方案详情
文
使用气相硅,CPEs展示了高的电导特性,机械强度和锂稳定的界面 , 气相硅的表面集团决定了CPE的力学性能,同时低分子量的 PEO 和锂盐决定了离子的传导特性,CPEs表明了作为新一代锂电池电解液的优点.
方案详情

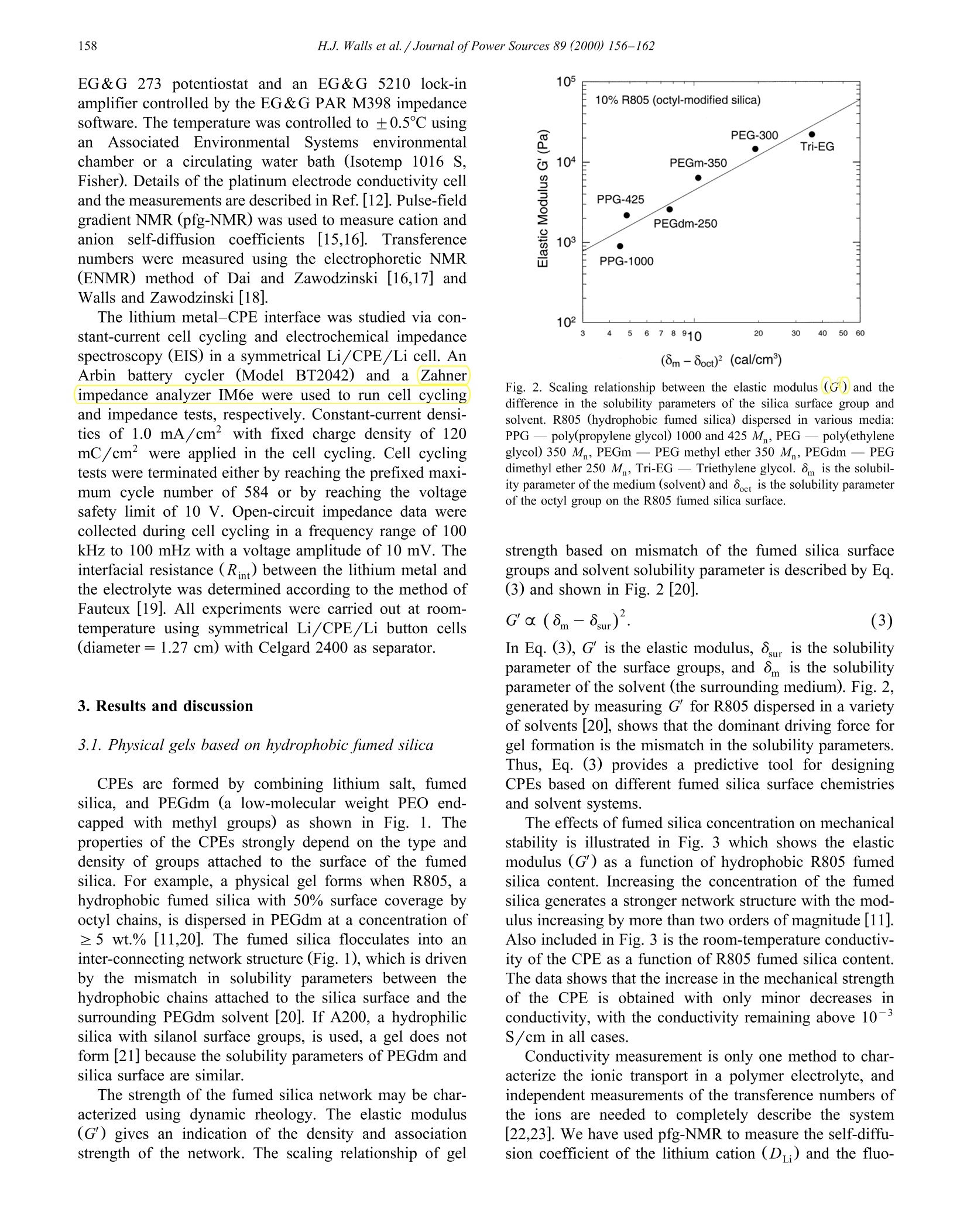
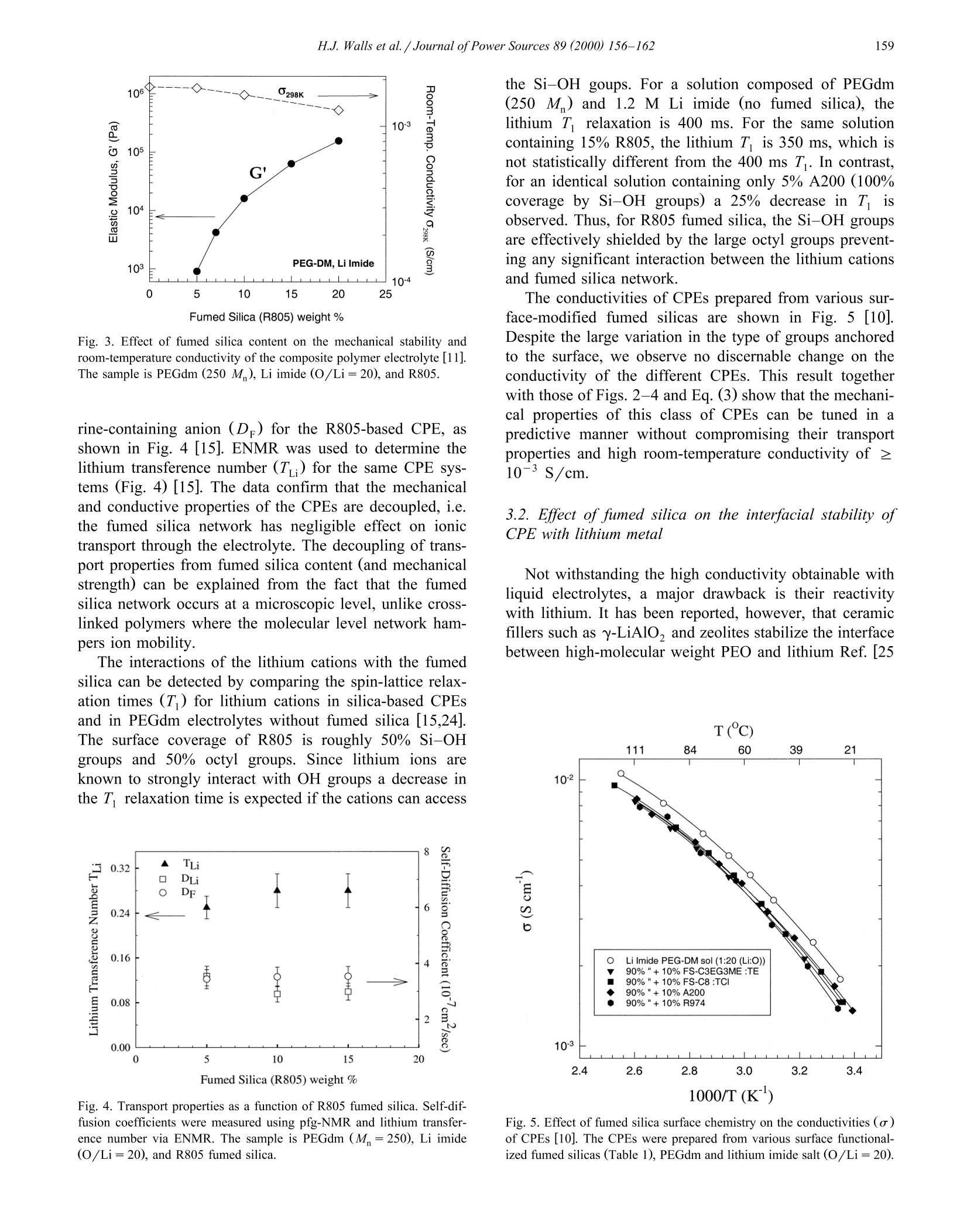
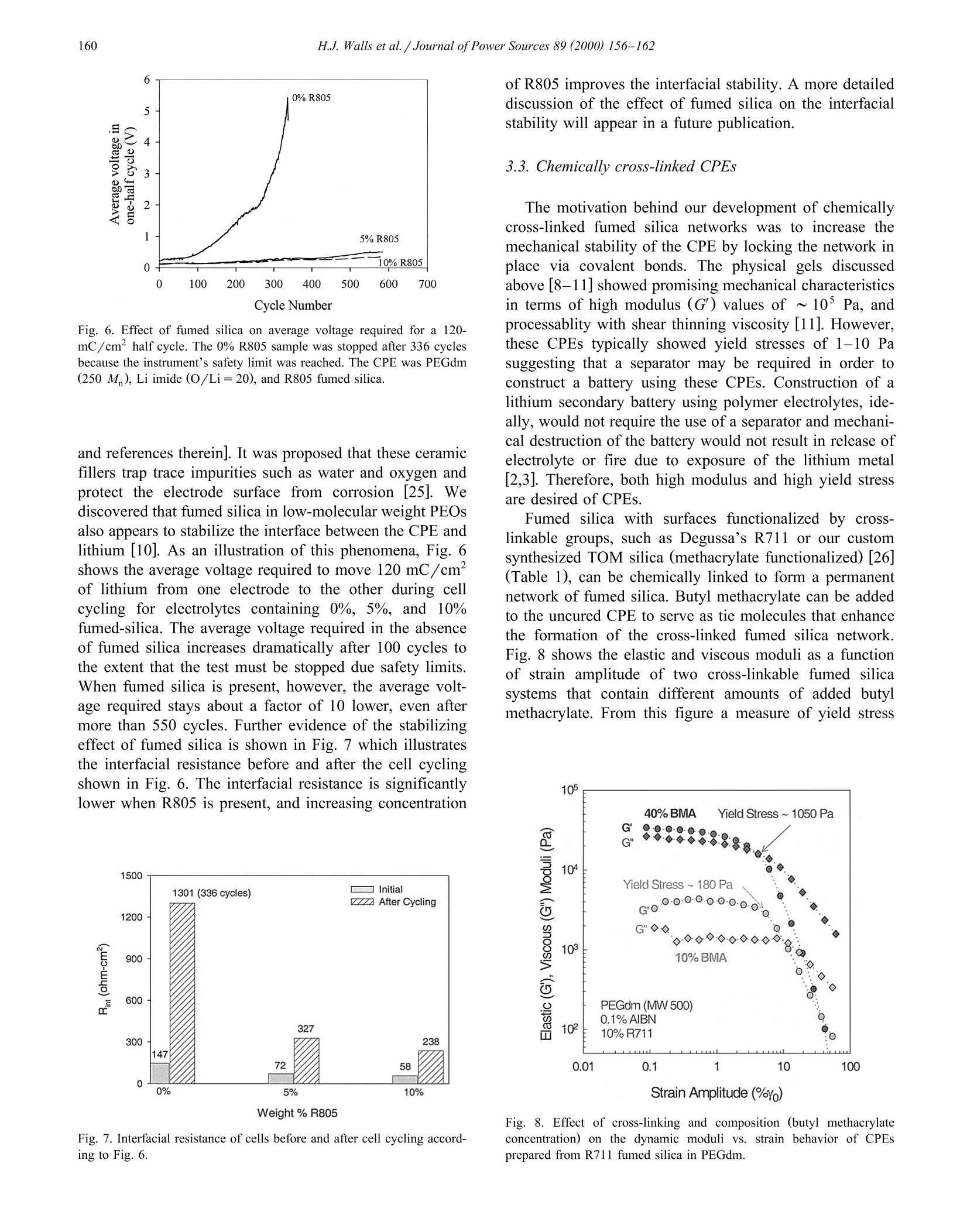
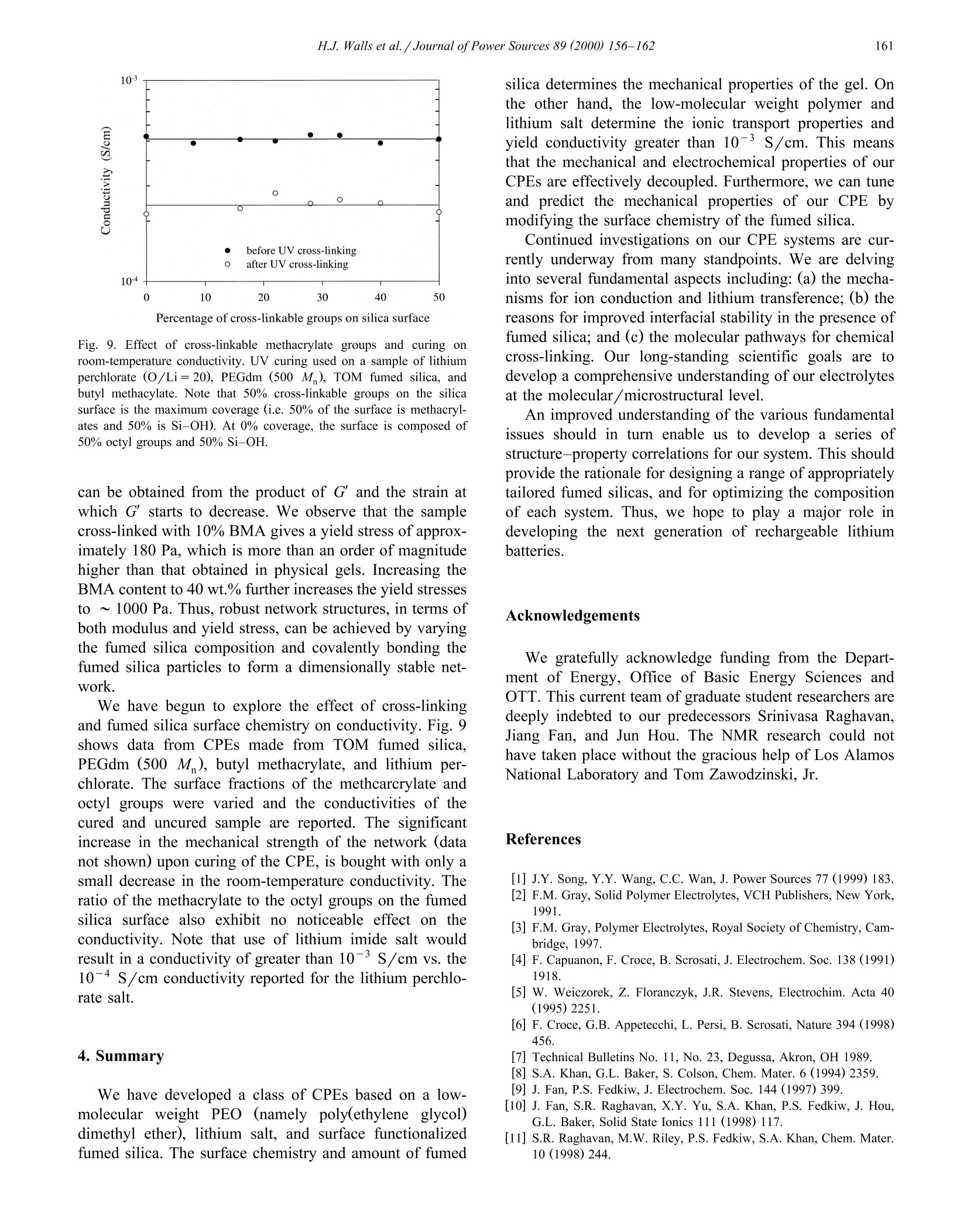
Journal of Power Sources 89 (2000) 156-162www.elsevier.com/locate/jpowsour 157H.J.Walls et al. /Journal ofPower Sources 89 (2000) 156-162 0378-7753/00/$ - see front matter @ 2000 Elsevier Science S.A. All rights reserved.PII: S0378-7753(00)00424-9 Fumed silica-based composite polymer electrolytes: synthesis, rheology,and electrochemistry H.J. Walls , Jian Zhou , Jeffrey A. Yerian, Peter S. Fedkiw a, Saad A. Khan a,*,Micah K. Stowe , Gregory L. Baker Department of Chemical Engineering, North Carolina State Uniuersity, Raleigh, NC 27695-7905, USA Department of Chemistry, Michigan State Uniuersity, East Lansing, MI 48224, USA Received 15 September 1999; accepted 13 December 1999 Abstract An overview of our research is presented on developing composite polymer electrolytes (CPEs) based on low-molecular weightpolyethylene oxide (PEO) (namely, poly(ethylene glycol) dimethyl ether), lithium salts (e.g. lithium triflate, lithium imide, etc.), andfumed silica. These CPEs demonstrate high room-temperature conductivites (>10-3 S/cm), mechanical strength, and form stableinterfaces with lithium metal as a result of the fumed silica. The surface groups on the fumed silica determine the mechanical propertiesof the CPE while the low-molecular weight PEO and lithium salt determine the ionic transport properties. These CPEs show promise aselectrolytes for the next generation of rechargeable lithium batteries. 2000 Elsevier Science S.A. All rights reserved. Keywords: Composite polymer electrolytes; Lithium secondary batteries 1. Introduction To date, the most common approaches to solid polymerelectrolytes for lithium batteries have employed high-molecular weight (M,> 10’) polymers based on the poly-ethylene oxide (PEO) structure [1-3]. When combinedwith lithium salts, linear PEO forms poorly conductivecrystalline complexes with room temperature conductivi-ties <10-5 S/cm [1-3]. The use of random, graft, andblock copolymers limits the crystallinity of the PEO seg-ments, but the room-temperature conductivity of most ofthese polymers rarely exceeds 10-4 S/cm, a value toolow for many applications [2,3]. To obtain high conductivi-ties, the normal operating temperature for these systemsmust be ≥80℃[1-3]. For more than a decade, researchers have recognizedthat adding inorganic fillers to PEO-based electrolytesimproves the conductivity of the electrolytes Refs. [1,4-6.Although initial attempts sought to improve the conductiv-ity of electrolytes through the use of conductive particles, ( Cor r esponding author. T e l.: + 1-919-515-4519; f ax: +1-919-515-3465. ) the dominant effect of inorganic fillers is to decrease thecrystallinty in samples prepared from high-molecularweight PEO, thus stabilizing the highly conductive amor-phous phase. Reports show that smaller particles are themost effective, presumably because their high-surface areainhibits crystallinity [4-6], and that the conductivity offilled electrolytes decreases only slightly for particle con-tents below 30 wt.% Ref. [1 and references therein]. A fundamentally different approach to the use of fillersis exemplified by the addition of fumed silica [7] to low-and moderate-molecular weight PEOs to yield materialswith the mechanical properties of solids, but having theprocessability of liquids [8-12]. The unique features offumed silica are its branched, primary structure consistingof fused SiO particles (Fig. 1) and the ability to tailor thesurface functionalities of the fumed silica. In compositeelectrolytes formed by combining hydrophobic fumed sil-ica, lithium salts and low-molecular weight PEO (Fig. 1),the filler and the electrolyte constitute separate phases,effectively decoupling the mechanical and conductiveproperties of the electrolyte [11]. In an extension of thisapproach, we showed that simple cross-linking reactionsallow conversion of the electrolytes into rubbery materialswith conductivities that rival liquid electrolytes [12]. In thiscommunication, we provide an overview of fumed silica- Fig. 1. Schematic route to CPE based on hydrophobic fumed silica(R805). The fumed silica particles are dispersed in a low-molecularweight PEO with a lithium salt. The fumed silica particles preferentiallyassociate to generate an interconnecting network with open cells contain-ing polymer electrolyte. based composite polymer electrolytes (CPEs) for use inrechargeable lithium and lithium-ion batteries, emphasiz-ing their mechanical and electrochemical properties. 2. Experimental 2.1. Preparation of CPEs Lithium bis(trifluoromethanesulfonylimideLiN-(CF,SO)2], lithium triflate [LiCF,SO;], and lithiumtris(trifluoromethanesulfonyl)methide [LiC(SO,CF,)]were gifts from 3M. The Aerosil fumed silicas were giftsfrom Degussa. All other chemicals were obtained fromAldrich. The lithium salts were dried at 80℃ under vac-uum overnight before use. Poly(ethylene glycol) dimethylether (PEGdm, M 250 and 500) was dried over 4 Amolecular sieves for 1 week. All fumed silicas were driedat 120C under vacuum for 3 days before use in makingCPEs. Table 1 shows the various fumed silicas used formaking CPEs. The CPEs were prepared from PEGdm solutions of thedesired lithium salt (0.3-1.2 M) in an argon-filled glovebox (moisture content <5 ppm). The fumed silica wasthen added to the PEGdm and dispersed into the elec-trolyte by use of a high shear mixer (Fig. 1). The moisturecontent of the CPEs was <50 ppm, as determined byKarl-Fischer titration. The preparation of cross-linkedCPEs are detailed elsewhere [12]. Briefly, fumed silicasmodified to contain surface-bound methacrylates (or simi-lar polymerizable groups) are combined with PEGdm, lithium salt, AIBN, and butyl methacrylate (used as a tiemolecule). This uncured CPE is then cross-linked ther-mally, or via UV radiation from a mercury lamp to formrubbery or hard solids. 2.2. Methods and measurements Rheological measurements were done using either aRheometrics Dynamic Stress Rheometer (DSR-II) or aRheometrics mechanical spectrometer (RMS 800; a straincontrolled rheometer). Details of the dynamic and steadyshear rheology are given elsewhere [11,13]. Here we brieflypresent the two parameters that are most useful for describ-ing the physical properties of the CPEs. Dynamic rheologyinvolves applying a low-amplitude sinusoidal deformation(y) to the sample at some frequency (ω) and maximumstrain amplitude (Y): The sinusoidal-stress response (r) of the sample may bewritten as an in-phase and out-of-phase component asshown in: The in-phase component is attributed to energy stored inthe sample and thus defines the elastic modulus G. Theout-of-phase component is attributed to energy dissipatedand thus defines the viscous modulus G". In short, for aliquid sample G" dominates, and for a solid sample G'dominates. The values of these moduli as a function offrequency are used to characterize materials [13,14]. Conductivity was measured using ac impedance spec-troscopy. Two different impedance spectroscopy setupswere used. One was an EG&G Princeton Applied Re-search 263A potentiostat and an EG& G Princeton AppliedResearch frequency response detector controlled by thePAR 398 impedance software. The second setup was an Table 1 Fumed silicas used to prepare CPEs. Designation is the name in theliterature or specified by us for the particular fumed silica. Note that theDegussa trade name for fumed silica is Aerosil and that Si-OH is the“‘native’surface group. For the modified fumed silicas (those containingsurface groups other then Si-OH), about 50% of the surface coverage isSi-OH and 50% is covered by the designated group(s) Designation Surface group(s) Type of surface Source A200 100% Si-OH hydrophilic Degussa R805 50% Si-C,H hydrophobic Degussa R974 50% Si-(CH3) hydrophobic Degussa R711 methacrylate cross-linkable Degussa TOM Si-CH17 hydrophobic in-house synthesis +Si-(CH2)CO2-cross-linkable FS-C8 CCH,CH, Si-CgH17 hydrophobic in-house synthesis FS-C3EG3ME Si-(CH,)s hydrophilic in-house synthesis -(CHO)3-OCH, EG& G 273 potentiostat and an EG&G 5210 lock-inamplifier controlled by the EG&G PAR M398 impedancesoftware. The temperature was controlled to ±0.5℃ usinganAssociated Environmental Systemssenvironmentalchamber or a circulating water bath (Isotemp 1016 S,Fisher). Details of the platinum electrode conductivity celland the measurements are described in Ref.[12]. Pulse-fieldgradient NMR (pfg-NMR) was used to measure cation andanionself-diffusion coefficients[15,16]. Transferencenumbers were measured using the electrophoretic NMR(ENMR) method of Dai and Zawodzinski [16,17] andWalls and Zawodzinski [18]. The lithium metal-CPE interface was studied via con-stant-current cell cycling and electrochemical impedancespectroscopy (EIS) in a symmetrical Li/CPE/Li cell. AnArbin battery cycler (Model BT2042) and aa(Zahner(impedance analyzer IM6e were used to run cell cyclingand impedance tests, respectively. Constant-current densi-ties of 1.0 mA/cm’ with fixed charge density of 120mC/cm’ were applied in the cell cycling. Cell cyclingtests were terminated either by reaching the prefixed maxi-mum cycle number of 584 or by reaching the voltagesafety limit of 10 V. Open-circuit impedance data werecollected during cell cycling in a frequency range of 100kHz to 100 mHz with a voltage amplitude of 10 mV. Theinterfacial resistance (R) between the lithium metal andthe electrolyte was determined according to the method ofFauteux [19]. All experiments were carried out at room-temperature using symmetrical Li/CPE/Li button cells(diameter = 1.27 cm) with Celgard 2400 as separator. 3. Results and discussion 3.1. Physical gels based on hydrophobic fumed silica CPEs are formed by combining lithium salt, fumedsilica, and PEGdm (a low-molecular weight PEO end-capped with methyl groups) as shown in Fig. 1. Theproperties of the CPEs strongly depend on the type anddensity of groups attached to the surface of the fumedsilica. For example, a physical gel forms when R805, ahydrophobic fumed silica with 50% surface coverage byoctyl chains, is dispersed in PEGdm at a concentration of≥ 5 wt.% [11,20]. The fumed silica flocculates into aninter-connecting network structure (Fig.1), which is drivenby the mismatch in solubility parameters between thehydrophobic chains attached to the silica surface and thesurrounding PEGdm solvent [20]. If A200, a hydrophilicsilica with silanol surface groups, is used, a gel does notform [21] because the solubility parameters of PEGdm andsilica surface are similar. The strength of the fumed silica network may be char-acterized using dynamic rheology. The elastic modulus(G) gives an indication of the density and associationstrength of the network. The scaling relationship of gel (8m-8oct)’ (cal/cm) Fig. 2. Scaling relationship between the elastic modulus () and thedifference in the solubility parameters of the silica surface group andsolvent. R805 (hydrophobic fumed silica) dispersed in various media:PPG -poly(propylene glycol) 1000 and 425 M,PEG - poly(ethyleneglycol) 350 M,PEGm- PEG methyl ether 350 M, PEGdm-PEGdimethyl ether 250 M, Tri-EG - Triethylene glycol. 8m is the solubil-ity parameter of the medium (solvent) and 8e is the solubility parameterof the octyl group on the R805 fumed silica surface. strength based on mismatch of the fumed silica surfacegroups and solvent solubility parameter is described by Eq.(3) and shown in Fig. 2[20]. In Eq. (3), G' is the elastic modulus, 8gur is the solubilityparameter of the surface groups, and 8m is the solubilityparameter of the solvent (the surrounding medium). Fig. 2,generated by measuring G' for R805 dispersed in a varietyof solvents [20], shows that the dominant driving force forgel formation is the mismatch in the solubility parameters.Thus, Eq. (3) provides a predictive tool for designingCPEs based on different fumed silica surface chemistriesand solvent systems. The effects of fumed silica concentration on mechanicalstability is illustrated in Fig. 3 which shows the elasticmodulus (G) as a function of hydrophobic R805 fumedsilica content. Increasing the concentration of the fumedsilica generates a stronger network structure with the mod-ulus increasing by more than two orders of magnitude [11].Also included in Fig.3 is the room-temperature conductiv-ity of the CPE as a function of R805 fumed silica content.The data shows that the increase in the mechanical strengthof the CPE is obtained with only minor decreases inconductivity, with the conductivity remaining above 10-S/cm in all cases. Conductivity measurement is only one method to char-acterize the ionic transport in a polymer electrolyte, andindependent measurements of the transference numbers ofthe ions are needed to completely describe the system[22,23]. We have used pfg-NMR to measure the self-diffu-sion coefficient of the lithium cation (Dui) and the fluo- Fig. 3. Effect of fumed silica content on the mechanical stability androom-temperature conductivity of the composite polymer electrolyte [11].The sample is PEGdm (250 M), Li imide (O/Li=20), and R805. rine-containing anion (D) for the R805-based CPE, asshown in Fig. 4 [15]. ENMR was used to determine thelithium transference number (Tui) for the same CPE sys-tems (Fig. 4) [15]. The data confirm that the mechanicaland conductive properties of the CPEs are decoupled, i.e.the fumed silica network has negligible effect on ionictransport through the electrolyte. The decoupling of trans-port properties from fumed silica content (and mechanicalstrength) can be explained from the fact that the fumedsilica network occurs at a microscopic level, unlike cross-linked polymers where the molecular level network ham-pers ion mobility. The interactions of the lithium cations with the fumedsilica can be detected by comparing the spin-lattice relax-ation times (T) for lithium cations in silica-based CPEsand in PEGdm electrolytes without fumed silica [15,24].The surface coverage of R805 is roughly 50% Si-OHgroups and 50% octyl groups. Since lithium ions areknown to strongly interact with OH groups a decrease inthe Tirelaxation time is expected if the cations can access Fig. 4. Transport properties as a function of R805 fumed silica. Self-dif-fusion coefficients were measured using pfg-NMR and lithium transfer-ence number via ENMR. The sample is PEGdm (M=250), Li imide(O/Li=20), and R805 fumed silica. the Si-OH goups. For a solution composed of PEGdm(250 M) and 1.2 M Li imide (no fumed silica), thelithium T relaxation is 400 ms. For the same solutioncontaining 15% R805, the lithium T is 350 ms, which isnot statistically different from the 400 ms T. In contrast,for an identical solution containing only 5% A200 (100%coverage by Si-OH groups) a 25% decrease in T isobserved. Thus, for R805 fumed silica, the Si-OH groupsare effectively shielded by the large octyl groups prevent-ing any significant interaction between the lithium cationsand fumed silica network. The conductivities of CPEs prepared from various sur-face-modified fumed silicas are shown in Fig. 5 [10].Despite the large variation in the type of groups anchoredto the surface, we observe no discernable change on theconductivity of the different CPEs. This result togetherwith those ofFigs. 2-4 and Eq. (3) show that the mechani-cal properties of this class of CPEs can be tuned in apredictive manner without compromising their transportproperties and high room-temperature conductivity of 10-3 S/cm. 3.2. Effect offumed silica on the interfacial stability ofCPE with lithium metal Not withstanding the high conductivity obtainable withliquid electrolytes, a major drawback is their reactivitywith lithium. It has been reported, however, that ceramicfillers such as y-LiAlO, and zeolites stabilize the interfacebetween high-molecular weight PEO and lithium Ref. [25 Fig.5. Effect of fumed silica surface chemistry on the conductivities (o)of CPEs [10]. The CPEs were prepared from various surface functional-ized fumed silicas (Table 1), PEGdm and lithium imide salt (O/Li=20). Fig. 6. Effect of fumed silica on average voltage required for a 120-mC/cm’ half cycle. The 0% R805 sample was stopped after 336 cyclesbecause the instrument’s safety limit was reached. The CPE was PEGdm(250 M ), Li imide (O/Li=20), and R805 fumed silica. and references therein]. It was proposed that these ceramicfillers trap trace impurities such as water and oxygen andprotect the electrode surface from corrosion [25]. Wediscovered that fumed silica in low-molecular weight PEOsalso appears to stabilize the interface between the CPE andlithium [10]. As an illustration of this phenomena, Fig. 6shows the average voltage required to move 120 mC/cmof lithium from one electrode to the other during cellcycling for electrolytes containing 0%, 5%, and 10%fumed-silica. The average voltage required in the absenceof fumed silica increases dramatically after 100 cycles tothe extent that the test must be stopped due safety limits.When fumed silica is present, however, the average volt-age required stays about a factor of 10 lower, even aftermore than 550 cycles. Further evidence of the stabilizingeffect of fumed silica is shown in Fig. 7 which illustratesthe interfacial resistance before and after the cell cyclingshown in Fig. 6. The interfacial resistance is significantlylower when R805 is present, and increasing concentration Fig. 7. Interfacial resistance of cells before and after cell cycling accord-ing to Fig. 6. of R805 improves the interfacial stability. A more detaileddiscussion of the effect of fumed silica on the interfacialstability will appear in a future publication. 3.3. Chemically cross-linked CPEs The motivation behind our development of chemicallycross-linked fumed silica networks was to increase themechanical stability of the CPE by locking the network inplace via covalent bonds. The physical gels ( cussedabove [8-11] showed promising mechanical characteristicsin terms of high modulus (G') values of ~10° Pa, andprocessablity with shear thinning viscosity [11]. However,these CPEs typically showed yield stresses of 1-10 Pasuggesting that a separator may be required in order toconstruct a battery using these CPEs. Construction of alithium secondary battery using polymer electrolytes, ide-ally, would not require the use of a separator and mechani-cal destruction of the battery would not result in release ofelectrolyte or fire due to exposure of the lithium metal[2,3]. Therefore, both high modulus and high yield stressare desired of CPEs Fumed silica with surfaces functionalized by cross-linkable groups, such as Degussa’s R711 or our customsynthesized TOM silica (methacrylate functionalized) [26](Table 1), can be chemically linked to form a permanentnetwork of fumed silica. Butyl methacrylate can be addedto the uncured CPE to serve as tie molecules that enhancethe formation of the cross-linked fumed silica network.Fig. 8 shows the elastic and viscous moduli as a functionof strain amplitude of two cross-linkable fumed silicasystems that contain different amounts of added butylmethacrylate. From this figure a measure of yield stress Fig. 8. Effect of cross-linking and composition (butyl methacrylateconcentration) on the dynamic moduli vs. strain behavior of CPEsprepared from R711 fumed silica in PEGdm. Fig. 9. Effect of cross-linkable methacrylate groups and curing onroom-temperature conductivity.UV curing used on a sample of lithiumperchlorate (O/Li=20), PEGdm (500 M), TOM fumed silica, andbutyl methacylate. Note that 50% cross-linkable groups on the silicasurface is the maximum coverage (i.e. 50% of the surface is methacryl-ates and 50% is Si-OH). At 0% coverage, the surface is composed of50% octyl groups and 50% Si-OH. can be obtained from the product of G and the strain atwhich G starts to decrease. We observe that the samplecross-linked with 10% BMA gives a yield stress of approx-imately 180 Pa, which is more than an order of magnitudehigher than that obtained in physical gels. Increasing theBMA content to 40 wt.% further increases the yield stressesto ~1000 Pa. Thus, robust network structures, in terms ofboth modulus and yield stress, can be achieved by varyingthe fumed silica composition and covalently bonding thefumed silica particles to form a dimensionally stable net-work. We have begun to explore the effect of cross-linkingand fumed silica surface chemistry on conductivity. Fig. 9shows data from CPEs made from TOM fumed silica.PEGdm (500 M), butyl methacrylate, and lithium per-chlorate. The surface fractions of the methcarcrylate andoctyl groups were varied and the conductivities of thecured and uncured sample are reported. The significantincrease in the mechanical strength of the network (datanot shown) upon curing of the CPE, is bought with only asmall decrease in the room-temperature conductivity. Theratio of the methacrylate to the octyl groups on the fumedsilica surface also exhibit no noticeable effect on theconductivity. Note that use of lithium imide salt wouldresult in a conductivity of greater than 10-3 S/cm vs. the10-4 S/cm conductivity reported for the lithium perchlo-rate salt. 4. Summary We have developed a class of CPEs based on a low-molecular weight PEO (namely poly(ethylene glycol)dimethyl ether), lithium salt, and surface functionalizedfumed silica. The surface chemistry and amount of fumed silica determines the mechanical properties of the gel. Onthe other hand, the low-molecular weight polymer andlithium salt determine the ionic transport properties andyield conductivity greater than 10-3S/cm. This meansthat the mechanical and electrochemical properties of ourCPEs are effectively decoupled. Furthermore, we can tuneand predict the mechanical properties of our CPE bymodifying the surface chemistry of the fumed silica. Continued investigations on our CPE systems are cur-rently underway from many standpoints. We are delvinginto several fundamental aspects including: (a) the mecha-nisms for ion conduction and lithium transference; (b) thereasons for improved interfacial stability in the presence offumed silica; and (c) the molecular pathways for chemicalcross-linking. Our long-standing scientific goals are todevelop a comprehensive understanding of our electrolytesat the molecular/microstructural level. An improved understanding of the various fundamentalissues should in turn enable us to develop a series ofstructure-property correlations for our system. This shouldprovide the rationale for designing a range of appropriatelytailored fumed silicas, and for optimizing the compositionof each system. Thus, we hope to play a major role indeveloping the next generation of rechargeable lithiumbatteries. Acknowledgements We gratefully acknowledge funding from the Depart-ment of Energy, Office of Basic Energy Sciences andOTT. This current team of graduate student researchers aredeeply indebted to our predecessors Srinivasa Raghavan,Jiang Fan, and Jun Hou. The NMR research could nothave taken place without the gracious help of Los AlamosNational Laboratory and Tom Zawodzinski, Jr. References [1] J.Y. Song, Y.Y. Wang, C.C. Wan, J. Power Sources 77(1999) 183. [2] F.M. Gray, Solid Polymer Electrolytes, VCH Publishers, New York,1991. [3] F.M. Gray, Polymer Electrolytes, Royal Society of Chemistry, Cam-bridge, 1997. [4] F. Capuanon, F. Croce, B. Scrosati, J. Electrochem. Soc. 138 (1991)1918. [5] W. Weiczorek, Z. Floranczyk, J.R. Stevens, Electrochim. Acta 40(1995) 2251. [6] F. Croce, G.B. Appetecchi, L. Persi, B. Scrosati, Nature 394 (1998)456. [7] Technical Bulletins No. 11, No. 23, Degussa, Akron, OH 1989. [8] S.A. Khan, G.L. Baker, S. Colson, Chem. Mater. 6(1994) 2359. [9] J. Fan, P.S. Fedkiw, J. Electrochem. Soc. 144 (1997) 399. [10] J. Fan, S.R. Raghavan,X.Y. Yu, S.A. Khan, P.S. Fedkiw, J. Hou,G.L. Baker, Solid State Ionics 111 (1998) 117. [11] S.R. Raghavan, M.W. Riley, P.S. Fedkiw, S.A. Khan, Chem. Mater.10 (1998) 244. ( [12] J . Hou, G .L. B aker, Chem. M a ter. 10 (1998) 33 1 1. ) ( [13] C.W. M acosko, R h eology: P r inciples, Measurements, and Applica- tions,VCH P u blisher, New Y o rk, 1 9 94. ) ( [ 1 4] J.D. F erry, V iscoelastic P roperties o f Polymers, 3rd ed n ., Wi l ey,New York, 1 980. ) ( [15] H.J. Walls, T.A. Z awodzinski, P . S. Fedkiw, S .A . Khan, Pr o ceed- ings, 1999 J oint I nternationa l Mtg. Of ECS, H onolulu, HA, Oct. 1 999 (submitted). ) ( [16] H . Dai, PhD Thesis, University of California at Santa Ba r bara, 1998.[ 1 7 ] H . Dai, T.A. Zawodzinski, J. Elecrochem. Soc. 143 (1996) L107. ) ( [ 1 8] H .J. W alls, T.A. Z a wodzinski, J. El e ctrochem. Soc. (2000) in pres s . [ 1 9] D . F auteux, S olid S tate Ionics 17 (1985) 1 33. ) [20] S.R. Raghavan,J. Hou, G.L. Baker, S.A. Khan, Langmuir 16 (2000)1066. ( [21] S .R. R aghavan, S . A. Khan, J. Colloid In t erface Sci. 1 8 5 (1997) 57 . ) ( [22] M. D oyl, T.F. F uller, J . Newman, E lectrochim. Acta 39 ( 1994) 2073. ) [23] Y. Ma, M. Doyle, T.F. Fuller, M.M. Doeff, L.C. De Jonghe, J.Newman, J. Electrochem. Soc. 142 (1995) 1859. ( [24] E. Fukushima, S.B.W. R oeder, Experimental Pulse N MR: A N uts and B olts Approach, Addison-Wesley, Reading, 1981. ) ( [25] G.B. Appetecchi et al., J. E lectrochem. Soc. 14 5 (1998) 4126. ) ( [ 26] J. Hou, G.L. Baker, ACS PMSE Pr o c. 80 (19 9 9) 614. )
确定



还剩5页未读,是否继续阅读?
香港环球分析测试仪器有限公司为您提供《气相二氧化硅的高分子电解液中合成,流变性和电化学特性检测方案(电化学工作站)》,该方案主要用于其他中电化学性能检测,参考标准--,《气相二氧化硅的高分子电解液中合成,流变性和电化学特性检测方案(电化学工作站)》用到的仪器有
相关方案
更多









