方案详情
文
纤维可用于复合材料、织物或非织物工艺甚至加强混领土结构。为给予纤维适当的表面性质,表面处理或涂层常被用于保护、功能化、润滑、色标和装饰。从接触角和表面张力计算的粘附功和界面张力数值研究厚度原因导致表面活性剂浓度的影响以及优化涂层性能。
方案详情

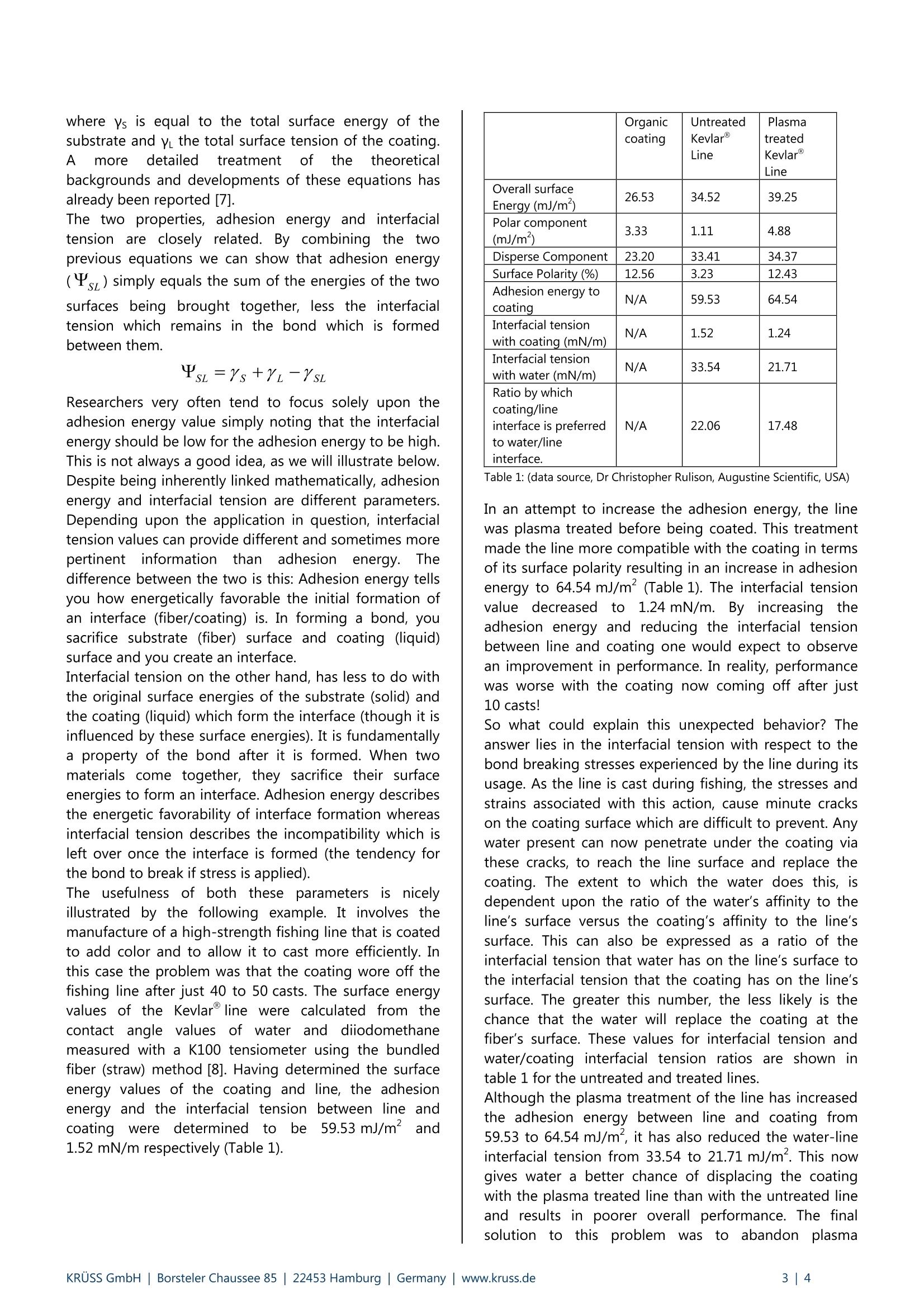
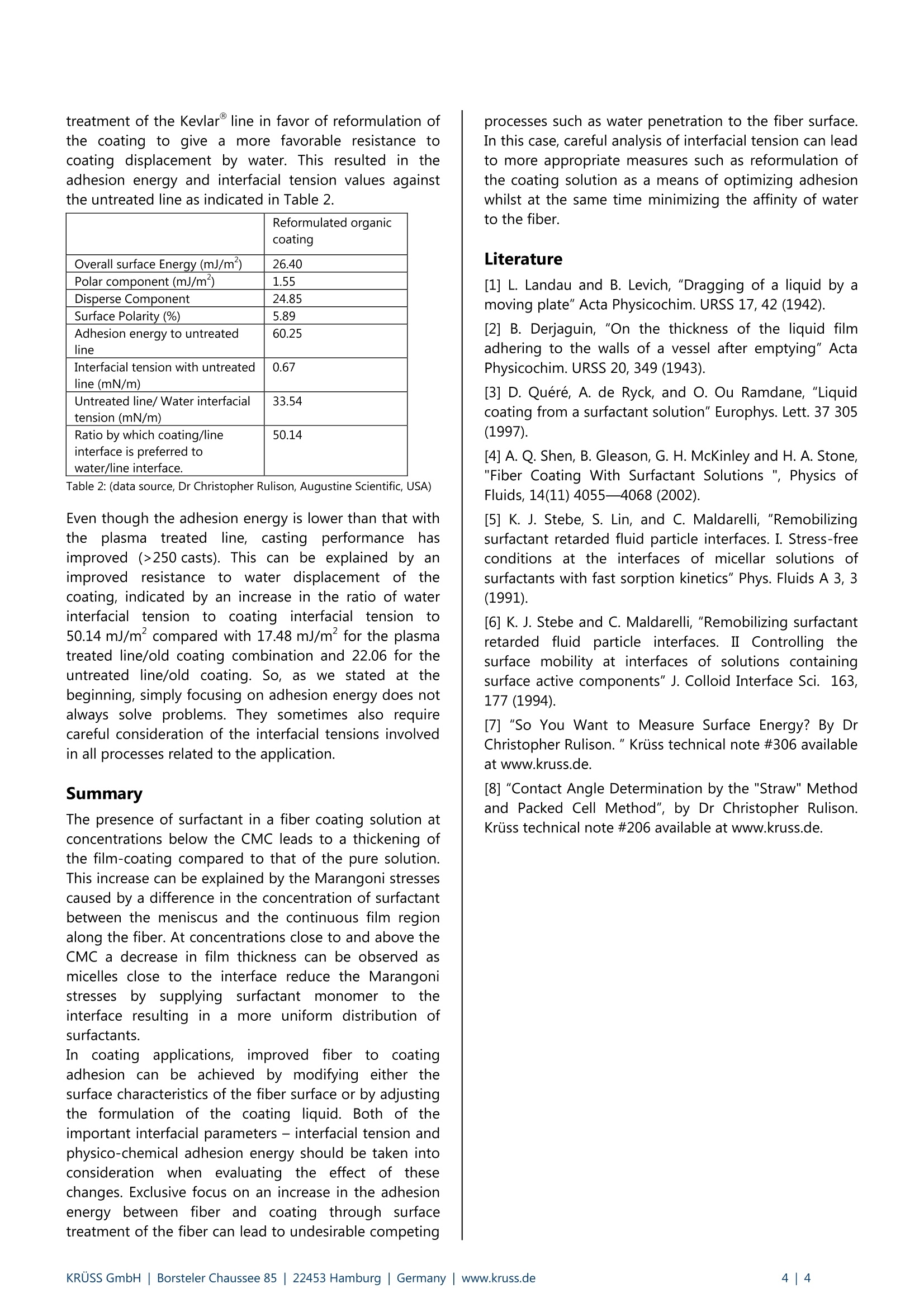
KRUSS Application Report The role of surfactants, adhesion energy and interfacial tensions in fibercoating applications Abstract Fibers affect all of us in one way or another during our daily lives. They may be used in the form of a composite material,a woven or non-woven technical textile or even in reinforced concrete structures. The surface properties of these fibersplay a key role in delivering the performance required by a particular process or final product. To impart the fiber with theappropriate surface properties, surface treatments or coatings are applied and most often serve to protect, functionalize,lubricate, color code and sometimes decorate the surface. This article focuses upon two important aspects of the surfacechemistry related to fibers: the influence of surfactant concentration in coating formulations upon the coating thicknessand the optimization of finished coating performance through the exploitation of fiber-coating adhesion energy andinterfacial tension values calculated from contact angle and surface tension measurements. Surface tension, CMC and coating thicknesson fibers Generally, coating is achieved by drawing a fiber at agiven speed through a bath containing the coatingliquid. As the fiber traverses the liquid air interface,a thinlayer of coating is deposited onto it. Early work on filmcoating problems was carried out by Landeau andLevich[1]and Derjaguin [2] who determined therelationship between substrate geometry (fiber)andspeed (U) in the low capillary number limit, where C is the capillary number, p is the fluid shearviscosity and y is the fluid surface tension. This so-called LLD relationship for the fiber coating filmthickness for Newtonian fluid in the absence of surfactantwas found to be: Where h is the coating thickness and b is the fiber radius.The wetting of fibers with surfactants at differentconcentrations both below and above the CMC has beenthe subject of a number of studies. It has been generallybeen observed that the presence of surfactant in asolution at concentrations below the CMC leads to athickening of the film-coating compared to that of thepure solution. The reason for this is illustrated in Figure 1. Fig. 1: Diagram of a fiber being drawn through a liquid bathin the presence of surfactant. The mechanism for the observed film thickening with asurfactant solution is due to stretching of the interface asit is dragged out from the meniscus by the fiber. Thisstretching alters the concentration of surfactants, whichresults in a surface tension gradient along the interface.Stress along the film resulting from this surface tensiongradient is commonly referred to as the Marangonieffect. In the case of a fiber being coated by a surfactantsolution, the Marangoni stress is caused by a differencein the concentration of surfactant between the meniscusand the continuous film region along the fiber. Thisresults in an additional traction along the direction ofpull towards the film and leads to a thickening of thefilm. The influence of surfactants is not restricted to a simpleincrease of the film thickness with increased surfactantconcentration. In fact, for surfactant concentrations closeto and above the CMC, the film thickness can decreasewith increasing surfactant concentration. This has beenreported for both surfactant solutions of SDS [3],[4] andTriton X100 [4]. This effect can be explained as follows. As the surfactantconcentration reaches and exceeds the value of CMC,micelles form in the solution. As these micelles are inconstant dynamic equilibrium with freee Ssurfactantmonomer in the bulk, monomers are being constantlyexchanged with micelles. In this way, micelles close to theinterface can act as reservoirs of free monomer that canadsorb at the interface resulting in a more uniformdistribution of surfactant at the surface compared withbulk .surfactant concentrationslS below theCMC.Consequently, the Marangoni effect is reduced and inturn the film thickness decreases. This effect has beenreferred by Stebe et al [5],[6] as surface re-mobilization.The extent to which the presence of surfactant affects thefilm thickness has been described as the thickeningfactor [4] and is given by: where h is the measured film thickness of the surfactantsolution and hup is the thickness predicted by the previously mentioned LLD relation. In the case of sodiumdodecyl sulfate (C12H25OSONa), a increases from a valueof 1 at zero concentration to a first maximum α= 2 at aconcentration of 0.4 xCMC. As the bulk concentrationsurpasses the CMC, a begins to decrease with increasingconcentration. This decrease is consistent withitthesurface re-mobilization effect described above. When the concentration reaches 10 x CMC, the film startsto thicken again and reaches a second maximum at25 xCMC at a value again of a=2.0 followed by a furtherdecrease to α1.6. These fluctuations in film thicknessare a result of the changing kinetics of micelle-monomerexchange with increase bulk concentration of surfactant.In other words, the stability characteristics of micelles -and thus their ability to exchange monomers to theinterface- are dependent upon the bulk concentration ofsurfactant. These kinetics and the stability characteristicsof micelles will of course vary for different surfactants ormixtures of surfactants. Consequently the variation in filmthickness with bulk concentration of surfactant will alsoexhibit different behavior. Keeping the coating on. Adhesion energy orinterfacial tension? In fiber coating applications, the aim is typically toimprove the wetting and adhesion between the fiber andthe coating liquid.This can be achieved by modifying thesurface characteristics of the fiber surface or by adjustingthe formulation of the coating liquid. In either case, thiswill lead to a change in the surface energy (and probablythe surface polarity) of the respective phase. Thesechanges can be calculated from contact angle andsurface tension data measured on the respective phases.From these surface energy values,two important,guiding, interfacial1 parametersare gained: physico-chemicaladhesionand coating/substrateiinnterfacialtension. These are described as follows. Assuming a simple two component (geometric mean)surface energy approach, such as Fowkes or Owens-Wendt, the predicted adhesion energy (sz) between aliquid (coating)and ssubstrate(fiber)isIsgivenbyFowkes/Dupre expression: whereYi and yys’are the disperse and polcomponents, respectively, of the substrate and y andy are the disperse and polar components of the liquid.The total surface energy of either material equals thesumof theecdispersee aandd polar surfaceenergycomponents. The surfacee polarity issgivenby thepercentage of the overall surface energy that is due tothe polar surface energy component.The interfacial tension (ysL) between the substrate and the coating is given by Good’s expression: where Ys is equal to the total surface energy of thesubstrate and y the total surface tension of the coating.A more detailed treatment of theie theoreticalbackgrounds and developments of these equations hasalready been reported [7]. The two properties, adhesion energy and interfacialtension are closely related. By combining the twoprevious equations we can show that adhesion energy(sz) simply equals the sum of the energies of the twosurfaces being brought together, less the interfacialtension which remains in the bond which is formedbetween them. Researchers very often tend to focus solely upon theadhesion energy value simply noting that the interfacialenergy should be low for the adhesion energy to be high.This is not always a good idea, as we will illustrate below.Despite being inherently linked mathematically, adhesionenergy and interfacial tension are different parameters.Depending upon the application in question, interfacialtension values can provide different and sometimes morepertinentiinformationn than adhesion1 energy.difference between the two is this: Adhesion energy tellsyou how energetically favorable the initial formation ofan interface (fiber/coating) is. In forming a bond, yousacrifice substrate (fiber) surface and coating (liquid)surface and you create an interface. Interfacial tension on the other hand, has less to do withthe original surface energies of the substrate (solid) andthe coating (liquid) which form the interface (though it isinfluenced by these surface energies). It is fundamentallya property of the bond after it is formed. When twomaterials come together, they sacrifice their surfaceenergies to form an interface. Adhesion energy describesthe energetic favorability of interface formation whereasinterfacial tension describes the incompatibility which isleft over once the interface is formed (the tendency forthe bond to break if stress is applied). The usefulness of both these parameterssisillustrated by the following example. It involves themanufacture of a high-strength fishing line that is coatedto add color and to allow it to cast more efficiently. Inthis case the problem was that the coating wore off thefishing line after just 40 to 50 casts. The surface energyvalues of the Kevlarline were calculated from thecontact angle values of water and diiodomethanemeasured with a K100 tensiometer using the bundledfiber (straw) method [8]. Having determined the surfaceenergy values of the coating and line, the adhesionenergy and the interfacial tension between line andcoatingwerel dCetermined tobee59.53 mJ/m and1.52 mN/m respectively (Table 1). Organiccoating UntreatedKevlarLine PlasmatreatedKevlarLine Overall surface Energy (mJ/m‘) 26.53 34.52 39.25 Polar component(mJ/m) 3.33 1.11 4.88 Disperse Component 23.20 33.41 34.37 Surface Polarity (%) 12.56 3.23 12.43 Adhesion energy tocoating N/A 59.53 64.54 Interfacial tensionwith coating (mN/m) N/A 1.52 1.24 Interfacial tensionwith water (mN/m) N/A 33.54 21.71 Ratio by whichcoating/line interface is preferredto water/lineinterface. N/A 22.06 17.48 Table 1: (data source, Dr Christopher Rulison, Augustine Scientific, USA) In an attempt to increase the adhesion energy, the linewas plasma treated before being coated. This treatmentmade the line more compatible with the coating in termsof its surface polarity resulting in an increase in adhesionenergy to 64.54 mJ/m(Table 1). The interfacial tensionvalue decreasedto 11.24 mN/m. By increasingttheadhesion energy and reducing the interfacial tensionbetween line and coating one would expect to observean improvement in performance. In reality, performancewas worse with the coating now coming off after just10 casts! So what could explain this unexpected behavior? Theanswer lies in the interfacial tension with respect to thebond breaking stresses experienced by the line during itsusage. As the line is cast during fishing, the stresses andstrains associated with this action, cause minute crackson the coating surface which are difficult to prevent. Anywater present can now penetrate under the coating viathese cracks, to reach the line surface and replace thecoating. The extent to which the water does this, isdependent upon the ratio of the water’s affinity to theline's surface versus the coating’s affinity to the line'ssurface. This can also be expressed as a ratio of theinterfacial tension that water has on the line's surface tothe interfacial tension that the coating has on the line'ssurface. The greater this number, the less likely is thechance that the water will replace the coating at thefiber's surface. These values for interfacial tension andwater/coating interfacial tension ratios are shown intable 1 for the untreated and treated lines. Although the plasma treatment of the line has increasedthe adhesion energy between line and coating from59.53 to 64.54 mJ/m, it has also reduced the water-lineinterfacial tension from 33.54 to 21.71 mJ/m. This nowgives water a better chance of displacing the coatingwith the plasma treated line than with the untreated lineand results in poorer overall performance. The finalsolution to thisproblem was to abandon plasma treatment of the Kevlar line in favor of reformulation ofthe coating to give a more favorable resistance tocoating displacement by water. This resulted in theadhesion energy and interfacial tension values againstthe untreated line as indicated in Table 2. Reformulated organiccoating Overall surface Energy (mJ/m) 26.40 Polar component(mJ/m) 1.55 Disperse Component 24.85 Surface Polarity (%) 5.89 Adhesion energy to untreatedline 60.25 Interfacial tension with untreatedline (mN/m) 0.67 Untreated line/ Water interfacialtension (mN/m) 33.54 Ratio by which coating/line interface is preferred to water/line interface. 50.14 Table 2: (data source, Dr Christopher Rulison, Augustine Scientific, USA) Even though the adhesion energy is lower than that withtheplasmaa: ttireateddline, casting performancehasimproved (>250 casts). This can be explained by animproved resistance tovwater displacement of thecoating, indicated by an increase in the ratio of waterinterfacial tension to coatinginterfacial tension to50.14 mJ/m² compared with 17.48 mJ/m² for the plasmatreated line/old coating combination and 22.06 for theuntreated line/old coating. So, as we stated at thebeginning, simply focusing on adhesion energy does notalways solve problems. They sometimes also requirecareful consideration of the interfacial tensions involvedin all processes related to the application. Summary The presence of surfactant in a fiber coating solution atconcentrations below the CMC leads to a thickening ofthe film-coating compared to that of the pure solution.This increase can be explained by the Marangoni stressescaused by a difference in the concentration of surfactantbetween the meniscus and the continuous film regionalong the fiber. At concentrations close to and above theCMC a decrease in film thickness can be observed asmicelles close to the interface reduce the Marangonistresses by supplyingsurfactantitmonomer to ttheinterface resulting in a more uniform distribution ofsurfactants. In coatingapplications, improveddfiber to coatingadhesion can be achieved by modifying either thesurface characteristics of the fiber surface or by adjustingthe formulation of the coating liquid. Both of theimportant interfacial parameters - interfacial tension andphysico-chemical adhesion energy should be taken intoconsideration when evaluating the effect of thesechanges. Exclusive focus on an increase in the adhesionenergy between fiber and coating through surfacetreatment of the fiber can lead to undesirable competing processes such as water penetration to the fiber surface.In this case, careful analysis of interfacial tension can leadto more appropriate measures such as reformulation ofthe coating solution as a means of optimizing adhesionwhilst at the same time minimizing the affinity of waterto the fiber. Literature [1] L. Landau and B. Levich,"Dragging of a liquid by amoving plate" Acta Physicochim. URSS 17, 42 (1942). [2] B. Derjaguin, "On the thickness of the liquid filmadhering to the walls of a vessel after emptying" ActaPhysicochim. URSS 20, 349 (1943). ( [3] D. Quere, A. de Ryck, and O. Ou Ramdane, "Liquidcoating from a surfactant solution" Europhys. Lett. 37 305 (1997). ) ( [4]A. Q. Shen, B . Gleason, G. H . McKinley and H. A. Stone,"Fiber Coating With S urfactant S olutions ", Physics of Fluids, 14(11) 4055—4068(2002). ) [5] K. J. Stebe, S. Lin, and C. Maldarelli, "Remobilizingsurfactant retarded fluid particle interfaces. I. Stress-freeconditions at the interfaces of micellar solutions ofsurfactants with fast sorption kinetics" Phys. Fluids A 3, 3(1991). [6]K. J. Stebe and C. Maldarelli, "Remobilizing surfactantretarded fluid particle interfaces. II Controlling thesurface mobility at interfaces of solutions containingsurface active components" J. Colloid Interface Sci. 163,177 (1994). [7]"So You Want to Measure Surface Energy? By DrChristopher Rulison." Kruss technical note #306 availableat www.kruss.de. [8] "Contact Angle Determination by the "Straw" Methodand Packed Cell Method", by Dr Christopher Rulison.Kruss technical note #206 available at www.kruss.de. KRUSS GmbH| Borsteler Chaussee Hamburg|Germany|www.kruss.de|
确定

还剩2页未读,是否继续阅读?
克吕士科学仪器(上海)有限公司为您提供《纤维中粘附,临界胶束浓度(CMC)检测方案(接触角测量仪)》,该方案主要用于纺织原料中粘附,临界胶束浓度(CMC)检测,参考标准--,《纤维中粘附,临界胶束浓度(CMC)检测方案(接触角测量仪)》用到的仪器有KRUSS DSA30研究型接触角测量仪、KRUSS K20型表面张力仪
推荐专场
相关方案
更多

















