锂离子电池具有工作电压高、能量密度大以及循环寿命长等优点,已经被广泛应用于各种便携式电子设备以及电动汽车等领域。然而,锂资源的稀缺性以及成本问题制约了锂离子电池在大规模储能系统中的应用。相对于锂而言,锌元素在地壳中的分布广泛、储量丰富,使得锌离子电池具有明显的资源优势以及价格优势。然而,带有多价态电子的锌离子在正极材料中嵌脱缓慢,动力学性能较差,严重影响了电池的比容量、循环稳定性和倍率性能。因此,仍需开发新型高性能正极材料。
方案详情

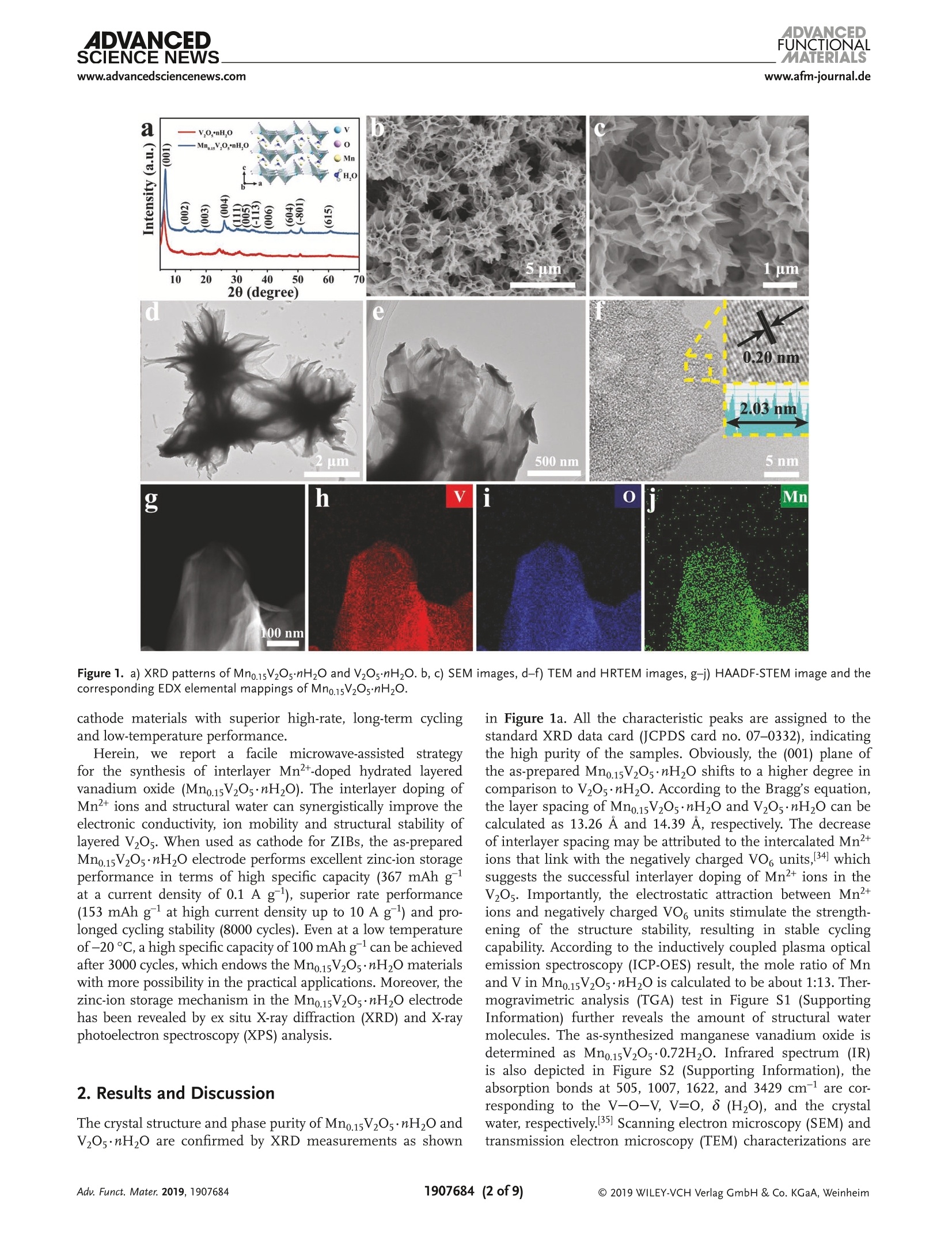
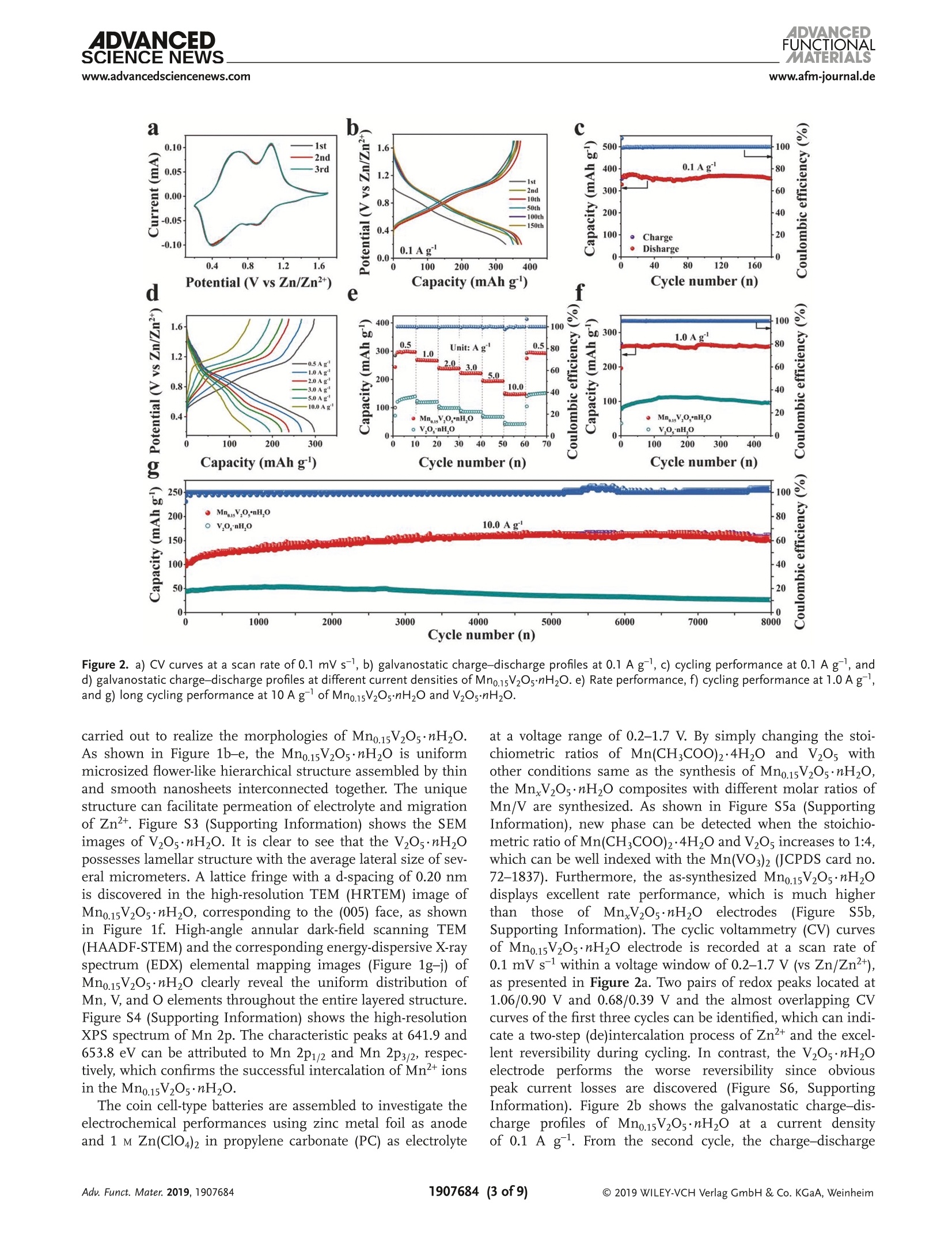
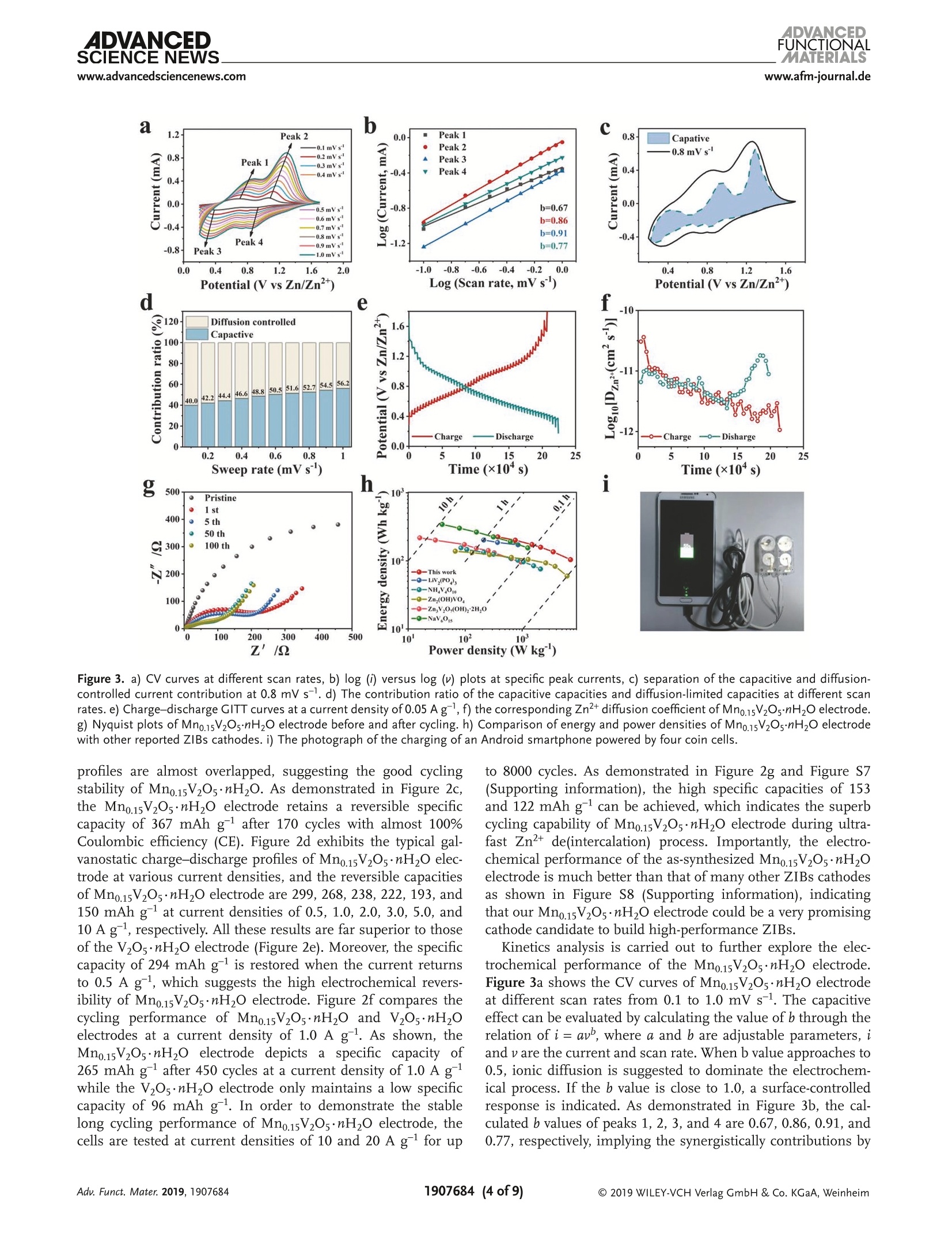
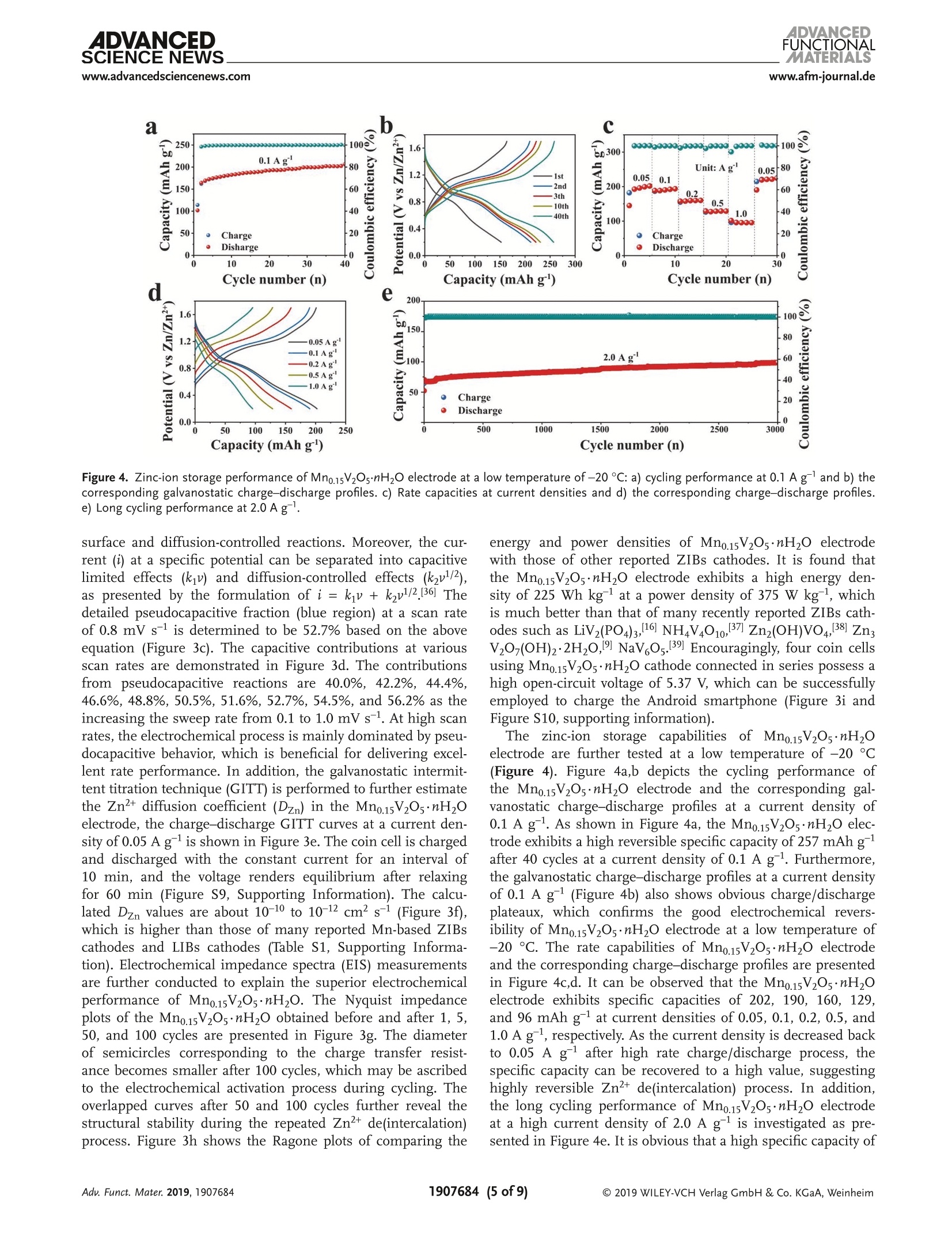
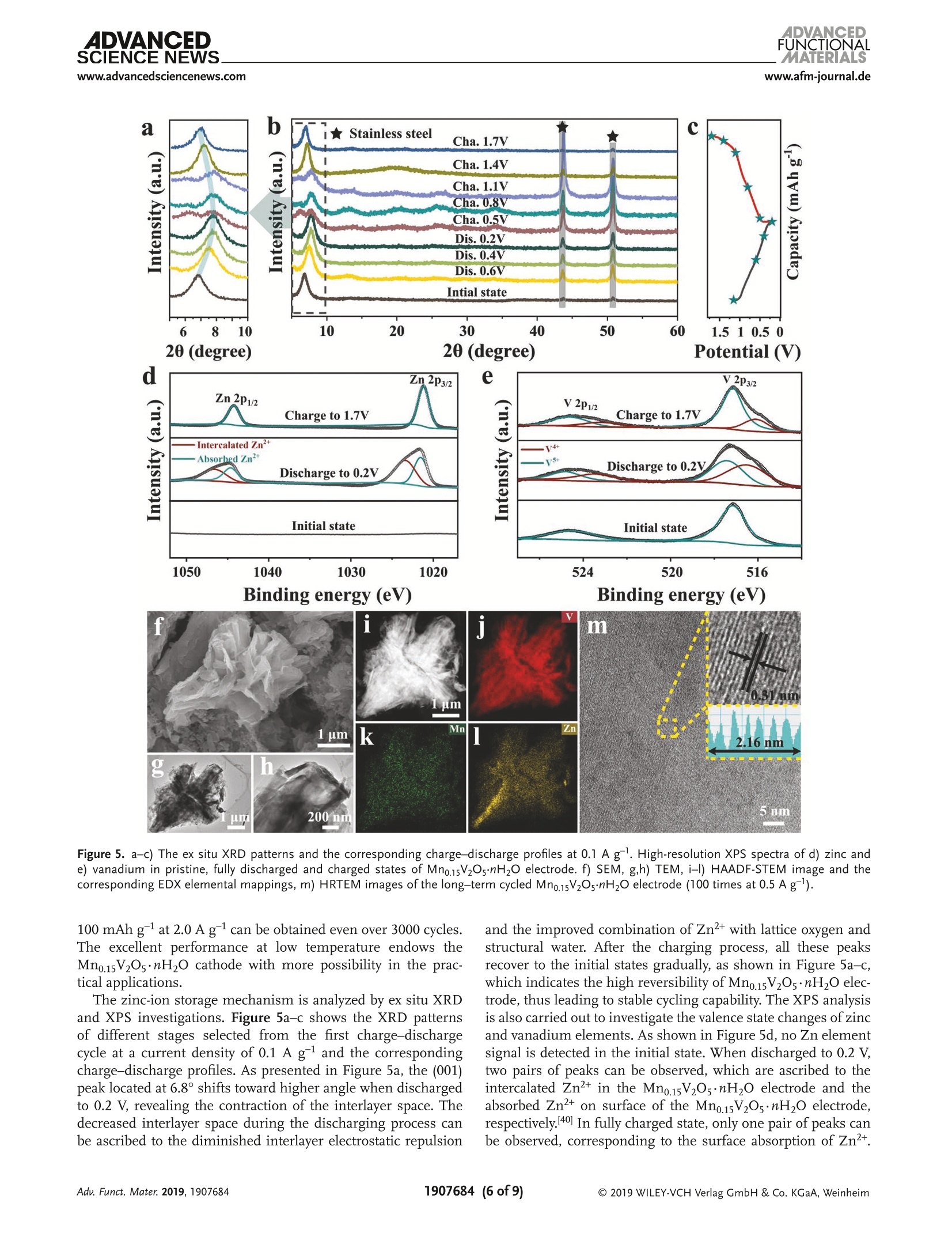
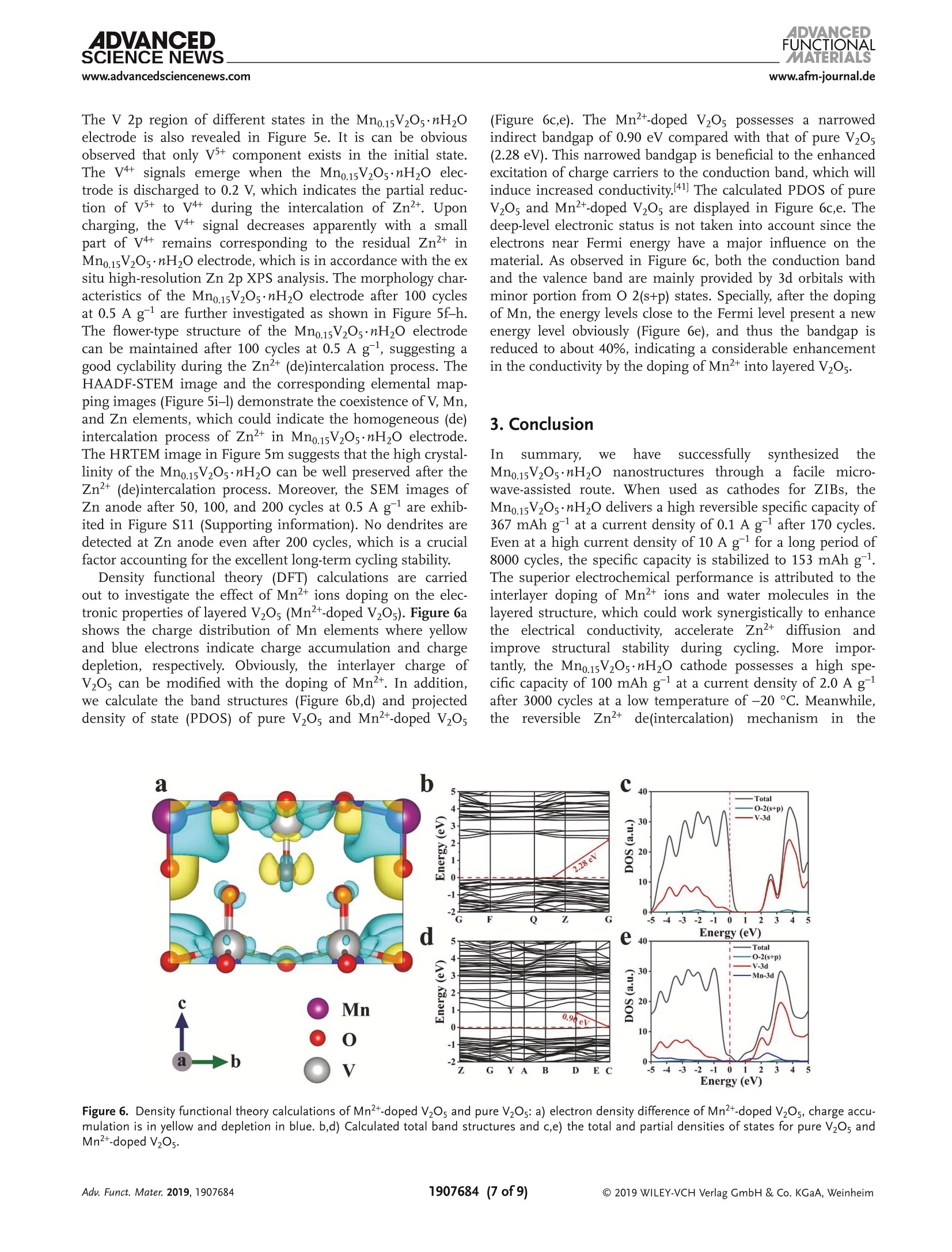
ADVANCEDFUNCTIONAMATERIALSFULL PAPERwww.afm-journal.d ADVANCEDSCIENCE NEWSs.www.advancedsciencenews.comwww.afm-journal.de Electronic Structure Regulation of Layered Vanadium Oxidevia Interlayer Doping Strategy toward Superior High-Rateand Low-Temperature Zinc-lon Batteries Hongbo Geng, Min Cheng, Bo Wang, Yang Yang, Yufei Zhang, and Cheng Chao Li* Currently, development of suitable cathode materials for zinc-ion batteries(ZIBs) is plagued by the sluggish kinetics of Zn+ with multivalent chargein the host structure. Herein, it is demonstrated that interlayer Mn2+-dopedlayered vanadium oxide (Mn0.15V2O5·nH2O) composites with narrowed directbandgap manifest greatly boosted electrochemical performance as zinc-ionbattery cathodes. Specifically, the Mn0.15V2Os·nH2O electrode shows a highspecific capacity of 367 mAh g-at a current density of 0.1 A g-as wellas excellent retentive capacities of 153 and 122 mAh gafter 8000 cyclesat high current densities up to 10 and 20 A g,respectively. Even at alow temperature of -20 C, a reversible specific capacity of 100 mAh g'can be achieved at a current density of 2.0 Agafter 3000 cycles. Thesuperior electrochemical performance originates from the synergisticeffects between the layered nanostructures and interlayer doping of Mn’+ions and water molecules, which can enhance the electrons/ions transportkinetics and structural stability during cycling. With the aid of various ex situcharacterization technologies and density functional theory calculations, thezinc-ion storage mechanism can be revealed, which provides fundamentalguidelines for developing high-performance cathodes for ZIBs. to their low cost and environmentallyfriendly, 11,12] among which zinc ion bat-teries (ZIBs) exhibit considerable advan-tagessiincludingrelativelylow Iredoxpotential of Zn²+/Zn (-0.76 V vs standardhydrogen electrode), high theoretical spe-cific capacity of Zn (820 mAh g) andhigh energy density.[13-15] Nevertheless,the large atom mass and strong electro-static interaction between divalent Zn+and the host lattice often cause sluggishtransport kinetics, thus leading to poorrate performance and inadequate cyclingstability.[16-18] Therefore, it is still neededto explore appropriate electrode mate-rials for ZIBs that can render enough ionstorage space and ensure reversible andfast Zn+de(intercalation). Recently, a series of cathode materials,such as polymorphs of MnO2, vanadium-based composites, Prussian blue analogs,metal sulfides and organic compounds,have been researched in ZIBs.[19-25] Amongthem, vanadium pentoxide (V20s) with lay- 1. Introduction The development of electronic energy storage systems (ESS)has attracted great attentions to keep up with the ever growingdemanddfor renewable energies.1-3] ILithium-ionbatteries(LIBs) are now one of the promising energy storage devicesbecause of their high energy and power densities.4-6]However,the low abundance and exorbitant cost of lithium resourceslimit its further applications in large-scale ESS.I7-10 As alter-natives, rechargeable batteries based on multivalent cations(Al+, Mg, Ca and Zn?+) are proposed by researchers due Dr. H. Geng, M. Cheng, B. Wang, Dr. Y. Yang, Dr. Y. Zhang, Prof. C. C. LiSchool of Chemical Engineering and Light IndustryGuangdong University of TechnologyGuangzhou 510006, ChinaE-mail: licc@gdut.edu.cnDr. H. Geng School of Chemistry and Materials Engineering Changshu Institute of Technology Changshu,Jiangsu 215500, China The ORCID identification number(s) for the author(s) of this articlecan be found under https://doi.org/10.1002/adfm.201907684. DOI: 10.1002/adfm.201907684 ered structure constructed by square pyramidal VO, units hasbeen proved to serve as the cathode for ZIBs due to the mul-tiple oxidation states of vanadium and its high abundance.126,27But V2Os suffers from low electrons/ions transfer dynamics andfragile structure during cycling, which will lead to rapid capacitydecay and inferior rate capability.[28] To tackle the above-men-tioned issue, significant research strategies have been attemptedto optimize the zinc-ion storage performance of V20s includingnanostructuring, structural modification, cations/moleculespillaring, etc. It is worth noting that the intercalation of metalions and water molecules in the interlayers of V20s could act aspillars to improve the structural stability and ensure fast elec-trochemical reaction dynamics, which is an emerging approachto improve the electrochemical performance of V2O5.[29,30]For instance, Nazar et al. have reported highly homogeneousZn0.25V2O ·nH20 nanobelts prepared through a scalable micro-wave hydrothermal route, which demonstrated a high reversiblespecific capacity of 250-300 mAh g-and good cycling stabilitywith capacity retention of more than 80% after 1000 cycles.31Particularly, Alshareef and co-workers synthesized layeredCa0.25V2Os·nH2O and MgV20s·nH20 that exhibited stablecycling performance and good rate capability.[32,33] Despite allthese efforts, the study of ZIBs is still in its primary stage, andit is hence highly desirable yet very challenging to develop ZIBs V.o,*nH,O 100 nm Figure 1. a) XRD patterns of Mn0.15V2Os·nH20 and V20s·nH2O. b, c) SEM images, d-f) TEM and HRTEM images, g-j) HAADF-STEM image and thecorresponding EDX elemental mappings of Mn0.15V2Os·nH2O. cathode materials with superior high-rate, long-term cyclingand low-temperature performance. Herein1., we report a facile microwave-assisted strategyfor the synthesis of interlayer Mn +-doped hydrated layeredvanadium oxide (Mno.15V2Os·nH2O). The interlayer doping ofMn+ions and structural water can synergistically improve theelectronic conductivity, ion mobility and structural stability oflayered V20s. When used as cathode for ZIBs, the as-preparedMn0.15V2Os·nH20 electrode performs excellent zinc-ion storageperformance in terms of high specific capacity (367 mAh gat a current density of 0.1 A g), superior rate performance(153 mAh gat high current density up to 10 Ag) and pro-longed cycling stability (8000 cycles). Even at a low temperatureof-20°℃, a high specific capacity of 100 mAhgcan be achievedafter 3000 cycles, which endows the Mno.15V2O5·nH20 materialswith more possibility in the practical applications. Moreover, thezinc-ion storage mechanism in the Mno.15V20s·nH2O electrodehas been revealed by ex situ X-ray diffraction (XRD) and X-rayphotoelectron spectroscopy (XPS) analysis. 2. Results and Discussion The crystal structure and phase purity of Mn0.15V2Os·nH2O andV2Os·nH20 are confirmed by XRD measurements as shown in Figure 1a. All the characteristic peaks are assigned to thestandard XRD data card (JCPDS card no. 07-0332), indicatingthe high purity of the samples. Obviously, the (001) plane ofthe as-prepared Mno.15V2Os·nH20 shifts to a higher degree incomparison to V205·nH2O. According to the Bragg’s equation,the layer spacing of Mn0.15V2Os·nH20 and V205·nH2O can becalculated as 13.26 A and 14.39 A, respectively. The decreaseof interlayer spacing may be attributed to the intercalated Mnions that link with the negatively charged VO6 units, 34 whichsuggests the successful interlayer doping of Mn²+ ions in theV2Os. Importantly, the electrostatic attraction between Mn"ions and negatively charged VO, units stimulate the strength-ening of the structure stability, resulting in stable cyclingcapability. According to the inductively coupled plasma opticalemission spectroscopy (ICP-OES) result, the mole ratio of Mnand Vin Mno.15V205·nH20 is calculated to be about 1:13. Ther-mogravimetric analysis (TGA) test in Figure S1 (SupportingInformation) further reveals the amount of structural watermolecules. The as-synthesized manganese vanadium oxide isdetermined as Mno.15V2O5·0.72H2O. Infrared spectrum (IR)is also depicted in Figure S2 (Supporting Information), theabsorption bonds at 505, 1007, 1622, and 3429 cm-are cor-responding to the V-O-V, V=0, 8 (H20), and the crystalwater, respectively.35] Scanning electron microscopy (SEM) andtransmission electron microscopy (TEM) characterizations are Figure 2. a) CV curves at a scan rate of 0.1 mV s-, b) galvanostatic charge-discharge profiles at 0.1 A g,c) cycling performance at 0.1 Ag, andd) galvanostatic charge-discharge profiles at different current densities of Mn0.15V2O5·nH2O. e) Rate performance, f) cycling performance at 1.0 A g,and g) long cycling performance at 10 A gof Mn0.15V2Os·nH20 and V2Os·nH2O. carried out to realize the morphologies of Mn0.15V2Os·nH2O.As shown in Figure 1b-e, the Mno.15V2Os·nH20 is uniformmicrosized flower-like hierarchical structure assembled by thinand smooth nanosheets interconnected together. The uniquestructure can facilitate permeation of electrolyte and migrationof Zn2+. Figure S3 (Supporting Information) shows the SEMimages of V2Os·nH2O. It is clear to see that the V2Os·nH2Opossesses lamellar structure with the average lateral size of sev-eral micrometers. A lattice fringe with a d-spacing of 0.20 nmis discovered in the high-resolution TEM (HRTEM) image ofMn0.15V2Os·nH2O, corresponding to the (005) face, as shownin Figure 1f. High-angle annular dark-field scanning TEM(HAADF-STEM) and the corresponding energy-dispersive X-rayspectrum (EDX) elemental mapping images (Figure 1g-j) ofMno.15V2Os·nH2O clearly reveal the uniform distribution ofMn, V, and O elements throughout the entire layered structure.Figure S4 (Supporting Information) shows the high-resolutionXPS spectrum of Mn 2p. The characteristic peaks at 641.9 and653.8 eV can be attributed to Mn 2p12 and Mn 2p32, respec-tively, which confirms the successful intercalation of Mn+ionsin the Mno.15V2Os·nH20. The coin cell-type batteries are assembled to investigate theelectrochemical performances using zinc metal foil as anodeand 1 M Zn(Cl04)2 in propylene carbonate (PC) as electrolyte at a voltage range of 0.2-1.7 V. By simply changing the stoi-chiometric ratios of Mn(CH,COO)2·4H20 and V20s withother conditions same as the synthesis of Mn0.15V2O5·nH2O,the MnV20s·nH20 composites with different molar ratios ofMn/V are synthesized. As shown in Figure S5a (SupportingInformation), new phase can be detected when the stoichio-metric ratio of Mn(CH,COO)2·4H2O and V20s increases to 1:4,which can be well indexed with the Mn(VO3)2 (JCPDS card no.72-1837). Furthermore, the as-synthesized Mno.15V20s·nH2Odisplays excellent rate performance, which is much higherthan those of MnV20s·nH2O electrodes(Figure SS5b.Supporting Information). The cyclic voltammetry (CV) curvesof Mn0.15V20s·nH2O electrode is recorded at a scan rate of0.1 mV s-l within a voltage window of 0.2-1.7 V (vs Zn/Zn²+),as presented in Figure 2a. Two pairs of redox peaks located at1.06/0.90 V and 0.68/0.39 V and the almost overlapping CVcurves ofthe first three cycles can be identified, which can indi-cate a two-step (de)intercalation process of Zn2+ and the excel-lent reversibility during cycling. In contrast, the V2Os·nH20electrodePperformstthe worse reversibilitsyince obviouspeak current losses are discovered (Figure S6, SupportingInformation). Figure 2b shows the galvanostatic charge-dis-charge profiles of Mn0.15V2Os·nH20 at a current densityof 0.1 A g. From the second cycle, the charge-discharge a 1.2 b C Peak 2 0.0 Peak 1 0.8 Capative -0.1mVs Peak 2 -0.8mVs 0.8 Peak 1 Peak 3 0.4 0.0 )_-0.4 -0.8 0.0 d 120 g500 400 300 N2200 100 Z'/Q Power density (Wkg) Figure 3. a) CV curves at different scan rates, b) log (i) versus log (v) plots at specific peak currents,c) separation of the capacitive and diffusion-controlled current contribution at 0.8 mVs-. d) The contribution ratio of the capacitive capacities and diffusion-limited capacities at different scanrates. e) Charge-discharge GITT curves at a current density of 0.05 Ag,f) the corresponding Zn?+ diffusion coefficient of Mno.15V2Os·mH2O electrode.g) Nyquist plots of Mn0.15V2Os·nH2O electrode before and after cycling. h) Comparison of energy and power densities of Mn0.15V2Os·nH2O electrodewith other reported ZIBs cathodes. i) The photograph of the charging of an Android smartphone powered by four coin cells. profiles are almost overlapped, suggesting the good cyclingstability of Mn0.15V20s·nH20. As demonstrated in Figure 2c,the Mno.15V20s·nH20 electrode retains a reversible specificcapacity of 367 mAh g after 170 cycles with almost 100%Coulombic efficiency (CE). Figure 2d exhibits the typical gal-vanostatic charge-discharge profiles of Mn0.15V20·nH20 elec-trode at various current densities, and the reversible capacitiesof Mno.15V2Os·nH2O electrode are 299, 268, 238,222,193, and150 mAh g-at current densities of 0.5, 1.0, 2.0, 3.0, 5.0, and10 A g, respectively. All these results are far superior to thoseof the V2Os·nH2O electrode (Figure 2e). Moreover, the specificcapacity of 294 mAh g-is restored when the current returnsto 0.5 A g, which suggests the high electrochemical revers-ibility of Mno.15V2Os·nH2O electrode. Figure 2f compares thecycling performance of Mno.15V20s·nH20 and V20s·nH20electrodes at a current density of 1.0 A g. As shown, theMno.15V2Os·nH2O electrode depicts a specific capacity of265 mAh gafter 450 cycles at a current density of 1.0 A gwhile the V205·nH2O electrode only maintains a low specificcapacity of 96 mAh g. In order to demonstrate the stablelong cycling performance of Mn0.15V20s·nH2O electrode, thecells are tested at current densities of 10 and 20 A gfor up to 8000 cycles. As demonstrated in Figure 2g and Figure S7(Supporting information), the high specific capacities of 153and 122 mAh g-can be achieved, which indicates the superbcycling capability of Mno.15V2Os·nH2O electrode during ultra-fast Zn²+ de(intercalation) process. Importantly, the electro-chemical performance of the as-synthesizedMn0.15V205·nH2Oelectrode is much better than that of many other ZIBs cathodesas shown in Figure S8 (Supporting information), indicatingthat our Mno.15V2Os·nH2O electrode could be a very promisingcathode candidate to build high-performance ZIBs. Kinetics analysis is carried out to further explore the elec-trochemical performance of the Mn0.15V2Os·nH2O electrode.Figure 3a shows the CV curves of Mn0.15V20s·nH2O electrodeat different scan rates from 0.1 to 1.0 mV s-. The capacitiveeffect can be evaluated by calculating the value of b through therelation of i =av, where a and b are adjustable parameters, iand v are the current and scan rate. When b value approaches to0.5, ionic diffusion is suggested to dominate the electrochem-ical process. If the b value is close to 1.0, a surface-controlledresponse is indicated. As demonstrated in Figure 3b, the cal-culated b values of peaks 1, 2, 3, and 4 are 0.67, 0.86, 0.91, and0.77, respectively, implying the synergistically contributions by Figure 4. Zinc-ion storage performance of Mn0.15V2O·nH2O electrode at a low temperature of-20 ℃: a) cycling performance at 0.1 Ag and b) thecorresponding galvanostatic charge-discharge profiles. c) Rate capacities at current densities and d) the corresponding charge-discharge profiles.e) Long cycling performance at 2.0 Ag surface and diffusion-controlled reactions. Moreover, the cur-rent (i) at a specific potential can be separated into capacitivelimited effects (kiu) and diffusion-controlled effects (kzu1/2),as presented by the formulation of i=kjv+ kzv1/2 [36] Thedetailed pseudocapacitive fraction (blue region) at a scan rateof 0.8 mV s-1 is determined to be 52.7% based on the aboveequation (Figure 3c). The capacitive contributions at variousscan rates are demonstrated in Figure 3d. The contributionsfrom pseudocapacitive reactions are 40.0%, 42.2%, 44.4%,46.6%, 48.8%,50.5%, 51.6%,52.7%,54.5%, and 56.2% as theincreasing the sweep rate from 0.1 to 1.0 mV s-1. At high scanrates, the electrochemical process is mainly dominated by pseu-docapacitive behavior, which is beneficial for delivering excel-lent rate performance. In addition, the galvanostatic intermit-tent titration technique (GITT) is performed to further estimatethe Zn"+ diffusion coefficient (Dzn) in the Mno.15V2Os·nH2Oelectrode, the charge-discharge GITT curves at a current den-sity of 0.05 A gis shown in Figure 3e. The coin cell is chargedand discharged with the constant current for an interval of10 min, and the voltage renders equilibrium after relaxingfor 60 min (Figure S9, Supporting Information). The calcu-lated Dzn values are about 10-10 to 10-12 cm² s-1 (Figure 3f),which is higher than those of many reported Mn-based ZIBscathodes and LIBs cathodes (Table S1, Supporting Informa-tion). Electrochemical impedance spectra (EIS) measurementsare further conducted to explain the superior electrochemicalperformance of Mno.15V205·nH2O. The Nyquist impedanceplots of the Mn0.15V2Os·nH20 obtained before and after 1, 5,50, and 100 cycles are presented in Figure 3g. The diameterof semicircles corresponding to the charge transfer resist-ance becomes smaller after 100 cycles, which may be ascribedto the electrochemical activation process during cycling. Theoverlapped curves after 50 and 100 cycles further reveal thestructural stability during the repeated Zn+ de(intercalation)process. Figure 3h shows the Ragone plots of comparing the energy and power densities of Mno.15V2Os·nH2O electrodewith those of other reported ZIBs cathodes. It is found thatthe Mno.15V2Os·nH2O electrode exhibits a high energy den-sity of 225 Wh kg at a power density of 375 W kg, whichis much better than that of many recently reported ZIBs cath-61 NHodes such as LiVz(PO4)3,16]NH4V4010,37Zn2(OH)VO4,38] ZngV207(OH)2·2H20, NaV,0s.39 Encouragingly, four coin cellsusing Mno.15V2Os·nH2O cathode connected in series possess ahigh open-circuit voltage of 5.37 V, which can be successfullyemployed to charge the Android smartphone (Figure 3i andFigure S10, supporting information). The zinc-ion storage capabilities of Mn0.15V205·nH20electrode are further tested at a low temperature of -20 C(Figure 4). Figure 4a,b depicts the cycling performance ofthe Mno.15V2Os·nH2O electrode and the corresponding gal-vanostatic charge-discharge profiles at a current density of0.1 Ag. As shown in Figure 4a, the Mno.15V2Os·nH20 elec-trode exhibits a high reversible specific capacity of257 mAh gafter 40 cycles at a current density of0.1 A g. Furthermore,the galvanostatic charge-discharge profiles at a current densityof 0.1 A g(Figure 4b) also shows obvious charge/dischargeplateaux, which confirms the good electrochemical revers-ibility ofMno.15V2Os·nH2O electrode at a low temperature of-20 °C. The rate capabilities of Mno.15V2Os·nH2O electrodeand the corresponding charge-discharge profiles are presentedin Figure 4c,d. It can be observed that the Mno.15V2Os·nH2Oelectrode exhibits specific capacities of 202, 190, 160, 129,and 96 mAh gat current densities of 0.05, 0.1, 0.2, 0.5, and1.0 A g,respectively. As the current density is decreased backto 0.05 A g after high rate charge/discharge process, thespecific capacity can be recovered to a high value, suggestinghighly reversible Zn² de(intercalation) process. In addition,the long cycling performance of Mno.15V205·nH2O electrodeat a high current density of 2.0 A gis investigated as pre-sented in Figure 4e. It is obvious that a high specific capacity of 1★ Stainless steel Figure 5. a-c) The ex situ XRD patterns and the corresponding charge-discharge profiles at 0.1 A g. High-resolution XPS spectra of d) zinc ande) vanadium in pristine, fully discharged and charged states of Mn0.15V2Os·nH2O electrode. f) SEM, g,h) TEM, i-l) HAADF-STEM image and thecorresponding EDX elemental mappings, m) HRTEM images of the long-term cycled Mn0.15V2Os·nH2O electrode (100 times at 0.5 Ag). 100 mAh gat 2.0 A gcan be obtained even over 3000 cycles.The excellent performance at low temperature endows theMno.15V205·nH2O cathode with more possibility in the prac-tical applications. The zinc-ion storage mechanism is analyzed by ex situ XRDand XPS investigations. Figure 5a-c shows the XRD patternsof different stages selected from the first charge-dischargecycle at a current density of 0.1 A g-and the correspondingcharge-discharge profiles. As presented in Figure 5a, the (001)peak located at 6.8° shifts toward higher angle when dischargedto 0.2 V, revealing the contraction of the interlayer space. Thedecreased interlayer space during the discharging process canbe ascribed to the diminished interlayer electrostatic repulsion and the improved combination of Zn²+ with lattice oxygen andstructural water. After the charging process, all these peaksrecover to the initial states gradually, as shown in Figure 5a-c,which indicates the high reversibility ofMno.15V2Os·nH2O elec-trode, thus leading to stable cycling capability. The XPS analysisis also carried out to investigate the valence state changes of zincand vanadium elements. As shown in Figure 5d, no Zn elementsignal is detected in the initial state. When discharged to 0.2V,two pairs of peaks can be observed, which are ascribed to theintercalated Zn²+ in the Mn0.15V20s·nH2O electrode and theabsorbed Zn²+on surface of the Mn0.15V205·nH2O electrode,respectively.[40] In fully charged state, only one pair of peaks canbe observed, corresponding to the surface absorption of Zn². The V 2p region of different states in the Mno.15V2Os·nH20electrode is also revealed in Figure 5e. It is can be obviousobserved that only V+ component exists in the initial state.The V4+ signals emerge when the Mno.15V2Os·nH2O elec-trode is discharged to 0.2 V, which indicates the partial reduc-tion of V+to V4+ during the intercalation of Zn’. Uponcharging, the V4+ signal decreases apparently with a smallpart of V4 remains corresponding to the residual Zn+ inMno.15V2Os·nH2O electrode, which is in accordance with the exsitu high-resolution Zn 2p XPS analysis. The morphology char-acteristics of the Mno.15V205·nH2O electrode after 100 cyclesat 0.5 A g are further investigated as shown in Figure 5f-h.The flower-type structure of the Mno.15V205·nH2O electrodecan be maintained after 100 cycles at 0.5 A g, suggesting agood cyclability during the Zn+(de)intercalation process. TheHAADF-STEM image and the corresponding elemental map-ping images (Figure 5i-l) demonstrate the coexistence of V,Mn,and Zn elements, which could indicate the homogeneous (de)intercalation process of Zn²+ in Mno.15V2Os·nH2O electrode.The HRTEM image in Figure 5m suggests that the high crystal-linity of the Mno.15V205·nH20 can be well preserved after theZn+(de)intercalation process. Moreover, the SEM images ofZn anode after 50, 100, and 200 cycles at 0.5 A gare exhib-ited in Figure S11 (Supporting information). No dendrites aredetected at Zn anode even after 200 cycles, which is a crucialfactor accounting for the excellent long-term cycling stability. Density functional theory (DFT) calculations are carriedout to investigate the effect of Mn+ions doping on the elec-tronic properties of layered V20s (Mn+-doped V20s). Figure 6ashows the charge distribution of Mn elements where yellowand blue electrons indicate charge accumulation and chargedepletion, respectively. Obviously, the interlayer charge ofV2Os can be modified with the doping of Mn²t. In addition,we calculate the band structures (Figure 6b,d) and projecteddensity of state (PDOS) of pure V20s and Mn+-doped V20s (Figure 6c,e). The Mn+-doped V20s possesses a narrowedindirect bandgap of 0.90 eV compared with that of pure V20s(2.28 eV). This narrowed bandgap is beneficial to the enhancedexcitation of charge carriers to the conduction band, which willinduce increased conductivity.[41] The calculated PDOS of pureV20s and Mn2+-doped V2Os are displayed in Figure 6c,e. Thedeep-level electronic status is not taken into account since theelectrons near Fermi energy have a major influence on thematerial. As observed in Figure 6c, both the conduction bandand the valence band are mainly provided by 3d orbitals withminor portion from O 2(s+p) states. Specially, after the dopingof Mn, the energy levels close to the Fermi level present a newenergy level obviously (Figure 6e), and thus the bandgap isreduced to about 40%, indicating a considerable enhancementin the conductivity by the doping of Mn+into layered V205. 3. Conclusion In summary, we have successfully SsVynthesized theMno15V2Os·nH,O nanostructures tthrough a facile micro-wave-assisted route. When used as cathodes for ZIBs, theMn0.15V20s·nH2O delivers a high reversible specific capacity of367 mAh gat a current density of 0.1 A g after 170 cycles.Even at a high current density of 10 A gfor a long period of8000 cycles, the specific capacity is stabilized to 153 mAh g.The superior electrochemical performance is attributed to theinterlayer doping of Mn²+ions and water molecules in thelayered structure, which could work synergistically to enhancethe electricalconductivity, accelerate Zndiffusion andimprove structural stability during cycling. More impor-tantly, the Mno.15V20s·nH20 cathode possesses a high spe-cific capacity of 100 mAh gat a current density of 2.0 A gafter 3000 cycles at a low temperature of -20 °C. Meanwhile,theereversible Znh2+de(intercalation)mechanismin. the Figure 6. Density functional theory calculations of Mn²+-doped V20s and pure V2Os: a) electron density difference of Mn+-doped V20s, charge accu-mulation is in yellow and depletion in blue. b,d) Calculated total band structures and c,e) the total and partial densities of states for pure V2Os andMn2+-doped V20s. Mno.15V2Os·nH2O electrode is investigated by the ex situ XRDand XPS analysis. Considering the excellent rate capability,good cycling stability and superior low-temperature perfor-mance, the as-synthesized Mn0.15V2Os·nH2O electrode holdsgreat potential in building high-performance ZIBs for large-scale energy storage applications. 4. Experimental Section Materials Preparation: In a typical procedure, 0.364 g V20s wasdissolved in 40 mL of deionized water, 0.082 g Mn(CH,COO)2·4HzOwas then added into the above orange solution under stirring. Afterstirring for 10 min, 5 mL H202 was instilled into the above slurry, andmagnetically stirred for another 30 min at room temperature. Afterward,the mixed solution were transferred into a 100 mL autoclave and placedin a microwave instrument (XH-800G), and maintained at 200 °℃ for3 h. Finally, the Mno.15V2Os·nH20 was washed by distilled water andethanol for several times. The VOs·nH0 was synthesized by a similarroute without adding manganese source. Material Characterizations: SEM (Hitach, Model SU8220) and TEM(FEl, Model Talos F200S) were used to investigate the morphology,XRDpatterns were recorded on a Bruke D8 with a Cu Ke radiation source. TGAunder nitrogen atmosphere was acquired by thermal gravimetric analyzer(STA409PC). XPS analyses were performed on X-ray photoelectronspectrophotometer (Thermo Fisher-Escalab 250Xi) with monochromaticAl-Ko X-ray source (excitation energy=1486 eV). The content ofelementswas determined by an ICP-OES spectrometer (SPECTRO BLUE SOP).Infrared spectra were recorded on Nicolet 6700 FTIR spectrometer. Electrochemical Measurements: The working electrode was prepared bymixing active material (70 wt%), super-P (20 wt%), and polyvinylidenefluoride (PVDF, 10 wt%) in N-methylpyrrolidone (NMP) to form ahomogeneous slurry. Then, the slurry was coated onto the stainlesssteel meshes, followed by vacuum drying at 50 ℃ for about 12 hand compression at 10 MPa. The mass loading of the active materialwas around 1.0 mg cm-2. The coin cells were assembled in air, usingzinc metal as anode and 1 M Zn(ClO4)2 in propylene carbonate (PC)as electrolyte. The electrochemical performances of these cells wereperformed in the voltage range of 0.2-1.7 V versus Zn/Zn2+ witha NEWARE multichannel battery test system. The electrochemicalimpedance spectrometry from 100 kHz to 10 mHz and cyclicvoltammetry were conducted with a GAMRY workstation. Supporting Information Supporting Information is available from the Wiley Online Library orfrom the author. Acknowledgements H.B.G., M.C., and B.W., contributed equally to this work. This workwas financially supported by the National Natural Science Foundationof China (Grant Nos. 51771058 and 51801030), the Natural ScienceFoundation of Guangdong Providence (Grant No. 2018A030310571), One-hundred Young Talents (Class A) of Guangdong UniversityTechnelogy (Grant No. 220413198), Pearl River Talent Program ofGuangdong Province (2017GC010030), and GuangdongProvinceUniversities and Colleges Pearl River Scholar Funded Scheme. Conflict of Interest The authors declare no conflict of interest. Keywords electronic structure regulation, high-rate, layered vanadium oxide, low-temperature performance, zinc-ion battery Received: September 17, 2019 Revised: October 24, 2019 ( Published online: ) ( [1] Y. X. Z eng, Z . Z . L a i, Y. H an, H . Z. Z h ang, S. L. X ie, X. H. Lu , Adv . Mater.2018,30,1802396. ) ( [2] Y. F . Dong, S. Li , K . N. Zhao, C. H. Han, W . C hen, B. L. W ang,L. Wang, B. A . Xu, Q. L . Wei, L. Z hang, X. Xu, L . Q . Mai, Energy Environ. Sci. 2015, 8 , 1 267. ) ( [3] D. Chen, X . H. R ui, Q. Zhang, H . B. G eng, L. Y. Gan, W. Zhang, C . C . Li, S . M. Huang, Y.Yu, Nano Energy 2019, 6 0, 1 71. ) ( [4] X. Guo, G. Z . F ang, W. Y. Zhang, J. Zhou, L. T . Shan, L . B. Wang,C. Wang, T. Q. Lin,Y. Tang, S. Q. Li a ng, Ad v . En e rgy Mater. 201 8 , 8, 1801819. ) ( [5] L . M. Z hou, Q. L i u, Z. H. Z h ang, K. Zh a ng, F. Y . Xiong, S. S. Ta n ,Q. Y . An, Y . M. K ang, Z . Z hou, L. Q. M a i, A d v. Mater. 2018, 30, 1801984. ) ( [6] P. He, M . Y . Yan, G. B . Zhang, R. M . Sun, L. N. Chen, Q. Y. An ,L . Q. Mai, Adv. Energy Mater. 2017, 7 ,1601920. ) ( [7] G. Z. Fang, J. Zhou, A. Q. P an, S . Q . L iang, ACS E n ergy Lett. 2018, 3,2480. ) ( [8] B. Y. Tang, G. Z. Fang, J . Zhou, L . B . W ang, Y . P . L e i, C. Wang,T. Q. Lin, Y. Tang, S. Q . Liang , Nano Energy 2018, 51,579. ) ( [9] C. Xia, J. Guo, Y. J. Lei, H. F . L iang, C. Zhao, H. N. Als h areef, Adv. Mater. 2018,30,1705580. ) ( [10] F . Liu, Z. Chen, G. Z. Fang, Z. Q. Wang, Y. S. Cai, B . Y.Tang,J. Zhou, S . Q. L iang, Nano-Micro L e tt.2 0 19, 11, 2 5 . ) ( [11] Z. G. Hou, X. Q. Zhang, X. N. Li , Y . C. Z hu, J . W. Liang, Y. T. Qian,J . Mater. Chem. A 2017, 5, 730. ) ( [12] G. Kasiri, R. Trocoli, A. B . Hashemi, F. L a Mantia, Electrochim. Acta 2016, 2 22, 7 4. ) ( [13] C. J. Xu, B. H. Li, H. D. D u, F. Y. K ang, Angew. Ch e m., Int. Ed. 201 2 , 51,933. ) [14] H. F. Li, C. J. Xu, C. P. Han, Y. Y. Chen, C. G. Wei, B. H. Li,F. Y. Kang,J. Electrochem. Soc. 2015, 162,A1439. [15] B. Lee, H. R. Lee, H. Kim, K. Y. Chung, B. W. Cho, S. H. Oh, Chem.Commun. 2015, 51,9265. ( [16] F. Wang, E. Y. Hu, W. S un, T . G ao, X. Ji, X. L. Fan, F. D. Han,X. Q. Yang, K . X u , C . S. Wa n g, Energy Environ. Sci . 20 1 8, 11, 3168. ) ( [17] P. Canepa, G. S. Gautam, D . C. Hannah, R. Malik, M. Liu , K. G . G allagher, K. A. Persson, G. C eder, C hem. R e v. 2017, 117, 4287. ) ( [18] P. He, G . B . Z h ang, X. B. Liao, M. Y. Yan , X. Xu, Q. Y . An, J . Li u , L. Q. Mai, Adv. Energy Mater.2018,8,1702463. ) ( [19] B. K. Wu, G . B . Z hang, M . Y . Y a n, T . F. X iong, P. He, L. He, X . Xu, L. Q. Mai, Small 2018, 14 , 1703850. ) ( [20] M. H . Alfaruqi, V. M athew, J. G i m, S. Kim, J. S ong, J. P. B ab o o, S . H. Choi, J. Kim, Chem. Mater. 2015, 27, 3609. ) ( [21] M. H . Alfaruqi, J. G im, S . K i m, J. Song, D. T. P ham, J. Jo, Z. Xiu, V. Mathew,J. Kim, Electrochem . Commun. 2015, 60,121. ) ( [22] V. Soundharrajan , BB. Sambandam, S. Kim, M . H. A l faruqi, D. Y . Putro,J. Jo, S. Kim, V. Mathew, Y . K . S un, J. K im, Nano Le t t. 2018,18, 2 402. ) ( [23] Z. Liu, G. Pulletikurthi, F. Endres, ACS Appl. Mater. I n terfaces 2016, 8, 1 2158. ) ( [24] F. Wan, L. L. Zhang, X. Dai, X. Y. Wang, Z. Q. N iu, J . Chen, N at. Commun. 2018, 9 , 1 656. ) ( [25] B. Sambandam, V. So u ndharrajan, S. K im , M. H . Alfar u qi, J. J o ,S. Kim, V . M athew, Y . K. Sun, J. K im, J. Mater. C hem. A 2018, 6, 15530. ) ( [26] Y. Q. Yang, Y. T ang, G. Z . F a ng, L. T. S h an, J. S. G u o, W. Y. Zh a ng,C. Wang, L. B . W ang,J. Zhou, S. Q. Liang, Energy Environ. S c i. 2018, 11,3157. ) ( [27] P. He, Y . L . Quan, X. Xu, M . Y. Yan, W. Ya n g, Q. Y. An, L. H e, L. Q. Mai, Small 2017, 1 3, 1 702551. ) ( [28] N. Zhang, Y. Dong, M . Jia , X. Bian, Y. Y. Wang, M. D . Q i u, j . Z. X u,Y. C. Liu, L. F . Jiao, F . Y. Cheng, ACS Energy Lett. 2018, 3 ,1366. ) ( [29] L. Y. Zhang, L . Chen, X. F. Z hou, Z. P . L i u, Adv. Energy Mater. 2015, 5,1400930. ) ( [30] M. Y . Yan, P. He, Y. Chen, S. Y. Wang, Q. L. W ei, K . N. Z hao, X. Xu, Q. Y. A n, Y . S huang, Y. Y. S h ao, K. T. Mu e ller, L. Q. Mai , J. Liu ,J . H. Yang, Adv. Mater. 2018, 30, 1 703725. ) ( [31] D. Kundu, B. D. Adams, V. Duffort, S . H. Vajargah, L . F. N azar, Nat. Energy 2016, 1,16119. ) ( [32] C. Xia,J. Guo, P. Li, X. X. Zhang, H. N. Alshareef, Angew. C hem., Int. Ed. 2018, 57,3943. ) ( [33] F. W. M ing, H . F . Liang, Y. J. Lei, S. Kandambeth, M . Eddaoudi, H. N. Alshareef, ACS Energy Lett. 2018, 3, 2602. ) ( [34] L. Q. Deng, X. G. Niu, G . S. M a , Z. Yang, L. Zeng, Y. J. Zhu, L. Guo, Adv. Funct. Mater. 2018,28,1800670. ) ( [35] Z. Peng, Q. L. W ei, S. S . T an, P . H e, W. L u o, Q . Y. An, L. Q . Mai, Chem. Commun. 2018, 54,4041. ) ( [36] J . L. Liu, J. Wang, C . H . Xu, H. Jia n g, C. Z. Li, L . L. Zhang, J. Y. Lin, Z . X. Shen, Adv. Sci. 2018, 5 , 1700322. ) ( [37] G. Z . Yang, T. Y . Wei, C. X . Wang, ACS Appl. Mater. Int e rfaces 2018, 10,35079. ) ( [38] D. L. Chao, C. Zhu, M. S ong, P. L iang, X. Zhang, N. H. T i ep,H. F. Z hao, J. Wang, R . M . Wang, H. Zha n g, H. J. F a n , Adv. Mat e r. 2018, 30,1803181. ) ( [39] S. Islam, M. H.Alfaruqi, B. Sambandam, D. Y. Putro, S . K i m, J . Jo, S . Kim, V. Mathew, J . Kim, Chem. Commun. 2019, 55,3793. ) ( [ 40] N. Z hang, M. Jia, Y . Do n g,Y. Y. Wang, J. Z. X u, Y. C. L iu , L . F. J iao,F. Y. Cheng, Adv. Funct . Mater. 2019, 29,1807331. ) ( [41] S. J. Peng, X. P. H an, L . L. Li, S . L . Chou, D . X . J i , H. J. Huang, Y . H. Du, J. Liu , S . Ramakrish n a, Adv. Energy Mater.2018,8,1800612. ) ( of )Adv. Funct. Mater. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim ( of )@ WILEY-VCH Verlag GmbH & Co. KGaA, WeinheimAdv. Funct. Mater. 通过层间掺杂策略调节层状氧化钒的电子结构,以实现卓越的高速率和低温锌离子电池锂离子电池具有工作电压高、能量密度大以及循环寿命长等优点,已经被广泛应用于各种便携式电子设备以及电动汽车等领域。然而,锂资源的稀缺性以及成本问题制约了锂离子电池在大规模储能系统中的应用。相对于锂而言,锌元素在地壳中的分布广泛、储量丰富,使得锌离子电池具有明显的资源优势以及价格优势。然而,带有多价态电子的锌离子在正极材料中嵌脱缓慢,动力学性能较差,严重影响了电池的比容量、循环稳定性和倍率性能。因此,仍需开发新型高性能正极材料。鉴于此,广东工业大学的李成超教授团队利用层间锰离子与水分子掺杂来协同提高五氧化二钒正极材料的锌离子传输动力学,并研究了其作为锌离子电池正极材料的电化学储锌性能和机理。结果表明,合成的Mn0.15V2O5·nH2O表现出优异的锌储存性能,其可逆容量高达367 mAh g-1(0.1 A g-1)。在10和20 A g-1的大电流密度下循环8000圈后,其比容量仍能保持在153和122 mAh g-1。即使在-20 oC的低温下,电流密度为2.0 A g-1时,循环2000圈,比容量可以稳定在100 mAh g-1。该材料优异的电化学主要归根于其层间掺杂锰离子与水分子的协同作用,不仅提高了电极材料电子导电性,而且有效增强了锌离子嵌入脱出动力学。并且由于离子柱撑作用以及局域电荷相互作用,该电极材料在循环过程中的结构稳定性得以大幅提升。该工作借助非原位XRD、TEM等表征技术,对Mn0.15V2O5·nH2O电极材料的物相结构、微观形貌在充放电过程中的变化进行了深入研究,揭示了其电化学储锌机理,为高倍率、长循环性能锌离子电池正极材料的设计合成提供了依据。相关论文“Electronic Structure Regulation of Layered Vanadium Oxide via Interlayer Doping Strategy toward Superior High-Rate and Low-Temperature Zinc-Ion Batteries”在线发表在Advanced Functional Materials上(DOI: 10.1002/adfm.201907684)。祥鹄仪器在文献中的使用过程:材料准备。在一个典型的过程中,0.364克V 2O5被溶解在40毫升的去离子水中,然后在搅拌0.082Mn(CH3COO)2-4H2O 然后在搅拌下加入上述橙色溶液中。搅拌10分钟后,将5mL H 2 O2灌入上述浆液中,然后,在室温下再磁力搅拌30分钟。之后混合后的溶液被转移到一个100毫升的高压锅中,并置于微波水热合成仪(XH-800G)中,并在200℃下保持3小时。最后,Mn 0.15V2O5-nH2O被蒸馏水和乙醇洗涤数次。V 2O5-nH2O是通过类似的方法合成的。不添加锰源。材料表征。SEM(Hitach, Model SU8220) 和 TEM (FEI, Model Talos F200S)用于研究其形态,XRD 图案是在Bruke D8上用CuKα辐射源记录的。TGA在氮气环境下,用热重分析仪(STA409PC)获得TGA数据。(STA409PC)。XPS分析是在X射线光电子分光光度计(Thermo)上进行的。分光光度计(Thermo Fisher-Escalab 250Xi)上进行,使用单色的Al-KαX射线源(激发能量=1486 eV)。元素的含量 用ICP-OES光谱仪(SPECTRO BLUE SOP)测定。红外线光谱在Nicolet 6700 FTIR光谱仪上记录。电化学测量。工作电极的制备方法是,将活性材料(70wt%)、超级P(20wt%)和聚偏二氟乙烯(PVDF,10wt%)混合制备。氟化物(PVDF,10wt%)在N-甲基吡咯烷酮(NMP)中混合,形成一个均匀的浆液。然后,该浆液被涂在不锈钢网上,然后在50℃下真空干燥约12小时,并在10MPa下压缩。活性材料的质量负荷 钮扣电池在空气中组装,使用金属锌作为阳极,并使用1毫升 Zn(ClO 4)2在碳酸丙烯酯(PC)中作为电解质。作为电解质。这些电池的电化学性能是在0.2-1.7V的电压范围内对Zn/Zn2+进行了测试。用NEWARE多通道电池测试系统对这些电池的电化学性能进行了测试。100千赫兹至10兆赫兹的电化学阻抗光谱法和环 伏安法是用GAMRY工作站进行的。图片原文END
确定



还剩7页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《锌离子电池中正极材料的电化学储锌性能和机理检测方案(微波合成仪)》,该方案主要用于其他中电化学性能检测,参考标准--,《锌离子电池中正极材料的电化学储锌性能和机理检测方案(微波合成仪)》用到的仪器有Nanocube 多功能微波水热平行合成仪、微波水热合成仪 Hydrocube XH-800SE型
推荐专场
相关方案
更多

















