方案详情
文
Laser-Induced Incandescence (LII) is investigated in laminar premixed flat ethylene/air flames at pressures up to 5 bar in a recently constructed high-pressure burner. Time-resolved LII signals were compared with a numerical model considering the influence of pressure on the LII signal decay. Peak particle temperatures after laser heatup were measured by the detection of the LII signal at two wavelenghts. Laser-induced emission spectra enabled to check for interfering signals. Gas-phase temperatures were requiered to compare the LII signal decay with the model and were measured using multi-line NO-LIF thermometry.
方案详情

Laser-induced incandescence and multi-line NO-LIF thermometry for soot diagnostics athigh pressures M. Hofmannl*, H. Kronemayer, B.F. Kock’, H. Jander’and C. Schulz? PCI, Physikalisch-Chemisches Institut, Universitat Heidelberg, Heidelberg, Germany IVG, Institut fuir Verbrennung und Gasdynamik, Universitat Duisburg-Essen, Duisburg,Germany Institut fur Physikalische Chemie, Universitat Gottingen, Gottingen, Germany Abstract Laser-Induced Incandescence (LII) is investigated in laminar premixed flat ethylene/air flames at pressures up to5 bar in a recently constructed high-pressure burner. Time-resolved LII signals were compared with a numericalmodel considering the influence of pressure on the LII signal decay. Peak particle temperatures after laser heatupwere measured by the detection of the LII signal at two wavelenghts. Laser-induced emission spectra enabled tocheck for interfering signals. Gas-phase temperatures were requiered to compare the LII signal decay with the modeland were measured using multi-line NO-LIF thermometry. Introduction Laser-induced incandescence (LII) has proven to bea powerful tool for soot diagnostics. It has been usedsuccessfully for measuring volume fractions of soot inflames [1-4] and exhaust gases [5,6] and particle sizeshave been deduced from the temporal behavior of theLII signal assuming monodisperse particles [7,8] as wellas considering a polydisperse lognormal particle sizedistribution [9,10]. Although LII seems to be quite well understood inatmopsheric-pressuresystems, only few systematicinvestigations have been performed at elevated pressure.Nevertheless, soot volume fraction measurements atpeak pressures up to 70 bar have been carried out inhigh-pressure environments (namely, Diesel engines)[11-14] and time-resolved LII measurements were car-ried out in shock-tube experiments up to 3 bar [15,16]and in a Diesel engine [17]. Recent publications pre-sented soot volume fraction measurements with LIIunder stable high-pressure conditions in laminar diffu-sion flames up to 5 bar [18] and 25 bar [19]. In [20] ageneral investigation is given of the pressure influenceon the LII signal in laminar premixed flames up to 15bar. In order to use LII as a tool for particle-size meas-urement it is important to apply models for data evalua-tion that describe the heat transfer from the laser heatedparticles to the surrounding gas in an appropriate way.A validation with experimental data is then required.The models require input data about peak particle tem-perature after laser heatup (to avoid the critical calcula-tion of peak temperatures from the particle’s absorptionproperties) and ambient gas temperatures. In this work we compare time-resolved LII signalsmeasured in laminar premixed ethylene/air flames up to 5 bar with the model of Kock and Roth [17]. The peakparticle temperature is measured by two-wavelengthdetection of the initial LII signal. Gas-phase tempera-tures are measured by multi-line NO-LIF thermometry. Background Laser-induced incandescence The LII signal is due to thermal radiation from parti-cles that are heated by intense laser radiation. Subse-quent cooling occurs due to several heat loss channelsuntil after a few hundreds of nanoseconds the particletemperature reaches ambient gas temperature again.Numerous models have been developed [17,21-26] topredict the temporal behavior of the LII signal. Thebasis for all models is an energy balance between ab-sorption of laser energy and heat loss due to vaporiza-tion of material from the surface, heat conduction to thesurrounding gas and radiation: where qabs is the rate of laser energy absorption, qint therate of increase of internal energy,qevap the rate of en-ergy loss by evaporation, qcond the rate of heat conduc-tion and qrad accounts for the cooling due to radiation.Various submodels for the different processes have beensuggested in the past. Solving the resulting differentialequation numerically yields the particle temperature as afunction of time which is then turned into LII signalintensities using Planck’s law. A more detailed modelfor soot particles including thermal annealing and oxi-dation has been developped by Michelsen [22]. In cur-rent LII models little attention has been paid to the in-fluence of pressure. Pressure affects the LII signalthrough the pressure-dependence of heat conduction andevaporation. ( * Corresponding author: max.hofmann@pci.uni-heidelberg.deProceedings of th e European Combustion Meeting 2005 ) Heat conduction depends on the Knudsen numberKn which is defined as where 4 is the mean free path of the gas molecules anda is the particle diameter. The mean free path length isapproximately where p is the gas pressure, Tg is the gas temperature, kgis the Boltzmann constant, and 2is the collisionalcross-section of the gas molecules. The heat conductioncan be devided into three regimes: The free molecularregime (Kn ≥ 10), the continuum regime (Kn<1) andthe transition regime (Kn ~ 1). In the free molecularcase heat conduction increases linearly with pressure,whereas it is independent on pressure in the continuumregime. In order to use the right expression for heatconduction in LII models at elevated pressure, Kn has tobe known. For typical sooting flames with a gas-phasetemperature of 1700 K and an assumed particle diameterof20 nm Kn can be estimated using the collisional crosssection of N2 of 0.43 nm²[27]. For 1 bar, Kn is~20, for5 bar ~4 and for typical peak pressures in Diesel en-gines of ~ 70 bar Kn is ~0.3. Hence, heat conduction inthe transition regime has to be taken into account atpressure higher than ~5- 10 bar. The model used in this study was developed byKock and Roth [17]. It accounts for the pressure-dependence both in the free molecular regime with alinear increase in heat conduction with pressure, and inthe transition regime, using an empirical function for theheat conduction given by Williams and Loyalka [28].Instead of monodisperse particles a polydisperse log-normal particle size distribution is assumed, which canbe characterized by the count median diameter CMDand the geometric standard deviation og. Evaporationeffects are not considered here due to low laser fluences. Temperature measurements in sooting flames There are different techniques available to measuretemperatures in flames [29]. Pyrometry can provideparticle-temperature information in flames by using thevisible emission of hot particles and their comparison toblack-body radiation. Gas-phase temperatures can beobserved by spectroscopic methods utilizing populationdifferences in quantum states of molecules or atomspresent in the flame. Frequently applied methods in-clude Coherent Anti-Stokes Raman scattering (CARS)for point measurements [18,30], diode laser absorptionfor line-of-sight integrated measurements and laser-induced fluorescence (LIF) techniques. Rayleigh ther-mometry, which is based on the change of density withtemperature, can usually not be used in particle ladenflames as the elastically scattered light off soot particlesis much stronger than the scattering off gas molecules,which is necessary for measuring the density unlessfiltered-Rayleigh techniques are used[31]. OH-LIF thermometry is impossible in sooting flames because ofthe low concentration of this intermediate species [32].Two-line atomic LIF techniques [33] can yield tempera-ture information as well as two-line nitric-oxide (NO)temperature imaging. NO is easily seeded to the freshgases, but is quickly reduced in rich flames due to re-burn reactions. We measured gas-phase temperatures insooting high-pressure flames with multi-line NO-LIF.This method has been previously developed [34] anddemonstrated in steady stoichiometric and sooting at-mospheric-pressure flames [35] as well as in non-sooting high-pressure flames up to 40 bar [36]. Multi-line NO-LIF thermometry In contrast to two-color LIF thermometry for gas-temperature measurements [33,37] the multi-line tech-nique yields absolute temperatures without calibrationand the technique can be applied even in systems withstrong scattering and fluorescence background. Thetechnique is based on the measurement of LIF excitationspectra of nitric oxide. Some hundreds to thousandsppm NO are added to the fuel/air mixture as a fluores-cent tracer. The laser is tuned over a part of the NOabsorption spectrum while individual images are takenwith an intensified CCD camera for each excitationwavelength. From the resulting stack of pictures (eachwith the laser tuned to the next wavelength) LIF excita-tion spectra can be extracted for each pixel. Simulatedspectra are then fitted to the experimental data withabsolute temperature, broad-band background and totalsignal intensity as free parameters. The best scan rangeis determined from a numerical analysis based on LIF-Sim [35,38]. The technique takes advantage of the shapeof the spectra (as a function of excitation wavelength)for each pixel within the observed area. In contrast totwo-line temperature imaging, broadband background(due to incompletely suppressed elastic scattering anddue to broadband fluorescence) does therefore not affectthe temperature evaluation. Experimental Setup High-pressure burner In order to stabilize a premixed, sooting high-pressure flame special requirements must be met. Toguarantee a stable, laminar flow of the premixed fuel/airgases small, regular channels are necessary. The chan-nels must have a small inner diameter to avoid aflashback of the premixed flame at high pressures. Theburner used in this study was installed inside a high-pressure chamber equipped with four quartz windowsfor optical access. The burner matrix consisted of a packof silver capillary tubes with an inner diameter of100 um and an outer diameter of 300 um, resulting in acircular matrix with a diameter of 20 mm. For stabiliza-tion, the central sooting flame (ethylene/air) was sur-rounded by a non-sooting methane/air flame (diameter56 mm). The two flames were surrounded by a coflowof air both for further stabilization of the flame and forkeeping the windows clean of soot and water. Picturesof the flame at 1, 2 and 5 bar are shown in Fig. 1. The equivalence ratio used in this study was o=2.1, corre-sponding to a C/O ratio of 0.7, with a gas velocity of8 cm/s for all flames. Fig. 1. Premixed ethylene-air flame with an equivalenceratio of d=2.1 at 1,2 and 5 bar. For the LII experiment the beam of a pulsedNd:YAG laser at 1064 nm (Puls duration: 7 ns FWHM)was aligned through the burner. A small portion of thebeam was cut out with an aperture and imaged onto thecenter of the burner in order to obtain a homogeneousenergy distribution within the beam (diameter 1.9 mm).The resulting energy density in the observed volumewas tuned in the range of 0.09-0.62 J/cm . Fig. 2. Experimental setup of the LII measurements.PMT, Photomultipier tube. ICCD, intensified CCDcamera. Signals were collected at right angle and focused onthe slit of an imaging spectrometer (Oriel). The result-ing images were detected with an intensified CCD cam-era (LaVision, FlameStar II) over a period of 50 nsstarting with the onset of the laser pulse (prompt detec-tion). Time-resolved LII signals were detected at rightangle at two different wavelengths (550 and 694 nm)with fast photomultipliers (Hamamatsu). The signalswere recorded by a digital storage oscilloscope at a sampling rate of1 Gs and transferred to a computer forfurther data evaluation. The setup is shown in Fig. 2 For thetemperature measurements atunableKrF* excimer laser (248 nm) was frequency-shifted to225 nm in a 10-bar hydrogen-filled Raman cell. Thelaser beam was focused to approximately 2×2 mm’andilluminated the region of interest. The NO moleculeswere excited in the A-X(0,0) band and the LIF-signalwas recorded with an intensified CCD camera. Elasti-cally-scattered light was suppressed by Schott UG5filters. An additional reflection band pass filter sepa-rated the 230-250 nm range for detection. The totalsetup is shown in Fig. 3. Fig. 3. Experimental setup of the multi-line NO-LIFthermometry. Results and Discussion . A. Spectrally-resolved laser-induced emission The wavelength-resolved detection of the LII signalenables to check for interferences from other species,e.g. fluorescence from the Czd’IIg→aIl Swan bands,which have been observed to interfere with LII signal[39,40]. Fig. 4shows LII spectra taken at pressures of 1,2 and 5 bar at different laser fluences. C2 emissionpeaks at 472 and 516 nm can be seen at all pressures,though decreasing with increasing pressure. Increasingpressure results in faster quenching of the electroni-cally-excited C2 molecules. With increasing laser flu-ence the C2 signal intensity increases. At high fluences ahigher evaporation rate of carbon particles is reached,producing more excited C2. Hence, detection of LIIaround 472 and 516 nm should be avoided, even at apressures of 5 bar. For the evaluation of particle sizes from time-resolved LII data the peak particle temperature T, mustbe known. T can be measured by detecting the LIIsignal at two different wavelengths [10,17].In this studywe simultaneously detected LII at 550 and 694 nm. Atthese wavelengths no significant interference from fluo-rescing species was observed in the spectrally-resolvedemission measurements. Fig. 4. Laser-induced emission spectra at 1, 2 and 5 barfor three different laser fluences. The spectra are notcorrected for spectral sensitivity of the detection system. B. Multi-line NO-LIF thermometry Temperatures in the sooting flame were measuredusing multi-line NO-LIF thermometry. The optimumscan range [35] was a 10 cm range with excitation at44407- 44417 cm²'(225.190-225.139 nm). 56 indi-vidual LIF images are taken at different wavelengthseach averaged over 100 laser shots. This gives a total of5600 laser shots for the excitation spectra. The spectraacquisition, therefore, takes about five minutes at arepetition rate of 25 Hz of the KrF* excimer laser sys-tem and the synchronized ICCD-camera. Gas tempera-tures were measured in rich ethylene/air flames withstoichiometries of o= 2.1 at total pressures of 1, 2 and 5bar. The gas temperature in the center (region of 10 mmwide x2 mm high) of the flame at 1 bar was 1644±30 K and 1600±20 K at 5 mm and 10 mm above theburner surface respectively. Temperatures at 2 bar are1665±20 K and 1710±20 K at 5 mm and 10 mm abovethe burner surface respectively. At 5 bar we find gastemperatures of 1850 ±100 K at 5 mm above the burnersurface. NO-LIF excitation spectra are shown in Fig. 5.The precision of the temperature measurements depends on the signal-to-noise ratio of the experimental excita-tion spectra. It can be improved by increasing signalaveraging and by using optimized detection filters. NOwas seeded to the premixed ethylene/air mixture inamounts of 0.5% at 1 and 2 bar and 2% NO at 5 bar.High NO concentrations were required to generate suf-ficient LIF signal due to strong reburn of added NO inrich flames. Spectral broadening additionally reducessignal intensities. In the present setup poor UV trans-mission of the windows further reduced the laser andsignal intensities. The optical setup will be improved infuture measurements.The elastically-scattered light waswell suppressed by the filters described above. Fig. 5. NO-LIF excitation spectra at 1 and 2 bar. Theblack dots are experimental data observed at 5 mmheight above burner. The red line shows the fitted spec-tra. C. Time-resolved LII detection A typical raw LII signal at 5 bar is shown in Fig. 6.Two signals are shown for both the 550 nm and the 694nm detection. The signals are averages over 40 lasershots and were taken at 5 mm height above the burnerwith a laser fluence of 0.16 J/cm. From the ratio of the peak intensities at the twowavelengths the peak particle temperature was calcu-lated. With the gas-phase temperatures obtained fromthe multi-line NO-LIF thermometry the numericalmodel was fitted to the LII signal decay curves. The fitsare shown in Fig. 7. Fits at 1 and 2 bar are given for LIIdecay curves at 10 mm height above the burner and at 5 mm height above the burner at 5 bar. Laser fluenceswere 0.09 J/cm², 0.22 J/cm’and 0.16 J/cm’ for 1, 2 and5bar respectively. Fluences had to be sufficiently Fig. 6. Typical raw LII signal at 5 bar for two differentdetection wavelengths (550 and 694 nm). low to avoid strong evaporation of soot. Fluences higherthan ~ 0.25 J/cmresulted in a poor fit for all pressures.Fits for 1 and2 bar were made using the model withfree molecular heat conduction. At 5 bar the transitionregime was used. Fig. 7: Numerical fits to the experimental LII decay.curves at 550 nm detection for 1,2 and 5 bar. The model and experiment are in good agreementfor all pressures. The CMD obtained at 1 and 2 bar(10 mm height above the burner) are 44 and 31 nmrespectively with a geometric standard deviation of 1.49and 1.54 respectively. At 5 bar (5 mm height above theburner) a smaller CMD of 10 nm with a o, of 1.84 isobtained. Here, particles are expected to be smaller assoot particles grow in size with increasing height abovethe burner in premixed flames. Conclusion In thiss workk we presented time-resolved laser-induced incandescence measurements in laminar, pre-mixed ethylene/air flames at pressures of 1, 2 and 5 bar.Experimental decay curves were compared with a nu-merical model taking into account the increased heatconduction with higher pressure. Therefore, gas-phasetemperatures were measured for the first time in sootinghigh-pressure flames with multi-line NO-LIF ther-mometry. Peak particle temperatures of the laser heatedsoot were measured by detecting the LII signal at twodifferent wavelengths. No significant interferences fromfluorescing species were observed at these wavelengthsin laser-induced emission spectra. Detection around 472and 516 nm should be avoided as fluorescence of elec-tronically excited C2 can interfere with LII at pressuresup to 5 bar. Comparison of the experimental data with the nu-merical model showed good agreement for all pressures.Hence, it is important to consider the pressure depend-ence on heat conduction in modeling laser-inducedincandescence. Further investigations will be necessary to comparesoot particle sizes obtained by LII at elevated pressureswith other techniques, e.g. TEM measurements. Acknowledgements Theauthorsthank theDeutscheForschungs-gemeinschaft (DFG) for the financial support withinSCHU1369/3, W. Weis and K. Schmidt (Heidelberg)for technical support and H. Gg. Wagner (Gottingen)and J. Wolfrum (Heidelberg) for their continuous inter-est and support. References 1. Ni, T.,Pinson,J. A., Gupta, S., and Santoro,R.J., Appl. Opt. 34:7083-7091 (1995). 2 Bryce,D. J., Ladommatos, N., and Zhao, H.,Appl.Opt. 39:5012-5022(2000). 3. Shaddix, C. R. and Smyth, K. C.,Combust.Flame 107:418-452(1996). 4. Appel, J., Jungfleisch, B., Marquardt, M.,Suntz, R., and Bockhorn, H., Proc. Comb. Inst26:2387-2395 (1996). 5. Snelling, D. R., Smallwood, G. J.,Sawchuk,R.A., Neill, W. S., Gareau, D., Clavel, D. J.,Chippior,W. L., Liu, F., and Giilder, O. L.,SAE Technical Paper Series No. 2000-01-1994(2000). ( Schraml, S., Will, S., and Leipertz, A., SAETechnical Paper Series No. 2000-01-2002 (2000). ) ( 7. Axelsson, B., Collin,R., and B engtsson, P.-E., Appl. Opt. 39:3683- 3 690 (2000). ) ( 8. Vander Wal, R. L . , Ticich, T. M.,an d Stephens, A. B., Combust. Flame 116:291-296 (1999). ) 9. Kock, B. F., Eckhardt, T., and Roth, P., Proc..Comb. Inst.29:2775-2781 (2002). 10. Lehre, T., Jungfleisch, B., Suntz, R., andBockhorn, H.,Appl. Opt.42:2021-2030(2003). 11. Dec,J. E., SAE Technical Paper Series No.920115(1992). 12. Wiltafsky, G., Stolz, W., Kohler, J., and Espey,C.,SAE Technical Paper Series No. 961200(1996). 13. Pinson, J. A.,Mitchell, D. L., Santoro, R. J.,and Litzinger, T. A., SAE Technical Paper Se-ries No. 932650(1993). 14. Bruneaux, G., Verhoeven, D., and Baritaud,T.,SAE Technical Paper Series No. 1999-01-3648(1999). 15. Starke, R. and Roth, P., Comb. and Flame127:2278-2285(2002). 16. Woiki, D., Giesen,A., and Roth, P., Proc.Comb. Inst. 28:2531-2537 (2000). 17. Kock, B. F. and Roth, P. "Two-color TR-LIIapplied to in-cylinder Diesel particle sizing," inProc. ofthe European Combustion Meeting(Orleans, 2003). 18. Geigle, K. P., Schneider-Kuhnle, Y., Tsurikov,M., Hadef, R.,Liickerath, R., Kriger, V., Stri-cker, W., and Aigner, M., Proc. Comb. Inst.30:in press (2004). 19. McCrain, L. L. and Roberts, W.L., Comb. andFlame 140:60-69 (2005). 20. Hofmann, M., Bessler, W. G., Schulz, C., andJander, H., Appl. Opt. 42:2052-2062 (2003). 21. Melton, L. A.,Appl.Opt. 23:2201-2208(1984). 22. Michelsen, H. A., J. Chem. Phys.118:7012-7045(2003). 23. Snelling, D. R., Liu, F., Smallwood, G.J., andGiilder, O. L. "Evaluation of the nanoscale heatand mass transfer model of LII: Prediction ofthe excitation intensity," in 34th National HeatTransfer Conference (Pittsburgh, 2000). 24. Bladh, H. and Bengtsson, P.-E.,Appl. Phys. B78:241-248(2004). 25. Will,S., Schraml, S., Bader, K., and Leipertz,A., Appl.Opt. 37:5647-5658(1998)). 26. Hofeldt, D. L., SAE Technical Paper SeriesNo. 930079(1993). 27. Reid,R., Prausnitz, J., and Poling, B., TheProperties ofGases and Liquids (McGraw-Hill, New York, 1987). ( 28. Williams, M. M . R. and Loyalka, S. K ., Aero-sol Science: Theory and Practice (Pergamon Press, O xford, 1 991) . ) ( 29. Laurendeau, N. M., Prog. Energy Combust.Sci. 14:147-170(1988) J . m ) ( 30. Brackmann, C., B o od, J., Bengtsson, P . -E., Seeger, T., Schenk, M., and Leipertz, A., Appl.Opt. 41:564-572(2002). ) ( 31. Hoffman, D., M i inch, K.-U., a n d Leipertz, A . ,Opt. Lett.21:525-527(1996). ) ( 32. Hildenbrand, F., Schulz, C., S ick, V ., J ander,H., and Wagner, H. G." A pplicability of KrF excimer laser induced fluorescence in sooting high-pressure flames," in VDI Flammentag Dresden, V DI Berichte 1492 (ISBN 3-18- 091492-0)(1999),269-274. ) ( 33. Engstrom, J., Nygren,J., Alden, M., and Ka- minski, C. F., Opt. Lett. 25:1469-1471 (2000). ) ( 34. Vyrodow, A. O., H einze, J.,Dillmann, M.,Meier, U. E . , and Stricker, W., Appl. Phys. B.61:409-414(1995). ) ( 35. Bessler,W. G . and Schulz, C.,Appl. Phys. B78:519-533 (2004). ) ( 36. Bessler, W . G.,Schulz, C., Le e , T., Shin, D. I., Hofmann, M., Jeffries,J. B .,W o lfrum, J., andHanson, R. K., Appl. Phys. B 75:97-102(2002). ) ( 37. Bessler, W. G., Hildenbrand,F., and Schulz,C., Appl. Opt. 40:748-756(2001). ) ( 38. Bessler, W . G., Schulz, C., Sick, V., and Daily,J. W. " A versatile modeling tool for nitric ox-ide LIF s pectra," in 3rd Joint meeting of the USsections o f the combustion institute (Chicago,2003). ) ( 39. Bengtsson,P.-E. and Alden, M., Appl. Phys. B60:51-59(1995). ) ( 40. Vander Wal, R. L., Appl. Phys. B 59:445-452(1994). )
确定



还剩4页未读,是否继续阅读?
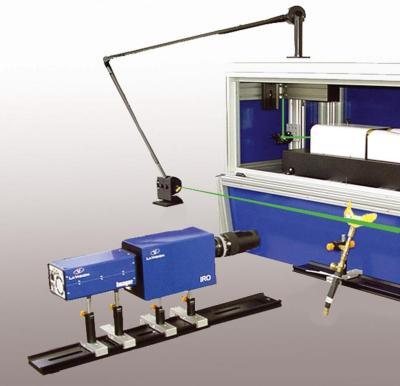
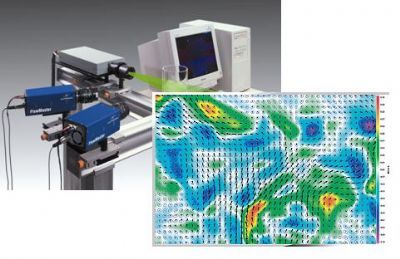
北京欧兰科技发展有限公司为您提供《碳烟中体积分数,初级粒径,温度场检测方案(烟气分析仪)》,该方案主要用于其他中体积分数,初级粒径,温度场检测,参考标准--,《碳烟中体积分数,初级粒径,温度场检测方案(烟气分析仪)》用到的仪器有激光诱导白炽光烟雾粒子成像分析仪(LII)、PLIF平面激光诱导荧光火焰燃烧检测系统、德国LaVision PIV/PLIF粒子成像测速场仪
推荐专场
烟气监测(CEMS)/烟气分析仪
更多
相关方案
更多
该厂商其他方案
更多