
方案详情
文
Pharm TOC通过高温催化氧化和VITA技术的完美结合成功的实现了医药用水总有机碳含量的准确测定
方案详情

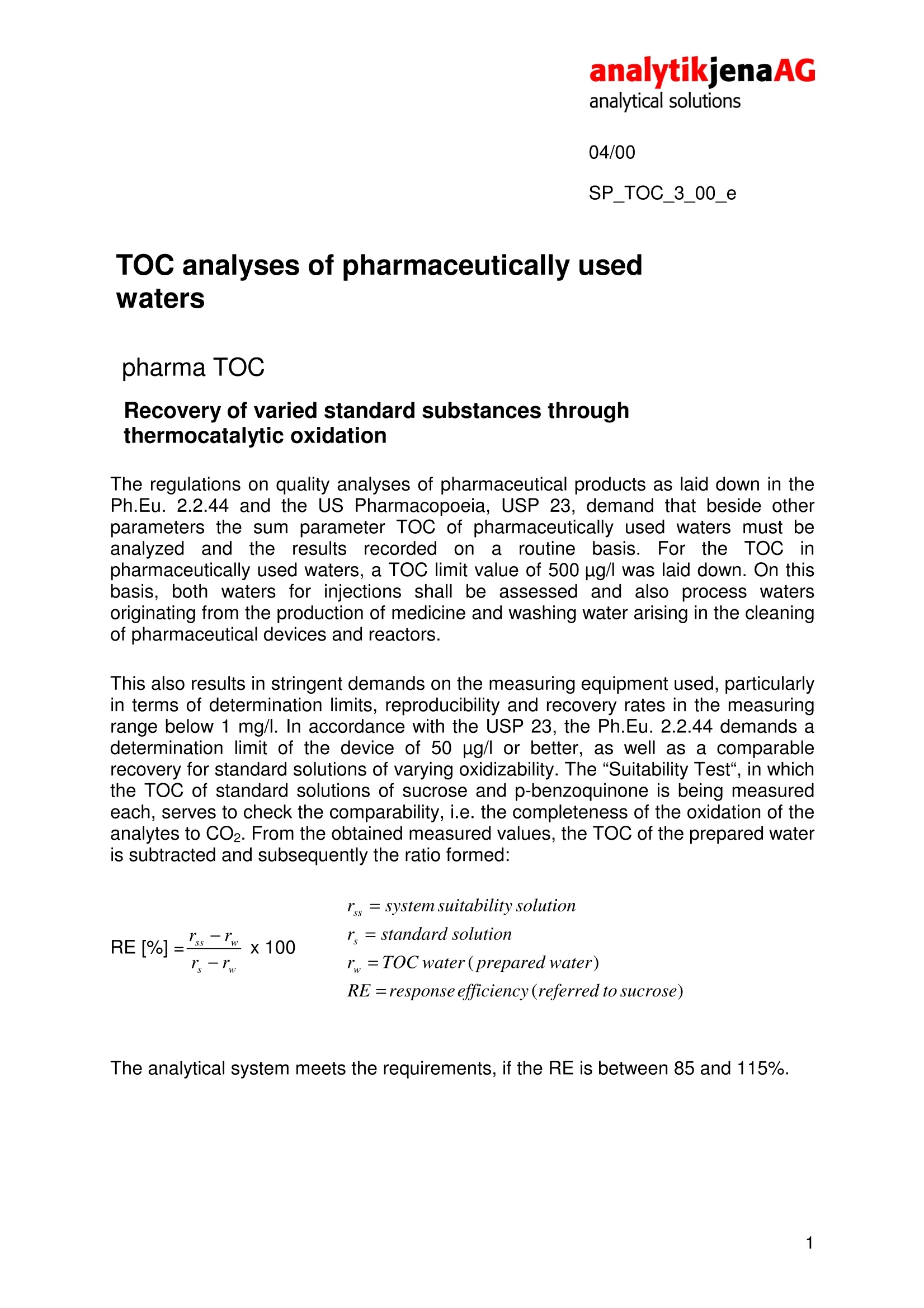
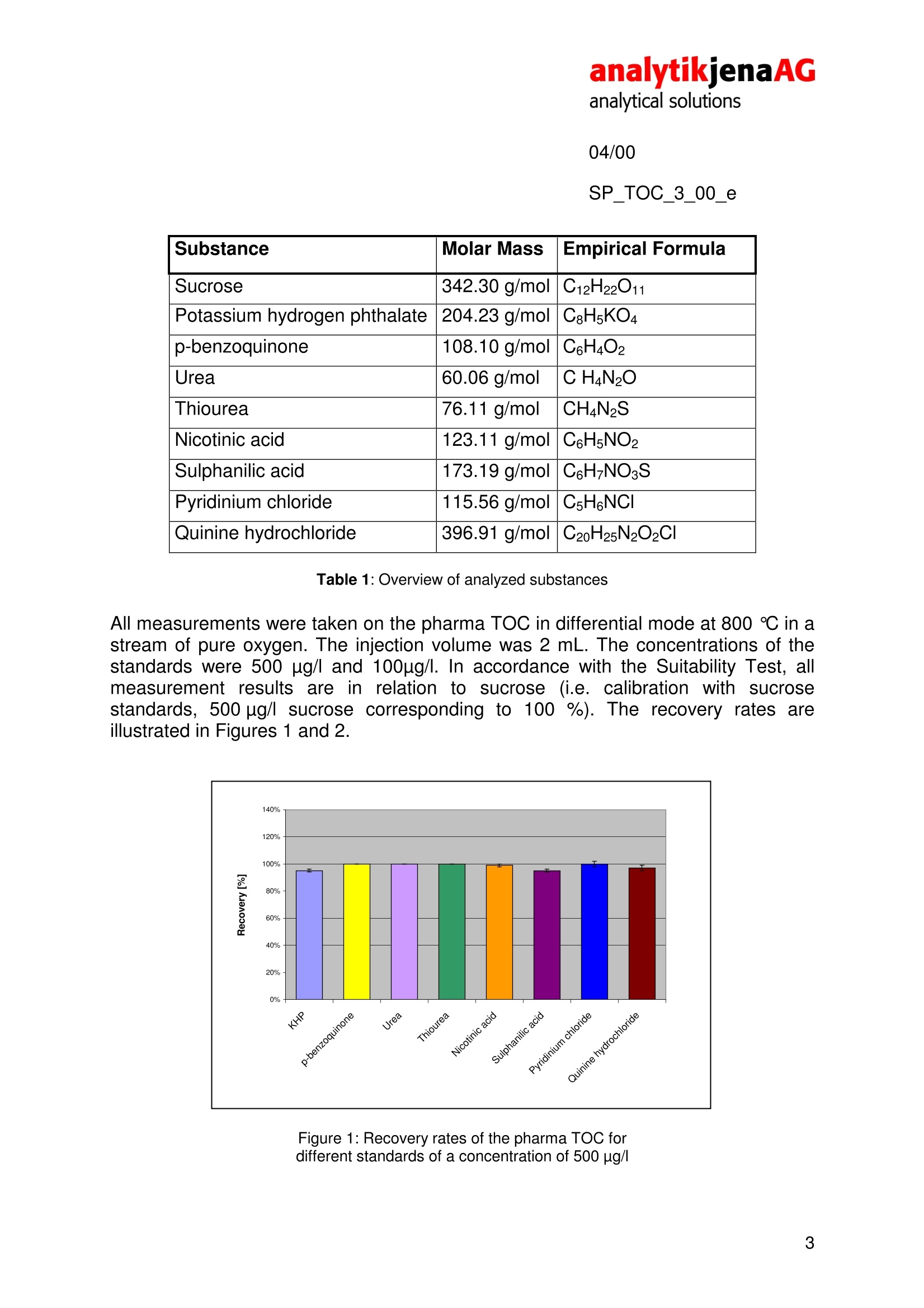
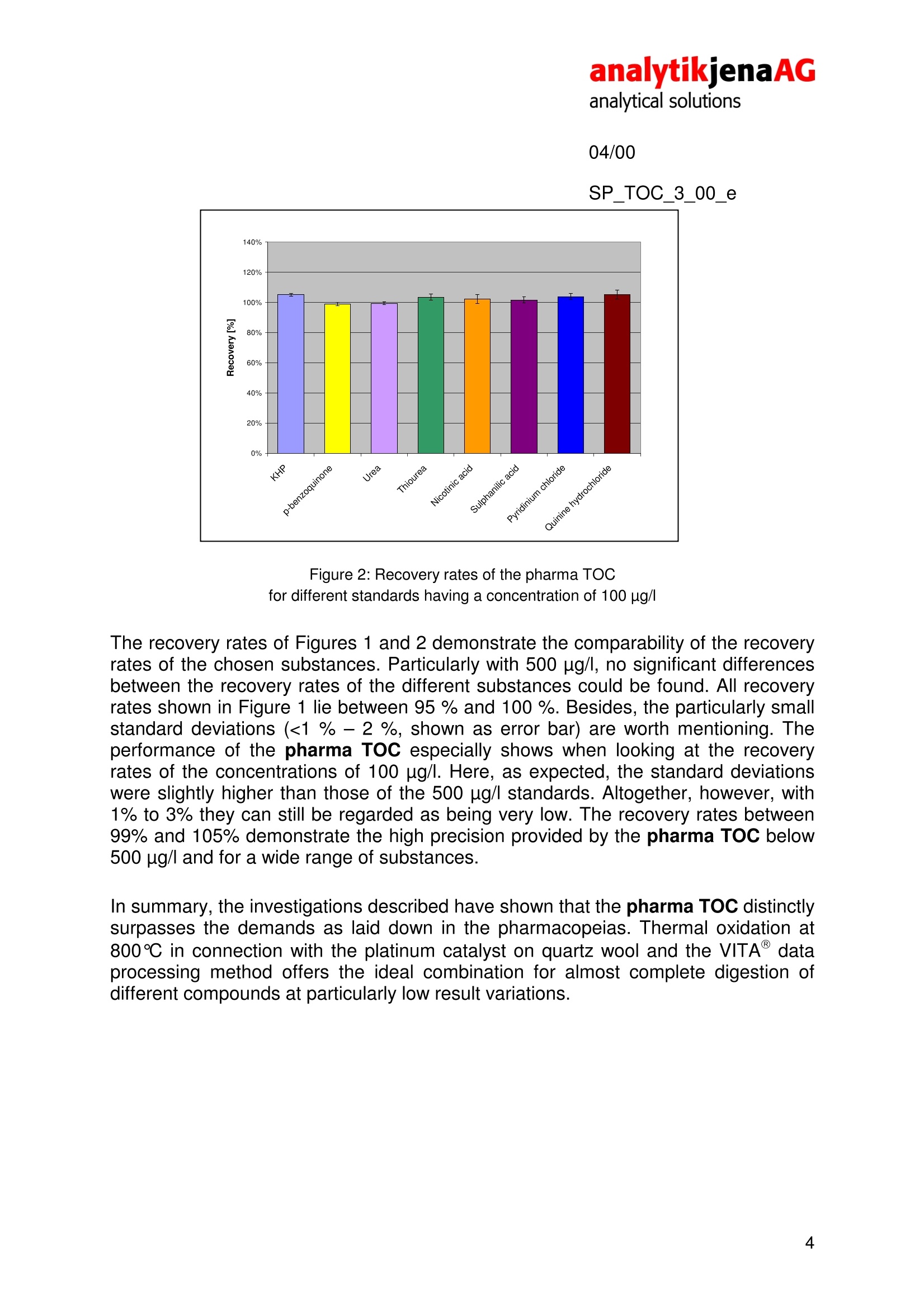
analytikjenaAGanalytical solutions04/00SP_TOC_3_00_e TOC analyses of pharmaceutically usedwaters pharma TOC Recovery of varied standard substances throughthermocatalytic oxidation The regulations on quality analyses of pharmaceutical products as laid down in thePh.Eu. 2.2.44 and the US Pharmacopoeia, USP 23, demand that beside otherparameters the sum parameter TOC of pharmaceutically used waters must beanalyzed and the results; recorded on aroutine basis. For theTOC inpharmaceutically used waters, a TOC limit value of 500 ug/l was laid down. On thisbasis, both waters for injections shall be assessed and also process watersoriginating from the production of medicine and washing water arising in the cleaningof pharmaceutical devices and reactors. This also results in stringent demands on the measuring equipment used,particularlyin terms of determination limits, reproducibility and recovery rates in the measuringrange below 1 mg/l. In accordance with the USP 23, the Ph.Eu. 2.2.44 demands adetermination limit of the device of 50 ug/l or better, as well as a comparablerecovery for standard solutions of varying oxidizability. The“Suitability Test", in whichthe TOC of standard solutions of sucrose and p-benzoquinone is being measuredeach, serves to check the comparability, i.e. the completeness of the oxidation of theanalytes to CO2. From the obtained measured values, the TOC of the prepared wateris subtracted and subsequently the ratio formed: The analytical system meets the requirements, if the RE is between 85 and 115%. 04/00 SP_TOC 3_00 e The analyses described here were performed on the pharma TOC. This analyzeroperates on the principle of thermocatalytic oxidation in combination with thepatented VITA@ method of signal analysis (VITA= dwell-time coupled integration forTOC analysis). Thermocatalytic oxidation at 800℃ in combination with a platinum catalyst in a pureoxygen flow ensures optimum recovery of sucrose and p-benzoquinone. Under theseconditions, typical RE values of the pharma TOC are between 98 % and 100 %.Thus, the demands of the System Suitability Test are easily met, which among othersis due to the selected combustion temperature and the catalyst used on thisanalyzer. The material of the catalyst is platinum-coated quartz wool, which has aparticularly large surface thus yielding a particularly high efficiency of the thermaloxidation of carbonaceous compounds to CO2 rhe use of the VITA method ensures especially high reproducibility in themeasurement of low TOC concentrations.. The VITA method was speciallydeveloped for the pharma TOC providing computer-controlled normalization of theflow variations (and thus the result variations) in the NDIR measuring cell usuallyarising in evaporation and condensation processes. For this, gas flow and NDIRsignals are measured simultaneously and then the signals normalized to a constantgas flow. Hence, the pharma TOC does not need a long sample loop to compensatefor occurring pressure variations. In combination with a high-quality NDIR detectorand a particularly precise injection needle, this ensures the user a determination limitof 15 pag/l in daily operation. For the Suitability Tests, the monographs of the pharmacopeias Ph.Eu. 2.2.44 andUSP 23 recommend the use of p-benzoquinone as substitutive substance for pooroxidizing compounds. As the spectrum of organic substances in pharmaceuticallyused waters is far more varied, in addition to the demands of the pharmacopeiasdifferent compounds were chosen to determine their recovery in TOC analysis withthe pharma TOC. In choosing the substances (Table 1), those substances wereconsidered that are frequently used in TOC analysis for the examination of theoxidation capacity of analytical instruments (urea, thiourea, sulphanilic acid andnicotinic acid). In addition, two compounds were chosen whose backbone is beingused in the production of many medicinal drugs (pyridium chloride and quininehydrochloride) and whose oxidizability on the pharma TOC is of interest, too. SP_TOC_3_00_e Substance Molar Mass Empirical Formula Sucrose 342.30 g/mol C12H22O11 Potassium hydrogen phthalate 204.23 g/mol C:H5KO4 p-benzoquinone 108.10 g/mol C6H4O2 Urea 60.06 g/mol C H4N2O Thiourea 76.11 g/mol CH4N2S Nicotinic acid 123.11 g/mol C6H5NO2 Sulphanilic acid 173.19 g/mol C6H7NO3S Pyridinium chloride 115.56 g/mol C5H6NCI Quinine hydrochloride 396.91 g/mol C20H25N2O2CI Table 1: Overview of analyzed substances All measurements were taken on the pharma TOC in differential mode at 800 ℃ in astream of pure oxygen. The injection volume was 2 mL. The concentrations of thestandards were 500 ug/l and 100ug/l. In accordance with the Suitability Test, allmeasurement results are in relation to sucrose (i.e. calibration with sucrosestandards, 500 ug/lsucrose corresponding to 100%). The recovery rates areillustrated in Figures 1 and 2. Figure 1: Recovery rates of the pharma TOC fordifferent standards of a concentration of 500 pg/ 04/00 SP_TOC_3_00_e Figure 2: Recovery rates of the pharma TOC for different standards having a concentration of 100 ug/l The recovery rates of Figures 1 and 2 demonstrate the comparability of the recoveryrates of the chosen substances. Particularly with 500 ug/l, no significant differencesbetween the recovery rates of the different substances could be found. All recoveryrates shown in Figure 1 lie between 95 %and 100%. Besides, the particularly smallstandard deviations (<1% -2 %, shown as error bar) are worth mentioning. Theperformance of the pharma TOC especially shows when looking at the recoveryrates of the concentrations of 100 ug/l. Here, as expected, the standard deviationswere slightly higher than those of the 500 ug/l standards. Altogether, however, with1% to 3% they can still be regarded as being very low. The recovery rates between99% and 105% demonstrate the high precision provided by the pharma TOC below500 pg/l and for a wide range of substances. In summary, the investigations described have shown that the pharma TOC distinctlysurpasses the demands as laid down in the pharmacopeias. Thermal oxidation at800℃ in connection with the platinum catalyst on quartz wool and the VITAdataprocessing method offers the ideal combination for almost complete digestion ofdifferent compounds at particularly low result variations.
确定

还剩2页未读,是否继续阅读?
耶拿分析仪器(北京)有限公司为您提供《医药用水中总有机碳检测方案(TOC分析仪)》,该方案主要用于洗涤用水中总有机碳检测,参考标准--,《医药用水中总有机碳检测方案(TOC分析仪)》用到的仪器有
相关方案
更多
该厂商其他方案
更多








