方案详情
文
Polarization null angle (PNA) method is an accurate alternative to the commonly used polarization intensity ratio method in
determination of molecular orientation at interfaces with sum frequency generation vibrational spectroscopy (SFG-VS). Here,
the accuracy and sensitivity of PNA method is tested on different experimental configurations. It is found that its accuracy and sensitivity
are more sensitive to the incident angle of the visible beam than the IR beam, and the range of the optimal experimental
configurations is identified. This development makes better understanding of the polarization measurement in SFG-VS, and should
find more applications for interface studies.
方案详情

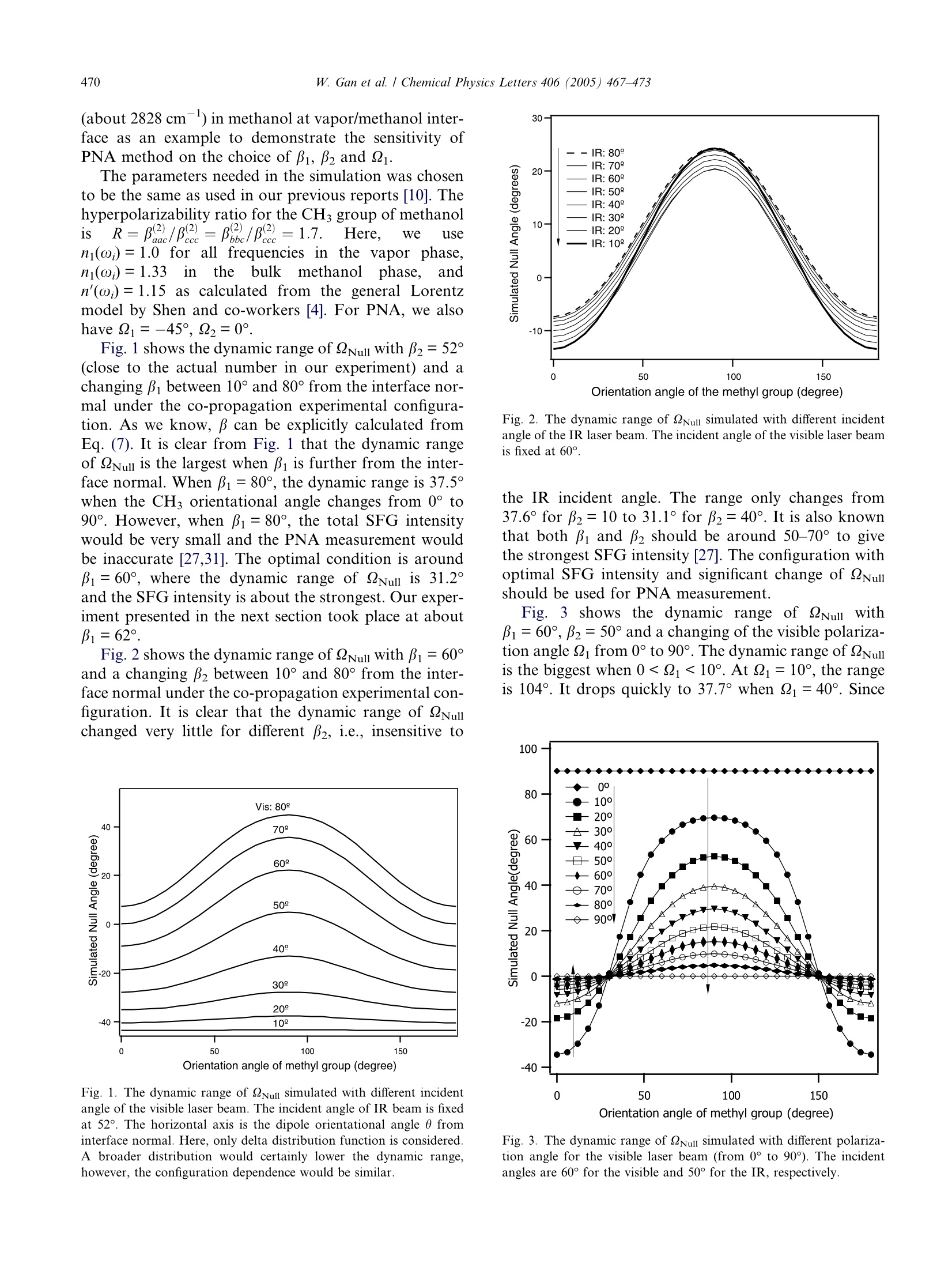
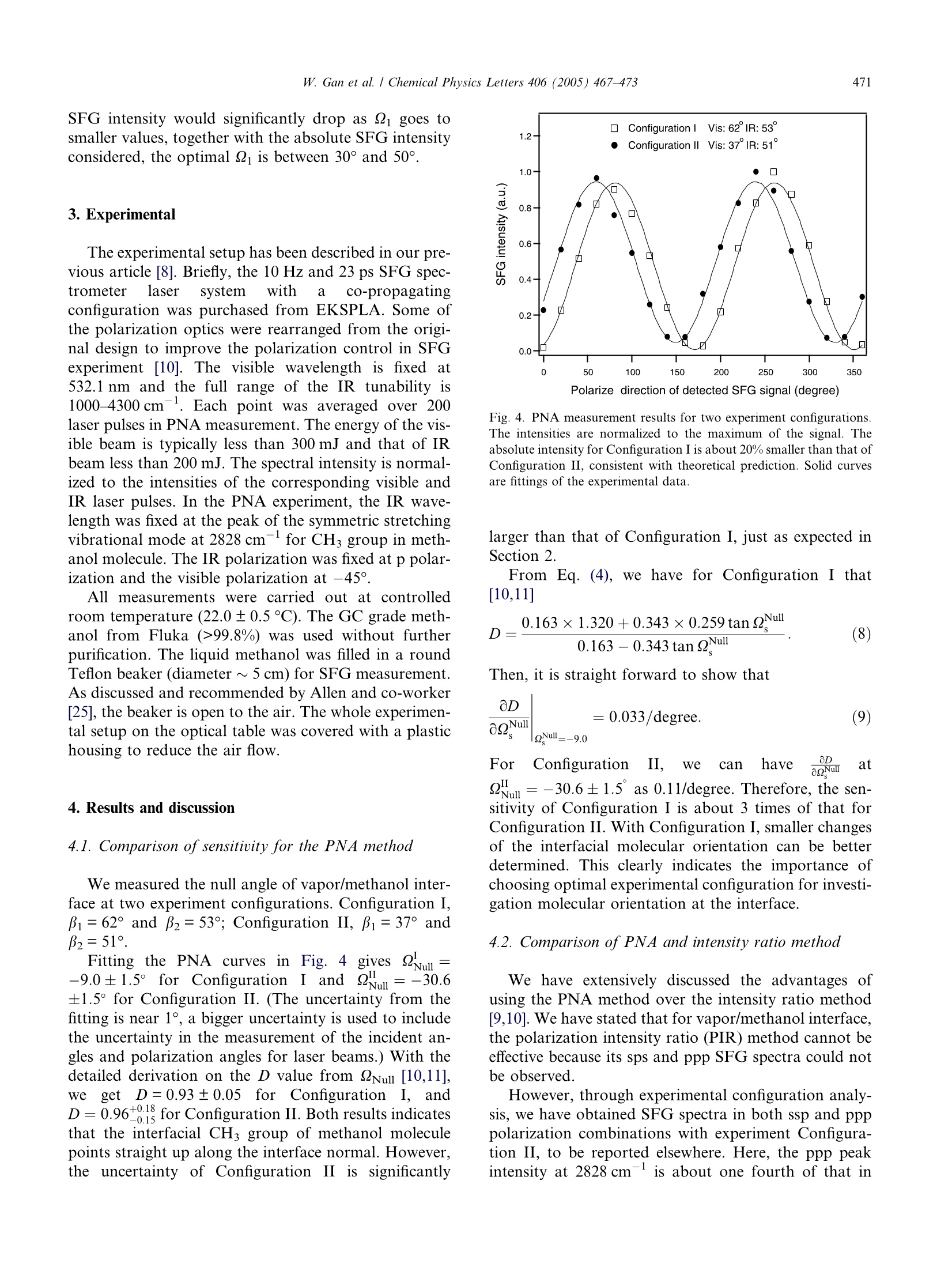
Available online at www.sciencedirect.comCHEMICALPHYSICSLETTERSChemical Physics Letters 406 (2005) 467-473www.elsevier.com/locate/cplett W. Gan et al. / Chemical Physics Letters 406 (2005)467-473468 Accuracy and sensitivity of determining molecular orientationat interfaces using sum frequency generation vibrational spectroscopy Wei Gan , Bao-hua Wul, Hua Chen, Yuan Guo, Hong-fei Wang* State Key Laboratory of Molecular Reaction Dynamics, Institute ofChemistry, Chinese Academy of Sciences, Beijing 100080, PR China Received 29 January 2005; in final form 10 March 2005Available online 29 March 2005 Abstract Polarization null angle (PNA) method is an accurate alternative to the commonly used polarization intensity ratio method indetermination of molecular orientation at interfaces with sum frequency generation vibrational spectroscopy (SFG-VS). Here,the accuracy and sensitivity of PNA method is tested on different experimental configurations. It is found that its accuracy and sen-sitivity are more sensitive to the incident angle of the visible beam than the IR beam, and the range of the optimal experimentalconfigurations is identified. This development makes better understanding of the polarization measurement in SFG-VS, and shouldfind more applications for interface studies. @2005 Elsevier B.V. All rights reserved. 1. Introduction Second harmonic generation (SHG) and sum fre-quency generation (SFG) have been used to probe thesurface/interface molecule with its unique surface selec-tivity and surface sensitivity [1-3]. In SHG and SFG,measurement of the SHG/SFG intensities in differentpolarization combination has been used to determinethe molecular orientation at the surface/interface [4-7].It has been elegantly shown that determination of inter-face molecular orientation and conformation dependson the ability to accurately obtain and analyze the rela-tive SFG intensities in different polarization combina-tions [4]. This is referred as the polarization intensityratio (PIR) method. Recently, we proposed that SFGintensities in different polarization combination can beused for determination of the symmetry property ofSFG vibrational peaks. This development indicates thatsum Ifrequencyagenerationn vibrationalspectroscopy Corresponding author. Fax: +86 10 62563167. ( E-mail address: h o ngfei@ m rdlab.icas.ac.cn (H.-f. W a ng). ) ( A l so t h e Graduate School of the Chinese Academy of Sciences. ) ( 0009-2614/$-se e front matter @ 2 005 Elsevier B.V. All rights reserved. doi:10.1016/j.cplett.2005.03.043 ) (SFG-VS) is not only an interface probe for measuringinterfacial vibrational spectra, but also a spectroscopictechnique for analysis and interpretation of the vibra-tional spectra [8]. These are all possible through quanti-tative polarization analysis and orientation analysis ofthe SFG-VS spectra. However, for some rather important molecular inter-faces, such as vapor/methanol, vapor/acetone, vapor/DMSO, vapor/acetonitrile interfaces, only one polariza-tion gives SFG signal which can be clearly detected. Thishandicaps the polarization and orientation analysis cru-cial for understanding of the molecular orientations andconformations of these interface because the PIR methodcannot be effectively applied. In order to solve this prob-lem, we recently proposed to use polarization null angle(PNA) method as ynan alternative method to overcomesuch shortcomings of PIR method [9,10]. It has beenshown that the PNA method can be very accurate andthus can provide crucial information on molecular ori-entation, structure and energetics of these interfaces[10-12]. As we have known, null methods have beenused for very accurate physical measurements, such asnull ellipsometry, magnetic null, accurate determination of Raman depolarization ratio, etc. [13-17]. PNA wasfirst used in SHG measurement early in 1983 [18]. How-ever, the application of PNA in SHG and SFG has beenonly occasionally reported since [9,10,19-21]. The air/methanol interface was the first air/liquidinterface investigated withSFC SFG [22], and it has beenintensively studied as a benchmark interface system inthe past decade [23-26]. With the PNA method, theaccuracy of the orientational parameter D=(cos0)/(cos’0) of the methyl groups at the air/methanol inter-face was greatly improved [10]. The accuracy of thePNA method enabled us to further investigate the smallorientational changes at the methanol aqueous solutioninterfaces with different bulk concentration [12]. Thisdevelopment have led to direct determination of thestructure and energetics of the Gibbs adsorption layersof the vapor/methanol-water mixture interface [12]. The accuracy and advantage of PNA method wasfurther revealed in the determination of surface molecu-lar orientation at the vapor/acetone interface [11]. In thiscase, the measured order parameter for the interfacialCH3 group is D<1, from which a new formulation ofD was developed to determine the orientation of surfacemolecules with more than one identical group. Such for-mulation shall enable analysis of complex interfaceswith multiple orientational configurations. It has been known in SFG-VS studies that the SFGspectral intensity would exhibit different feature underdifferent experimental configuration. However, unlikeon SHG [27-30], systematic experimental configurationanalysis has not been performed on SFG-VS, except adiscussion of four incident angle sets in the co-propa-gating geometry in reflective SFG-VS experiment ofSAM on gold surface by Tanaka et al. [31]. In thiswork, we shall discuss how the experimental configura-tion influence the sensitivity of PNA measurement inSFG-VS. We shall show that the accuracy and sensitiv-ity of PNA method are more sensitive to the incidentangle of the visible (Vis) beam than the IR beam,and the range of the optimal experimental configura-tions can be identified. This is especially useful for sen-sitive detection of the small orientation changes of thesurface/interface molecules. aswe shall report else-where [11,12]. 2. Theory of PNA method in SFG and its sensitivity onexperimental configuration 2.1. PNA method in SFG-VS The intensity of the SFG or SHG signal reflectedfrom the interface can be expressed as [4] Here, ω, ω and 2 are the frequencies of the SF signal,visible and IR laser beam, respectively. n(ω;) is therefractive index of bulk medium j at frequencyω;; Bi isthe incident or reflection angle from interface normalof the beam with frequency ω;; I(ω;) is the intensity ofthe SFG signal or the input laser beams, respectively;e(ω;) is the unit electric field vector for frequency ω;atthe interface; L(ω;) is the tensorial fresnel factor at ω;Xik is one of Xijkthe seven nonzero macroscopic susceptibil-ity tensors of a rotationally isotropic interface (Ccovsymmetry),i.e., for SFG-VS thelye ya are (2)=y2)re aVZx2=x2,x2=×2,x2[4,8]. So from Eq. (2) we have The parameters Q, and 2 are polarization angles ofthe SFG signal, visible and IR laser beam, respectively.It is so defined that the xy plane in the laboratory coor-dinates system A(x,y,z) is the plane of interface with z asthe surface normal; all the light beams propagate in thexz plane; p denotes the polarization of the optical fieldin the xz plane, while s the polarization perpendicularto the xz plane [4,8]. Lxx,Lyy and Lz are fresnel factorsgiven by Shen and co-workers [4]. It is important to know that Xerr contains all the ori-entational, polarization as well as strength informationof the SFG-VS measurement. Usually, four indepen-dent polarization combinations of Xers are measuredin SFG-VS [4], namely, ssp,ppp, sps and pss polariza-tions. Here ssp, represents the following polarizationcombinations: SF signal is s polarized, Visible beamis s polarized, and IR beam is p polarized, and soforth. To our knowledge, the complete expression ofXeffin Eq. (3) has not been written out in previousSFG-VS literatures. This expression in Eq. (3) is forthe co-propagating experimental geometry, where thevisible and IR beams are in the same quarter of thexz plane. For counter-propagating geometry, the fifthand sixth terms in Eq.(3) shall interchange their signs.The coherent nature of Xer, which the PNA method is based on, enables accurate analysis of molecular orien-tation in an ordered media [9,32]. As we have demonstrated recently [9,10], in the PNAmethod with SFG-VS, if we fix the experimental config-urations, i.e., the incident angle ps, and two of thepolarization angles, e.g., i and 2 for the visible andIR beams, we can always find a polarization QNull forthe SFG signalto satisfy =0.Usually, with21=-45°(positive sign denotes clockwise directionwhen facing the coming direction of the beam), and22=0°, i.e., p polarization, we have the following: Here, a common constant factor of √2/2 is omitted inEq. (4). It is known that xin is the ensemble average of themicroscopic (molecular)hyperpolarizabilitytensor. ijItk has been demonstrated that for both SFG andSHG [9] where r(0) is called the orientational field functional,which contains all molecular orientational informationat a given SFG experimental configuration; while thedimensionless parameter c is called the general orienta-tional parameter, which determines the orientationalresponse of r(0) to the molecular orientation angle O;and d is the susceptibility strength factor, which is a con-stant in a certain experimental polarization configura-tion with a given molecular system. The d and c canbe derived from Eq. (3) with known expressions for, which has been worked out for molecular groupswith different symmetry types, for example, CH2 withC2v symmetry, CH with C3v, as well as C-H, C=O withCoov, etc. [5,8,10,33,34]. It is easy to show that c can beexplicitly calculated from the values of i, Qi, n(;),n'(ω;) and hyperpolarizability ratio R=Brxe/Bede Therefore, the orientational parameter D=(cos0)/(cos’0) can be directly obtained from calculation of cowith the measured Q=2Nul from Eq. (4). This is calledthe PNA for determination of the orientational param-eter D. Since =Null generally exists and can be accu-rately measured, this PNA approach not only can have much better sensitivity, but also can be generally usedwhere the intensity ratio method fails [9,10]. 2.2. Experimental configuration influences on SFGpolarization analysis and sensitivity ofPNA method We have shown previously that accurate determina-tion of the Null value can improve the accuracy of theD value [9,10]. However,the dynamic range of QNull alsodetermines the accuracy of the D value. We shall shownthat this range depends on the experimental configura-tion, i.e., the choosing of Bi, and 2 values. The big-ger thisrange, the better accuracy for D valuemeasurement. For general discussion of the PNA method, we need tolook into the details of Eqs. (3) and (4). Setting 22=0°i.e., IR beam at p polarization, is absolutely necessaryfor the following two reasons. Firstly, it simplifies Eq.(3) by making the coefficients of the Xyzy and Xzyy termszero. Secondly, since we have to scan the IR wavelength,experimentally it is extremely difficult to accurately con-trol 22 other than s and p polarization. The s polarizationfor IR, i.e.,Q2=90°, would make the first and the lastfour terms in Eq. (3) disappear, which makes PNA mea-surement impossible. With Q2=0°,Eq. (3) has only the ssp term, i.e., thefirst term, and the ppp terms, i.e., the last four terms,left. We can then derive the following very importantexperimental implications for polarization analysis inSFG-VS. That is, the single resonance co-propagatingexperimental configuration is the best way for effectivepolarization analysis in SFG-VS. Under this condition,the Xxzxand Xzxx terms essentially cancels each other, be-cause of the fact that Li(@)=Li(@i) and B= B1. There-fore, there are only Xyyz, Xxxz and Xzzz terms left in Eq.(3). All these three terms have the same Lzz(@2) factor,so the ratio between them are essentially independentto the refractive indexes of the IR wavelengths. Thus,a perfect condition for polarization analysis includingPNA calculations. Under double resonance or coun-ter-propagationeexperimentalconfigurations,bothLi(@)=Lii(@i) and β=Bi will be violated, and thepolarization analysisshall become much morecomplicated. SFG is a coherent three wave mixing process andshould satisfy the following phase matching condition[35]: Because k for the visible light is usually several timesbigger than k2 for the IR, B follows the value of βi muchmore strongly than on β2. Therefore, one should expectthat the SFG polarization measurement depends moreon the choosing of Bi than B2. Here, we use the symmet-ric stretching vibrational mode of the methyl group (about 2828 cm-) in methanol at vapor/methanol inter-face as an example to demonstrate the sensitivity ofPNA method on the choice of Bi, B2 and Q1. The parameters needed in the simulation was chosento be the same as used in our previous reports [10]. Thehyperpolarizability ratio for the CH3 group of methanolis Here. we usenj(ω;)=1.0 for all frequencies in the vapor phase,nj(@;)=1.33inthebulk methanol)1phase,~,andn'(ω;)=1.15 as calculated from the general Lorentzmodel by Shen and co-workers [4]. For PNA, we alsohave Q1=-45°,Q2=0°. Fig. 1 shows the dynamic range of QNull with β2=52°(close to the actual number in our experiment) and achanging Bi between 10° and 80° from the interface nor-mal under the co-propagation experimental configura-tion. As we know, B can be explicitly calculated fromEq. (7). It is clear from Fig. 1 that the dynamic rangeof QNull is the largest when Bi is further from the inter-face normal. When pi=80°, the dynamic range is 37.5°when the CH orientational angle changes from 0°to90°. However, when i= 80°, the total SFG intensitywould be very small and the PNA measurement wouldbe inaccurate [27,31].The optimal condition is aroundBi = 60°, where the dynamic range of QNull is 31.2°and the SFG intensity is about the strongest. Our exper-iment presented in the next section took place at abouti=62°. Fig.2 shows the dynamic range of Null with Bi=60°and a changing B2 between 10° and 80° from the inter-face normal under the co-propagation experimental con-figuration. It is clear that the dynamic range of Nullchanged very little for different B2, i.e., insensitive to Fig. 1. The dynamic range of QNull simulated with different incidentangle of the visible laser beam. The incident angle of IR beam is fixedat 52°. The horizontal axis is the dipole orientational angle 0 frominterface normal. Here, only delta distribution function is considered.A broader distribution would certainly lower the dynamic range,however, the configuration dependence would be similar. Fig.2. The dynamic range of QNull simulated with different incidentangle of the IR laser beam. The incident angle of the visible laser beamis fixed at 60°. the IR incident angle. The range only changes from37.6° for β2=10 to 31.1° for β2= 40°. It is also knownthat both Bi and B2should be around 50-70°to givethe strongest SFG intensity [27]. The configuration withoptimal SFG intensity and significant change of Nullshould be used for PNA measurement. Fig. 3 shows the dynamic range of 2Null withβ1=60°,B2=50° and a changing of the visible polariza-tion angle 2 from 0° to 90°. The dynamic range of QNuliis the biggest when 099.8%) was used without furtherpurification. The liquid methanol was filled in a roundTeflon beaker (diameter ~5 cm) for SFG measurement.As discussed and recommended by Allen and co-worker[25], the beaker is open to the air. The whole experimen-tal setup on the optical table was covered with a plastichousing to reduce the air flow. 4. Results and discussion 4.1. Comparison of sensitivity for the PNA method We measured the null angle of vapor/methanol inter-face at two experiment configurations. Configuration I,1=62°and B2=53°; Configuration II, Bi=37°andB2=51°. Fitting the PNA curves iinn FFiigg.. 44 g gives 2ul=-9.0±1.5° for Configuration1I aunndd Qyull=-30.6±1.5°for Configuration II. (The uncertainty from thefitting is near 1°, a bigger uncertainty is used to includethe uncertainty in the measurement of the incident an-gles and polarization angles for laser beams.) With thedetailed derivation on the D value from QNul [10,11],We getLD=0.93±0.05 for Configuration I, andD=0.96+0.18 for Configuration II. Both results indicatesthat the interfacial CHs group of methanol moleculepoints straight up along the interface normal. However,the uncertainty of Configuration II issignificantly Polarize direction of detected SFG signal (degree) Fig. 4. PNA measurement results for two experiment configurations.The intensities are normalized to the maximum of the signal. Theabsolute intensity for Configuration I is about 20% smaller than that ofConfiguration II, consistent with theoretical prediction. Solid curvesare fittings of the experimental data. larger than that of Configuration I, just as expected inSection 2. From Eq. (4), we have for Configuration I that[10,11] Then, it is straight forward to show that 0D ForCConfigurationⅡI. we can have a Q Nu l ats2wll=-30.6±1.5° as 0.11/degree. Therefore, the sen-sitivity of Configuration I is about3 times of that forConfiguration II. With Configuration I, smaller changesof the interfacial molecular orientation can be betterdetermined.This clearly indicates the importance ofchoosing optimal experimental configuration for investi-gation molecular orientation at the interface. 4.2. Comparison of PNA and intensity ratio method We have extensively discussed the advantages ofusing the PNA method over the intensity ratio method[9,10]. We have stated that for vapor/methanol interface,the polarization intensity ratio (PIR) method cannot beeffective because its sps and ppp SFG spectra could notbe observed. However, through experimental configuration analy-sis, we have obtained SFG spectra in both ssp and ppppolarization combinations with experiment Configura-tion II, to be reported elsewhere. Here, the ppp peakintensity at 2828 cm-is about one fourth of that in ssp spectra. Therefore, the ratio of SFG intensity inthese two polarization combinations can be used toobtain D value through PIR method. We, thus, obtainedXssp/Xppp=-2.0±0.3, where the positive sign is physi-cally impossible as discussed by Shen and co-workers2ind2], aaccordingly thee orderpparameter iD=1.6_. The large e-0.6°rror comes from the uncertaintyfor fitting the weaker ppp peak. Compare to the D valueobtained from the PNA method under the two configu-rations, we immediately see that PNA method is morethan an order of magnitude more accurate than PIRmethod. Recently, Rao et al. have pointed out that the PNAmethod involves calculation of only one c values, whilethe PIR method should involve calculating at least twoand some times three c values [9]. Therefore, this differ-ence between the accuracy of PNA and PIR is intrinsicIn general practice of PIR method, the intensity ratiobetween the sps and ssp polarization has been often used[22,24]. As we discussed above, using the sps polariza-tion cannot avoid the uncertainty form the unknowndielectric constants across the IR wavelength. This isanother intrinsic disadvantage of the PIR method. PNA would fail when the contributions to the spectrapeak under measurement have multiple origins or spec-tral interference. Under such circumstances, carefu]spectra fitting of SFG spectra at different polarizationangle shall be used to obtain the 2Null for a specificvibrational mode [10]. With the same argument as Raoet al. have pointed out, PNA will still be much moreaccurate than the PIR approach, which involves mea-surement of SFG spectra in only two polarizationsTherefore, it is suggested to always use PNA or it equiv-alent treatment for obtaining molecular orientationalparameters. It should be noted that in order to get accurate and.reliable D values, the knowledge of the Raman depolar-ization ratio of a know group at certain wavelength iscrucial. Independent measurement of the Raman depo-larization ratio is important for SFG studies. 5. Conclusion Polarization analysis is essential for determination ofthe interfacial molecular orientation and orientational.order. In this report, we discussed the effective experi-mental configuration for better polarization analysis inSFG-VS studies. We pointed out that the single reso-nance co-propagationexperimentalslconfigurationshould be independent from the complex dielectric con-stants of the IR wavelength, and thus ideal for accurateand effective polarization analysis. We further discussedthe accuracy and sensitivity of the polarization null an-gle (PNA) method under different experimental configu-ration.Our analysis shows that the incident angle of the visible laser beam and the polarization angle of the vis-ible laser beam can be optimized to get better sensitivityand accuracy for the measurement of the orientationalparameter of interfacial molecular groups. We furthercompared the accuracy of the PNA method and the gen-erally used polarization intensity ratio (PIR) method.We conclude that the PNA method has an order of mag-nitude better accuracy for determination of the orienta-tional parameter D than the PIR method. Acknowledgments H.F.W. thanks support from the Chinese Academyof Sciences, the Natural Science Foundation of China(NSFC Nos. 20274055,20425309), and the ChineseMinistry of Science anddTechnology (MOST No.G1999075305). ( References ) ( [1] P.B. Miranda,Y . R. Shen, J. Phys. Chem. B 1 0 3 (17) (19 9 9) 3292. ) ( [2] Y.R. Shen, N ature 3 3 7 (1989) 519. ) ( [3] K .B. Eisenthal, C hem. R e v. 96 (1996) 1343. ) ( [4] X. Zhuang, P .B. Miranda, D. K i m, Y. R . Sh e n, Phys. Rev. B 5 9 (1999) 1 2632. ) [5] N. Watanabe, H. Yamamoto, A. Wada, K. Domen, C. Hirose,Spectrochim. Acta A 50 (8-9) (1994) 1529. [6] M.J. Shultz, C. Schnitzer, D. Simonelli, S. Baldelli, Int. Rev.Phys. Chem. 19 (1) (2000)123. [7] C.M. Johnson, E. Tyrode, S. Baldelli, M.W. Rutland, C. Leygraf,J. Phys. Chem. B 109 (2005) 328. [8] R. Lu, W. Gan, B.H. Wu, H. Chen, H.F. Wang, J. Phys. Chem.B108 (2004) 7297. ( [9] Y. Rao, Y.S. Tao, H .F. Wang, J. C h em. Phys. 11 9 (2 0 03) 5226. ) ( [10] R. Lu, W. Gan, H .F. Wang, Chin. Sci. Bull. 48 ( 20) (2003) 2 183,and the letter on 4 9(9) (2004) 8 99. ) [11] H. Chen, W. Gan, B.H. Wu, D. Wu, Y. Guo, H.F. Wang, J. Phys.Chem. B 109 (17)(2005) (in press). [12] H. Chen, W. Gan, R. Lu, Y. Guo, H.F. Wang, J. Phys. Chem. B109(17)(2005) (in press). [13] R.M. Esquerra, R.A. Goldbeck, D.B. Kim-Shapiro, D.S. Kliger,J. Phys. Chem. A 102 (1998) 8740. [14] R.A. Goldbeck, T.D. Dawes, O. Einarsdottir, W.H. Woodruff,D.S. Kliger, Biophys. J. 60 (1991) 125. [15] J.G. Jason, Y.L. Christopher, G. Xue, I.K. Mann, D. Zhang,S.Y. Wang, F.W. Harris, Z.D. Stephen-Cheng, S.C. Hong,X.W. Zhuang, Y.R. Shen, J. Am. Chem. Soc. 123 (2001) 5768. ( [16] T. McMillan, P. Taborek, J.E. R u tledge, Rev. Sc i . Instr. 75 (11)(2004) 5005. ) [17] Y. Saito, T. Ishibashi, H. Hamaguchi, J. Raman. Spectrosc. 31(2000) 725. ( [18] T.F. Heinz, H.W.K. Tom, Y.R. S h en, Phys. R e v. A 28 (1 9 83)1883. ) [19] D. Zhang, J. Gutow, K.B. Eisenthal, J. Phys. Chem. 98 (1994)13729. [20] T.G. Zhang, C.H. Zhang, G.K. Wong, J. Opt. Soc. Am. B 7 (6)(1990)902. [21]M.A. Polizzi, R.M. Plocinik, G.J. Simpson, J. Am. Chem. Soc.126 (2004) 5001, with corrections in 127 (2005) 1058. [22] R. Superfine, J.Y. Huang, Y.R. Shen, Phys. Rev. Lett. 66 (8)(1991)1066. ( [23] C.D. Stanners, Q. Du, R.P. Chin, P . Cremer, G .A. Somorjai,Y.R. Shen, C hem. P h ys. Lett. 2 32 ( 1 995) 407. ) ( [24] K. Wolfrum, H. Graener, A. Laubereau, Chem. Ph y s. Lett.21 3 (1- 2 )(1993) 41. ) ( [25] M. Gang, H.C. Allen, J. P hys. Chem. B 1 0 7 ( 2 6) (2003) 6343. ) ( [26] J.Y. Huang, M .H. W u , Phys. Rev. E 5 0 (5 ) (1994) 3737. ) ( [27] B. Dick, A. Gierulski, G. Marowsky, G.A. R eider, A ppl. P hys. B 38(1985) 1 07. ) ( [28] G.J. Simpson, K.L. Rowlen, Anal. Chem. 7 2 (2000) 3 399. ) ( [29] R. Adachi,T . Inoue, T. O gawa, Anal. Sci. 1 7 (2001) 449. ) ( [30] R.W.J. Hollering, J. Opt. Soc. Am. B 8 (2) ( 1991)374. ) ( [31] Y. Tanaka, S. Lin, M. Aono, T. S uzuki, A ppl. Phys. B 68 (1999)713. ) ( [32] H .F. Wang, C h in. J . Chem.Phys. 17 (3)(2004) 36 2 . ) ( [33] C.Y. Wang, H . G roenzin, M . J. S h ultz, J . Phys. Chem. B 1 0 8 (2004)265. ) ( [34] Q. Du, E . F reysz, Y .R. S hen, P h ys. Rev. L e t t . 7 2 (1994) 238. ) ( [35] Y .R. Shen, T h e Principles o f Nonlinear Optics, W i ley, New Yor k , 1985. )
确定





还剩5页未读,是否继续阅读?
北京欧兰科技发展有限公司为您提供《界面中分子取向,精度和灵敏度分析检测方案(其它光谱仪)》,该方案主要用于其他中分子取向,精度和灵敏度分析检测,参考标准--,《界面中分子取向,精度和灵敏度分析检测方案(其它光谱仪)》用到的仪器有Ekspla SFG 表面和频光谱分析系统、Ekspla PL2230型高能量皮秒激光器、Ekspla CARS 相干反斯托克斯拉曼显微光谱仪
推荐专场
其它光谱仪
更多
相关方案
更多
该厂商其他方案
更多