
方案详情
文
肾脏患者的皮肤组织重塑和非渗透性钠储存 荷兰格罗宁根大学医学中心肾内科Dermal tissue remodeling and non-osmotic sodium storage in kidney patients
方案详情

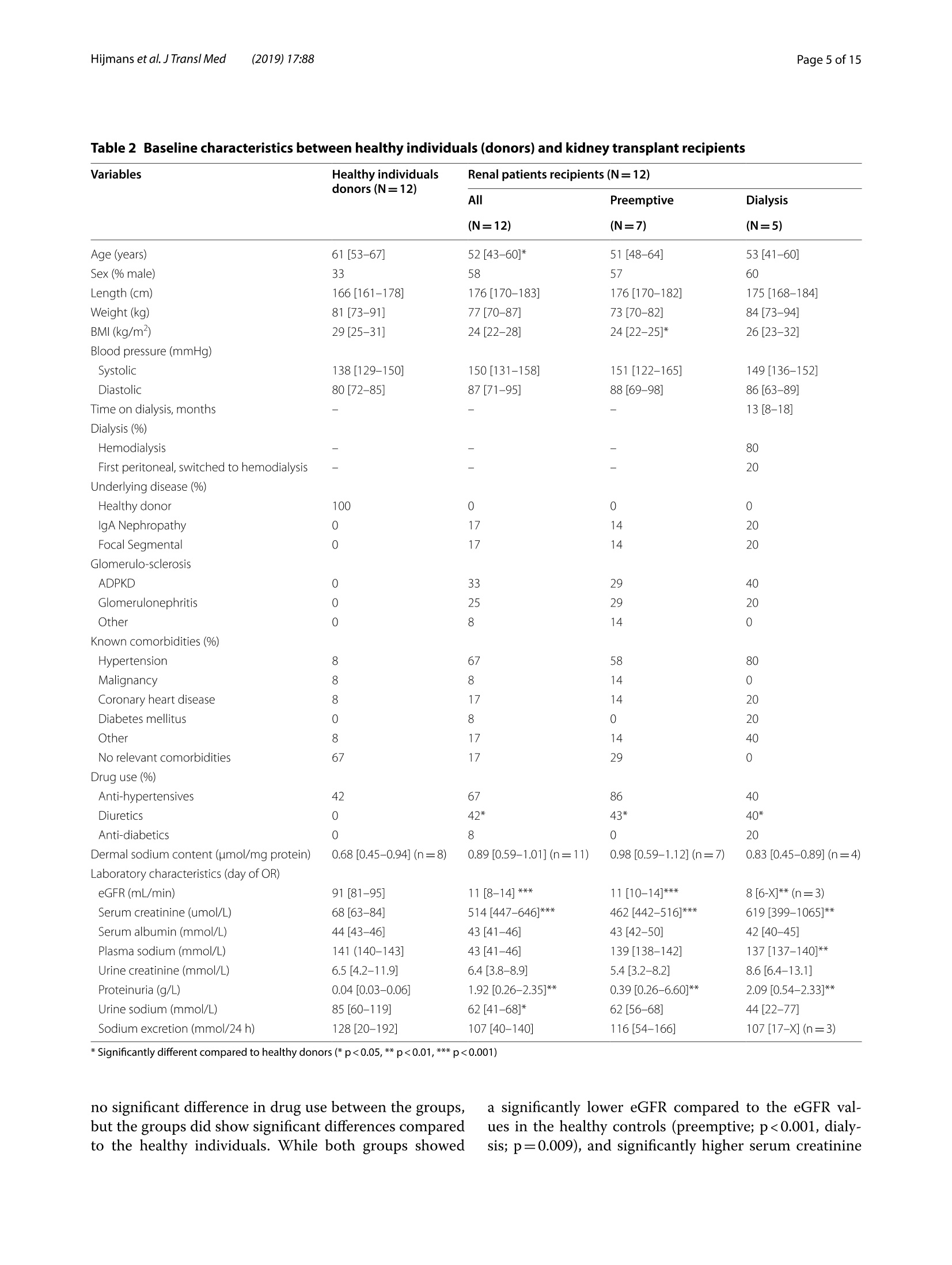
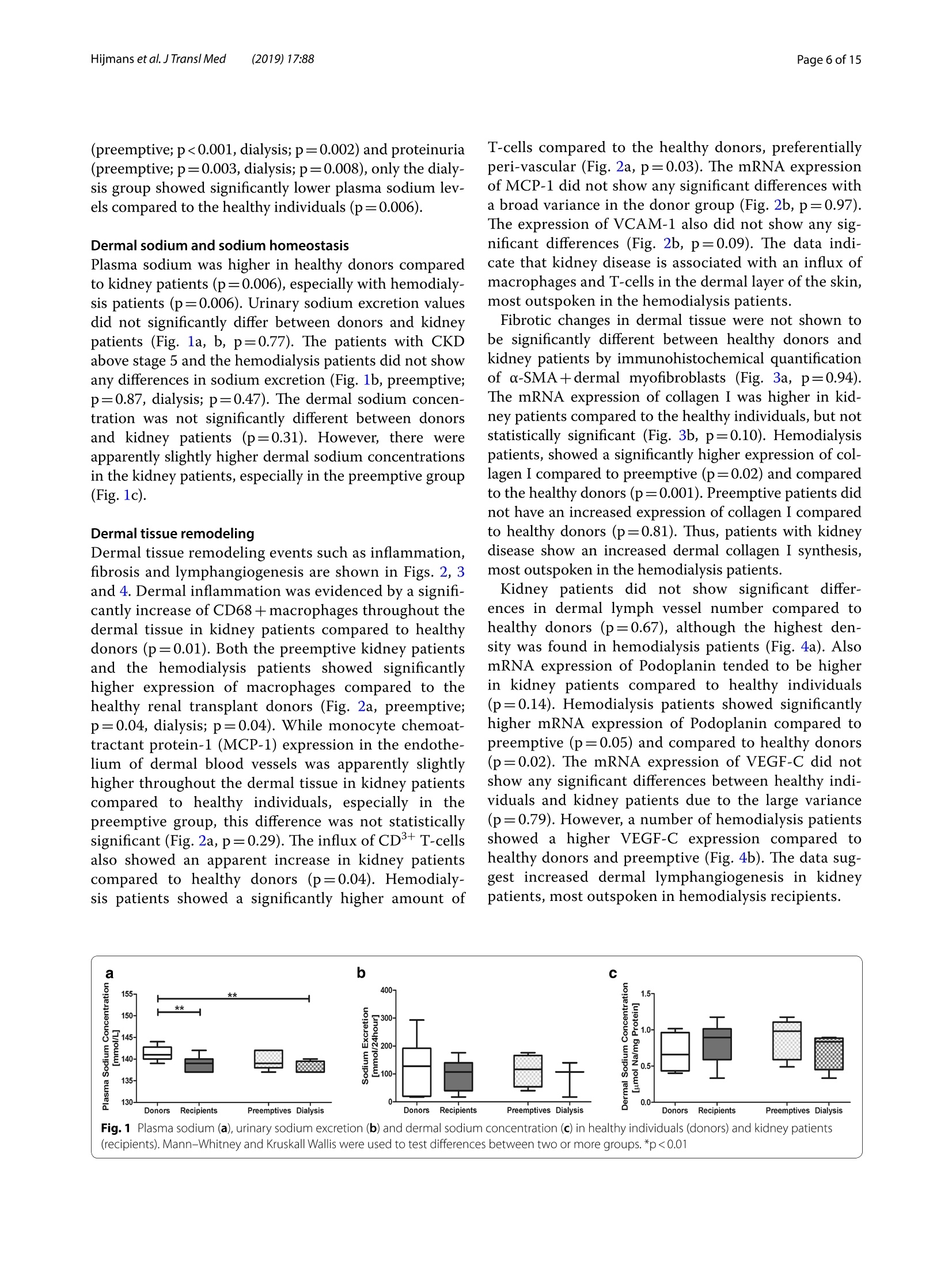
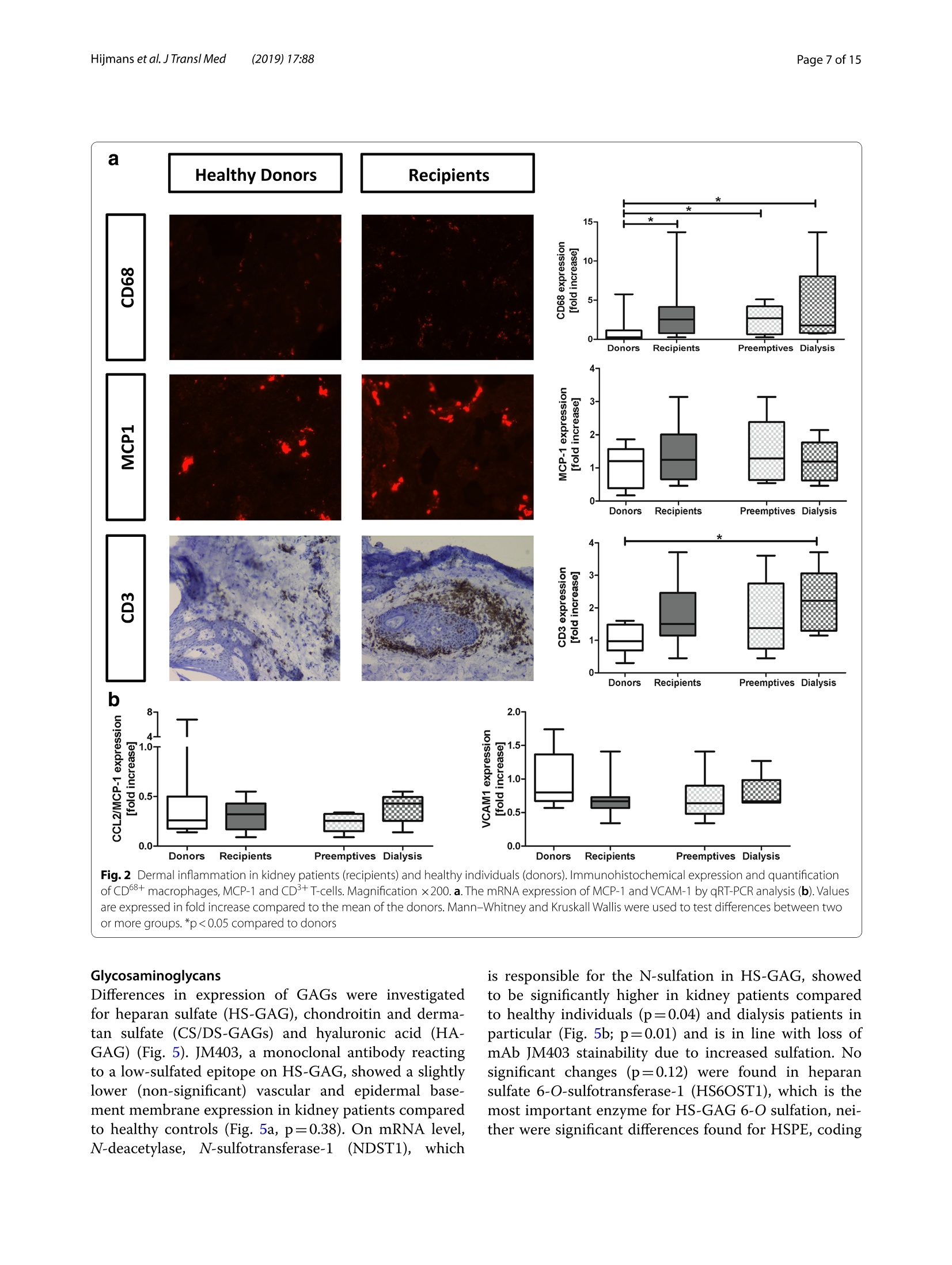
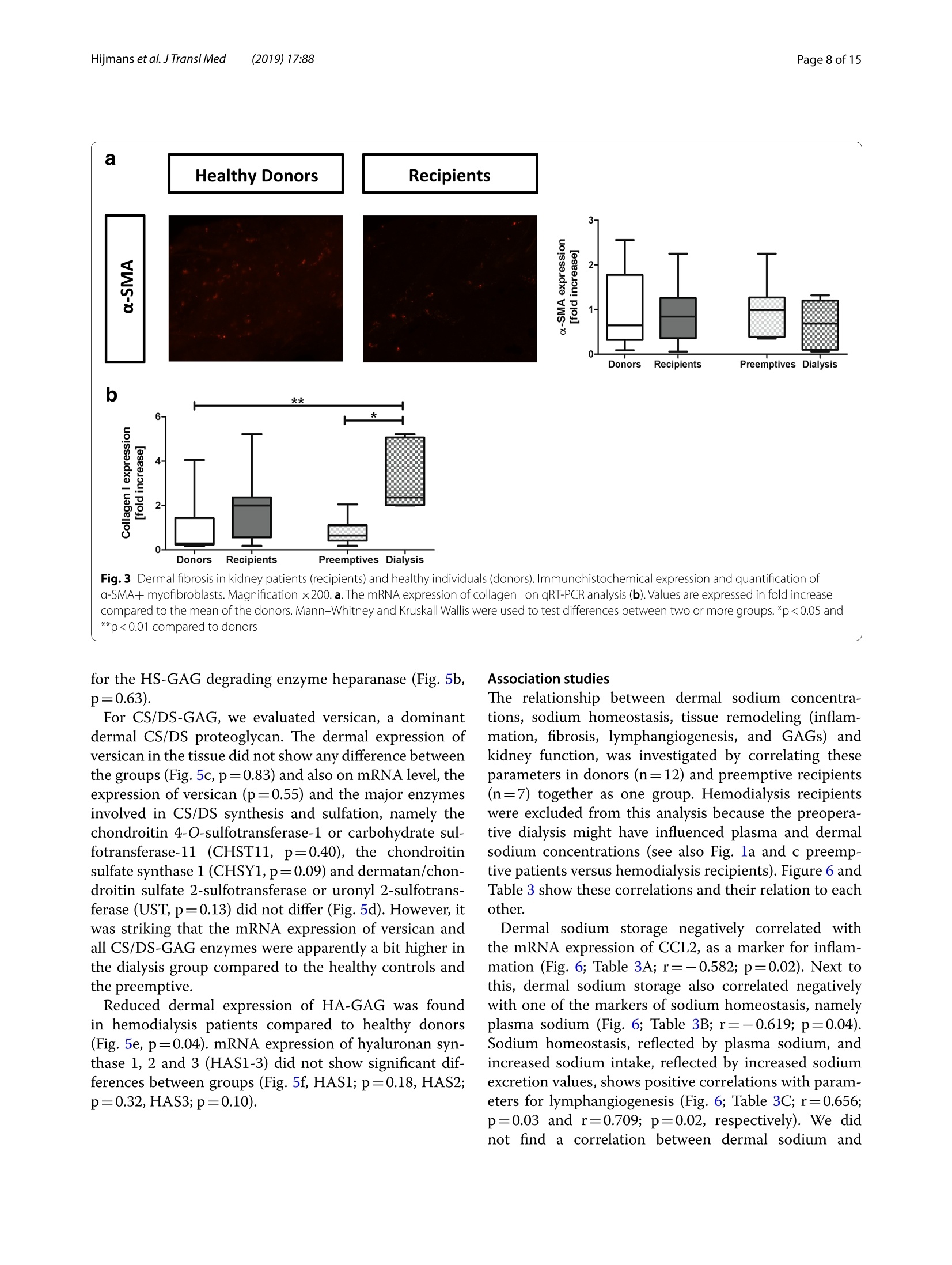
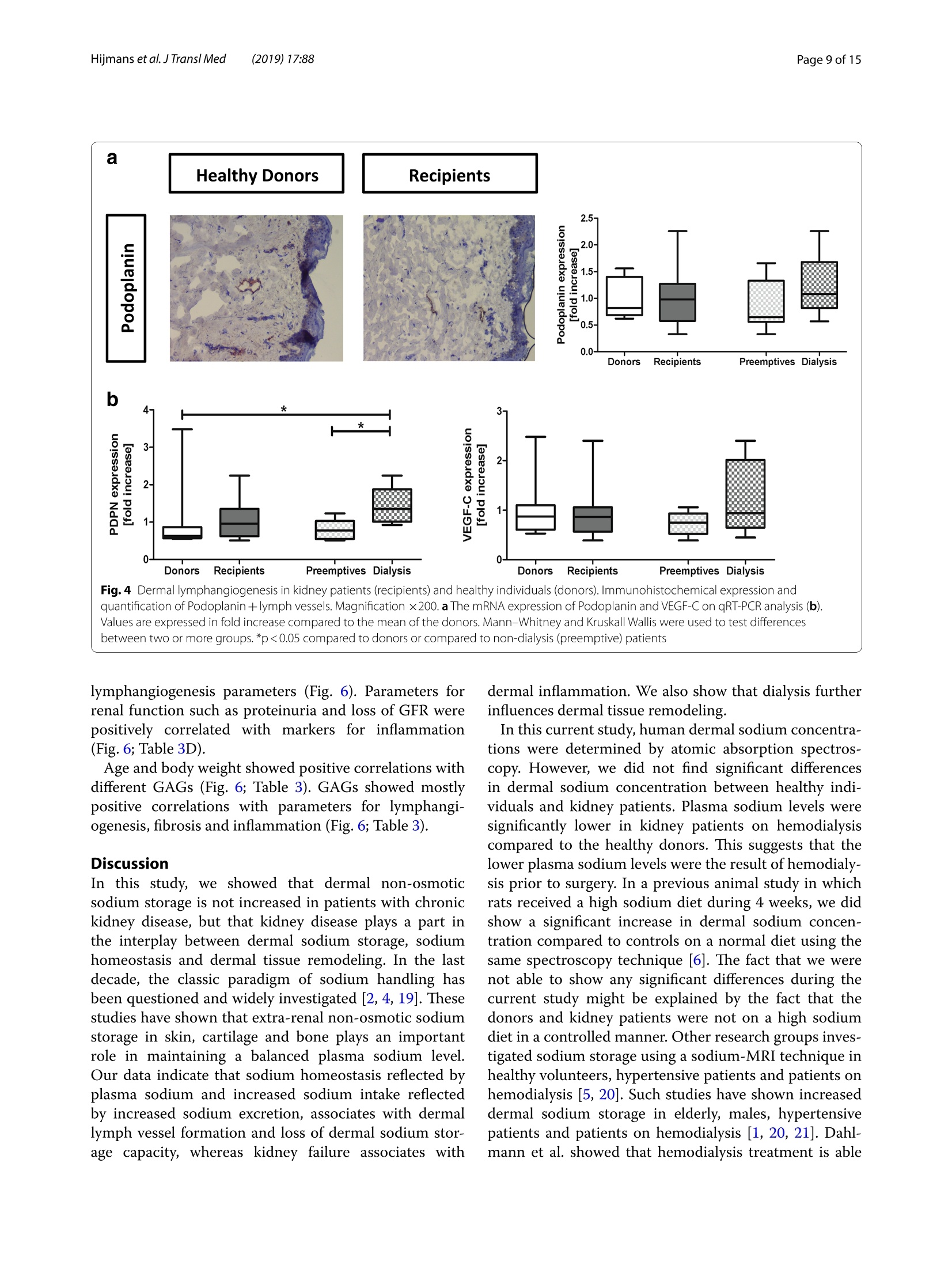
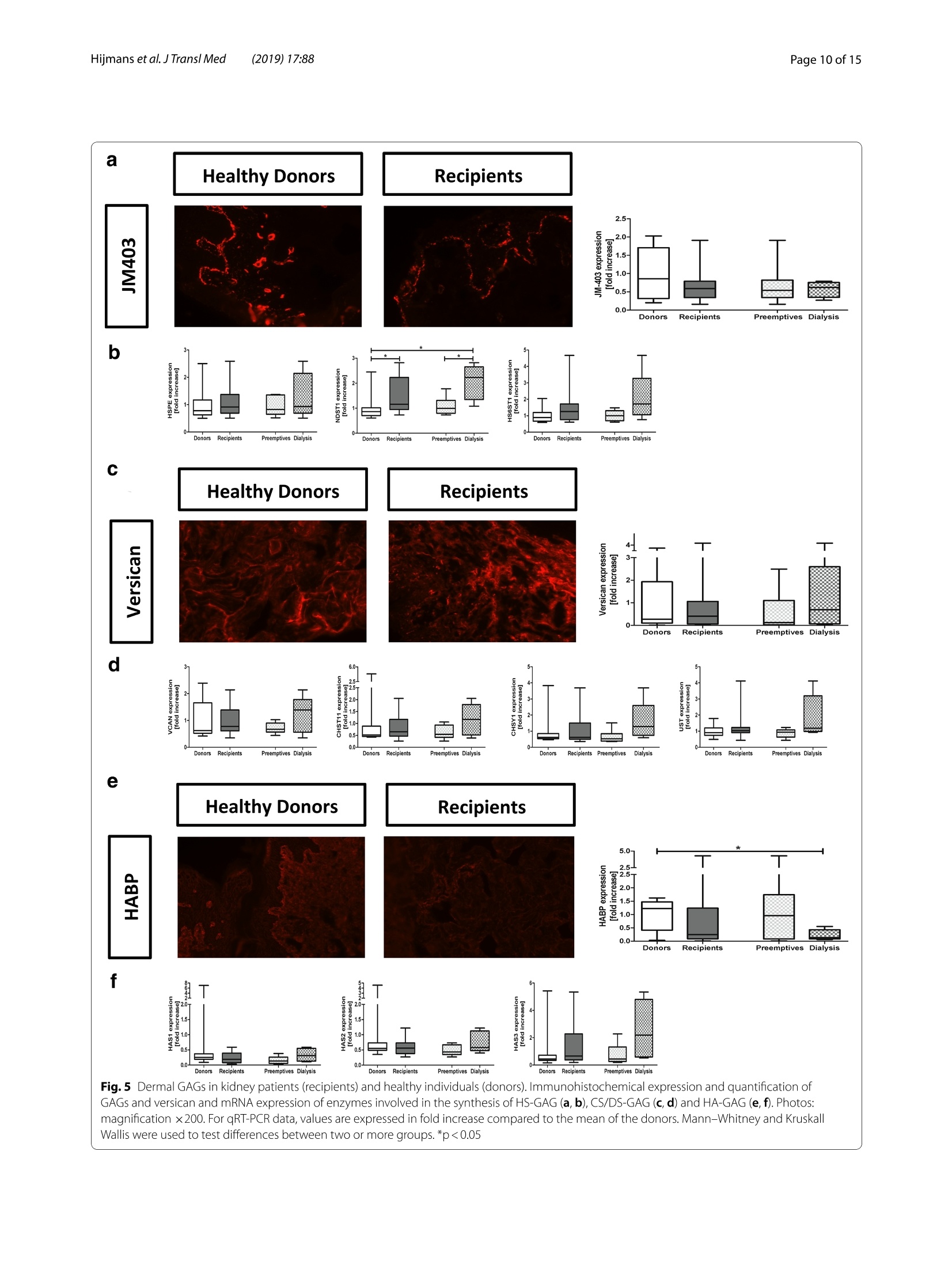
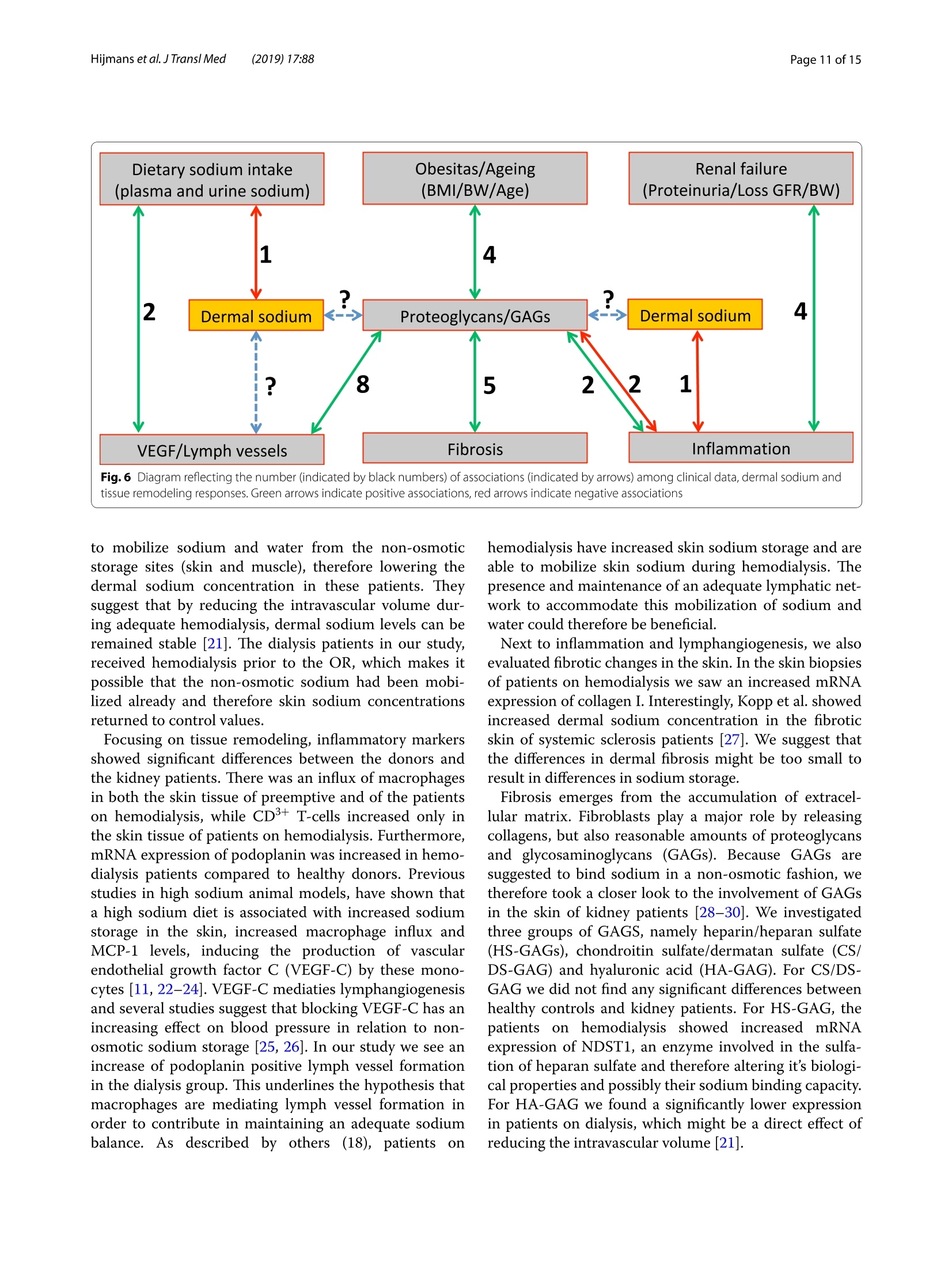
肾脏患者的皮肤组织重塑和非渗透性钠储存 荷兰格罗宁根大学医学中心肾内科Dermal tissue remodeling and non-osmotic sodium storage in kidney patientsUMCG医学中心附属于格罗宁根大学。它是世界上最大的医院之一,提供跨地区三级保健到荷兰北部的一部分。该医疗中心的员工近17,000人,数量1,400张病床。它是世界上最大的移植手术中心之一。所有可能的器官移植手术都能在UMCG进行,包括多器官联合移植在一次手术中完成。Hijmans et al. J Transl Med (2019) 17:88https://doi.org/10.1186/s12967-019-1815-5Journal ofTranslational Medicine肾脏患者的皮肤组织重塑和非渗透性钠储存RESEARCH Hijmans et al. J Transl Med (2019) 17:88Page 2 of 15 Dermal tissue remodeling and non-osmoticsodium storage in kidney patients Ryanne S. Hijmans1,2,3*, Marco van Londen1, Kwaku A. Sarpong1, Stephan J. L. Bakker1, Gerjan J. Navis1,Twan T. R. Storteboom4, Wilhelmina H. A. de Jong4, Robert A. Pol2 and Jacob van den Born1 Abstract Background: Excess dietary sodium is not only excreted by the kidneys, but can also be stored by non-osmotic bind-ing with glycosaminoglycans in dermal connective tissue. Such storage has been associated with dermal infalmma-tion and lymphangiogenesis. We aim to investigate if skin storage of sodium is increased in kidney patients and if this storage is associated with clinical parameters of sodium homeostasis and dermal tissue remodeling. Methods: Abdominal skin tissue of 12 kidney patients (5 on hemodialysis) and 12 healthy kidney donors wasobtained during surgery. Skin biopsies were processed for dermal sodium measurement by atomic absorption spec-troscopy, and evaluated for CD68+ macrophages, CD3+ T-cells, collagen I, podoplanin + lymph vessels, and glycosami-noglycans by qRT-PCR and immunohistochemistry. Results: Dermal sodium content of kidney patients did not difefr from healthy individuals, but was inversely associ-ated with plasma sodium values (p < 0.05). Compared to controls, kidney patients showed dermal tissue remodelingby increased CD68+ macrophages, CD3+ T-cells and Collagen I expression (all p < 0.05). Also, both N- and O-sulfation of heparan sulfate glycosaminoglycans were increased (all p < 0.05), most outspoken in hemodialysis patients. Plasma and urinary sodium associates with dermal lymph vessel number (both p < 0.05), whereas loss of eGFR, proteinuria and high systolic blood pressure associated with dermal macrophage density (all p < 0.05). Conclusion: Kidney patients did not show increased skin sodium storage compared to healthy individuals. Results do indicate that kidney failure associates with dermal infalmmation, whereas increased sodium excretion and plasma sodium associate with dermal lymph vessel formation and loss of dermal sodium storage capacity. Trial registration The cohort is registered at clinicaltrials.gov as NCT (September 6, 2017). NCT, NCT03272841. Regis-tered 6 September 2017—Retrospectively registered, https: //clini caltr ials.gov Keywords: Sodium, Transplantation, Kidney, Skin, Remodeling Background Over the past decades, the classical paradigm of sodiumhandling by the human body has been widely questioned [1–3]. New evidence suggests that besides via regulationof sodium excretion by the kidneys, sodium can also be stored in a non-osmotic manner in bone, cartilage and 荷兰格罗宁根大学医学中心肾内科 *Correspondence: r.s.hijmans@umcg.nl 1 Department of Internal Medicine, Division of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands Full list of author information is available at the end of the article skin tissue [2, 4]. Especially skin has been shown to func-tion as an extra compartment for sodium storage [5]. In a previous animal study, we showed increased der-mal sodium concentrations in rats who received a high sodium diet for 4 weeks [6]. However, human dermaltissue remodeling responses such as lymphangiogenesis,fbirosis and infalmmation have not been investigated,especially not in relation to dermal sodium concentra-tions. A recent study showed that the sodium storagein human tissues, such as arteries, skin and muscle, is mediated by glycosaminoglycans (GAGs) [7]. While ithas been shown that XYLT-1, an enzyme involved in the synthesis of GAGs, is increased, that study did not inves-tigate in greater detail, which GAGs are involved. In previous studies, our group showed that uponvarious noxi, renal proteoglycans and their covalently attached GAG side chains (such as heparan sulfate,HS) can be converted into pro-infalmmatory media-tors orchestrating macrophage infulx, T-cell infulx, andcontribute to fbirosis and lymphangiogenesis [6, 8]. Our studies, among others, have shown that this conversioncan also be induced by a high sodium diet [6, 9–11]. In order to investigate these phenomena, we focusedon the growing group of patients with end stage renal dis-ease (ESRD) and who were in need of renal replacementtherapy (RRT) [12]. Tehse patients are sufefring from CKD and are matched up with healthy renal transplantdonors. While early transplantation (CKD above stage 5;preemptive) has several beneftis in terms of patient andgrafts survival [13, 14], the majority of patients sufefr-ing from ESRD are dialysis dependent and awaiting renaltransplantation. Multiple studies have shown high car-diovascular morbidity and mortality in dialysis patients,mainly associated by systemic infalmmation which can be induced by uremia, the underlying renal disease, dialy-sis-related factors and comorbidities [15–17]. In order to decrease cardiovascular morbidity andmortality, the driving forces of systemic infalmmation in patients with chronic kidney disease, and especially indialysis patients, need further investigation. In this study, we aim to investigate whether skin stor-age of sodium is increased in kidney patients (i.e. both hemodialysis patients and preemptive patients), compar-ing them to healthy individuals (kidney donors). Second,we hypothesize that skin storage of sodium is associatedwith clinical parameters of sodium homeostasis, such as plasma sodium regulation and sodium excretion. Next,we hypothesize that dermal sodium storage is associ-ated with tissue remodeling responses such as changes inGAGs, infalmmation, lymphangiogenesis and fbirosis. Methods Study population For this study, full thickness skin biopsies were obtainedfrom 12 healthy controls (kidney donors) and 12 kidney patients at the time of renal transplantation (recipients),undergoing surgery at the University Medical Center Groningen (UMCG) between 14th February and 22ndMarch 2017. Of the 12 kidney patients, 5 were on hemo-dialysis (HD). From all patients, blood and urine wereanalyzed for sodium (Ion Selective Electrode by Roche Modular, Roche, Mannheim, Germany) and creatinineconcentration (Roche Modular Enzymatic method,Roche, Mannheim, Germany). For all renal patients, clin-ical data were obtained from electronic patient flies and the urine and plasma were collected on the day of theoperation (OR) or 1 day before the OR. For the healthy donors, clinical data were also obtained from their elec-tronic flies. Urine and plasma were collected 1 day before OR or a week before OR. All study participants providedwritten informed consent prior to study and are enrolled in the Transplantlines Biobank and Cohort study(TXLINES01). Teh cohort is registered at clinicaltrials.gov as NCT03272841. Teh study protocol is in accordance with the DutchMedical Research Involving Human Subjects Act (WMO), and approved by the Medical Ethics Commit-tee of the University Medical Center Groningen (METc 2014/077). All procedures were conducted in accordancewith the declarations of Helsinki and Istanbul. Study protocol Pre-operatively, both kidney donors and kidney patientsreceived intravenous antibiotic profylaxis (2 g Cefalozlin and 500vmg Metronidazole) 30omin prior to the OR. Nextto this all patients received 200 mL of intravenous 15%Mannitol at the time of induction. Renal patients (recipi-ents) also received intravenous immunosuppressive medication pre-OR (40img Solumedrol®, 0.075emg/kgTacrolimus, 2000 mg Cellcept® and 20omg Basiliximab).In case of identical HLA-typing, the Basiliximab wasnot given. All patients who were on dialysis received HD 1 day before surgery. One of the patients received plas-mapheresis the day before the OR for immuno-absorp-tion because of ABO-incompatibility. Of the 5 patientson hemodialysis, 1 received ultraflitration to 1 kg above goal weight during the last dialysis. Abdominal full-thick-ness skin tissue was obtained from kidney donors and kidney patients during transplant surgery after incision.Immediately after skin biopsies were taken under dry and sterile conditions, they were placed in a tin containerkept cool on ice (0°C) with the precaution of avoiding any contact of the skin with water or saline. Teh skinsamples were then transported to the research laboratory for processing. Tehre, the skin tissue was pinned on asterile, falt surface and 1 or 2 biopsies (depending on size of harvested skin) were taken with a biopsy punch (Stie-fel Biopsy Punch, 6 mm; SmithKline Beecham, UK) for immunohistochemistry. Teh remainder of the tissue wasdivided in halve and the halves were placed in two sterile Eppendorf tubes for future qPCR and sodium measure-ments after which all processed skin samples were stored in a minus 80°°C freezer. Measurement of dermal sodium Teh skin samples for determination of sodium contentwere cut into two equal parts and wet weights were measured. Both halves were then dried overnight in an oven at 80 °C after which their dry weight was measured.One of the samples was then dissolved in a destruction solution made up of a 4:1 mixture of perchloric acid andpure nitric acid (Sigma-Aldrich, St Louis, USA) at 60°C for 3 h. To 0.5 mL sample solution, 4.5 mL of water wasadded to obtain a 5 mL stock sample solution. Teh sample solution was then diluted 1:1000 after which the sodiumcontent was measured by atomic absorption (falme)spectrometry using the Tehrmo M Series AA Spectrom-eter (Tehrmo Fisher Scientifci, Waltham, USA). Teh sec-ond half of the sample was used to calculate the amountof protein per sample by measuring the nitrogen content using a Dumas method (Dumatherm Nitrogen/Proteinanalyser, C. Gerhardt UK Ltd, Northamptonshire, UK).Sodium concentrations were expressed as µmol sodiumper mg protein. Immunohistochemistry Immunohistochemical staining was performed on4-um-thick cryo sections cut from skin biopsies with a Leica CM1950 cryostat (Leica Biosystems, Wetzlar,Germany) followed by acetone or 4% paraformaldehyde fxiation for 10emin. Endogenous peroxidase activity wasblocked by incubating with 0.03% hydrogen peroxide (in phosphate bufefred saline; PBS). Endogenous biotin bind-ing sites were blocked by an Avidin/Biotin blocking step in case of biotin-labeled reagents. Skin cryo sections wereincubated for 1 h with the following primary antibod-ies/reagents: mouse anti-human α-smooth muscle actin(SMA; clone 1A4, Sigma-Aldrich, St Louis, USA), rabbit anti-human CD3 (clone A0452, Dako, Glostrup, Den-mark), biotinylated hyaluronan binding protein (HABP,Seikagaku, Tokyo, Japan), mouse anti heparan sulphatemAB JM403 [18], mouse anti-human podoplanin (Clone D240, TehrmoFisher, Rockford, USA), mouse anti-humanCD68 (clone ED1, AbD Serotec, Oxford, UK), rabbit anti-versican (ITK Diagnostics, B.V., Uithoorn, Teh Nether-lands) and mouse anti-human MCP-1 (Peprotech, Rocky Hill, USA) diluted in PBS/1% Bovine Serum Albumin(BSA). Binding of primary antibodies was detected by incubating the sections for 30imin with either a second-ary or both secondary and tertiary antibodies diluted in PBS/1% BSA (and 1% normal human serum in somecases). We used rabbit anti-mouse Ig horseradish peroxi-dase (HRP), goat anti-rabbit Ig HRP, goat anti-mouse IgHRP, rabbit anti-goat Ig HRP, (all from Dako, Heverlee,Belgium) in PBS/1% BSA. As negative controls, the pri-mary antibodies were replaced by PBS/1% BSA and were all found to be negative. Bound antibodies were visual-ized by aminoethylcarbazole (AEC) counterstained with diluted hematoxylin or by the TSA TM tetramethylrho-damine system (PerkinElmer Life Sciences Inc., Waltham,USA) (10 min) for HRP antibodies. In the detection of CD3 antigen immunoreactivity was visualized using3,3′-diaminobenzidine (DAB) solution. Biotinylated HABP was visualized using Cy3 conjugated streptavidin(Invitrogen, Carlsbad, USA). DAPI solution (Vector labo-ratories, Burlingame, USA) was applied to the sectionsand incubated for 10 min for nuclear staining and sub-sequently mounted in either Citifulor mounting medium(fulorescence) or Aquatex mounting medium. Teh whole staining procedure was carried out at room temperature. Quantifciation of immunohistochemistry Stainings were evaluated on a Leica DM4000B (LeicaBiosystems Wetzlar, Germany) equipped for immuno-fulorescence, and with a DFX345FX camera using a LASsoftware package. At least 5 pictures at 20 × magnifcia-tion per skin sample were taken followed by digital quan-tifciation using ImageJ 1.46r (Rasband, W.S., US National Institutes of Health) and expressed as % positively stainedarea (for macrophages, glycosaminoglycans and collagen I). D2-40 podoplanin positive lymphvessles and CD3-positive T-cells were quantifeid manually by two inde-pendent researchers and the mean of both scorings wereused, expressed per standardized tissue area. Gene expression RNA was isolated from frozen skin tissue by the Favor-Prep Tissue Total RNA Mini Kit (Favorgen Biotech Corp, Vienna, Austria) according to the manufacturer’sprotocol. Teh total amount of RNA after isolation was measured by a nanodrop UV-spectrometer (NanodropTechnologies, Wilminton, DE, USA) at 260/280 nm. From 700 ng RNA, cDNA was synthesized using theQuantitect reverse transcription kit (Qiagen, Venlo, the Netherlands) in accordance with the manufacturer’sprotocol. Teh following solutions were added to 1 ng/µL RNA: 2 µL genomic DNA wipeout bufefr, 1 µL Quan-tiscript Reverse Transcriptase (RT), 4eµL 5× RT Bufefr,1 µL RT Primer Mix and RNase-free water (up to 20 µL).Teh samples were placed in a MyCycler™wTehrmal Cycler (Bio-Rad Laboratories) to start the cDNA synthesis with1 cycle of 42 °C/15 min and 95 °C/3 min. Afterwards, the cDNA samples were diluted with 130 µL of RNAse-freewater and stored at 44°C (for use shortly). For quantita-tive reverse transcription-polymerase chain reaction(qRT-PCR), 3 µL cDNA (diluted 3× from stock) and 7 µL SYBR Green-Primer- Water mix (consisting of 5 µLSYBR Green Supermix (BioRad, Veenendaal, Teh Neth-erlands), 0.08 µL gene specifci primer set (0.5 mM) and1.92nµL MilliQ water) were pipetted into a 384 wells plate (Applied Biosystems, Foster City, CA). All reac-tions were performed in triplicate. Teh plate was covered with an adhesive cover and centrifuged for 30 s. Prim-ers were ordered from Sigma and the sequences of the oligonucleotides are shown in Table 1 below. Amplifcia-tion was performed using an ABI7900HT Tehrmal cycler (Applied Biosystems) with the cycle procedure as follows:10 min at 955°C, with 40 repeats of a 15 s denaturation step at 95 °C and a 40 s extension and annealing step at600°C. Data analysis was performed using science detec-tion software 2.4 (Applied Biosystems). To determine dif-ferences in expression of gene of interest, Ct-values were normalized against mean Ct-values of β-Actin as house-keeping gene. Statistics Data are shown as median (interquartile range) and com-parisons between groups were performed by Mann–Whitney U test. Spearman Rank correlation coefcfieinton the Z-scores of various parameters was used for asso-ciation studies. Statistical analysis were performed usingSPSS 23.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5.0 (GraphPad Software Inc., La Jolla, CA, USA)was used to construct graphs and fgiures. P values below 0.05 were considered statistically signifciant. Results Clinical characteristics Descriptive data are given for healthy individuals (donors,n = 12) and kidney patients (recipients, n = 12) in Tabler 2. Teh kidney patients in this study were younger comparedto the healthy donors (p = 0.05), and there were more male recipients compared to male donors (although notsignifciantly difefrent, p = 0.23). Healthy individuals and kidney patients in this study are well matched for length(p = 0.05) and weight (p = 0.78). Blood pressure did not signifciantly difefr between the two groups (systolicp = 0.23 and diastolic p = 0.25). Kidney patients sufefred from various renal diseases and had more comorbidity,such as hypertension, coronary heart disease and diabe-tes mellitus. Tehrefore, they also used more anti-hyper-tensive, diuretic and anti-diabetic medication. In terms of kidney function eGFR (p < 0.001), plasma sodium(p = 0.006) and urine sodium (p = 0.02) were signifciantly decreased in kidney patients compared to healthy indi-viduals, while proteinuria was signifciantly higher in kid-ney patients compared to the healthy donors (p = 0.001). No signifciant difefrences were found between kid-ney patients who were on dialysis (n = 5) and patients in higher CKD stages (preemptive, n = 7) for the clinicalcharacteristics. Compared to healthy controls, there were no signifciant difefrences in age, sex, length, weight andblood pressure (Table 2, right column). Teh median time on dialysis prior to renal transplantation was 13 (8–18)months. Twenty percent of the dialysis patients had been on peritoneal dialysis prior to hemodialysis. Tehre was Table 1 Primer oligonucleotide sequences (forward and reverse) used in RT-qPCR Primer Forward sequence Reverse sequence A. Infalmmation CCL2 5′-AGA CTA ACC CAG AAA CAT CC-3′ 5′-ATT GAT TGC ATC TGG CTG -3′ VCAM 5′-TCC TGA GCT TCT CGT GCT CTATT-3′ 5′-TGA CCC CTT CAT GTT GGC TT-3′ B. Fibrosis COL1A1 5′-GGG ATT CCC TGG ACC TAA AG-3′ 5′-GGA ACA CCT CGC TCT CCA -3′ C. Lymphangiogenesis VEGFC 5′-CTG GCT CAG GAA GAT TTT ATG-3′ 5′-TGT TTT TAC AGA CAC ACT GG-3′ PDPN 5′-AAG ATG GTT TGT CAA CAG TG-3′ 5′-GTA CCT TCC CGA CAT TTT TC-3′ D. Proteoglycan related VCAN 5′-CCA GTG TGA ACT TGA TTT TG-3′ 5′-CAA CAT AAC TTG GAA GGC AG-3′ NDST1 5′-CGT GAC GCG ACC TAG CGA -3′ 5′-TCA TAG GTG GAG TGA TTT GAC TGG -3′ HS6ST1 5′-AGG AAG TTC TAC TAA CAT CACC-3 5′-CCC ATC ACA CAT ATG CAA C-3′ HSPE 5′-CCT TGC TAT CCG ACA CCT TT-3′ 5′-GGC TGA CAG GCC CAA TTT A -3′ CHYSY1 5′-AGA CTT TCA GCA AAA TCC AG-3′ 5′-GTT TGA GAG AAA GGA CAA GG-3′ HAS1 5′-TCC ACT GTG TAT CCT GCA TC-3′ 5′-CCC CAA AAG TAT CCT GCA TC -3′ HAS2 5′-GAT GCA TTG TGA GAG GTT TC-3′ 5′-CCG TTT GGA TAA ACT GGT AG-3′ HAS3 5′-CTT GAA GAT TAA TGT AGG ATG ACA GGCT-3′ 5′-AAA GTT GAC GAC CAC AGT GCAA-3′ UST 5′-GAA CGT GAA TGA AAA CTT CC-3′ 5′-TCT GGG TCT TTG TAG ATA CTG-3′ CHST11 5′-TAT TTC CAA ATC ATG CGG AG-3′ 5′-ATT GGG TTG TAG AGT TCC TG-3′ E. Housekeeping β-Actin 5′-CCA ACC GCG AGA AGA TGA -3′ 5′-CCA GAG GCG TAC AGG GAT AG-3′ Table 2 Baseline characteristics between healthy individuals (donors) and kidney transplant recipients Variables Healthy individuals Renal patients recipients (N = 12) donors (N = 12) All Preemptive Dialysis (N = 12) (N = 7) (N = 5) Age (years) 61 [53–67] 52 [43–60]* 51 [48–64] 53 [41–60] Sex (% male) 33 58 57 60 Length (cm) 166 [161–178] 176 [170–183] 176 [170–182] 175 [168–184] Weight (kg) 81 [73–91] 77 [70–87] 73 [70–82] 84 [73–94] BMI (kg/m2) 29 [25–31] 24 [22–28] 24 [22–25]* 26 [23–32] Blood pressure (mmHg) Systolic 138 [129–150] 150 [131–158] 151 [122–165] 149 [136–152] Diastolic 80 [72–85] 87 [71–95] 88 [69–98] 86 [63–89] Time on dialysis, months – – – 13 [8–18] Dialysis (%) Hemodialysis – – – 80 First peritoneal, switched to hemodialysis – – – 20 Underlying disease (%) Healthy donor 100 0 0 0 IgA Nephropathy 0 17 14 20 Focal Segmental 0 17 14 20 Glomerulo-sclerosis ADPKD 0 33 29 40 Glomerulonephritis 0 25 29 20 Other 0 8 14 0 Known comorbidities (%) Hypertension 8 67 58 80 Malignancy 8 8 14 0 Coronary heart disease 8 17 14 20 Diabetes mellitus 0 8 0 20 Other 8 17 14 40 No relevant comorbidities 67 17 29 0 Drug use (%) Anti-hypertensives 42 67 86 40 Diuretics 0 42* 43* 40* Anti-diabetics 0 8 0 20 Dermal sodium content (µmol/mg protein) 0.68 [0.45–0.94] (n = 8) 0.89 [0.59–1.01] (n = 11) 0.98 [0.59–1.12] (n = 7) 0.83 [0.45–0.89] (n = 4) Laboratory characteristics (day of OR) eGFR (mL/min) 91 [81–95] 11 [8–14] *** 11 [10–14]*** 8 [6-X]** (n = 3) Serum creatinine (umol/L) 68 [63–84] 514 [447–646]*** 462 [442–516]*** 619 [399–1065]** Serum albumin (mmol/L) 44 [43–46] 43 [41–46] 43 [42–50] 42 [40–45] Plasma sodium (mmol/L) 141 (140–143] 43 [41–46] 139 [138–142] 137 [137–140]** Urine creatinine (mmol/L) 6.5 [4.2–11.9] 6.4 [3.8–8.9] 5.4 [3.2–8.2] 8.6 [6.4–13.1] Proteinuria (g/L) 0.04 [0.03–0.06] 1.92 [0.26–2.35]** 0.39 [0.26–6.60]** 2.09 [0.54–2.33]** Urine sodium (mmol/L) 85 [60–119] 62 [41–68]* 62 [56–68] 44 [22–77] Sodium excretion (mmol/24 h) 128 [20–192] 107 [40–140] 116 [54–166] 107 [17–X] (n = 3) * Significantly difefrent compared to healthy donors (* p < 0.05, ** p < 0.01, *** p < 0.001) no signifciant difefrence in drug use between the groups,but the groups did show signifciant difefrences compared to the healthy individuals. While both groups showed a signifciantly lower eGFR compared to the eGFR val-ues in the healthy controls (preemptive; p < 0.001, dialy-sis; p = 0.009), and signifciantly higher serum creatinine (preemptive; p < 0.001, dialysis; p = 0.002) and proteinuria(preemptive; p = 0.003, dialysis; p = 0.008), only the dialy-sis group showed signifciantly lower plasma sodium lev-els compared to the healthy individuals (p = 0.006). Dermal sodium and sodium homeostasis Plasma sodium was higher in healthy donors comparedto kidney patients (p = 0.006), especially with hemodialy-sis patients (p = 0.006). Urinary sodium excretion valuesdid not signifciantly difefr between donors and kidney patients (Fig.i 1a, b, p = 0.77). Teh patients with CKDabove stage 5 and the hemodialysis patients did not show any difefrences in sodium excretion (Fig. 1b, preemptive;p = 0.87, dialysis; p = 0.47). Teh dermal sodium concen-tration was not signifciantly difefrent between donorsand kidney patients (p = 0.31). However, there were apparently slightly higher dermal sodium concentrationsin the kidney patients, especially in the preemptive group (Fig..1c). Dermal tissue remodeling Dermal tissue remodeling events such as infalmmation,fbirosis and lymphangiogenesis are shown in Figs. 2, 3and 4. Dermal infalmmation was evidenced by a signif-icantly increase of CD68 + macrophages throughout the dermal tissue in kidney patients compared to healthydonors (p = 0.01). Both the preemptive kidney patients and the hemodialysis patients showed signifciantlyhigher expression of macrophages compared to the healthy renal transplant donors (Fig. 2a, preemptive;p = 0.04, dialysis; p = 0.04). While monocyte chemoat-tractant protein-1 (MCP-1) expression in the endothe-lium of dermal blood vessels was apparently slightly higher throughout the dermal tissue in kidney patientscompared to healthy individuals, especially in the preemptive group, this difefrence was not statisticallysignifciant (Fig.o 2a, p = 0.29). Teh infulx of CD3+ T-cells also showed an apparent increase in kidney patientscompared to healthy donors (p = 0.04). Hemodialy-sis patients showed a signifciantly higher amount of T-cells compared to the healthy donors, preferentiallyperi-vascular (Fig. 2a, p = 0.03). Teh mRNA expression of MCP-1 did not show any signifciant difefrences witha broad variance in the donor group (Fig.f 2b, p = 0.97).Teh expression of VCAM-1 also did not show any sig-nifciant difefrences (Fig.12b, p = 0.09). Teh data indi-cate that kidney disease is associated with an influx ofmacrophages and T-cells in the dermal layer of the skin,most outspoken in the hemodialysis patients. Fibrotic changes in dermal tissue were not shown tobe signifciantly difefrent between healthy donors and kidney patients by immunohistochemical quantifciationof α-SMA + dermal myofbiroblasts (Fig. 3a, p = 0.94).Teh mRNA expression of collagen I was higher in kid-ney patients compared to the healthy individuals, but not statistically signifciant (Fig.e 3b, p = 0.10). Hemodialysispatients, showed a signifciantly higher expression of col-lagen I compared to preemptive (p = 0.02) and comparedto the healthy donors (p = 0.001). Preemptive patients did not have an increased expression of collagen I comparedto healthy donors (p = 0.81). Tuhs, patients with kidney disease show an increased dermal collagen I synthesis,most outspoken in the hemodialysis patients. Kidney patients did not show signifciant difefr-ences in dermal lymph vessel number compared to healthy donors (p = 0.67), although the highest den-sity was found in hemodialysis patients (Fig. 4a). Also mRNA expression of Podoplanin tended to be higherin kidney patients compared to healthy individuals (p = 0.14). Hemodialysis patients showed signifciantlyhigher mRNA expression of Podoplanin compared to preemptive (p = 0.05) and compared to healthy donors(p = 0.02). Teh mRNA expression of VEGF-C did not show any signifciant difefrences between healthy indi-viduals and kidney patients due to the large variance (p = 0.79). However, a number of hemodialysis patientsshowed a higher VEGF-C expression compared to healthy donors and preemptive (Fig. 4b). Teh data sug-gest increased dermal lymphangiogenesis in kidney patients, most outspoken in hemodialysis recipients. (recipients). Mann–Whitney and Kruskall Wallis were used to test differences between two or more groups. *p < 0.01 Glycosaminoglycans Difefrences in expression of GAGs were investigatedfor heparan sulfate (HS-GAG), chondroitin and derma-tan sulfate (CS/DS-GAGs) and hyaluronic acid (HA-GAG) (Fig. 5). JM403, a monoclonal antibody reacting to a low-sulfated epitope on HS-GAG, showed a slightlylower (non-signifciant) vascular and epidermal base-ment membrane expression in kidney patients comparedto healthy controls (Fig.k 5a, p = 0.38). On mRNA level,N-deacetylase, N-sulfotransferase-1 (NDST1), which is responsible for the N-sulfation in HS-GAG, showedto be signifciantly higher in kidney patients compared to healthy individuals (p = 0.04) and dialysis patients inparticular (Fig.u 5b; p = 0.01) and is in line with loss of mAb JM403 stainability due to increased sulfation. Nosignifciant changes (p = 0.12) were found in heparan sulfate 6-O-sulfotransferase-1 (HS6OST1), which is themost important enzyme for HS-GAG 6-O sulfation, nei-ther were signifciant difefrences found for HSPE, coding compared to the mean of the donors. Mann–Whitney and Kruskall Wallis were used to test difefrences between two or more groups. *p < 0.05 and **p < 0.01 compared to donors for the HS-GAG degrading enzyme heparanase (Fig. 5b,p = 0.63). For CS/DS-GAG, we evaluated versican, a dominantdermal CS/DS proteoglycan. Teh dermal expression of versican in the tissue did not show any difefrence betweenthe groups (Fig.n 5c, p = 0.83) and also on mRNA level, the expression of versican (p = 0.55) and the major enzymesinvolved in CS/DS synthesis and sulfation, namely the chondroitin 4-O-sulfotransferase-1 or carbohydrate sul-fotransferase-11 (CHST11, p = 0.40), the chondroitin sulfate synthase 1 (CHSY1, p = 0.09) and dermatan/chon-droitin sulfate 2-sulfotransferase or uronyl 2-sulfotrans-ferase (UST, p = 0.13) did not difefr (Fig.n 5d). However, itwas striking that the mRNA expression of versican and all CS/DS-GAG enzymes were apparently a bit higher inthe dialysis group compared to the healthy controls and the preemptive. Reduced dermal expression of HA-GAG was foundin hemodialysis patients compared to healthy donors (Fig.m 5e, p = 0.04). mRNA expression of hyaluronan syn-thase 1, 2 and 3 (HAS1-3) did not show signifciant dif-ferences between groups (Fig. 5f, HAS1; p = 0.18, HAS2;p = 0.32, HAS3; p = 0.10). Association studies Teh relationship between dermal sodium concentra-tions, sodium homeostasis, tissue remodeling (infalm-mation, fbirosis, lymphangiogenesis, and GAGs) andkidney function, was investigated by correlating these parameters in donors (n = 12) and preemptive recipients(n = 7) together as one group. Hemodialysis recipients were excluded from this analysis because the preopera-tive dialysis might have infulenced plasma and dermal sodium concentrations (see also Fig. 1a and c preemp-tive patients versus hemodialysis recipients). Figure 6 and Table 3 show these correlations and their relation to each othDeerr.mal sodium storage negatively correlated with the mRNA expression of CCL2, as a marker for infalm-mation (Fig.R 6; Tablen 3A; r = − 0.582; p = 0.02). Next to this, dermal sodium storage also correlated negativelywith one of the markers of sodium homeostasis, namely plasma sodium (Fig.t 6; Tables 3B; r = − 0.619; p = 0.04).Sodium homeostasis, refelcted by plasma sodium, and increased sodium intake, refelcted by increased sodiumexcretion values, shows positive correlations with param-eters for lymphangiogenesis (Fig. 6; Table 3C; r = 0.656;p = 0.03 and r = 0.709; p = 0.02, respectively). We did not find a correlation between dermal sodium and Fig. 4 Dermal lymphangiogenesis in kidney patients (recipients) and healthy individuals (donors). Immunohistochemical expression and quantifciation of Podoplanin + lymph vessels. Magnifciation ×200. a The mRNA expression of Podoplanin and VEGF-C on qRT-PCR analysis (b).Values are expressed in fold increase compared to the mean of the donors. Mann–Whitney and Kruskall Wallis were used to test difefrences between two or more groups. *p < 0.05 compared to donors or compared to non-dialysis (preemptive) patients lymphangiogenesis parameters (Fig. 6). Parameters forrenal function such as proteinuria and loss of GFR were positively correlated with markers for infalmmation(Fig. 6; Table 3D). Age and body weight showed positive correlations withdifefrent GAGs (Fig. 6; Table 3). GAGs showed mostly positive correlations with parameters for lymphangi-ogenesis, fbirosis and infalmmation (Fig. 6; Table 3). Discussion In this study, we showed that dermal non-osmoticsodium storage is not increased in patients with chronic kidney disease, but that kidney disease plays a part inthe interplay between dermal sodium storage, sodium homeostasis and dermal tissue remodeling. In the lastdecade, the classic paradigm of sodium handling has been questioned and widely investigated [2, 4, 19]. Tehsestudies have shown that extra-renal non-osmotic sodium storage in skin, cartilage and bone plays an importantrole in maintaining a balanced plasma sodium level.Our data indicate that sodium homeostasis refelcted byplasma sodium and increased sodium intake refelcted by increased sodium excretion, associates with dermallymph vessel formation and loss of dermal sodium stor-age capacity, whereas kidney failure associates with dermal infalmmation. We also show that dialysis furtherinfulences dermal tissue remodeling. In this current study, human dermal sodium concentra-tions were determined by atomic absorption spectros-copy. However, we did not find signifciant difefrencesin dermal sodium concentration between healthy indi-viduals and kidney patients. Plasma sodium levels weresignifciantly lower in kidney patients on hemodialysis compared to the healthy donors. Tish suggests that thelower plasma sodium levels were the result of hemodialy-sis prior to surgery. In a previous animal study in whichrats received a high sodium diet during 4lweeks, we did show a signifciant increase in dermal sodium concen-tration compared to controls on a normal diet using the same spectroscopy technique [6]. Teh fact that we werenot able to show any signifciant difefrences during the current study might be explained by the fact that thedonors and kidney patients were not on a high sodium diet in a controlled manner. Other research groups inves-tigated sodium storage using a sodium-MRI technique in healthy volunteers, hypertensive patients and patients onhemodialysis [5, 20]. Such studies have shown increased dermal sodium storage in elderly, males, hypertensivepatients and patients on hemodialysis [1, 20, 21]. Dahl-mann et al. showed that hemodialysis treatment is able Fig.5 Derma l GAG s in kidney patients (r ec i pient s ) and h e a l thy in d i viduals (don o r s ). I mm u no h is t oche mi cal expression a n d quan tif icatio n of GAGs and ver s ica n and mR N A expression of enzymes i nvolved i n t h e s yn th esi s of H S-GAG (a,b), C S /D S -GAG (c, d) a n d H A-GAG (e, f ). Photos:ma g n if i cation×200. F or q R T-P CR data , va lu es a r e expressed in fold increase compa r ed t o t h e m ea n of t h e donors. Man n -W h itney and Krusk a ll Wallis we r e u sed t o tes t dif f erences be t ween two or more groups.*p<0.05 tissue remodeling responses. Green arrows indicate positive associations, red arrows indicate negative associations to mobilize sodium and water from the non-osmoticstorage sites (skin and muscle), therefore lowering the dermal sodium concentration in these patients. Tehysuggest that by reducing the intravascular volume dur-ing adequate hemodialysis, dermal sodium levels can beremained stable [21]. Teh dialysis patients in our study,received hemodialysis prior to the OR, which makes itpossible that the non-osmotic sodium had been mobi-lized already and therefore skin sodium concentrationsreturned to control values. hemodialysis have increased skin sodium storage and areable to mobilize skin sodium during hemodialysis. Teh presence and maintenance of an adequate lymphatic net-work to accommodate this mobilization of sodium and water could therefore be benefciial. Next to infalmmation and lymphangiogenesis, we alsoevaluated fbirotic changes in the skin. In the skin biopsies of patients on hemodialysis we saw an increased mRNAexpression of collagen I. Interestingly, Kopp et al. showed increased dermal sodium concentration in the fbiroticskin of systemic sclerosis patients [27]. We suggest that the difefrences in dermal fbirosis might be too small toresult in difefrences in sodium storage. Fibrosis emerges from the accumulation of extracel-lular matrix. Fibroblasts play a major role by releasing collagens, but also reasonable amounts of proteoglycansand glycosaminoglycans (GAGs). Because GAGs are suggested to bind sodium in a non-osmotic fashion, wetherefore took a closer look to the involvement of GAGs in the skin of kidney patients [28–30]. We investigatedthree groups of GAGS, namely heparin/heparan sulfate (HS-GAGs), chondroitin sulfate/dermatan sulfate (CS/DS-GAG) and hyaluronic acid (HA-GAG). For CS/DS-GAG we did not find any signifciant difefrences betweenhealthy controls and kidney patients. For HS-GAG, the patients on hemodialysis showed increased mRNAexpression of NDST1, an enzyme involved in the sulfa-tion of heparan sulfate and therefore altering it’s biologi-cal properties and possibly their sodium binding capacity.For HA-GAG we found a signifciantly lower expressionin patients on dialysis, which might be a direct efefct of reducing the intravascular volume [21]. Table 3 Correlations between clinical data, tissue remodeling responses, and dermal sodium Variables p-value Variables R A. Dietary sodium intake and lymphangiogenesis Plasma sodium vs. podoplanin expression 0.656 0.028 Urine sodium vs. podoplanin expression 0.709 0.022 B. Dietary sodium intake and dermal sodium Plasma sodium vs. dermal sodium concentration − 0.619 0.042 C. Obesity, age and proteoglycans Age vs. mRNA expression of HAS3 0.530 0.042 Body weight vs. hyaluronan expression 0.503 0.047 BMI vs. versican expression 0.562 0.024 BMI vs. mRNA expression of CHST11 0.589 0.021 D. Proteoglycans and lymphangiogenesis mRNA expression of CHSY1 vs. mRNA expression of VEGF-C 0.571 0.026 mRNA expression of CHSY1 vs. mRNA expression of podoplanin 0.546 0.035 mRNA expression of UST vs. mRNA expression of VEGF-C 0.679 0.005 mRNA expression of UST vs. mRNA expression of Podoplanin 0.625 0.001 mRNA expression of HAS2 vs. mRNA expression of VEGF-C 0.757 0.001 mRNA expression of VCAN vs. mRNA expression of podoplanin 0.600 0.018 mRNA expression of NDST1 vs. mRNA expression of podoplanin 0.704 0.003 mRNA expression of CHST11 vs. mRNA expression of podoplanin 0.671 0.006 E. Proteoglycans and fibrosis mRNA expression of VCAN vs. mRNA expression of collagen I 0.557 0.031 mRNA expression of NDST1 vs. mRNA expression of collagen I 0.629 0.012 mRNA expression of CHST11 vs. mRNA expression of collagen I 0.546 0.035 mRNA expression of UST vs. mRNA expression of collagen I 0.589 0.021 mRNA expression of HS6ST1 vs. mRNA expression of collagen I 0.514 0.050 F. Proteoglycans and infalmmation mRNA expression of HSPE vs. CD68 expression 0.539 0.038 mRNA expression of HAS2 vs. MCP1 expression − 0.554 0.032 mRNA expression of UST vs. MCP1 expression − 0.621 0.013 Heparan sulfate expression (JM403) vs. CD3 expression 0.518 0.048 G. Renal failure and infalmmation Proteinuria vs. CD68 expression 0.730 0.007 Proteinuria vs. MCP-1 expression 0.614 0.034 Systolic blood pressure vs. CD68 expression 0.649 0.007 eGFR vs. CD68 expression 0.577 0.019 H. Dermal sodium and infalmmation Dermal sodium storage vs. mRNA expression of CCL2 − 0.582 0.023 Correlations are performed on the Z-scores of the values of donors and preemptive patients In order to unravel the possible interplay between allabove described phenomena, we performed association studies. Our data indicates that kidney disease and der-mal sodium associates with dermal infalmmation, while sodium homeostasis and sodium intake (refelcted byincreased plasma sodium and sodium excretion, respec-tively) is mainly associated with lymph vessel density inthe skin. We were not able to find a direct link between infalmmation and lymphangiogenesis in this study. Bothloss of kidney function, as well as an increased sodium We acknowledge that the present study has its limita-tions. Firstly, the groups were very small, since we wanted to investigate if we were able to find any changes between the groups. While both the nitrogen and the sodiummeasurements are sensitive and robust, the margin of errors is increasing when using smaller size skin biopsiessince both sodium and nitrogen are calculated per mg dry weight. Another disadvantage of the small group sizes isthe inability to perform multivariable linear regression or Cox regression analyses. Next to this, we cannot rule outany efefct of the immunosuppressive medication that has been given to the kidney patients pre-OR. While kidneypatients did show more tissue remodeling and therefore infalmmation, the difefrences with the healthy groupmight have been more outspoken without immunosup-pressive medication. Jantsch et al. have shown previouslythat antibiotics (Gentamycin) reduces sodium storage in the skin by in vitro [33]. In our study we used Cefazolinand Metronidazole in a in vivo model, namely humans,who were all given prophylactic antibiotic treatmentprior to surgery (30 min before incision). While we do not expect the antibiotics to have such an direct efefcton skin sodium concentrations whitin 30 min in a physi-ological model, in theory, we can not exclude that anti-biotic profylaxis in all patients explains why there were no difefrences found in skin sodium concentrations. Tehsame holds true for Mannitol, an osmotic diuretic, given maximum 30 min prior to incision. While it is theoreti-cally possible that its diuretic properties increased uri-nary sodium excretion, we do not expect this to havemajor efefcts on non-osmotic sodium binding in the skin,in 30 min from infusion to taking the skin biopsies rightafter incision. Teh group of Titze have shown signifciant correlationsbetween gender, age and skin sodium concentrations [34, 35]. In this study, we tried to age-match patients anddonors as much as possible; however, in transplantation donors are selected based on HLA-type and health. Wethus could not avoid patients to be signifciantly older compared to donors. However, Wang etgal. showed a sig-nifciant increase of skin sodium concentration in elderly people compared to younger people [34]. Since we didnot see a signifciant difefrence in skin sodium concentra-tion of donors and patients, we do not expect that whencorrected for age, patients would have lower skin sodium content compared to their healthy counterparts. Tehrewere no signifciant difefrences in age and gender between the patient groups themselves (preemptive and dialysispatients). Future studies should take into account the possible efefcts of gender and age on skin sodium con-centration in transplant patients. While we did not find a signifciant difefrence in skinsodium concentrations, we did find difefrences in lym-phangiogenesis and infalmmation. Difefrent pathwaysmight explain these difefrences and other immunologi-cal pathways could be induced or tempered by years of chronic kidney disease and/or dialysis [36]. One inter-esting alternative factor of importance in CKD, is the pathway of uremic infalmmation. Uremic specifci causessuch as abnormalities of the phosphate-Klotho axis play a crucial role in CKD, having a direct efefct on cellular andtissue function [37, 38]. Furthermore, recent studies have shown indoxyl sulfate (IS) to be one of the most potenturemic toxins involved in CKD progression, by induc-ing infalmmation and oxidative stress [39, 40]. Nakanoet al. even showed that clinically relevant concentrations of indoxyl sulfate induced proinfalmmatory responsesof macrophages and the infulenced the roles of organic anion transporters and organic anion transporting poly-peptides [39]. Next to uremic infalmmation, the innate immunesystem is also known to play a crucial role in disease progression in CKD. While we hypothesise that gly-cosaminoglycans are able to bind sodium non-osmot-ically, our group has also shown that they can interactwith complement factors [41]. Poppelaars et al. investi-gated the role of complement specifcially in patients onhemodialysis. Tehir group showed a complement medi-ated increased cardiovascular risk in dialysis patientsand experimental complement inhibition revealed a pro-infalmmatory response secondary to complementactivation [16, 42, 43]. Tish might explain why the most profound difefrences in skin lymphatic vessels, GAGsand infalmmation in our study were found in the dialy-sis patients. Further research is warranted to investigatethese alternative (or parallel) processes in renal trans-plant patients. We used unique material of chronic kidney diseasepatients; both hemodialysis patients and preemptive patients before transplantation and their age-matchedhealthy donors. It’s the frist study investigating difefr-ences in dermal sodium concentration, sodium homeo-stasis and tissue remodeling in these groups of ESRD patients. With our spectroscopy technique we were ableto use small skin biopsies and objectively quantify the exact sodium concentration, finding a robust way tomeasure sodium in the skin. We performed an extensive analysis comparing dermal sodium concentrations, withtissue remodeling and difefrent groups of GAGs, creating a starting point for further research. Conclusion In conclusion, our data suggest that there is an inter-play among dermal sodium storage, sodium homeo-stasis (refelcted by plasma sodium) and sodium intake(refelcted by sodium excretion), dermal tissue remod-eling and kidney function, although the causal rela-tionships and GAG involvement is not clear from our work. Teh exact mechanisms behind these phenomena Abbreviations CD68/ED1: cluster of difefrentiation 68, pan-macrophage marker; CD3:cluster of difefrentiation 3 (T-cell); eGFR: estimated glomerular flitration rate;N-Sulfation: nitrogen-sulfation; O-Sulfation: oxygen-sulfation; ESRD: end-stage renal disease; RRT : renal replacement therapy; CKD: chronic kidney disease;GAG : glycosaminoglycan; HS: heparan sulfate; XYLT-1: xylosyltransferase 1;HD: hemodialysis; OR: operation; WMO: Wet Maatschappelijke Ondersteuning (Dutch law); METc: Medisch Etische Toetsings Commissie (Dutch Ethical Com-mittee); HLA: human leukocyte antigen; PBS/BSA: phosphate bufefr saline/bovine serum albumin; α-SMA: alpha-smooth muscle actin; HABP: hyaluronan binding protein; JM403: anti-heparan sulfate antibody; MCP-1/CCL: monocyte chemoattractant protein-1; Ig HRP: immunoglobulin horseradish peroxidase;DAB/AEC: aminoethylcarbazole/peroxidase substrate 3,3′-diaminobenzidine;DAPI: 4′,6′-diamidino-2-phenylindole Hydrochloride; RT: reverse transcriptase;VCAM1: vascular cell adhesion protein 1; VEGF-C: vascular endothelial growth factor C; CS: chondroitin sulfate; DS: dermatan sulfate; HA: hyaluronic acid;NDST1: N-deacetylase, N-sulfotransferase-1; HS6OST1: heparan sulfate 6-O-sul-fotransferase-1; CHST11: chondroitin 4-O-sulfotransferase-1 or carbohydrate sulfotransferase-11; CHSY1: chondroitin sulfate synthase 1; UST: uronyl 2-sul-fotransferase; HAS: hyaluronan synthase; VCAN: versican; IS: indoxyl sulfate. Authors’ contributions Conception and design of research: RSH, GN, WHAdJ, SJLB, RAP, JvdB.Performed experiments: RSH, KAS, TTRS. Analyzed data: RSH, KAS, MvL. Inter-preted results of experiments: RSH, MvL, SJLB, GN, RAP, JvdB. Prepared figures:RSH. Drafted manuscript: RSH. Edited and revised manuscript: RSH, MvL, SJLB,WHAdJ, GN, RAP, JvdB. All authors read and approved the final manuscript. Author details 1 Department of Internal Medicine, Division of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.2 Department of Surgery, Division of Transplantation Surgery, University Medi-cal Center Groningen, University of Groningen, Groningen, The Netherlands.3 Present Address: Surgical Department, Martini Hospital Groningen, Gronin- gen, The Netherlands. 4 Department of Laboratory Medicine, University Medi-cal Center Groningen, University of Groningen, Groningen, The Netherlands. Acknowledgements The authors thank Wendy Dam for her help with the experiments. Competing interests The authors declare that they have no competing interests. Availability of data and materials The authors agree to the terms of the BioMed Central Copyright and License Agreement and, where applicable, Open Data policy. Consent for publication All authors of the manuscript have read and agreed to its content and are accountable for all aspects of the accuracy and integrity of the manuscript in accordance with ICMJE criteria. Ethics approval and consent to participate The study protocol is in accordance with the Dutch Medical Research Involving Human Subjects Act (WMO), and approved by the Medical Ethics Committee of the University Medical Center Groningen (METc 2014/077). All procedures were conducted in accordance with the declarations of Helsinki and Istanbul. Funding None of the authors received additional funding for this project. Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in pub-lished maps and institutional aflifatiions. References 1. Titze J, Rakova N, Kopp C, Dahlmann A, Jantsch J, Luft FC. Balancing wob-bles in the body sodium. Nephrol Dial Transpl. 2016;31:1078–81. 2. Hofmeister LH, Perisic S, Titze J. Tissue sodium storage: evidence for kid-ney-like extrarenal countercurrent systems? Eur J Physiol. 2015;467:551–8. 3. Olde Engberink RHG, Rorije NMG, van der Homan Heide JJ, van den Born B-JH, Vogt L. Role of the vascular wall in sodium homeostasis and salt sensitivity. J Am Soc Nephrol. 2015;26:777–83. 4. Titze J. Water-free sodium accumulation. Semin Dial. 2009;22:253–5. 5. Linz P, Santoro D, Renz W, Rieger J, Ruehle A, Ruf fJ, et al. Skin sodiummeasured with 23Na MRI at 7.0 T. NMR Biomed. 2015;28:54–62. 6. Hijmans RS, Shrestha P, Sarpong KA, Yazdani S, el Masri R, de Jong WHA,et al. High sodium diet converts renal proteoglycans into pro-inflamma-tory mediators in rats. PLoS ONE. 2017;12:e0178940. 7. Fischereder M, Michalke B, Schmöckel E, Habicht A, Kunisch R, Pavelic I,et al. Sodium storage in human tissues is mediated by glycosaminogly-can expression. Am J Physiol Physiol. 2017;313:F319–25. 8. Celie JWAM, Rutjes NWP, Keuning ED, Soininen R, Heljasvaara R, Pihla-janiemi T, et al. Subendothelial heparan sulfate proteoglycans become major l-selectin and monocyte chemoattractant protein-1 ligands upon renal ischemia/reperfusion. Am J Pathol. 2007;170:1865–78. 9. Titze J, Lang R, Ilies C, Schwind KH, Kirsch KA, Dietsch P, et al. Osmoti-cally inactive skin Na+ storage in rats. Am J Physiol Ren Physiol.2003;285:1108–17. 10. Schafhfulber M, Volpi N, Dahlmann A, Hilgers KF, Maccari F, Dietsch P, et al.Mobilization of osmotically inactive Na+ by growth and by dietary salt restriction in rats. Am J Physiol Ren Physiol. 2007;292:1490–500. 11. Slagman MCJ, Kwakernaak AJ, Yazdani S, Laverman GD, van den Born J,Titze J, et al. Vascular endothelial growth factor C levels are modulated by dietary salt intake in proteinuric chronic kidney disease patients and in healthy subjects. Nephrol Dial Transplant. 2012;27:978–82. 12. Brück K, Stel VS, Gambaro G, Hallan S, Volzke H, Arnlo VJ, et al. CKD preva-lence varies across the european general population. J Am Soc Nephrol.2016;27:2135–47. 13. Abramowicz D, Hazzan M, Maggiore U, Peruzzi L, Cochat P, Oberbauer R, et al. Does pre-emptive transplantation versus post start of dialysis transplantation with a kidney from a living donor improve outcomes after transplantation? A systematic literature review and position state-ment by the Descartes Working Group and ERBP. Nephrol Dial Transplant.2016;31:691–7. 14. Witczak BJ, Leivestad T, Line PD, Holdaas H, Reisaeter AV, Jenssen TG, et al.Experience from an active preemptive kidney transplantation Pro-gram—809 cases revisited. Transplantation. 2009;88:672–7. 15. Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griftfh iJL, et al.Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–15. 16. Poppelaars F, Faria B, da Gaya Costa M, Franssen CFM, van Son WJ, Berger SP, et al. The Complement System in Dialysis: a Forgotten Story? Front Immunol. 2018;9:71. 17. Jofre R, Rodriguez-Benitez P, Lopez-Gomez JM, Perez-Garcia R. Inflam-matory syndrome in patients on hemodialysis. J Am Soc Nephrol.2006;17:S274–80. 18. van den Born J, Gunnarsson K, Bakker MA, Kjellén L, Kusche-Gullberg M,Maccarana M, et al. Presence of N-unsubstituted glucosamine units in native heparan sulfate revealed by a monoclonal antibody. J Biol Chem.1995;270:31303–9. 19. Nguyen MK, Kurtz I. Is the osmotically inactive sodium storage pool fixed or variable? J Appl Physiol. 2007;90095:445–7. 20. Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Müller DN, et al.23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension. 2015;61:635–40. 21. Dahlmann A, Dörfelt K, Eicher F, Linz P, Kopp C, Mössinger I, et al.Magnetic resonance-determined sodium removal from tissue stores in hemodialysis patients. Kidney Int. 2015;87:434–41. 22. Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothe-lial growth factor (VEGF) in renal pathophysiology. Kidney Int.2004;65:2003–17. 23. Nykänen AI, Sandelin H, Krebs R, Keränen MAI, Tuuminen R, Kärpänen T, et al. Targeting lymphatic vessel activation and CCL21 production by vascular endothelial growth factor receptor-3 inhibition has novel immunomodulatory and antiarteriosclerotic efefcts in cardiac allografts.Circulation. 2010;121:1413–22. 24. Yazdani S, Navis GJ, Hillebrands JL, van Goor H, van den Born J. Lym-phangiogenesis in renal diseases: passive bystander or active partici-pant?. Med: Expert Rev Mol; 2014. 25. Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Derer W, et al.Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent bufefring mechanism.Nat Med. 2009;15:545–52. 26. Selvarajah V, Mäki-Petäjä KM, Pedro L, Bruggraber SFA, Burling K, Good-hart AK, et al. Novel mechanism for bufefring dietary salt in humans novelty and significance. Hypertension. 2017;70:930–7. 27. Kopp C, Beyer C, Linz P, Dahlmann A, Hammon M, Jantsch J, et al. Na+deposition in the fibrotic skin of systemic sclerosis patients detected by23Na-magnetic resonance imaging. Rheumatology. 2016;56:kew371. 28. Silver L, Christie R, Dahl L. Connective tissue as a major sodium reservoir.Fed. Proc. 1957;16:372. 29. Sugár D, Agócs R, Tatár E, Tóth G, Horváth P, Sulyok E, et al. The contribu-tion of skin glycosaminoglycans to the regulation of sodium homeostasis in rats. Physiol Res. 2017;67:777–85. 30. Farber SJ, Schubert M, Schuster N. The binding of cations by chondroitin sulfate. J Clin Invest. 1957;36:1715–22. 31. Titze J, Shakibaei M, Schafhfulber M, Schulze-tanzil G, Porst M, Schwind KH, et al. Glycosaminoglycan polymerization may enable osmoti-cally inactive Na+ storage in the skin. Am J Physiol Hear Circ Physiol.2004;287:203–8. 32. Schnabelrauch M, Scharnweber D, Schiller J. Sulfated glycosaminogly-cans as promising artificial extracellular matrix components to improve the regeneration of tissues. Curr Med Chem. 2013;20:2501–23. 33. Jantsch J, Schatz V, Friedrich D, Schröder A, Kopp C, Siegert I, et al.Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab.2015;21:493–501. 34. Wang P, Deger MS, Kang H, Ikizler TA, Titze J, Gore JC. Sex difefrences in sodium deposition in human muscle and skin. Magn Reson Imaging.2017;36:93–7. 35. Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Muller DN, et al.23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension. 2013;61:635–40. 36. Mihai S, Codrici E, Popescu ID, Enciu A-M, Albulescu L, Necula LG, et al.Inflammation-related mechanisms in chronic kidney disease prediction,progression, and outcome. J Immunol Res. 2018;2018:1–16. 37. Kooman JP, Dekker MJ, Usvyat LA, Kotanko P, van der Sande FM, Schalk-wijk CG, et al. Inflammation and premature aging in advanced chronic kidney disease. Am J Physiol Physiol. 2017;313:F938–50. 38. Kuro-o M. The Klotho proteins in health and disease. Nat Rev Nephrol.2019;15:27–44. 39. Nakano T, Katsuki S, Chen M, Decano JL, Halu A, Lee LH, et al. Uremic toxin indoxyl sulfate promotes proinflammatory macrophage activa-tion via the interplay of OATP2B1 and Dll4-notch signaling. Circulation.2019;139:78–96. 41. Zaferani A, Talsma D, Richter MKS, Daha MR, Navis GJ, Seelen MA, et al.Heparin/heparan sulphate interactions with complement–a possible target for reduction of renal function loss? Nephrol Dial Transplant.2014;29:515–22. 42. Poppelaars F, da Gaya Costa M, Berger SP, Assa S, Meter-Arkema AH, Daha MR, et al. Strong predictive value of mannose-binding lectin levels for cardiovascular risk of hemodialysis patients. J Transl Med. 2016;14:236. 43. Poppelaars F, da Gaya Costa M, Faria B, Berger SP, Assa S, Daha MR, et al.Intradialytic complement activation precedes the development of car-diovascular events in hemodialysis patients. Front Immunol. 2018;9:2070. Ready to submit your research? Choose BMC and benefit from: · fast , co n venient on l in e sub m ission · th o r o u gh peer review by experie n ce d researchers i n you r fi eld · r ap id publication on acceptance · s up p o rt for resear c h d a ta, inc lu din g large and comp le x d at a t ype s · gold Open Access w h ich fosters wider col l a b oration an d i ncreased citations · m aximum visibi l i ty f or yo u r r esea r ch : over 100M website views per yea r At BMC , research is always i n progress . Learn more biomedcentral .com/s u bmiss i ons BMC
确定






还剩13页未读,是否继续阅读?
中国格哈特为您提供《肾脏患者的皮肤组织蛋白质含量的检测》,该方案主要用于内脏器官中生化检验检测,参考标准--,《肾脏患者的皮肤组织蛋白质含量的检测》用到的仪器有格哈特杜马斯定氮仪DT N Pro
推荐专场















