
方案详情
文
From each patient one biopsy was thawed. The thawed tissue was cut into two comparable parts and the wet weight of both parts was measured. Both parts were dried overnight at 100?C, after which they were weighed again in order to measure dry weights. One part was dissolved in nitric acid and consequentially diluted, and sodium content of this solution was measured by way of flame spectrometry. The other part was ashed to measure nitrogen via thermal conduction (Dumatherm Nitrogen/Protein analyzer, C. Gerhardt). Nitrogen content of the ashed biopsies was measured as a parameter for protein content of the tissue. This protein content was used to correct for subcutaneous fat, since the assumption was that sodium is stored in skin instead of in fat, and protein is largely absent from fat tissue. Sodium content of the skin is expressed as mmol sodium per mg dry
weight or per mg protein.
从每个患者中解冻一个活检。将解冻的组织切割成两个可比较的部分,并测量两个部分的湿重。两个部分在100摄氏度干燥过夜,之后再次称重以测量干重。将一部分溶解在硝酸中并随后稀释,并且通过火焰光谱法测量该溶液的钠含量。另一部分用于通过热传导测量氮(格哈特公司 杜马森Dumatherm燃烧法全自动氮/蛋白质测定仪)。测量灰化后的活检的氮含量作为组织蛋白质含量的参数。这种蛋白质含量用于皮下脂肪,因为假设钠储存在皮肤中而不是脂肪中,并且脂肪组织中基本上不存在蛋白质。皮肤的钠含量表示为每mg干重或每mg蛋白质的mmol钠。
方案详情

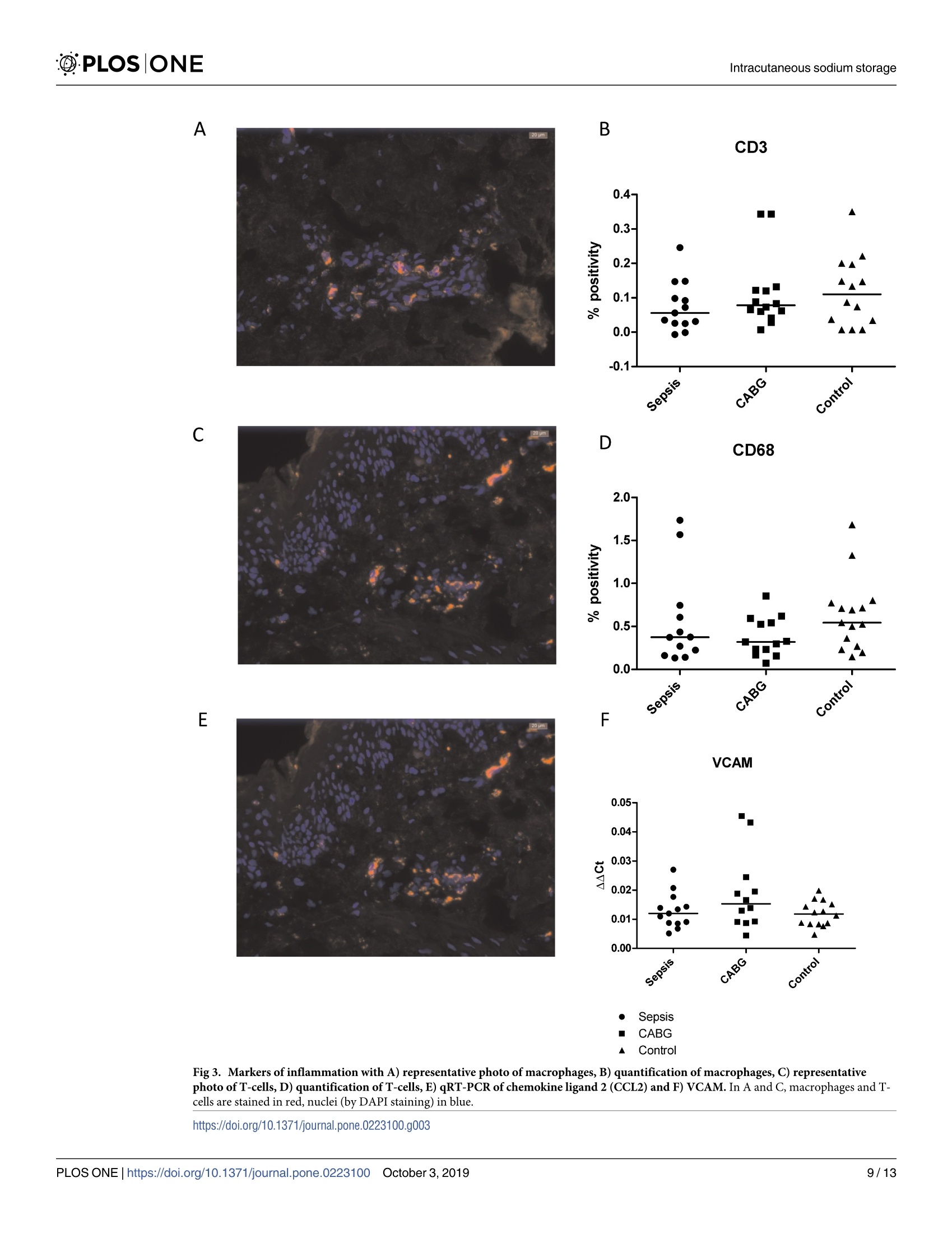
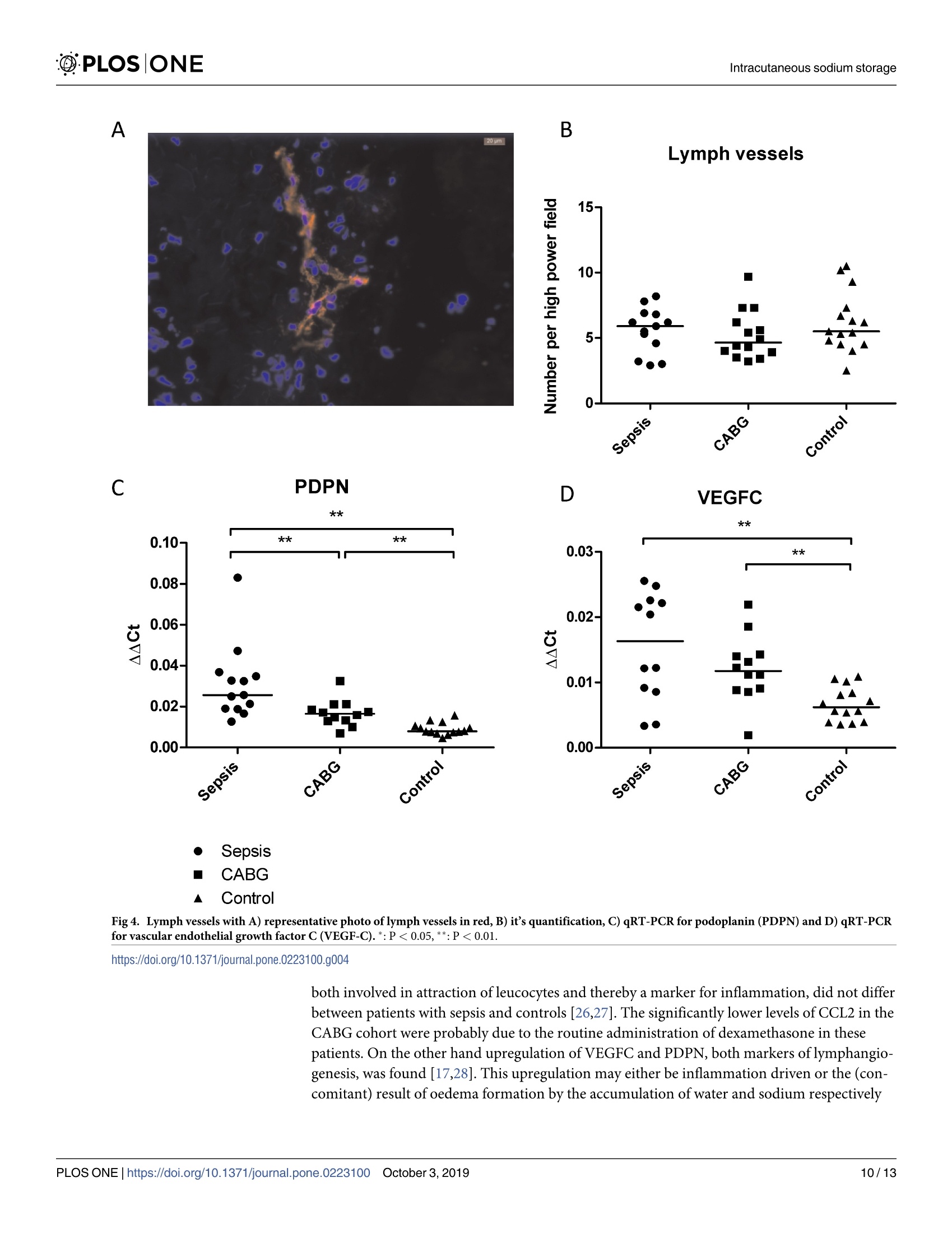
The development of ICU-acquired sodium disturbances is not fully understood. Alterations in non-osmotic skin sodium storage, hypothetically inflammation-driven, could play a role. To investigate this in critically ill patients we conducted a patient-control study with skin punch biopsies in patients with sepsis (n = 15), after coronary artery bypass grafting (CABG, n = 15) and undergoing total hip arthroplasty (THA-controls, n = 15) respectively, together representing a range in severity of systemic inflammation. Biopsies were taken within 24 hours (sepsis) and within 2 hours (CABG) after ICU-admission, and prior to arthroplasty. Biopsies were analysed for sodium content. In addition immunostainings and quantitative real time PCR were performed. The primary aim of this study was to detect possible differences in amounts of cutaneous sodium. The secondary aims were to quantify inflammation and lymphangiogenesis with concomitant markers. The highest amounts of both water and sodium were found in patients with sepsis, with slightly lower values after CABG and the lowest amounts in THA-controls. Correlation between water and sodium was 0.5 (p<0.01). In skin biopsies in all groups comparable amounts of macrophages, T-cells and lymph vessels were found. In all groups comparable expression of inflammation markers were found. However, higher mRNA transcript expression levels of markers of lymphangiogenesis were found in patients with sepsis and after CABG. The conjoint accumulation of water and sodium points towards oedema formation. However, the correlation coefficient of 0.5 leaves room for alternative explanations, including non-osmotic sodium storage. No signs of dermal inflammation were found, but upregulation of markers of lymphangiogenesis could indicate future lymphangiogenesis.ICU获得的钠紊乱的发展尚未完全了解。非渗透皮肤钠储存的变化,假设是炎症驱动的,可能起作用。为了在危重患者中研究这一点,我们分别对脓毒症患者(n=15),冠状动脉旁路移植(CABG,n=15)和进行全髋关节成形术(THA对照,n=15)进行了皮肤穿刺活检的患者对照研究,共同代表全身炎症严重程度的范围。在ICU入院后24小时内(败血症)和2小时内(CABG)以及关节成形术前进行活检。活检分析钠含量。此外,进行免疫染色和定量实时PCR。本研究的主要目的是检测皮肤钠量的可能差异。次要目的是用伴随标志物量化炎症和淋巴管生成。在脓毒症患者中发现水和钠的最高量,CABG后值略低,THA对照中的最低量。水与钠的相关性为0.5(p<0.01)。在滑雪@PLOS|ONE @PLOSIONEIntracutaneous sodium storage 门OPEN ACCESS Citation: van IJzendoorn M. van den Born J.Hijmans R, Bodde R, Buter H, Dam W, et al.(2019)An observational study on intracutaneous sodiumstorage in intensive care patients and controls.PLoS ONE 14(10): e0223100. https://doi.org/10.1371/journal.pone.0223100 Editor: Linda L. Maerz, Yale University, UNITEDSTATES Received: April 16,2019 Accepted: September 13, 2019 Published: October 3,2019 Copyright: @ 2019 van IJzendoorn et al. This is anopen access article distributed under the terms ofthe Creative Commons Attribution License, whichpermits unrestricted use, distribution,andreproduction in any medium, provided the originalauthor and source are credited. Data Availability Statement: The raw databasefrom this study has been uploaded to Zenodo (DOI:10.5281/zenodo.3345288). Funding: Funding for laboratory fees concerninganalysis of the biopsies (measurements of sodiumand nitrogen, immunostainings and qRT-PCR) wasprovided by a local hospital fund (StichtingIntensive Care Onderzoek Friesland). Funders didnot play any role in the study design, datacollection and analysis, decision to publish or 重症监护患者和对照组皮内钠储存的观察研究 RESEARCH ARTICLE An observational study on intracutaneoussodium storage in intensive care patients andcontrols Marjolein van IJzendoorno1,2*, Jacob van den Born?, Ryanne Hijmans?, Rianne Bodde²Hanneke Buter, Wendy Dam², Peter Kingma, Gwendolyn Maes, Tsjitske van der Veen,Wierd Zijlstra’, Baukje Dijkstra, Gerjan Navis?, Christiaan Boerma1 1 Intensive Care, Medical Centre Leeuwarden, Friesland, the Netherlands, 2 Department of InternalMedicine-Nephrology, University Medical Centre Groningen, Groningen, the Netherlands, 3 Department ofOrthopaedic Surgery, Medical Centre Leeuwarden, Friesland, the Netherlands * vanijzendoorn@kpnmail.nl Abstract The development of ICU-acquired sodium disturbances is not fully understood. Alterationsin non-osmotic skin sodium storage, hypothetically inflammation-driven, could play a role.To investigate this in critically ill patients we conducted a patient-control study with skinpunch biopsies in patients with sepsis (n=15), after coronary artery bypass grafting (CABG,n = 15) and undergoing total hip arthroplasty (THA-controls,n=15) respectively, togetherrepresenting a range in severity of systemic inflammation. Biopsies were taken within 24hours (sepsis) and within 2 hours (CABG) after ICU-admission, and prior to arthroplasty.Biopsies were analysed for sodium content. In addition immunostainings and quantitativereal time PCR were performed. The primary aim of this study was to detect possible differ-ences in amounts of cutaneous sodium. The secondary aims were to quantify inflammationand lymphangiogenesis with concomitant markers. The highest amounts of both water andsodium were found in patients with sepsis, with slightly lower values after CABG and thelowest amounts in THA-controls. Correlation between water and sodium was 0.5 (p<0.01).In skin biopsies in all groups comparable amounts of macrophages, T-cells and lymph ves-sels were found. In all groups comparable expression of inflammation markers were found.However, higher mRNA transcript expression levels of markers of lymphangiogenesis werefound in patients with sepsis and after CABG. The conjoint accumulation of water andsodium points towards oedema formation. However, the correlation coefficient of 0.5 leavesroom for alternative explanations, including non-osmotic sodium storage. No signs of dermalinflammation were found, but upregulation of markers of lymphangiogenesis could indicatefuture lymphangiogenesis. Introduction Different mechanisms are involved in human sodium homeostasis. Some of these mechanismsare well-known, such as for instance fluid intake by stimulation of thirst and renal sodium and preparation of the manuscript and in this way noconflict of interest applies. Competing interests: The authors have declaredthat no competing interests exist. water retention and excretion. Lately, newly-found evidence has indicated that sodium storagemight also play an important role in this process. Sodium storage occurs with concomitantwater accumulation (osmotically active sodium storage), but it also occurs also non-osmoti-cally. This non-osmotically active storage takes place in the so called third compartment. Thiscompartment was already described early in the 20th century [1-3]. Skin is part of the third compartment. Recently Titze et al. demonstrated and extensivelydescribed a subcutaneous compartment where sodium is non-osmotically stored [4-7]. In thiscompartment sodium is non-osmotically bound to glycosaminoglycans (GAGs), by pathwaysinvolving macrophages as well as lymphangiogenesis [8]. Both macro1Cphages and lymphangio-genesis play a role in the process of inflammation [9]. Consequently, it is known that inflam-mation has modifying effects on GAGs [10-12]. Inflammation is common in critically illpatients, as are derangements in sodium homeostasis[13]. We hypothesize that inflammation in critically ill patients alters cutaneous sodium storageand thereby contributes to the development of sodium disorders. However, no literature aboutcutaneous sodium storage in critically ill patients in itself is available. Therefore we designed astudy to investigate cutaneous sodium storage in critically ill patients. In the present study wemeasured skin sodium content and inflammatory pathways in intensive care patients with sep-sis, in patients after coronary artery bypass grafting (CABG), and otherwise healthy patientsundergoing primary total hip arthroplasty (THA) for arthrosis (controls). These groups wereselected to represent a range of severity of inflammation, with the most severe cases beingfound in patients suffering from sepsis and the least severe ones in patients after CABG andabsent in THA-controls. Previous studies on intracutaneous sodium storage in humans made use of MRI to visualizeand assess sodium [7,14]. This technique requires MRI with a strong magnetic field with spe-cialized software and a specific coil, limiting its availability. In the experimental setting quanti-fication of sodium storage in full skin appeared to be successful [5]. However, this techniquerequires a substantial amount of tissue, which prompted us to make use of a recently developedmethod which allows for the quantifying of sodium concentration in far smaller tissue samples12,15]. The primary aim of this study was to detect differences in amounts of cutaneous sodiumstorage between patients with sepsis and the control groups. Said aim allowed us to divide ourinterest, making way for secondary aims which were as follows: to investigate the density oflymph capillaries, and presence and incidence of macrophage influx in skin biopsies of allaforementioned groups. Materials and methods Design and setting We conducted a single center observational patient-control study in three groups of patients.The primary study population consisted of patients with sepsis. All consecutive patients withsepsis admitted to the ICU were screened for their eligibility based on the in- and exclusioncriteria as presented in Table 1. Patients in this group were included within 24 hours afteradmission to our 20-bed mixed medical and surgical ICU. Sepsis was defined according to thesepsis-3 definition: patients had life-threatening organ dysfunction (increase in sequentialorgan failure assessment (SOFA) score of ≥ 2 points), caused by a dysregulated host responseto infection [16]. After inclusion of a patient with sepsis two control patients (1 CABG, 1 con-trol) were recruited, with matched gender and who fell within an age range not exceeding fiveyears either senior or junior. These criteria were based on the previous findings that intracuta-neous sodium storage is different between males and females and alters with ageing [17,18].In Inclusion Exclusion Sepsis group: 50-80 years Absence of both upper legs CABG and THA: 45-85 years Cutaneous disease that makes biopsy of healthy skin impossible Sepsis group: fulfilling the sepsis-3 criteria Use of dermatocorticosteroids on the total surface of both upper legswithin the previous 2 weeks Controls: no medical history of chronic and/ or systemic diseases Fully tattooed surface of both upper legs Use of diuretics in the previous month Current dependency on renal replacement therapy CABG: Coronary artery bypass grafting, THA: Total hip arthroplasty CABG: Coronary artery bypass grafting, THA: Total hip arthroplastyhttps://doi.org/10.1371/journal.pone.0223100.t001 all subjects two full-thickness punch skin biopsies (diameter 3mm) were performed in eitherthe right or left hip region. In patients with sepsis biopsies were obtained within 24 hours afterICU-admission. Patients with sepsis were initially resuscitated with early goal directed therapyfollowing the guidelines from the Surviving Sepsis Campaign. No corticosteroids were admin-istered. In patients that underwent CABG biopsies were taken within 2 hours after completionof the surgical procedure, primarily due to the fact that during this period patients were stillanesthetized. In orthopedic patients the study procedure was performed after induction ofregional or general anesthesia but before start of surgery. No complications of skin punchbiopsies were reported. Furthermore a spot urine sample and a blood sample were collected,with the latter one being used to measure levels of sodium, urea and creatinine. In urinesodium and creatinine were measured. Fractional excretion of sodium (FEna) was calculatedaccording to the following formula: Eq 1: Fractional sodium excretion uNa: urinary sodium concentration, sCreat: plasma creatinine concentration, sNa: serumsodium concentration, uCreat: urine creatinine concentration Informed consent was obtained from patients or their next of kin according to applicableDutch laws. In patients with sepsis informed consent was obtained after ICU-admission, incontrols this was already done previous to either a CABG or a hip replacement. All patientswere treated in accordance with the declaration of Helsinki. The study protocol was approvedby the local ethics board (RTPO, Regionale Toetsingscommissie Patientgebonden Onderzoek,NL 56729.099.16) and registered at clinicaltrials.gov (NCT02912299). The study was fundedby the Stichting Intensive Care Onderzoek Friesland. Data collection Several parameters were collected from all subjects: age, gender, prescribed drugs, medical his-tory, blood pressure before surgery or septic episode (if available), length, weight, serum andurine electrolytes and data concerning kidney function. In both patients with sepsis andpatients after CABG scores on severity of illness (APACHE IV and SOFA) were calculated.Furthermore, these same groups were used to perform bioelectrical impedance analyses (BIA)in order to estimate fluid status. Due to the known inaccuracy of the registration of adminis-tration of fluids in the emergency room and in conventional hospital wards, no data aboutactual fluid balances since admission were available. In patients suffering from sepsis, the source of sepsis and severity of illness were registered. Skin biopsies were weighed (XS204Analytical Balance, Mettler-Toledo International Inc, USA), after which they were packed perunit in tin cans and stored at -80°C. From the spot urine samples two 2ml cups were stored at-80C. These cups were stored in order to allow for the possibility of performing additionalanalyses. Samples were destroyed after completion of all analyses. Bioelectrical impedance analysis BIA is a non-invasive method to estimate body composition. Two electrodes are placed on botha hand and a foot on one side of the body. A data analyzer (BIA 101 Anniversary, Akern, Ger-many) produces an alternating current between these electrodes. Measured decrease in voltageand delay in flow of these current are measured as resistance (R), reactance (Xc) and phaseangle (PhA) [19]. From these values both fluid and nutritional state of a patient can be deducted.R and Xc are related to a patient’s length and the determination of PhA is gender dependent. Sodium measurement in skin biopsies From each patient one biopsy was thawed. The thawed tissue was cut into two comparableparts and the wet weight of both parts was measured. Both parts were dried overnight at 100°C, after which they were weighed again in order to measure dry weights. One part was dis-solved in nitric acid and consequentially diluted, and sodium content of this solution was mea-sured by way of flame spectrometry [12,15]. The other part was ashed to measure nitrogen viathermal conduction (DumathermNitrogen/Protein analyzer, C..Gerhardt UK Ltd Northamp-tonshire, UK). Nitrogen content of the ashed biopsies was measured as a parameter for proteincontent of the tissue. This protein content was used to correct for subcutaneous fat, since theassumption was that sodium is stored in skin instead of in fat, and protein is largely absentfrom fat tissue [20]. Sodium content of the skin is expressed as mmol sodium per mg dryweight or per mg protein. Staining and qRT-PCR The remaining biopsies were used for cryo sections using a cryostat (Leica CM1950). Thosesections were 4um for immunostainings and 5um for quantitative real time polymerase chainreactions (qRT-PCR). Sections for immunostainings were dried for one hour and thereafterstored at -80°C. Samples were stained for lymphatic endothelium (Podoplanin and lymph ves-sels), CD3+T-lymphocytes and macrophages (CD68). Details of the staining procedures aregiven in Table 2. Sections were assessed using a fluorescent microscope (Leica DM 400B). Pho-tos were taken with the Leica DFX345FX camera and LAS software. Counting of lymph vesselswas done blinded and manually by two researchers. T-cells and macrophages were analyzed bydigital image analyses using Image J. Sections for PCR were used for RNA isolation using theRneasy microkit (Qiagen, Venlo, The Netherlands) followed by complementary DNA (cDNA)synthesis using the Quantitect kit (Qiagen). This cDNA was used for qRT-PCR with differentprimer pairs. Used primers were chemokine ligand 2 (CCL2), vascular cell adhesion molecule(VCAM), vascular endothelial growth factor (VEGFC), podoplanin (PDPN) and the house-keeping gene B-actin. Details of the primers are given in Table 3. PCR was run using the Fas-tStart Universal Sybr green (ROX) master mix (Roche, Basel, Switzerland). Statistical analysis To our knowledge, skin sodium in critically ill patients was never investigated before, whichconsequently did not allow us to perform a power calculation. We expected small variations in @PLOSIONE Table 2. Details of the immunofluorescence stainings. Cells Pre-treatment First antibody Secondary and tertiary antibodies Goat anti-Rb IgG-HRP 1:100 in PBS/ Visualization T-cells 100% acetone fixation+0.03%H2O2 block+1% BSA block Rb anti-human CD3 1:200 inPBS/1%BSA (A04522 DAKO) 1%HS (DAKO)+Rb anti-goatIgG-HRP 1:100 in PBS/1%BSA(DAKO) Tetramethylrhodamine-TRITC 1:50 (PerkinElmer)+ DAPI 1:5000+ Cityfluormounting medium Macrophages 100% acetone fixation+0.03% HO, block+1% BSA block Mouse anti-human CD681:1000 in PBS/1%BSA (EBM11DAKO) Rb anti-mouse IgG-HRP 1:100 in PBS/1%HS (DAKO)+ Goat anti-RbIgG-HRP 1:100 in PBS/1%HS (DAKO) Tetramethylrhodamine-TRITC 1:50 (PerkinElmer)+DAPI 1:5000 +Cityfluormounting medium Lymph endothelial cells 100% acetone fixation+0.03% HO2 block+1% BSA block Mouse anti-human podoplanin1:100 in PBS/1%BSA (D240ThermoFisher) Rb anti-mouse IgG-HRP 1:100 in PBS/1%HS (DAKO)+Goat anti-Rb IgG-HRP 1:100 in PBS/1%HS (DAKO) Tetramethylrhodamine-TRITC 1:50 (PerkinElmer)+DAPI 1:5000 +Cityfluor mounting medium https://doi.org/10.1371/journal.pone.0223100.t002 the investigated variables within groups and rather more considerable variations between thethree groups as separate entities. In previous animal studies, in which skin sodium was investi-gated, group sizes ranged from 6 to 20 animals per group [5,21-24]. Because of this study weexpected that 15 patients per group would be sufficient in order to detect statistically signifi-cant differences. Statistical analyses were performed with SPSS 24 and 25 (IBM, New York,USA). Due to the small populations we used non-parametric tests (Mann-Whitney U test,Kruskal-Wallis test) to compare groups. Results are expressed as median with interquartileranges. The correlation between sNa and cutaneous sodium concentration (cNa) was testedwith chi-square and Spearman’s r coefficient (p) and corrected for group analysis. For all statis-tical analyses a p-value of <0.05 is considered statistically significant. ResultsPatient characteristics Patients were included in the three aforementioned groups between November 2016 and Sep-tember 2017. Characteristics of the included patients are given in Table 4. This table showsthat patients with sepsis were severely ill, according to their APACHE IV and SOFA scores. Allincluded patients had normal sNa. According to the difference in (fractional) sodium excre-tion patients with sepsis did retain sodium when compared to patients after CABG and THA-controls. Patients with sepsis had markedly lower urinary sodium excretion with concomitantlow FEna' Skin sodium and water content and their clinical correlates Water and sodium content were higher both in patients with sepsis and CABG patients incomparison with THA-controls (Fig 1). Water content was 50 [38-58]% in patients with sep-sis, 41 [36-53]% in patients after CABG and 27 [18-44]%(p<0.01) in THA-controls. Sodium Table 3. Primers used for qRT-PCR. Process Primer Forward Reverse Inflammation CCL2 5'-AGACTAACCCAGAAACATCC-3' 5\-ATTGATTGCATCTGGCTG-3' VCAM 5'-TCCTGAGCTTCTCGTGCTCTATT-3' 5`-TGACCCCTTCATGTTGGCTT-3' Lymphangio-genesis VEGFC 5'-CTGGCTCAGGAAGATTTTATG-3 5'-TGTTTTTACAGACACACTGG-3' PDPN 5'-AAGATGGTTTGTCAACAGTG-3' 5'-GTACCTTCCCGACATTTTTC-3' Housekeeping Gene B-actine 5'- CCAACCGCGAGAAGATGA-3' 5'- CCAGAGGCGTACAGGGATAG- 3' https://doi.org/10.1371/journal.pone.0223100.t003 Table 4. Characteristics of included patients. Patients with sepsis included within 24 hours after ICU admission, patients after CABG included within 2 hours afterICU admission and patients with hip replacement included before start of surgery. Variable Sepsis(n=15) CABG(n=15) Hip replacement (n=15) P-value Age, years 63[60-75] 68[63-74] 63[58-72] 0.33 Male,n (%) 9(60) 9(60) 9(60) 1 BMI 2422-26 28[25-30] 2624-30 0.13 Source of sepsis Abdominal 8(53) NA NA NA Pulmonary 3(20) NA NA NA Other 4(27) NA NA NA APACHEIV,score 7762-90] 4743-54] NA <0.01* APACHE IV,% 2315-49 0.80.3-1.2 NA <0.01* SOFA,admission 9[6-101 4[3-5] NA <0.01* SBP,mmHg 135[128-146] 134123-162 141[131-153] 0.63 DBP,mmHg 7672-78] 78[68-84] 84[82-94] 0.01* sNa,mmol/1* 139137-141 138[137-141] 139[138-141] 0.52 sCreat, umol/1* 75[54-113] 80[72-87】 71[60-83] 0.46 BUN,mmol/1* 8.76.5-10.8] 5[4.3-5.8 5.5[3.8-6.6] <0.01* uNa,mmol/l* 23[10-76] 8257-96 10172-151 <0.01* uCreat,mmol/l* 8.7[5.9-11.7] 2.8[2.4-5.7] 8.9[4.7-12.8] <0.01* FEna,%* 1.5[0.5-6.6] 9.6[7.5-12.4] 6.3[4.3-9.9] 0.01* CRP on admission, mg/l* 132[112-218] NA NA NA Phase angle,°* Men 4.4[4-4.6] 5.2[4.5-5.4] NA 0.11 Women 3.1[2.9-6.4] 5.66[4.8-6.2] NA 0.25 Resistance,Q/m* 238206-267 268 [249-296] NA 0.05 Reactance,Q/m* 18[14-21] 25[22-30] NA <0.01* Use of ACEI/ARB,n (%) 3(20) 4(27) 5(33) 0.71 Use of NSAID、 n(%) ASA excluded 0(0) 1(7) 6(40) <0.01* ASA included 4(27) 13(87) 9(60) <0.01* Data are expressed as median [IQR], unless otherwise stated. P-value < 0.05 is considered statistically significant. Significant values are flagged with *. CABG: Coronaryartery bypass grafting, BMI: Body mass index, APACHE IV: Acute physiology and chronic health evaluation-version 4 (%= predicted mortality), SOFA: Sequentialorgan failure assessment, SBP: Systolic blood pressure,DBP: Diastolic blood pressure, sNa: Serum sodium concentration, sCreat: Serum creatinine concentration, BUN:Blood urea nitrogen / serum urea concentration, uNa: Urine sodium concentration, uCreat: Urine creatinine concentration, ACEI: Angiotensin converting enzymeinhibitor, ARB: Angiotensin II receptor blocker, ASA: acetylsalicylic acid, NA: Not available *Measured around time point of skin biopsies https://doi.org/10.1371/journal.pone.0223100.t004 content, expressed as mmol sodium per mg protein, was 1.6[1-3.1],1.4[0.9-1.8] and 0.9 [0.8-1] (p=0.02) in respectively septic patients, patients after CABG and THA-controls. Sodiumconcentrations in dry weight biopsies were 1 [0.9-1.5],1[0.6-1.4] and 0.7 [0.6-0.8] mmol/mg(p < 0.01) in respectively patients with sepsis, patients after CABG and THA-controls. Sodiumconcentration in biopsies, expressed in mmol per mg wet weight, did not differ betweengroups either. Pearson’s correlation between sodium content per mg of protein and fluid percentage inbiopsies, filtered by group, was 0.5 (p<0.01). This correlation is visualized in Fig 2. No signifi-cant correlations between sNa and cNa or uNa, or between sNa, cNa, uNa and body fluid con-tent as measured with BIA were found. R and Xc, both corrected for length, were lower inpatients with sepsis compared to CABG(p<0.01). These findings indicate higher body fluid ASodium content per mg protein B Fluid content in biopsies Fig 1. A) Sodium and B) water content in skin biopsies.*: P <0.05,**:P<0.01. https://doi.org/10.1371/journal.pone.0223100.g001 levels. FEna was 1.5 [0.5-6.6] % in patients with sepsis, 9.6 [7.5-23.4] % in patients after CABGand 6.3 [4.3-9.9] % in THA-controls (p <0.05 between all groups). As described previously,no adequate total in hospital fluid balances were available. Total ICU-fluid intake and excre-tion (fluid balances) previous to the moment of inclusion were only available for patients with Fig 2. Correlation between sodium content in mmol / mg protein and fluid percentage in biopsies.https://doi.org/10.1371/journal.pone.0223100.g002 sepsis and patients after CABG. Patients in these groups had comparable fluid balances(patients with sepsis 1.3 [0.7-3] L positive, patients after CABG 1.5 [1.4-2] L positive,p=0.87). Skin inflammatory parameters In 4 cases (2 samples from patients with sepsis and 1 sample from both a patient after CABGand a control) not enough tissue was available for staining. In 3 additional cases not enoughmaterial was available for qRT-PCR (2 CABG, 1 control). To asses dermal inflammation weevaluated macrophages by CD68 and T-cells by CD3 staining. Both stainings showed scatteredoccasional macrophages and T-cells in the dermal layer of the skin biopsies. Quantificationrevealed no significant differences in macrophage and T-cell density among the three groups(Fig 3A-3D). We also evaluated the mRNA expression of the CCL2 (MCP1), a potent che-moattractant for monocytes/macrophages, and of VCAM-1, an endothelial adhesion moleculeinvolved in leukocyte recruitment. qRT-PCR analyses showed no differences between patientswith sepsis and THA-controls (Fig 3E and 3F). C-reactive protein (CRP), as a marker of gener-alized inflammation, was only measured in patients with sepsis. CRP on admission was 132[112-218] mg/l and 236 [123-398] mg/l after 24 hours. Furthermore we evaluated lymph vessel density by podoplanin, and lymphangiogenesis byVEGF-C, since lymph vessels have been described to be involved in dermal sodium handling.Staining of podoplanin revealed lymph vessels to be found in the dermal layer of the skin11,)slightly concentrated towards the epidermal/dermal junction. Manual counting of the lymphvessels in all biopsies (expressed per high power field) did not reveal differences among thethree groups (Fig 4A and 4B). qRT-PCR for PDPN and VGEF-C clearly showed both tran-scripts to be increased in the CABG group and even more in the sepsis group, indicatinglymphangiogenesis in both groups (Fig 4C and 4D). Nine patients with sepsis developedhypernatremia (defined as serum sodium concentration of ≥ 143mmol/l). No differences werefound in cutaneous sodium storage, nor in markers of inflammation compared to the patientswho did not develop hypernatremia. Discussion Our findings suggest that a substantial proportion of cutaneous sodium content was osmoti-cally active due to the fact that the observed differences in dermal sodium content were associ-ated with differences in fluid content. In other words: oedema and salt accumulation go handin hand. This hypothesis is supported by 2 findings. Firstly, the amount of sodium expressedas concentration in wet-weight samples did not differ between groups. Secondly, BIA alsorevealed higher body fluid levels in patients with higher sodium and fluid content in their skin.Clearly, this is in line with the current literature. Already in the first century before Christ Cel-cus described edema ("tumor’) as one of the characteristics of inflammation. It is well knownthat both in patients with sepsis and in patients after CABG a systemic inflammatory responseis present [25]. This response is reflected by the high CRP-level in patients with sepsis. Unfor-tunately, no CRP-levels were available for the other study groups. Inflammation in the controlgroup is unlikely because study procedures were to be conducted before start of surgery andno THA was conducted whenever clinical signs of inflammation were present. It is of note thatthis inflammatory process was not reflected in the skin by macrophage or T-lymphocyte influx,nor in upregulation of inflammatory markers. In a recent study skin biopsies of healthy sub-jects were compared with biopsies of patients with chronic kidney disease [15]. In these chron-ically ill patients no changes in non-osmotic sodium storage were found. However, bothinflammation and lymphangiogenesis were present. In our study levels of VCAM and CCL2, A 20pmB CD3 C 20pm Sepsis CABG Control Fig 3. Markers of inflammation with A) representative photo of macrophages, B) quantification of macrophages, C) representativephoto of T-cells, D) quantification of T-cells, E) qRT-PCR of chemokine ligand 2 (CCL2) and F) VCAM. In A and C, macrophages and T-cells are stained in red, nuclei (by DAPI staining) in blue. A BLymph vessels C PDPN ● Sepsis ■ CABG Control Fig 4. Lymph vessels with A) representative photo of lymph vessels in red, B) it's quantification, C) qRT-PCR for podoplanin (PDPN) and D) qRT-PCRfor vascular endothelial growth factor C (VEGF-C).*:P<0.05,**:P<0.01. https://doi.org/10.1371/journal.pone.0223100.g004 both involved in attraction of leucocytes and thereby a marker for inflammation, did not differbetween patients with sepsis and controls [26,27]. The significantly lower levels of CCL2 in theCABG cohort were probably due to the routine administration of dexamethasone in thesepatients. On the other hand upregulation of VEGFC and PDPN, both markers of lymphangio-genesis, was found [17,28]. This upregulation may either be inflammation driven or the (con-comitant) result of oedema formation by the accumulation of water and sodium respectively [18,19]. Lymphangiogenesis in inflammation is controlled by cells from the mononuclearphagocyte system (MPS) [19]. In addition, sodium itself influences lymphangiogenesis via anMPS-mediated, but inflammation-independent, pathway [19]. Data regarding sodium intakein our study population was not available but previous studies showed high sodium intake incritically ill patients [29,30]. A sign of a potential positive sodium balance in patients with sep-sis in this study is a markedly lower FEna in comparison to patients after CABG and THA-controls. However, the correlation coefficient between fluid and sodium content per mg protein (p=0.5, corrected for group division) suggests a moderate relationship, whilst also suggesting thatnot all sodium was osmotically active. If this would have been the case, a stronger correlationcould be expected. Our data are not sufficient to determine why this correlation is this farbelow 1. (Part of) an explanation could be an interindividual difference in nature of sodiumstorage, which would be either osmotically or non-osmotically active. From our data it becomes clear that the developed technique is able to quantify sodiumcontent in small skin biopsies of various patient categories. In each patient category sodiumconcentrations were within narrow limits, despite the absolute low values. In addition, therewas a clear separation between groups. The main limitation of this study is that we did not detect changes in non-osmotic sodiumstorage with this particular method. Another limitation turned out to be the fact that skinbiopsies in patients with sepsis were taken in the early phase of severe disease. The inflamma-tion found in patients with chronic kidney disease suggests that, on a longer term, significantchanges in skin biopsies of critically ill patients might be found. The markedly lower FEna inpatients with sepsis, and the development of hypernatremia in a substantial part of thesepatients, suggest sodium accumulation. Perhaps, during the course of a septic period, changesin cutaneous sodium storage might become visible as well. Conclusions Both sepsis and CABG patients had significantly higher levels of fluid and sodium content incomparison to THA-controls. The modest association between sodium and water accumula-tion leaves space for alternative explanations instead of simply oedema formation. Besides, sys-temic inflammation was not reflected in skin, at least not in the early phase of sepsis. With thecurrent methodology we were able to detect differences in sodium storage in small sample skinbiopsies, applicable in the clinical setting. These data allows for further research on sodiumhandling in critically ill patients, for example with consecutive skin biopsies during the courseof critical illness. Acknowledgments We thank Twan Storteboom for his work on the protocol for measuring sodium and nitrogenin skin biopsies, as well as for the actual execution of these experiments. We also thank MattyKoopmans for her contribution to the protocol and her unrelenting help with statistical issues. Author Contributions Conceptualization: Marjolein van IJzendoorn, Jacob van den Born, Ryanne Hijmans, Han-neke Buter, Gwendolyn Maes, Tsjitske van der Veen, Wierd Zijlstra, Baukje Dijkstra, Ger-jan Navis, Christiaan Boerma. Data curation: Marjolein van IJzendoorn. Formal analysis: Marjolein van IJzendoorn, Jacob van den Born, Gerjan Navis, ChristiaanBoerma. Investigation: Marjolein van IJzendoorn, Rianne Bodde, Wendy Dam, Gwendolyn Maes,Tsjitske van der Veen, Wierd Zijlstra, Baukje Dijkstra, Christiaan Boerma. Methodology: Marjolein van IJzendoorn, Jacob van den Born, Ryanne Hijmans, HannekeButer, Peter Kingma, Gerjan Navis, Christiaan Boerma. Project administration: Marjolein van IJzendoorn, Baukje Dijkstra. Supervision: Jacob van den Born, Hanneke Buter, Wendy Dam, Peter Kingma, Gerjan Navis,Christiaan Boerma. Validation: Marjolein van IJzendoorn, Wendy Dam, Christiaan Boerma. Visualization: Rianne Bodde, Wendy Dam. Writing - original draft: Marjolein van IJzendoorn, Hanneke Buter, Christiaan Boerma. Writing - review & editing: Marjolein van IJzendoorn, Jacob van den Born, Ryanne Hijmans,Rianne Bodde, Wendy Dam, Peter Kingma, Gwendolyn Maes, Tsjitske van der Veen, Wierd Zijlstra, Baukje Dijkstra, Gerjan Navis, Christiaan Boerma. References ( 1. Padtberg J . Uber die Bedeutung der Haut als Chlordepot. Archiv fur experimentelle Pathologie undPharmakologie. 1910;63(1):60-79. ) 2. Garnett ES, Ford J, Golding PL, Mardell RJ, Whyman AE. The mobilizaton of osmotically inactivesodium during total starvation in man. Clin Sci. 1968;35(1):93-103. PMID: 4878417 3. Wahlgren V. Uber die Bedeutung der Gewebe als Chlordepots. Archiv fur experimentelle Pathologieund Pharmakologie. 1909;61(2):97-112. 4. Titze J. Water-free sodium accumulation. Semin Dial.2009;22(3):253-255. https://doi.org/10.1111/j1525-139X.2009.00569.xPMID: 19573004 5. Titze J, Lang R, llies C, Schwind KH, Kirsch KA,Dietsch P, et al. Osmotically inactive skin Na+ storagein rats. Am J Physiol Renal Physiol. 2003;285(6):F1108-1117.https://doi.org/10.1152/ajprenal.00200.2003 PMID: 12888617 6. Titze J, Maillet A, Lang R, Gunga HC, Johannes B, Gauquelin-Koch G, et al. Long-term sodium balancein humans in a terrestrial space station simulation study. Am J Kidney Dis. 2002;40(3):508-516. https://doi.org/10.1053/ajkd.2002.34908 PMID: 12200802 ( 7. Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Muller DN, et al . 23Na magnetic resonance imag-ing-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension. 2013;61(3):635-640. h ttp s:/ / d o i . org / 1 0.1 1 61 / HYPERTENS I O N AHA. 11 1 . 00566 PMID: 23339 1 69 ) ( 8. Reitsma S, Slaaf DW, Vink H, van Zandvoort MAMJ, oude Egbrink MGA. The endothelial glycocalyx:composition, functions, and visualization. Pflugers Arch. 2007;454(3):345-59. http s : // doi. o r g/ 1 0.1007 / s00424 - 007 - 0212 - 8 PMID: 1 7 2 56154 ) ( 9. Mouta C, Heroult M. Inflammatory triggers of l ymphangiogenesis.Lymphat Res Biol.2003;1(3):201- 218. PMID: 15 624 4 38 ) ( 10. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organfailure and guidelines for the use of i nnovative therapies in sepsis. The ACCP/SCCM Consensus Con-ference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest.1992;101(6):1644-1655. h ttps: / / do i . o rg/1 0.1378/ che st . 101 . 6.1644 PMID: 1303622 ) ( 11. Celie JW, Rutjes NW, Keuning ED, Soininen R, Heljasvaara R, Pihlajaniemi T, et al . Subendothelialheparan sulfate proteoglycans become major L-selectin and monocyte chemoattractant protein-1ligands upon renal ischemia/reperfusion. Am J Pathol. 2007;170(6):1865-1878. htt ps : // d o i . o r g /1 0.2353/ajpath.2007.07006 1 PMID: 17525255 ) ( 12. Hijmans RS, Shrestha P, Sarpong KA , Yazdani S, El Masri R, de Jong WHA, et al. High sodium dietconverts renal proteoglycans into pro-inflammatory mediators i n rats. PloS one.2017;12(6):e0178940.Available from h t t ps: // d o i . or g / 10 . 1 371 / journal . po n e.0 1 78940 PMID: 285948 4 9 ) 13. Lindner G, Funk GC. Hypernatremia in critically ill patients. J Crit Care. 2013;28(2):216.e11-20. 14. Linz P, Santoro D, Renz W, Rieger J, Ruehle A, Ruff J, et al. Skin sodium measured with 23Na MRI at7.0 T. NMR Biomed. 2015;28(1)54-62. 15. Hijmans RS, van Londen M, Sarpong KA, Bakker SJL, Navis GJ, Storteboom TTR, et al. Dermal tissueremodelling and non-osmotic sodium storage in kidney patients. J Transl Med. 2019;17:88.Availablefrom https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-019-1815-5.PMID:30885222 16. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third Interna-tional consensus definitions for aepsis and aeptic ahock (Sepsis-3).JAMA. 2016;315(8):801-810.https://doi.org/10.1001/jama.2016.0287 PMID: 26903338 17. Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, et al. Hyperplasia of lymphatic vesselsin VEGF-C transgenic mice. Science.1997;276(5317):1423-1425. https://doi.org/10.1126/science.276.5317.1423 PMID: 9162011 18. Kerjaschki D. The crucial role of macrophages in lymphangiogenesis. J Clin Invest. 2005;115(9):2316-2319. https://doi.org/10.1172/JCI26354 PMID: 16138185 19. Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, et al. Macrophages regulatesalt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffer-ing mechanism. Nat Med. 2009; 15(5):545-552.https://doi.org/10.1038/nm.1960 PMID: 19412173 20. Crescenzi R, Marton A, Donahue PM, Mahany HB, Lants SK, Wang P, et al. Tissue aodium xontent iselevated in the skin and subcutaneous adipose tissue in women with lipedema. obesity. Silver Spring.2018;26(2):310-317. 21. Titze J, Shakibaei M, Schafflhuber M, Schulze-Tanzil G, Porst M, Schwind KH, et al. Glycosaminogly-can polymerization may enable osmotically inactive Na+ storage in the skin. Am J Physiol Heart CircPhysiol. 2004;287(1):H203-208.https://doi.org/10.1152/ajpheart.01237.2003 PMID: 14975935 22. Titze J, Krause H, Hecht H, Dietsch P, Rittweger J, Lang R, et al. Reduced osmotically inactive Na stor-age capacity and hypertension in the Dahl model. Am J Physiol Renal Physiol. 2002;283(1):F134-141.https://doi.org/10.1152/ajprenal.00323.2001 PMID: 12060595 23. Titze J, Bauer K, Schafflhuber M, Dietsch P, Lang R, Schwind KH, et al. Internal sodium balance inDOCA-salt rats: a body composition study. Am J Physiol Renal Physiol. 2005;289(4):F793-802.https://doi.org/10.1152/ajprenal.00096.2005 PMID: 15914779 24. Schafflhuber M, Volpi N, Dahlmann A, Hilgers KF, Maccari F, Dietsch P, et al. Mobilization of osmoti-cally inactive Na+ by growth and by dietary salt restriction in rats. Am J Physiol Renal Physiol. 2007;292(5):F1490-1500. https://doi.org/10.1152/ajprenal.00300.2006 PMID: 17244896 25. Day J, Taylor K. The systemic inflammatory response syndrome and cardiopulmonary bypass. Interna-tional Journal of Surgery.2005;3(2):129-140. https://doi.org/10.1016/j.ijsu.2005.04.002 PMID:17462274 26. Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration.Annu Rev Physiol. 1995;57:827-872. https://doi.org/10.1146/annurev.ph.57.030195.004143 PMID:7778885 27. Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts asaT-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91:3652-3656. https://doi.org/10.1073/pnas.91.9.3652 PMID:8170963 ( 28. Fu J, Gerhardt H, McDaniel JM, Xia B, Liu X, Ivanciu L, et al. Endothelial cell O-glycan deficiencycauses blood/lymphatic misconnections and consequent fatty liver disease in mice. J Clin Invest. 2008;118(11):3725-3737. h t t ps: / / doi . or g/ 1 0. 11 72/ J CI36077 PMID: 1 8924607 ) 29. van IJzendoorn MCO, Buter H, Kingma WP, Navis GJ, Boerma EC. The development of intensive careunit acquired hypernatremia is not explained by sodium overload or water deficit: a retrospective cohortstudy on water balance and sodium handling. Critical care research and practice. 2016;2016:9571583.Available from https://www.hindawi.com/journals/ccrp/2016/9571583/. https://doi.org/10.1155/2016/9571583 PMID:27703807 ( 30. Bihari S, Peake SL, Seppelt I, Williams P, Bersten A, George Institute for Global Health, et al. Sodiumadministration in critically i ll patients in Australia and New Zealand: a multicentre point prevalencestudy. Crit Care Resusc.2013;15(4):294-300. PMID: 24289511 ) PLOS ONE| https://doi.org/journal.pone.October ,
确定







还剩11页未读,是否继续阅读?
中国格哈特为您提供《皮肤中钠储存量检测方案(定氮仪)》,该方案主要用于其他中生化检验检测,参考标准--,《皮肤中钠储存量检测方案(定氮仪)》用到的仪器有格哈特杜马斯定氮仪DT N Pro
推荐专场















