方案详情
文
使用美国戴安公司离子色谱测定食品(牛奶)/海水/尿液中碘离子的三篇方法文献.该方法具有灵敏度高,方法简单可靠的特点.
方案详情

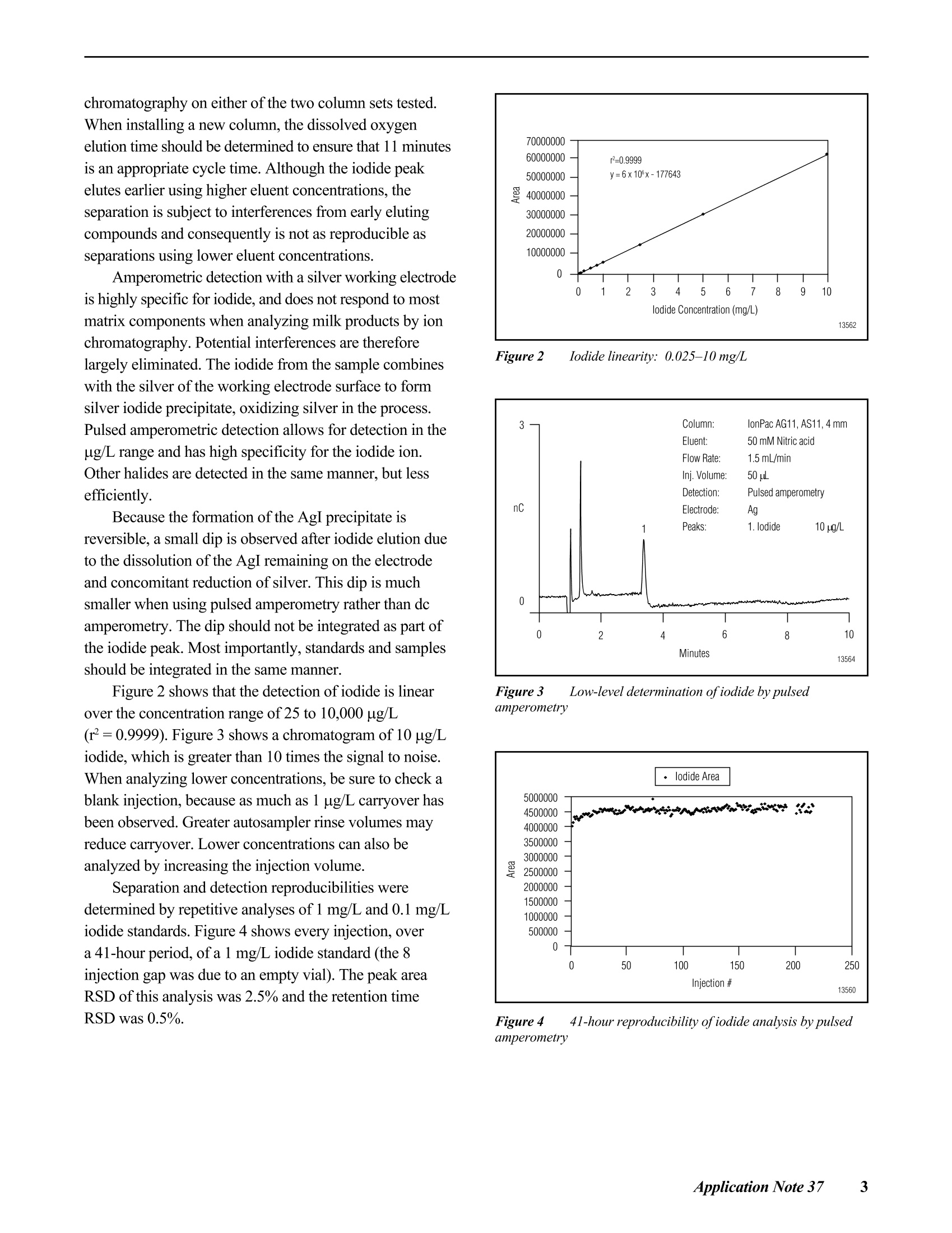
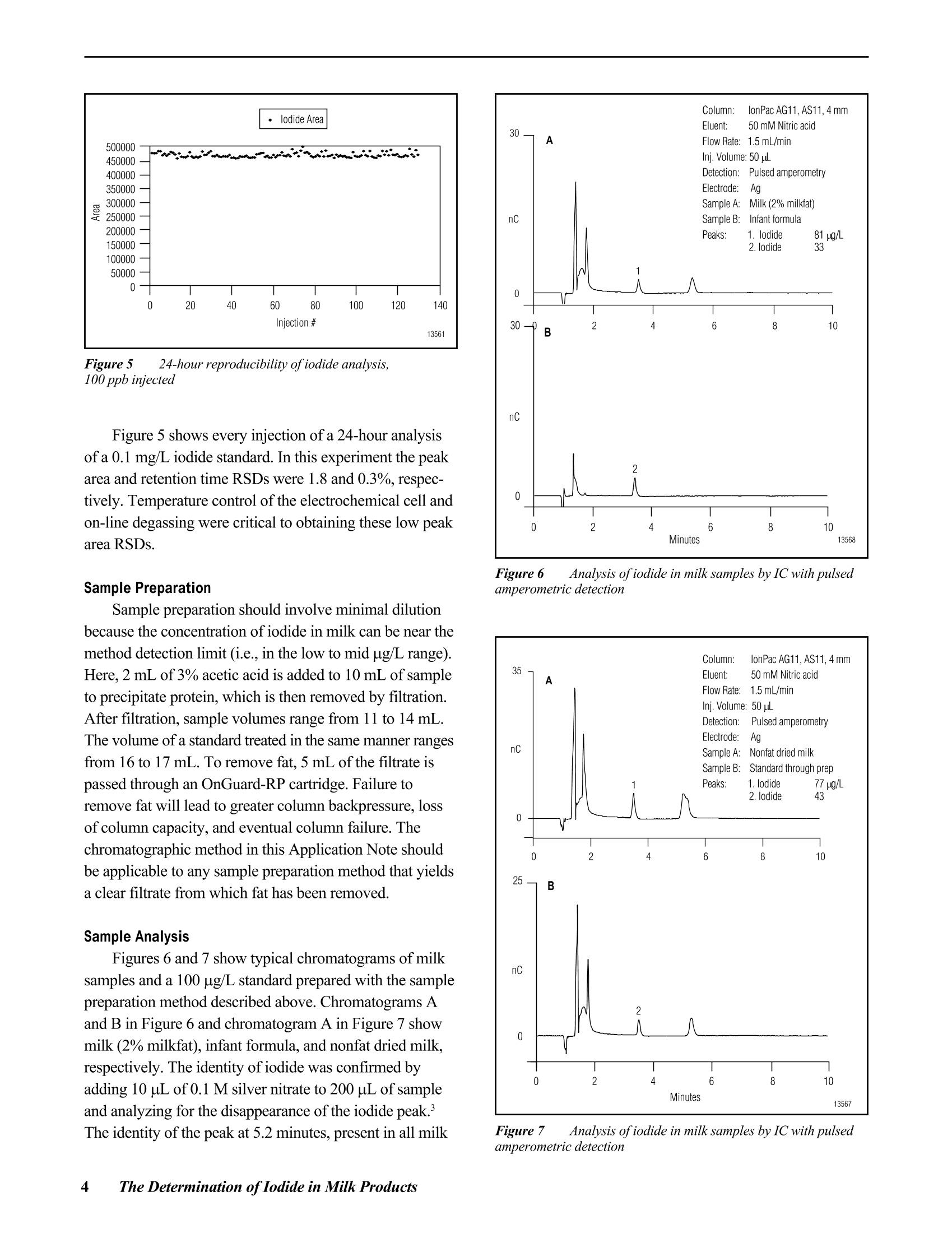
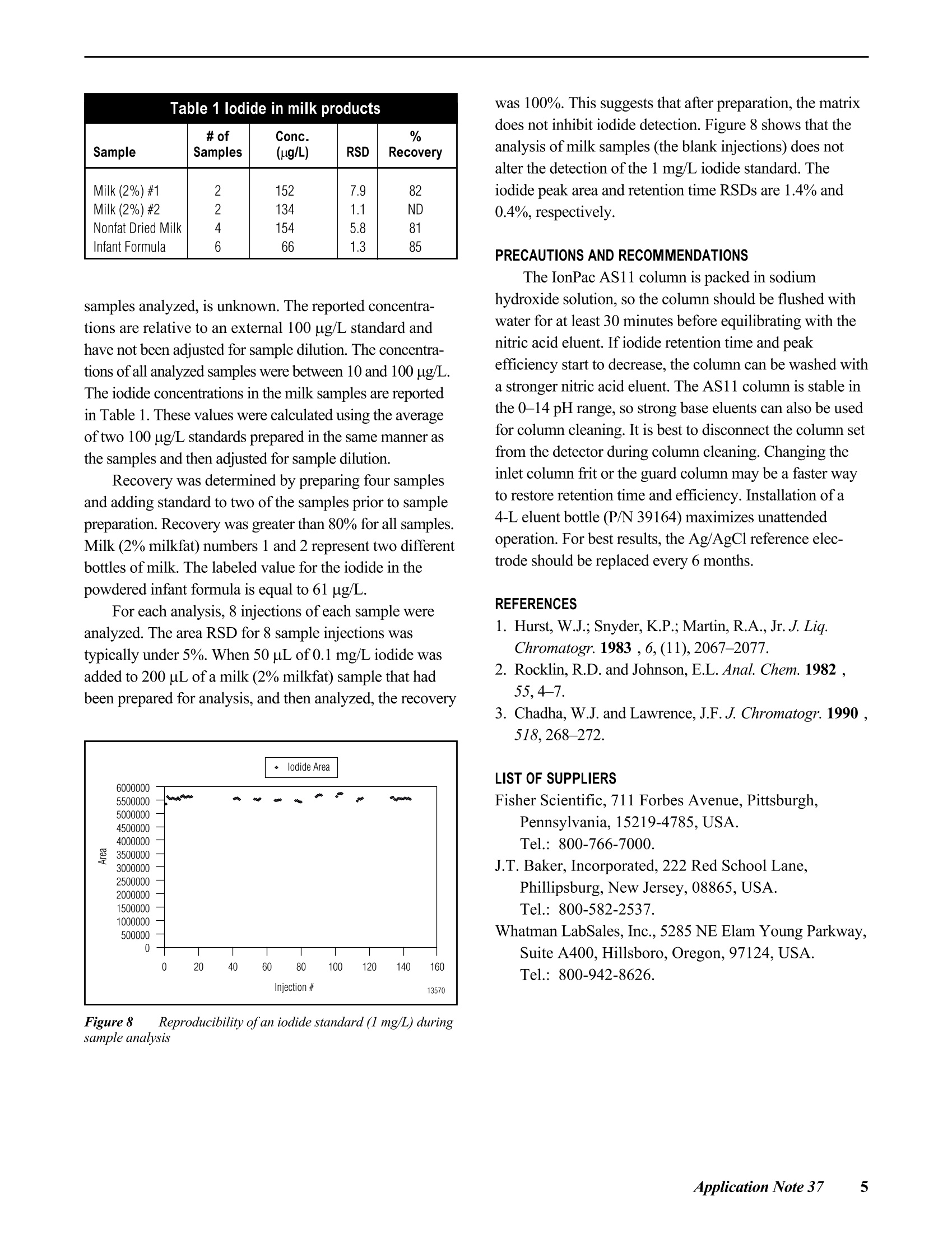
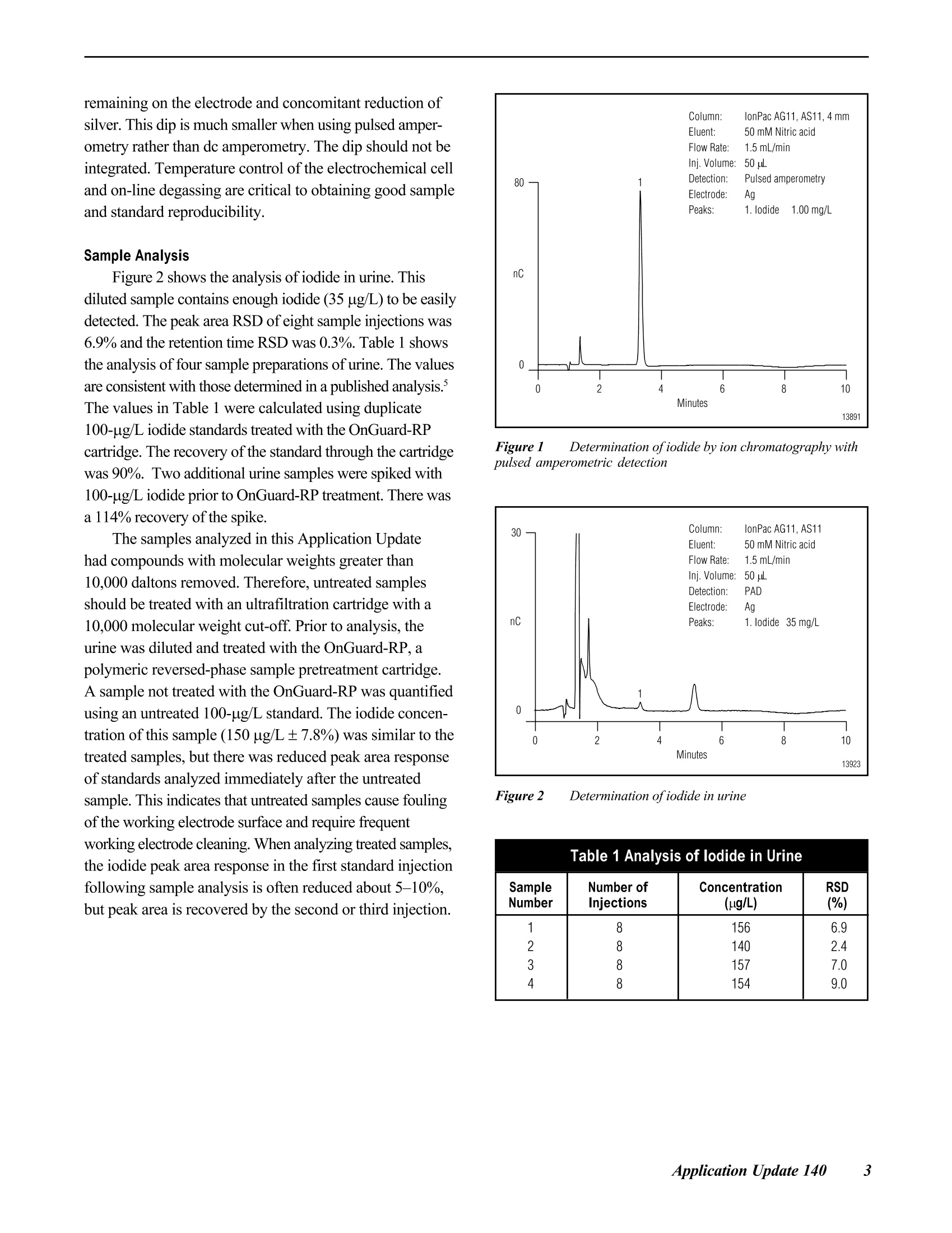
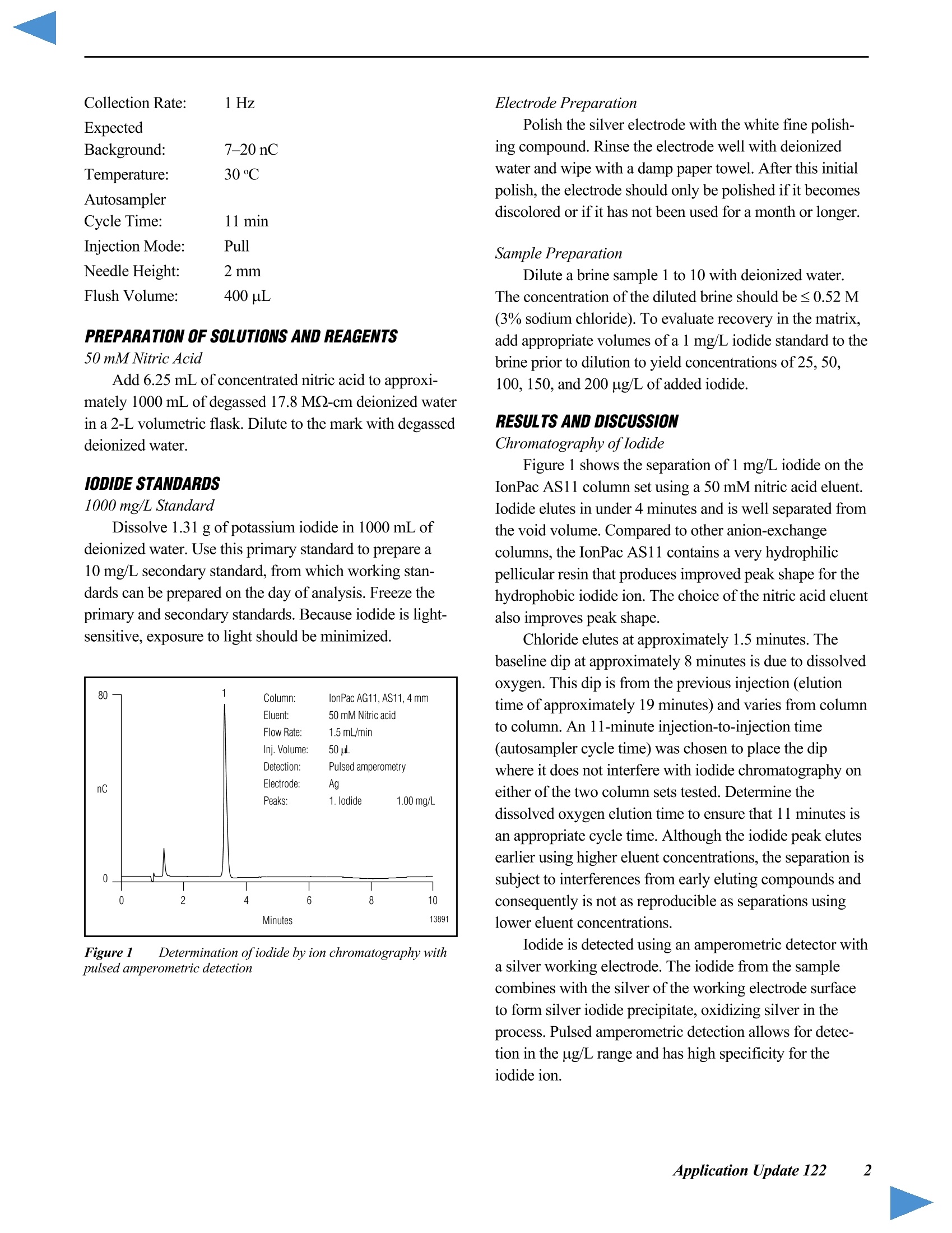
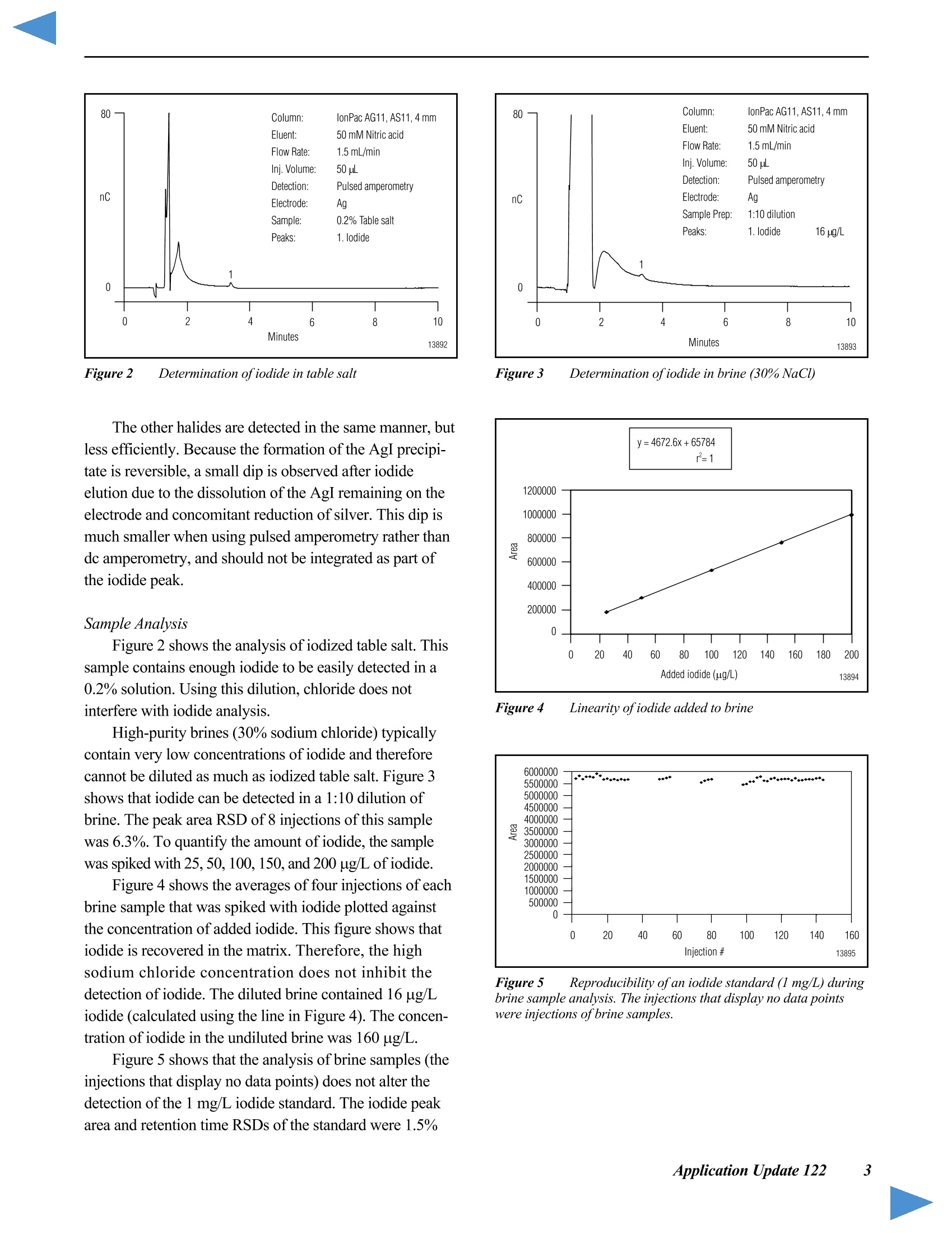
D DIONEX The Determination of Iodide in Milk Products INTRODUCTION Trace levels of iodide are necessary for normalphysical and mental development; however, excess iodidecan lead to thyroid disorders. Common sources of iodideinclude iodized table salt and seafood, but other foodproducts also contain iodide. Within the dairy industry,iodophors are used as disinfectants, which can also lead toincreased iodide consumption by the public. A concernover high iodide levels in the diet has led to a nutritionallabeling requirement for iodide/iodine. In this Application Note, ion chromatography coupledwith pulsed amperometric detection is used to determineiodide in milk products. This method is specific, sensitive,and rapid. Iodide is separated on the IonPac@AS11column, which contains a hydrophilic, anion-exchangeresin that is well suited to the chromatography oftherelatively hydrophobic iodide anion. Using a nitric acideluent, the iodide ion elutes from the column in less than5 minutes. Although iodide can be detected by directcurrent (dc) amperometry on a silver working electrode, apulsed amperometric waveform is used in this ApplicationNote to improve the reproducibility of iodide analysis.’Like dc amperometry, the detection limit of iodide usingpulsed amperometric detection is in the low ug/L range. EQUIPMENT Dionex DX-500 Chromatography system consisting of:GP40 Gradient Pump with vacuum degas optionLC25 or LC30 Liquid Chromatography ModuleED40 Electrochemical DetectorEO1 Eluent OrganizerAS3500 Autosampler Dionex PeakNet Chromatography WorkstationWhatman 2V Filters, 185 mm (Whatman)OnGuard -RP Sample Pretreatment Cartridges(Dionex P/N 39595) REAGENTS AND STANDARDSDeionized water, 17.8 MQ-cm resistivity or betterConcentrated nitric acid, ultrapure (J.T. Baker)Glacial acetic acid (J.T.Baker)Potassium iodide (Fisher Scientific) CONDITIONSColumns: IonPac AS11 Analytical, 4 x 250 mm(P/N 44076)IonPac AG11 Guard,4 x 50 mm(P/N 44078)Expected OperatingPressure: 6.5 MPa (950 psi)Degas Interval: 10 minInjection Volume: 50 uLInjection Loop: 100 uLEluent: 50 mM Nitric acidFlow Rate: 1.5 mL/minDetection: Pulsed amperometry, silver workingelectrode,Ag/AgCl reference Waveform for the ED40 Detector: Time (sec) Potential (V) 0.00 +0.1 0.20 +0.1 0.90 +0.1 0.91 -0.8 0.93 -0.3 1.00 -0.3 Collection Rate: 1Hz Background: 7-20 nC (typical) Temperature: 30°℃ Autosampler:11 min cycle timeInjection Mode:Pull Needle Height: 2 mm Flush Volume: 400 pL PREPARATION OF SOLUTIONS AND REAGENTS50 mM Nitric Acid Add 6.25 mL of concentrated nitric acid to approxi-mately 1000 mL of degassed 17.8 MQ-cm deionized waterin a 2-L volumetric flask. Dilute to the mark with degasseddeionized water. lodide Standards Prepare a 1000 mg/L standard by dissolving 1.31 gofpotassium iodide in 1000 mL of deionized water. Thisprimary standard was used to prepare a 10 mg/L secondarystandard, which was appropriately diluted for linearitystudies. Both the primary and secondary standards werestored frozen. Because iodide is light-sensitive, exposureto light should be minimized. All standards prepared fromthe 10 mg/L stock solution should be used on the day theyare prepared. Electrode Preparation Polish the silver electrode with the white fine polish-ing compound. Rinse the electrode well with deionizedwater and wipe with a damp paper towel. After this initialpolish, the electrode should only be polished if it becomesdiscolored or if it has not been used for a month or longer. SAMPLE PREPARATION OnGuard-RP Preparation Pass 5 mL of methanol, followed by 10 mL ofdeionized water, through the cartridge at 4 mL/min. Tosave time, up to 12 cartridges can be prepared at one timeusing the OnGuard Sample Prep Station (P/N 39599). Milk Sample Preparation Prepare the infant formula as suggested for feeding.Prepare the nonfat dried milk as recommended for serving(10 mL of water for every 0.95 g of milk powder). Pipet 10 mL of milk product into a 100-mL polypro-pylene beaker. Add 2 mL of 3% acetic acid and mix. Add8 mL of deionized water and mix. Pass the sample througha Whatman 2V filter. Measure the filtrate volume and pass 5 mL of sample through the OnGuard-RP cartridge at4 mL/min, discarding the first 3 mL of sample. Collectthe remaining filtrate and inject an aliquot into the chro-matograph. If the filtrate is cloudy, it should not be used.A cloudy filtrate suggests that a different sample prepara-tion method is necessary. To determine recovery, add 1 mL of 1 mg/L iodide tothe sample prior to the addition of acetic acid and add only7 mL of water prior to filtration. Calibration standardswere prepared by subjecting them to the sample prepara-tion procedure. 10 mL of 0.1 mg/L iodide was prepared induplicate for each experiment. RESULTS AND DISCUSSION Chromatography of lodide Figure 1 shows the separation of 1 mg/L iodide on theIonPac AS11 column set using a 50 mM nitric acid eluent.Iodide elutes in less than 4 minutes and is well separatedfrom the void volume. Compared to other ion-exchangecolumns, the IonPac AS11 contains a very hydrophilicpellicular resin that improves the peak shape of the hydro-phobic iodide ion. The nitric acid eluent also improvespeak shape. Figure 1 Determination ofiodide by ion chromatography withpulsed amperometric detection Fluoride, chloride. bromide.and iodate elutewell before iodide. Chloride elutes at approximately1.5 minutes. The dip in the baseline at approximately8 minutes is due to dissolved oxygen. This dip is from theprevious injection (elution time of approximately19 minutes) and varies from column to column. An11-minute injection-to-injection time (autosampler cycletime) places the dip where it does not interfere with iodide chromatography on either of the two column sets tested.When installing a new column, the dissolved oxygenelution time should be determined to ensure that 11 minutesis an appropriate cycle time. Although the iodide peakelutes earlier using higher eluent concentrations, theseparation is subject to interferences from early elutingcompounds and consequently is not as reproducible asseparations using lower eluent concentrations. Amperometric detection with a silver working electrodeis highly specific for iodide, and does not respond to mostmatrix components when analyzing milk products by ionchromatography. Potential interferences are thereforelargely eliminated. The iodide from the sample combineswith the silver of the working electrode surface to formsilver iodide precipitate, oxidizing silver in the process.Pulsed amperometric detection allows for detection in theug/L range and has high specificity for the iodide ion.Other halides are detected in the same manner, but lessefficiently. Because the formation of the AgI precipitate isreversible, a small dip is observed after iodide elution dueto the dissolution of the AgI remaining on the electrodeand concomitant reduction of silver. This dip is muchsmaller when using pulsed amperometry rather than dcamperometry. The dip should not be integrated as part ofthe iodide peak. Most importantly, standards and samplesshould be integrated in the same manner. Figure 2 shows that the detection of iodide is linearover the concentration range of 25 to 10,000 pg/L(r²=0.9999). Figure 3 shows a chromatogram of 10 ug/Liodide, which is greater than 10 times the signal to noise.When analyzing lower concentrations, be sure to check ablank injection, because as much as 1 ug/L carryover hasbeen observed. Greater autosampler rinse volumes mayreduce carryover. Lower concentrations can also beanalyzed by increasing the injection volume. Separation and detection reproducibilities weredetermined by repetitive analyses of 1 mg/L and 0.1 mg/Liodide standards. Figure 4 shows every injection, overa 41-hour period, of a 1 mg/L iodide standard (the 8injection gap was due to an empty vial). The peak areaRSD of this analysis was 2.5% and the retention timeRSD was 0.5%. Figure 2 Iodide linearity: 0.025-10 mg/L Figure 3 Low-level determination ofiodide by pulsedamperometry Figure 4 41-hour reproducibility ofiodide analysis by pulsedamperometry Figure 5 24-hour reproducibility ofiodide analysis,100 ppb injected Figure 5 shows every injection of a 24-hour analysisof a 0.1 mg/L iodide standard. In this experiment the peakarea and retention time RSDs were 1.8 and 0.3%,respec-tively. Temperature control of the electrochemical cell andon-line degassing were critical to obtaining these low peakarea RSDs. Sample Preparation Sample preparation should involve minimal dilutionbecause the concentration of iodide in milk can be near themethod detection limit (i.e., in the low to mid ug/L range).Here, 2 mL of 3% acetic acid is added to 10 mL of sampleto precipitate protein, which is then removed by filtration.After filtration, sample volumes range from 11 to 14 mL.The volume of a standard treated in the same manner rangesfrom 16 to 17 mL. To remove fat. 5 mL of the filtrate ispassed through an OnGuard-RP cartridge. Failure toremove fat will lead to greater column backpressure, lossofcolumn capacity, and eventual column failure. Thechromatographic method in this Application Note shouldbe applicable to any sample preparation method that yieldsa clear filtrate from which fat has been removed. Sample Analysis Figures 6 and 7 show typical chromatograms of milksamples and a 100 pg/L standard prepared with the samplepreparation method described above. Chromatograms Aand B in Figure 6 and chromatogram A in Figure 7 showmilk (2% milkfat), infant formula, and nonfat dried milk,respectively. The identity of iodide was confirmed byadding 10 uL of0.1 M silver nitrate to 200 pL of sampleand analyzing for the disappearance of the iodide peak.The identity of the peak at 5.2 minutes, present in all milk Figure 6 Analysis ofiodide in milk samples by IC with pulsedamperometric detection Figure 7 Analysis ofiodide in milk samples by IC with pulsedamperometric detection Table 1 lodide in milk products Table 1 lodide in milk products Sample # ofSamples Conc.(ug/L) RSD Recovery Milk (2%)#1Milk (2%) #2Nonfat Dried MilkInfant Formula 2246 15213415466 7.91.15.81.3 82ND8185 samples analyzed, is unknown. The reported concentra-tions are relative to an external 100 ug/L standard andhave not been adjusted for sample dilution. The concentra-tions of all analyzed samples were between 10 and 100 pg/L.The iodide concentrations in the milk samples are reportedin Table 1. These values were calculated using the averageof two 100 pg/L standards prepared in the same manner asthe samples and then adjusted for sample dilution. Recovery was determined by preparing four samplesand adding standard to two of the samples prior to samplepreparation. Recovery was greater than 80% for all samples.Milk (2% milkfat) numbers 1 and 2 represent two differentbottles of milk. The labeled value for the iodide in thepowdered infant formula is equal to 61 ug/L. For each analysis, 8 injections of each sample wereanalyzed. The area RSD for 8 sample injections wastypically under 5%. When 50 uL of 0.1 mg/L iodide wasadded to 200 uL ofa milk (2% milkfat) sample that hadbeen prepared for analysis, and then analyzed, the recovery Figure 8 Reproducibility of an iodide standard (1 mg/L) duringsample analysis was 100%. This suggests that after preparation, the matrixdoes not inhibit iodide detection. Figure 8 shows that theanalysis of milk samples (the blank injections) does notalter the detection of the 1 mg/L iodide standard. Theiodide peak area and retention time RSDs are 1.4% and0.4%, respectively. PRECAUTIONS AND RECOMMENDATIONS The IonPac AS11 column is packed in sodiumhydroxide solution, so the column should be flushed withwater for at least 30 minutes before equilibrating with thenitric acid eluent. Ifiodide retention time and peakefficiency start to decrease, the column can be washed witha stronger nitric acid eluent. The AS11 column is stable inthe 0-14 pH range, so strong base eluents can also be usedfor column cleaning. It is best to disconnect the column setfrom the detector during column cleaning. Changing theinlet column frit or the guard column may be a faster wayto restore retention time and efficiency. Installation of a4-L eluent bottle (P/N 39164) maximizes unattendedoperation. For best results, the Ag/AgCl reference elec-trode should be replaced every 6 months. REFERENCES 1. Hurst, W.J.; Snyder, K.P.; Martin, R.A., Jr. J. Liq.Chromatogr. 1983 ,6,(11),2067-2077. 2. Rocklin,R.D. and Johnson,E.L. Anal. Chem. 1982 ,55,4-7. 3. Chadha, W.J. and Lawrence, J.F.J. Chromatogr. 1990 ,518,268-272. LIST OF SUPPLIERS Fisher Scientific, 711 Forbes Avenue, Pittsburgh,Pennsylvania, 15219-4785,USA.Tel.: 800-766-7000. J.T. Baker, Incorporated, 222 Red School Lane,Phillipsburg, New Jersey, 08865, USA.Tel.: 800-582-2537. Whatman LabSales, Inc., 5285 NE Elam Young Parkway,Suite A400, Hillsboro, Oregon, 97124, USA. Tel.: 800-942-8626. ( P rinted on recycled a n d recyclable paper. ) Dionex U.S. Regional OfficesSunnyvale, CA(408) 737-8522Westmont,IL (630)789-3660Houston, TX (281)847-5652Atlanta, GA (770)432-8100Marlton, NJ (856) 596-0600 Dionex International Subsidiaries Italy (06) 66 51 50 52 Japan(06)6885-1213 The Netherlands (0161) 434303 Switzerland (062) 205 99 66 United Kingdom(01276) 691722 *Designed, developed, and manufactured under an NSAl registered ISO 9001 Quality System. Application Update 140 The Determination of Iodide in Urine Measuring the concentration of iodide in urine isuseful for the diagnosis of transient thyroid dysfunctionand iodine-induced hyperthyrosis. Patients with iodine-induced hyperthyrosis have 10- to 100-fold more urinaryiodide than healthy patients. In this Application Update, ion chromatographycoupled with pulsed amperometric detection is used todetermine iodide in urine. This method is specific, sensi-tive, and rapid. Sample preparation involves removal ofurine components with molecular weights greater than10,000 daltons, sample dilution, and treatment with apolymeric, reversed-phase cartridge (OnGuard "-RP). Iodide, a relatively hydrophobic anion, is separatedon the IonPac@ AS11 column, which contains a hydrophilicresin that is well suited to the chromatography of iodide.Using a nitric acid eluent, the iodide ion elutes from thecolumn in under 5 minutes. Although iodide can bedetected by direct current (dc) amperometry on a silverworking electrode,a pulsed amperometric waveformimproves reproducibility.The linearity and reproducibilityof iodide detection by pulsed amperometry has beendemonstrated. Because the detection limit of iodide usingpulsed amperometric detection is in the low ug/L range,this technique can be used to determine iodide in urine. EQUIPMENT Dionex DX-500 Chromatography system consisting of:GP50 Gradient Pump with vacuum degas optionLC25 or LC30 Liquid Chromatography ModuleED40 Electrochemical DetectorEO1 Eluent OrganizerAS3500 Autosampler Dionex PeakNet Chromatography Workstation REAGENTS AND STANDARDSDeionized water, 17.8 MQ-cm resistivity or betterConcentrated nitric acid (16 M), ultrapure (J.T. Baker)Potassium iodide (Fisher Scientific) CONDITIONS Waveform for the ED40 Detector: Collection Rate: 1 Hz Expected Background: 7-20 nC Temperature: 30℃ Autosampler Cycle Time: 11 min Injection Mode: Pull Needle Height: 2 mm Flush Volume: 400 pL PREPARATION OF SOLUTIONS AND REAGENTSEluent Preparation 50 mM Nitric Acid Add 6.25 mL of concentrated nitric acid to approxi-mately 1000 mL of degassed 17.8 MQ-cm deionized waterin a2-L volumetric flask. Dilute to the mark with degasseddeionized water. lodide Standards Dissolve 1.31 g of potassium iodide in 1000 mL ofdeionized water to prepare a 1000-mg/L standard. Use thisprimary standard to prepare a 10-mg/L secondary standardfrom which working standards can be prepared on the dayof analysis. Freeze and store the primary and secondarystandards. Because iodide is light-sensitive, minimizeexposure to light. Electrode Preparation Polish the silver electrode with fine white polishingcompound. Rinse the electrode well with deionized waterand wipe with a damp paper towel. After this initial polish-ing, the electrode should only be polished if it becomesdiscolored or if it has not been used for a month or longer. Sample Preparation OnGuard-RP Preparation Pass 5 mL of methanol, followed by 10 mL of deionizedwater, through the cartridge at 4 mL/min. Up to 12samples at a time can be prepared using the OnGuardSample Prep Station (P/N 39599). Urine Sample Preparation Dilute 1 mL of human male urine from which compo-nents with molecular weights greater than 10,000 daltonshave been removed (Sigma, P/N U6378) with 4 mL ofwater. Pass this sample through an OnGuard-RP cartridge at 4 mL/min, discarding the first 3 mL. Inject this filtrateinto the chromatograph. To determine recovery, add 0.5 mLof 1-mg/L iodide to the sample prior to dilution with water(add 3.5 mL of water). Prepare a5-mL sample of 0.1-mg/Liodide using the above sample preparation procedure tocalculate the iodide content of each sample. Prepare thisstandard in duplicate for each experiment. Analysis of Electroactive Anions To determine the retention times of other electroactiveanions, analyze 1-mg/L solutions of the sodium salts ofsulfite, thiosulfate, cyanate, thiocyanate, and sulfide. RESULTS AND DISCUSSIONChromatography of lodide Figure 1 shows the separation of 1-mg/L iodide on theAS11 column set using a 50 mM nitric acid eluent. Iodideelutes in under 4 min and is well separated from the voidvolume. Unlike other anion-exchange columns, the IonPacAS11 column contains a very hydrophilic, pellicular resin thatproduces improved peak shape for the hydrophobic iodideion. The choice of a nitric acid eluent also improves peak shape.Chloride elutes at approximately 1.5 min. The dip in thebaseline at approximately 8 min is due to dissolved oxygen.This dip is from the previous injection (elution time ofapproximately 19 min) and varies from column to column.An 11-min injection-to-injection time (autosampler cycletime) was chosen to place the dip where it does not interferewith iodide chromatography on either of the two column setstested. Determine the dissolved oxygen elution time to ensurethat 11 min is an appropriate cycle time. Although the iodidepeak elutes earlier using higher eluent concentrations, theseparation is subject to interferences from early-elutingcompounds and consequently is not as reproducible asseparations using lower eluent concentrations. Iodide is detected using an amperometric detector witha silver working electrode. The iodide from the samplecombines with the silver of the working electrode surfaceto form silver iodide precipitate, oxidizing silver in theprocess. Pulsed amperometric detection has high specific-ity for the iodide ion and allows for detection in the ug/Lrange. The other halides are detected in the same manner,but less efficiently. The formation of the silver iodideprecipitate is reversible, so a small dip is observed afteriodide elution due to the dissolution of the silver iodide remaining on the electrode and concomitant reduction ofsilver. This dip is much smaller when using pulsed amper-ometry rather than dc amperometry. The dip should not beintegrated. Temperature control of the electrochemical celland on-line degassing are critical to obtaining good sampleand standard reproducibility. Sample Analysis Figure 2 shows the analysis of iodide in urine. Thisdiluted sample contains enough iodide (35 ug/L) to be easilydetected. The peak area RSD of eight sample injections was6.9% and the retention time RSD was 0.3%. Table 1 showsthe analysis of four sample preparations of urine. The valuesare consistent with those determined in a published analysis.The values in Table 1 were calculated using duplicate100-ug/L iodide standards treated with the OnGuard-RPcartridge. The recovery ofthe standard through the cartridgewas 90%. Two additional urine samples were spiked with100-pg/L iodide prior to OnGuard-RP treatment. There wasa 114% recovery of the spike. The samples analyzed in this Application Updatehad compounds with molecular weights greater than10,000 daltons removed. Therefore, untreated samplesshould be treated with an ultrafiltration cartridge with a10,000 molecular weight cut-off. Prior to analysis, theurine was diluted and treated with the OnGuard-RP, apolymeric reversed-phase sample pretreatment cartridge.A sample not treated with the OnGuard-RP was quantifiedusing an untreated 100-pg/L standard. The iodide concen-tration of this sample (150 pg/L±7.8%) was similar to thetreated samples, but there was reduced peak area responseof standards analyzed immediately after the untreatedsample. This indicates that untreated samples cause foulingof the working electrode surface and require frequentworking electrode cleaning. When analyzing treated samples,the iodide peak area response in the first standard injectionfollowing sample analysis is often reduced about 5-10%,but peak area is recovered by the second or third injection. Figure 1 Determination ofiodide by ion chromatography withpulsed amperometric detection Figure 2 Determination ofiodide in urine Table 1 Analysis of lodide in Urine SampleNumber Number ofInjections Concentration(ug/L) RSD(%) 1234 888 8 156 140157 154 6.92.47.09.0 Analysis of Other Electroactive Anions Sulfite and cyanide were not eluted or detected withthis method. Thiocyanate eluted at 5.2 min, approximatelythe same time as an unknown peak in the urine sample.Thiosulfate eluted at approximately 13 min. Sulfide,whichusually is not present in urine, coeluted with iodide but wasonly detected with about one-fiftieth the sensitivity. PRECAUTIONS AND RECOMMENDATIONS The IonPac AS11 column is packed in sodium hydrox-ide. The column should be flushed with water for at least30 min before equilibrating with the nitric acid eluent. Ifthere is a loss of iodide retention time or peak efficiency,the column can be washed with a stronger nitric acideluent. The AS11 column is stable in the 0-14 pH range,so strong base eluents can also be used for column clean-ing. It is best to disconnect the column set from thedetector cell during column cleaning. Changing the inletcolumn frit or the guard column may also restore retentiontime and efficiency. Installation of a 4-L eluent bottle (P/N 39164) allowsunattended operation for longer periods. Dionex recom-mends replacement of the Ag/AgCl reference electrodeevery 6 months. REFERENCES 1. Mura, P.; Papet, Y; Sanchez, A.; and Piriou, A. J.Chromatogr. B, 1995, 664,440-443. ( 2. Rocklin, R. D. and Johnson, E. L. Anal. Chem. 1982,55,4-7. ) 3. Dionex Application Note 37. 4. Han, K.; Koch, W. F.; and Pratt, K. W. Anal.Chem.1987,59,731-736. 5. Heurtebise, M. and Ross, W.J. Anal. Chem. 1971, 43,1438-1441. LIST OF SUPPLIERS Fisher Scientific, 711 Forbes Avenue,Pittsburgh, Pennsylvania,15219-4785, USA.Tel.1-800-766-7000. J.T. Baker, Incorporated, 222 Red SchoolLane,Phillipsburg, New Jersey 08865,USA.Tel. 1-800-582-2537. Sigma Chemical Company, P.O. Box 14508, St. Louis,Missouri,63178,USA.Tel. 1-800-325-3010. The Determination of Iodide in Brine Feed brines for chlor-alkali cells must be relativelyfree of alkaline earth metals and iodide. Brines high inalkaline earth metals shorten membrane life due to theinteraction of these metals with iodide at the membranesurface. In this Application Update, ion chromatographycoupled with pulsed amperometric detection is used todetermine iodide in brine (approximately 30% sodiumchloride). This method is specific, sensitive, and rapid.Sample preparation is a simple sample dilution. Iodide isseparated on the IonPac@ AS11 column, which contains ahydrophilic resin that is well suited to the chromatographyof iodide, a relatively hydrophobic anion. Using a nitricacideluent, the iodide ion elutes from the column in underfive minutes.Although iodide can be detected by directcurrent (dc) amperometry on a silver working electrode,a pulsed amperometric waveform is used to improvethe reproducibility of iodide analysis. The linearity andreproducibility of iodide detection by pulsed amperometryhave been demonstrated.The detection limit of iodideusing pulsed amperometric detection is in the low ug/Lrange. EQUIPMENT Dionex DX-500 Chromatography system consisting of:GP40 Gradient Pump with vacuum degas optionLC25 or LC30 Liquid Chromatography ModuleED40 Electrochemical DetectorEO1 Eluent OrganizerAS3500 Autosampler Dionex PeakNet Chromatography Workstation REAGENTS AND STANDARDS Deionized water, 17.8 MQ-cm resistivity or betterConcentrated nitric acid (16 M), ultrapure (J.T. Baker)Potassium iodide (Fisher Scientific) CONDITIONS Column: IonPac AS11 Analytical,4 x 250 mm (P/N 44076) IonPac AG11 Guard, 4x 50 mm (P/N 44078) Expected OperatingPressure: 6.5 MPa (950 psi)Degas Interval: 10 minInjection Volume: 50 uLInjection Loop: 100 uLEluent: 50 mM Nitric acidFlow Rate: 1.5 mL/minDetection: Pulsed amperometry, silver workingelectrode, Ag/AgCl reference Waveform for the ED40 Detector: Collection Rate: 1 Hz Expected Background: 7-20 nC Temperature: 30℃ Autosampler Cycle Time: 11 min Injection Mode: Pull Needle Height: 2 mm Flush Volume: 400 uL PREPARATION OF SOLUTIONS AND REAGENTS 50 mM Nitric Acid Add 6.25 mL of concentrated nitric acid to approxi-mately 1000 mL of degassed 17.8 MQ-cm deionized waterin a 2-L volumetric flask. Dilute to the mark with degasseddeionized water. IODIDE STANDARDS 1000 mg/L Standard Dissolve 1.31 g of potassium iodide in 1000 mL ofdeionized water. Use this primary standard to prepare a10 mg/L secondary standard, from which working stan-dards can be prepared on the day of analysis. Freeze theprimary and secondary standards. Because iodide is light-sensitive, exposure to light should be minimized. Figure 1 Determination ofiodide by ion chromatography withpulsed amperometric detection Electrode Preparation Polish the silver electrode with the white fine polish-ing compound. Rinse the electrode well with deionizedwater and wipe with a damp paper towel. After this initialpolish, the electrode should only be polished if it becomesdiscolored or if it has not been used for a month or longer. Sample Preparation Dilute a brine sample 1 to 10 with deionized water.The concentration of the diluted brine should be≤ 0.52 M(3% sodium chloride). To evaluate recovery in the matrix,add appropriate volumes of a 1 mg/L iodide standard to thebrine prior to dilution to yield concentrations of 25, 50,100, 150, and 200 ug/L of added iodide. RESULTS AND DISCUSSION Chromatography of lodide Figure 1 shows the separation of1 mg/L iodide on theIonPac AS11 column set using a 50 mM nitric acid eluent.Iodide elutes in under 4 minutes and is well separated fromthe void volume. Compared to other anion-exchangecolumns, the IonPac AS11 contains a very hydrophilicpellicular resin that produces improved peak shape for thehydrophobic iodide ion. The choice of the nitric acid eluentalso improves peak shape. Chloride elutes at approximately 1.5 minutes. Thebaseline dip at approximately 8 minutes is due to dissolvedoxygen. This dip is from the previous injection (elutiontime of approximately 19 minutes) and varies from columnto column. An 11-minute injection-to-injection time(autosampler cycle time) was chosen to place the dipwhere it does not interfere with iodide chromatography oneither of the two column sets tested. Determine thedissolved oxygen elution time to ensure that 11 minutes isan appropriate cycle time. Although the iodide peak elutesearlier using higher eluent concentrations, the separation issubject to interferences from early eluting compounds andconsequently is not as reproducible as separations usinglower eluent concentrations. Iodide is detected using an amperometric detector witha silver working electrode. The iodide from the samplecombines with the silver of the working electrode surfaceto form silver iodide precipitate, oxidizing silver in theprocess. Pulsed amperometric detection allows for detec-tion in the ug/L range and has high specificity for theiodide ion. Figure 2 Determination ofiodide in table salt The other halides are detected in the same manner, butless efficiently. Because the formation of the AgI precipi-tate is reversible, a small dip is observed after iodideelution due to the dissolution of the AgI remaining on theelectrode and concomitant reduction of silver. This dip ismuch smaller when using pulsed amperometry rather thandc amperometry, and should not be integrated as part ofthe iodide peak. Sample Analysis Figure 2 shows the analysis of iodized table salt. Thissample contains enough iodide to be easily detected in a0.2% solution. Using this dilution, chloride does notinterfere with iodide analysis. High-purity brines (30% sodium chloride) typicallycontain very low concentrations of iodide and thereforecannot be diluted as much as iodized table salt. Figure 3shows that iodide can be detected in a 1:10 dilution ofbrine. The peak area RSD of 8 injections of this samplewas 6.3%. To quantify the amount of iodide, the samplewas spiked with 25, 50,100,150, and 200 pg/L of iodide. Figure 4 shows the averages of four injections of eachbrine sample that was spiked with iodide plotted againstthe concentration of added iodide. This figure shows thatiodide is recovered in the matrix. Therefore, the highsodium chloride concentration does not inhibit thedetection of iodide. The diluted brine contained 16 pg/Liodide (calculated using the line in Figure 4). The concen-tration of iodide in the undiluted brine was 160 ug/L. Figure 5 shows that the analysis of brine samples (theinjections that display no data points) does not alter thedetection of the 1 mg/L iodide standard. The iodide peakarea and retention time RSDs of the standard were 1.5% Figure 3 Determination ofiodide in brine (30%NaCl) Figure 4 Linearity ofiodide added to brine Figure 5 Reproducibility ofan iodide standard (1mg/L) duringbrine sample analysis. The injections that display no data pointswere injections of brine samples. and 0.5%, respectively. In another experiment, 16 injec-tions of a 1 mg/L standard were followed by 56 injectionsof brine, and then 16 additional injections of the standard.There was no significant change in the standard peak arearesponse after 56 injections of brine. Both experimentsshow that repetitive analysis of diluted brine samples doesnot inhibit the response of iodide. Temperature control ofthe electrochemical cell and on-line degassing were criticalto obtaining these low peak area RSDs. PRECAUTIONS AND RECOMMENDATIONS The IonPac AS11 column is packed in a sodiumhydroxide solution. The column should be flushed withwater for at least 30 minutes before equilibrating with thenitric acid eluent. If there is a loss of iodide retention timeand/or peak efficiency, the column can be washed with astronger nitric acid eluent. The AS11 column is stable inthe 0-14 pH range, so strong base eluents can also be usedfor column cleaning. It is best to disconnect the column setfrom the detector during column cleaning. Changing theinlet column frit or the guard column may be a faster wayto restore retention time and efficiency. Installation of a4-L eluent bottle (P/N 39164) maximizes unattendedoperation. For best results, the Ag/AgCl reference elec-trode should be replaced every 6 months. REFERENCES 1. Rocklin, R.D. and Johnson, E.L.Anal. Chem.1982, 55, 4-7. 2. Dionex Corporation, The Determination of Iodide inMilk Products; Application Note 37; Sunnyvale,California. LIST OF SUPPLIERS Fisher Scientific, 711 Forbes Avenue,Pittsburgh, Pennsylvania, 15219-4785, USA, Tel: 1-800-766-7000. J.T. Baker, Incorporated, 222 Red School Lane,Phillipsburg, New Jersey, 08865,USA,Tel:1-800-582-2537. Dionex Corporation Dionex Corporation Dionex U.S. Regional Offices Dionex International Subsidiaries 1228 Titan Wav Salt Lake City Technical Center ( Sunn y vale, CA (408) 737-8522 ) P.O. Box 3603 1515 West 2200 South, Suite A Sunnyvale, CA Salt Lake City, UT 94088-3603 84119-1484 ( Sunn y vale, CA (408) 737-8522Westmont, I l (630) 789 - 3660 Houston, TX ( 281) 8 47 - 5652At la nta, GA (770)432-8100 ) LPN 034075-01 3M 6/98 (408) 737-0700 (801)972-9292 Marlton, NJ (609)596-0600 http://www.dionex.com pplication Note The Determination of Iodide in Milk Products
确定








还剩12页未读,是否继续阅读?
赛默飞色谱与质谱为您提供《尿液中碘离子检测方案(离子色谱仪)》,该方案主要用于尿液中碘离子检测,参考标准--,《尿液中碘离子检测方案(离子色谱仪)》用到的仪器有赛默飞戴安ICS-2100离子色谱系统
推荐专场
相关方案
更多
该厂商其他方案
更多