方案详情
文
文章介绍采用FIAS400-Optima 5300DV在线分析铬形态物,结果表明:方法简单,操作方便,灵敏度高及重现性好。
方案详情

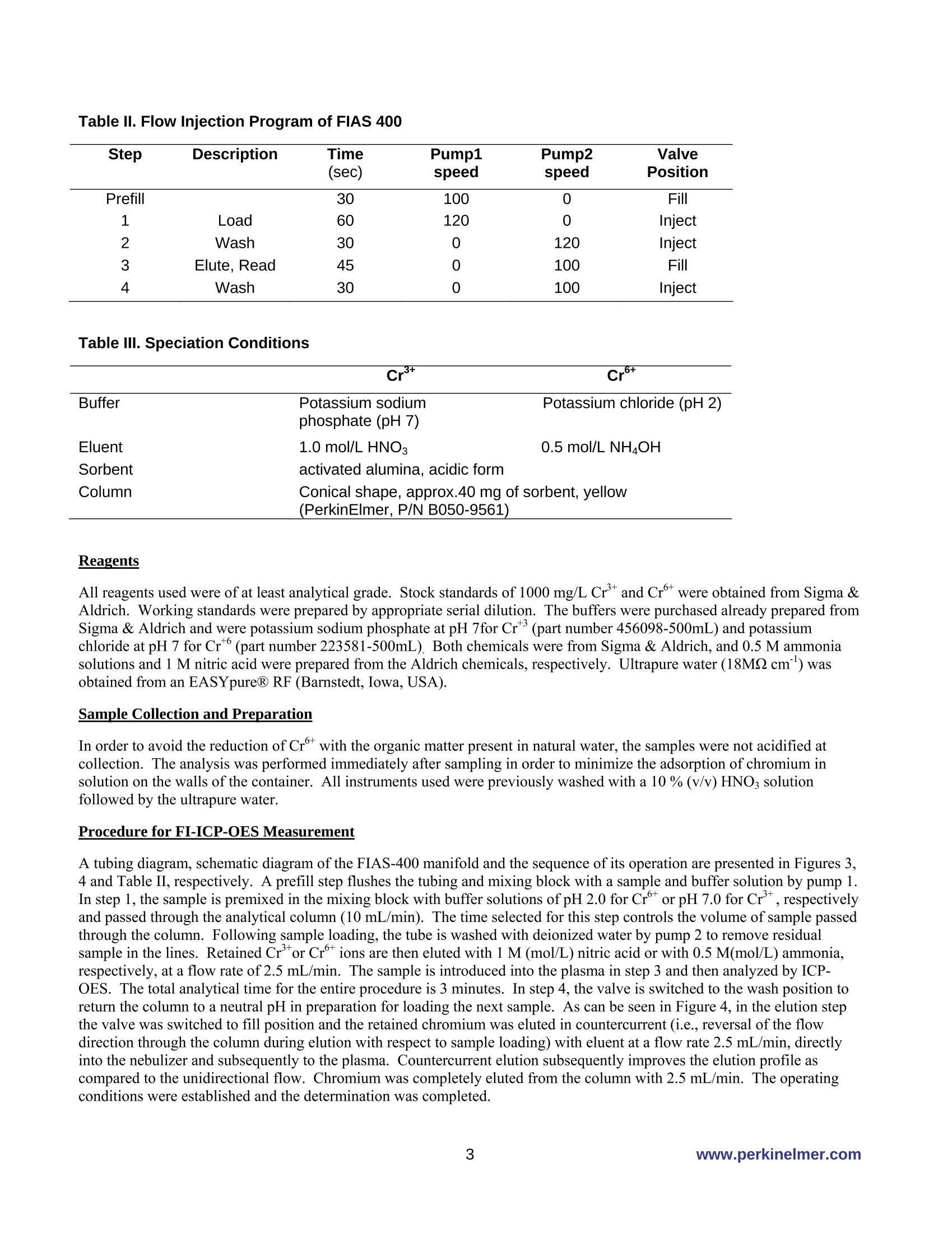
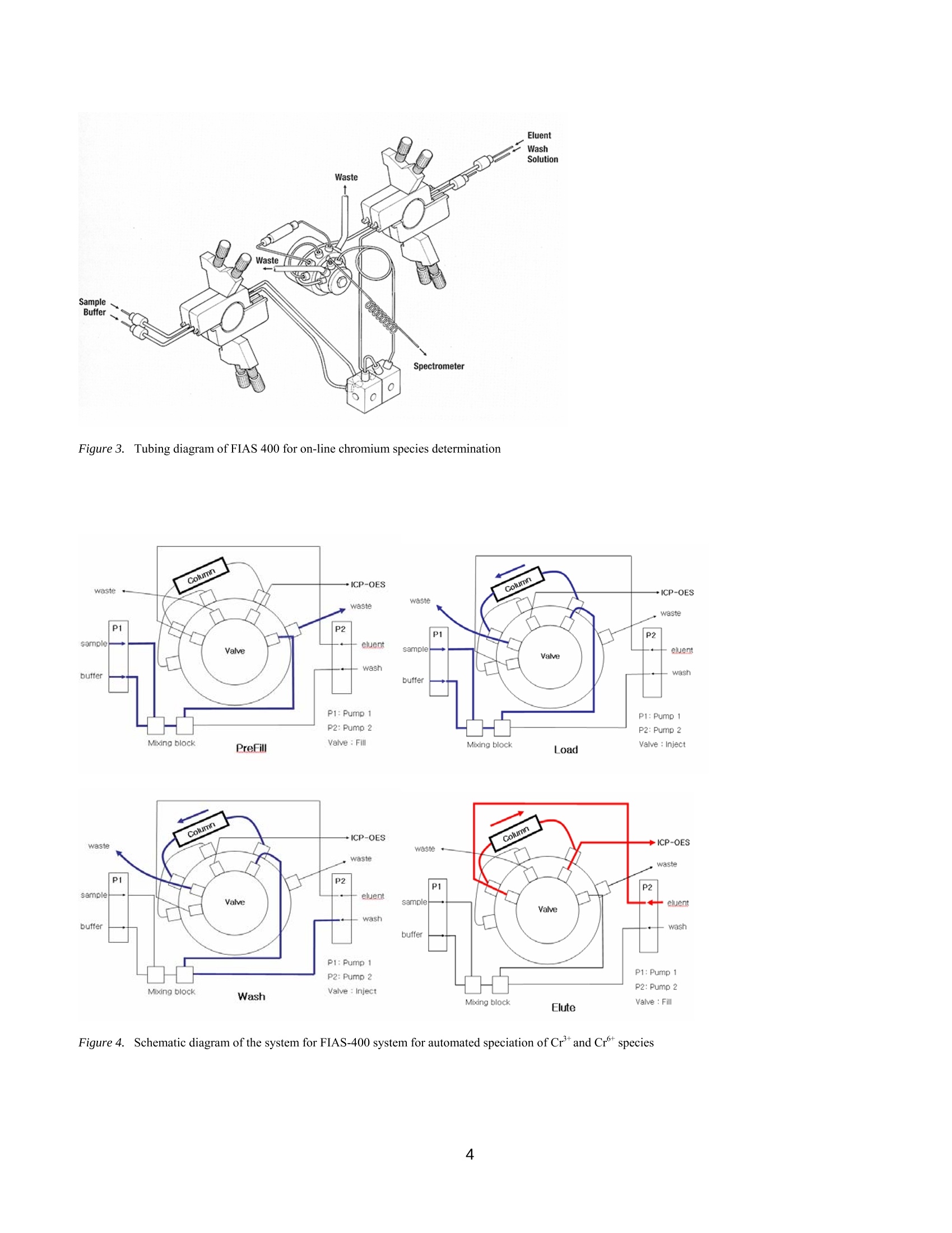
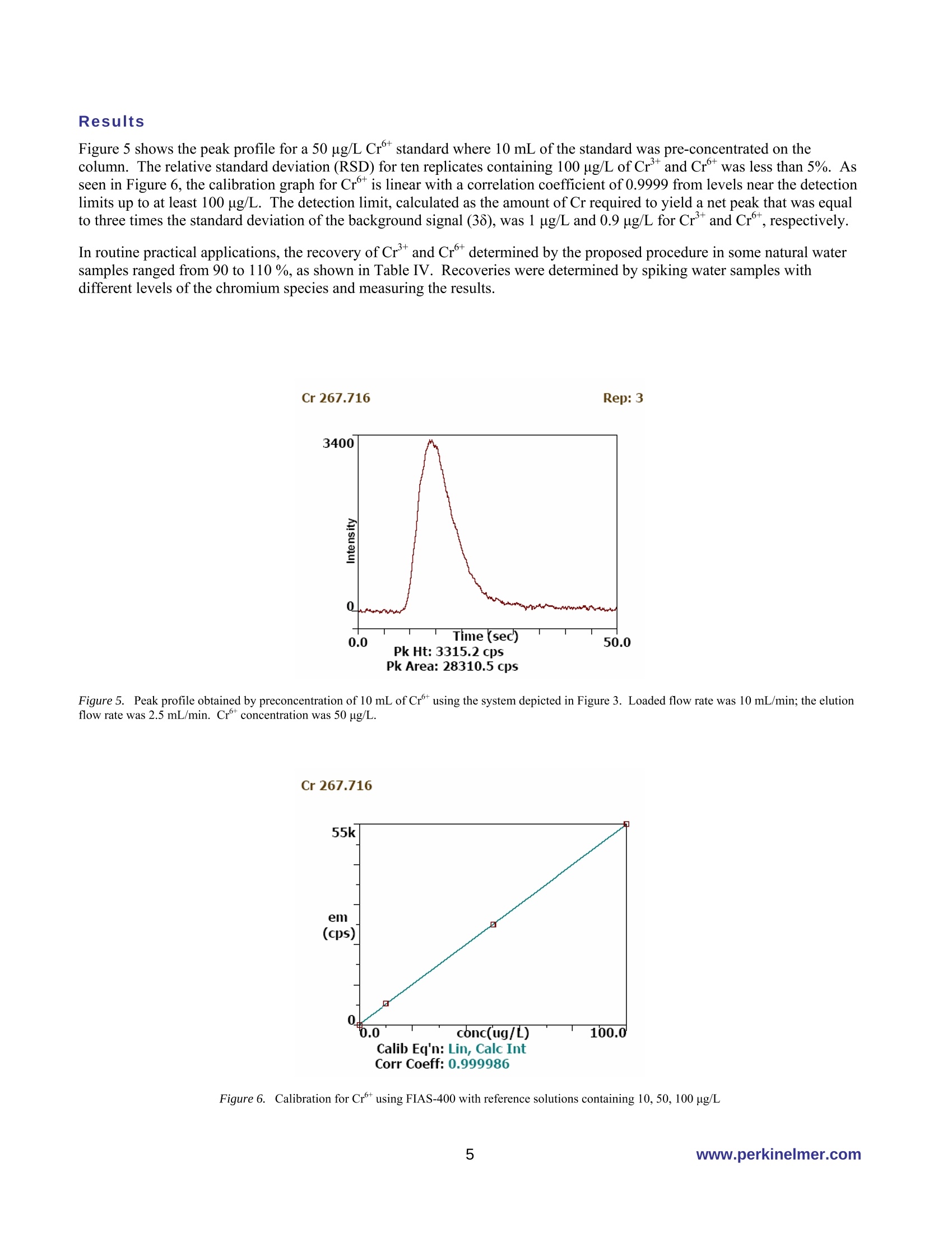
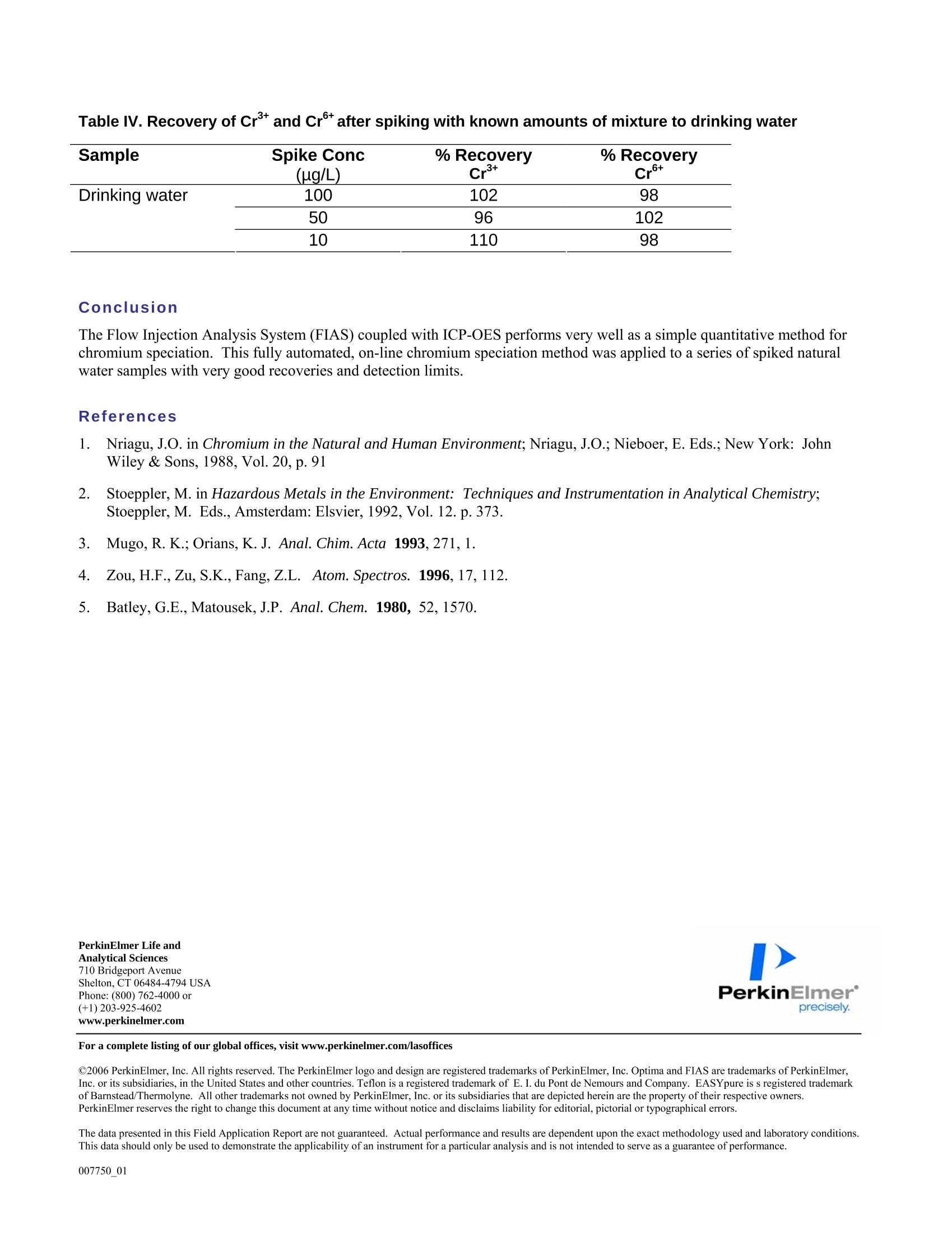
PerkinElmer FIELDAPPLICATIONFREPORTICPOPTICALEMISSION On-Line Chromium (Cr3+ / Cr6+)Speciation by FI-ICP-OES Authors: Eui Sung Hwang, Sung Hyun Nam, Nam Hoon KimPerkinElmer Korea Ltd.Co. Seoul, South Korea Introduction Over the past several years, numerous papers concerning the determination of Cr’and Cr in various samples have beenpublished. The reason for this interest is governed by the fact that chromium toxicity depends critically on its oxidation state.The two primary oxidation states of chromium (Crand Cr) in natural waters differ significantly in biological,geochemical and toxicological properties(1-2). While Cr’is considered essential in mammals for the maintenance ofglucose, lipid, and protein metabolism, Cris known to be toxic to humans because of its ability to oxidize other species andits undesirable effects on the lung, liver and kidney. Because of different toxicities and bio-availability of chromium species,the analysis of total chromium does not give full information about possible health hazards. Therefore, it is important tomonitor the concentration of the individual chromium species as well as the total content in the environment. Traditionalmethods for the speciation of inorganic chromium are relatively time-consuming, involving species separation based onsolvent extraction (3), co-precipitation (4), electrochemical separation (5), ion exchange, solid-phase extraction,or selectivevolatilization in combination with graphite furnace atomic absorption spectroscopy. In the present work, an on-line FI-ICP-OES method is proposed for the pre-concentration of Crand Cr. The determination was performed by ICP-OESassociated with a FI methodology. The goal of this work was to establish a fully automated method for the determination ofCrand Cr which is easily controlled, has high sensitivity, and is reproducible. Experimental Instrumentation A PerkinElmer OptimaTM 5300 DV ICP-OES (inductively coupled plasma optical emission spectrometer) (PerkinElmer Lifeand Analytical Sciences, Shelton, CT, USA) with WinLab32TM v3.1 software was used. A PerkinElmer FIASTM-400 systemwas used as the flow injection accessory, which is also controlled with the WinLab32 v3.1 software. The FIAS-400 system, equipped with an AS-91 autosampler, was connected directly to the Optima 5300 DV nebulizer bymeans of narrow-bore Teflon tubing and used for on-line pre-concentration of chromium. The automatic operation of theinjection valve and two multi-channel peristaltic pumps were programmed using the spectrometer software (PerkinElmerICP-OES WinLab32 v3.1). The internal gas flow of the FIAS-400 system was deactivated. Tygon peristaltic tubing (1.52mm i.d.) was used to pump the sample and buffer, while 0.76 mm i.d tubing was used for eluent; PTFE tubing (0.3 mm i.d.)was used for all connections in order to minimize dead volume. A conically shaped activated alumina microcolumn(PerkinElmer, PN B050-9561, yellow) was used for speciation of Crand Cr. Photos of the FIAS system and the columnappear in Figures 1 and2. Figure 1. FI-ICP-OES with FIAS-400, Autosampler 91 and Optima 5300 DV ICP-OES Figure 2. FIAS-400 and activated alumina mini column (yellow) Time-resolved signals of chromium were displayed on the computer monitor along with peak area and integrated intensityvalues. A Scott spray chamber and cross-flow nebulizer were used for sample introduction into the ICP-OES. The operatingconditions for FI-ICP-OES are summarized in Table I, and the flow injection program for FIAS 400 is shown in Table II.Using this program, a total sample volume of approximately 15 mL is used for the analysis: the flow rate is about 10 mL/min, with the prefill step lasting 30 seconds and the load step requiring 60 seconds. All measurements were made using thetransient scan mode with integration time of 50 ms for each Cr-species and 3 points per peak. Because transient signals areused, the peak area mode was used for all quantification work. Speciation conditions are shown in Table III. Table I. FI-ICP-OES Operating Conditions Plasma Gas 15 L/min Auxiliary Gas 0.2 L/min Nebulizer Gas 0.8 L/min RF Power 1300 W Plasma View Axial View Peak Processing Peak Area Signal processing Transient Points per peak 3 Integration time 50 ms Wavelength 267.7 nm Replicates 3 Table ll. Flow Injection Program of FIAS 400 Step Description Time Pump1 Pump2 Valve (sec) speed speed Position Prefill 30 100 0 Fill 1 Load 60 120 0 Inject 2 Wash 30 0 120 Inject 3 Elute, Read 45 0 100 Fill 4 Wash 30 0 100 Inject Table III. Speciation Conditions Cr+ Cr+ Buffer Potassium sodium Potassium chloride (pH 2) phosphate (pH7) Eluent 1.0 mol/L HNO: 0.5 mol/L NH4OH Sorbent activated alumina, acidic form Column Conical shape, approx.40 mg of sorbent, yellow (PerkinElmer, P/N B050-9561) Reagents All reagents used were ofat least analytical grade. Stock standards of 1000 mg/L Crand Cr were obtained from Sigma &Aldrich. Working standards were prepared by appropriate serial dilution. The buffers were purchased already prepared fromSigma & Aldrich and were potassium sodium phosphate at pH 7for Cr(part number 456098-500mL) and potassiumchloride at pH 7 for Cr(part number 223581-500mL). Both chemicals were from Sigma & Aldrich, and 0.5 M ammoniasolutions and 1 M nitric acid were prepared from the Aldrich chemicals, respectively. Ultrapure water (18MQ cm ') wasobtained from an EASYpureR RF (Barnstedt, Iowa, USA). Sample Collection and Preparation In order to avoid the reduction of Cr with the organic matter present in natural water, the samples were not acidified atcollection. The analysis was performed immediately after sampling in order to minimize the adsorption of chromium insolution on the walls of the container. All instruments used were previously washed with a 10 % (v/v) HNO3 solutionfollowed by the ultrapure water. Procedure for FI-ICP-OES Measurement A tubing diagram, schematic diagram of the FIAS-400 manifold and the sequence of its operation are presented in Figures 3,4 and Table II, respectively. A prefill step flushes the tubing and mixing block with a sample and buffer solution by pump 1.In step 1, the sample is premixed in the mixing block with buffer solutions of pH 2.0 for Cr°or pH 7.0 for Cr, respectivelyand passed through the analytical column (10 mL/min). The time selected for this step controls the volume of sample passedthrough the column. Following sample loading, the tube is washed with deionized water by pump 2 to remove residualsample in the lines. Retained Cr’or Crtions are then eluted with 1 M (mol/L) nitric acid or with 0.5 M(mol/L) ammonia,respectively, at a flow rate of 2.5 mL/min. The sample is introduced into the plasma in step 3 and then analyzed by ICP-OES. The total analytical time for the entire procedure is 3 minutes. In step 4, the valve is switched to the wash position toreturn the column to a neutral pH in preparation for loading the next sample. As can be seen in Figure 4, in the elution stepthe valve was switched to fill position and the retained chromium was eluted in countercurrent (i.e., reversal of the flowdirection through the column during elution with respect to sample loading) with eluent at a flow rate 2.5 mL/min, directlyinto the nebulizer and subsequently to the plasma. Countercurrent elution subsequently improves the elution profile ascompared to the unidirectional flow. Chromium was completely eluted from the column with 2.5 mL/min. The operatingconditions were established and the determination was completed. Eluent Figure 3..TTubing diagram of FIAS 400 for on-line chromium species determination Figure 44.. SSchematic diagram of the system for FIAS-400 system for automated speciation of Crand Crspecies Results Figure 5 shows the peak profile for a 50 ug/L Cr standard where 10 mL of the standard was pre-concentrated on thecolumn. The relative standard deviation (RSD) for ten replicates containing 100 ug/L ofCr’and Cr was less than 5%. Asseen in Figure 6, the calibration graph for Cris linear with a correlation coefficient of 0.9999 from levels near the detectionlimits up to at least 100 pg/L. The detection limit, calculated as the amount of Cr required to yield a net peak that was equalto three times the standard deviation of the background signal (38), was 1 ug/L and 0.9 ug/L for Crand Cr, respectively. In routine practical applications, the recovery of Crand Crdetermined by the proposed procedure in some natural watersamples ranged from 90 to 110 %, as shown in Table IV. Recoveries were determined by spiking water samples withdifferent levels of the chromium species and measuring the results. Figure 5. Peak profile obtained by preconcentration of 10 mL of Crusing the system depicted in Figure 3. Loaded flow rate was 10 mL/min; the elutionflow rate was 2.5 mL/min. Cr concentration was 50 ug/L. Cr 267.716 Figure 6. Calibration for Cr using FIAS-400 with reference solutions containing 10, 50, 100 ug/L Table IV. Recovery of Crand Cr after spiking with known amounts of mixture to drinking water Sample Spike Conc % Recovery (ug/L) cr+ cr6+ Drinking water 100 102 98 50 96 102 10 110 98 Conclusion The Flow Injection Analysis System (FIAS) coupled with ICP-OES performs very well as a simple quantitative method forchromium speciation. This fully automated, on-line chromium speciation method was applied to a series of spiked naturalwater samples with very good recoveries and detection limits. References .1Nriagu, J.O. in Chromium in the Natural and Human Environment; Nriagu, J.O.; Nieboer, E. Eds.; New York: JohnWiley & Sons, 1988, Vol. 20, p.91 2. Stoeppler, M. in Hazardous Metals in the Environment: Techniques and Instrumentation in Analytical Chemistry;Stoeppler, M. Eds., Amsterdam: Elsvier, 1992,Vol.12. p. 373. 3. Mugo,R. K.; Orians, K. J. Anal. Chim. Acta 1993,271,1. 4. Zou, H.F., Zu, S.K.,Fang, Z.L. Atom. Spectros.1996,17,112. 5. Batley, G.E., Matousek, J.P. Anal. Chem. 1980, 52,1570. PerkinElmer Life andAnalytical Sciences710 Bridgeport AvenueShelton, CT 06484-4794 USAPhone: (800) 762-4000 or(+1) 203-925-4602 precisely. www.perkinelmer.com For a complete listing of our global offices, visit www.perkinelmer.com/lasoffices ( O 2006 PerkinElmer, In c . All r i ghts reserved. The PerkinElmer logo and design are r e gistered trademarks of PerkinElmer, I n c. Optima and FIAS are tr a demarks of PerkinElme r ,Inc. or its subsidiaries, i n the United States and other countries. Tefl o n is a registered trademark o f E . I. du Pont de Nemours and Company. EASYpure is s registered tra d emarkof Barnstead/Thermolyne. All o ther trademarks not ow n ed by PerkinEl m er, Inc. or its subsidiaries that are depicted herein are the p r ope r ty of their respective owners. ) PerkinElmer reserves the right to change this document at any time without notice and disclaims liability for editorial, pictorial or typographical errors. The data presented in this Field Application Report are not guaranteed. Actual performance and results are dependent upon the exact methodology used and laboratory conditions.This data should only be used to demonstrate the applicability of an instrument for a particular analysis and is not intended to serve as a guarantee of performance. www.perkinelmer.com
确定


还剩4页未读,是否继续阅读?
珀金埃尔默企业管理(上海)有限公司为您提供《采用FI-ICP-OES在线分析铬 (Cr3 + Cr6 +)形态》,该方案主要用于其他中--检测,参考标准--,《采用FI-ICP-OES在线分析铬 (Cr3 + Cr6 +)形态》用到的仪器有
相关方案
更多
该厂商其他方案
更多









