
Comparison of three methods for extracting Liuhuanggou bituminous coal
方案详情

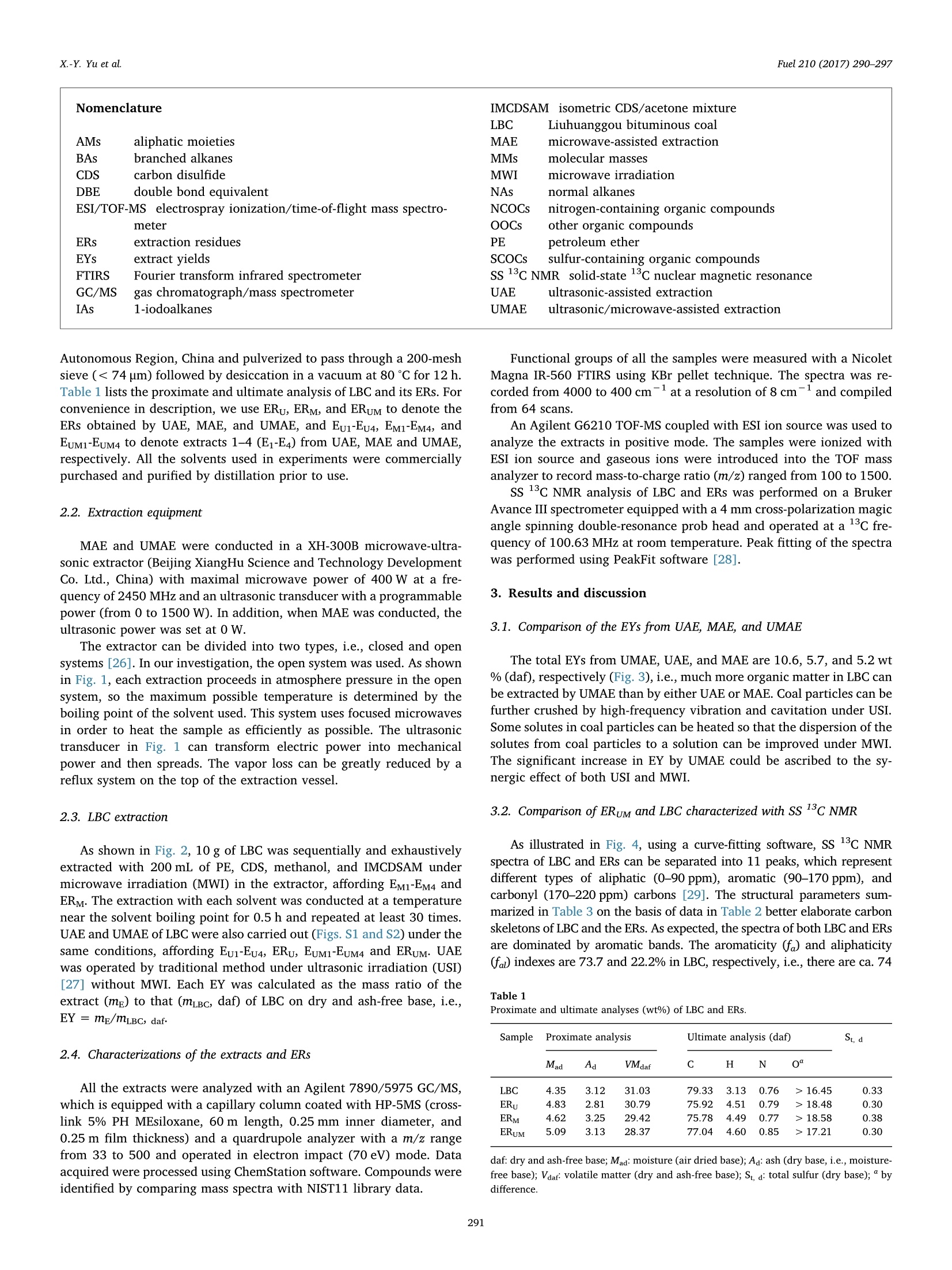
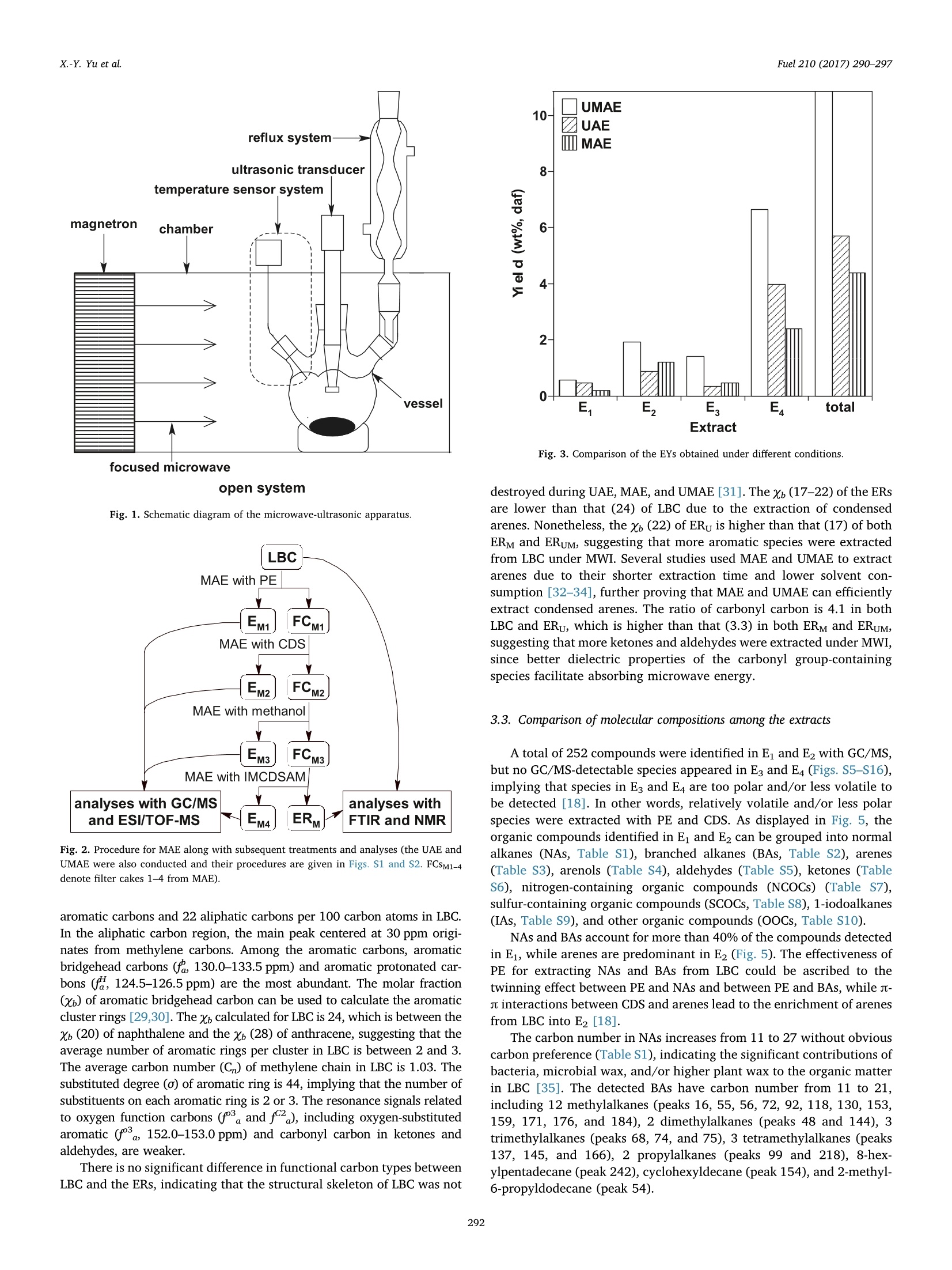
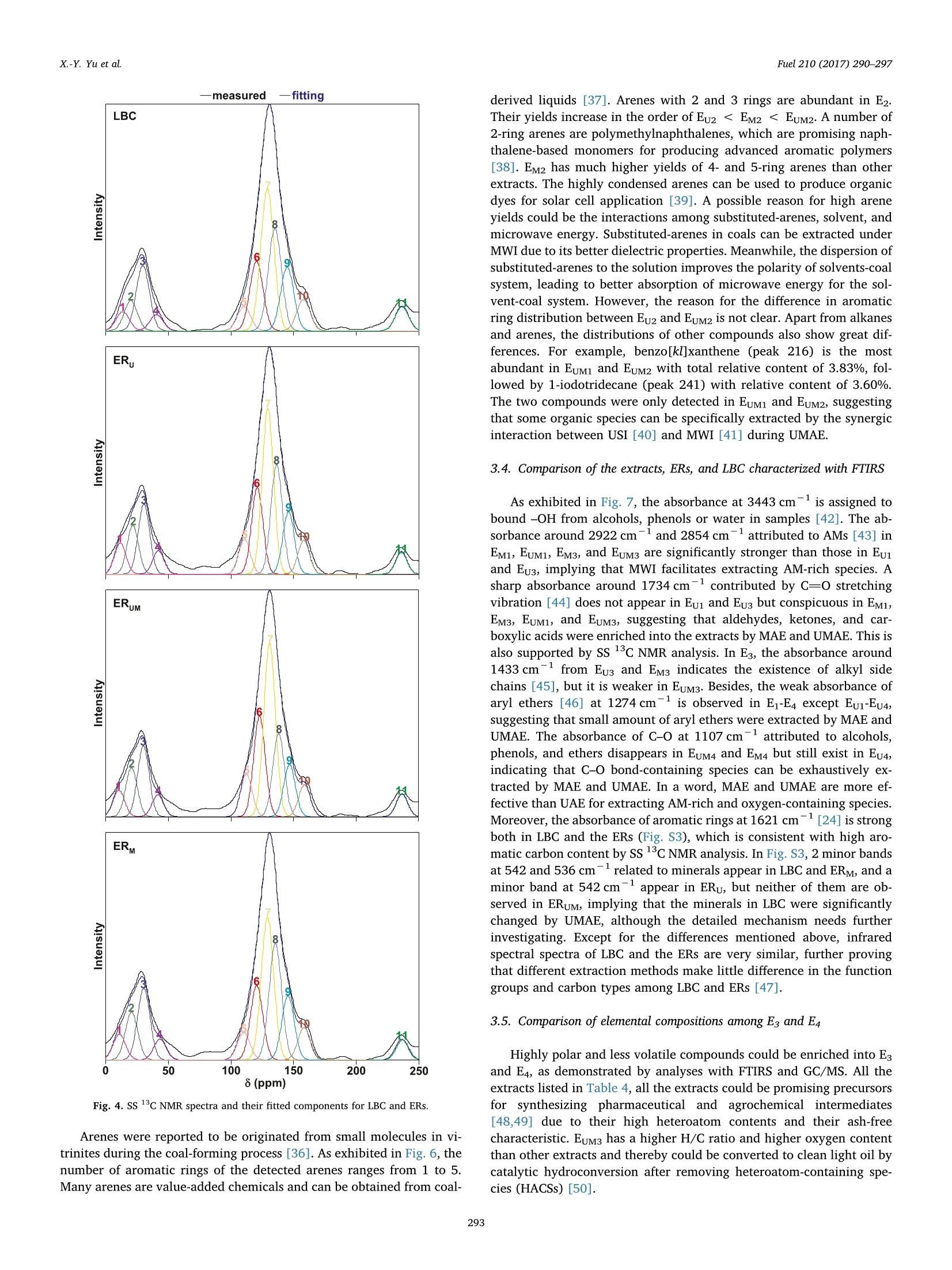
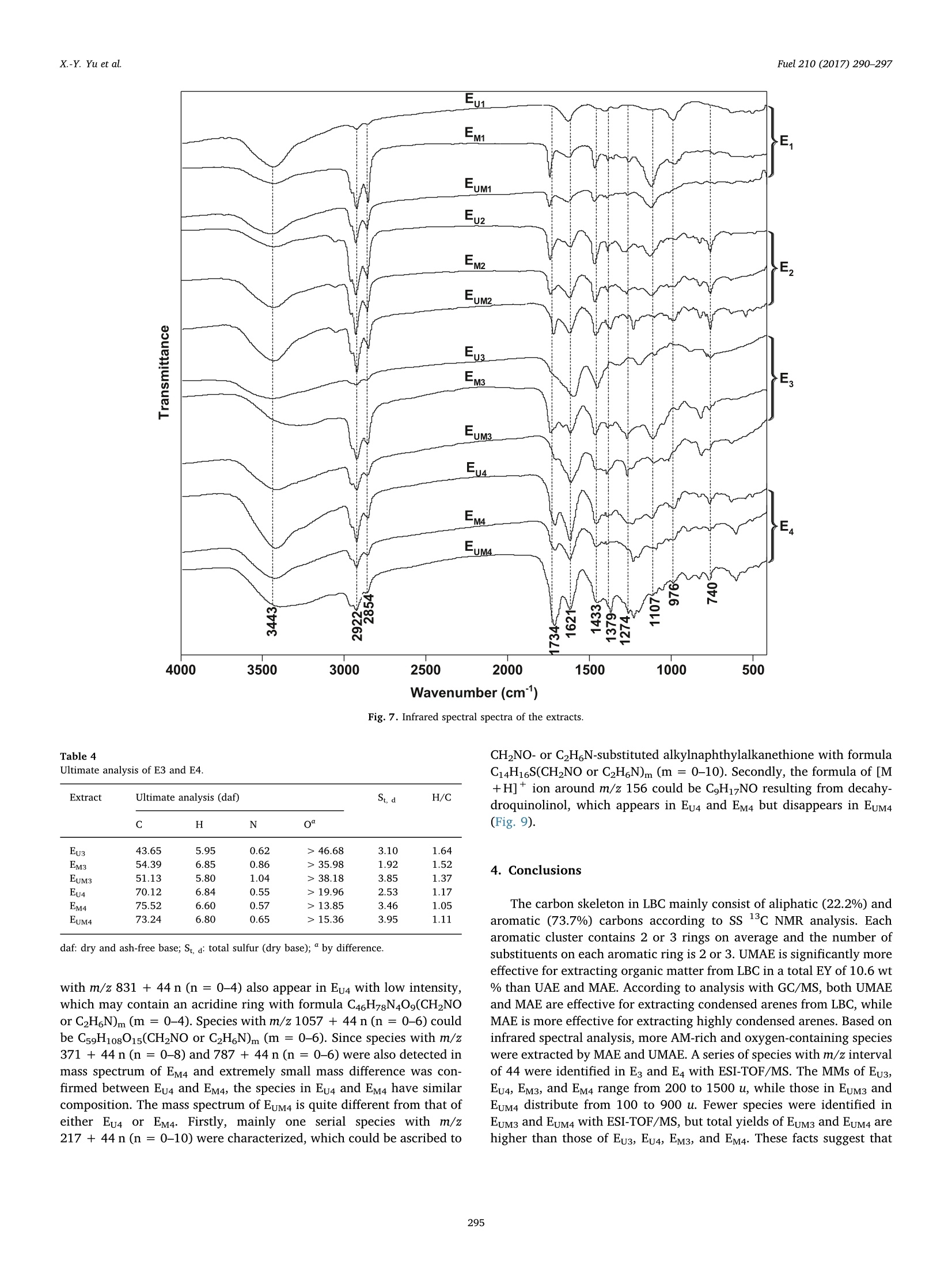
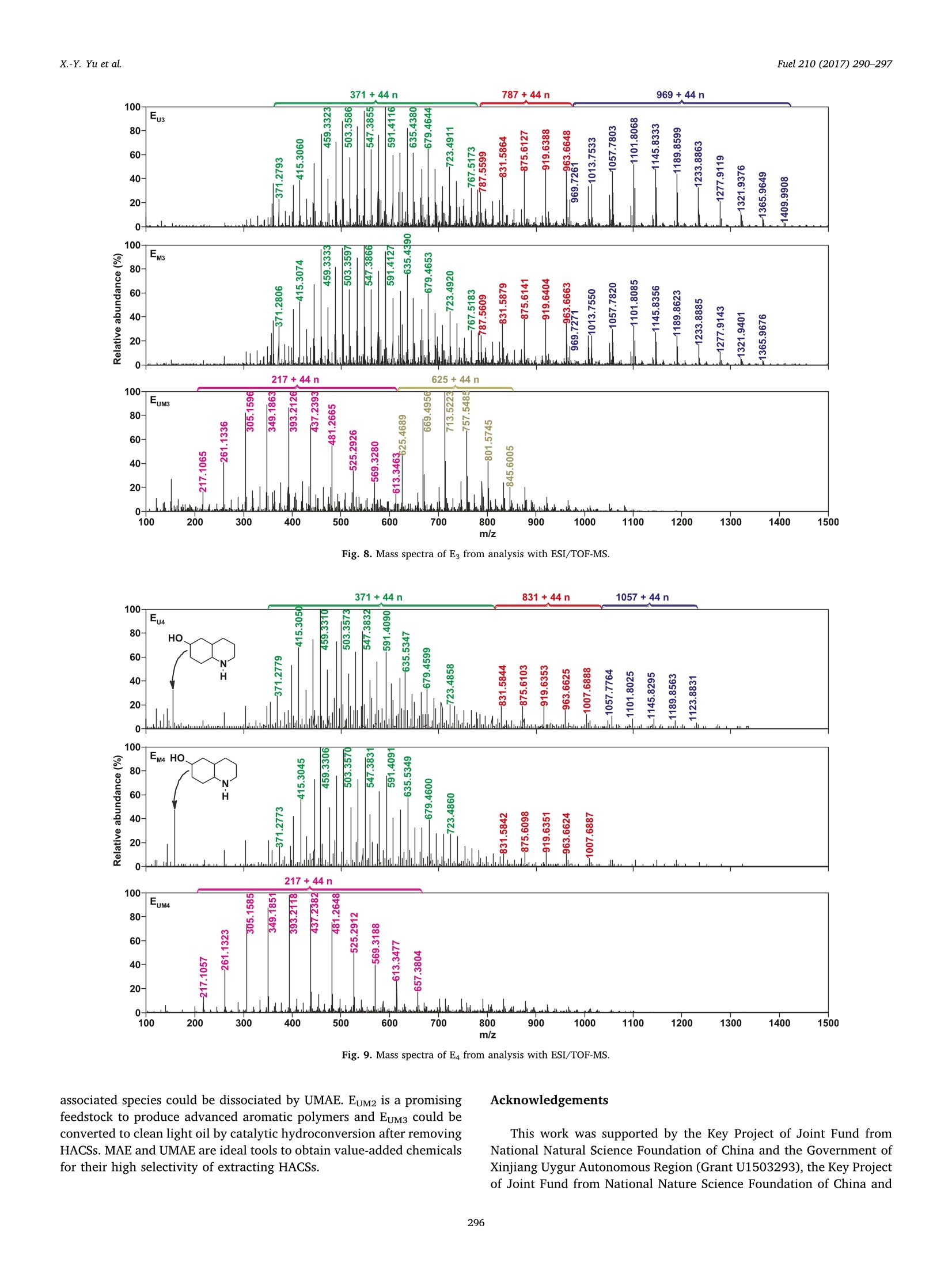
Fuel 210 (2017) 290-297Contents lists available at ScienceDirect X.-Y. Yu et al.Fuel 210 (2017)290-297 Fuel journal homepage: www.elsevier.com/locate/fuel Full Length ArticleComparison of three methods for extracting Liuhuanggou bituminous coal Xin-Yue Yu, Xian-Yong Wei *, Zhan-Ku Li, Yi Chen, Zhi-Min Zong, Feng-Yun Ma,Jing-Mei Liu" Key Laboratory of Coal Processing and Efficient Utilization, Ministry of Education, China University of Mining & Technology, Xuzhou 221116, Jiangsu, ChinaKey Laboratory of Coal Cleaning Conversion and Chemical Engineering Process, Xinjiang Uyghur Autonomous Region, College of Chemistry and Chemical Engineering,Xinjiang University, Urumqi 830046, Xinjiang, China ARTICLEINFO ABSTRACT Keywords:Microwave-assisted extractionUltrasonic-assisted extractionUltrasonic/microwave-assisted extractionSequential extraction Microwave-assisted extraction (MAE), ultrasonic-assisted extraction (UAE), and ultrasonic/microwave-assistedextraction (UMAE) were separately conducted on the extraction of Liuhuanggou bituminous coal (LBC) se-quentially using petroleum ether, carbon disulfide (CDS), methanol, and isometric CDS/acetone mixture assolvents to afford extracts 1-4(E1-E4) from MAE (EM1-EM4), UAE (Eu1-Eu4), and UMAE (EuM1-EuM4). The highesttotal extract yield of 10.6 wt% was obtained by UMAE, while MAE is the most effective for extracting highlycondensed arenes from LBC. The carbon types in LBC mainly consist of aliphatic (22.2%) and aromatic (73.7%)carbons. Each aromatic cluster of LBC contains 2 or 3 aromatic rings on average. The molecular masses of Eu3,Eu4, EM3, and EM4 range from 200 to 1500 u, while those in EuM3 and EuM4 distribute from 100 to 900 u. EuM2 is apromising feedstock to produce advanced aromatic polymers and EuM3 could be converted to clean light oil bycatalytic hydroconversion after removing heteroatom-containing species (HACSs). MAE and UMAE are idealtools to obtain value-added chemicals for their high selectivity of extracting HACSs. 1. Introduction Extract yields (EYs) of coals vary considerably, depending on coalproperties [1], extraction temperature [2,3], solvent [4,5], and ex-traction method [1,2,5]. Under mild conditions, solvents that ex-tract > 20% of organic matter from coals disrupt hydrogen bondswithin the coals to a large extent without covalent bond cleavage [6,7]. Microwave-assisted extraction (MAE) showed a better ability toextract biomass [8-10],soil [11], sediment [12], and waste water [13]with less solvent consumption [14]. It has been developed to vacuummicrowave-assisted extraction, dynamic microwave-assisted extraction,and ultrasonic/microwave-assisted extraction (UMAE) [15]. Ultrasonic-assisted extraction (UAE) was used for coal extraction with carbondisulfide (CDS)/N-methyl-2-pyrrolidinone (NMP) mixed solvent [1-4],but the comparison of the effect on coal extraction among MAE, UMAE,and UAE was seldom reported. High EYs from bituminous coals can be obtained using solvents withstrong electron-donor and -acceptor properties like CDS/NMP [16], butunderstanding the molecular compositions of the extracts is difficultdue to the complex interactions between the solvents and extracts [17].Sequential extraction with low boiling-point solvents and subsequentanalyses proved to be effective for understanding the molecular com-positions of extractable species in coals [18,19]. However, there are ( * Corresponding author. ) ( E-mail address: wei _ xia n yo ng@ 16 3. c om ( X . -Y. Wei). ) ( h ttp ://d x . d oi.or g / 1 0. 1 016/j . f u e l . 2 017. 0 8. 07 1 R eceived 20 April 2017; Received in revised form 16 August 2 017; A ccepted 18 August 2017 ) relatively few studies devoted to the comparison about the effect ofdifferent methods on coal extraction. Gas chromatograph/mass spectrometer (GC/MS) is widely applied toidentifying relatively volatile, less polar, and thermally stable organic spe-cies in complex mixtures [18,20,21]. Electrospray ionization (ESI) massspectrometer has advantage in ionizing polar, less volatile, and thermallylabile compounds with high molecular mass (MMs) [22,23]. Some usefulinformation on coal structures can be provided with direct characterizationtools, such as Fourier transform infrared spectrometer (FTIRS) [24] andsolid-state 13c nuclear magnetic resonance (SS 13CNMR) [25]. In the present investigation, we compared the effects of MAE, UAE,and UMAE on the sequential extraction of Liuhuanggou bituminouscoal (LBC, a Chinese bituminous coal), using petroleum ether (PE), CDS,methanol, and isometric CDS/acetone mixture (IMCDSAM) as solvents.The related samples, including the extracts and extraction residues(ERs), were characterized with GC/MS, ESI/time-of-flight mass spec-trometer (ESI/TOF-MS), FTIRS, and SS 13C NMR. 2. Experimental 2.1. Materials LBC was collected from Liuhuanggou Coal Mine, Xinjiang Uyghur Nomenclature IMCDSAM isometric CDS/acetone mixture LBC Liuhuanggou bituminous coal AMs aliphatic moieties MAE microwave-assisted extraction BAs branched alkanes MMs molecular masses CDS carbon disulfide MWI microwave irradiation DBE double bond equivalent NAs normal alkanes ESI/TOF-MS electrospray ionization/time-of-flight mass spectro- NCOCs nitrogen-containing organic compounds meter OOCs other organic compounds ERs extraction residues PE petroleum ether EYs extract yields SCOCs sulfur-containing organic compounds FTIRS Fourier transform infrared spectrometer SS 13C NMR solid-state 3c nuclear magnetic resonance GC/MS gas chromatograph/mass spectrometer UAE ultrasonic-assisted extraction IAs 1-iodoalkanes UMAE ultrasonic/microwave-assisted extraction Autonomous Region, China and pulverized to pass through a 200-meshsieve (<74 um) followed by desiccation in a vacuum at 80 ℃ for 12 h.Table 1 lists the proximate and ultimate analysis of LBC and its ERs. Forconvenience in description, we use ERu, ERM, and ERuM to denote theERs obtained by UAE, MAE, and UMAE, and Eui-Eu4, EM1-EM4, andEuM1-EuM4 to denote extracts 1-4 (E1-E4) from UAE, MAE and UMAE,respectively. All the solvents used in experiments were commerciallypurchased and purified by distillation prior to use. 2.2. Extraction equipment MAE and UMAE were conducted in a XH-300B microwave-ultra-sonic extractor (Beijing XiangHu Science and Technology DevelopmentCo. Ltd., China) with maximal microwave power of 400 W at a fre-quency of 2450 MHz and an ultrasonic transducer with a programmablepower (from 0 to 1500 W). In addition, when MAE was conducted, theultrasonic power was set at 0 W. The extractor can be divided into two types, i.e., closed and opensystems [26]. In our investigation, the open system was used. As shownin Fig. 1, each extraction proceeds in atmosphere pressure in the opensystem, so the maximum possible temperature is determined by theboiling point of the solvent used. This system uses focused microwavesin order to heat the sample as efficiently as possible. The ultrasonictransducer in Fig. 1 can transform electric power into mechanicalpower and then spreads. The vapor loss can be greatly reduced by areflux system on the top of the extraction vessel. 2.3. LBC extraction As shown in Fig. 2, 10 g of LBC was sequentially and exhaustivelyextracted with 200 mL of PE, CDS, methanol, and IMCDSAM undermicrowave irradiation (MWI) in the extractor, affording EM1-EM4 andERM. The extraction with each solvent was conducted at a temperaturenear the solvent boiling point for 0.5 h and repeated at least 30 times.UAE and UMAE of LBC were also carried out (Figs.S1 and S2) under thesame conditions, affording Eu1-Eu4, ERu, EuM1-EuM4 and ERuM. UAEwas operated by traditional method under ultrasonic irradiation (USI)[27] without MWI. Each EY was calculated as the mass ratio of theextract (mg) to that (mLBc, daf) of LBC on dry and ash-free base, i.e.,EY=mg/mLBC, daf. .4. Characterizations of the extracts and ERs All the extracts were analyzed with an Agilent 7890/5975 GC/MS,which is equipped with a capillary column coated with HP-5MS (cross-link 5% PH MEsiloxane, 60 m length, 0.25mm inner diameter, and0.25 m film thickness) and a quardrupole analyzer with a m/z rangefrom 33 to 500 and operated in electron impact (70eV) mode. Dataacquired were processed using ChemStation software. Compounds wereidentified by comparing mass spectra with NIST11 library data. Functional groups of all the samples were measured with a NicoletMagna IR-560 FTIRS using KBr pellet technique. The spectra was re-corded from 4000 to 400 cm-at a resolution of 8 cm-;and compiledfrom 64 scans. An Agilent G6210 TOF-MS coupled with ESI ion source was used toanalyze the extracts in positive mode. The samples were ionized withESI ion source and gaseous ions were introduced into the TOF massanalyzer to record mass-to-charge ratio (m/z) ranged from 100 to 1500. ss 13c NMR analysis of LBC and ERs was performed on a BrukerAvance III spectrometer equipped with a 4 mm cross-polarization magicangle spinning double-resonance prob head and operated at a 3C fre-quency of 100.63 MHz at room temperature. Peak fitting of the spectrawas performed using PeakFit software [28]. 3. Results and discussion 3.1. Comparison of the EYs from UAE, MAE, and UMAE The total EYs from UMAE, UAE, and MAE are 10.6, 5.7, and 5.2 wt% (daf), respectively (Fig.3), i.e.,much more organic matter in LBC canbe extracted by UMAE than by either UAE or MAE.Coal particles can befurther crushed by high-frequency vibration and cavitation under USI.Some solutes in coal particles can be heated so that the dispersion of thesolutes from coal particles to a solution can be improved under MWI.The significant increase in EY by UMAE could be ascribed to the sy-nergic effect of both USI and MWI. 3.2. Comparison of ERuM and LBC characterized with SS 13C NMR As illustrated in Fig. 4, using a curve-fitting software, SS 13c NMRspectra of LBC and ERs can be separated into 11 peaks, which representdifferent types of aliphatic (0-90 ppm), aromatic (90-170 ppm), andcarbonyl (170-220 ppm) carbons [29]. The structural parameters sum-marized in Table 3 on the basis of data in Table 2 better elaborate carbonskeletons of LBC and the ERs. As expected, the spectra of both LBC and ERsare dominated by aromatic bands. The aromaticity (fa) and aliphaticity(fa) indexes are 73.7 and 22.2% in LBC,respectively, i.e., there are ca. 74 Table 1Proximate and ultimate analyses (wt%) of LBC and ERs. Sample F Proximate analysis Ultimate analysis (daf) St, d Mad Aa VMdaf C H N LBC 4.35 3.12 31.03 79.33 3.13 0.76 >16.45 0.33 ERu 4.83 2.81 30.79 75.92 4.51 0.79 >18.48 0.30 ERM 4.62 3.25 29.42 75.78 4.49 0.77 >18.58 0.38 ERuM 5.09 3.13 28.37 77.04 4.60 0.85 > 17.21 0.30 daf: dry and ash-free base; Mad: moisture (air dried base); Aj: ash (dry base, i.e., moisture-free base); Vdaf: volatile matter (dry and ash-free base); St, d: total sulfur (dry base); “bydifference. focused microwave open system Fig. 1. Schematic diagram of the microwave-ultrasonic apparatus Fig. 2. Procedure for MAE along with subsequent treatments and analyses (the UAE andUMAE were also conducted and their procedures are given in Figs. S1 and S2. FCSM1-4denote filter cakes 1-4 from MAE). aromatic carbons and 22 aliphatic carbons per 100 carbon atoms in LBC.In the aliphatic carbon region, the main peak centered at 30 ppm origi-nates from methylene carbons. Among the aromatic carbons, aromaticbridgehead carbons (,130.0-133.5 ppm) and aromatic protonated car-bons (fa, 124.5-126.5 ppm) are the most abundant. The molar fraction(Xb) of aromatic bridgehead carbon can be used to calculate the aromaticcluster rings [29,30]. The Xb calculated for LBC is 24, which is between theXb (20) of naphthalene and the xb (28) of anthracene, suggesting that theaverage number of aromatic rings per cluster in LBC is between 2 and 3.The average carbon number (Cn) of methylene chain in LBC is 1.03. Thesubstituted degree (0) of aromatic ring is 44, implying that the number ofsubstituents on each aromatic ring is 2 or 3. The resonance signals relatedto oxygen function carbons (fa and fa), including oxygen-substitutedaromatic (fa 152.0-153.0ppm) and carbonyl carbon in ketones andaldehydes, are weaker. There is no significant difference in functional carbon types betweenLBC and the ERs, indicating that the structural skeleton of LBC was not Fig. 3. Comparison of the EYs obtained under different conditions. destroyed during UAE,MAE, and UMAE [31]. The xb(17-22) of the ERsare lower than that (24) of LBC due to the extraction of condensedarenes. Nonetheless, the xb (22) of ERy is higher than that (17) of bothERM and ERuM, suggesting that more aromatic species were extractedfrom LBC under MWI. Several studies used MAE and UMAE to extractarenes due to their shorter extraction time and lower solvent con-sumption [32-34], further proving that MAE and UMAE can efficientlyextract condensed arenes. The ratio of carbonyl carbon is 4.1 in bothLBC and ERu, which is higher than that (3.3) in both ERM and ERuM,suggesting that more ketones and aldehydes were extracted under MWI,since better dielectric properties of the carbonyl group-containingspecies facilitate absorbing microwave energy. 3.3. Comparison of molecular compositions among the extracts A total of 252 compounds were identified in Ei and E2 with GC/MS,but no GC/MS-detectable species appeared in Es and E4 (Figs. S5-S16),implying that species in Es and E4 are too polar and/or less volatile tobe detected [18]. In other words, relatively volatile and/or less polarspecies were extracted with PE and CDS. As displayed in Fig. 5, theorganic compounds identified in Ei and E2 can be grouped into normalalkanes (NAs, Table S1), branched alkanes (BAs, Table S2), arenes(Table S3), arenols (Table S4), aldehydes (Table S5), ketones (TableS6), nitrogen-containing organic compounds (NCOCs) (Table S7),sulfur-containing organic compounds (SCOCs, Table S8), 1-iodoalkanes(IAs, Table S9), and other organic compounds (OOCs, Table S10). NAs and BAs account for more than 40% of the compounds detectedin Ei, while arenes are predominant in E2 (Fig. 5). The effectiveness ofPE for extracting NAs and BAs from LBC could be ascribed to thetwinning effect between PE and NAs and between PE and BAs, while n-n interactions between CDS and arenes lead to the enrichment of arenesfrom LBC into E2 [18]. The carbon number in NAs increases from 11 to 27 without obviouscarbon preference (Table S1), indicating the significant contributions ofbacteria, microbial wax, and/or higher plant wax to the organic matterin LBC [35]. The detected BAs have carbon number from 11 to 21,including 12 methylalkanes (peaks 16, 55,56,72,92, 118,130,153,159, 171, 176, and 184), 2 dimethylalkanes (peaks 48 and 144), 3trimethylalkanes (peaks 68, 74, and 75), 3 tetramethylalkanes (peaks137, 145, and 166), 2 propylalkanes (peaks 99 and 218), 8-hex-ylpentadecane (peak 242), cyclohexyldecane (peak 154), and 2-methyl-6-propyldodecane (peak 54). 0 50 100 150 200 250 8 (ppm) Fig. 4. SS 13c NMR spectra and their fitted components for LBC and ERs. Arenes were reported to be originated from small molecules in vi-trinites during the coal-forming process [36]. As exhibited in Fig. 6, thenumber of aromatic rings of the detected arenes ranges from 1 to 5.Many arenes are value-added chemicals and can be obtained from coal- derived liquids [37]. Arenes with 2 and 3 rings are abundant in E2.Their yields increase in the order of Eu2 < EM2 46.68 3.10 1.64 EM3 54.39 6.85 0.86 > 35.98 1.92 1.52 EuM3 51.13 5.80 1.04 >38.18 3.85 1.37 Eu4 70.12 6.84 0.55 >19.96 2.53 1.17 EM4 75.52 6.60 0.57 >13.85 3.46 1.05 EuM4 73.24 6.80 0.65 >15.36 3.95 1.11 daf: dry and ash-free base; St, : total sulfur (dry base);“by difference. with m/z 831 + 44n (n = 0-4) also appear in Eu4 with low intensity,which may contain an acridine ring with formula C46H78N40g(CH2NOor C2H6N)m(m = 0-4). Species with m/z 1057 + 44n (n = 0-6) couldbe C59H108015(CH2NO or C2H N)m(m=0-6). Since species with m/z371 + 44n(n = 0-8) and 787 + 44n (n=0-6) were also detected inmass spectrum of EM4 and extremely small mass difference was con-firmed between Eu4 and EM4, the species in Eu4 and EM4 have similarcomposition. The mass spectrum of EuM4 is quite different from that ofeither Eu4 or EM4. Firstly, mainly one serial species with m/z217 + 44 n (n = 0-10) were characterized, which could be ascribed to CH2NO-or C2H N-substituted alkylnaphthylalkanethione with formulaC14H16S(CH2NO or C2H N)m(m=0-10). Secondly, the formula of [M+H]+ ion around m/z 156 could be CgH17NO resulting from decahy-droquinolinol, which appears in Eu4 and EM4 but disappears in EuM4(Fig. 9). 4. Conclusions The carbon skeleton in LBC mainly consist of aliphatic (22.2%) andaromatic (73.7%) carbons according to SS 13c NMR analysis. Eacharomatic cluster contains 2 or 3 rings on average and the number ofsubstituents on each aromatic ring is 2 or 3. UMAE is significantly moreeffective for extracting organic matter from LBC in a total EY of 10.6 wt% than UAE and MAE. According to analysis with GC/MS, both UMAEand MAE are effective for extracting condensed arenes from LBC, whileMAE is more effective for extracting highly condensed arenes. Based oninfrared spectral analysis, more AM-rich and oxygen-containing specieswere extracted by MAE and UMAE. A series of species with m/z intervalof 44 were identified in Eg and E4 with ESI-TOF/MS. The MMs of Eu3,Eu4, EM3, and EM4 range from 200 to 1500 u, while those in EuM3 andEuM4 distribute from 100 to 900 u. Fewer species were identified inEuM3 and EuM4 with ESI-TOF/MS, but total yields of EuM3 and EuM4 arehigher than those of Eu3, Eu4, EM3, and EM4. These facts suggest that Fig. 8. Mass spectra of E from analysis with ESI/TOF-MS. Fig. 9. Mass spectra of E4 from analysis with ESI/TOF-MS Acknowledgements This work was supported by the Key Project of Joint Fund fromNational Natural Science Foundation of China and the Government ofXinjiang Uygur Autonomous Region (Grant U1503293), the Key Projectof Joint Fund from National Nature Science Foundation of China and associated species could be dissociated by UMAE.EuM2 is a promisingfeedstock to produce advanced aromatic polymers and EuM3 could beconverted to clean light oil by catalytic hydroconversion after removingHACSs. MAE andUMAE are ideal tools to obtain value-added chemicalsfor their high selectivity of extracting HACSs. the Government of Shanxi Province (Grant U1610223), and the NaturalScientific Foundation of China (Grant 21576280). Appendix A. Supplementary data Supplementary data associated with this article can be found, in theonline version, at http://dx.doi.org/10.1016/j.fuel.2017.08.071. ( References ) ( [ 1 ] I ino M, T aka n ohas h i T , O h suga H , T o da K. E x t ract i on o f c o als wit h CS 2- N-me t h y l - 2 - p y r ro l i d inone m i x e d s o lven t a t roo m t e mpe r at u r e . Fuel 1 988 ;6 7( 1 2): 1 6 3 9 - 47. ) ( [2] W ei XY , S h en JL , T a kan ohash i T , I i n o M. E f f e ct o f e x t ra c ta bl e su b s ta nce s on co a l d i ss o l u tion. Us e of C S 2 - N-m e t h y l - 2-p y rr o lid i none m ix e d so l vent for d i ssol u t i on re- a ct i on p roducts. E n e rg y F ue l 1 9 89;3 ( 5 ):5 7 5 - 9 . ) ( [3] T a k ano ha shi T, X i ao F , T a kah i r o Y o sh id a A , S a i t o I. D i ff e r ence i n e x tra ct io n y i e l ds between CS2/ N M P an d N M P for upper free p ort c o al. E nergy F uel s 2 003; 1 7( 1 ) :25 5 - 6 . ) ( [ 4] T a k anoh a shi T , T a k a y u ki Y a n agi da A , I i no M, M a i nw a r in g D E . E x tr action a nd s wel l ing o f l ow- r ank c o al s wi t h v a rious solvents at ro o m temperatur e . E n e rgy F u e ls 1 996; 1 0( 5 ) : 1 1 28 -3 2. ) ( [5] L u H Y, W ei X Y , Y u R , P e n g Y L , Qi X Z ,Q i e L M , et a l . S e quential therma l di s s o l ution o f Huol i ng u ole l i g n ite i n m et hanol a n d i n e t ha n ol. E n e r gy F ue l 2 0 11 ; 2 5 (6 ) :2 7 41- 5 . ) ( [6] 1 I s hiz uk a T , T a k anoh a shi T , I t o O, L in o M . E ffects of additiv es an d oxy g e n on e x - t r a c ti on yie l d w it h C S 2- N M P mi x e d sol v ent f o r a r g on n e pr e m ium c oal s a mples. Fu e l 1993; 7 2( 4 ):579 - 80. ) ( N is h ioka M . M u l t i s t ep e x tra ct ion of c oal . F u e l 1991 ; 70(12) : 141 3 - 9 . P a n X , N i u G, L i u H . M icr ow av e-as s iste d e x t ra c t io n o f t e a poly p henols a nd t e a ) ( c a f f e i ne f r o m g reen t e a l e a v e s. C h em Eng P r ocess 20 03 ; 4 2( 2 ) : 1 2 9 - 3 3. [9] R osta g n o MA , Pa l ma M , Ba r r o so C G. M i cr owave as s i s ted ext r act i on of soy is o - f l a v o ne s. A na l Chim A c ta 2 00 7 ;58 8(2) :274 -82. ) ( [10] C hen Y , G u X , H ua n g SQ, Li J , Wa ng X, T a n g J . O pt i mizati o n o f u l t r asoni c/ m i - c r owave a ss i ste d e x traction ( U MAE) o f po l ys a c c ha r i de s fro m Inonotus obliquus and e va l uation of i t s a n ti- t u m o r a c tiv i t ies. In t J Bi o l M a cromol 2 0 10;46(4 ): 42 9 -3 5 . ) ( [11] 1 Eg i z ab al A , Z u lo aga O , E t x eb a rri a N, Fern a nde z L A, M ad ari a g a J M . C o mpa r iso n of m icr ow a ve - a s sist ed e x tractio n a n d Sox h let e xtr a c t i o n f or p h eno ls i n so i l sam p le s u si ng e xp er i men ta l de s i g ns . A n al y s t 1 99 8;123 ( 8):16 7 9-84. ) ( [12 ] P astor A , V a z quez E , C i s ca r R , G u ar d ia MD L . Ef f i ci e ncy o f t h e m icro w av e - a ssiste d e x trac t ion o f hy d roca r bons a n d p e s t ici de s f rom s e d i me n t s . A n al Chi m Act a 19 9 7; 3 44 ( 3):241 -9 . ) ( [13] O nusk a F I , T e rry K A. M i cr owav e e x t ra ct i o n i n an a ly t ic al c he mi st ry of p o llu ta nt s: p olychlorin a ted b iphenyl s . J H i gh R es o lut C hromatogr 1 9 9 5;18 ( 7 ):4 1 7 -2 1 . ) ( [14] M and a l V, M oh a n Y , H e m a l ath a S . Mi c ro wa v e a ss ist e d e xt r a ct i on - a n i n n ov a t iv e a nd pr o m i si n g e x t ra c t i o n t ool f or medici n a l p l an t re sea rch . Pha r ma c o g n o s y R e v 2 00 6 ;1(1) :7-1 8. ) ( [15] C h a n C H , Y u so f f R , N g o h GC , K u n g F W . Mic ro wav e -a s si st e d extr a c tion s of a c t i ve i ng re d ie n t s fr om p l an t s. J C h r o m a to g r A 2 0 1 1 ; 12 1 8 ( 37):6 2 13- 2 5. ) ( [16 ] S hui H , W a ng Z , G ao J. E x a mi na t i o n of the r o le o f CS 2 in t he CS 2 / NM P m ix e d s o lve n t s t o coal ex tr a c t ion . Fue l Pr o c es s Technol 2 0 06;87(3) : 1 8 5 - 90 . ) ( [17] L i u C M, Z on g Z M , Ji a JX, L iu GF, W ei X Y . A n e v i den c e f o r the str o n g a ssoc ia t i o n of N -meth yl- 2 -p yr r ol i dinone w i th so m e o r ga n ic s p ec i es in th r e e C hi n ese bi t umi n ous c oa l s. Chin Sc i B ul l 2008 ; 53 ( 8 ): 1 1 5 7 - 6 4 . ) ( [18] S hi D L , W e i X Y , F a n X , Zon g ZM , C h e n B, Zh a o Y P, e t a l . C h a r a c te r iz a t ion s o f t h e e x trac t s f ro m G e t i n g b it um inou s c oal b y sp e ctrom et ries. E n e rgy F u e l 2 013 ; 27( 7 ): 3 709- 1 7 . ) ( [19] L i u FJ, Wei X Y, G ui J , Wa n g YG, L i P , Z o n g Z M. Ch a r a c t er iz a t ion o f b i om a r ke r s an d s t r uc t u r a l f ea tures of con den sed a rom a ti c s i n X i a n feng lign ite . E ne rg y F u e l 2013 ;2 7(12) : 7 3 6 9-7 8. ) ( [20 ] H a n L , Z ong Z M , J i n X , W an g YQ, W ang F , Y u T T, e t al . So lu b il ity o f d a g a ng v a c u u m r e s i d u e a n d m o le c u l ar co m p o si t io n o f the solu b le fr a c t i o n s in d i ffe r e n t sol v e nt s . F u e l 2 008; 8 7( 2 ):2 6 0 - 3 . ) ( [21] T an g S R ,Z o n g ZM, Zho u L, Z ha o W , L i X B , P e n g Y L , et a l . M o l ec u l ar c o m p ositi o n of s ol ub le f r a c t i on fr o m de p olymer i z e d cornst a l k p o w de r i n s u p e r criti ca l m et h anol an d ethanol. Rene w E n e rg y 2010;35(5) : 946- 5 1. ) ( [22 ] L i Z K , Zo n g Z M, Y an H L , W a ng Y G, Ni H X , We i XY , et a l . C h aract e riz at i on o f a c i di c s p e ci e s in ethanol-s o lubl e p o r t i o n f r o m Z h a o to n g li g ni t e et h a noly si s b y n eg a tive -i o n e l e ctrospra y i oniz a tion F o u r i e r tr ansfor m i o n c y c lo t ron r e s o n a n c e ma s s s pect ro. 5 - m et ry . F u e l P ro c ess T ec h nol 201 4 ; 1 2 8 :2 9 7-3 0 2 . ) ( [23] W an g M, F a n X , W e i X Y , Ca o J P , Z ha o YP , W ang SZ , et a l. Ch a r a c t e r i z a t io n of t h e o xidation p rodu c ts o f S hengl i l igni t e us in g m ass s pectro m et e rs wit h “ h ard”“ s o f t ” a nd a m bien t i o n source s . F u e l 2 016; 1 83 : 1 1 5- 2 2 . ) ( [24] L i Z K , W ei X Y , Ya n HL, Z on g ZM . I n s i g ht i n t o th e st r u ctu r al f eatu re s o f Z h a o t ong l i gnite usi n g m u ltiple techni q ue s. F uel 2015 ; 1 53:17 6 - 82 . ) ( [25 ] L i u F J , Wei XY, F a n M, Z o ng Z M . S e p a r ati o n and str uct ura l ch a r ac t er izat ion of the v a l u e - a d de d chem ica l s f r o m m i l d d egr ad a ti o n of li gn it e s: a r ev i ew. A ppl E nergy ) ( 2 016; 1 70: 4 1 5-3 6 . ) ( [26] C am e l V . M i c ro w a v e -as s i st ed s o lv e nt e x t rac t io n of e nv i r on me n tal s a m p le s. T r AC, T ren d s A n a l C h em 2 000 ; 1 9 ( 4):22 9 -48. ) ( [27] K 1 on gJ , Wei X Y , Y a n HL, Li ZK , Zha o M X , Li Y , e t a l. A na l ysis of e x t ra c ta bl e b a s ic n itro g en c o mpoun d s i n B u l i a ng o u su b b it u mino u s c o al b y p o s it i v e-io n E S I FT-I C R M S . F u e l 2 0 1 5; 1 59 :38 5 - 91. ) ( [28]W an g Y G , W e i X Y , X i e RL , L i u F J , Li P, Z o ng ZM. St r u ctu ral c h ar a cter i zation o f t y p i c a l or g a n ic s pecies i n J i nc h eng N o . 1 5 a nth r acite. E n e r g y Fu e ls 2 015 ;29 ( 2 ) : 595 - 6 0 1 . ) ( [29] L i u F J , W e i X Y, X ie R L, W an g Y G , Li WT , L i Z K , e t a l . C h a ra c t e r iza t ion o f oxy ge n - c ont a i nin g s p ec ie s i n m e t h an o l ys is p r od u c t s o f t he e x tra ct i on re s idue f r o m X ian f e ng lig nite w i th neg a t i v e -ion e le c tro sp ray i on iz a t i o n F our i e r t rans f or m i o n cy c l o tr on r e s o n ance m ass spectromet r y. E n e rg y F u els 2 0 14 ; 28( 9 ): 5 596 -6 05. ) ( [30]T o n g J , Ha n X , W a ng S , Ji ang X. E v a lu a ti on of s t ructura l c h a ra c t e r ist ic s o f H u a di an o i l s h a le kerogen u si n g d i r ec t t e c h n iques ( s o lid-st ate 3 c N MR , X P S , FT- I R, and X R D ). E nerg y F u els 2 011; 2 5(9) :40 06- 1 3 . ) ( [31] E sk i l s son C S , B j o rkl u nd E . A n alyt i ca l - s c a le mi c rowave- as sis ted e xt r ac tion. J C h ro m a t o gr A 20 00; 9 0 2 (1 ) :2 27 - 50 . ) ( [32] : S hu Y Y ,L a o RC , C h i u CH , Tur l e R. A n aly s i s o f p o l y c yc l i c a r om a t ic h y dr o c arbons in se d imen t refe r e n ce m ater ial s by microwav e - as si s ted ex tr a ction. Ch e mospher e 200 0 ; 4 1(1 1 ): 1 709 - 16. ) ( [33] 1 F l o t ro n V , H o ues so u J , B os io A , Delte i l C, Bermond A , C a me lV. R a p i d de t e rm i - n a t i o n o f p ol y cyc l ic a rom at ic h yd ro c a r bons i n s e w a g e s l u d g es u s i ng mi cr ow a ve- a s si s t e d sol ven t e x trac ti on : c o m p a r i so n w i t h o t h e r e x t r a c tio n me t ho d s . J C h romato g r A 20 0 3 ; 999( 1 - 2): 1 75-8 4 . ) ( [34] P u rc a ro G , M oret S , C o n t e L S . O p t imi sa tion o f m i crowave ass i ste d e x tr a ctio n ( MA E ) f o r p oly c ycl i c a ro m ati c h yd ro car bo n (P A H ) de t ermina t ion in sm o ke d m eat. M e a t Sci 2 009;81(1 ): 27 5- 80. ) ( [ 3 5] P eters K E , M oldowa n JM . Th e b i om arker gu id e : in t e r p r e ti n g mo l ecular fo s s i ls in p et r o leum a n d a n c i ent s e d i ment s. E n g le w ood Clif fs , N J ( U n i t e d S t at e s ) : P r enti c e Ha ll; 1 993. ) ( [36] D a vi s MR, A b bott J M , Ga i n es A F . C h e m ical str u ctures of te l ocollinite s a n d s po r- i nite s . 1 . Di f f e r e n tiat i on between te l oco l linites a n d s p orin ite s b y t h e a r om a ti c s t ruc t u re s p res en t in t he i r py rid i n e e xt r a c t s . F u e l 1985;64(10) : 1 3 6 2 -9. ) ( [37] S c hob e rt HH, S o ng C . Ch em ic als an d m a t e r ia l s fr o m co al i n the 2 1s t c e ntur y . F u el 2 002;81(1 ) : 1 5 - 3 2 . ) ( [38] H i r a mot o M, K is h i g a m i Y , Y ok o ya m a M . D o p ing ef f e ct o n t he t w o -laye r o rg a ni c so l ar c e ll . Chem L e tt 1990 ; 19:119 -22 . ) ( [39 ] Y u CC, J ia n g K J , H u a ng J H, Z hang F , B a o X , Wa n g F W, et a l. No v el py r en e -based do n or - accep to r or ga nic d ye s f o r s ol a r c ell a p p l i ca t i o n . O r g E lec t ron 2 013 ; 14 ( 2 ):445 -5 0 . ) ( [40] Y ang B , Zh a o MM, S h i J, Y a ng N, J i a n g Y M . E f f e c t o f ul t r as o nic t r e at men t o n the r e c o v er y a nd D P PH rad i c a l s ca v eng i n g act i vi t y of p ol ys a c c h ari d e s fr om longan fruit per i c a rp . F o od Chem 2 008 ;1 06 ( 2) :6 85- 9 0 . ) ( [41] K i ss GA C , F org ac s E , C se r ha t i T , Mota T , M or ai s H , R am os A , et a l. O pt i mis at ion o f t he mi c r owa v e-as sis t ed e x t r ac t io n of p igme n ts from pa p r i k a (C a p s i c u m an n uum L. ) p ow de rs. J C h romatogr A 2 0 00;8 8 9(1-2 ): 4 1 -9 . ) ( [42] W ang Y G, Wei X Y , W a n g S K, Li ZK, Li P , L iu F J , e t al . S truc t ur a l e va lu a tion of X i a o lon g tan li g ni t e b y d i r e ct c ha r a c te r i zat i o n and py ro l ytic an a l y s i s. F u e l P r o c e s s T echn ol 2 0 1 6; 144 : 248 - 54 . ) ( [43] L i u F J , W e i X Y, Li W T, G ui J, Li P , Wa n g Y G , e t a l . M ethanolys i s o f ex trac t ion r e si du e f ro m X ianf e ng l i g ni t e w i th N a OH a n d produ c t c h a r a c t e r i z at i o n s wi t h dif - f erent s pectrom et r ies. F u e l Proc ess T echnol 2014 ; 1 3 6 : 8- 1 6. ) ( [44] N Ni u Z , Gu i j i a n , Y in H , L iu H . I n v esti ga tion o f ef f e ct o f KB H4 o n s tructure of extr a cts , Gu j ian,4 o f a p e rh y d r ou s b it um i nou s c o a l wi t h d i ff e ren t sol v e n t s. Fre s e ni u s E n v i r o n Bull 2016; 2 5(8):3221-8 . ) ( [45 ] W ang YG , Wei XY , Yan H L , Sh i D L , L i u FJ, L i P , et al . Stru c tural feat u res o f e x - t r a c t ion re sid ues f r o m s upe r c ri ti c a l me t ha n olys i s o f tw o C hinese l i gn i te s. En e rgy F u els 20 1 3; 2 7(8) : 46 32 -8. ) ( [46] C hen B . We i X Y , Zo ng ZM , Y a n g ZS , Qin g Y, L iu C. D if fere n c e i n che mi c al c o m -positi o n o f s u p e rcritic a l meth a nolys is p r oduct s bet w e en tw o lign i tes. Ap p l Energ y 2011 ;8 8(1 2): 4570 - 6. ) ( [47] W an g Y N , W ei XY , Li Z K , Y a n H L , W a n g T M , Zh a ng YY , et al . E x tractio n a n d thermal dis s ol u tio n o f P i l iq i ng subbi t u m inous co al. F u el 2 01 7 ; 2 0 0:2 8 2-9. ) ( [48] H i ga sio Y S , S h oj i T . H e t e r o c yc l i c c o mp o und s su c h as py r rol e s , p yr id ines, p yr - o l l id i ns, p i p erdines, in dole s , imidaz ol a nd p y r azin s . A p pl Cat a l A 2 001 ;22 1(1) : 1 97-2 07 . ) ( [49] L I e n tin i G , B runo C , C a t a l an o A , C a r o cc i A , Fra n c h i ni C, T o r t o r e ll a V . F a c i l e ent r y t o e thyl tetrahy d ro- 1 H-p y rr o lizin- 7 a (5 H)- yl a ceta t e: a v e rsatile p h arm a ceu t ical i nte r -med i a t e. He t eroc y cle s 2 0 08 ; 75(9) : 2193 - 2 0 0. ) ( [ 50] L i Z K , W ei X Y , Y a n HL, Y u X Y , Zo n g ZM. I n s ig ht int o t h e c h e m i c a l co m p lexi t y o f e tha n o l y si s p r od u cts f r om ex t r a ction resid u e of Zh a ot on g l ign i te. Fu e l 2 016; 1 74:287- 9 5. ) ( [ 51] B a e E , N a JG, C h u ng SH, K i m HS , Ki m S. I de n ti fi c at i on of a bo ut 30 0 0 0 c h emical compone n ts i n s h a le oils b y el e c tr o s p ra y io n izat i on (E S I) and atm o sph er ic pr e ss ur e p ho t oionization (A P P I ) c o u ple d w i t h 15 T F o u r ier tr an s form i on c y c l o t r o n r e - s on a n c e m a ss s p ec t r ometr y (F T - I C R M S ) a nd a c omp aris o n t o co nv e n tional oil . E n er g y F u e l 2 010; 2 4(4):256 3- 9 . )
确定


还剩6页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《煤中提取过程检测方案(微波合成仪)》,该方案主要用于煤炭中提取过程检测,参考标准--,《煤中提取过程检测方案(微波合成仪)》用到的仪器有电脑微波超声波组合合成萃取仪 Soniwave XH-300A+型 、智能温压双控微波消解仪、电脑微波超声波紫外光组合合成萃取仪
推荐专场
相关方案
更多























