方案详情
文
润湿性和粘附性与拒水据污一样取决于表面能。疏水纺织品表面分子层的表面能可以通过光学接触角的方法测得。而清洁过程非常复杂,固体表面的润湿再润湿过程受到液体相污物的溶解性质影响。以经验方法评价合适表面活性剂非常不精确。本文基于污物和清洁溶液表面张力提供了一个切实可行的评价方法用来预测清洁效果。
方案详情

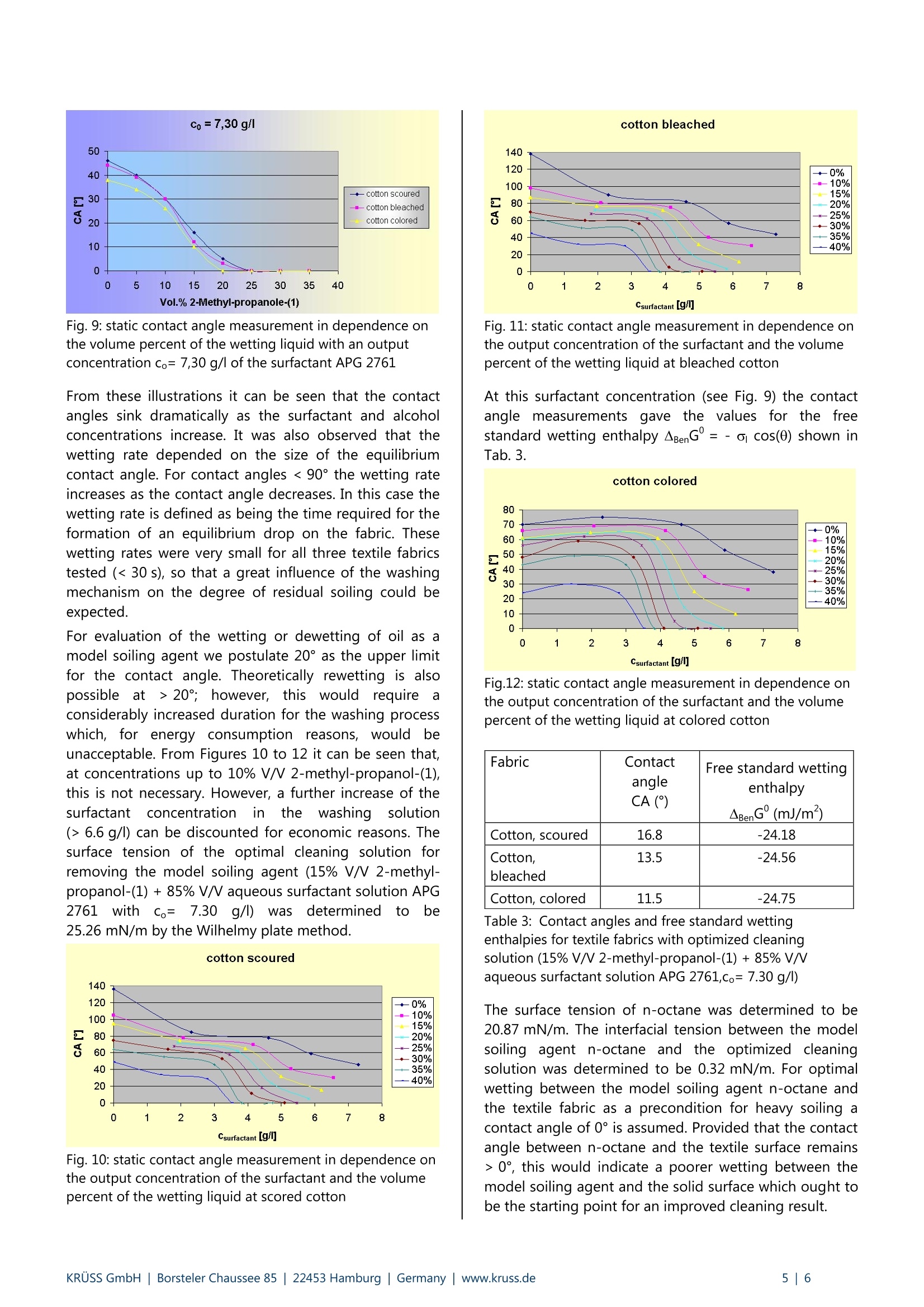
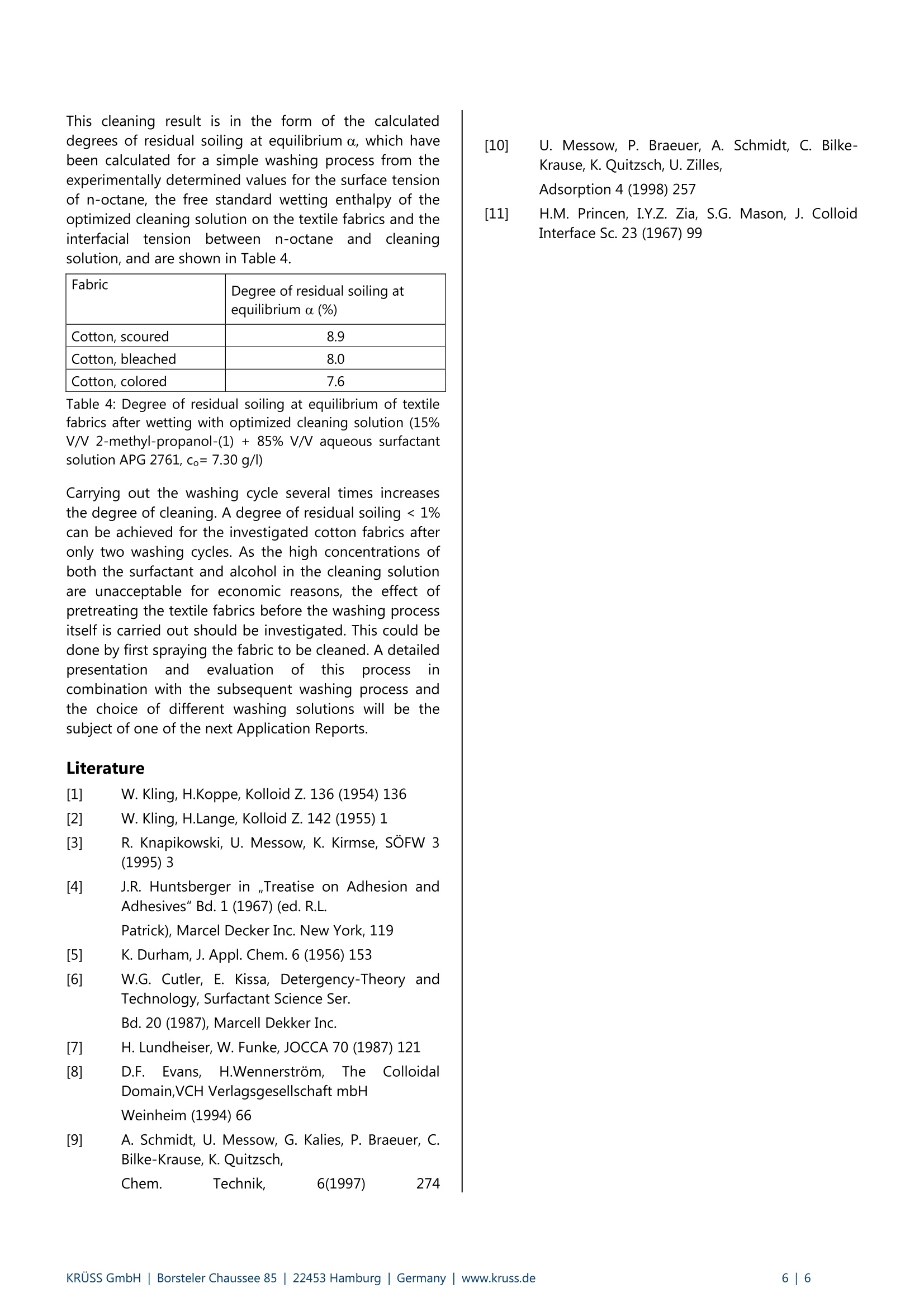
KRUSS Application Report Cleaning of textiles Application report: AR231e Industry section: Textiles Author: C. Bilke-Krause Date: 01/2003 Method: Force Tensiometer-K100 Drop Shape Analysis SystemDSA10 Spinning-Drop-Tensiometer SITE 04 Keywords: Textile cleaning, washing, rewetting, surfactant, cotton, interfacial tension Assessing the cleaning ability of aqueous surfactant solutions on soiledhydrophobic textile fabrics by using contact angle and surface tensionmeasurements Low costs - great eftects! Abstract Wettability and adhesion as well as water- or dirt-repellent properties are determined by surface energy quantities. Theseplay a key role in problems concerning hydrophilicity/hydrophobicity and optimization of the cleaning process. Thesurface energy of the uppermost monolayers of hydrophobic textile structures can be determined by optical contactangle methods. The suitability of surfactants as cleaning components is usually evaluated by empirical methods, as anexplicit evaluation of the cleaning process is difficult from a theoretical viewpoint. The reason is that cleaning processesare very complex and the wetting and rewetting processes on solid surfaces are overlayered by the solubility properties ofthe soiling agent in the liquid volume phase. This article presents a practical way of making an estimation based onsurface tension measurements between dirt components and the cleaning solution as well as on contact anglemeasurements made on hydrophobic textiles; this makes it possible to predict the cleaning effect. The basis for the mechanism of many technical washingand cleaning processes, such as soil removal, wetting,dispersing and soil carrying properties, is given by theadsorption of ssurfactants. As aresultofttheirasymmetricalpolar/non-polar molecular construction,surfactantsisbbecome enriched aatboundaries. Inanaqueous phase such processes, in which the hydrophobicand not the hydrophilic surfactant component are moreor less forced out of the water, are energetically favored.The resultant adsorption depends to a large extent onthe class of surfactant and the concentration of thehydrophilic and hydrophobic molecule components. Withaqueous solutions the adsorption of surfactants whichoccurs at the solid/liquid interface of largely nonpolarsolids takes place in such a way that the hydrophilicgroups of the surfactant molecules contained in theadsorption layer are directed toward the aqueous phase.The adhesion of the surfactant molecules to the solidsubstrate can usually be explained byvan der Waalsinteractive forcesacross the hydrophobic moleculecomponent. In this way an originally hydrophobic surfacebecomes more or less strongly hydrophilized. Fig.1: Drops of water on hydrophobic fabric The central problem in the evaluation of the cleaningeffectton solidsurfaces byssolutionscontainingsurfactants is the formulation of the Gibbs'enthalpy forrewetting, as under isothermal-isobaric conditions thisquantity provides tlthebasic statements aabout thethermodynamic equilibrium. With the aid of the freewetting enthalpies and the interfacial tension betweenthe oil phase (soiling agent) and aqueous phase (cleaningsolution containing surfactants) it is possible to predictthe cleaning effect; this is usually understood to be theratio of the soil-free, i.e. cleaned surface, to the totalsurface of the solid. The introduced degree of cleannessor rewetting therefore links the possible rewetting, givenby the free standard rewetting enthalpy, with the soiledand cleaned surface fractions which are in equilibrium. In order to depict the wetting and rewetting of a solidfrom the point of view of the interfacialil energeticconsiderations, the cleaning effect of an aaqueoussolution of a nonionic surfactant (alkylpolyglycoside) ontextile fabrics soiled with n-octane has been investigated. In the description of cleaning processes Koppe, Kling andLangehave introducedthetermrrewetting; [1], [2]. Rewetting processes can be discussed from both athermodynamic and a kinetic viewpoint. The kineticinfluences include the rewetting kinetics itself, the flowbehavior in the washing liquid, the rate of soil bindingonto the adsorbents contained in the cleaning agents aswell asthe transport of these adsorbents to thecorresponding interface. In the following observations wehave limited ourselvess toprocesses for which therewetting mechanism is determined purelythermodynamically [3]. Theory The adhesion of a substance, e.g. a soiling agentcontaining fat or oil, to a solid surface is determined byinteractive forces. With a large number of points ofcontact, small forces are sufficient to achieve a highdegree of adhesion [4]. Such relatively "weak" forcesrepresent the purely disperse van der Waals interactiveforces, with which a nonpolar soiling agent is retained onthe solid surface[5]. The larger these forces are, the moredifficult is the cleaning process and the lower the degreeofthermodynamic cleaning.. iThe diewetting ordetachment of the soil from the surface can be describedby both thermodynamic [6] and hydrodynamic models[7]. Young’s equation is used as a basis for deriving athermodynamic relationship for the rewetting process; inorder for it to be valid conditions of thermodynamicequilibrium are assumed: ag: interfacial tension solid/liquid as: surface tension of solid o: surface tension of liquid 0 : contact or wetting angle The free standard wetting enthalpy is defined by Gibbs asfollows [8]: Under the assumption that a soil component l_ is locatedon a level, homogeneous solid and that this solid isinitially completely covered by it, the rewetting with aliquid (cleaning solution) l2 can be described according to[3]. As a result a mathematical description for the degreeof soiling a, can be obtained;this gives the ratio of thesolid surface covered with the soiling agent to the totalsolid surface: a can have values between 0 (complete rewetting) and 1(no rewetting). If the soiling agent is oil and a completespreading of the oil phase on the aqueous phase isassumed then, according to [3], the following relationshipapplies to the participating surfaces at thermodynamicequilibrium: This means that for the degree of residual soiling atequilibrium on a level solid the following mathematicalequation can be postulated: Equation 5 represents the basis for the estimation of thedegree of residual soiling at equilibrium (or the degree ofcleaning at equilibrium) from the quantities interfacialtension and free standard wetting enthalpy, which areeasily accessible experimentally (determined from surfacetensionsandI contact anglermeasurements).). Thedependency of the free standard wetting enthalpy on theconcentration is given by the Gibbs’ adsorption isothermand is discussed in detail in [9] and [10]. Methods and substances Measurements of the surface tensions of the liquids usedwere made with a KRUSS Force Tensiometer - K100(Fig.2) using the Wilhelmy plate method. Fig. 2: KRUSS Force Tensiometer-K100 The very small interfacial tension of the surfactant/n-octane system used (<1 mN/m) was measured with theSITE 04 Spinning Drop Tensiometer (Fig.3). In this casethe approximation according to Princen et al.[11] wasused asthis allows the interfacialtension to bedetermined from the diameter of a rotating drop of thelighter phase in the heavier phase under certain limitingconditions. Fig.3: KRUSS Spinning-Drop-Tensiometer SITE 04 Contact angle measurements on the hydrophobic textilesurfaces were carried out with the DSA10DO4 DropShape Analysis System using the sessile drop method(Fig.4). Fig.4: KRUSS drop shape analysis system DSA10 For the preparation of the cleaning solution a surfactantof the alkylpolyglycoside group (APG 2761, Huls) wasused as the wetting agent and 2-methyl-propanol-(1) asthe auxiliary wetting agent. n-Octane was used for the preparation of a definedmodel soiling agent on the textile fabric. Scoured,bleached and colored cotton were used as the textilefabrics. The hydrophobicity of the various cotton fabrics used was evaluated before the start of the investigation bymaking contact angle measurements with water (Tab.1).Static contact angle measurements were made with anaqueous surfactant solution at a low initial concentration(Tab.2). The suitableeaauxiliary wetting agentWasdetermined by making contact angle measurements withvarious alcohols. The textile fabrics were intensivelywetted with the model soiling agent n-octane. Staticcontact angle measurements were carried out withaqueous surfactant solutions at various initial surfactantconcentrations on the cotton fabrics coated with themodel soiling agent in order to optimize the cleaningsolution (Fig. 6-9). The contact angle values obtained with the optimizedcleaning solution and the textile fabrics (Tab. 3) togetherwith the free standard wetting enthalpies determinedfrom surface andinterfacial tension measurements(Tab. 3) were used to calculate the degree of residualsoiling after the use of the optimized cleaning solutionfor a washing cycle (Tab.4). Results The static contact angle measurements carried out onthe textile fabrics with water show that the textilesurfaces have a definite hydrophobic property. Fabric Contact angle CA (°) Cotton, scoured 136.1 Cotton, bleached 138.3 Cotton, colored 116.2 Table 1: Static contact angle measurements with water ontextile fabrics As a result of the high hydrophobicity of the textilefabrics it is necessary to be able to achieve a largerewetting effect with a relatively small difference betweenthe wetting potentials. This is why very small interfacialtensions are required between the soiling and cleaningagents. Aqueous solutions of alkylpolyglycosides with theaddition of alcohols do have such low interfacialtensions. This is why the alkylpolyglycosides, a somewhatunusual class of surfactants for use in washing processes,are used for evaluating the degree of cleaning (or degreeof residual soiling) on hydrophobic cotton fabrics. Fabric Contact angle (°) Cotton, scoured 85.3° Cotton, bleached 90.1° Cotton,colored 74.9° Table 2: Static contact angle measurements with an aqueoussurfactant solution (APG 2761, co=2.31 g/l) on textile fabrics In order to select a suitable auxiliary wetting agent forimproving the effectiveness of the APG 2761, variousalcohols (n-propanol, i-propanol, n-butanol, 2-methyl-propanol-(1) anddecanol))Vwere testedbby makingcontact angle measurements. Only tertiary butanol (2-methyl-propanol-(1)) showed the rapid adsorption onthe textile fabrics required for a suitable auxiliary wettingagent. All other alcohols tested showed static equilibriumangles on the test fabrics as the results of the contactangle measurements, or had a very slow adsorption rateon the textile structure. This is why 2-methyl-propanol-(1)wasselected aass tthe auxiliary wetting agent.Optimization of the volume fraction of tertiary butanol inthe aqueous solution was carried out by making contactangle measurements with the binary mixture asafunction of the volume concentration of the alcohol. Theresults of these measurements are shown in Fig. 5. Fig.5: static contact angle measurement with a binary blend2-methyl-propanol-(1)/water in dependence on the volumebreach The results of the contact angle measurements carriedout for the optimization of the cleaning solution as afunction of the initial surfactant concentration and theconcentration of the wetting agent are shown in Figures6 to 12. Fig.6: static contact angle measurement in dependence onthe volume percent of the wetting liquid with an outputconcentration co= 2,31 g/l of the surfactant APG 2761 Fig.7: static contact angle measurement in dependence onthe volume percent of the wetting liquid with an outputconcentration co=4,62 g/l of the surfactant APG 2761 Fig. 8: static contact angle measurement in dependence onthe volume percent of the wetting liquid with an outputconcentration co= 5,90 g/l of the surfactant APG 2761 Fig.9: static contact angle measurement in dependence onthe volume percent of the wetting liquid with an outputconcentration co= 7,30 g/l of the surfactant APG 2761 From these illustrations it can be seen that the contactangles sink dramatically as the surfactant and alcoholconcentrations increase. It was also observed that thewetting rate depended on the size of the equilibriumcontact angle. For contact angles < 90°the wetting rateincreases as the contact angle decreases. In this case thewetting rate is defined as being the time required for theformation of an equilibrium drop on the fabric. Thesewetting rates were very small for all three textile fabricstested (<30 s), so that a great influence of the washingmechanism on the degree of residual soiling could beexpected. For evaluation of the wetting or dewetting of oil as amodel soiling agent we postulate 20°as the upper limitfor the contact angle. Theoretically rewetting is alsopossible at > 20°; however, thiswould requireaconsiderably increased duration for the washing processwhich, for energy consumption reasons, wouldbeunacceptable. From Figures 10 to 12 it can be seen that,at concentrations up to 10% V/V 2-methyl-propanol-(1),this is not necessary. However, a further increase of thesurfactantconcentration inthelewashinga solution(> 6.6 g/l) can be discounted for economic reasons. Thesurface tension of the optimal cleaning solution forremoving the model soiling agent (15% V/V 2-methyl-propanol-(1)+85% V/V aqueous surfactant solution APG2761 with co=7.30 g/)) was determined tobe25.26 mN/m by the Wilhelmy plate method. cotton scoured Fig. 10: static contact angle measurement in dependence onthe output concentration of the surfactant and the volumepercent of the wetting liquid at scored cotton Fig. 11: static contact angle measurement in dependence onthe output concentration of the surfactant and the volumepercent of the wetting liquid at bleached cotton At this surfactant concentration (see Fig. 9) the contactangle measurements gave thee values for the freestandard wetting enthalpy ABenG'=-acos(0) shown inTab.3. Csurfactant [g/] Fig.12: static contact angle measurement in dependence onthe output concentration of the surfactant and the volumepercent of the wetting liquid at colored cotton Fabric ContactangleCA(°) Free standard wetting enthalpyABenG'(mJ/m) Cotton, scoured 16.8 -24.18 Cotton, bleached 13.5 -24.56 Cotton, colored 11.5 -24.75 Table 3: Contact angles and free standard wettingenthalpies for textile fabrics with optimized cleaningsolution (15%V/ 2-methyl-propanol-(1)+85% V/Vaqueous surfactant solution APG 2761,co=7.30 g/l) The surface tension of n-octane was determined to be20.87 mN/m. The interfacial tension between the modelsoiling agent n-octane and the optimized cleaningsolution was determined to be 0.32 mN/m. For optimalwetting between the model soiling agent n-octane andthe textile fabric as a precondition for heavy soiling acontact angle of 0° is assumed. Provided that the contactangle between n-octane and the textile surface remains>0°, this would indicate a poorer wetting between themodel soiling agent and the solid surface which ought tobe the starting point for an improved cleaning result. This cleaning result is in the form of the calculateddegrees of residual soiling at equilibrium a, which havebeen calculated for a simple washing process from theexperimentally determined values for the surface tensionof n-octane, the free standard wetting enthalpy of theoptimized cleaning solution on the textile fabrics and theinterfacialtension betweenn-octaneanddcleaningsolution, and are shown in Table 4. ( [10] U. Messow, P. B r aeuer, A . Schmidt, C. Bi l ke- Krause, K . Quitzsch,U. Zilles, Adsorption 4 ( 1998) 257 ) ( [111 H.M. P rincen, I.Y.Z. Zia, S.G. Mason, J. C olloid Interface Sc. 23 (1967) 99 ) Fabric Degree of residual soiling atequilibrium α (%) Cotton,scoured 8.9 Cotton, bleached 8.0 Cotton, colored 7.6 Table 4: Degree of residual soiling at equilibrium of textilefabrics after wetting with optimized cleaning solution (15%V/V 2-methyl-propanol-(1)+85% V/ aqueous surfactantsolution APG 2761, co=7.30 g/l) Carrying out the washing cycle several times increasesthe degree of cleaning. A degree of residual soiling < 1%can be achieved for the investigated cotton fabrics afteronly two washing cycles. As the high concentrations ofboth the surfactant and alcohol in the cleaning solutionare unacceptable for economic reasons, the effect ofpretreating the textile fabrics before the washing processitself is carried out should be investigated. This could bedone by first spraying the fabric to be cleaned. A detailedpresentation andevaluationi ofthis process incombination with the subsequent washing process andthe choice of different washing solutions will be thesubject of one of the next Application Reports. Literature W. Kling, H.Koppe, Kolloid Z. 136 (1954) 136 W. Kling, H.Lange, Kolloid Z. 142 (1955) 1 R. Knapikowski, U. Messow, K. Kirmse, SOFW 3(1995) 3 [41 J.R. Huntsberger in ,,Treatise on Adhesion andAdhesives" Bd. 1 (1967) (ed. R.L. ( Patrick), Marcel Decker Inc. N ew York, 119 ) K. Durham, J. Appl. Chem. 6 (1956) 153 ( W.G. C utler, E. Kissa, Detergency-Theory and Technology, Surfactant Science Ser. Bd. 20 (1987), Marcell Dekker Inc. ) ( H. Lundheiser, W. Funke, JOCCA 70 (1987) 1 2 1 ) ( D.F. .Evans, H.Wennerstrom, The C olloidal Domain,VCH V e rlagsgesellschaft mbH Weinheim (1994) 66 ) [91 A. Schmidt, U.Messow, G. Kalies, P. Braeuer, C.Bilke-Krause, K. Quitzsch,Chem. Technik, 6(1997) 274 KRUSS GmbH|Borsteler Chaussee Hamburg| Germany|lwww.kruss.de|
确定



还剩4页未读,是否继续阅读?
克吕士科学仪器(上海)有限公司为您提供《纺织品中清洁能力检测方案(表面张力仪)》,该方案主要用于纺织品/服装/帽中清洁能力检测,参考标准--,《纺织品中清洁能力检测方案(表面张力仪)》用到的仪器有KRUSS DSA100接触角测量仪
推荐专场
相关方案
更多