2001 (Figure 4c). On July 24 and30 there was a negative correlation between oxygensaturation and soil water content in the control plot(P < 0.05) but not in the Carex stand (P= 0.08and P > 0.1, respectively). On September 14 oxygenvalues in both plots correlated significantly with soilwater content(P <0.05). Again no correlation wasfound for soil temperature and the climatic parametersair temperature, air humidity and water vapor deficit.During all three periods with hourly measurements, 02 control [%] Figure 2. Mean oxygen saturation under Carex rostrata and corresponding values in the control plot. The dashed line gives the 1:1 relationshipbetween the two plots. Figure 3. Relationship between soil water content and oxygen saturation for C. rostrata (closed symbols) and control (open symbols). oxygen saturation under C. rostrata was significantlyhigher than in the control plot (P<0.01), althoughwater content was higher under Carex. Discussion The site studied was characterized by a dense stand ofC. rostrata. With 445 shoots m- shoot density wasin the same range of 440-466 shoots m-reportedas maximum values by Saarinen (1998), who studiedthe same species in a boreal mesotrophic fen. With48.3%of aerenchyma, shoots of C. rostrata were char-acterized by a high percentage of aerenchymous tissuecompared to 22% and 35% in leaves and leafless culmsrespectively of C. acutiformis (Busch,2000), a speciesthat colonizes similar habitats. As most wetland plants increase the percentage of aerenchymous area underwaterlogged conditions (Busch, 2000; Smirnoff andCrawford,1983) it appears that C. rostrata was mor-phologically well adapted to anaerobic soil conditions.This is confirmed by the results of Visser et al. (2000),who reported an aerenchymous percentage of 40% inthe roots of C. rostrata. They found that among sixspecies of the genus Carex, C. rostrata produced thehighest amount of root and shoot biomass. However,Barclay and Crawford (1982) found that rhizomes ofC. rostrata survive under anoxic conditions for onlyfour days when separated from aboveground organs,which shows low physiological resistance of theseorgans to anoxia. This explains why C. rostrata dis-appeared after increasing water level in a Swedishlake (Sjoberg and Danell, 1983). Thus C. rostratais physiologically not adapted to long lasting anoxic Figure 4. 12 h courses of oxygen saturation under C. rostrata (left) and in the control plot (right). Measurements were performed on 24.07.2001(a), in the night of 30/31.07.2001 (b) and on 14.08.2001 (c). Data points are means from four sensors per plot. Note different scaling of axes. conditions; rather it avoids them by a high proportionof aerenchymous tissue. This morphological adaptation is essential for surviving anoxic soil conditionsand enables a high shoot density as well as soiloxygenation. The oxygen levels found in the peat mirror a netbalance of oxygen input and oxygen consumption. Hy-poxia or even anoxia in wetland soils result from lowoxygen diffusion rates that are caused by high watercontent (e.g., Brandle, 1996; Ponnamperuma, 1972).Appearance of molecular oxygen indicates that oxy-gen input exceeds consumption and aerobic metabolicprocesses are at least partially present (Gambrell et al.,1991). Even though soil oxygen supply can be in-fluenced by mass flow of air, such processes onlycontribute to a small proportion of soil aeration; andtherefore diffusion is the main process of air renewalin soils (Armstrong, 1979; Henderson and Patrick.1982), in particular in the cases of waterlogged peatsoils. As soil texture in the control plot remained un-changed, oxygen values measured there can mainly beattributed to oxygen diffusion rate; and oxygen satu-ration mirrors general diffusion conditions in the peatTherefore strongly fluctuating oxygen saturation in the control plot indicated that oxygen diffusion rate variedconsiderably. Differences found between the two plots (cf. Fig-ures 1 and 2) are effected by the presence of C. ros-trata. However, almost parallel courses of oxygensaturation in both plots (Figure 1) and the marked re-lationship between oxygen saturation under the vege-tation covered plot and control (Figure 3), showed thatsoil aeration under C. rostrata still strongly dependedon general diffusion conditions. The pronounced rapiddecline of oxygen saturation with an increase of onlyabout 1.5% water content in both plots indicated im-paired diffusion through water logging. Such rapidchanges in terms of oxygen diffusion rate have beenreported to take place in terrestrial soils within a spanof 3 to 8% water content (Feng et al., 2002). Previous studies already proved that ROL of wet-land and submersed plants increased soil redox poten-tial (Wium-Andersen and Andersen, 1972; Tessenowand Baynes 1978) or even provided evidence for oxy-gen enrichment in the rooting zone under laboratoryconditions (Callaway and King, 1996; McDonaldet al., 2002; Pedersen et al., 1995; Sand-Jensen et al.,1982; Sorell, 1999; Waters et al., 1989). This study could confirm a soil aeration effect by ROL under fieldconditions. The presence of C. rostrata had conse-quences regarding the interaction between soil watercontent and soil oxygen. The soil water content fordeclining oxygen saturation was higher under the veg-etation cover than in the vegetation-free control plotas indicated by different inflexion points c of the re-gression (1) (68.5% water content under C. rostratacompared to 67.5% in the control plot). Such a smalldifference is substantial considering that the mean wa-ter contents varied between 67 and 69% during themeasurement period from June to October (cf. Fig.ure 1b). This underlines the effect of C. rostrata onthe soil properties, as oxygen diffusion rates shifted ina similar narrow range of water contents when com-paring sandy loam and loamy sand (Feng et al., 2002).Furthermore in periods of high water content oxygensaturation under Carex was still above zero. Thesedifferences emphasize the additional source of oxy-gen from the ROL of C. rostrata. In addition to thespecific influence of C. rostrata on the relationshipbetween soil oxygen and water content due to ROL(Figure 2), transpiration may also temporarily reducethe soil water content and thus open up a possibilityfor a higher oxygen diffusion rate. Thus in contrastto the partly anaerobic conditions in the control plotaerobic metabolism was at least partially possible un-der C. rostrata during the whole period studied. Thesefindings are strongly supported by Allen et al. (2002),who found C. rostrata among several studied speciesto be best capable of improving the removal of dis-solved organic matter in wastewater, thereby increas-ing the redox potential significantly. They concludedthat oxygen supply from the roots of C. rostrata mayhave exceeded respiratory demands. For peat soils low in iron, up to 94% of oxygenconsumption can be attributed to heterotrophic respi-ration (Watson et al., 1997); but in eutrophic habitatssuch as the investigated nutrient rich fen, the highestactivity of methanotrophic bacteria was found (Sorrellet al., 2002). As a further implication increased oxygen supply mediated through C. rostrata may there-fore decrease methane production in wetlands (VanBodegom et al., 2001). Different dynamics of each single sensor, leadingto the partial high standard deviation in Figure 1a,reflect the fact that oxygen was not homogeneouslydistributed under Carex. The reason might be thatC. rostrata releases oxygen only at strictly confinedlocations on its roots (Conlin and Crowder, 1989).In anaerobic environments oxygen released is rapidly diluted and consumed with increasing distance fromits source (Armstrong et al., 2000;Christensen et al.,1994) as indicated by thin iron plaques around theroots of C. rostrata (Crowder and MacFie, 1986). Asmicrooptodes reflect only a very small point, differ-ences among the sensors might be caused by unequaldistances from the oxygen sources and diffusion ratesbetween them. Therefore as the placing of sensorsunder field conditions can be done only randomly,it is likely that oxygen saturation was still higherin the close vicinity of the roots. Moreover, thespatio-temporal variability of oxygen saturation canbe caused by growing roots thereby moving oxygensources (Van Bodegom et al., 2001) or by induciblebarriers to ROL in the hypodermis/exodermis of roots(Colmer, 2003). The latter was postulated for plantsgrowing on transiently water logged soils. Several studies have shown a diurnal change ofoxygen saturation within the cormus of some wetlandspecies (Laing, 1940), or at least a rapid response ofROL due to photosynthetic activity (Christensen et al.,1994; Pedersen and Sand-Jensen, 1995). Moreover,many species studied are known to create a pressurizedaeration driven either by a temperature gradient be-tween leaves and atmosphere (thermal transpiration’;Dacey, 1987; Grosse et al., 1989, 1991) or by a dif-ference in air humidity between the inner atmosphereof the leaves and air (humidity induced convection';e.g., Bendix et al.,1994). These processes should re-sult in a diurnal course of oxygen release, showinghigh oxygen saturation during the day and decreasetowards the evening and during the night. However inthis study oxygen saturation was found to increase al-most linearly during the 12 h courses, even in the night(Figure 4b) when photosynthesis and pressurized ven-tilation cease. More detailed studies on substrate oxy-genation combining photosynthesis with parametersdriving pressurized ventilation are necessary to clarifythe role of photosynthesis and pressurized ventilationin soil aeration. In the present study no correlationwas found for climatic parameters other than watercontent confirming the strong influence on diffusionconditions. Neither the 12h courses nor data evaluat-ing the whole period studied gave direct evidence thatphotosynthesis or pressurized ventilation were respon-sible for higher oxygen saturation under Carex. Thecurrent data rather suggest that the high percentageof aerenchymous tissue in combination with a highshoot density C. rostrata provides a soil/atmosphereinterface that enhances oxygen diffusion into the soil. We are indebted to the Arbeitsgemeinschaft Schwa-bisches Donaumoos for permission to work on pro-tected areas and for their technical support. We thankthe Zweckverband Landeswasserversorgung for theprecipitation data and Mrs Ellen Salzer for her helpat the electron microscope. We are very grateful toDr Hartmut Lanzinger and Prof. Dr Ulrich Stadtmiillerfor their help in non-linear regression fitting. References Allen W C, Hook P B, Biederman J A and Stein O R 2002 Temper-ature and wetland plant species effects on wastewater treatmentand root zone oxidation. J. Environ. Qual. 31, 1010-1016. Armstrong J, Armstrong W and Becket P M 1992 Phragmites aus-tralis: Venturi- and humidity-induced pressure flows enhancerhizome aereation and rhizosphere oxidation. New Phytol. 120,197-207. Armstrong W and Boatman D J 1967 Some field observations re-lating the growth of bog plants to conditions of soil aeration. J.Ecol. 55, 101-110. Armstrong W 1979 Aeration in higher plants. Adv. Bot. Res. 7, 226-332. Armstrong W, Cousins D, Armstrong J, Turner D W and Beck-ett P M 2000 Oxygen distribution in wetland plant roots andpermeability barries to gas-exchange with the rhizosphere: Amicroelectrode and modeling study with Phragmites australis.Ann. Bot. 86,687-703. Barclay A M and Crawford R M M 1982 Plant growth and survivalunder strict anaerobiosis. J. Exp. Bot. 33,541-549. Bendix M, Tornbjerg T and Brix H 1994 Internal gas transport inTypha latifolia L. and Typha angustifolia L. 1. Humidity-inducedpressurization and convective throughflow. Aquat. Bot. 49, 75-89. Brandle R 1996 Uberflutung und Sauerstoffmangel. In Stress beiPflanzen. Eds. Ch Brunold, A Riegsegger and R Brandle.pp. 133-148. UTB fur Wissenschaft, Bern, Stuttgart, Wien. Busch J 2000 Gaswechsel und strukturelle Anpassungen einheim-iher Seggen unter dem EinfluB unterschiedlicher edaphischerund atmospharischer Standortsbedingungen, Cuvillier VerlagGottingen. Callaway R M and King L 1996 Temperature-driven variationin substrate oxygenation and the balance of competition andfacilitation. Ecology 77,1189-1195. Chabbi A, McKee K L and Mendelssohn I A 2000 Fate of oxy-gen losses from Typha domingensis (Typhaceae) and Cladiumjamaicense (Cyperaceae) and consequences for root metabolism.Am. J. Bot. 87,1081-1090. Colmer T D 2003 Long-distance transport of gases in plants: A per-spective on internal aeration and radial oxygen loss from roots.Plant Cell Environ. 26.17-36. Conlin T S S and Crowder A A 1989 Location of radial oxygen lossand zones of potential iron uptake in a grass and two nongrassemergent species. Can.J. Bot. 67,717-722. Conway V M 1937 Studies in the autecology of Cladium mariscus.R. Br. Part III. Ibid. 36, 64-96. Christensen P O, Revsbach N P and Sand-Jensen K 1994 Mi-crosensor analysis of oxygen in the rhizosphere of the aquaticmacrophyte Littorella uniflora (L.) Ascherson. Plant Physiol.105,847-852. Crowder A A and MacFie S M 1986 Seasonal deposition of ferrichydroxide plaque on roots of wetland plants. Can. J. Bot. 64,2120-2124. Dacey J W H 1981 Pressurized ventilation in the yellow water lily.Ecology 62, 1137-1147. Dacey J W H 1987 Knudsen-transitional flow and gas pressurizationin leaves of Nelumbo. Plant Physiol. 85, 199-203. Ernst W H O 1990 Ecophysiology of plants in waterlogged andflooded environments. Aquat. Bot. 38, 73-90. Fagerstedt K V 1992 Development of aerenchyma in roots andrhizomes of Carex rostrata (Cyperaceae). Nord. J. Bot. 12,115-120. Feng G, Wu L and Letey J 2002 Evaluating aeration criteria by si-multaneous measurement of oxygen diffusion rate and soil-waterregime. Soil Sci. 167,495-503. Flessa H 1994 Plant-induced changes in the redox potential of therhizosphere of the submerged vascular macrophytes Myriophyl-lum verticillatum L. and Ranunculus circinatus L. Aquat. Bot.47, 119-129. Gambrell P, Delaune R D and Patrick jr. W H 1991 Redox processesin soils following oxygen depletion. In Plant life under oxy-gen deprivation. Eds. M B Jackson, D D Dacies and H Lam-bers. pp. 101-117. Academic Publishing bv. The Hague, TheNetherlands. Gansert D, Burgdorf M and Losch R 2001 A novel approach tothe in situ measurement of oxygen saturations in the sapwoodof woody plants. Plant Cell Environ. 24, 1055-1064. Griese J 1998 Cyperaceae (Sauergraser) und Typhaceae (Rohrkol-bengewachse). In Die Farn- und Bliitenpflanzen Baden-Wurttembergs. Band 8. Ed. O Sebald. Ulmer Verlag. Grosse W 1989 Thermosmotic air transport in aquatic plants affect-ing growth activities and oxygen diffusion to wetland soils. InConstructed wetlands for wastewater treatment: Municipal, in-dustrial and agricultural. Proceeding from the First InternationalConference on Constructed Wetlands for Wastewater Treatment.Chatanooga TN. 1988. Ed. D A Hammer. pp. 469-476. LewisPublishers, Chelsea, MI. Grosse W, Buchel H B and Tiebel H 1991 Pressurized ventilation inwetland plants. Aquat. Bot. 39,89-98. Hacker S D and Bertness M D 1995 Morphological and physio-logical consequences of a positive plant interaction. Ecology 76,2165-2175. Henderson R E and Patrick jr H 1982 Soil aeration and plant pro-ductivity. In Handbook of agriculture productivity. Vol. 1 PlantProductivity. Ed. M Rechagl. pp 51-69, CRC Press, Boca Raton. Holst G, Glud R N, Kihl M and Klimant L 1997 A microoptodearray for fine scale measurement of oxygen distribution. Sensor.Actuat. B-Chem. 38-39, 122-129. Jones G H 1992 Plants and Microclimate. Cambridge UniversitiyPress, Cambridge, New York, Melbourne. Kludze HK and DeLaune R D 1996 Soil redox intensity effects onoxygen exchange and growth of cattail and sawgrass. Soil Sci.Soc. Am. J. 60,616-621. Klimant I, Meyer V and Kiihl M 1995 Fiber-optic oxygen mi-crosensors, a new tool in aquatic biology. Limnol. Oceanogr.40,1159-1165. Laing H E 1940 The composition of the internal atmosphereof Nuphar advenum and other water plants. Am. J. Bot. 27,861-867. Mc Donald M P, Galwey N W and Colmer T D 2002 Similarity anddiversity in adventitious root anatomy as related to root aerationamong a range of wetland and dryland grass species. Plant CellEnviron. 25, 441-451. Pedersen O, Sand-Jensen K and Revsbech N P 1995 Diel pulsesof O2 and CO2 in sandy lake sediments inhabited by Lobeliadortmanna. Ecology 76, 1536-1545. Ponnamperuma F N 1972 The chemistry of submerged soils. Adv.Argon. 24, 29-96. Saarinen T 1996 Biomass and production of two vascular plants ina boreal mesotrophic fen. Can. J.Bot. 74, 934-938. Sand-Jensen K, Prahl C and Stokholm H 1982 Oxygen release fromroots of submerged aquatic macrophytes. Oikos 38, 349-354. Sjoberg K and Danell K 1983 Effects of permanent flooding onCarex - Equisetum wetlands in Northern Sweden. Aquat. Bot.15,275-286. Smirnoff N and Crawford RNN 1983 Variation in the structure andresponse to flooding of root aerenchyma in some wetland plants.Ann. Bot. 51,237-249. Saarinen T Demography of Carex rostrata in a boreal mesotrophicfen: Shoot dynamics and biomass development. Ann. Bot. Fenn.35,203-209. Sorrell B K 1999 Effect of external oxygen demand on radial oxy-gen loss by Juncus roots in titanium citrate solutions. Plant CellEnviron. 22, 1587-1593. Sorrell B K, Downes M T and Stanger C L 2002 Methanotrophicbacteria and their activity on submerged aquatic macrophytes.Aquat. Bot. 72, 107-119. Steed JE, DeWald L E and Kolb T E 2002 Physiological and growthresponses of riparian sedge transplants to groundwater depth. Int.J. Plant Sci. 163,925-936. Tessenow U and Baynes Y 1978 Redoxchemische Einfluissevon Isoetes lacustris L. im Litoralsediment des Feldsees(Hochschwarzwald). Arch. Hydrobiol. 82, 20-48. Van Bodegom P, Goudriaan J and Leffelaar P 2001 A mechanisticmodel on methane oxidation in a rice rhizosphere. Biogeochem-istry 55, 145-177. Visser E J W,Bogemann G M, Van de Steeg HM,Pierik R and BlomW P M 2000 Flooding tolerance of Carex species in relation tofield distribution and aerenchyma formation. New Phytol. 148,93-103. Waters I, Armstrong W, Thompson C J, Setter T L, Adkins S,Gibbs J and Greenway H 1989 Diurnal changes in radial oxy-gen loss and ethanol metabolism in roots of submerged andnon-submerged rice seedlings. New Phytol.113,439-451. Watson A, Stephen KD, Nedwell D B and Arah J A 1997 Oxidationof methane in peat: Kinetics of CH4 and O2 removal and the roleof plant roots. Soil Biol. Biochem. 29, 1257-1267. WieBner A, Kuschk P and Stottmeister U 2002 Oxygen releaseby roots of Typha latifolia and Juncus effusus in laboratoryhydroponic systems. Acta Biotechnol. 22, 209-216. Wium-Andersen S and Andersen J M 1972 The influence of vege-tation on the redox profile of the sediment of Grane Langso aDanish Lobelia lake. Limnol.Oceanogr. 17, 948-952. Section editor: H. Lambers
确定







还剩8页未读,是否继续阅读?
产品配置单
科艺仪器有限公司为您提供《土壤中美洲鳗苔草对土壤含氧量和湿度关系的影响检测方案(氧分析仪)》,该方案主要用于土壤中生物检测,参考标准--,《土壤中美洲鳗苔草对土壤含氧量和湿度关系的影响检测方案(氧分析仪)》用到的仪器有光学O2、pH 和 CO2测量仪、光纤土壤测氧仪
推荐专场
相关方案
更多

















